Long-term stimulation with isoproterenol augments macrophage CD14 expression and increases E. coli phagocytosis by cAMP-dependent protein kinase signaling mechanism.
Keywords: bone marrow, H-89, siRNA, CD14−/−, TLR4, MD-2, catecholamine
Abstract
CD14 is a glycoprotein that binds bacterial LPS in MØ. It is an essential component of the phagocytic system and is increased in septic shock. Critical injury and sepsis result in elevated endogenous CA levels. CAs have a significant impact on MØ inflammatory functions. We tested the hypothesis that β-adrenergic stimulation regulates CD14 expression and bacterial phagocytosis in BMØ. Murine BMØ stimulated with isoproterenol (>8 h) induced a dose-dependent increase in cell surface CD14 expression. Specific PKA inhibitor (H-89) and gene-silencing (siRNA) studies demonstrated the role of cAMP-dependent PKA in mediating this response. In addition, we observed a correlation between an isoproterenol-mediated increase in CD14 expression and live Escherichia coli uptake in BMØ. Further, the essential role of CD14 in an isoproterenol-mediated increase in E. coli uptake was highlighted from experiments using CD14−/− mice. Moreover, the dose response of isoproterenol stimulation to CD14 expression and E. coli phagocytosis overlapped with similar EC50. Additionally, isoproterenol-mediated E. coli phagocytosis was prevented by H-89, suggesting that β-adrenergic stimulus in BMØ increases CD14 expression and live E. coli phagocytosis through a common signaling pathway. Our studies indicate the potential impact of β-adrenergic agents on important innate immune functions.
Introduction
Increased CD14 is associated with high mortality in gram-negative septic shock [1]. Bacterial sepsis is ranked among the top 10 causes of death in adults and neonates [2]. As a primordial response to stressful events such as sepsis, plasma CA epinephrine and norepinephrine are increased more than 10-fold via sympathetic activation of the adrenal medulla and release from nerve terminals [3, 4]. Although the metabolic and cardiovascular actions of CA are well defined, their influence on the function of innate immune cells, such as monocytes and MØ, are less well understood. Although norepinephrine has high specificity to α-adrenergic receptors, epinephrine acts primarily through β-adrenergic receptors present on target cells. β-Adrenergic agents activate adenylate cyclase predominantly, thereby increasing intracellular cAMP levels in MØ [5]. There is sufficient evidence that the second messenger signal cAMP can modulate MØ function [6] and cell surface antigen expressions [7]. We have reported previously that addition of epinephrine during in vitro differentiation of ER-MP-20+ BM progenitors increased membrane-bound CD14 expression in BMØ through β2-adrenergic receptor stimulation [8], but its effect on mature MØ is not known.
The innate immune receptor CD14 is expressed by MØ, and its role as a LPS signaling molecule is well established. CD14 mediates inflammatory responses and cytokine production through transcriptional regulation of NF-κB following LPS binding [9]. However, our reports demonstrate that epinephrine attenuated LPS-stimulated, proinflammatory cytokine responses in MØ [10, 11]. Bergmann and Sautner [12] have reviewed several similar findings by other investigators. Another potential function for CD14 is phagocytosis. CD14-dependent phagocytosis of apoptotic cells and Gram-negative bacteria by MØ have been reported in human and murine systems [13, 14]. It is evident that the CD14 molecule in the soluble form present in serum or the GPI-anchored membrane-bound form present on monocytes binds and promotes Gram-negative bacterial phagocytosis [15]. Therefore, it is conceivable that β-adrenergic stimulation could promote bacterial phagocytosis through increased membrane CD14 expression in MØ.
To our knowledge, there are no reports about β-adrenergic regulation of MØ cell surface CD14 expression or the implications of increased CD14 expression in MØ bacterial phagocytosis. Moreover, the short-term versus long-term effects of CA or any stress hormones can vary. Therefore, it is important to delineate time- and dose-dependent effects of β-adrenergic stimulation on MØ phenotype and function. Here, we tested the hypothesis that β-adrenergic agonist stimulation increases cell surface CD14 expression and live Escherichia coli phagocytosis in MØ.
MATERIALS AND METHODS
Animal models
Adult male (6–8 weeks) B6D2F1, B6.129S-CD14tm1Frm/J (CD14−/−), and C57/BL /6J mice (Jackson Laboratories, Bar Harbor, ME, USA) were allowed to acclimatize for 7 days at our Comparative Medicine Facility, according to the Association for Assessment and Accreditation of Laboratory Animal Care International guidelines. The Institutional Animal Care and Use Committee of Loyola University Medical Center (Maywood, IL, USA) approved all of the experimental protocols.
Isolation and in vitro differentiation of BMØ
Total nucleated BM cells (5×107) were labeled with rat anti-mouse anti-ER-MP-20 mAb (Accurate Scientific, Westbury, NY, USA), followed by microbead-conjugated anti-rat IgG (20 μl), and separated by positive selection using MACS magnetic separation columns (Miltenyi Biotec, Auburn, CA, USA). BM ER-MP20+ cells (106), isolated from B6D2F1, CD14−/−, or C57/BL/6J (background strain), were differentiated into BMØ in a tetrafluoroethylene-lined dish with 1 ml IMDM containing 50 ng/ml M-CSF at 37°C for 12–14 days. BMØs were characterized by F4/80 expression.
Isolation of PMØ
Casein-elicited murine PMØ were isolated by peritoneal lavage 48 h after sodium caseinate challenge. The purity of PMØ was 90–95%, as verified by F4/80 expression.
Treatment of BMØ and PMØ
PMØ and BMØ were incubated with epinephrine, norepinephrine, or isoproterenol from 0 to 18 h with varying concentrations as mentioned. For blocking studies, H-89, SB 203580, or ICI 118551 [8] was added 30 min before adding isoproterenol. MØ were then washed with PBS and then stimulated with a TLR4 agonist (ultra-pure LPS; 100 ng/ml) or GFP-E. coli (25×106 CFU bacteria/106 MØ) at 37°C and 5% CO2 for 30 min–1 h.
Cell staining for flow cytometry
BMØ (106 cells) and PMØ (106 cells) were labeled with the fluorochrome-conjugated primary antibodies to F4/80+, CD14, and TLR4-MD-2 (BD Biosciences, San Diego, CA, USA) for 30 min. MØ were gated on F4/80-positive expression. Of the F4/80+ MØ, those subsets expressing CD14, the TLR4-MD-2 complex, or coexpression of CD14 and TLR4-MD-2 were identified. Unstained and fluorescence-conjugated isotype controls were included.
PKA enzyme activity assay
Relative enzyme activity (PKA) was determined using a commercially available ELISA kit (Assay Designs, Ann Arbor, MI, USA). Briefly, lysates were prepared from BMØ following isoproterenol stimulation, with and without H-89 pretreatment, and protein content was determined (BCA protein assay kit, Pierce, Rockford, IL, USA) before PKA assay [16].
siRNA transfection
siRNA gene-silencing experiments were carried out using a commercially available siRNA kit (Cell Signaling Technology, Danvers, MA, USA). Briefly, BMØ (106 cells) were transfected with 40 μmoles siRNA or control siRNA in IMDM for 5–7 h. Intracellular PKA C-α was labeled with PKA-specific fluorescent antibody. PKA expression was detected by FACS and by confocal microscopy.
Quantification of phagocytosis by FACS analysis
Isoproterenol-stimulated BMØ (106) and PMØ (106) were incubated with GFP-E. coli (at a MØ:bacteria ratio of 1:25) in 10% serum containing IMDM at 37°C for 1 h with constant rotation. MØ were washed with ice-cold PBS, excess bacteria were removed by centrifugation, and the membrane-bound bacteria were cleaved with lysozyme (1 mg/ml) for 20 min. The percentage of F4-80+ cells expressing GFP E. coli was determined by FACS analysis. PI was calculated by multiplying the percentage of phagocytosing MØ with the MFI of GFP-E. coli.
RESULTS AND DISCUSSION
Membrane CD14 expression is up-regulated by β-adrenergic stimulation
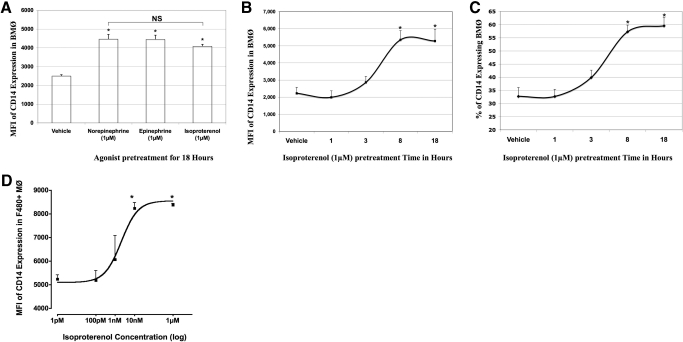
Addition of epinephrine to BM progenitors during differentiation influenced the cell surface CD14 expression in BMØ in a β2- adrenergic receptor-dependent mechanism [8]. Here, we tested the influence of CAs and isoproterenol (a pan β-adrenergic agonist) stimulation on mature BMØ phenotype. BMØ, stimulated with and without norepinephrine, epinephrine, and isoproterenol (1 μM) overnight, were analyzed for CD14 expression by flow cytometry. A significant increase in the MFI of CD14 expression was noted in BMØ treated with CAs and the pharmacologic agent isoproterenol in comparison with vehicle control (norepinephrine=4466±250; epinephrine=4454±220; isoproterenol=4084±103 vs. vehicle=2499±172; P<0.05; Fig. 1A). According to these results, the β-adrenergic agonist isoproterenol and the CAs epinephrine and norepinephrine had similar effects on BMØ CD14 expressions. Therefore, we used the pharmacologic agent isoproterenol in time- and dose-response experiments to study β-receptor-mediated effects in BMØ.
Figure 1. BMØ CD14 expression following adrenergic stimulation.
BMØ were stimulated with varying concentrations of adrenergic agonists for the specified times and labeled with antibodies to F4/80-FITC and CD14-APC and analyzed by FACS. (A) Bar graph comparing the MFI of CD14 expression in BMØ stimulated with 1 μM norepinephrine, epinephrine, or isoproterenol for 18 h. (B) Graph representing time course of isoproterenol stimulation on the x-axis and the MFI of CD14 expressed by BMØ on y-axis. (C) Graph representing time course of isoproterenol stimulation on the x-axis and the percentage of BMØ expressing CD14 on y-axis. (D) Dose response curve of the MFI of CD14 expression in the F4/80+CD14+ fraction of BMØ (y-axis) to varying concentrations of isoproterenol (x-axis logarithmic scale) stimulation for 18 h. Data are given as mean ± sem. *P < 0.05 versus vehicle; n = 4.
Briefly, BMØ were treated with isoproterenol (1 μM) and analyzed for CD14 expression by flow cytometry at the end of 1, 3, 8, and 18 h. A vehicle treatment was included as control. We noticed a significant increase in the MFI of CD14 expression in BMØ treated with isoproterenol starting at 8 h and reached a plateau at 18 h (5356±539 and 5282±691 vs. 2231±342; P<0.05; Fig. 1B). Similarly, a significant increase in the percentage of CD14+ expression in F4/80+ BMØ was noted with isoproterenol treatment at 8 and 18 h in comparison with vehicle control (57.3±2.5% and 59.6±3.2% vs. 32.8±3.3%; P<0.05; Fig. 1C) with no significant changes at 1- and 3-h stimulations. A maximal induction of CD14 mRNA was reported in RAW 264.7 cells treated with 8-Br-cAMP after 6 h of incubation, indicating a similar time-dependent increase in CD14 transcription [17].
Next, we tested concentrations ranging from 1 pM to 1 μM isoproterenol in a dose- response experiment. This range of CA concentrations is reported to produce discernible effects in various functions of MØ [18, 19]. The MFI of CD14 expression in the F4/80+CD14+ fraction of BMØ was recorded by flow cytometry. A sigmoidal dose response of CD14 expression in BMØ (y-axis) to varying doses of isoproterenol treatment (x-axis) was noted (Fig. 1D). A significant increase was noted at 1 nM (6069±1006) versus vehicle treatment (3184±632), and a maximal response was reached at 10 nM and 1 μM isoproterenol (8241±244 and 8380±57, respectively), with a half-maximal stimulatory concentration (EC50) at 2.054 nM of the agonist concentration. Lower concentrations of isoproterenol (1 pM and 100 pM) promoted a modest increase in MFI of CD14 expression (5229±183 and 5177±418, respectively) in comparison with vehicle treatment. It is evident from dose-response studies that supraphysiologic levels of adrenergic hormones resulting from trauma and sepsis may have an influence on mature BMØ CD14 expression.
Isoproterenol acts via PKA to increase membrane CD14 expression in BMØ
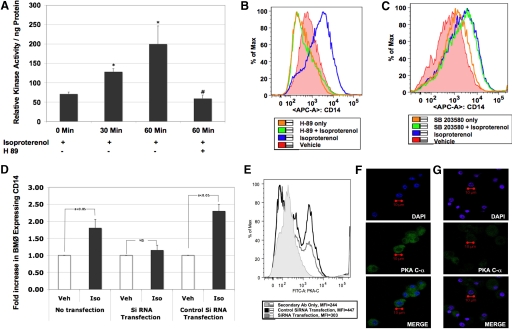
β-Adrenergic receptor engagement predominantly activates the PKA signaling pathway [20]. PKA is the primary regulatory enzyme that mediates the action of cAMP by catalyzing phosphorylation in response to hormone stimuli. Therefore, we measured the intracellular enzyme activity in BMØ following isoproterenol stimulation. Briefly, BMØ (106) were stimulated with isoproterenol (0–60 min), and the relative kinase activity of the lysates was determined following the instructions of a commercially available kit [16]. As expected, kinase activity was increased significantly with β-adrenergic stimulation (71±5, 128±8, and 200±47/ng protein at 0, 30, and 60 min, respectively; P<0.05 vs. 0 time-point). Further, pretreatment with H-89 30 min before isoproterenol stimulation inhibited the kinase activity completely (59±9/ng protein) in BMØ, emphasizing the potential role of PKA following isoproterenol stimulation (Fig. 2A).
Figure 2. Isoproterenol acts via PKA.
Relative PKA enzyme activity by ELISA. Briefly, BMØ (106) stimulated with isoproterenol (1 μM) from 0 to 60 min, with and without H-89 (added 30 min prior to addition of agonist), were lysed immediately in the presence of protease inhibitors. The protein concentration in the cell lysate was determined using the BCA protein assay kit (Pierce). (A) Bar graph showing relative PKA activity/ng protein. Data are given as mean ± sem. *P < 0.05 versus 0 min; #P < 0.05 versus isoproterenol stimulation; n = 4. Histograms representing the MFI of CD14 expression in BMØ pretreated with H-89 (B) or SB203580 (C), 30 min before stimulating with isoproterenol. RNA degradation of PKA with siRNA transfection. BMØ were transfected with siRNA targeted to PKA C-α or control, scrambled siRNA. (D) Bar graph showing fold increase in CD14 expression on the y-axis and the different treatment groups on the x-axis. Data given in bar graph are mean ± sem. Confirmation of gene silencing. Veh, Vehicle; Iso, isoproterenol. (E) Histogram of PKA C-α protein expression following control and siRNA transfection resulting from FACS analysis. (F and G) Confocal images of BMØ showing nuclear staining (blue), PKA protein expression (green), and merge images with control transfection and siRNA transfection, respectively. A Zeiss LSM 510 confocal microscope was used. Original image resolution, 1024 × 1024; n= 4.
Next, to test whether isoproterenol-induced up-regulation of CD14 acts through PKA in BMØ, we pretreated BMØ with a cell-permeable PKA inhibitor H-89 for 30 min before isoproterenol stimulation (18 h) and analyzed for membrane CD14 expression by flow cytometry. As seen in the histogram (Fig. 2B), the MFI of CD14 expression was increased significantly by isoproterenol stimulation (blue) in comparison with vehicle (red) control (4236±406 vs. 1631±324; P<0.05); H-89 completely abrogated isoproterenol-stimulated up-regulation of CD14 expression (green, 1488±214); and H-89 alone did not change CD14 expression (orange, 1604±307).
cAMP has been reported to activate p38 MAPK, and H-89 has been shown to inhibit kinase activity of mitogen- and stress-activated kinase 1, acting downstream of the p38 MAPK [21]. To address the role of p38 MAPK in isoproterenol-induced CD14 expression, we pretreated BMØ with SB 203580, a highly selective inhibitor of p38 MAPK. The histogram in Fig. 2C demonstrates the MFI of CD14 expression in BMØ, with and without SB 203580 pretreatments [vehicle: 2499±72 (red); isoproterenol: 4084±105 (blue); isoproterenol+ SB 203580: 3939±152 (green); SB 203580: 2815±107 (orange)]. SB 203580 did not reverse isoproterenol-induced CD14 up-regulation, as did H-89, suggesting that an isoproterenol-mediated increase in BMØ CD14 expression is regulated via PKA signaling and not the p38 MAPK pathway.
To confirm further the role of PKA in isoproterenol-mediated CD14 expression in BMØ, we used the method of targeted gene silencing. If β-adrenergic stimulation of BMØ with isoproterenol signaled through PKA to augment CD14 expression, then the targeted disruption of PKA transcription prior to incubation with isoproterenol should prevent the increase in cell surface CD14. To test this, gene expression of cAMP-dependent PKA (PKA C-α) was silenced temporarily in BMØ with siRNA targeted to PKA C-α or control, scrambled siRNA. BMØ were then incubated with isoproterenol or vehicle overnight, and the cells were washed and stained for F4/80 and CD14. The bar graph in Fig. 2D demonstrates the CD14 expression (fold increase over vehicle control) on the y-axis and the different treatment groups on the x-axis. Vehicle treatment was normalized to 1 in each treatment group for comparison. siRNA transfection of PKA C-α abolished the isoproterenol-induced increase in BMØ CD14 expression, and the control siRNA did not. These results confirm that PKA signaling regulates an isoproterenol-mediated increase in BMØ CD14 expression. Transfection efficiency was verified by staining of the targeted protein PKA C-α. According to the flow cytometry results in Fig. 2E, the MFI of PKA protein expression was significantly lower in the BMØ transfected with siRNA (298±5) in comparison with the scrambled, control siRNA (440±7). Separate staining with FITC-conjugated secondary antibody alone was included to account for nonspecific binding, and accordingly, there was a 75% inhibition in PKA protein expression with siRNA transfection in BMØ. Flow results were confirmed further with confocal images, where the PKA expression (green) is more pronounced with control transfection (Fig. 2F) and is reduced significantly with siRNA transfection (Fig. 2G).
Isoproterenol stimulation augments CD14/TLR4-MD-2 surface expression in MØ
Although CD14 presents LPS to TLR4 [22], LPS is recognized by TLR4 and a small glycosylated protein MD-2 [23], which is a requisite protein for LPS signaling of TLR4 [24]. The mAb MTS510 preferentially binds to TLR4, which is associated with MD-2 [25], and several reports indicate a close interaction of CD14 with TLR4 and MD-2 [26, 27]. Given that β-adrenergic stimulation reduces a LPS-mediated TNF-α response [11], we tested the effect of isoproterenol on TLR4-MD-2 complex formation and the coexpression of CD14 in BMØ.
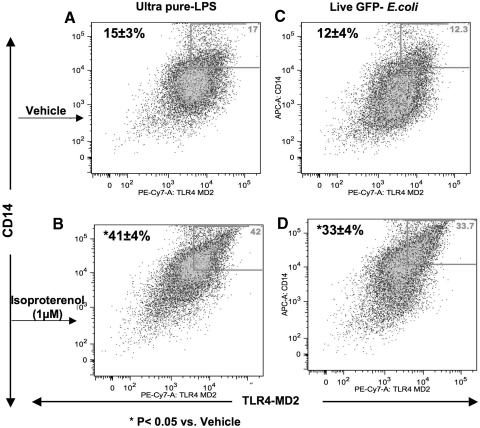
BMØ were pretreated with isoproterenol (1 μM for 18 h), followed by a specific TLR4 agonist (ultra-pure LPS) or live, Gram-negative bacteria for 30 min, and the coexpression of CD14 and TLR4-MD-2 complex formation was measured by flow cytometry. The percentage of F4/80+ BMØ coexpressing CD14 and TLR4-MD-2 was enhanced significantly with isoproterenol pretreatment compared with vehicle control following ultra-pure LPS (vehicle: 15±3% and isoproterenol: 41±4%; P<0.05; Fig. 3A and B), as well as with live E. coli stimulations (vehicle: 12±4% and isoproterenol: 33± 4%; P<0.05; Fig. 3C and D).
Figure 3. CD14/TLR4-MD-2 complex coexpression.
BMØ (106) were stimulated with isoproterenol or vehicle, washed, and treated with ultra-pure LPS or live E. coli for 30 min before labeling with F4/80-FITC, TLR4-MD-2-PE-Cy7, and CD14-APC mAb. Dot plots obtained from FACS analyses show those BMØ gated for F4/80+, expressing CD14 and the TLR4-MD-2 complex in the upper-right quadrants. (A) Vehicle + LPS; (B) isoproterenol + LPS; (C) vehicle + live E. coli; and (D) isoproterenol + live E. coli treatments, respectively. Data are given as mean ± sem. *P < 0.05 versus vehicle; n = 4.
When CD14 is expressed on the surface of immune cells, it has no inherent signaling capacity. However, CD14 is an essential molecule that enhances LPS binding to MD-2 [28]. In addition, CD14 can bind the whole bacteria as well as the cell-wall component LPS [29]. In line with these observations, our results demonstrate that β-adrenergic stimulation can significantly increase the percentage of BMØ coexpressing the CD14/TLR4-MD-2 complex after stimulation with LPS or live E. coli.
β-Adrenergic stimulation of BMØs facilitates a time- and dose-dependent phagocytic response
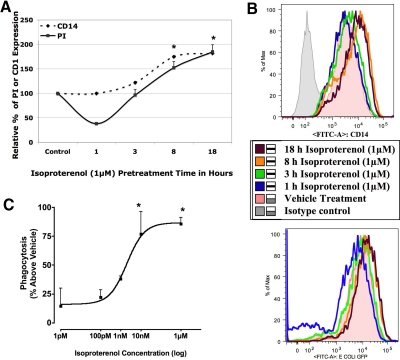
CD14 is involved in the phagocytosis of heat-killed whole bacteria in different cell types [29, 30] and plays a prominent role in endotoxic shock following exposure to LPS or Gram-negative bacteria [31]. Considering that septic shock is associated with increased CD14 [1], one of the implications of isoproterenol-induced membrane CD14 expression in BMØ could be bacterial phagocytosis. Therefore, we tested whether isoproterenol may also influence phagocytosis of live bacteria. BMØ were pretreated with isoproterenol for a period of 1, 3, 8, and 18 h. At the end of each pretreatment time, the BMØ were washed with fresh medium and incubated with live GFP-E. coli, and the phagocytic responses were measured by flow cytometry. Supplemental Fig. 1A is a representative dot plot, where the upper-right quadrant shows the percentage of F4/80+ BMØ taking up GFP-E. coli; the histogram in Supplemental Fig. 1B shows the MFI of the phagocytosed E. coli, and the bar graph in Supplemental Fig. 1C is the calculated PI as detailed in Materials and Methods. Supplemental Fig. 1D shows no change in MFI of GFP-E. coli, with and without trypan blue quenching following lysozyme treatment. The phagocytic response (y-axis) to time-dependent β-adrenergic stimulation of BMØ (x-axis) is shown in Fig. 4A. MFI of CD14 expression by BMØ (y-axis) to β-adrenergic stimulation is also included for comparison. Vehicle control is taken as 100% for PI and CD14 expression. Accordingly, we noticed a transient decrease in E. coli uptake by BMØ when pretreated with isoproterenol for 1 h (short time), which returned to normal levels after 3 h of pretreatment (PI: 38.33±2.3% and 96.6±11.6% at 1 and 3 h vs. vehicle control as 100%). CD14 expression remained unchanged during this time. Thereafter, we noticed a steady increase in E. coli uptake over 8 and 18 h of isoproterenol stimulation, during which time, CD14 expression was also increased correspondingly as compared with vehicle control (PI: 152.5±12.6% and 185±14.6%; CD14: 175±2.5% and 185±3.18% after 8 and 18 h of isoproterenol stimulation). These results demonstrate that β-adrenergic stimulation of BMØ promotes a biphasic phagocytic response. Fig. 4B illustrates the histograms of the MFI of CD14 (upper panel) expression and GFP-E. coli (lower panel) up-take by F/480+ BMØ following isoproterenol treatment for 1, 3, 8, and 18 h.
Figure 4. Time- and dose-dependent phagocytic response to isoproterenol stimulation.
(A) The graph shows the percent PI (solid line) to the time course of isoproterenol treatment (x-axis). Vehicle treatment is taken as 100%, and CD14 expression (dotted line) is shown alongside for comparison. (B) Histograms of MFI of CD14 expression (upper panel) and GFP-E. coli (lower panel) in BMØ treated with isoproterenol over the indicated time course. (C) The sigmoidal dose-response curve (variable slope) as a function of logarithm of agonist concentrations (for 18 h). Data are given as mean ± sem. *P < 0.05 versus vehicle; n = 4.
Next, we investigated BMØ E. coli phagocytosis to different doses of isoproterenol stimulation over an extended period of 18 h. Fig. 4C illustrates the dose-response curve of the PI of BMØ stimulated with varying concentrations (1 pM–1 μM) of isoproterenol. There was a dose-dependent increase in PI with increasing concentrations of isoproterenol. A marginal increase in comparison with vehicle treatment was noted at lower concentrations of isoproterenol (1 pM and 100 pM), with a maximal increase in phagocytic response at 10 nM with no further change at higher concentrations up to 1 μM. Half-maximal stimulatory concentration (EC50) calculated from the sigmoidal dose-response curve was 2 nM. This would favor the argument that CAs are likely to have a significant effect, not only during homeostasis at physiological levels (0.1 nM–1 nM) but also during stress conditions parallel to a CD14 dose-response curve when CA levels are elevated in the supraphysiological range (10 nM–1 μM) during stress, such as an infection and trauma.
Results from dose-response studies indicate that low physiological levels (EC50=2 nM) of isoproterenol are required for the cell surface CD14 expression and E. coli phagocytosis in BMØ and that β-adrenergic effects are increased significantly at higher concentrations (10 nM–1 μM), which are often associated with severe trauma, infections, and sepsis [3, 4]. Moreover, genetically altered mice lacking in epinephrine and norepinephrine (dbh −/−) are highly susceptible to infections and often die of systemic dissemination of the pathogen [32], suggesting a fundamental, biological role for CA in host response to infections. Further, the results presented here underscore the need for understanding the adrenergic regulation of an innate immune response in light of the wide use of adrenergic agonists and antagonists as therapeutic modalities.
Although the data presented here demonstrate that β-adrenergic stimulation enhances bacterial phagocytosis in BMØ, norepinephrine was shown to have a contrasting effect in wound MØ obtained from skin of BALB/c mice [33]. Stellate Langerhans cells present in the epidermal layer of the skin also express F4/80 antigen [34]. Given that F4/80 antigen cannot discriminate among resident MØ, recruited monocytes, and differentiated DCs [35] and that the study did not exclude DCs with CD11c antigen, it is plausible that the wound MØ obtained from the sponge implant may not be a homogeneous MØ population, whereas in the present study, monocyte-committed ER-MP20+ BM progenitors were maintained under standard culture conditions, and the growth factor M-CSF for MØ differentiation eliminated any CD11c+ DC heterogeneity.
Isoproterenol-induced E. coli phagocytosis is CD14-dependent in BMØ
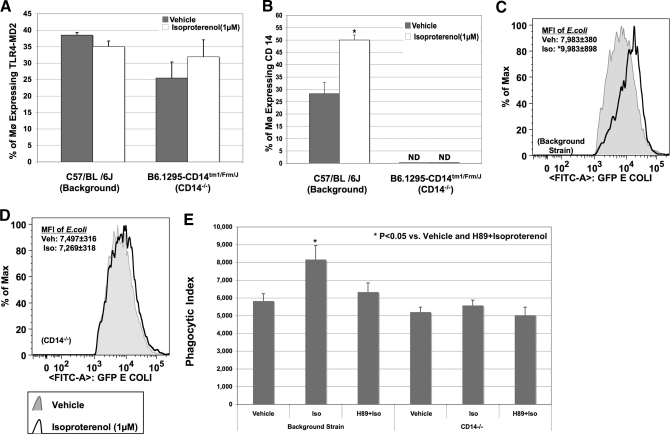
β-Adrenergic receptor-mediated, live E. coli phagocytosis and the synergistic stimulation with LPS/E. coli augmenting CD14/TLR4-MD-2 complex formation prompted us to delineate further the essential role of CD14 and the TLR4-MD-2 complex in this process. BMØ were obtained from CD14−/− mice, and CD14 intact background strain and cell surface expressions of F4/80, CD14, and TLR4-MD-2 were determined. As expected, CD14 was not present in BMØ from CD14−/− mice. Therefore, TLR4-MD-2 expression following LPS stimulation was determined instead of CD14/TLR4-MD-2 coexpression. The TLR4-MD-2 complex remained unaltered in isoproterenol-treated BMØ, obtained from CD14-intact or CD14−/− mice (Fig. 5A), indicating that isoproterenol does not influence TLR4-MD-2 complex formation. In contrast, isoproterenol significantly increased the percentage of CD14 expressing BMØ in the background strain but not in CD14−/− mice (Fig. 5B). Similarly, isoproterenol pretreatment of BMØ obtained from background mice exhibited a significant increase in E. coli phagocytosis (MFI of phagocytosed E. coli: 9983±898 vs. 7983±380; P<0.05; Fig. 5C). In contrast, BMØ obtained from CD14−/− mice demonstrated no change in E. coli phagocytosis with isoproterenol pretreatment (MFI of phagocytosed E. coli: 7497±316 vs. 7269±318; Fig. 5D). Our results indicate that isoproterenol changes the expression of CD14 but not TLR4-MD-2 to augment E. coli phagocytosis. However, increased CD14 expression by isoproterenol is likely to enhance the coexpression of CD14/TLR4/MD-2 complex formation, thereby promoting E. coli phagocytosis.
Figure 5. CD14 and not TLR4-MD-2 complex is altered by isoproterenol.
BMØ from CD14−/− mice and background strain stimulated with isoproterenol or vehicle were incubated with ultra-pure LPS or E. coli for 30 min. Staining with F4/80-FITC, TLR4-MD-2-PE-Cy7, and CD14-APC was followed by FACS analysis. Percentage of all F4/80+ BMØ expressing the TLR4-MD-2 complex or membrane CD14 was determined by FACS analyses. (A) Percentage of F480+ BMØ expressing the TLR4-MD-2 complex is not altered by isoproterenol in CD14−/− mice or the background strain. (B) Isoproterenol treatment augments CD14 expression in BMØ obtained from background strain and not CD14−/− mice. Data are given as mean ± sem. *P < 0.05 versus vehicle; n = 4. (C and D) Histograms representing the MFI of phagocytosed GFP-E. coli in F480+ BMØ from the background strain and CD14−/− mice, respectively. Black lines represent isoproterenol treatment, and shaded areas represent vehicle control. *P < 0.05 versus vehicle. (E) Isoproterenol-induced, live E. coli phagocytosis is mediated by PKA signaling. BMØ, obtained from CD14-intact and CD14−/− mice, were pretreated with H-89 (25 μM) before adding isoproterenol (1 μM) or vehicle overnight, and E. coli phagocytosis was determined. The bar graph is the representation of the PI (mean±sem). Blocking with H-89 completely abrogated an isoproterenol-mediated increase in PI in BMØ obtained from CD14-intact background mice, and neither isoproterenol nor H-89 had any effect on CD14−/− mice. *P < 0.05 versus vehicle; n = 4.
One potential explanation for the role of isoproterenol in E. coli phagocytosis is that by changing the expression of CD14, the stoichiometry of CD14/TLR4-MD-2 complex formation is favored, leading to an increase in bacterial binding to this complex and eventual phagocytosis [36]. This theory is strengthened further by the report from Ulevitch's group [27], demonstrating that LPS binds to TLR4 and MD-2 only when coexpressed with CD14. Garcia et al. [19] have reported that norepinephrine (10 μM) did not modify adherence but modulated the phagocytic process of MØ, which can be blocked by the β-antagonist propranolol, thereby eliminating the possibility of increased bacterial adherence in the present study. Similar to our finding that isoproterenol augments membrane CD14 in a PKA-dependent mechanism, PGE2 and 8-Br-cAMP have been shown to stimulate CD14 gene expression in RAW 264.7 cells, a MØ cell line, and primary cultures [17, 37]. More importantly, we demonstrate the essential role of CD14 in isoproterenol-mediated E. coli phagocytosis, with BMØ obtained from CD14−/− mice. Moreover, anti-CD14 mAb administration following i.v. E. coli injections resulted in bacteremia with significant tissue dissemination [38]. Nonetheless, the physiologic significance of isoproterenol-induced MØ CD14 expression and bacterial phagocytosis is still not clear. However, increased plasma levels of catecholamine and neuroendocrine hormones are evident in patients ensuing trauma, burns, sepsis, and septic shock [3, 4, 39] and warrant further investigations.
Finally, we questioned whether isoproterenol-induced, live E. coli phagocytosis is also mediated through PKA signaling. We carried out live E. coli phagocytosis experiments in BMØ obtained from CD14−/− mice pretreated with H-89 prior to the addition of isoproterenol. PI results are represented as a bar graph in Fig. 5E. H-89 completely abrogated an isoproterenol-mediated increase in PI in BMØ obtained from background mice (vehicle: 5825±410; isoproterenol: 8164±789; H-89+isoproterenol: 6336±514; P<0.05 vs. vehicle and H-89+isoproterenol), and neither isoproterenol nor H-89 had any effect on CD14−/− mice (vehicle: 5207±278; isoproterenol: 5575±304; H-89+isoproterenol: 5027±459). These results demonstrate that in parallel to CD14 expression, isoproterenol-induced E. coli phagocytosis is also mediated via PKA signaling in BMØ. Nonetheless, in vivo CD14 inhibition studies in different models of sepsis are inconclusive [38, 40, 41] and warrant further investigation.
CD14 is an essential but not a vital comolecule for LPS-mediated cellular responses, as CD14−/− mice are able to generate cellular responses to LPS at high doses. Gram-negative bacteria are recognized specifically by TLR4 receptors, and TLR4 is implicated in directing MØ phagocytosis of bacteria by p38 MAPK-mediated signaling [42]. Monocytes have also been shown to phagocytose Gram-negative bacteria in a CD14-dependent mechanism [29]. Based on our results, BMØ subjected to short incubation periods (<1 h) with the β-adrenergic agonist reduced a phagocytic response significantly, whereas longer incubations (>8 h) surprisingly increased live E. coli uptake in BMØ, correlating with increased CD14 expression at later time-points. Therefore, extended (18 h) pretreatment with isoproterenol, resulting in increased E. coli uptake, may be dependent on CD14, whereas the short-time (1 h) inhibition response may be mediated by other signals independent of CD14 expression. This biphasic, phagocytic response to live E. coli uptake with time-dependent β-adrenergic stimulation of BMØ is interesting and novel. In fact, results from short-term incubations with isoproterenol compare with other reports that show inhibition of phagocytic responses [43], suggestive of a disparity in adrenergic regulation of phagocytosis. Emerging evidences demonstrating an opposing inflammatory response in MØ to different stimuli [44] and a cAMP-dependent dual effect on splenic MØ phagocytosis [45] challenge the central dogma on β-adrenergic influence in immune cells. Here, we report that stimulation of BMØ with isoproterenol for 8–18 h augmented E. coli phagocytosis, and 1-h stimulation decreased E. coli phagocytosis, emphasizing the possibility of a time-dependent dual effect.
Catecholamines influence E. coli phagocytosis in BMØ
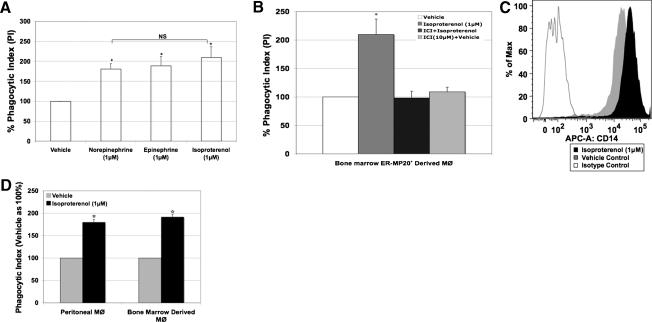
Our results demonstrating that an isoproterenol-mediated increase in E. coli phagocytosis corresponds to CD14 expression prompted us to test whether the CAs norepinephrine and epinephrine will have similar effects. BMØ were stimulated with and without norepinephrine, epinephrine, and isoproterenol (1 μM) overnight, and phagocytosis experiments were carried out using live E. coli and analyzed by flow cytometry. The percent PI was calculated as detailed in Materials and Methods. BMØ treated with norepinephrine and epinephrine for 18 h exhibited a significant increase in PI, similar to the pharmacologic agent isoproterenol in comparison with vehicle control (norepinephrine: 181±13%; epinephrine: 189±23%; isoproterenol: 210±27 vs. vehicle: 100%; P<0.05; Fig. 6A).
Figure 6. (A and B) Isoproterenol-induced increase in BMØ E. coli phagocytosis is mediated through β2-adrenergic receptors.
(A) Percent PI of BMØ stimulated with 1 μM norepinephrine, epinephrine, or isoproterenol for 18 h. (B) Percent PI of BMØ pretreated with a selective β2-adrenergic antagonist ICI118551 (ICI) compound 30 min before the addition of isoproterenol. (C and D) Elicited PMØ response to isoproterenol stimulation. Sodium caseinate-elicited PMØ, obtained by Ficol density gradient, were stimulated with isoproterenol (1 μM) or vehicle overnight and stained with antibodies for anti-CD14-APC and anti-F4/80-FITC or were subjected to E. coli phagocytosis, stained with F4/80-PE, and analyzed by flow cytometry. (C) Representative histogram of CD14 expression in F4/80+ PMØ with MFI on the x-axis and percentage of cells on the y-axis. The area under the gray line is the isotype control. (D) Bar graph represents the PI (mean±sem) of PMØ and compared with BMØ (from B). Data are given as mean ± sem. *P < 0.05 versus vehicle; n = 4.
Next, to test the premise that if β2-adrenergic receptors are involved in isoproterenol-induced E. coli uptake then, BMØ were pretreated with a specific β2-adrenergic antagonist 30 min before isoproterenol stimulation [8]. The ICI118551 compound (β2-adrenergic receptor antagonist) inhibited isoproterenol-induced phagocytosis completely (Fig. 6B), implying the engagement of β2-adrenergic receptors.
PMØs demonstrate similar responses to isoproterenol stimulation
To ascertain that MØ, isolated from a different tissue bed, will similarly influence CD14 expression and E. coli phagocytosis, we included studies using elicited PMØ. β-Adrenergic stimulation of PMØ with isoproterenol shifts the MFI of CD14 cell surface expression in comparison with vehicle treatment (34,910±1394 vs.19,235±977; P<0.05; Fig. 6C). In parallel, isoproterenol also augmented PMØ E. coli phagocytosis by 80% (Fig. 6D). Results outlined from this study not only rule out the polarizing effects as a result of standard ex vivo culture conditions used in BMØ but also demonstrate the universal effect of isoproterenol stimulation on CD14 expression and bacterial phagocytosis in elicited PMØ as well.
Our results indicating enhanced, live E. coli phagocytosis following adrenergic stimulation in BMØ and elicited PMØ are in line with the observation of Garcia et al. [19], who noticed an increase in latex bead uptake by PMØ stimulated with norepinephrine. Similarly, an independent investigation reports that dibutyryl cAMP-elevating agents can augment phagocytosis of opsonized sheep erthrocytes in PMØ [46]. In contrast, Zetterlund et al. [47] claim that the β-agonist terbutaline had no effect on phagocytosis of FITC-labeled E. coli by alveolar MØ. The discrepancy may be a result of the fact that these investigators used complement-opsonized, FITC-labeled E. coli for their phagocytosis studies, engaging a different phagocytic system [48]. Unlike live bacteria, opsonized sheep erythrocytes and FITC-labeled E. coli uptake may not involve TLR4 or CD14. Our phagocytosis measurements made with live E. coli cultured overnight at 37°C are more clinically relevant and distinct from other studies.
In summary, this study establishes a time- and dose-dependent β-adrenergic augmentation of membrane CD14 in MØ via PKA signaling. The data provided here support an isoproterenol-induced, time-dependent, biphasic E. coli phagocytic response in BMØ. β-Adrenergic stimulation of BMØ for >8 h increased E. coli uptake through enhanced CD14 expression via PKA signaling, and less than a 1-h incubation decreased the BMØ phagocytic response by an unknown mechanism.
Supplementary Material
ACKNOWLEDGMENTS
Funding from National Institutes of Health grants R03 AI 079530-01 and RO1 GM 42577 and Dr. Ralph and Marion C. Falk Medical Research Trust supported this work.
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
- 8-Br-cAMP
- 8-bromoadenosine cAMP
- APC
- allophycocyanin
- BMØ
- bone marrow-derived macrophage
- CA
- catecholamine
- MØ
- macrophage
- MD-2
- myeloid-differentiation protein 2
- MFI
- mean fluorescence intensity
- PI
- phagocytic index
- PKA C-α
- α isoform of the catalytic subunit C
- PMØ
- peritoneal macrophage
- siRNA
- small interfering RNA
REFERENCES
- 1.Landmann R., Zimmerli W., Sansano S., Link S., Hahn A., Glauser M. P., Calandra T. (1995) Increased circulating soluble CD14 is associated with high mortality in gram-negative septic shock. J. Infect. Dis. 171, 639–644 [DOI] [PubMed] [Google Scholar]
- 2.Angus D. C., Linde-Zwirble W. T., Lidicker J., Clermont G., Carcillo J., Pinsky M. R. (2001) Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit. Care Med. 29, 1303–1310 [DOI] [PubMed] [Google Scholar]
- 3.Goodall M., Stone C., Haynes B. W., Jr. (1957) Urinary output of adrenaline and noradrenaline in severe thermal burns. Ann. Surg. 145, 479–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benedict C. R., Grahame-Smith D. G. (1978) Plasma noradrenaline and adrenaline concentrations and dopamine-β-hydroxylase activity in patients with shock due to septicemia, trauma and hemorrhage. Q. J. Med. 47, 1–20 [PubMed] [Google Scholar]
- 5.Welscher H. D., Cruchaud A. (1978) Conditions for maximal synthesis of cyclic AMP by mouse macrophages in response to β-adrenergic stimulation. Eur. J. Immunol. 8, 180–184 [DOI] [PubMed] [Google Scholar]
- 6.Bourne H. R., Lichtenstein L. M., Melmon K. L., Henney C. S., Weinstein Y., Shearer G. M. (1974) Modulation of inflammation and immunity by cyclic AMP. Science 184, 19–28 [DOI] [PubMed] [Google Scholar]
- 7.Thivierge M., Le Gouill C., Tremblay M. J., Stankova J., Rola-Pleszczynski M. (1998) Prostaglandin E2 induces resistance to human immunodeficiency virus-1 infection in monocyte-derived macrophages: downregulation of CCR5 expression by cyclic adenosine monophosphate. Blood 92, 40–45 [PubMed] [Google Scholar]
- 8.Muthu K., Deng J., Romano F., He L. K., Gamelli R., Shankar R., Jones S. B. (2005) Thermal injury and sepsis modulates β-adrenergic receptors and cAMP responses in monocyte-committed bone marrow cells. J. Neuroimmunol. 165, 129–138 [DOI] [PubMed] [Google Scholar]
- 9.Ulevitch R. J., Tobias P. S. (1999) Recognition of gram-negative bacteria and endotoxin by the innate immune system. Curr. Opin. Immunol. 11, 19–22 [DOI] [PubMed] [Google Scholar]
- 10.Deng J., Muthu K., Gamelli R., Shankar R., Jones S. B. (2004) Adrenergic modulation of splenic macrophage cytokine release in polymicrobial sepsis. Am. J. Physiol. Cell Physiol. 287, C730–C736 [DOI] [PubMed] [Google Scholar]
- 11.Muthu K., Deng J., Gamelli R., Shankar R., Jones S. B. (2005) Adrenergic modulation of cytokine release in bone marrow progenitor-derived macrophage following polymicrobial sepsis. J. Neuroimmunol. 158, 50–57 [DOI] [PubMed] [Google Scholar]
- 12.Bergmann M., Sautner T. (2002) Immunomodulatory effects of vasoactive catecholamines. Wien. Klin. Wochenschr. 114, 752–761 [PubMed] [Google Scholar]
- 13.Devitt A., Moffatt O. D., Raykundalia C., Capra J. D., Simmons D. L., Gregory C. D. (1998) Human CD14 mediates recognition and phagocytosis of apoptotic cells. Nature 392, 505–509 [DOI] [PubMed] [Google Scholar]
- 14.Schlegel R. A., Krahling S., Callahan M. K., Williamson P. (1999) CD14 is a component of multiple recognition systems used by macrophages to phagocytose apoptotic lymphocytes. Cell Death Differ. 6, 583–592 [DOI] [PubMed] [Google Scholar]
- 15.Jack R. S., Grunwald U., Stelter F., Workalemahu G., Schutt C. (1995) Both membrane-bound and soluble forms of CD14 bind to gram-negative bacteria. Eur. J. Immunol. 25, 1436–1441 [DOI] [PubMed] [Google Scholar]
- 16.Flynn J. M., Lannigan D. A., Clark D. E., Garner M. H., Cammarata P. R. (2008) RNA suppression of ERK2 leads to collapse of mitochondrial membrane potential with acute oxidative stress in human lens epithelial cells. Am. J. Physiol. Endocrinol. Metab. 294, E589–E599 [DOI] [PubMed] [Google Scholar]
- 17.Liu S., Morris S. M., Jr., Nie S., Shapiro R. A., Billiar T. R. (2000) cAMP induces CD14 expression in murine macrophages via increased transcription. J. Leukoc. Biol. 67, 894–901 [DOI] [PubMed] [Google Scholar]
- 18.Garcia J. J., del Carmen Saez M., De la Fuente M., Ortega E. (2003) Noradrenaline and its end metabolite 3-methoxy-4-hydroxyphenylglycol inhibit lymphocyte chemotaxis: role of α- and β-adrenoreceptors. Mol. Cell. Biochem. 254, 305–309 [DOI] [PubMed] [Google Scholar]
- 19.Garcia J. J., del Carmen Saez M., De la Fuente M., Ortega E. (2003) Regulation of phagocytic process of macrophages by noradrenaline and its end metabolite 4-hydroxy-3-metoxyphenyl-glycol. Role of α- and β-adrenoreceptors. Mol. Cell. Biochem. 254, 299–304 [DOI] [PubMed] [Google Scholar]
- 20.Insel P. A. (1996) Seminars in medicine of the Beth Israel Hospital, Boston. Adrenergic receptors—evolving concepts and clinical implications. N. Engl. J. Med. 334, 580–585 [DOI] [PubMed] [Google Scholar]
- 21.Thomson S., Clayton A. L., Hazzalin C. A., Rose S., Barratt M. J., Mahadevan L. C. (1999) The nucleosomal response associated with immediate-early gene induction is mediated via alternative MAP kinase cascades: MSK1 as a potential histone H3/HMG-14 kinase. EMBO J. 18, 4779–4793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kitchens R. L., Wang P., Munford R. S. (1998) Bacterial lipopolysaccharide can enter monocytes via two CD14-dependent pathways. J. Immunol. 161, 5534–5545 [PubMed] [Google Scholar]
- 23.Miyake K. (2004) Endotoxin recognition molecules, Toll-like receptor 4-MD-2. Semin. Immunol. 16, 11–16 [DOI] [PubMed] [Google Scholar]
- 24.Shimazu R., Akashi S., Ogata H., Nagai Y., Fukudome K., Miyake K., Kimoto M. (1999) MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J. Exp. Med. 189, 1777–1782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akashi S., Shimazu R., Ogata H., Nagai Y., Takeda K., Kimoto M., Miyake K. (2000) Cutting edge: cell surface expression and lipopolysaccharide signaling via the Toll-like receptor 4-MD-2 complex on mouse peritoneal macrophages. J. Immunol. 164, 3471–3475 [DOI] [PubMed] [Google Scholar]
- 26.Nagai Y., Akashi S., Nagafuku M., Ogata M., Iwakura Y., Akira S., Kitamura T., Kosugi A., Kimoto M., Miyake K. (2002) Essential role of MD-2 in LPS responsiveness and TLR4 distribution. Nat. Immunol. 3, 667–672 [DOI] [PubMed] [Google Scholar]
- 27.Da Silva Correia J., Soldau K., Christen U., Tobias P. S., Ulevitch R. J. (2001) Lipopolysaccharide is in close proximity to each of the proteins in its membrane receptor complex. Transfer from CD14 to TLR4 and MD-2. J. Biol. Chem. 276, 21129–21135 [DOI] [PubMed] [Google Scholar]
- 28.Visintin A., Latz E., Monks B. G., Espevik T., Golenbock D. T. (2003) Lysines 128 and 132 enable lipopolysaccharide binding to MD-2, leading to Toll-like receptor-4 aggregation and signal transduction. J. Biol. Chem. 278, 48313–48320 [DOI] [PubMed] [Google Scholar]
- 29.Grunwald U., Fan X., Jack R. S., Workalemahu G., Kallies A., Stelter F., Schutt C. (1996) Monocytes can phagocytose Gram-negative bacteria by a CD14-dependent mechanism. J. Immunol. 157, 4119–4125 [PubMed] [Google Scholar]
- 30.Schiff D. E., Kline L., Soldau K., Lee J. D., Pugin J., Tobias P. S., Ulevitch R. J. (1997) Phagocytosis of gram-negative bacteria by a unique CD14-dependent mechanism. J. Leukoc. Biol. 62, 786–794 [DOI] [PubMed] [Google Scholar]
- 31.Haziot A., Ferrero E., Kontgen F., Hijiya N., Yamamoto S., Silver J., Stewart C. L., Goyert S. M. (1996) Resistance to endotoxin shock and reduced dissemination of gram-negative bacteria in CD14-deficient mice. Immunity 4, 407–414 [DOI] [PubMed] [Google Scholar]
- 32.Alaniz R. C., Thomas S. A., Perez-Melgosa M., Mueller K., Farr A. G., Palmiter R. D., Wilson C. B. (1999) Dopamine β-hydroxylase deficiency impairs cellular immunity. Proc. Natl. Acad. Sci. USA 96, 2274–2278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gosain A., Muthu K., Gamelli R. L., DiPietro L. A. (2007) Norepinephrine suppresses wound macrophage phagocytic efficiency through α- and β-adrenoreceptor dependent pathways. Surgery 142, 170–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hume D. A., Robinson A. P., MacPherson G. G., Gordon S. (1983) The mononuclear phagocyte system of the mouse defined by immunohistochemical localization of antigen F4/80. Relationship between macrophages, Langerhans cells, reticular cells, and dendritic cells in lymphoid and hematopoietic organs. J. Exp. Med. 158, 1522–1536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leon B., Lopez-Bravo M., Ardavin C. (2005) Monocyte-derived dendritic cells. Semin. Immunol. 17, 313–318 [DOI] [PubMed] [Google Scholar]
- 36.Latz E., Visintin A., Lien E., Fitzgerald K. A., Monks B. G., Kurt-Jones E. A., Golenbock D. T., Espevik T. (2002) Lipopolysaccharide rapidly traffics to and from the Golgi apparatus with the Toll-like receptor 4-MD-2-CD14 complex in a process that is distinct from the initiation of signal transduction. J. Biol. Chem. 277, 47834–47843 [DOI] [PubMed] [Google Scholar]
- 37.Iwahashi H., Takeshita A., Hanazawa S. (2000) Prostaglandin E2 stimulates AP-1-mediated CD14 expression in mouse macrophages via cyclic AMP-dependent protein kinase A. J. Immunol. 164, 5403–5408 [DOI] [PubMed] [Google Scholar]
- 38.Opal S. M., Palardy J. E., Parejo N., Jasman R. L. (2003) Effect of anti-CD14 monoclonal antibody on clearance of Escherichia coli bacteremia and endotoxemia. Crit. Care Med. 31, 929–932 [DOI] [PubMed] [Google Scholar]
- 39.Berczi I. (1998) Neurohormonal host defense in endotoxin shock. Ann. N. Y. Acad. Sci. 840, 787–802 [DOI] [PubMed] [Google Scholar]
- 40.Frevert C. W., Matute-Bello G., Skerrett S. J., Goodman R. B., Kajikawa O., Sittipunt C., Martin T. R. (2000) Effect of CD14 blockade in rabbits with Escherichia coli pneumonia and sepsis. J. Immunol. 164, 5439–5445 [DOI] [PubMed] [Google Scholar]
- 41.Thorgersen E. B., Hellerud B. C., Nielsen E. W., Barratt-Due A., Fure H., Lindstad J. K., Pharo A., Fosse E., Tonnessen T. I., Johansen H. T., Castellheim A., Mollnes E. (2010) CD14 inhibition efficiently attenuates early inflammatory and hemostatic responses in Escherichia coli sepsis in pigs. FASEB J. 24, 712–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blander J. M., Medzhitov R. (2004) Regulation of phagosome maturation by signals from Toll-like receptors. Science 304, 1014–1018 [DOI] [PubMed] [Google Scholar]
- 43.Kamanova J., Kofronova O., Masin J., Genth H., Vojtova J., Linhartova I., Benada O., Just I., Sebo P. (2008) Adenylate cyclase toxin subverts phagocyte function by RhoA inhibition and unproductive ruffling. J. Immunol. 181, 5587–5597 [DOI] [PubMed] [Google Scholar]
- 44.Szelenyi J., Selmeczy Z., Brozik A., Medgyesi D., Magocsi M. (2006) Dual β-adrenergic modulation in the immune system: stimulus-dependent effect of isoproterenol on MAPK activation and inflammatory mediator production in macrophages. Neurochem. Int. 49, 94–103 [DOI] [PubMed] [Google Scholar]
- 45.Roy B., Rai U. (2004) Dual mode of catecholamine action on splenic macrophage phagocytosis in wall lizard, Hemidactylus flaviviridis. Gen. Comp. Endocrinol. 136, 180–191 [DOI] [PubMed] [Google Scholar]
- 46.Vogel S. N., Weedon L. L., Oppenheim J. J., Rosenstreich D. L. (1981) Defective Fc-mediated phagocytosis in C3H/HeJ macrophages. II. Correction by cAMP agonists. J. Immunol. 126, 441–445 [PubMed] [Google Scholar]
- 47.Zetterlund A., Larsson P. H., Muller-Suur C., Palmberg L., Larsson K. (1998) Budesonide but not terbutaline decreases phagocytosis in alveolar macrophages. Respir. Med. 92, 162–166 [DOI] [PubMed] [Google Scholar]
- 48.Underhill D. M., Ozinsky A. (2002) Phagocytosis of microbes: complexity in action. Annu. Rev. Immunol. 20, 825–852 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.