IFNγ produced by intraperitoneal myeloid and NK cells during cecal ligation/puncture-induced septic shock facilitates myeloid cell activation yet blockade of IFNγ does not improve survival.
Keywords: shock, natural killer cells, myeloid cells, inflammation
Abstract
Several studies indicate that IFN-γ facilitates systemic inflammation during endotoxin-induced shock. However, the pathobiology of IFN-γ in clinically relevant models of septic shock, such as CLP, is not well understood. In this study, the role of IFN-γ in the pathogenesis of CLP-induced septic shock was evaluated by examining IFN-γ production at the tissue and cellular levels. The impact of IFN-γ neutralization on systemic inflammation, bacterial clearance, and survival was also determined. Following CLP, concentrations of IFN-γ in plasma and peritoneal lavage fluid were low in comparison with concentrations of IL-6 and MIP-2, as was IFN-γ mRNA expression in liver and spleen. The overall percentage of IFN-γ+ splenocytes was <5% after CLP and not statistically different from control mice. Intracellular IFN-γ was present in a large proportion of peritoneal exudate cells after CLP, primarily in infiltrating myeloid cells and NK cells. i.p. myeloid cell activation was decreased in IFN-γKO mice, and plasma concentrations of IL-6 and MIP-2 were significantly lower in IFN-γKO mice and in mice treated with anti-IFN-γ compared with controls, but bacterial clearance was not affected. IFN-γKO mice were resistant to CLP-induced mortality when treated with systemic antibiotics. However, neutralization of IFN-γ with blocking antibodies did not improve survival significantly. These studies show that IFN-γ facilitates the proinflammatory response during CLP-induced septic shock. However, neutralization of IFN-γ did not improve survival uniformly.
Introduction
IFN-γ, the sole member of the type II IFN family, is a homodimeric protein classically produced by NK cells and T lymphocytes, although cells of the monocyte/macrophage lineage are also a source of IFN-γ [1–3]. Its production by NK and T cells is regulated primarily by the actions of myeloid cell-derived IL-12 and IL-18, as well as by antigen presentation to Th1 and Tc1 lymphocytes [4, 5]. The functions of IFN-γ during bacterial infections and sepsis are multifaceted. Experimental studies show that IFN-γ is essential for an effective host response to a variety of pathogens including vaccinia virus, Leishmania major, Toxoplasma gondii, Listeria monocytogenes, and minimally pathogenic Mycobacteria [6–9]. Similar to findings in mouse studies, humans with IFN-γR mutations show increased susceptibility to Mycobacteria species, some viruses, and intracellular bacteria such as Listeria [10–12]. However, increased susceptibility to infection with common bacterial and fungal pathogens has not been reported in IFN-γ-deficient humans, although decreased neutrophil mobilization and NK cell activation have been observed [13]. Interestingly, IFN-γ polymorphisms have been associated with increased longevity in humans, presumably as a result of the decreased predominance of inflammation-associated diseases such as atherosclerosis, neurodegeneration, and diabetes [14, 15].
IFN-γ is necessary for induction of some LPS-responsive genes such as iNOS, and it facilitates the production of several proinflammatory cytokines and chemokines [16, 17]. The systemic response to LPS and the development of LPS-induced shock are facilitated by IFN-γ, and mice deficient of IFN-γ or IFN-γR are resistant to LPS-induced toxicity [18–21]. In addition, antibody-mediated blockade of IFN-γ attenuates systemic inflammation and improves survival in mice challenged with an otherwise lethal dose of LPS [22]. Other studies indicate that IFN-γ contributes to systemic inflammation during more clinically relevant models of sepsis such as CLP. Miles et al. [23] showed that systemic administration of IFN-γ after CLP worsens systemic inflammation and increases mortality. Other studies indicate that IFN-γ contributes to CLP-induced pulmonary inflammation and that antibody-mediated blockade of IFN-γ will improve survival after CLP [24, 25]. However, our recent studies show that the plasma concentrations of IFN-γ observed after CLP are markedly lower than those reported after systemic LPS administration, which raised questions as to the mechanisms of IFN-γ-facilitated inflammation during CLP-induced shock. Therefore, we examined IFN-γ production at the systemic, local, and cellular levels and the impact of IFN-γ on the activation of specific leukocyte populations to dissect mechanisms of IFN-γ-facilitated inflammation during CLP-induced septic shock. The effects of IFN-γ blockade on systemic inflammation, bacterial clearance, and survival were also assessed.
MATERIALS AND METHODS
Mice
Female, 8- to 10-week-old C57BL/6J WT and IFN-γKO (B6.129S7-IFN-gtm1tsγ/J) mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). All studies were approved by the Institutional Animal Care and Use Committee at the University of Texas Medical Branch (Galveston, TX, USA) and met National Institutes of Health guidelines for the care and use of experimental animals. For IFN-γ neutralization, mice received i.v. injection with 200 μg azide-free, functional-grade neutralizing anti-IFN-γ (eBioscience, San Diego, CA, USA; clone XMG1.2) at 1 h before and 24 h after CLP. Control mice received an injection of nonspecific IgG in the same regimen.
CLP
Mice were anesthetized with 2% isoflurane in oxygen via facemask and presented to the surgeon in a blinded and random manner to minimize experimental bias. A 1- to 2-cm midline incision was made through the abdominal wall. The cecum was identified and ligated 1.5 cm from the tip with a 3–0 silk tie. A double puncture of the cecum was performed using a 20-gauge needle. Great care was taken to avoid ligation-induced obstruction of flow between the ileum and colon. The cecum was returned to the abdominal cavity, and the incision was closed with surgiclips. All mice were resuscitated by i.p. injection with 1 ml LR solution alone or LR solution containing imipenem/cilistatin (Primaxin, Merck and Co., Whitehouse Station, NJ, USA; 25 mg/kg) immediately after CLP and twice daily thereafter. Control mice did not receive surgical manipulation.
ELISA
Heparinized blood was obtained by carotid laceration in mice anesthetized with 2% isoflurane, and plasma was harvested from centrifuged blood (1200 g for 10 min). Peritoneal lavage was performed with 5 ml unsupplemented RPMI-1640 medium. Peritoneal cells were removed by centrifugation, and the supernatant was harvested for analysis. IL-6 and MIP-2 concentrations in plasma and peritoneal lavage fluid were measured using an ELISA, according to the manufacturer's protocol (eBioscience).
Microbiology
Bacterial counts were performed on aseptically harvested blood and peritoneal fluid. All fluid and tissues were harvested under 2% isoflurane anesthesia. Blood was obtained by carotid laceration. Peritoneal fluid was harvested by injection and aspiration of 5 ml sterile PBS into the peritoneal cavity after aseptic preparation of the abdominal wall. Samples were serially diluted in sterile PBS and cultured on tryptic soy agar plates, which were incubated (37°C) for 48–72 h, and colony counts were performed. Anaerobic conditions were achieved using an anaerobic chamber and the BBL GasPak Plus anaerobic system (Becton Dickinson, Sparks, MD, USA).
Flow cytometry
For splenocyte isolation, spleens were excised and placed in cold (4°C) RPMI-1640 medium containing 10% FBS (complete RPMI). Spleens were dissociated with the rubber tip of a 1-ml syringe plunger, and erythrocytes were lysed by incubation in erythrocyte lysis buffer (R&D Systems, Minneapolis, MN, USA). Splenocytes were then washed with cold complete RPMI-1640 medium supplemented with 10% FBS and stored on ice prior to antibody labeling. Peritoneal leukocytes were harvested by peritoneal lavage with cold complete RPMI-1640 medium. Leukocytes were incubated with anti-mouse CD16/32 (eBioscience) at a concentration of 1 μg/1 million cells for 30 min at 4°C to block nonspecific binding of labeling antibodies to FcRs. Cells (1×106/tube) were placed in polystyrene tubes containing labeling antibodies or isotype controls (0.5–1 μg antibody/tube), incubated (4°C) for 30 min, and washed with 2 ml cold PBS. For intracellular staining, cells were incubated with Cytofix/Cytoperm buffer (BD Biosciences, San Diego, CA, USA) prior to incubation with antibodies. In most cases, cells were fixed with 250 μl 1% paraformaldehyde. Samples were analyzed with a FACSCanto flow cytometer (BD Biosciences). Data were analyzed using FlowJo software (Tree Star, Inc., Ashland, OR, USA).
For multispectral imaging flow cytometric analysis of nuclear morphology, peritoneal leukocytes were harvested and prepared as described above. Cells were surface-labeled with fluorochrome-conjugated antibodies against F4-80, Gr1, Ly6C, and/or Ly6G (eBioscience). The cells were fixed in 1% paraformaldehyde and shipped overnight to Amnis Corp. (Seattle, WA, USA). Upon arrival, the cells were stained with PI, and images were collected on more than 10,000 cells/sample using the ImageStream flow cytometer. Single-color controls were collected and used to calculate a spectral crosstalk compensation matrix to compensate the imagery. Using algorithms in the IDEAS image analysis software (Amnis Corp.), the cells were immunophenotyped, and the nuclei were analyzed for morphological characteristics.
RNase protection assay
Spleen, heart, liver, and lung were harvested and flash-frozen in liquid nitrogen. Samples were stored at −80°C until used. Total RNA was isolated using Tri-Reagent (Molecular Research Center, Cincinnati, OH, USA). The RNase protection assay was performed using the Riboquant system (BD PharMingen, San Diego, CA, USA), per the manufacturer's instructions. Briefly, radiolabeled RNA probes were synthesized from DNA template sets using T7 RNA polymerase, 32P-UTP, and pooled, nonradiolabeled nucleotides. Isolated total RNAs (20 μg/sample) were hybridized with the purified riboprobes and subjected to RNase digestion. Protected RNA species were separated on 5% polyacrylamide sequencing gels using 0.5× Tris-borate-EDTA running buffer. Gels were run at 50 W constant power for 70 min and dried under vacuum, and the protected fragments were visualized using autoradiography.
Statistics
All data were analyzed using GraphPad Prism software (GraphPad Software, San Diego, CA, USA). Survival curves were compared using the Log Rank test. For temperature, acid base, and cytokine measurements, the mean and sem were calculated. Data from multiple group experiments were analyzed using one-way ANOVA followed by a post-hoc Tukey test to compare groups. For measurements of bacterial CFU, groups were compared using a nonparametric Kruskal-Wallis test, followed by a post-hoc Dunn's test. A value of P < 0.05 was considered statistically significant for all experiments. All values are presented as the mean ± se, except for bacterial counts, which are presented as scatter plots, and the median value is designated.
RESULTS
Local and systemic IFN-γ production during CLP-induced septic shock
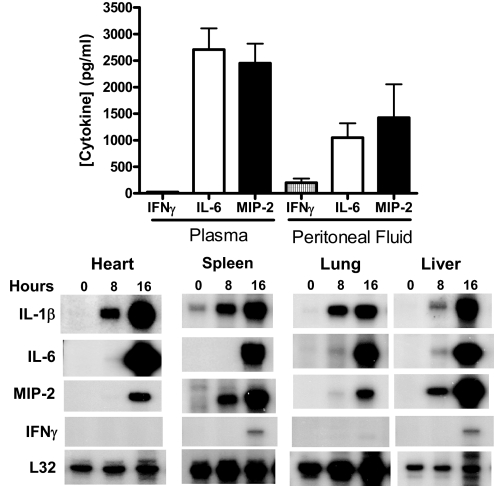
Studies were undertaken to assess tissues that are remote from the primary site of infection (Fig. 1). Examination of plasma at 16 h after CLP showed that IFN-γ was present in relatively low concentrations (<30 pg/ml) compared with IL-6 and MIP-2 (Fig. 1). Concentrations of IFN-γ in peritoneal lavage fluid were higher than in plasma at 16 h after CLP but were lower than IL-6 and MIP-2 concentrations. Evaluation of earlier time-points after CLP showed that IFN-γ concentrations at 4 and 8 h after CLP were lower than at the 16-h time-point (data not shown). Examination of proinflammatory cytokine mRNA expression in solid tissues showed that IL-1β, IL-6, and MIP-2 mRNAs were expressed at high levels in heart, spleen, lung, and liver after CLP. However, IFN-γ mRNA expression was not detectable in heart and lung and compared with the other cytokines analyzed, was expressed at relatively low levels in spleen and liver (Fig. 1).
Figure 1. Local and systemic IFN-γ, IL-6, and MIP-2 expression after CLP.
Plasma and peritoneal lavage fluid were harvested at 16 h after CLP. IL-6 and MIP-2 concentrations were measured by ELISA. For measurement of cytokine mRNA expression, heart, lung, spleen, and liver were harvested prior to CLP (0 h) and at 8 and 16 h after CLP. Total tissue RNA was isolated, and RNase protection assays were performed. Autoradiographs are typical of results obtained from 3 separate experiments. L32 served as a loading control.
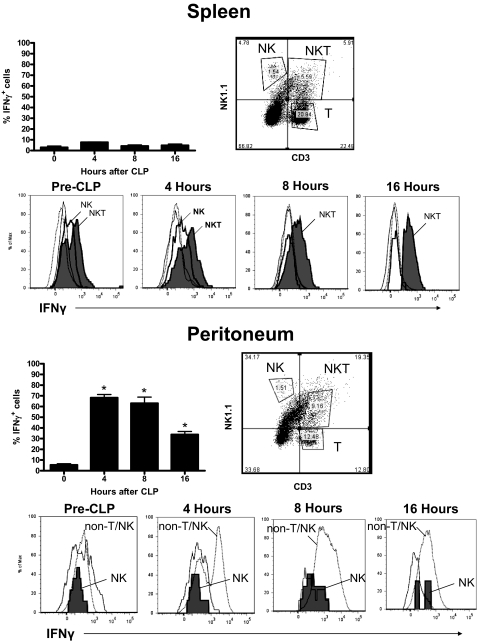
Analysis of intracellular IFN-γ in leukocytes from spleen showed that the percentage of IFN-γ+ splenocytes at 4, 8, and 16 h after CLP was not significantly different from control mice (Time 0), which did not undergo CLP (Fig. 2). Temporal analysis of intracellular IFN-γ in splenocytes showed that IFN-γ expression was predominantly unchanged at 4, 8, and 16 h after CLP compared with control (pre-CLP) mice but might be weakly expressed by NKT (NK1.1+CD3+) cells.
Figure 2. Intracellular IFN-γ in splenic and peritoneal leukocytes after CLP.
Splenic and peritoneal leukocytes were harvested from mice at 0, 4, 8, and 16 h after CLP, and intracellular IFN-γ production was determined by flow cytometry after labeling with fluorochrome-tagged anti-IFN-γ. Specific cell populations were identified by labeling with anti-NK1.1 and anti-CD3. Histograms were generated from the designated cell populations. n = 5 mice/group. *P < 0.05 compared to 0 hours.
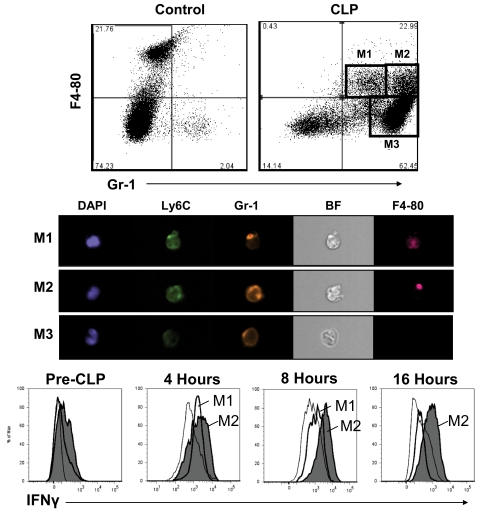
Analysis of IFN-γ-producing cells in the peritoneal cavity after CLP showed that the percentage of i.p. leukocytes that were positive for IFN-γ peaked at 4–8 h (Fig. 2). IFN-γ+ cells in the peritoneal cavity consisted of NK cells and a large proportion of cells that was negative for NK and T cell markers (NK1.1−CD3−). The number of NK cells that were positive for intracellular IFN-γ peaked at 8 h after CLP (59% positive, 3.6±0.7×106 total). Further analysis of NK1.1−CD3− cells showed that CLP caused the recruitment of numerous Gr-1+ cells into the peritoneal cavity (Fig. 3). Further examination of i.p. myeloid cells showed that nonseptic mice (control) possess significant numbers of F4-80+Gr-1− resident peritoneal macrophages and relatively small numbers of F4-80−Gr-1+ neutrophils (Fig. 3). At 16 h after CLP, 3 distinct myeloid cell populations were present in the peritoneal cavity. These consisted of F4-80+Gr-1lo (population M1, 6.0±0.4% of total), F4-80+ Gr-1hi (population M2, 9.1±0.7% of total), and F4-80–Gr-1+ (population M3, 59.7±6.2% of total) cells (Fig. 3). Analysis of these myeloid cell populations using a multispectral flow cytometer showed that populations M1 and M2 had kidney-shaped nuclei, typical of monocyte/macrophages, whereas population M3 had segmented nuclei that are characteristic of neutrophils (Fig. 3, DAPI stain). Multispectral analysis of surface F4-80 and Gr-1 was consistent with the expression pattern observed using conventional flow cytometry. In addition, populations M1 and M2 showed more avid binding of anti-Ly6C than population M3 (Fig. 3). Temporal analysis of intracellular IFN-γ among those cell populations using conventional flow cytometry showed that a large proportion of populations M1 (57.3±3.2%) and M2 (63.1±5.5%) was positive for IFN-γ at 4 h after CLP, whereas the proportion of IFN-γ+ cells in population M3 (8.0±0.5%) was relatively low (Fig. 3). A large proportion of population M2 remained positive for intracellular IFN-γ, out to 16 h after CLP (Fig. 3). The presented results were obtained by preincubating cells with fixation buffer containing saponin and brefeldin A prior to intracellular antibody labeling. As a control, myeloid cells were incubated with anti-IFN-γ without prior incubation with saponin- and brefeldin A-containing fixative. The number of IFN-γ+ myeloid cells was <4% in samples not treated with saponin and brefeldin A (data not shown).
Figure 3. Characterization of peritoneal myeloid cells after CLP.
Peritoneal leukocytes were harvested from control mice and from mice at 16 h after CLP (top panel). For phenotypic analysis, cells were surface-labeled with anti-Gr-1 and anti-F4-80, followed by analysis using a conventional flow cytometer. Nuclear morphology and visualization of surface antigens among specific cell populations were determined using an ImageStream flow cytometer (Amnis Corp.; middle panel). BF, Bright field. Intracellular IFN-γ content was determined in the identified myeloid cell populations by antibody labeling and flow cytometry (bottom panel). Histograms were generated from the designated cell populations. Results are typical of at least 4 different experiments.
Peritoneal myeloid cell activation is attenuated in IFN-γ-deficient mice
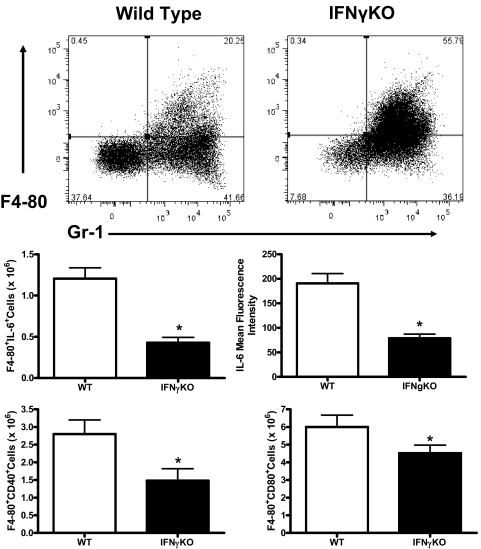
Analysis of peritoneal myeloid cells after CLP showed that the percentages of true neutrophils (F4-80−Gr-1+) were not significantly different when comparing WT and IFN-γKO mice (39±7% vs. 37±4%; Fig. 4). However, the percentage of F4-80+Gr-1+ myeloid cells was significantly (P<0.05) lower in WT mice compared with IFN-γKO mice (22±5% vs. 52±8) at 16 h after CLP (Fig. 4). Myeloid cell activation was attenuated in IFN-γ-deficient mice compared with WT controls, as indicated by decreased numbers of IL-6+ cells and decreased IL-6 mean fluorescence intensity (Fig. 4). The numbers of CD40+ and CD80+ myeloid cells were also significantly lower in IFN-γ-deficient mice compared with WT mice (Fig. 4).
Figure 4. Myeloid cell recruitment and activation in WT and IFN-γ-deficient mice.
Peritoneal leukocytes were harvested from mice at 16 h after CLP, and phenotypic analysis was determined by labeling with anti-Gr-1 and anti-F4-80, followed by flow cytometry. Cellular activation was determined by measurement of intracellular IL-6 and surface CD40 and CD80 expression using antibody-labeling and flow cytometry. n = 5 mice/group; *P < 0.05 compared with WT.
Effects of IFN-γ deficiency and blockade on survival during CLP-induced septic shock
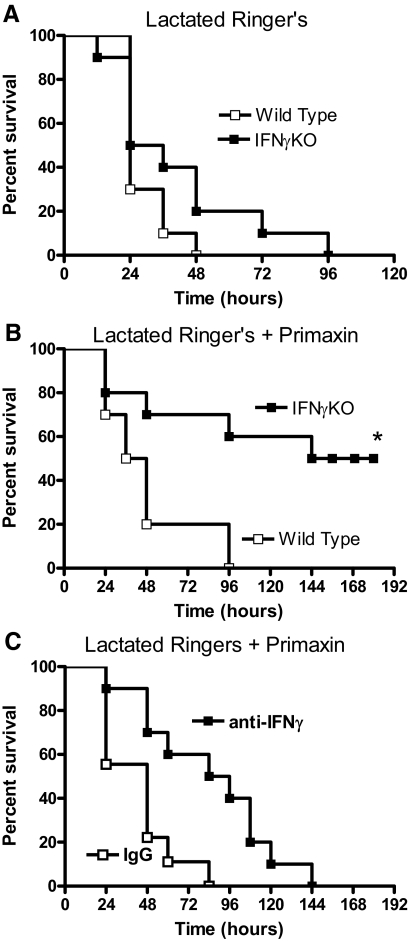
Studies were undertaken to assess sepsis-induced mortality in IFN-γKO mice and in WT mice treated with anti-IFN-γ (Fig. 5). In mice that did not receive antibiotic treatment, there was no significant difference in survival when comparing WT and IFN-γKO mice (Fig. 5A). However, IFN-γKO mice showed significantly improved survival compared with WT mice when mice were treated with fluids and systemic antibiotics (Fig. 5B). Mice that survived until 192 h remained viable and active at 3 weeks after CLP, at which time, the study was terminated. Long-term survival was not improved significantly in mice receiving i.v. treatment with anti-IFN-γ (10 mg/kg×2) compared with mice treated with nonspecific IgG (Fig. 5C). The median survival time was 48 h in IgG-treated mice compared with 84 h in mice receiving anti-IFN-γ. The time to 100% mortality was 84 versus 144 h in IgG and anti-IFN-γ-treated mice, respectively (Fig. 5B). Mice receiving IgG and anti-IFN-γ were treated with fluids and systemic antibiotics.
Figure 5. Survival of mice after CLP.
(A) Survival of WT and IFN-γKO mice after CLP. Mice were treated with LR solution alone and did not receive Primaxin. (B) Survival of WT and IFN-γKO mice after CLP. Beginning immediately after CLP, all mice received i.p. injections of LR containing imipenem/cilistatin (Primaxin, 25 mg/kg). LR and antibiotic treatment were continued twice daily throughout the observation period. *P < 0.05 compared with WT. (C) Survival of WT mice treated with i.p. IgG or anti-IFN-γ (10 mg/kg) 1 h prior to and 24 h after CLP. All mice received i.p. injections of LR containing imipenem/cilistatin (Primaxin, 25 mg/kg). LR and antibiotic treatment were continued twice daily throughout the observation period; n = 10 mice/group.
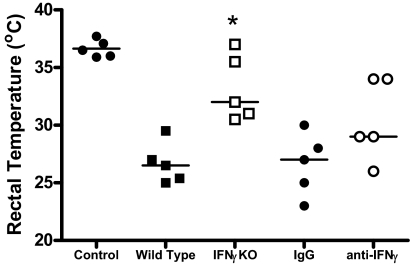
Rectal temperature was also measured in mice as an indicator of the severity of sepsis at 24 h after CLP (Fig. 6). Core body temperature was significantly higher in IFN-γKO mice compared with WT controls (Fig. 6). Rectal temperature was not statistically different when comparing WT mice treated with anti-IFN-γ and nonspecific IgG (Fig. 6).
Figure 6. Rectal temperature in control and IFN-γ-deficient mice after CLP.
In all experiments, rectal temperature was measured at 24 h after CLP. In antibody-treatment experiments, mice received i.v. treatment with IgG or anti-IFN-γ (10 mg/kg) 1 h prior to CLP. *P < 0.05 compared with WT controls; n = 5 mice/group.
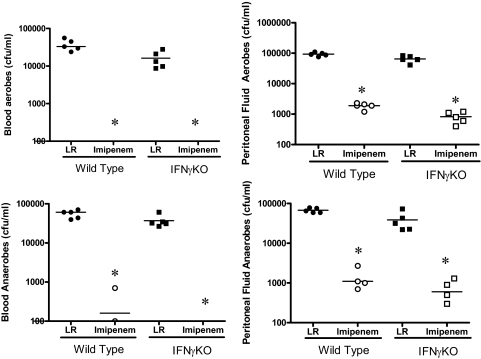
Bacterial clearance was not altered, but systemic inflammation was attenuated in IFN-γ-deficient mice during septic peritonitis
Evaluation of bacterial burden in blood and peritoneal fluid showed that the numbers of bacterial CFUs, aerobes and anaerobes, were not significantly different when comparing WT and IFN-γKO mice, regardless of whether mice were treated with imipenem (Fig. 7). Imipenem treatment decreased bacterial counts in blood and peritoneal fluid by >99% in WT and IFN-γKO mice. In mice treated with anti-IFN-γ, blood bacterial counts were not significantly different from mice receiving nonspecific IgG (data not shown).
Figure 7. Bacterial counts in blood and peritoneal lavage fluid after CLP.
All mice received i.p. injections of LR solution, alone or LR containing imipenem/cilistatin (Primaxin, 25 mg/kg) immediately after CLP. Blood and peritoneal lavage fluid were harvested at 18 h after CLP, serially diluted, and cultured on tryptic soy agar plates. *P < 0.05 compared with LR; n = 5 mice/group.
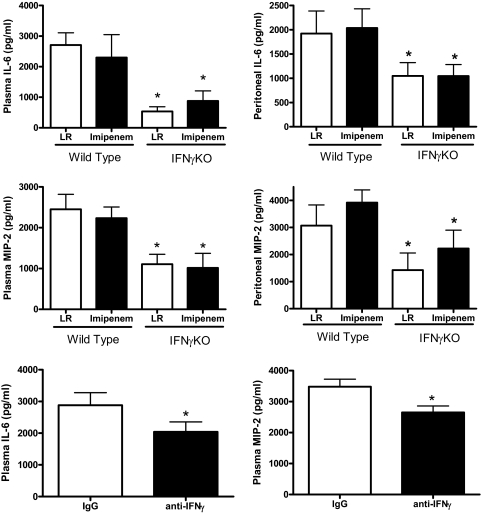
To assess systemic inflammation, plasma IL-6 and MIP-2 concentrations were measured in control and IFN-γ-deficient mice (Fig. 8). Concentrations of IL-6 and MIP-2 in plasma and peritoneal lavage fluid were significantly lower in IFN-γKO mice compared with WT controls (Fig. 8). Imipenem treatment did not significantly alter plasma or peritoneal fluid concentrations of IL-6 or MIP-2 compared with mice receiving LR solution. i.v. treatment with anti-IFN-γ also caused a significant decrease in plasma IL-6 and MIP-2 concentrations compared with mice treated with nonspecific IgG at 24 h after CLP (Fig. 8).
Figure 8. Cytokine concentrations in control and IFN-γ-deficient mice.
All mice received i.p. injections of LR solution, alone or LR containing imipenem/cilistatin (Primaxin, 25 mg/kg) immediately after CLP. Plasma and peritoneal lavage fluid were harvested 18 h after CLP. IL-6 and MIP-2 concentrations were measured by ELISA. For mice treated with anti-IFN-γ, antibody was given i.v. 1 h prior to CLP, and mice were treated with Primaxin beginning at the time of the CLP procedure. *P < 0.05 compared with WT or IgG control; n = 6 mice/group.
DISCUSSION
The results of the present study show that IFN-γ facilitates the local inflammatory response within the peritoneal cavity during CLP-induced septic shock. Infiltrating myeloid and NK cells produce IFN-γ, which appears to be an important factor for myeloid cell activation within the infected and inflamed peritoneal cavity. Blockade of IFN-γ also decreased systemic inflammation, as indicated by decreased plasma concentrations of IL-6 and MIP-2 in the plasma of IFN-γKO mice and mice treated with anti-IFN-γ. The findings of this study confirm and extend previous observations regarding the role of IFN-γ in the pathogenesis of sepsis. The proinflammatory potential of IFN-γ during CLP-induced sepsis has been recognized previously. Miles et al. [23] reported that administration of exogenous IFN-γ to mice after CLP increases mortality in a dose-dependent manner. They concluded that administration of IFN-γ worsens systemic inflammation and facilitates tissue injury in septic mice. Conversely, a study reported by Yin and colleagues [24] showed that inhibition of IFN-γ activity by treatment with neutralizing antibodies improved survival and decreased i.p. concentrations of the proinflammatory mediator high mobility group protein B-1 in rats. Similarly, Lainee et al. [26] showed that delayed neutralization of IFN-γ by antibody treatment in monkeys, rendered septic by systemic Escherichia coli infusion, resulted in decreased production of several proinflammatory cytokines and improved overall survival. In the present study, we did not observe a significant improvement in long-term survival when comparing mice treated with nonspecific IgG or anti-IFN-γ. The differences in survival seen after antibody-mediated neutralization of IFN-γ in our study compared with those of Yin et al. [24] and Lainee et al. [26] are likely to be a result of differences in sepsis models. The CLP model used in our study caused 100% mortality in WT mice within 72–96 h. Yin and colleagues [24] used a less-severe model of CLP-induced sepsis, in which control rats showed 30–70% long-term survival. The study by Lainee and colleagues [26] was performed in nonhuman primates using a model of E. coli-induced sepsis, in which 100% mortality was induced. Therefore, there are significant differences in the severity of sepsis and the mechanism of sepsis induction and in the species used in the respective studies. The antibody dose used in our study (10 mg/kg) was more than 6 times that used by Yin et al. [24] (1.2 mg/kg) and was identical to that used by Lainee and colleagues [26]. Furthermore, we treated mice 1 h before and 24 h after CLP using the 10-mg/kg dose, whereas Lainee et al. [26] administrered a single dose of antibody. Although it is possible that we did not achieve complete IFN-γ neutralization using our treatment protocol, this seems unlikely, given that we administered more total antibody than given in the studies reported previously. Furthermore, our measurements showed IFN-γ to be present at relatively low concentrations in plasma and peritoneal lavage fluid, and the large dose of antibody given should have been sufficient to neutralize circulating IFN-γ.
In the present study, proinflammatory cytokine concentrations were decreased in plasma and peritoneal lavage fluid from IFN-γKO mice after CLP, regardless of whether systemic antibiotics were given. IFN-γ-deficient mice treated with fluids and systemic antibiotics showed improved survival compared with WT controls. However, survival was not improved significantly in IFN-γKO mice that did not receive antibiotics. Primaxin treatment caused a >99% decrease in bacterial counts in blood and peritoneal fluid in WT and IFN-γKO mice. Cytokine production was decreased significantly in IFN-γKO mice, regardless of whether mice were treated with antibiotics. This leads us to conclude that IFN-γ facilitates systemic inflammation during CLP-induced sepsis and that sepsis-induced inflammation is decreased significantly in mice with a congenital lack of IFN-γ. It is likely that severe bacteremia contributed to mortality in mice that did not receive antibiotic treatment. We hypothesize that removal of bacterial burden by treatment with Primaxin uncovered the beneficial effects of attenuated inflammation in IFN-γKO mice. Interestingly, Martignoni et al. [27] reported that IFN-γKO mice are more susceptible to CLP-induced mortality than WT mice, which is in direct opposition to our findings and to those of Yin et al. [24] and Lainee and colleagues [26]. The reasons for the differing outcome in the Martignoni study [27] compared with our results are not clear but may be related to differences in experimental models.
The differences in survival noted between IFN-γKO mice and antibody-treated mice in this study could be explained by several potential mechanisms. First, antibody treatment may have been inadequate for complete neutralization of circulating IFN-γ. As noted above, this seems unlikely. However, treatment with anti-IFN-γ caused significant decreases in plasma IL-6 and MIP-2 concentrations at 24 h after CLP, although the magnitude of inhibition was not as great as that observed in IFN-γKO mice. Therefore, complete neutralization of IFN-γ at the cellular level may not have been achieved after antibody treatment. IFN-γ was produced by large numbers of myeloid cells within the peritoneal cavity, and its presence was associated with myeloid cell activation. This would suggest the presence of local autocrine and/or paracrine mechanisms of action that might not be blocked by antibodies but would be lost in mice that are genetically incapable of producing IFN-γ such as IFN-γKO mice. IFN-γ-mediated activation of myeloid cells by lymphoid cells, such as T and NK, may also be mediated through interactions that are bridged by the immune synapse, which may be impervious to an exogenous antibody [28]. Finally, it is possible that cells that require IFN-γ for differentiation, such as Th1 and Tc1 lymphocytes, facilitate CLP-induced inflammation through IFN-γ-independent mechanisms during CLP-induced sepsis. Those cells would be absent in IFN-γKO mice but not in mice receiving acute treatment with neutralizing IFN-γ antibodies. Our previous studies show that CD8+, but not CD4+, T cells amplify the CLP-induced inflammatory response [29]. This would raise the possibility of Tc1 lymphocytes being an active factor during CLP-induced inflammation. However, that conjecture remains to be proven. Taken together, these findings indicate that IFN-γ facilitates systemic inflammation during CLP-induced septic shock, directly or indirectly.
Our findings extend our prior reports by showing that the peritoneal cavity is a major site of IFN-γ-facilitated inflammation during CLP-induced sepsis. IFN-γ concentrations were highest within the peritoneal cavity. Lesser concentrations of IFN-γ were observed in plasma. Spleen and liver were identified as tissue sources of IFN-γ mRNA expression during sepsis, which is in agreement with previous reports [30]. In the present study, NK cells and infiltrating myeloid cells were the primary i.p. sources of IFN-γ. We have reported that large numbers of NK cells are recruited into the peritoneal cavity after CLP [31]. In that study and in the present study, we show that a large proportion of i.p. NK cells produces IFN-γ. NK cell depletion, as reported in our earlier studies [31], and IFN-γ deficiency decrease systemic inflammation, attenuate peritoneal myeloid cell activation, and improve survival during CLP-induced septic shock. Taken together, these findings indicate that NK cell-derived IFN-γ is one factor that facilitates CLP-induced inflammation. Inflammatory monocytes are another apparent i.p. source of IFN-γ identified in this study. Large numbers of myeloid cells infiltrate the peritoneal cavity after CLP, which are primarily F4-80+Gr-1+ and F4-80–Gr-1+. Three major subpopulations were identified based on surface antigen expression and nuclear morphology. These included F4-80+Gr-1lo cells that had a kidney-shaped nucleus typical of monocytes (population M1). A second population (M2) was F4-80+Gr-1hi and also had a kidney-shaped nuclear morphology. Both of those myeloid cell populations produce IFN-γ. The third population (M3), typical of neutrophils, was F4-80–Gr-1+, which had segmented nuclei and did not produce appreciable amounts of IFN-γ. Taken together, these findings indicate that IFN-γ-producing peritoneal myeloid cells are of the macrophage/monocyte lineage. Although the 2 IFN-γ-producing myeloid cell populations showed differences in Gr-1 and Ly6G expression, the nuclear morphology in both groups was typical of monocytes, and both populations expressed the macrophage/monocyte marker F4-80. The variation in surface antigen expression between these populations is likely a result of differences in maturity and level of differentiation. The ability of macrophage/monocytes to produce IFN-γ has been reported previously in experimental models of autoimmune disease and infection [32–34]. Martignoni et al. [27] also reported that IFN-γ+Gr-1+ cells were present in the peritoneal cavities of mice after CLP. They concluded that those cells were neutrophils. However, they did not perform a detailed analysis of myeloid cell markers and nuclear morphology.
The findings from the present study suggest that infiltrating myeloid cells, monocyte-derived IFN-γ, may function through autocrine mechanisms during CLP-induced sepsis, as peritoneal monocytes produce IFN-γ and monocyte/macrophage activation was decreased significantly in IFN-γ-deficient mice, as indicated by decreased production of IL-6 and surface expression of CD40 and CD80. Our recent studies [31] show that IL-6-producing i.p. myeloid cells are F4-80+Gr-1+, which is characteristic of the IFN-γ-producing monocyte populations observed in the present study. Therefore, autocrine-mediated mechanisms are likely to be present. It is possible that the intracellular IFN-γ, noted within myeloid cells in our study, is a result of detection of IFN-γ, which has been internalized after receptor binding. To assess this, we performed anti-IFN-γ labeling in the presence and absence of saponin and brefeldin A, which increases cellular permeability and prevents protein secretion, respectively. In the absence of saponin and brefeldin A, <4% of peritoneal myeloid cells were positive for IFN-γ, whereas >50% were positive when preincubated with buffer containing saponin and brefeldin A. These results make it likely that IFN-γ was derived by synthesis within peritoneal myeloid cells rather than by surface binding and internalization. One would expect a large amount of surface IFN-γ labeling to be present if a significant level of IFN-γR binding were present.
Bacterial counts in blood and peritoneal lavage fluid were not significantly different between WT and IFN-γ-deficient mice in the present study. Therefore, bacterial clearance in this model of septic shock is not altered by the absence of IFN-γ. This is in agreement with our studies reported previously, in which clearance of Pseudomonas aeruginosa was not altered during primary infection in IFN-γ-deficient mice compared with WT mice and treatment with rIFN-γ, and amplifying cytokine secretion did not alter Pseudomonas clearance [35, 36]. Numerous previous studies have shown that IFN-γ is important for the host response to intracellular pathogens such as Mycobacteria, Salmonella, Leishmania, and Listeria [5, 8, 9]. Humans with IFN-γ deficiency are commonly infected with intracellular microbes, particularly mycobacteria, but infection with ordinary extracellular pathogens does not appear to be more common than in the general population [10–13]. Some investigators have reported that the presence of IFN-γ is deleterious in experimental models of infection with extracellular bacteria. McLoughlin et al. [37] reported that the leukocyte environment generated by T cell-derived IFN-γ during infection with Staphylococcus aureus is deleterious and results in increased host injury. Other studies by Yin and colleagues [24, 25] have shown that IFN-γ deficiency decreases bacterial burden in rats after CLP. The authors of the latter study proposed that the decreased bacterial burden seen in IFN-γ-deficient animals is caused by enhanced fibrin deposition and improved local containment of microorganisms. Regardless of mechanism, the lack of IFN-γ does not appear to impair the host response to common extracellular pathogens.
In conclusion, the present study shows that IFN-γ deficiency attenuates systemic inflammation independently of bacterial clearance and that congenital IFN-γ deficiency improves overall survival during induced CLP, directly or indirectly. These observations were made using a model of acute intra-abdominal sepsis, which is a common clinical presentation in patients with intra-abdominal pathology, such as perforated appendicitis, diverticulitis, and ischemic bowel [38], Many patients present to emergency departments in the early phases of severe sepsis. Recent studies indicate that early, goal-directed therapy improves long-term outcomes significantly in those patients [39]. Currently, goal-directed approaches for the treatment of sepsis are centered on hemodynamic resuscitation and oxygen delivery. However, inflammation is an important part of pathophysiology during early sepsis, but little is known about the benefits of modulating inflammation during early sepsis in the clinical setting. The results of this study and others indicate that IFN-γ participates in the early, proinflammatory response. It is unclear currently whether IFN-γ is an attractive target for anti-inflammatory intervention during acute sepsis. The present study did not show survival benefit from treatment with anti-IFN-γ antibodies, which is in conflict with some reports published previously, in which IFN-γ neutralization improved survival in septic rats and nonhuman primates [25, 26]. Nevertheless, the present study shows that IFN-γ neutralization decreases systemic inflammation during sepsis. IFN-γ neutralization has not been reported for the treatment of acute sepsis in humans.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health R01 GM66885, grant 8780 from the Shriners of North America, The James F. Arens Endowed Chair, and The John Sealy Distinguished Chair in M.D. Ph.D. Studies in Honor of Dr. Truman G. Blocker. The authors thank Raymond Kong and Dr. Tad George at Amnis Corp. for assistance with multispectral imaging flow cytometry.
Footnotes
- CLP
- cecal ligation and puncture
- KO
- knockout
- LR
- lactated Ringer's
- Tc
- cytotoxic T lymphocyte
AUTHORSHIP
C.R.R. made major contributions to the design and interpretation of experiments and was actively involved in performing flow cytometry, surgical procedures, and antibody treatments. He and D.S.H. contributed equally to this work and should be considered co-first authors. D.S.H. was actively involved in the design and interpretation of experiments and was actively involved in flow cytometry and surgical procedures. A.E. was actively involved in all experiments performed using IFN-γKO mice, including surgical procedures, microbiology, and cytokine measurements. He was actively involved in experimental design and interpretation. J.N. worked closely with A.E. in the design, execution, and interpretation of experiments performed using IFN-γKO mice. R.M. worked closely with A.E. in the design, execution, and interpretation of experiments performed using IFN-γKO mice. G.F. was actively involved in performing RNase protection assays, ELISAs, microbiological analyses, and surgical procedures. She was instrumental in interpreting findings and trouble-shooting technical aspects of the study. E.D.M. was actively involved in the conceptualization, design, and interpretation of experiments. He was actively involved in performing microbiological evaluations. T.T-K. was actively involved in the conceptualization, design, and interpretation of experiments. She was actively involved in supervising and assisting with flow cytometry experiments. E.R.S. is the Principal Investigator and is responsible for all conceptual and technical aspects of this study.
REFERENCES
- 1.Ogasawara K., Takeda K., Hashimoto W., Satoh M., Okuyama R., Yanai N., Obinata M., Kumagai K., Takada H., Hiraide H., Seki S. (1998) Involvement of NK1+ T cells and their IFN-γ production in the generalized Shwartzman reaction. J. Immunol. 160, 3522–3527 [PubMed] [Google Scholar]
- 2.Feng C. G., Kaviratne M., Rothfuchs A. G., Cheever A., Hieny S., Young H. A., Wynn T. A., Sher A. (2006) NK cell-derived IFN-γ differentially regulates innate resistance and neutrophil response in T cell-deficient hosts infected with Mycobacterium tuberculosis. J. Immunol. 177, 7086–7093 [DOI] [PubMed] [Google Scholar]
- 3.Robinson C. M., O'Dee D., Hamilton T., Nau G. J. (2009) Cytokines involved in interferon-γ production by human macrophages. J. Innate Immun. 2, 56–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bastos K. R., Barboza R., Sardinha L., Russo M., Alvarez J. M., Lima M. R. (2007) Role of endogenous IFN-γ in macrophage programming induced by IL-12 and IL-18. J. Interferon Cytokine Res. 27, 399–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hunter C. A., Chizzonite R., Remington J. S. (1995) IL-1 β is required for IL-12 to induce production of IFN-γ by NK cells. A role for IL-1 β in the T cell-independent mechanism of resistance against intracellular pathogens. J. Immunol. 155, 4347–4354 [PubMed] [Google Scholar]
- 6.Wang X., Suzuki Y. (2007) Microglia produce IFN-γ independently from T cells during acute toxoplasmosis in the brain. J. Interferon Cytokine Res. 27, 599–605 [DOI] [PubMed] [Google Scholar]
- 7.Alimohammadian M. H., Darabi H., Malekzadeh S., Mahmoodzadeh-Niknam H., Ajdary S., Khamesipour A., Bahonar A., Mofarrah A. (2007) Exposure to Leishmania major modulates the proportion of CD4+ T cells without affecting cellular immune responses. Microbiol. Immunol. 51, 1003–1011 [DOI] [PubMed] [Google Scholar]
- 8.Singh U. P., Singh R., Singh S., Karls R. K., Quinn F. D., Taub D. D., Lillard J. W., Jr. (2008) CXCL10+ T cells and NK cells assist in the recruitment and activation of CXCR3+ and CXCL11+ leukocytes during Mycobacteria-enhanced colitis. BMC Immunol. 9, 25–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D'Orazio S. E., Troese M. J., Starnbach M. N. (2006) Cytosolic localization of Listeria monocytogenes triggers an early IFN-γ response by CD8+ T cells that correlates with innate resistance to infection. J. Immunol. 177, 7146–7154 [DOI] [PubMed] [Google Scholar]
- 10.Dorman S. E., Uzel G., Roesler J., Bradley J. S., Bastian J., Billman G., King S., Filie A., Schermerhorn J., Holland S. M. (1999) Viral infections in interferon-γ receptor deficiency. J. Pediatr. 135, 640–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jouanguy E., Altare F., Lamhamedi S., Revy P., Emile J. F., Newport M., Levin M., Blanche S., Seboun E., Fischer A., Casanova J. L. (1996) Interferon-γ-receptor deficiency in an infant with fatal bacille Calmette-Guerin infection. N. Engl. J. Med. 335, 1956–1961 [DOI] [PubMed] [Google Scholar]
- 12.Pierre-Audigier C., Jouanguy E., Lamhamedi S., Altare F., Rauzier J., Vincent V., Canioni D., Emile J. F., Fischer A., Blanche S., Gaillard J. L., Casanova J. L. (1997) Fatal disseminated Mycobacterium smegmatis infection in a child with inherited interferon γ receptor deficiency. Clin. Infect. Dis. 24, 982–984 [DOI] [PubMed] [Google Scholar]
- 13.Su S. B., Grajewski R. S., Luger D., Agarwal R. K., Silver P. B., Tang J., Tuo J., Chan C. C., Caspi R. R. (2007) Altered chemokine profile associated with exacerbated autoimmune pathology under conditions of genetic interferon-γ deficiency. Invest. Ophthalmol. Vis. Sci. 48, 4616–4625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lio D., Scola L., Crivello A., Bonafe M., Franceschi C., Olivieri F., Colonna-Romano G., Candore G., Caruso C. (2002) Allele frequencies of +874T → Α single nucleotide polymorphism at the first intron of interferon-γ gene in a group of Italian centenarians. Exp. Gerontol. 37, 315–319 [DOI] [PubMed] [Google Scholar]
- 15.Pes G. M., Lio D., Carru C., Deiana L., Baggio G., Franceschi C., Ferrucci L., Oliveri F., Scola L., Crivello A., Candore G., Colonna-Romano G., Caruso C. (2004) Association between longevity and cytokine gene polymorphisms. A study in Sardinian centenarians. Aging Clin. Exp. Res. 16, 244–248 [DOI] [PubMed] [Google Scholar]
- 16.Heremans H., Billiau A. (1989) The potential role of interferons and interferon antagonists in inflammatory disease. Drugs 38, 957–972 [DOI] [PubMed] [Google Scholar]
- 17.Heremans H., Dillen C., Groenen M., Matthys P., Billiau A. (2000) Role of interferon-γ and nitric oxide in pulmonary edema and death induced by lipopolysaccharide. Am. J. Respir. Crit. Care Med. 161, 110–117 [DOI] [PubMed] [Google Scholar]
- 18.Heremans H., Dillen C., van Damme J., Billiau A. (1994) Essential role for natural killer cells in the lethal lipopolysaccharide-induced Shwartzman-like reaction in mice. Eur. J. Immunol. 24, 1155–1160 [DOI] [PubMed] [Google Scholar]
- 19.Billiau A., Heremans H., Vandekerckhove F., Dillen C. (1987) Anti-interferon-γ antibody protects mice against the generalized Shwartzman reaction. Eur. J. Immunol. 17, 1851–1854 [DOI] [PubMed] [Google Scholar]
- 20.Shimizu Y., Margenthaler J. A., Landeros K., Otomo N., Doherty G., Flye M. W. (2002) The resistance of P. acnes-primed interferon γ-deficient mice to low-dose lipopolysaccharide-induced acute liver injury. Hepatology 35, 805–814 [DOI] [PubMed] [Google Scholar]
- 21.Car B. D., Eng V. M., Schnyder B., Ozmen L., Huang S., Gallay P., Heumann D., Aguet M., Ryffel B. (1994) Interferon γ receptor deficient mice are resistant to endotoxic shock. J. Exp. Med. 179, 1437–1444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dinges M. M., Schlievert P. M. (2001) Role of T cells and γ interferon during induction of hypersensitivity to lipopolysaccharide by toxic shock syndrome toxin 1 in mice. Infect. Immun. 69, 1256–1264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miles R. H., Paxton T. P., Dries D. J., Gamelli R. L. (1994) Interferon-γ increases mortality following cecal ligation and puncture. J. Trauma 36, 607–611 [DOI] [PubMed] [Google Scholar]
- 24.Yin K., Gribbin E., Wang H. (2005) Interferon-γ inhibition attenuates lethality after cecal ligation and puncture in rats: implication of high mobility group box-1. Shock 24, 396–401 [DOI] [PubMed] [Google Scholar]
- 25.Yin K., Hock C. E., Lai P. S., Ross J. T., Yue G. (1999) Role of inter-feron-γ in lung inflammation following cecal ligation and puncture in rats. Shock 12, 215–221 [DOI] [PubMed] [Google Scholar]
- 26.Lainee P., Efron P., Tschoeke S. K., Elies L., De Winter H., Lorre K., Moldawer L. L. (2005) Delayed neutralization of interferon-γ prevents lethality in primate Gram-negative bacteremic shock. Crit. Care Med. 33, 797–805 [DOI] [PubMed] [Google Scholar]
- 27.Martignoni A., Tschop J., Goetzman H. S., Choi L. G., Reid M. D., Johannigman J. A., Lentsch A. B., Caldwell C. C. (2008) CD4-expressing cells are early mediators of the innate immune system during sepsis. Shock 29, 591–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bollyky P. L., Evanko S. P., Wu R. P., Potter-Perigo S., Long S. A., Kinsella B., Reijonen H., Guebtner K., Teng B., Chan C. K., Braun K. R., Gebe J. A., Nepom G. T., Wight T. N. (2010) Th1 cytokines promote T-cell binding to antigen-presenting cells via enhanced hyaluronan production and accumulation at the immune synapse. Cell. Mol. Immunol. 7, 211–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sherwood E. R., Enoh V. T., Murphey E. D., Lin C. Y. (2004) Mice depleted of CD8+ T and NK cells are resistant to injury caused by cecal ligation and puncture. Lab. Invest. 84, 1655–1665 [DOI] [PubMed] [Google Scholar]
- 30.Hu C. K., Venet F., Heffernan D. S., Wang Y. L., Horner B., Huang X., Chung C. S., Gregory S. H., Ayala A. (2009) The role of hepatic invariant NKT cells in systemic/local inflammation and mortality during polymicrobial septic shock. J. Immunol. 182, 2467–2475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Etogo A. O., Nunez J., Lin C. Y., Toliver-Kinsky T. E., Sherwood E. R. (2008) NK but not CD1-restricted NKT cells facilitate systemic inflammation during polymicrobial intra-abdominal sepsis. J. Immunol. 180, 6334–6345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fenton M. J., Vermeulen M. W., Kim S., Burdick M., Strieter R. M., Kornfeld H. (1997) Induction of γ interferon production in human alveolar macrophages by Mycobacterium tuberculosis. Infect. Immun. 65, 5149–5156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salins S., Newton C., Widen R., Klein T. W., Friedman H. (2001) Differential induction of γ interferon in Legionella pneumophila-infected macrophages from BALB/c and A/J mice. Infect. Immun. 69, 3605–3610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robinson B. W., McLemore T. L., Crystal R. G. (1985) γ Interferon is spontaneously released by alveolar macrophages and lung T lymphocytes in patients with pulmonary sarcoidosis. J. Clin. Invest. 75, 1488–1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murphey E. D., Fang G., Varma T. K., Sherwood E. R. (2007) Improved bacterial clearance and decreased mortality can be induced by LPS tolerance and is not dependent upon IFN-γ. Shock 27, 289–295 [DOI] [PubMed] [Google Scholar]
- 36.Murphey E. D., Herndon D. N., Sherwood E. R. (2004) γ Interferon does not enhance clearance of Pseudomonas aeruginosa but does amplify a proinflammatory response in a murine model of postseptic immunosuppression. Infect. Immun. 72, 6892–6901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McLoughlin R. M., Lee J. C., Kasper D. L., Tzianabos A. O. (2008) IFN-γ regulated chemokine production determines the outcome of Staphylococcus aureus infection. J. Immunol. 181, 1323–1332 [DOI] [PubMed] [Google Scholar]
- 38.Podnos Y. D., Jimenez J. C., Wilson S. E. (2002) Intra-abdominal sepsis in elderly persons. Clin. Infect. Dis. 35, 62–68 [DOI] [PubMed] [Google Scholar]
- 39.Rivers E. P., Coba V., Whitmill M. (2008) Early goal-directed therapy in severe sepsis and septic shock: a contemporary review of the literature. Curr. Opin. Anaesthesiol. 21, 128–140 [DOI] [PubMed] [Google Scholar]