Differential mechanisms between live and heat-killed R. akari in engaging TLR2 and TLR4 to active NF-κB, p38 MAP kinase and induce cytokine expression.
Keywords: innate immunity, Toll-like receptors, signal transduction, cell activation, monocytes/macrophages
Abstract
A better understanding of the pathogenesis of rickettsial disease requires elucidation of mechanisms governing host defense during infection. TLRs are primary sensors of microbial pathogens that activate innate immune cells, as well as initiate and orchestrate adaptive immune responses. However, the role of TLRs in rickettsia recognition and cell activation remains poorly understood. In this study, we examined the involvement of TLR2 and TLR4 in recognition of Rickettsia akari, a causative agent of rickettsialpox. Transfection-based complementation of TLR2/4-negative HEK293T cells with human TLR2 or TLR4 coexpressed with CD14 and MD-2 enabled IκB-α degradation, NF-κB reporter activation, and IL-8 expression in response to heat-killed (HK) R. akari. The presence of the R753Q TLR2 or D299G TLR4 polymorphisms significantly impaired the capacities of the respective TLRs to signal HK R. akari-mediated NF-κB reporter activation in HEK293T transfectants. Blocking Ab against TLR2 or TLR4 markedly inhibited TNF-α release from human monocytes stimulated with HK R. akari, and TNF-α secretion elicited by infection with live R. akari was reduced significantly only upon blocking of TLR2 and TLR4. Live and HK R. akari exerted phosphorylation of IRAK1 and p38 MAPK in 293/TLR4/MD-2 or 293/TLR2 stable cell lines, whereas only live bacteria elicited responses in TLR2/4-negative HEK293T cells. These data demonstrate that HK R. akari triggers cell activation via TLR2 or TLR4 and suggest use of additional TLRs and/or NLRs by live R. akari.
Introduction
Macrophages, DCs, and neutrophils sense invading microbial pathogens by nonclonally distributed PRRs to activate innate host defense mechanisms rapidly and to promote and orchestrate adaptive immune responses [1–3]. TLRs are principal membrane-associated innate sensors that recognize conserved PAMPs at the cell surface (TLR2, TLR4, TLR5, TLR11) or in the intracellular endosomal compartment (TLR3, TLR7–9) [1, 4]. TLR4, the predominant signal-transducing receptor for Gram-negative bacterial LPS [5], is critical for host defense against Gram-negative bacterial pathogens, as has been demonstrated in studies with TLR4-deficient mice [6]. In addition to LPS, TLR4 recognizes other structurally unrelated microbial PAMPs, including the F protein of RSV [7], chlamydial heat shock proteins 60 and 70 [8], and pneumolysin [9]. MD-2, an extracellular protein, is essential for conferring LPS sensitivity to TLR4 [10], and CD14 enhances LPS responses by facilitating LPS binding to MD-2, enables MyD88-independent signaling pathways by LPS, and enhances TLR responses to other microbial PAMPs, e.g., lipoarabinomannan [11, 12]. TLR2 recognizes lipoproteins and lipopeptides from Gram-positive bacteria, mycoplasma, and mycobacteria [13] in cooperation with TLR1 (triacylated lipopeptides) or TLR6 (diacylated lipopeptides) [1, 14, 15]. TLR5 is the main membrane-associated sensor of bacterial flagellin [16], whereas endosomal TLR3 and TLR7/8 detect double-stranded and single-stranded, viral RNA, respectively [17–19]. TLR9 responds to unmethylated CpG motifs present in bacterial and viral DNA and recognizes fungal pathogens Candida albicans and Aspergillus fumigatus [4, 20–22]. Microbial recognition leads to TLR dimerization and conformation changes, creating docking platforms within their TIR intracellular domains to enable recruitment of adapters and kinases that mediate transcription factor activation and expression of inflammatory and costimulatory molecules [23].
In humans, the D299G and T399I SNPs in the ectodomain of TLR4 have been associated with decreased LPS responsiveness in primary airway epithelial cells and alveolar macrophages [24]. The D299G SNP has also been linked to increased incidence of Gram-negative bacterial and RSV infections, tuberculosis, Crohn's disease, and predisposition to septic shock [25–29]. SNPs within TLR2 have been associated with an increased incidence of certain infectious diseases, such as tuberculosis, and decreased capacity of TLR2 to signal cell activation [30–33]. The R753Q TLR2 mutation was reported to determine susceptibility to staphylococcal infections, and cells obtained from individuals with this TLR2 mutation showed diminished responses to bacterial lipopeptides obtained from Borellia burgdorferi and Treponema pallidum [34]. These results suggest that TLR2 and TLR4 polymorphisms are associated with susceptibility to infectious diseases.
Rickettsia akari is an intracellular bacterium that is transmitted to humans by mites causing rickettsialpox [35]. The mechanisms regulating cell activation during R. akari infection are poorly defined. LPS-unresponsive C3H/HeJ mice expressing the P712H point mutation that renders TLR4 nonfunctional are the most susceptible to R. akari among the 24 mouse strains studied [36]. The D299G TLR4 SNP was linked to Mediterranean spotted fever caused by Rickettsia conorii [37], suggesting TLR4 as an important sensor of rickettsia. However, it is unknown to what extent TLR4 recognition of R. akari is affected by TLR4 SNPs and whether TLR4 is the sole sensor. This study was undertaken to determine involvement of TLR2 and TLR4 in recognition and cell activation in response to R. akari. Transfection-based complementation of TLR2/4-negative HEK293T cells with WT or mutant TLR2 or TLR4 variants, combined with Ab-mediated blocking of TLR2 or TLR4 in human monocytes, has revealed that TLR2 and TLR4 are important sensors of R. akari. Our data demonstrate that live R. akari also uses TLR2/4-independent mechanisms of cell activation, suggesting the use of additional TLRs or cytosolic PRRs.
MATERIALS AND METHODS
Reagents and cell culture
Ab against IκB-α, β-actin, tubulin, and IRAK1 were from Santa Cruz Biotechnology (Santa Cruz, CA, USA); anti-p-p38, anti-p-IRAK1, and anti-p38 Ab were purchased from Cell Signaling (Danvers, MA, USA); and anti-TLR2 and anti-TLR4 Ab TL2.1 and HTA125 were from eBioscience (San Diego, CA, USA). SuperFect transfection reagent and EndoFree plasmid purification kits were from Qiagen (Valencia, CA, USA). Ultrapure Escherichia coli 0111:B4 LPS and Pam3Cys were obtained from Invivogen (San Diego, CA, USA), and African green monkey kidney (Vero) cells were obtained from American Type Culture Collection (Manassas, VA, USA). R. akari (the MK strain) was obtained as a clinical isolate from a human patient and was propagated in African green monkey kidney cells (Vero) at respective concentrations of 106 PFU/ml as described previously [38]. After growing for 4 days at 35°C in the gas atmosphere of 5% CO2, cells were scraped off, centrifuged, resuspended in PBS, and subjected to 5 cycles of freezing-thawing for lysis, followed by differential centrifugation (1000 g for 10 min) to remove the cell debris. The supernatant containing R. akari was then centrifuged for 20 min at 15,000 g at 4°C. The pellet containing 109 PFU (determined by Vero cell infection and limiting dilution plaque formation, as described [38]) was resuspended in PBS and used for preparation of HK bacteria or for cell infection with live R. akari. R. akari were boiled for 10 min at 100°C and used as a source of HK bacteria. HEK293T cells were described previously [7, 39–43], and HEK293 cell lines stably transfected with YFP huTLR4 and Flag-tagged huMD-2 (293/TLR4/MD-2) or huYFP-TLR2 (293/TLR2) were kindly provided by Dr. Douglas T. Golenbock (University of Massachusetts Medical School, Worchester, MA, USA). Cells were cultured in DMEM (Mediatech, Inc., Manassas, VA, USA), supplemented with 2 mM L-glutamine, 10% FBS (HyClone, Logan, UT, USA), 100 U/ml penicillin, and 100 μg/ml streptomycin (Invitrogen, San Diego, CA, USA; complete DMEM) in the absence (HEK293T) or presence (293/TLR4/MD-2 and 293/TLR2) of G418 (0.5 mg/ml, Sigma Chemical Co., St. Louis, MO, USA). Human monocytes were isolated from whole blood by counter-flow centrifugal elutriation from PBMCs that were obtained by leukapheresis of blood from healthy human volunteers at the Department of Transfusion Medicine, NIH (Bethesda, MD, USA), as described previously [42–44]. Monocytes were cultured in RPMI-1640 medium (Mediatech, Inc.), supplemented with 2 mM L-glutamine, 100 u/ml penicillin, 100 μg/ml streptomycin (Invitrogen), 5 × 10−5 M β-ME (Sigma Chemical Co.), and 5% FBS (HyClone). Studies with human cells were approved by the Institutional Review Board, University of Maryland (Baltimore, MD, USA).
Recombinant plasmids and transient transfection
Expression vectors pCDNA3-huYFP-TLR4, pCDNA3-huYFP-TLR2, pCDNA3-huCD14, pEFBOS-HA-huMD-2, pELAM-luciferase, and pCMV-β-galactosidase were described previously [41–43] and obtained from Dr. Golenbock (University of Massachusetts Medical School). Point mutations D299G and T399I in huTLR4 protein were created by site-directed mutagenesis of pCDNA3-YFP-TLR4 using a QuikChange mutagenesis kit (Stratagene, La Jolla, CA, USA), according to the manufacturer's instructions. The following primer pairs were used: D299G, nucleotide change, A896 → G: forward primer, 5′-ttagactactacctcgatggtattattgacttattt-3′, reverse primer, 5′-aaataagtcaataataccatcgaggtagtagtctaa-3′; T399I, nucleotide change, C1196 → T: forward primer, 5′-ctgttctcaaagtgattttgggacaatcagcctaaagtatttagatctgagc-3′, reverse primer: 5′-gctcagatctaaatactttaggctgattgtcccaaaatcactttgagaacag-3′. The point mutation R753Q in human TLR2 protein was introduced into pCDNA3-YFP-TLR2 by site-directed mutagenesis using the following primers: 5′-gcgcttctgcaagctgcagaagataatgaacacca-3′, forward, and 5′-tggtgttcattatcttctgcagcttgcagaagcgc-3′, reverse. Nucleotide sequencing (Biopolymer/Genomic Core Facility, University of Maryland, Baltimore) of each construct was carried out using Big Dye Terminator Cycle Sequencing Kit, v. 3.0 (Applied Biosystems, Foster City, CA, USA), according to the manufacturer's protocol, to verify the presence of the introduced SNPs and the absence of random mutations as a result of PCR errors. For transient transfections, HEK293T cells were cultured overnight in 6-well plates (2×106 cells/well, protein and RNA studies) or 24-well plates (2×106 cells/well, for reporter assays) and cotransfected for 3 h with expression vectors as described in the figure legends using Superfect transfection reagent as described [42, 43]. Cellular extracts were prepared as reported previously [43] and used for immunoblotting, and reporter assays were conducted as described below.
Luciferase reporter assays
For NF-κB reporter assays, HEK293T, HEK293T transient transfectants, or 293/TLR4/MD-2 and 293/TLR2 stable transfectans were plated in 24-well plates (2×105 cells/well) and grown overnight. For transient transfections, HEK293T cells were cotransfected using Superfect (Qiagen) with pCDNA3-YFP-TLR4 encoding WT [45] or D299G variants (50 ng/well each), pCDNA3-CD14 (20 ng/well), pEFBOS-MD-2 (2 ng/well), pELAM-luciferase (200 ng/well), and pCMV-β-galactosidase (50 ng/well), and total plasmid DNA amount was adjusted to 1 μg/well with pCDNA3. Overexpression of WT or mutant (R753Q) TLR2 species was achieved by transient transfection as described for TLR4, replacing TLR4-encoding plasmids with pCDNA3-YFP-TLR2 vectors encoding WT or R753Q variants. Following transfections, cells were recovered for 24 h, treated as indicated in figure legends, and lysed in a passive lysis buffer (Promega, Madison, WI, USA), and firefly luciferase versus β-galactosidase activities were measured with a luciferase reporter assay system (Promega) and β-galactosidase assay kit (Galacto-Light system, Tropix Inc., Bedford, MA, USA) on a LB9507 luminometer (Berthold Technologies, Bad Wildbad, Germany).
Immunoblotting
Cell extracts were prepared using a lysis buffer containing 20 mM Tris-HCl (pH 7.4), 1% Triton X-100, 150 mM NaCl, 12.5 mM β-glycerophosphate, 50 mM sodium fluoride, 1 mM DTT, 1 mM sodium orthovanadate, 2 mM EDTA, 1 mM PMSF, and protease inhibitor cocktail (Roche, Indianapolis, IN, USA). Protein concentration was determined using a protein assay kit (Bio-Rad, Hercules, CA, USA), and samples were resuspended in Laemmli buffer (Bio-Rad) and loaded (20 μg/lane) onto 4–20% polyacrylamide minigels (Invitrogen). Proteins were separated by electrophoresis and electrotransferred to Immobilon-P membranes (Millipore, Bedford, MA, USA). Membranes were blocked and probed with corresponding Ab as described previously [42, 43].
Isolation of RNA, PCR, and real-time PCR
Total RNA was isolated with RNeasy kits (Qiagen), followed by DNase digestion and repurification, as recommended by the manufacturer. cDNA was prepared from 1 μg RNA using a RT system (Promega). To determine R. akari loads, RNA, prepared from cells infected with bacteria, was subjected to RT-PCR to amplify R. akari-specific 16S RNA as reported [36], and relative quantities of human β-actin mRNA (a housekeeping gene) were also analyzed [44]. The following primers were used: R. akari 16S RNA forward, 5′-GTTCGGAATTACTGGGCGTA-3′, R. akari 16S RNA reverse, 5′-AATTAAACCGCATGCTCCAC-3′; β-actin forward, 5′-GATGATATCGCCGCGCTCGT-3′, β-actin reverse, 5′-GTAGATGGGCACAGTGTGGGTG-3′. The following PCR procedure was used for both sets of primers: 95°C, 5 min (denaturing); 30 amplification cycles of 95°C for 30 s, 56°C for 30 s, and 72°C for 2 min; and the final cycle of 72°C for 10 min. To analyze cytokine gene expression, cDNA was subjected to quantitative real-time PCR analysis on a MyIQ real-time PCR system (Bio-Rad). The following primers were used: huHPRT, forward, 5′-ACCAGTCAACAGGGGACATAAAAG-3′, reverse, 5′-GTCTGCATTGTTTTGCCAGTGTC-3′; huIL-8, forward, 5′-CACCGGAAGGAACCATCTCACT-3′, reverse, 5′-TGCACCTTCACACAGAGCTGC-3′. Data were processed using the 2–ΔΔcomparative threshold method [46]. Purified and sequenced PCR products were used as standards and positive controls, and melting curve analysis was used additionally to ensure the specificity of PCR amplification.
Analysis of cytokine secretion by ELISA
Levels of TNF-α and IL-8 in supernatants were determined by ELISA in the University of Maryland, Baltimore, Cytokine Core Laboratory using commercial Ab and standards obtained from R&D Systems (Minneapolis, MN, USA; TNF-α) and Biosource (Camarillo, CA, USA; IL-8), respectively, with the lower detection limit of 3.9 pg/ml.
Statistical analysis
Statistical analysis was performed using the GraphPad Prism 5 program for Windows (GraphPad Software, San Diego, CA, USA). Statistical differences among experimental groups were evaluated by a one-way ANOVA with repeated measures, followed by post hoc comparisons with Tukey's multiple paired comparison test. Values are expressed as mean ± sd.
RESULTS
Overexpression of TLR2 or TLR4 imparts NF-κB activation in HEK293 cells in response to stimulation with HK R. akari
To examine whether TLR2 and/or TLR4 are involved in cell activation elicited by HK R. akari, we first assessed activation of the transcription factor NF-κB, a principal transcription regulator of a variety of genes encoding cytokines, chemokines, adhesion, and costimulatory molecules. To this end, we first measured protein levels of IκB-α, an inhibitor sequestering NF-κB dimers in the cytoplasm of unstimulated cells that undergoes receptor-triggered proteasomal degradation, allowing NF-κB to translocate into the nucleus and to bind κB sites in promoter elements, initiating gene expression. TLR2/4-negative HEK293T cells or HEK293 cells stably expressing TLR2 (293/TLR2) or the TLR4/MD-2 cassette (293/TLR4/MD-2) were treated with medium or stimulated with HK R. akari for 15 and 30 min. As controls for TLR specificity, cells were stimulated with TLR1/2 and TLR4 agonists, Pam3Cys, and LPS, respectively. Immunoblot analyses of whole cell lysates demonstrate prominent IκB-α degradation in response to stimulation with HK R. akari in 293/TLR2 and 293/TLR4/MD-2 cells, whereas no degradation was observed in untransfected HEK293T cells (Fig. 1, top panel). In line with the literature, LPS caused IκB-α degradation only in 293/TLR4/MD-2 cells but not in 293/TLR2 or HEK293T cells, whereas Pam3Cys elicited the response only in 293/TLR2 cells (Fig. 1, 2nd and 3rd panels from top). The differences in IκB-α levels were not a result of variations in protein loading, as comparable levels of β-actin were observed in all samples analyzed (Fig. 1, bottom panel, and data not shown).
Figure 1. Overexpression of TLR2 or TLR4 in HEK293 cells confers IκB-α degradation in response to HK R. akari.
HEK293T cells and 293/TLR2 or 293/TLR4/MD-2 stable transfectants were treated for the indicated time periods with medium (0 time-point), 1 μg/ml Pam3Cys, 100 ng/ml LPS, or HK R. akari (prepared based on bacteria equivalent used for heat inactivation/HEK cell ratio 50:1). Cell extracts were prepared and analyzed by immunoblotting with Ab against IκB-α or β-actin to determine IκB-α degradation and protein loading. Shown are results of a representative (n=3) experiment.
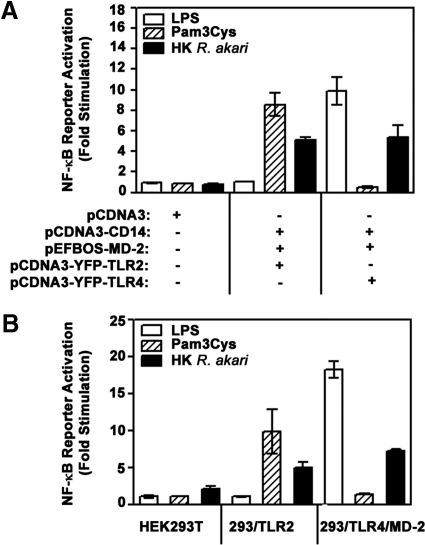
To confirm that HK R. akari-inducible, TLR-mediated degradation of IκB-α is linked to increased transactivation potential of NF-κB, we studied activation of NF-κB-dependent reporter pELAM-luciferase that results in the expression of a luciferase reporter gene. HEK293T cells were transiently transfected with a control vector (pCDNA3) or complemented with YFP-TLR2 or YFP-TLR4 in combination with MD-2 and CD14 following transfection of the corresponding plasmids and with pELAM-luciferase together with pCMV-β-galactosidase (used for normalization; Fig. 2A). As a second approach, we compared activation of the NF-κB-dependent pELAM-luciferase reporter transfected in HEK293T cells or stable cell lines 293/TLR2 or 293/TLR4/MD-2 (Fig. 2B). Cells were treated for 5 h with medium, HK R. akari, or established TLR2 and TLR4 agonists, Pam3Cys and LPS, to confirm the specificity of TLR stimulation. Activation of the NF-κB reporter, pELAM-luciferase, was measured based on expression of firefly luciferase, normalized to that of galactosidase, and fold stimulation was calculated by dividing values in cells incubated with stimuli by those detected in medium-treated cultures. As shown in Fig. 2A, HK R. akari induced 5.6- to 5.9-fold activation of pELAM-luciferase in HEK293T cells overexpressing TLR2 or TLR4 along with CD14 and MD-2, whereas no stimulation (fold activation=1) was seen in cells transfected with pCDNA3 only. In accordance with previous results reported by us and others [39, 41, 47], cell transfection with pCDNA3-CD14 or pEFBOS-MD-2 without addition of TLR2/4-encoding vectors failed to elicit NF-κB reporter activation (data not shown). As shown in Fig. 2B, we observed 4.9- and 7.2-fold pELAM-luciferase induction by HK R. akari in 293/TLR2 and 293/TLR4/MD-2 stable cell lines, whereas only twofold induction was seen in HEK293T cells. As expected, LPS stimulated activation of pELAM-luciferase reporter only in HEK293 cells overexpressing TLR4 but not in cells expressing TLR2 or transfected with pCDNA3 only (Fig. 2A and B). Fig. 2 also shows that only TLR2-expressing HEK293 cells showed NF-κB reporter activation in response to stimulation with Pam3Cys, and no induction was detected in TLR4-expressing cells or HEK293T cells transfected with pCDNA3 only or expressing CD14/MD-2 without TLRs (data not shown). Taken collectively, these results indicate that introduction of TLR2 or TLR4 by transfection into TLR2/4-deficient HEK293T cells enables NF-κB activation in response to stimulation with HK R. akari.
Figure 2. Overexpression of TLR2 or TLR4 enables NF-κB reporter activation in HEK cells stimulated with HK R. akari.
(A) HEK293T cells were transiently transfected with empty vector (pCDNA3) or combinations of pCDNA3-CD14 (20 ng/well), pEFBOS-HA-MD-2 (2 ng/well), and pCDNA3-YFP-TLR4 (50 ng/well) encoding WT, D299G, or T399I TLR4 species, as indicated. Total level of plasmid DNA was adjusted to 1 μg/well with empty (pCDNA3) vector. (B). HEK293T cells or 293/TLR2 or 293/TLR4/MD-2 stable transfectants were plated into 24-well plates and grown for 20 h. (A and B) Cells were cotransfected with pELAM-luciferase (200 ng/well) and pCMV-β-galactosidase (50 ng/well), recovered for 24 h, and treated for 5 h with medium, 1 μg/ml Pam3Cys, 100 ng/ml LPS, or HK R. akari (prepared based on bacteria equivalent used for heat inactivation/HEK cell ratio 50:1). Cell lysates were prepared and assayed for firefly luciferase versus β-galactosidase activities, and results were expressed as fold stimulation calculated by dividing firefly luciferase/β-galactosidase observed in wells with stimuli by those detected in medium-alone wells. Shown are data (mean±sd) of a representative (n=3) experiment.
Overexpression of TLR2 or TLR4 in HEK293T cells enables IL-8 gene expression and secretion in response to stimulation with HK R. akari
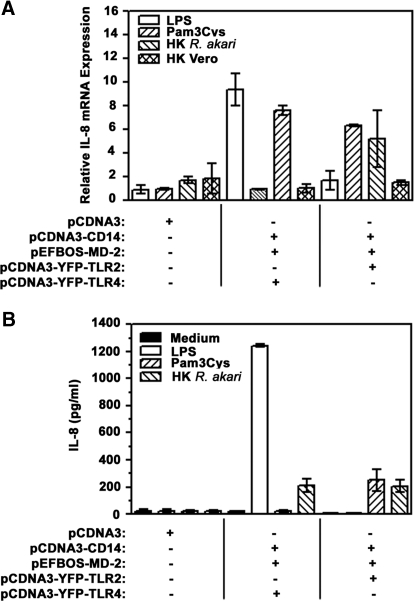
In view of importance of NF-κB in mediating gene expression [4], we next asked whether TLR-mediated activation of this transcription factor translates into induction of cytokine mRNA and protein expression. As IL-8 is the most inducible cytokine expressed by HEK293 cells [7, 13, 41], we compared IL-8 mRNA expression and secretion in HEK293T cells transiently transfected with a pCDNA3 empty vector (negative control) versus cells expressing TLR2 or TLR4, along with coreceptors CD14 and MD-2, upon cell stimulation with HK R. akari. Similar to NF-κB reporter studies described above, we used Pam3Cys and LPS, known TLR1/2 and TLR4 agonists, to control for the specificity of cell activation via corresponding TLRs. Real-time PCR analyses demonstrated that HK R. akari caused 7.6 and 5.2-fold induction of IL-8 gene expression in HEK293T/CD14/MD-2 cells coexpressing TLR4 and TLR2, respectively, whereas no response was observed in HEK293T cells transfected with pCDNA3 only (Fig. 3A). Of note, exposure of either cell line to HK control, uninfected Vero cells (infected Vero cells were used to generate R. akari preparations), failed to activate IL-8 mRNA (Fig. 3A). Similar to IL-8 mRNA data, only HEK293T/CD14/MD-2 cells expressing TLR2 or TLR4 responded to exposure to HK R. akari by increases in secreted IL-8 levels from 22 pg/ml (unstimulated cells incubated with medium) to 220 pg/ml and 206 pg/ml, respectively, and pCDNA3-transfected HEK293T cells did not respond to any stimuli (Fig. 3B). As expected, stimulation of HEK293T/CD14/MD-2 cells with LPS resulted in 9-fold up-regulation of IL-8 mRNA and increased levels of secreted IL-8 from 22 pg/ml to 1242 pg/ml, whereas no responses were observed in TLR2-expressing cells or cells transfected with pCDNA3 vector only (Fig. 3A and B). Reciprocally, Pam3Cys caused a 6-fold increase in IL-8 mRNA levels and enhanced production of IL-8 from 22 pg/ml to 282 pg/ml only in HEK293 cells expressing TLR2 and exhibiting no activity in TLR4-expressing cells or cells transfected with pCDNA3 only (Fig. 3A and B). These results show acquisition of sensitivity of HEK293T cells to HK R. akari upon their complementation with transfected TLR2 or TLR4, along with CD14 and MD-2.
Figure 3. Transfection-based complementation of HEK293T cells with TLR2 or TLR4 elicits IL-8 gene expression and secretion upon activation with HK R. akari.
HEK293T cells were plated in 6-well plates (3×106 cells/well), grown overnight, and transiently transfected with pCDNA3, alone or in combination with pCDNA3-CD14 (200 ng/well), pEFBOS-MD-2 (20 ng/well), and pCDNA3-YFP-TLR2 or pCDNA3-YFP-TLR4 (500 ng/well each). After recovery for 20 h, cells were treated for 3 h (A) or 24 h (B) with medium, 1 μg/ml Pam3Cys, 100 ng/ml LPS, HK R. akari (prepared based on bacteria equivalent used for heat inactivation/HEK cell ratio 50:1), or HK lysates of Vero cells (used in equivalent amounts and processed identically compared with lysates of Vero cells infected with R. akari). (A) RNA was isolated and converted into cDNA, and samples were subjected to real-time PCR analyses of HPRT and IL-8 mRNA expression using gene-specific primers. Shown are data of a representative (n=4) experiment. (B) Amounts of secreted IL-8 were analyzed in cell-free supernatants by ELISA. Shown are results (mean±sd) of a representative (n=3) experiment.
D299G TLR4 and R753Q TLR2 mutations impair the ability of TLR2 and TLR4 to mediate NF-κB reporter activation in response to HK R. akari
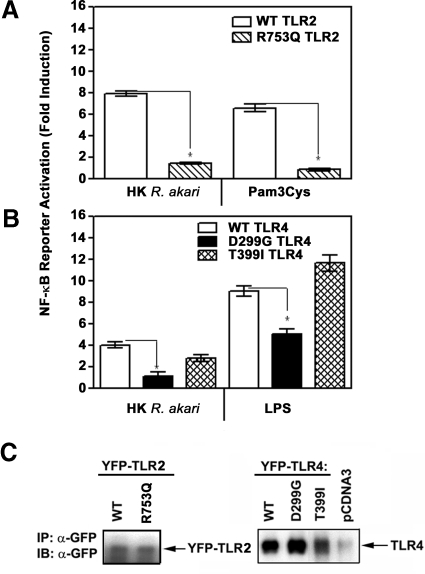
To further confirm use of TLR2 and TLR4 by R. akari for cell activation, we took advantage of known mutations present in these TLRs, the D299G, T399I TLR4, and R753Q TLR2 SNPs, that have been associated with impaired receptor signaling and predisposition to a number of infectious diseases [24–28, 31, 33, 37]. These mutations were introduced into expression vectors encoding WT TLR2 or TLR4 by site-directed mutagenesis, and their presence was confirmed by sequencing. Subsequently, the effect of the R753Q TLR2 and D299G TLR4 mutations on TLR-mediated activation of NF-κB reporter in response to stimulation with HK R. akari was examined. To this end, TLR2/4-negative HEK293T cells were transfected with expression plasmids encoding WT or mutant versions of TLR2 or TLR4, along with vectors encoding CD14 and MD-2, as well as with the NF-κB reporter pELAM-luciferase and pCMV-β-galactosidase (used for normalization). After recovery, cells were treated for 5 h with medium or incubated with HK R. akari, LPS, or Pam3Cys, and NF-κB reporter activation was measured in cell extracts relative to β-galactosidase expression. Overexpression of WT TLR2 and TLR4 in HEK293T resulted in ∼6.7- and 8.5-fold induction of the NF-κB reporter in response to Pam3Cys and LPS, respectively, and rendered cells sensitive to HK R. akari, as evidenced by ∼8- and 4-fold activation of pELAM-luciferase (Fig. 4A and B). The presence of the R753Q mutation resulted in 70–85% inhibition of pELAM-luciferase activation mediated by HK R. akari and Pam3Cys (Fig. 4A), and the D299G SNP led to 49–67% decreases in induction of the NF-κB reporter by LPS and HK R. akari, respectively (Fig. 4B). In contrast, comparable pELAM-luciferase reporter activation was induced by HK R. akari or LPS in cells expressing WT TLR4 or the T399I TLR4 (Fig. 4B). Comparable levels of WT or mutant YFP-TLR2 and -TLR4 were detected by immunoblot analyses of cell extracts with anti-GFP Ab that detect the YFP tag [40] (Fig. 4C). Thus, impaired capacities of the R753Q TLR2 and D299G TLR4 to elicit NF-κB reporter activation could not be attributed to their lower expression levels. These data indicate that the R753Q TLR2 and D299G TLR4 mutations significantly impair the capacity of the respective TLRs to mediate NF-κB activation in response to HK R. akari.
Figure 4. The D299G TLR4 and R753Q TLR2 mutations inhibit the capacities of TLR2 and TLR4 to signal NF-κB-reported activation elicited by stimulation of HEK transfectants with HK R. akari.
HEK293T cells were transfected with pCDNA3-CD14, pEFBOS-MD-2, pELAM-luciferase, pCMV-β-galactosidase, and pCDNA3-YFP-TLR2 encoding WT or R753Q species (A) or pCDNA3-YFP-TLR4 encoding WT, D299G, or T399I variants (B), as described in Fig. 2. Cells were treated for 5 h with medium, 100 ng/ml LPS, 1 μg/ml Pam3Cys, and HK R. akari (prepared based on bacteria equivalent used for heat inactivation/HEK cell ratio 50:1). Firefly versus β-galactosidase activities were measured in cell lysates, and data were expressed as described in Fig. 2. *P < 0.05. (C) Cell lysates from cells transfected with expression vectors encoding WT or mutant YFP-TLR2, YFP-TLR4, or control pCDNA3 plasmid were subjected to immunoblot (IB) analyses with anti-GFP Ab. The data (mean±sd) of a representative (n=3) experiment are depicted. IP, Immunoprecipitation.
Blocking Ab against TLR2 or TLR4 inhibit TNF-α secretion by human monocytes stimulated with HK R. akari
In our next series of experiments, we extended our data about involvement of TLR2 and TLR4 in cell activation by HK R. akari from the HEK293 overexpression system that uses forced TLR overexpression to human monocytes. In doing so, we used Ab against TLR2 (TL2.1) and TLR4 (HTA125) that inhibit secretion of cytokines by monocytes treated with the respective TLR agonists [39]. The presence of anti-TLR2 Ab led to ∼63% reduction of TNF-α secretion in response to Pam3Cys compared with cytokine levels detected in Pam3Cys-stimulated monocytes treated with anti-TLR4 or isotype control Ab, while not affecting the LPS response (Fig. 5A). Reciprocally, anti-TLR4 Ab reduced LPS-mediated TNF-α release by 49% compared with LPS-induced cytokine levels observed in cultures treated with anti-TLR2 Ab or isotype control Ab (Fig. 5A). Stimulation of monocytes for 24 h with HK R. akari increased TNF-α secretion by ∼11-fold, addition of anti-TLR2 or anti-TLR4 Ab decreased this response by 37–48%, and their simultaneous addition led to 58% inhibition of TNF-α release (Fig. 5A). These results indicate that Ab-mediated blocking of TLR2 or TLR4 inhibits TNF-α production by human monocytes stimulated with HK R. akari.
Figure 5. The effect of blocking anti-TLR2 and anti-TLR4 Ab on secretion of TNF-α by human monocytes stimulated with LPS, Pam3Cys, or R. akari.
Human monocytes were pretreated for 30 min on ice with isotype control Ab or Ab against TLR2 (TL2.1), TLR4 (HTA125), or both (10 μg/ml each). (A) Cells were incubated for 24 h with medium, 100 ng/ml LPS, 1 μg/ml Pam3Cys, or HK R. akari (prepared based on bacteria equivalent used for heat inactivation/HEK cell ratio 50:1). (B) Monocytes were incubated for 24 h with medium, 10 ng/ml LPS, and 100 ng/ml Pam3Cys or infected with live R. akari at MOI 10 and 50. Cell-free supernatants were collected and used for measurements of secreted TNF-α levels by ELISA. *P < 0.05. (C) RNA was prepared from R. akari-infected monocytes (triplicate samples for each treatment), reverse-transcribed, and subjected to PCR analysis with primers specific for 16S R. akari RNA or β-actin mRNA. Shown are results of a representative experiment.
Infection with live R. akari results in cell activation via TLR2/4-dependent and independent mechanisms
To examine whether live R. akari signals cell activation via TLR2 or TLR4, we first studied the effect of pretreatment of human monocytes with blocking Ab against TLR2 and TLR4 on TNF-α release triggered by infection with live R. akari. Addition of anti-TLR2 Ab did not affect TNF-α production elicited by live R. akari regardless of MOI used, as compared with bacteria-infected cells exposed to isotype control Ab (Fig. 5B). Anti-TLR4 Ab did not cause statistically significant changes in the TNF-α response evoked by live R. akari infection at MOI 50, while yielding 24% down-regulation of the cytokine response triggered by live bacteria at MOI 10 (Fig. 5B). Of note, the presence of anti-TLR4 Ab led to 48% inhibition of TNF-α release upon stimulation of monocytes with the dose of HK R. akari equivalent to MOI 50 (Fig. 5A). Simultaneous addition of Ab against both receptors resulted in 36.5% inhibition of TNF-α secretion elicited by live R. akari (MOI 10), which is lower compared with the inhibitory effect of these Ab on TNF-α release induced by HK bacteria used at the 5-times higher dose equivalent (58%; Fig. 5A and B). PCR analyses showed similar expression levels of R. akari 16S RNA in groups of bacteria-infected cells treated with different Ab and equal expression of β-actin mRNA (Fig. 5C), revealing comparable bacterial loads and sample loading.
As IRAK1 and p38 kinases are essential in induction of TLR signaling cascades [1, 48, 49], we next compared activation of IRAK1 and p38 kinases in TLR2/4-negative HEK293T cells or HEK293 cells stably expressing TLR2 or TLR4/MD-2 after coincubation with live R. akari for 2 h. Activation of IRAK1 and p38 was judged by their phosphorylation, which is a prerequisite for induction of kinase activities of IRAK1 and p38 [1, 48–50]. Immunoblot analyses showed the presence of phosphorylated IRAK1 and p38 species in whole cell lysates obtained from HEK293T, 293/TLR2, and 293/TLR4/MD-2 cells upon infection with live R. akari but not in medium-treated cells, and a higher degree of phosphorylation was observed in cells expressing TLR2 or TLR4 (Fig. 6A, top and middle panels). The noted differences were not a result of variations in protein loading, as similar levels of tubulin were detected in all samples analyzed (Fig. 6A, bottom panel). Comparable expression levels of R. akari 16S RNA were seen in infected HEK293T cells and 293/TLR2 and 293/TLR4/MD-2 transfectants (Fig. 6B), indicating that the noted differences were not a result of variations in the bacterial loads. These data show that whereas live R. akari can signal IRAK1 and p38 activation via TLR2/4-independent mechanisms, overexpression of TLR2 or TLR4 enhances R. akari-mediated responses. These data indicate that live R. akari induces signaling by triggering TLR2/4-independent mechanisms.
Figure 6. The effect of infection of HEK293T, 293/TLR2, and 293/TLR4/MD-2 cells with live R. akari on activation of IRAK1 and p38.
HEK293T cells and 293/TLR2 and 293/TLR4/MD-2 stable transfectants were incubated for 2 h with medium or infected with R. akari at MOI = 50. (A) Cell lysates were prepared and subjected to immunoblot analyses with Ab against p-IRAK1 and p-p38 to analyze activation of these kinases based on their phosphorylation, and anti-tubulin Ab was used to control for protein loading. (B) Bacterial loads were estimated based on PCR analysis of 16S R. akari RNA in samples of infected cells. No RT indicates PCR reaction on the RNA sample isolated from R. akari-infected HEK293T cells in the absence of RT. Shown are data of a representative experiment.
DISCUSSION
Elucidation of mechanisms by which rickettsia activate innate immune cells is important for rational drug design and development of new therapeutic approaches to treat rickettsial infections, including Rocky Mountain spotted fever, Mediterranian spotted fever, and typhus. Previous studies showed that C3H/HeJ mice expressing nonfunctional P712H TLR4 develop fatal R. conorii infection upon inoculation with the dose of R. conorii that was well controlled and nonfatal in TLR4-competent C3H/HeN mice [36]. The development of R. conorii infection in C3H/HeJ mice was associated with higher bacterial burdens, impaired production of proinflammatory cytokines, and decreased protective Th1 responses [36]. The lack of functional TLR4 led to a decreased proportion of activated NK cells in the spleen of R. conorii-infected mice and impaired secretion of IFN-γ and NK cytotoxicity and abolished the capacity of rickettsia-infected DCs to activate NK cells upon their adoptive transfer into TLR4-competent mice [51]. Furthermore, the prevalence in expression of the D299G TLR4 SNP was found in patients with Mediterranean spotted fever caused by R. conorii [37], suggesting TLR4 as an important sensor of rickettsiae. However, studies in patients with African tick bite fever caused by Rickettsia africae revealed TLR2 as the major receptor mediating activation of platelets, and the R. africae-mediated procoagulant effects and sCD40 expression in endothelial cells were TLR4-dependent [52]. Thus, the use of TLR-dependent and independent mechanisms by rickettsiae remains poorly understood.
Macrophages are important in host defense against intracellular microbial pathogens, including rickettsia, through bacteria phagocytosis, destruction of intracellular bacteria via the production of reactive oxygen and reactive nitrogen intermediates, and cytokine secretion [53]. R. akari targets macrophages and endothelial cells, where proliferation of rickettsia contributes to vascular permeability and expression of cytokines and adhesion molecules [38, 54]. In contrast to C3H/HeN, C3H/HeJ mice expressing the P712H signaling-deficient TLR4 mutant were found to succumb to R. akari, and their histopathological examination revealed the presence of R. akari in many macrophages and circulating monocytes [36, 55], and mice with deficient macrophage functions show increased susceptibility to R. akari [56]. C3H/HeJ mice expressing the nonfunctional P712H TLR4 are the most susceptible to R. akari among the 24 mouse strains studied [36] and exhibit impaired serum levels of proinflammatory cytokines TNF-α, IL-1β, and IL-6 after infection with R. akari in vivo (unpublished observation). As R. akari expresses lipoproteins, lipopeptides, and LPS, which have been documented as TLR2 and TLR4 agonists for other bacteria [4, 5, 13], we systematically delineated involvement of TLR2 and TLR4 in cell activation induced by R. akari.
To the best of our knowledge, this paper shows for the first time that TLR2 and TLR4 act as sensors of live and HK R. akari and mediate activation of IRAK1, p38, NF-κB, and expression of TNF-α and IL-8. Several lines of evidence indicate involvement of TLR2 and TLR4 in cell activation induced by R. akari. First, transfection-based complementation of TLR2/4-negative HEK293T cells with TLR2 or TLR4, along with coreceptors CD14 and MD-2, enabled cell responsiveness to HK R. akari, as judged by degradation of IκB-α, induction of NF-κB reporter activity, and activation of IL-8 gene expression and secretion. Likewise, HEK293 cells stably expressing TLR2 or TLR4/MD-2 also exhibited NF-κB reporter activation and IL-8 gene expression and secretion in response to HK R. akari, whereas TLR2/4-negative cells failed to respond.
Second, introduction of the mutations in TLR2 (R753Q) and TLR4 (D299G), which have been associated previously with impaired TLR responsiveness and increased incidence of certain infections [24–28, 31, 33, 37], resulted in a significant loss of the capacities of the respective TLRs to mediate NF-κB reporter activation in response to HK R. akari. The R753Q mutation is localized to the signaling TIR domain of TLR2 and has been linked to a number of infectious diseases, including atopic dermatitis [57], severe ulcerative colitis [58], and tuberculosis [31]. Mutagenesis of the TIR region containing the R753 residue was reported to impair TLR1-TLR2 association and abolish recruitment of MyD88 [59]. In this regard, it is of interest that in contrast to WT TLR2, the R753Q TLR2 mutant fails to recruit MyD88 upon stimulation with irradiated Mycobacterium tuberculosis (unpublished observation). Thus, the inhibitory effect of the R753Q mutation on TLR2-mediated NF-κB activation in response to HK R. akari may occur as a result of interference with TLR2 heterodimerization with TLR1 and/or inhibition of MyD88 recruitment to TLR2. Studies are in progress to address this question. On the other hand, the D299G and T399I SNPs are located in the ectodomain of TLR4, and between the two, it is the D299G SNP that has the greatest signaling deficiency [24, 47] and has been reported to be associated with a number of infectious and inflammatory diseases. Indeed, the D299G TLR4 SNP has been linked to increased incidence of Gram-negative bacteremia and sepsis [24, 25], malaria [28], RSV bronchiolitis in full-term, healthy infants [26, 60], and increased occurrence of infections with Gardnerella vaginalis and Gram-negative rods [61]. In the Tunisian population, subjects expressing the D299G TLR4 SNP had a greater risk of developing active pulmonary tuberculosis [62], and it is also more prevalent in patients with Crohn's disease [29]. Our data also demonstrate a profound signaling deficiency of the D299G TLR4 variant in mediating NF-κB activation in response to HK R. akari and LPS, whereas the T399I species elicited the response similar to that induced by WT TLR4.
The mechanisms of inhibitory effect of the D299G mutation remain incompletely understood. Decreased TLR4 expression in carriers of the D299G SNP was suggested initially to account for blunted responses of airway cells to inhaled LPS [24]. However, Rallabhandi et al. [47] found comparable protein levels of WT and mutant TLR4 in 293/CD14/MD-2 cells transfected with the corresponding TLR4 species, yet cells expressing the D299G TLR4 showed suppressed LPS-induced NF-κB activation. The computer-assisted modeling of the TLR4 ectodomain showed the D299G as located in leucine-rich repeat 10, which forms a putative ligand-binding or coreceptor site, suggesting that this SNP interferes with ligand binding or TLR4 interactions with coreceptors CD14 and/or MD-2 [47]. Elucidation of the exact molecular mechanism by which the D299G affects TLR4 signaling awaits further studies about crystal structures of WT versus D299G TLR4 ectodomains, alone and in complex with TLR4 agonists, and analyses of dimerization patterns of TLR4 variants and their interaction with CD14 and MD-2. It is of importance that carriers of the D299G TLR4 variant showed increased incidence and severity of Mediterranean spotted fever caused by R. conorii [37], suggesting TLR4 as an important sensor of rickettsiae. Of note, Ozen et al. [63] reported an elevated frequency of the R753Q TLR2 SNP in patients manifesting increased severity of familial Mediterranean spotted fever. TLR2 was found as the major receptor eliciting activation of platelets in patients with African tick bite fever caused by R. africae, whereas the R. africae-mediated, procoagulant effects and sCD40 expression in endothelial cells were TLR4-dependent [52], suggesting combined and cooperative involvement of different PRRs in rickettsia recognition. No data are currently available regarding a possible association between the frequency of occurrence of TLR2/TLR4 SNPs and infectious diseases caused by R. akari. Such studies would help to reveal functional roles of PRRs in determining susceptibility to rickettsiosis.
HEK293 cells do not represent natural targets for R. akari, and in contrast to monocytes and macrophages expressing many TLR-inducible cytokines [64], HEK293 cells express a limited repertoire (IL-8, RANTES, and IFN-β) [40–43]. Therefore, we sought to extend our results about the use of TLR2 and TLR4 by HK R. akari to human monocytes, using blocking Ab against TLR2 or TLR4. The 3rd line of evidence shows that blocking of TLR2 or TLR4 markedly decreased secretion of TNF-α by human monocytes stimulated with HK R. akari, and addition of both Ab led to an even greater inhibitory effect on cytokine secretion. To the best of our knowledge, this paper is the first demonstration that HK R. akari engages TLR2 and TLR4 for eliciting cytokine production in human monocytes. Previous studies have demonstrated unambiguously that engagement of TLR2 and TLR4 and their respective agonists, e.g., mycoplasma-activating lipoprotein 2 and LPS, results in additive or synergistic effects on TLR-inducible responses, including MAPK and NF-κB activation, cytokine gene expression, and cytokine release [65]. Our results extend these data to putative, structural components of R. akari, strongly suggesting that TLR2- and TLR4-reacting ligands within the HK R. akari preparations engage the respective TLR, leading to an additive or synergistic effect. Characterization of TLR2 and TLR4 agonists expressed by R. akari is the subject of our ongoing studies. These rickettsial components, by analogy to other bacterial ligands, may include R. akari-derived lipoproteins as ligands for TLR2, and rickettsial LPS and heat shock proteins may represent TLR4 ligands.
TLR2 and TLR4 are known activators of chemokines and proinflammatory cytokines [1, 4, 53], and this paper shows TLR2/4-mediated induction of IL-8 and TNF-α expression upon recognition of R. akari. Thus, detection of R. akari by TLR2 and TLR4 expressed on endothelial cells, macrophages, and DCs would be predicted to mediate recruitment and activation of neutrophils and monocytes to infection loci, contributing to resolution of rickettsial infection. TLR2 and TLR4 have been reported to induce expression of antimicrobial peptides in response to various microbial pathogens, including Chlamydia pneumoniae, Staphylococcus aureus, and M. tuberculosis [66–68], which could also be involved in host immune defense against rickettsial infections. Indeed, cecropin A and ceratotoxin A were reported to cause a lethal effect on Rickettsia monacensis and Rickettsia peacockii [69]. Further experiments are required to address the question of the role for TLR-dependent induction of antimicrobial peptides by innate immune cells in mediating host defense against R. akari.
R. akari replicates intracellularly in macrophages and endothelial cells, suggesting that infection with live bacterium may engage endosomal TLRs as well as intracellular sensors of bacterial PAMPs such as NLRs, as rickettia have been reported as capable of escaping from phagosome and penetrating in the cytoplasm [70]. The identity of other TLRs and NLRs involved in recognition of live R. akari is presently unknown, but it is plausible that endosomal TLR9 [20] could recognize hypomethylated CpG motifs present in rickettsial DNA, whereas endosomal TLR7/8 and cytoplasmic NLRP3 may sense R. akari RNA, similar to their reported involvement in the recognition of E. coli RNA [71, 72]. Detection of R. akari by inflammasome-activating NLRs, such as NLRP3, would then result in secretion of IL-1β and IL-18, which play a central role in activation of various innate and adaptive cellular networks. Notably, NLRP3 and other inflammasome-activating NLRs can trigger pyroptosis, limiting R. akari replication inside macrophages and endothelial cells. Further studies in mice deficient for TLRs and NLRs, implicated in host defense against rickettsial infections, are required to identify definitively innate sensors of live R. akari.
Interestingly, live B. burgdorferi was reported to induce a larger range of cytokine genes compared with lysed bacteria, including IFN-β and IFN-stimulated genes Mx1, IFIT1, IFIT2, IFIT3, IFI16, and RSD2, genes encoding matrix metallopeptidases MMP1, MMP10, MMP19, and genes involved in protein ubiquitination or ubiquitin-like functions, ISG15, HERC5, UBE2L6, and USP18 [73]. Furthermore, live but not lysed B. burgdorferi elicited activation of caspase-1 and IL-1β maturation and secretion [73], suggesting the involvement of NLRs, such as NLRP3. These data indicate that in addition to lipoproteins acting through TLR2 that cannot activate type I IFN expression [74], live B. burgdorferi express additional ligands or induce “danger” signals that can activate type I IFN and other genes via engagement of other TLRs and NLRs [73]. We demonstrate herein that infection with live R. akari induces activation of IRAK1 and p38 MAPKs, as judged by their phosphorylation, not only in HEK293 cells expressing TLR2 or TLR4 but also in the TLR2/4-negative, parental HEK293T cell line, although overexpression of TLR2 or TLR4 potentiated IRAK1 and p38 activation. To confirm results obtained in HEK293 overexpression studies in more physiological settings with macrophage-like cells, we used infection of human monocytes with live R. akari in the presence of isotype control and anti-TLR2 or anti-TLR4 Ab and compared levels of secreted TNF-α in these cell cultures. Interestingly, we observed a significantly reduced capacity of blocking Ab against TLR2 and TLR4 to inhibit TNF-α secretion elicited by monocyte infection with live R. akari compared with their significant inhibitory effects on this response triggered by HK R. akari. Furthermore, infection of WT and TLR2- or TLR4-deficient mouse-immortalized macrophage cell lines with live R. akari at MOI 10 and 50 led to very small differences in levels of secreted TNF-α, and only 12% and 25% inhibition was observed (data not shown), and deficiencies in TLR2 or TLR4 ablated macrophage activation by the respective agonists Pam3Cys (TLR2) or LPS (TLR4). Thus, similar to reported results with live versus lysed B. burgdorferi, our data suggest the use of other TLRs and possibly NLRs by live R. akari and imply cooperation of signaling pathways elicited by other putative TLRs/NLRs with those induced by TLR2 or TLR4 in fine-tuning the innate host response against R. akari.
In summary, this paper demonstrates for the first time that TLR2 and TLR4 are innate immune sensors closely involved in recognition of HK and live R. akari. This conclusion is supported by several complementing lines of evidence. These include enabling cell responsiveness to HK R. akari upon complementation of TLR2/4-negative HEK293T cells with TLR2 or TLR4, compromised ability of mutants TLR2 and TLR4 to mediate NF-κB activation in response to HK R. akari, and inhibition of HK R. akari-elicited TNF-α secretion in human monocytes treated with blocking TLR2 or TLR4 Ab. In contrast to HK R. akari using only TLR2 and TLR4, infection with live R. akari activated IRAK1 and p38 MAPK in TLR2/4-negative HEK293T cells and showed lower TLR2/4 dependence in human monocytes and mouse macrophages, indicating the use of TLR2, TLR4, and other TLRs and/or NLRs for recognition. Experiments are ongoing to identify putative TLR2 and TLR4 agonists expressed by R. akari and to delineate innate immune sensors involved in the recognition of live bacterium.
ACKNOWLEDGMENTS
This work was supported by NIH grant R21 AI067468 and University of Maryland, Baltimore (UMB)–University of Maryland College Park (UMCP) Intramural NIH seed grant (to A.E.M.). We are thankful to Dr. Douglas T. Golenbock and Katherine Fitzgerald (University of Massachusetts School of Medicine) for providing us with expression vectors and cell lines.
Footnotes
- HEK
- human embryonic kidney
- HK
- heat-killed
- HPRT
- hypoxanthine-guanine phosphoribosyltransferase
- hu
- human
- IRAK
- IL-1R-associated kinase
- MD-2
- myeloid differentiation protein 2
- NIH
- National Institutes of Health
- NLR
- nucleotide-binding and oligomerization domain-like receptor
- NLRP3
- nucleotide-binding and oligomerization domain-like receptor family
- pyrin domain-containing 3, p
- phospho
- Pam3Cys
- tripalmitoyl-S-glyceryl cysteine
- PAMPs
- pathogen-associated molecular patterns
- PRRs
- pattern recognition receptors
- RSV
- respiratory syncytial virus
- RT
- reverse transcription
- s
- soluble
- SNP
- single nucleotide polymorphism
- TIR
- Toll/IL-1R
AUTHORSHIP
A.E.M. conceived the idea, designed the experiments, oversaw the entire project, and prepared the manuscript; M.A.Q-D. performed real-time PCR, immunoblot, and NF-κB reporter assays, and conducted experiments with live R. akari; C.S. constructed and sequenced expression vectors encoding TLR2 and TLR4 SNPs and conducted NF-κB reporter and immunoblot assays; Y.X. performed experiments in human monocytes to determine the effect of pretreatment with blocking anti-TLR2/4 Ab on R. akari-induced TNF-α and isolated RNA and conducted PCR analyses of 16S R. akari RNA and β-actin mRNA expression; H.C. helped with immunoblot, ELISA, and real-time PCR experiments; L.M.W. isolated and provided human monocytes; and S.R. directed experiments with live R. akari and helped with data interpretation and manuscript preparation.
REFERENCES
- 1.O'Neill L. A. (2006) How Toll-like receptors signal: what we know and what we don't know. Curr. Opin. Immunol. 18, 3–9 [DOI] [PubMed] [Google Scholar]
- 2.Ting J. P., Willingham S. B., Bergstralh D. T. (2008) NLRs at the intersection of cell death and immunity. Nat. Rev. Immunol. 8, 372–379 [DOI] [PubMed] [Google Scholar]
- 3.Kawai T., Akira S. (2008) Toll-like receptor and RIG-I-like receptor signaling. Ann. N. Y. Acad. Sci. 1143, 1–20 [DOI] [PubMed] [Google Scholar]
- 4.Doyle S. L., O'Neill L. A. (2006) Toll-like receptors: from the discovery of NF-κB to new insights into transcriptional regulations in innate immunity. Biochem. Pharmacol. 72, 1102–1113 [DOI] [PubMed] [Google Scholar]
- 5.Poltorak A., He X., Smirnova I., Liu M. Y., Van Huffel C., Du X., Birdwell D., Alejos E., Silva M., Galanos C., Freudenberg M., Ricciardi-Castagnoli P., Layton B., Beutler B. (1998) Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282, 2085–2088 [DOI] [PubMed] [Google Scholar]
- 6.Hoshino K., Takeuchi O., Kawai T., Sanjo H., Ogawa T., Takeda Y., Takeda K., Akira S. (1999) Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the LPS gene product. J. Immunol. 162, 3749–3752 [PubMed] [Google Scholar]
- 7.Kurt-Jones E. A., Popova L., Kwinn L., Haynes L. M., Jones L. P., Tripp R. A., Walsh E. E., Freeman M. W., Golenbock D. T., Anderson L. J., et al. (2000) Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat. Immunol. 1, 398–401 [DOI] [PubMed] [Google Scholar]
- 8.Bulut Y., Faure E., Thomas L., Karahashi H., Michelsen K. S., Equils O., Morrison S. G., Arditi M. (2002) Chlamydial heat shock protein 60 activates macrophages and endothelial cells through Toll-like receptor 4 and MD2 in a MyD88-dependent pathway. J. Immunol. 168, 1435–1440 [DOI] [PubMed] [Google Scholar]
- 9.Malley R., Henneke P., Morse S. C., Cieslewicz M. J., Lipsitch M., Thompson C. M., Kurt-Jones E., Paton J. C., Wessels M. R., Golenbock D. T. (2003) Recognition of pneumolysin by Toll-like receptor 4 confers resistance to pneumococcal infection. Proc. Natl. Acad. Sci. USA 100,1966–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shimazu R., Akashi S., Ogata H., Nagai Y., Fukudome K., Miyake K., Kimoto M. (1999) MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J. Exp. Med. 189, 1777–1782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pugin J., Heumann D., Tomasz A., Kravchenko V. V., Akamatsu Y., Nishijima M., Glauser M. P., Tobias P. S., Ulevitch R. J. (1994) CD14 as a pattern recognition receptor. Immunity 1, 509–516 [DOI] [PubMed] [Google Scholar]
- 12.Jiang Z., Georgel P., Du X., Shamel L., Sovath S., Mudd S., Huber M., Kalis C., Keck S., Galanos C., Freudenberg M., Beutler B. (2005) CD14 is required for MyD88-independent LPS signaling. Nat. Immunol. 6, 565–570 [DOI] [PubMed] [Google Scholar]
- 13.Lien E., Sellati T. J., Yoshimura A., Flo T. H., Rawadi G., Finberg R. W., Carroll J. D., Espevik T., Ingalls R. R., Radolf J. D., Golenbock D. T. (1999) Toll-like receptor 2 functions as a pattern recognition receptor for diverse bacterial products. J. Biol. Chem. 274, 33419–33425 [DOI] [PubMed] [Google Scholar]
- 14.Bulut Y., Faure E., Thomas L., Equilis O., Arditi M. (2001) Cooperation of Toll-like receptor 2 and 6 for cellular activation by soluble tuberculosis factor and Borrelia burgdorferi outer surface protein A lipoprotein: role of Toll-interacting protein and IL-1 receptor signaling molecules in Toll-like receptor 2 signaling. J. Immunol. 167, 987–994 [DOI] [PubMed] [Google Scholar]
- 15.Hajjar A. M., O'Mahony D. S., Ozinsky A., Underhill D. M., Aderem A., Klebanoff S. J., Wilson C. B. (2001) Cutting edge: functional interactions between Toll-like receptor (TLR) 2 and TLR1 or TLR6 in response to phenol-soluble modulin. J. Immunol. 166, 15–19 [DOI] [PubMed] [Google Scholar]
- 16.Gewirtz A. T., Navas T. A., Lyons S., Godowski P. J., Madara J. L. (2001) Cutting edge: bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J. Immunol. 167, 1882–1885 [DOI] [PubMed] [Google Scholar]
- 17.Alexopoulou L., Holt A. C., Medzhitov R., Flavell R. A. (2001) Recognition of double-stranded RNA and activation of NFκB by Toll-like receptor 3. Nature 413, 732–738 [DOI] [PubMed] [Google Scholar]
- 18.Lund J. M., Alexopoulou L., Sato A., Karow M., Adams N. C., Gale N. W., Iwasaki A., Flavell R. A. (2004) Recognition of pneumolysin by Toll-like receptor 4 confers resistance to pneumococcal infection. Proc. Natl. Acad. Sci. USA 101,5598–560315034168 [Google Scholar]
- 19.Heil F., Hemmi H., Hochrein H., Ampenberger F., Kirschning C., Akira S., Lipford G., Wagner H., Bauer S. (2004) Species-specific recognition of single-stranded RNA via Toll-like receptor 7 and 8. Science 303, 1526–1529 [DOI] [PubMed] [Google Scholar]
- 20.Hemmi H., Takeuchi O., Kawai T., Kaisho T., Sato S., Sanjo H., Matsumoto M., Hoshino K., Wagner H., Takeda K., Akira S. (2000) A Toll-like receptor recognizes bacterial DNA. Nature 408, 740–745 [DOI] [PubMed] [Google Scholar]
- 21.Abe T., Hemmi H., Miyamoto H., Moriishi K., Tamura S., Takaku H., Akira S., Matsuura Y. (2005) Involvement of the Toll-like receptor 9 signaling pathway in the induction of innate immunity by baculovirus. J. Virol. 79, 2847–2858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hochrein H., Schlatter B., O'Keeffe M., Wagner C., Schmitz F., Schiemann M., Bauer S., Suter M., Wagner H. (2004) Herpes simplex virus type-1 induces IFN-b production via Toll-like receptor 9-dependent and -independent pathways. Proc. Natl. Acad. Sci. USA 101,11416–11421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vogel S. N., Fitzgerald K. A., Fenton M. J. (2003) TLRs: differential adapter utilization by Toll-like receptors mediates TLR-specific patterns of gene expression. Mol. Interv. 3, 466–477 [DOI] [PubMed] [Google Scholar]
- 24.Arbour N. C., Lorenz E., Schutte B. C., Zabner J., Kline J. N., Jones M., Frees K., Watt J. L., Schwartz D. A. (2000) TLR4 mutations are associated with endotoxin hyporesponsiveness in humans. Nat. Genet. 25, 187–191 [DOI] [PubMed] [Google Scholar]
- 25.Lorenz E., Mira J. P., Frees K. L., Schwartz D. A. (2002) Relevance of mutations in the TLR4 receptor in patients with Gram-negative septic shock. Arch. Intern. Med. 162, 1028–1032 [DOI] [PubMed] [Google Scholar]
- 26.Awomoyi A. A., Rallabhandi P., Pollin T. I., Lorenz E., Sztein M. B., Boukhvalova M. S., Hemming V. G., Blanco J. C., Vogel S. N. (2007) Association of TLR4 polymorphisms with symptomatic respiratory syncytial virus infection in high-risk infants and young children. J. Immunol. 179, 3171–3177 [DOI] [PubMed] [Google Scholar]
- 27.Ferwerda B., Kibiki G. S., Netea M. G., Dolmans W. M., van der Ven A. J. (2007) The Toll-like receptor 4 Asp299Gly variant and tuberculosis susceptibility in HIV-infected patients in Tanzania. AIDS 21, 1375–1377 [DOI] [PubMed] [Google Scholar]
- 28.Mockenhaupt F. P., Cramer J. P., Hamann L., Stegemann M. S., Eckert J., Oh N. R., Otchwemah R. N., Dietz E., Ehrhardt S., Schröder N. W., Bienzle U., Schumann R. R. (2006) Toll-like receptor (TLR) polymorphisms in African children: common TLR-4 variants predispose to severe malaria. J. Commun. Dis. 38, 230–245 [PubMed] [Google Scholar]
- 29.Franchimont D., Vermeire S., El Housni H., Pierik M., Van Steen K., Gustot T., Quertinmont E., Abramowicz M., Van Gossum A., Deviere J., Rutgeerts P. (2004) Deficient host-bacteria interactions in inflammatory bowel disease? The Toll-like receptor (TLR)-4 Asp299Gly polymorphism is associated with Crohn's disease and ulcerative colitis. Gut 53, 987–992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ben-Ali M., Barbouche M. R., Bousnina S., Chabbou A., Dellagi K. (2004) Toll-like receptor 2 Arg677Trp polymorphism is associated with susceptibility to tuberculosis in Tunisian patients. Clin. Diagn. Lab. Immunol. 11, 625–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ogus A. C., Yoldas B., Ozdemir T., Uguz A., Olcen S., Keser I., Coskun M., Cilli A., Yegin O. (2004) The Arg753GLn polymorphism of the human Toll-like receptor 2 gene in tuberculosis disease. Eur. Respir. J. 23, 219–223 [DOI] [PubMed] [Google Scholar]
- 32.Yim J. J., Lee H. W., Lee H. S., Kim Y. W., Han S. K., Shim Y. S., Holland S. M. (2006) The association between microsatellite polymorphisms in intron II of the human Toll-like receptor 2 gene and tuberculosis among Koreans. Genes Immun. 7, 150–155 [DOI] [PubMed] [Google Scholar]
- 33.Thuong N. T., Hawn T. R., Thwaites G. E., Chau T. T., Lan N. T., Quy H. T., Hieu N. T., Aderem A., Hien T. T., Farrar J. J., Dunstan S. J. (2007) A polymorphism in human TLR2 is associated with increased susceptibility to tuberculous meningitis. Genes Immun. 8, 422–428 [DOI] [PubMed] [Google Scholar]
- 34.Lorenz E., Mira J. P., Cornish K. L., Arbour N. C., Schwartz D. A. (2000) A novel polymorphism in the Toll-like receptor 2 gene and its potential association with staphylococcal infection. Infect. Immun. 68, 6398–6401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brouqui P., Parola P., Fournier P. E., Raoult D. (2007) Spotted fever rickettsioses in Southern and Eastern Europe. FEMS Immunol. Med. Microbiol. 49, 2–12 [DOI] [PubMed] [Google Scholar]
- 36.Jordan J. M., Woods M. E., Olano J., Walker D. H. (2008) The absence of Toll-like receptor 4 signaling in C3H/HeJ mice predisposes them to overwhelming rickettsial infection and decreased protective Th1 responses. Infect. Immun. 76, 3717–3724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Balistreri C. R., Candore G., Lio D., Colonna-Romano G., Di Lorenzo G., Mansueto P., Rini G., Mansueto S., Cillari E., Franceschi C., Caruso C. (2005) Role of TLR4 receptor polymorphisms in Boutonneuse fever. Int. J. Immunopathol. Pharmacol. 18, 655–660 [DOI] [PubMed] [Google Scholar]
- 38.Radulovic S., Price P. W., Beier M. S., Gaywee J., Macaluso J. A., Azad A. (2002) Rickettsia-macrophage interactions: host cell responses to Rickettsia akari and Rickettsia typhi. Infect. Immun. 70, 2576–2582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Flo T. H., Ryan L., Latz E., Takeuchi O., Monks B. G., Lien E., Halaas O., Akira S., Skjak-Braek G., Golenbock D. T., Espevik T. (2002) Involvement of Toll-like receptor (TLR) 2 and TLR4 in cell activation by mannuronic acid polymers. J. Biol. Chem. 277, 35489–35495 [DOI] [PubMed] [Google Scholar]
- 40.Visintin A., Latz E., Monks B. G., Espevik T., Golenbock D. T. (2003) Lysines 128 and 132 enable lipopolysaccharide binding to MD-2, leading to Toll-like receptor-4 aggregation and signal transduction. J. Biol. Chem. 278, 48313–48320 [DOI] [PubMed] [Google Scholar]
- 41.Medvedev A. E., Vogel S. N. (2003) Overexpression of CD14, TLR4, and MD-2 in HEK 293T cells does not prevent induction of in vitro endotoxin tolerance. J. Endotoxin Res. 9, 60–64 [DOI] [PubMed] [Google Scholar]
- 42.Medvedev A. E., Piao W., Shoenfelt J., Rhee S. H., Chen H., Basu S., Wahl L. M., Fenton M. J., Vogel S. N. (2007) Role of TLR4 tyrosine phosphorylation in signal transduction and endotoxin tolerance. J. Biol. Chem. 282, 16042–16053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Piao W., Song C., Chen H., Wahl L. M., Fitzgerald K. A., O'Neill L. A., Medvedev A. E. (2008) Tyrosine phosphorylation of MyD88 adapter-like (Mal) is critical for signal transduction and blocked in endotoxin tolerance. J. Biol. Chem. 283, 3109–3119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Medvedev A. E., Lentschat A., Wahl L. M., Golenbock D. T., Vogel S. N. (2002) Dysregulation of LPS-induced Toll-like receptor 4-MyD88 complex formation and IL-1 receptor-associated kinase 1 activation in endotoxin-tolerant cells. J. Immunol. 169, 5209–5216 [DOI] [PubMed] [Google Scholar]
- 45.Newton S., Ding Y., Chung C. S., Chen Y., Lomas-Neira J. L., Ayala A. (2004) Sepsis-induced changes in macrophage co-stimulatory molecule expression: CD86 as a regulator of anti-inflammatory IL-10 response. Surg. Infect. (Larchmt) 5, 375–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Livak K. J., Schmittgen T. D. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(–Δ Δ C(T)) method. Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]
- 47.Rallabhandi P., Bell J., Boukhvalova M. S., Medvedev A., Lorenz E., Arditi M., Hemming V. G., Blanco J. C., Segal D. M., Vogel S. N. (2006) Analysis of TLR4 polymorphic variants: new insights into TLR4/MD-2/CD14 stoichiometry, structure, and signaling. J. Immunol. 177, 322–332 [DOI] [PubMed] [Google Scholar]
- 48.Kawagoe T., Sato S., Matsushita K., Kato H., Matsui K., Kumagai Y., Saitoh T., Kawai T., Takeuchi O., Akira S. (2008) Sequential control of Toll-like receptor-dependent responses by IRAK1 and IRAK2. Nat. Immunol. 9, 684–691 [DOI] [PubMed] [Google Scholar]
- 49.Li X., Commane M., Jiang Z., Stark G. R.IL-1-induced NFa B and c-Jun N-terminal kinase (JNK) activation diverge at IL-1 receptorassociated kinase (IRAK). (2001) Proc. Natl. Acad. Sci. USA 98, 4461–4465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Payne D. M., Rossomando A. J., Martino P., Erickson A. K., Her J. H., Shabanowitz J., Hunt D. E., Weber M. J., Sturgill T. W. (1991) Identification of the regulatory phosphorylation sites in pp42/mitogen-activated protein kinase (MAP kinase). EMBO J. 10, 885–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jordan J. M., Woods M. E., Soong L., Walker D. H. (2009) Rickettsiae stimulate dendritic cells through Toll-like receptor 4, leading to enhanced NK cell activation in vivo. J. Infect. Dis. 199, 236–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Damas J. K., Jensenius M., Ueland T., Otterdal K., Yndestad A., Froland S. S., Rolain J. M., Myrvang B., Raoult D., Aukrust P. (2006) Increased levels of soluble CD40L in African tick bite fever: possible involvement of TLRs in the pathogenic interaction between Rickettsia africae, endothelial cells, and platelets. J. Immunol. 177, 2699–2706 [DOI] [PubMed] [Google Scholar]
- 53.Mosser D. M., Edwards J. P. (2008) Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 8, 958–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shi R. J., Simpson-Haidaris P. J., Lerner N. B., Marder V. J., Silverman D. J., Sporn L. A. (1998) Transcriptional regulation of endothelial cell tissue factor expression during Rickettsia rickettsii infection: involvement of the transcription factor NF-κB. Infect. Immun. 66, 1070–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Anderson G. W., Jr., Osterman J. V. (1980) Host defenses in experimental rickettsialpox: resistance of C3H mouse sublines. Acta Virol. 24, 294–296 [PubMed] [Google Scholar]
- 56.Meltzer M. S., Nacy C. A. (1980) Macrophages in resistance to rickettsial infection: susceptibility to lethal effects of Rickettsia akari infection in mouse strains with defective macrophage function. Cell. Immunol. 54, 487–490 [DOI] [PubMed] [Google Scholar]
- 57.Niebuhr M., Langnickel J., Draing C., Renz H., Kapp A., Werfel T. (2008) Dysregulation of Toll-like receptor-2 (TLR-2)-induced effects in monocytes from patients with atopic dermatitis: impact of the TLR-2 R753Q polymorphism. Allergy 63, 728–734 [DOI] [PubMed] [Google Scholar]
- 58.Podolsky D. K., Gerken G., Eyking A., Cario E. (2009) Colitis-associated variant of TLR2 causes impaired mucosal repair because of TFF3 deficiency. Gastroenterology 137, 209–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gautam J. K., Ashish Comeau L. D., Krueger J. K., Smith M. F. (2006) Structural and functional evidence for the role of the TLR2 DD loop in TLR1/TLR2 heterodimerization and signaling. J. Biol. Chem. 281, 30132–30142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tal G., Mandelberg A., Dalal I., Cesar K., Somekh E., Tal A., Oron A., Itskovich S., Ballin A., Houri S., Beigelman A., Lider O., Rechavi G., Amariglio N. (2004) Association between common Toll-like receptor 4 mutations and severe respiratory syncytial virus disease. J. Infect. Dis. 189, 2057–2063 [DOI] [PubMed] [Google Scholar]
- 61.Genc M. R., Vardhana S., Delaney M. L., Onderdonk A., Tuomala R., Norwitz E., Witkin S. S. (2004) Relationship between a Toll-like receptor-4 gene polymorphism, bacterial vaginosis-related flora and vaginal cytokine responses in pregnant women. Eur. J. Obstet. Gynecol. Reprod. Biol. 116, 152–156 [DOI] [PubMed] [Google Scholar]
- 62.Ferwerda B., Kibiki G. S., Netea M. G., Dolmans W. M., van der Ven A. J. (2007) The Toll-like receptor 4 Asp299Gly variant and tuberculosis susceptibility in HIV-infected patients in Tanzania. AIDS 21, 1375–1377 [DOI] [PubMed] [Google Scholar]
- 63.Ozen S., Berdeli A., Türel B., Kutlay S., Yalcinkaya F., Arici M., Besbas N., Bakkaloglu A., Yilmaz E. (2006) Arg753Gln TLR-2 polymorphism in familial Mediterranean fever: linking the environment to the phenotype in a monogenic inflammatory disease. J. Rheumatol. 33, 2498–2500 [PubMed] [Google Scholar]
- 64.Chen H., Cowan M. J., Hasday J. D., Vogel S. N., Medvedev A. E. (2007) Tobacco smoking inhibits expression of proinflammatory cytokines and activation of IL-1R-associated kinase, p38, and NF-κB in alveolar macrophages stimulated with TLR2 and TLR4 agonists. J. Immunol. 179, 6097–6106 [DOI] [PubMed] [Google Scholar]
- 65.Sato S., Nomura F., Kawai T., Takeuchi O., Muhlradt P. F., Takeda K., Akira S. (2000) Synergy and cross-tolerance between Toll-like receptor (TLR) 2- and TLR4-mediated signaling pathways. J. Immunol. 165, 7096–7101 [DOI] [PubMed] [Google Scholar]
- 66.Romano Carratelli C., Mazzola N., Paolillo R., Sorrentino S., Rizzo A. (2009) Toll-like receptor-4 (TLR4) mediates human β-defensin-2 (HBD-2) induction in response to Chlamydia pneumoniae in mononuclear cells. FEMS Immunol. Med. Microbiol. 57, 116–124 [DOI] [PubMed] [Google Scholar]
- 67.Hussain T., Nasreen N., Lai Y., Bellew B. F., Antony V. B., Mohammed K. A. (2008) Innate immune responses in murine pleural mesothelial cells: Toll-like receptor-2 dependent induction of β-defensin-2 by staphylococcal peptidoglycan. Am. J. Physiol. Lung Cell. Mol. Physiol. 295, L461–L470 [DOI] [PubMed] [Google Scholar]
- 68.Rivas-Santiago B., Hernandez-Pando R., Carranza C., Juarez E., Contreras J. L., Aguilar-Leon D., Torres M., Sada E. (2008) Expression of cathelicidin LL-37 during Mycobacterium tuberculosis infection in human alveolar macrophages, monocytes, neutrophils, and epithelial cells. Infect. Immun. 76, 935–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Baldridge G. D., Kurtti T. J., Munderloh U. G. (2005) Susceptibility of Rickettsia monacensis and Rickettsia peacockii to cecropin A, ceratotoxin A, and lysozyme. Curr. Microbiol. 51, 233–238 [DOI] [PubMed] [Google Scholar]
- 70.Teysseire N., Boudier J. A., Raoult D. (1995) Rickettsia conorii entry into Vero cells. Infect. Immun. 63, 366–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kariko K., Buckstein M., Ni H., Weissman D. (2005) Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity 23, 165–175 [DOI] [PubMed] [Google Scholar]
- 72.Kanneganti T. D., Ozoren N., Body-Malapei M., Am A., Park J. H., Franchi L., Whitfield J., Barchet W., Colonna M., Vandenabeele P., Bertin J., Coyle A., Grant E. P., Akira S., Nunez G. (2006) Bacterial RNA and small antiviral compounds activate capspase-1 through cryopyrin/Nalp3. Nature 440, 233–236 [DOI] [PubMed] [Google Scholar]
- 73.Salazar J. C., Duhnam-Ems S., La Vake C., Cruz A. R., Moore M. W., Caimano M. J., Velez-Climent L., Shupe J., Krueger W., Radolf J. D. (2009) Activation of human monocytes by live Borrelia burgdorferi generates TLR2-dependent and -independent responses which include induction of IFN-β. PLoS Pathog. 5e1000444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hirschfeld M., Weis J. J., Toshchakov V., Salkowski C. A., Cody M. J., Ward D. C., Qureshi N., Michalek S. M., Vogel S. N. (2001) Signaling by Toll-like receptor 2 and 4 agonists results in differential gene expression in murine macrophages. Infect. Immun. 69, 1477–1482 [DOI] [PMC free article] [PubMed] [Google Scholar]