Abstract
A main challenge in tissue engineering and regenerative medicine is achieving local and efficient growth factor release to guide cell function. Gelatin is a denatured form of collagen that cells can bind to and degrade through enzymatic action. In this study, gelatin microspheres were used to release bone morphogenetic protein 2 (BMP2). Spherical microparticles with diameters in the range of 2–6 µm were created by an emulsification process and were stabilized by crosslinking with the small molecule genipin. The degree of crosslinking was varied by controlling the incubation time in genipin solution. Loading rate studies, using soy bean trypsin inhibitor as a model protein, showed rapid protein uptake over the first 24 h, followed by a levelling off and then a further increase after approximately 3 days, as the microspheres swelled. Growth factor release studies using microspheres crosslinked to 20%, 50% and 80% of saturation and then loaded with BMP2 showed that higher degrees of crosslinking resulted in higher loading efficiency and slower protein release. After 24 h, the concentration profiles produced by all microsphere formulations were steady and approximately equal. Microspheres incubated with adult human mesenchymal stem cells accumulated preferentially on the cell surface, and degraded over time in culture. BMP2-loaded microspheres caused a three- to eight-fold increase in expression of the bone sialoprotein gene after 14 days in culture, with more crosslinked beads producing a greater effect. These results demonstrate that genipin-crosslinked gelatin microspheres can be used to deliver growth factors locally to cells in order to direct their function.
Keywords: drug delivery, controlled release, growth factor, gelatin, genipin
1. Introduction
Delivery of growth factors and other soluble bioactive cues is an important aspect of achieving tissue regeneration. A variety of approaches to controlled release of such molecules have been developed, using a wide range of materials (Silva et al., 2007; Tessmar and Göpferich, 2007). Delivery of proteins from microspheres has emerged as a promising option because the high surface area : volume ratio of the spherical geometry allows efficient loading and release from these matrices. Generally, microspheres are loaded with a protein or drug of interest, and then are either injected directly to the site of interest as a slurry of particles (Adhirajan et al., 2007; Hoshino et al., 2006) or are incorporated into a scaffold as a mechanism to provide soluble biochemical cues (Habraken et al., 2008; Kawai et al., 2000). Release from microspheres offers the advantage that the bioactive substance is delivered locally and efficiently, properties that are especially important for expensive and potent drugs and growth factors. In the case of highly biodegradable microparticles, the matrix itself is eroded as the bioactive agent is released, leaving no trace of the delivery system.
Gelatin is a hydrolysed form of collagen Type I that is used widely in the food and medical industries, including tissue-engineering applications (Tabata and Ikada, 1998; Young et al., 2005). It has binding sites for cell attachment, and it is well established that it can be broken down by cellular action through the secretion of specific matrix metalloproteinases. This enzymatic breakdown process does not produce toxic by-products and gelatin matrices can be completely degraded by cells. One important property of gelatin is that it can be dissolved in aqueous solutions and then reconstituted into a hydrogel at temperatures that are not damaging to proteins. In contrast, many delivery systems using synthetic polymers require the use of organic solvents or toxic initiators of polymerization, which can adversely affect both the bioactivity of growth factors and interactions with cells (Sivadas et al., 2008).
Different processing methods lead to gelatin materials with different chemical properties. Gelatin isolated using an acidic process is commonly called type A and has an isoelectric point (pI) of approximately 9, which is close to that of native collagen. Type A carries a net positive charge at physiological pH (~ 7.4). In contrast, type B gelatin is isolated using an alkaline process, which results in hydrolysis of amide groups and an increased number of negatively charged carboxyl groups. Type B gelatin has pI ≈ 5, and therefore at physiological pH this material has abundant negative charges. The charged moieties in gelatin can sequester proteins via polyionic complexing, and either negatively or positively charged molecules can be sequestered in gelatin matrices, depending on the pH and the type of gelatin used. This concept has been used widely to create gelatin-based drug and growth factor delivery systems (Patel et al., 2008a; Tabata et al., 1998)
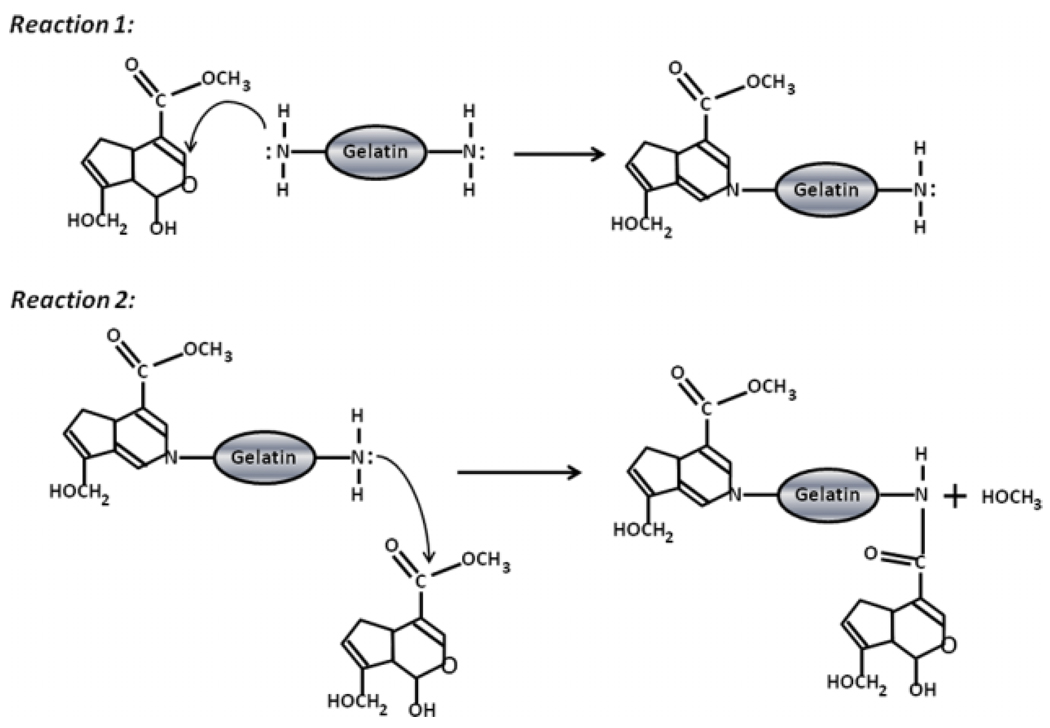
Lyophilized gelatin is commonly dissolved in aqueous media at temperatures of 60–80°C and solid hydrogels form when these solutions are cooled to temperatures of 15–35°C, depending on gelatin type and solution conditions. However, because gelatin has a melting point near 37°C it melts when heated to physiological temperatures. As a consequence of its low melting point, gelatin needs to be stabilized for controlled release applications. Glutaraldehyde crosslinking has been used to stabilize gelatin matrices (McGuigan and Sefton, 2007; Yamamoto et al., 1998) but residual toxicity is a concern (Sisson et al., 2009). Genipin is a 226 Da molecule extracted from the fruit of the gardenia plant that has been used to crosslink a variety of protein and polysaccharide matrices, including gelatin for drug-delivery applications (Liang et al., 2003; Wei et al., 2007; Cruz et al., 2008; Dare et al., 2009; Ko et al., 2009). The reaction mechanism is a two-step process requiring two distinct reactions, as shown in Figure 1. The first reaction occurs when the genipin molecule undergoes a nucleophilic attack by the primary amines of gelatin, resulting in the heterocyclic linking of genipin to the gelatin amine (Butler et al., 2003). The second reaction occurs when the ester group on genipin undergoes a nucleophilic substitution (Butler et al., 2003). The resultant covalent crosslinks between the primary amine residues (Liang et al., 2004) leave very minimal residual toxicity (Tsai et al., 2000). In addition, it can form ionic complexes with charged molecules (Tsai et al., 2001) and therefore may aid in sequestering proteins.
Figure 1.
Crosslinking mechanism of genipin resulting in intermolecular crosslinking of gelatin
The purpose of this study was to create and characterize a growth factor delivery method using naturally-derived materials: gelatin stabilized by genipin crosslinking. Such a system has the advantage that cells can recognize and bind to the gelatin matrix, and also can potentially degrade it through enzymatic action. An emulsification–gelation process was used to create gelatin microspheres, which subsequently were crosslinked with genipin. The degree of crosslinking could be varied by controlling the incubation time in genipin, and the loading rate and efficiency of matrices crosslinked to different degrees were measured. In addition, the swelling behaviour of the microspheres and the release of a model protein were characterized. Finally, the interaction of gelatin microspheres with adult human mesenchymal stem cells (hMSCs) was examined, including the effects of controlled release of recombinant human bone morphogenetic protein-2 (rhBMP-2) on cell phenotype. A fully degradable growth factor release system that is activated on cellular demand would enhance current strategies aimed at tissue regeneration.
2. Materials and methods
2.1. Materials
Dulbecco’s modified Eagle medium: Ham’s F-12 nutrient medium (DMEM : F12), fetal bovine serum (FBS), penicillin–streptomycin, l-glutamine, trypsin–EDTA, glycine, ninhydrin, acetic acid, sodium hydroxide (NaOH) and agarose were all purchased from Fisher Scientific (Fair Lawn, NJ, USA). Lyophilized collagen type I was purchased from MP Biomedicals (Solon, OH, USA). Types A and B gelatin, phosphate-buffered saline (PBS), ethanol, BMP-2 primary antibody, citric acid, tin chloride dehydrate, 2-methoxyethanol, glutaraldehyde and formalin were all purchased from Sigma-Aldrich (St. Louis, MO, USA). hMSCs were purchased from Lonza (Valais, Switzerland). Calcein AM, ethidium homodimer, Alexafluor-488 conjugated soy bean trypsin inhibitor (SBTI), recombinant human BMP-2 (rhBMP-2), Alexafluor-488 phalloidin, 4′,6-diamidino-2-phenylindole (DAPI) and Alexafluor secondary antibodies were purchased from Invitrogen (Eugene, OR, USA). Polydimethylsiloxane (PDMS) was purchased from GE Silicones (Waterford, NY, USA) and Pluronic L101 surfactant was obtained from BASF (Florham Park, NJ, USA). Collagenase II and genipin were purchased from Wako Chemicals (Richmond, VA, USA) and the BSA Standard was purchased from Peirce Biotechnology (Rockford, IL, USA). All other stock chemicals were all purchased from Sigma-Aldrich.
2.2. Microsphere preparation
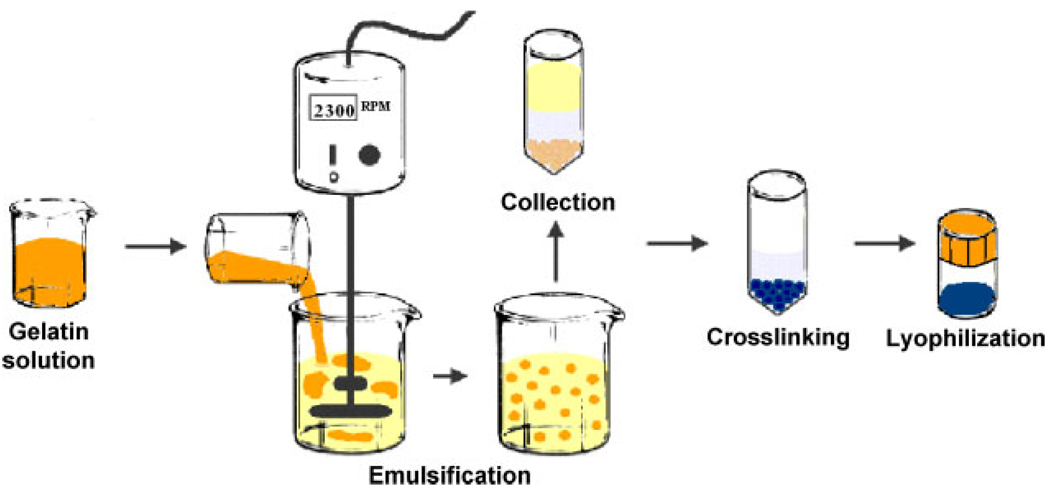
Microspheres were prepared using a water-in-oil emulsion technique shown schematically in Figure 2. A 6.0% working volume of gelatin was prepared at pH 7.2 and warmed to 37 °C. Preparations of types A and B gelatin were handled the same way. The emulsification bath consisted of 70 ml polydimethylsiloxane (PDMS) in a 100 ml beaker and was also warmed to 37°C. A custom-made impeller was used to stir the PDMS at 2300 rpm and 3.0 ml gelatin solution was added. The gelatin was allowed to emulsify for a total of 4 min, with short periods of no mixing to allow redistribution of the emulsion phase in the bath, so that all larger droplets were fully broken up. The entire emulsification bath was then surrounded by an ice-water bath with continuous stirring at 2300 rpm for 30 min. The gelatin was allowed to cool below the Tg, subsequently solidifying, resulting in a suspension of microspheres suspended in the PDMS. The contents of the emulsification vessel was then separated into two 50 ml conical tubes (ca. 37 ml/tube) and PBS containing 100 ppm Pluronic L101 surfactant was added until 50 ml total volume was reached. The tubes were vigorously shaken, then centrifuged at 1000 rpm for 5 min at 4°C. Approximately three-quarters of the top layer of PDMS was then aspirated and the total volume was again brought to 50 ml with PBS-L101. The tubes were vigorously shaken and again centrifuged at 1000 rpm for 5 min at 4°C. The remaining solution was aspirated, leaving a pellet of microspheres.
Figure 2.
Schematic diagram of microsphere preparation process. Gelatin is emulsified in an oil phase, gelled, collected and subsequently crosslinked with genipin
2.3. Genipin crosslinking
A 1.0% solution of genipin in sterile phosphate-buffered saline (PBS) was prepared and kept at room temperature (RT). The gelatin matrix was crosslinked by adding 6.0 ml genipin solution to the pellet of formed microspheres collected from the emulsification process. The microspheres were allowed to incubate in the genipin solution at 23°C for the time required to achieve the desired level of crosslinking. The crosslinking time varied for each gelatin type, and the times required to reach 20%, 50% and 80% crosslinking are shown in Table 1. The microspheres turned progressively more blue as their levels of crosslinking increased. At the end of the incubation, excess genipin solution was aspirated and 15.0 ml 100% ethanol was added and allowed to stand for 1 h. The microspheres were then washed twice more with fresh 100% ethanol. The final microsphere preparation was then placed in a −80°C freezer for 2 h before being lyophilized.
Table 1.
Incubation time in 1.0% genipin solution to achieve desired degree of crosslinking in gelatin microspheres. Type A gelatin crosslinked more rapidly than type B
| Incubation time required (h) |
||
|---|---|---|
| Degree of crosslinking (%) | Type A gelatin | Type B gelatin |
| 20 | 1.5 | 6.6 |
| 50 | 4.8 | 21.8 |
| 80 | 12.9 | 96.0 |
2.4. Crosslinking analysis
A ninhydrin assay was used to measure the percentage of free amino groups in gelatin samples, which can be converted to the degree of crosslinking by comparing to uncrosslinked gelatin. The ninhydrin reagent consisted of two solutions. The first contained 1.05 g citric acid, 0.4 g NaOH and 0.04 g SnCl•H2O dissolved in 25.0 ml diH2O. The second solution was made by mixing 1.0 g ninhydrin with 25.0 ml ethylene glycol monomethyl ether (Yuan et al., 2007). These two solutions were combined and stirred for 45 min in the dark at 23°C. A stock glycine standard was prepared for generating a standard curve (Liang et al., 2003; Yuan et al., 2007). Microspheres to be assayed were allowed to crosslink for different periods of time (0, 6, 12, 24, 48 and 72 h) and were subsequently rinsed and lyophilized. Samples consisted of 1.5 mg lyophilized microspheres, which were placed in separate 2 ml microcentrifuge tubes. Each sample was rehydrated in 100 µl diH2O for 1 h and a set of standards was prepared by rehydrating in the appropriate glycine solution. 1.0 ml ninhydrin solution was then added to all the samples and standards, followed by incubation at 100°C for 20 min. 5.0 ml 50% isopropanol was added to all samples and absorbance at 570 nm was read on a microplate reader (Liang et al., 2003; Yuan et al., 2007).
2.5. Protein loading rate and efficiency
Soybean trypsin inhibitor (SBTI) conjugated with Alexa fluor 488 was used as a marker protein to characterize protein loading into gelatin microspheres. This labelled protein has a molecular weight of 21 kDa, which approximates the size of BMP (26 kDa) and can be easily detected using fluorescence microscopy. The isoelectric point (pI) of SBTI is approximately 4.2 and therefore type A gelatin (pI ≈ 9) was used in this study. SBTI was dissolved at 100 µg/ml and 300 µl of this solution was added to 3.0 mg samples of gelatin microspheres. The samples were incubated on an orbital shaker at 37°C and were imaged using confocal microscopy (Zeiss, Thornwood, NY, USA) every 24 h for a total period of 6 days. Fluorescence images were separated into two channels, one containing the Alexafluor 488 signal and the other containing gelatin autofluorescence. The resulting micrographs were converted to binary images and the pixels in each channel were summed. The degree of protein loading was determined by the ratio of the sum of the dye pixels to the sum of the gelatin pixels. These images also were used to measure microsphere diameters to monitor the swelling of the particles. All images were analysed using MATLAB.
For studies using rhBMP-2, type B gelatin matrices with pI ≈ 5 were used because they form polyionic complexes with the positively charged rhBMP-2 molecule (pI ≈ 8.5) at physiological pH. The loading efficiency was determined by soaking type B gelatin microspheres in rhBMP-2 for 1 week and then degrading them and assaying for rhBMP-2 levels. The microspheres were rinsed three times in diH2O and before being incubated in collagenase II solution at 37°C until the microspheres were fully degraded. The samples were diluted 1 : 1000 and the rhBMP-2 concentration was determined by means of a sandwich ELISA assay (Quantikine BMP-2 ELISA kit, R&D Systems). Briefly, 50 µl samples and standards were added to a microwell plate and 100 µl assay diluent was added and incubated for 2 h on an orbital shaker. The plate was washed four times and 200 µl BMP-2 conjugate was added to each well and incubated for 2 h at room temperature on an orbital shaker. The wells were again washed and substrate solution was added to each well and incubated for 30 min, protected from light. Finally, 50 µl stop solution was added to each well and the plate was read using a spectrophotometer. The reading at 450 nm was subtracted from the reading at 540 nm and analyte concentration was determined by comparison with the standard curve. The degree to which the microspheres were loaded was determined by dividing the rhBMP-2 mass in the particles by the total protein mass in the initial solution added to 1 mg dry microspheres.
2.6. Growth factor release studies
For studies evaluating the release of rhBMP-2 in the presence of hMSCs, cells were plated at a density of 104 cells/cm2 onto tissue culture-treated 6-well plates with 3.0 ml medium. hMSCs were cultured in DMEM/F-12 culture medium supplemented with 10% FBS, 1% penicillin–streptomycin and 2 mM l-glutamine. The cells were plated 24 h prior to beginning growth factor release studies, and all cultures were kept in a humidified incubator at 37°C with 5% CO2. hMSCs in this study were used between passages 6 and 10.
Gelatin microspheres were loaded with growth factor by soaking them in a solution of 100 ng/ml rhBMP-2. A volume of 100 µl BMP solution was used for each 1.0 mg gelatin, and the microspheres were incubated overnight on an orbital shaker at 37°C. Samples of microspheres crosslinked to either 20%, 50% or 80% were used. For each sample type, 1.0 mg of the appropriate microsphere types was added to a well containing hMSCs. For growth factor release studies, 150 µl of the supernatant was removed hourly for the first 8 h and then every 24 h for a period of 5 days. An equivalent volume of fresh warm medium was replaced in order to maintain constant volume and sink conditions, and the samples were fed with fresh standard medium at day 3. Studies examining the effect of growth factor release on hMSC function were run for 14 days, with the medium changed every 4 days. For these studies, control cultures were established to isolate the effect of controlled growth factor release. Blank (BLK) cultures used only culture medium with no growth factor addition. ‘Burst’ cultures were given a single bolus of 150 ng/ml rhBMP-2 at day 1 and were then fed with standard culture medium. ‘Continuous’ fed cultures were provided with standard medium containing 150 ng/ml rhBMP-2 at each feeding point. hMSC experiments were repeated a minimum of three times with separate cell batches.
2.7. Imaging of microspheres and cells
Gelatin autofluoresces at approximately 600 nm and this property was used to image the microspheres under fluorescence without the need for additional staining. The average size and size distribution of hydrated gelatin microspheres was determined by analysing digital images of microsphere populations. Five random fields of view were imaged, using a confocal microscope (Zeiss), and the diameters of all microspheres in each image were determined, using image analysis software (Media Cybernetics, Bethesda, MD, USA). In experiments involving hMSCs, cell morphology was also imaged using confocal microscopy. The cells were fixed in 3.7% formalin and were permeabilized in in 0.1% Triton X-100. The actin cytoskeleton was labelled with Alexafluor 488-conjugated phalloidin and the nucleus with DAPI.
Gelatin microspheres were prepared for SEM by dehydrating for 4 h in 100% ethanol, followed by lyophilization for 24 h. The microspheres were then attached to the SEM mounting stub, using carbon tape, sputter-coated with gold and imaged on a high-resolution field emission SEM (Zeiss). For SEM imaging of cell interaction with the microspheres, hMSCs were grown on glass coverslips. After incubation with microspheres, the samples were fixed in a 2 : 2% gluteraldahyde : paraformaldehyde mixture for 2 h at 23°C. The samples were then dehydrated by successive ethanol washes and dried using a critical point dryer (Tousimis, Rockville, MD, USA), followed by sputtercoating with gold and SEM imaging.
2.8. Quantitative RT-PCR
hMSCs were harvested and RNA was isolated using TRIzol Reagent (Invitrogen, Carlsbad, CA, USA) after 14 days in culture. Isolation was performed according to the manufacturer’s instructions and total isolated RNA was dissolved in RNase/DNase-free water and stored at −20 °C. Total RNA was quantified using a spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA). Quantitative reverse transcription RT–PCR was performed using the LightCycler® 480 Real-Time PCR System (Roche, Pleasonton, CA, USA) and the QuantiTect® SYBR® Green RT–PCR Kit with HotStar Taq DNA Polymerase. Primers used for amplification of differentiation marker genes were designed using OligoPerfect™ (Invitrogen) and purchased through IDT Technologies (Coralville, IA, USA). RT–PCR was performed according to the following protocol defined by the manufacturer: RT, 20 min at 50°C, 20°C/s ramp; PCR activation, 15 min at 95°C, 20°C/s ramp; then 35–55 cycles of (denaturation 15 s at 94°C, 20/s ramp, annealing 20–30 s at 50–60°C, 20°C/s ramp, extension 30 s 72°C, 2°C/s ramp). All samples were loaded in duplicate and normalized to total RNA content and to the performance of the housekeeping gene GAPDH. Fold differences in gene expression were relative to hMSCs cultured on tissue culture plastic and calculated using the ΔΔCt method (Livak and Schmittgen, 2001). The results were compared using a two-sample t-test, assuming equal variances.
3. Results and discussion
3.1. Bead preparation and processing
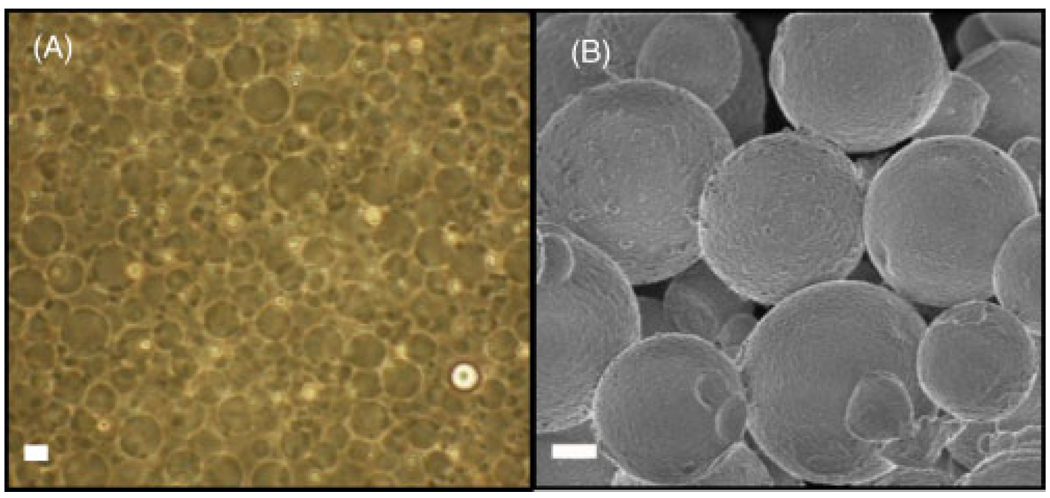
The oil-in-water emulsion technique used to fabricate gelatin particles yielded spherical microparticles which could easily be collected. Light microscope and scanning electron microscope images of gelatin microspheres are shown in Figure 3. The process parameters used in this study resulted in microspheres with an average diameter of 3.9 µm, with a range of approximately 2–6 µm. The size of the microspheres could be controlled by the rate at which the gelatin–oil slurry was mixed (data not shown); however, our goal was to create microspheres in the 3–5 µm diameter range. Formed and crosslinked microspheres had a tendency to aggregate, but could be easily separated using a tissue homogenizer. Once separated, the microspheres could be dehydrated and lyophilized, and the reconstituted bead mixture would no longer aggregate.
Figure 3.
(A) Light microscope image of gelatin microspheres before crosslinking. (B) SEM image of 20% cross-linked gelatin microspheres. Scale bars = 2 µm
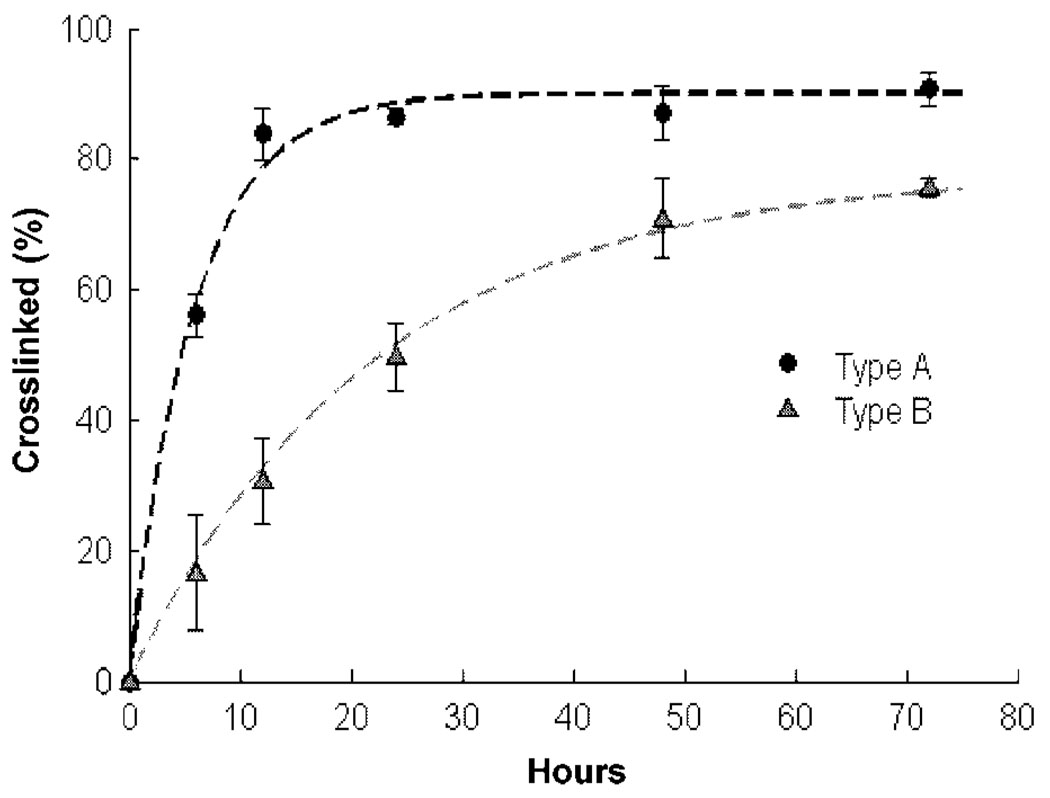
The small molecule genipin was used to crosslink the gelatin matrix by incubating the microspheres in a 1.0% solution of genipin in PBS. Figure 4 shows the degree of crosslinking of gelatin microspheres achieved over time in the genipin solution. Preparing genipin in PBS provided a means of introducing a nucleophilic agent that can initiate the crosslinking reaction of genipin with itself, thereby forming an oligomeric crosslinking agent (Liang et al., 2004) that could produce a higher degree of intermolecular binding, without residual toxicity to cells. Another benefit of using PBS is that the crosslinking activity of genipin has been shown to be the most potent when it is at pH 7.4 (Nickerson et al., 2006). In order to create a controllable reaction, the reaction was carried out at 23°C, such that the reaction could be stopped before all available amino groups had been crosslinked. The ninhydrin assay results showed that type A gelatin was crosslinked rapidly, reaching a plateau value of approximately 90% after a little more than 24 h (Figure 4A). Type B gelatin reacted more slowly and reached a plateau value of approximately 80% after more than 72 h (Figure 4B). The differences in the rate of crosslinking of the two gelatin types is probably caused by the larger number of primary amine groups on the type A gelatin molecule, which means that more crosslinking sites on adjacent gelatin molecules are available. It is probable that the lower number of primary amine groups on type B gelatin hinders the formation of intermolecular crosslinks. This rate also could be modulated by changing the pH at which the crosslinking is achieved (Sung et al., 2000); however, this parameter was not investigated. The crosslinking rate data provided a means to crosslink gelatin matrices to a desired degree (20%, 50% or 80% in this study), simply by controlling the incubation time in genipin.
Figure 4.
Degree of crosslinking with incubation time in genipin for types A and B gelatin microspheres. Type A gelatin crosslinks more rapidly and reaches a plateau, while type B gelatin crosslinks more gradually
3.2. Growth factor loading and release
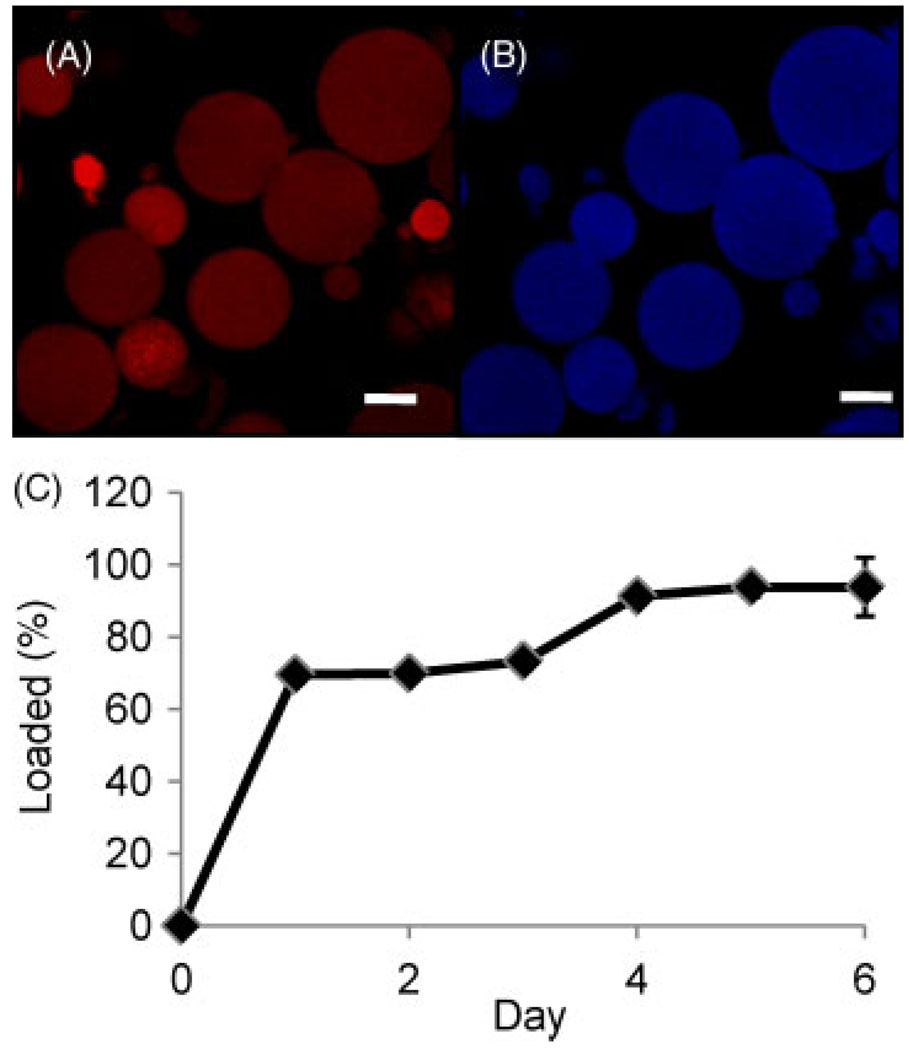
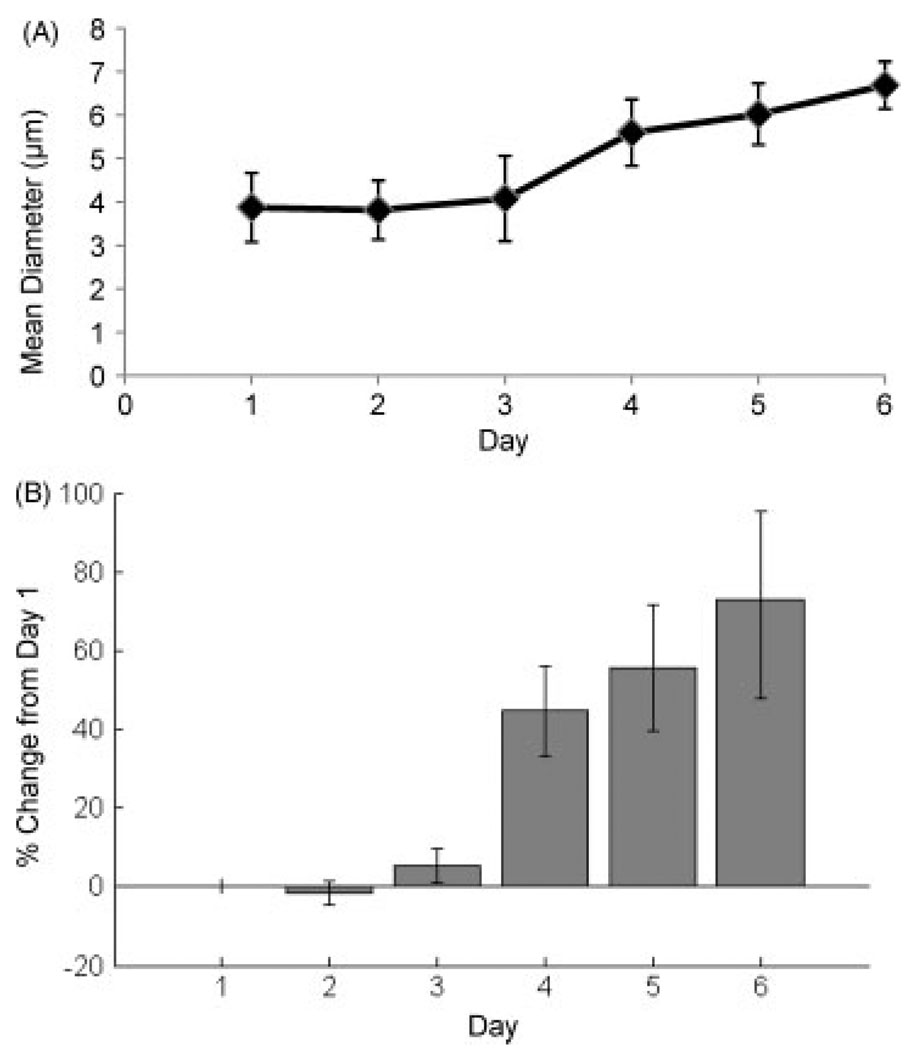
The rate and degree of protein loading into crosslinked gelatin microspheres was assessed by quantifying the amount of fluorescently labelled soy bean trypsin inhibitor (SBTI) protein taken up over time. These experiments were performed using type A gelatin microspheres, due to the isoelectric point of SBTI (pI ≈ 4.5), which allows it to form polyionic complexes with acidic gelatin. The analysis was based on fluorescence images of microspheres, and representative images of the SBTI channel and the gelatin channel are shown in Figure 5A, B. Digital image analysis was used to quantify the amount of SBTI loaded, as shown in Figure 5C, as well as the diameter of the microspheres over time, shown in Figure 6A. Loading of SBTI reached an initial plateau value of approximately 70% after 24 h of incubation. This value increased stepwise to approximately 90% loading at day 4 and plateaued at this level. The sudden increase in SBTI loading corresponded with a statistically significant stepwise increase in microsphere diameter from around 4 µm to approximately 5.5 µm between days 3 and 4 (Figure 6). Swelling of the microspheres may be caused by hydrolysis of the genipin crosslinks (Boral et al., 2006; Ofner and Bubnis, 1996), resulting in increased porosity of the matrix, a higher osmotic influx and higher protein loading. The microspheres continued to swell gradually after day 4, and diameter changes after day 4 were not statistically significant.
Figure 5.
Fluorescence microscopy images of (A) dye channel and (B) gelatin channel of crosslinked type A microspheres loaded with SBTI. Scale bar = 2 µm. (C) Loading rate of SBTI with incubation time
Figure 6.
Swelling of gelatin microspheres over time, expressed as (A) mean diameter and (B) percentage change relative to original size. Increased swelling was observed after day 3
Loading of rhBMP-2 into crosslinked type B gelatin microspheres showed that loading efficiency increased with the degree of crosslinking (Table 2). Crosslinked genipin monomers have up to four sites that can participate in hydrogen bonding. rhBMP-2 is a heavily glycosylated molecule and therefore has the ability to associate with a matrix through hydrogen bonding. The increase in hydrogen binding potential to the network could enhance the ability of the matrix to interact with sugar residues on the rhBMP-2, ultimately increasing the retention of the protein. In addition, it is likely that intermolecular binding caused by genipin crosslinking increased the tortuosity of the matrix, thereby increasing the retention of rhBMP-2.
Table 2.
Loading efficiency of rhBMP-2 in type B gelatin microspheres increased with degree of crosslinking, reported as %/mg gelatin microspheres
| Degree of crosslinking (%) | Loading efficiency (%) |
|---|---|
| 20 | 17.4 |
| 50 | 60.0 |
| 80 | 96.6 |
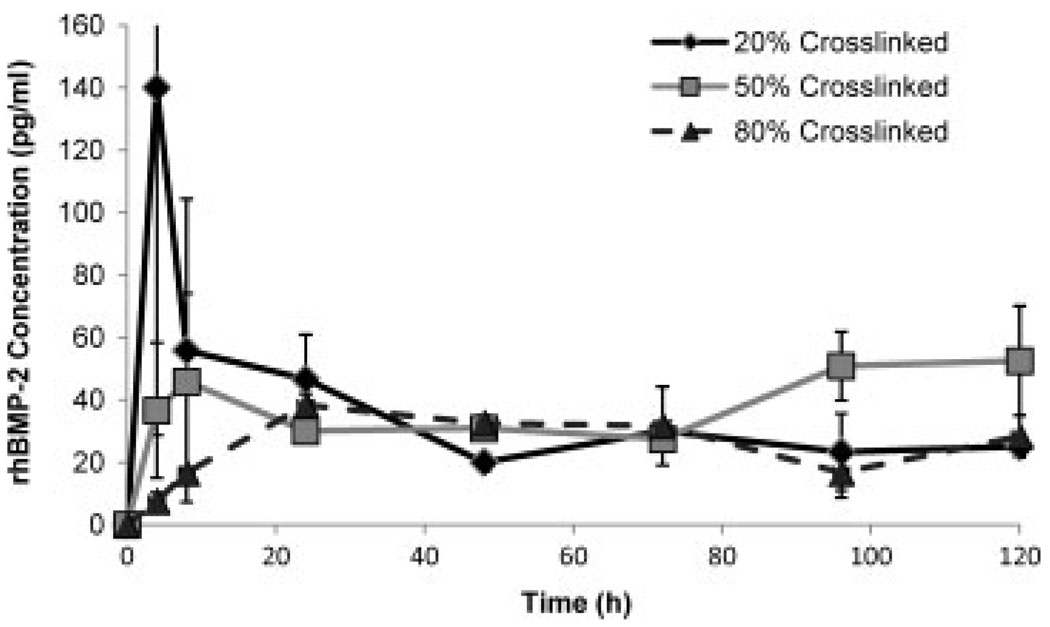
Release of rhBMP-2 was measured using an ELISA method to quantify the amount of released protein. Importantly, no rhBMP-2 was released from the gelatin microspheres in the absence of cells (data not shown), in spite of a change in the ionic environment. This suggests that this growth factor is tightly bound in the type B gelatin matrix (pI ≈ 5) and requires cellular action to be released. Figure 7 shows the release of rhBMP-2 over time in culture with hMSCs, expressed as the concentration of growth factor released into the medium over time. The degree of initial (days 0–1) growth factor release was inversely related to the degree of crosslinking. The 20% crosslinked microspheres showed an initial spike in release to about 140 pg/ml at 4 h, and the growth factor concentration subsequently dropped. The 50% crosslinked microspheres initially released approximately 45 pg/ml rhBMP-2 at 8 h, and also showed decreased release thereafter. In contrast, the 80% crosslinked microspheres produced a steady increase in growth factor concentration, which peaked at 24 h and then remained relatively steady for the duration of the 5 day experiment. For all microsphere types, the plateau concentration of rhBMP-2 was in the range 20–40 pg/ml, although the 50% crosslinked microspheres showed a modest increase to around 50 pg/ml after day 3. The decreased burst release in microspheres with higher crosslink density is probably related to increased tortuosity in the diffusion paths and a higher degree of ionic complexation in these microspheres. Previous work in which glutaraldehyde was used as a crosslinking agent showed a large burst release from gelatin microspheres in the first hour (Yamamoto et al., 2001). Other studies using glutaraldehyde-crosslinked gelatin microspheres did not observe a large burst release (Patel et al., 2008b); however, they did observe release in the absence of cells. It is possible that genipin crosslinking provides more complete sequestration of the rhBMP-2 because of the hydrogen-bonding potential of genipin oligomers, and therefore cellular action is required to release the protein.
Figure 7.
Release of rhBMP-2 from 20%, 50% and 80% type B gelatin microspheres incubated with cultured hMSCs. Initial release rate decreased with increasing degree of crosslinking
3.3. hMSC interactions with gelatin microspheres
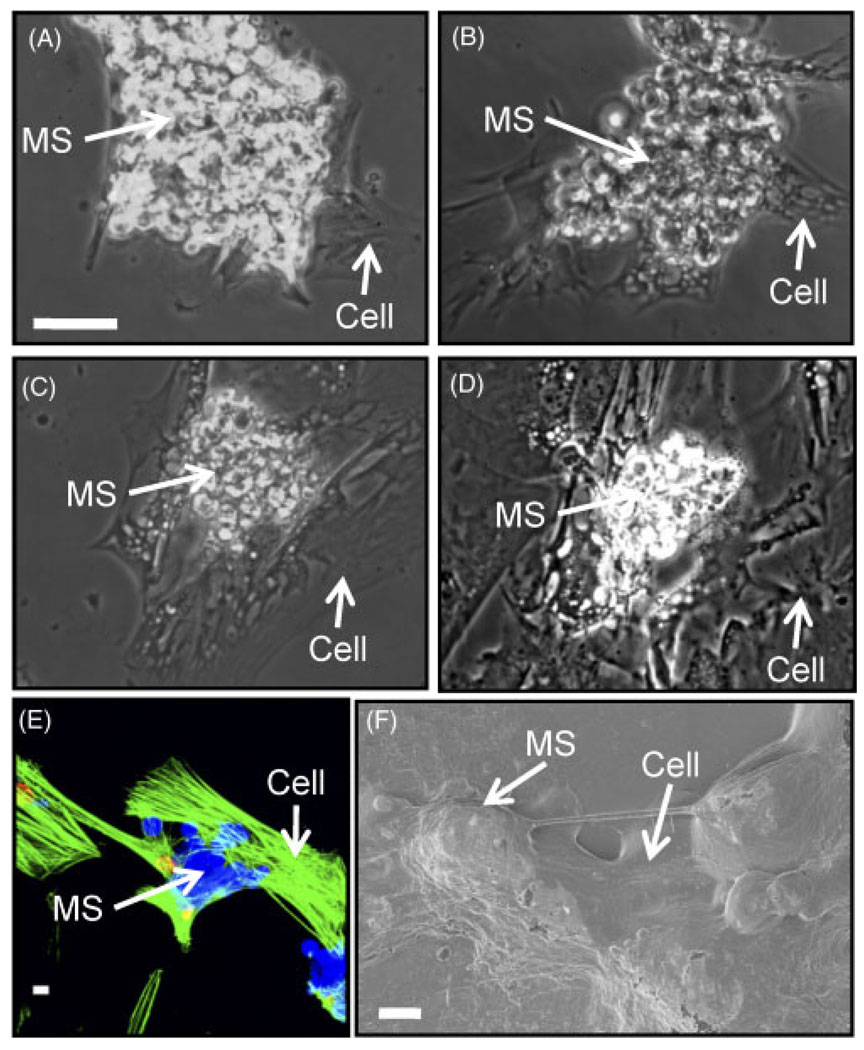
Figure 8 shows images of gelatin microspheres in contact with hMSC in culture. When microspheres were added to wells containing non-confluent monolayers of hMSCs, they clearly accumulated preferentially on top of the cells, as opposed to on the vacant polystyrene culture surface (Figure 8A). This phenomenon occurred within 2 h of microsphere addition, and the microspheres remained adhered to the hMSC as the culture progressed. In contrast, microspheres added to wells with no cells remained homogeneously distributed over the culture surface. We investigated whether the cells were phagocytosing gelatin microspheres by imaging, using confocal microscopy (Figure 8E) and scanning electron microscopy (Figure 8F). It was clear that the hMSCs strongly interacted with the microspheres but did not appear to engulf them. Gelatin is a denatured form of the extracellular matrix protein collagen, and it is not clear whether the form of the protein affects uptake up by cells. In addition, it was noted that the cells extended pseudopodia to interact with the gelatin. These data suggest that hMSCs can recognize and bind to the gelatin matrix of crosslinked microspheres but do not ingest them.
Figure 8.
Light micrographs of hMSCs cultured with 20% crosslinked type B gelatin microspheres at (A) 1, (B) 5, (C) 9 and (D) 12 days after the microspheres were added. Gelatin microspheres degraded with time in culture. Scale bar (A–D) = 10 µm. (E) Fluorescent and (F) scanning electron micrographs of hMSCs interacting with type B gelatin microspheres 24 h after addition of the microspheres. hMSCs interacted with, but did not engulf, the gelatin microspheres. Scale (E, F) = 2 µm
The gelatin microspheres remained in contact with the cells over the 2 week culture period, but degraded over time. Figure 8A – D shows that microsphere number and size decreased over 12 days in culture. Microspheres that were not in contact with cells did not break down, suggesting a cell-mediated degradation mechanism. It is well established that cells within a 3D microenvironment can remodel and reshape the surrounding matrix (Stegemann and Nerem, 2003; Hong et al., 2007). Cells reshape their environment through the action of integrin binding and subsequent release of matrix metalloproteinases (MMPs), enzymes with an intrinsic ability to degrade the surrounding matrix. hMSCs have been shown to express a variety of MMPs that aid them in migrating from the bone marrow to the site of injury (Schneider et al., 2009), including MMPs that degrade gelatin. Particle degradation and release of growth factor therefore is dependent on MSC-facilitated degradation of the gelatin matrix.
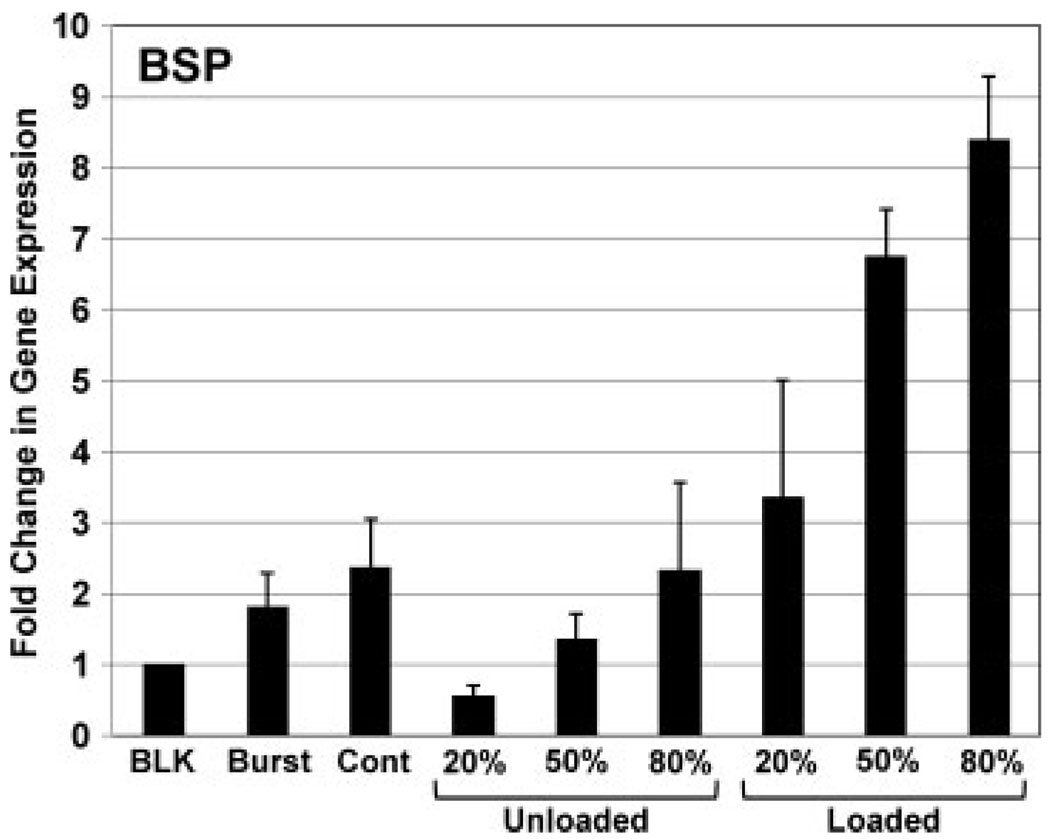
Evidence that microspheres were releasing rhBMP-2 with an effect on hMSC phenotype is shown in Figure 9. Compared to hMSCs kept in standard culture medium (BLK), BMP-loaded microspheres caused a three- to eightfold increase in the expression of the bone sialoprotein (BSP) gene. The effect increased with increasing crosslink density, with the 50% and 80% crosslinked microspheres having statistically higher gene expression (p < 0.001), probably because higher crosslinking and an increased load of rhBMP-2 caused sustained release of growth factor, as evidenced by a slower degradation rate. Interestingly, unloaded gelatin microspheres also had a modest effect on BSP expression, with 80% crosslinked microspheres slightly increasing BSP expression and 20% microspheres causing a slight decrease. These effects may be caused by hMSCs binding to the microspheres and subsequent changes in integrin signalling, although these effects were not specifically investigated in this study. The response of hMSCs to loaded microspheres with 50% and 80% crosslinking was also markedly greater (p < 0.05) than adding a bolus of rhBMP-2 (‘Burst’) or periodic replenishment of rhBMP-2 at feeding (‘Cont’), reinforcing that microsphere-mediated release of growth factors can provide improved differentiation signals.
Figure 9.
Expression of bone sialoprotein (BSP) gene by hMSCs after 14 days in culture with unloaded and BMP-loaded gelatin microspheres with different crosslink densities, as compared to bolus administration of BMP at day 1 (Burst), continuous BMP-administration (Cont) and blank unsupplemented medium (BLK). BMP release from microspheres increased BSP gene expression and the effect was dependent on crosslink density
3.4. Conclusions
The process for creating gelatin microparticles using a water-in-PDMS emulsion technique is simple and robust, and the microspheres can be subsequently stabilized by crosslinking with genipin. The crosslink density can be easily controlled by varying the incubation time in genipin, and the resulting gelatin microspheres can be used for protein delivery. The release rate of protein is dependent on the degree of crosslinking, and constant growth factor release profiles can be achieved at higher crosslink densities. hMSCs clearly interact with the gelatin matrix and degrade it over time to release entrapped growth factor, which acts as a soluble cue to direct cell differentiation. Such degradable microspheres have potential utility in tissue-engineering applications where cell-demanded release of growth factors is desirable. The microspheres could be delivered separately, or as part of a scaffold or defined microenvironment to control cell function (Lund et al., 2008).
Acknowledgements
This work was supported in part by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, through Grant No. R01-AR053231. This study used only cultured cells and adhered to all institutional and national ethical standards.
References
- Adhirajan N, Shanmugasundaram N, Babu M. Gelatin microspheres cross-linked with EDC as a drug delivery system for doxycyline: development and characterization. J Microencapsul. 2007;24(7):647–659. doi: 10.1080/02652040701500210. [DOI] [PubMed] [Google Scholar]
- Boral S, Gupta AN, Bohidar HB. Swelling and de-swelling kinetics of gelatin hydrogels in ethanol–water marginal solvent. Int J Biol Macromol. 2006;39:240–249. doi: 10.1016/j.ijbiomac.2006.03.028. [DOI] [PubMed] [Google Scholar]
- Cruz DM, Ivirico JL, Gomes MM, et al. Chitosan microparticles as injectable scaffolds for tissue engineering. J Tissue Eng Regen Med. 2008;2(6):378–380. doi: 10.1002/term.106. [DOI] [PubMed] [Google Scholar]
- Butler M, Ng YF, Pudney P. Mechanism and kinetics of the crosslinking reaction between biopolymers containing primary amine groups and genipin. J Polym Sci A Polym Chem. 2003;41(24):3941–3953. [Google Scholar]
- Dare EV, Griffith M, Poitras P, et al. Genipin crosslinked fibrin hydrogels for in vitro human articular cartilage tissue-engineered regeneration. Cells Tissues Organs. 2009 doi: 10.1159/000209230. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Kawai K, Suzuki S, Tabata Y, et al. Accelerated tissue regeneration through incorporation of basic fibroblast growth factor-impregnated gelatin microspheres into artificial dermis. Biomaterials. 2000;21(5):489–499. doi: 10.1016/s0142-9612(99)00207-0. [DOI] [PubMed] [Google Scholar]
- Ko CS, Huang JP, Huang CW, et al. Type II collagen–chondroitin sulfate–hyaluronan scaffold cross-linked by genipin for cartilage tissue engineering. J Biosci Bioeng. 2009;107(2):177–182. doi: 10.1016/j.jbiosc.2008.09.020. [DOI] [PubMed] [Google Scholar]
- Habraken WJ, Boerman OC, Wolke JG, et al. In vitro growth factor release from injectable calcium phosphate cements containing gelatin microspheres. J Biomed Mater Res A. 2008 doi: 10.1002/jbm.a.32263. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Hong H, McCullough CM, Stegemann JP. The role of ERK signaling in protein hydrogel remodeling by vascular smooth muscle cells. Biomaterials. 2007;28(26):3824–3833. doi: 10.1016/j.biomaterials.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino K, Kimura T, De Grand AM, et al. Three catheter-based strategies for cardiac delivery of therapeutic gelatin microspheres. Gene Ther. 2006;13(18):1320–1327. doi: 10.1038/sj.gt.3302793. [DOI] [PubMed] [Google Scholar]
- Liang HC, Chang WH, Lin KJ, et al. Genipin-crosslinked gelatin microspheres as a drug carrier for intramuscular administration: in vitro and in vivo studies. J Biomed Mater Res A. 2003;65(2):271–282. doi: 10.1002/jbm.a.10476. [DOI] [PubMed] [Google Scholar]
- Liang HC, Chang WH, Liang HF, et al. Crosslinking structures of gelatin hydrogels crosslinked with genipin or a water-soluble carbodiimide. J Appl Polym Sci. 2004;91:4017–4026. [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lund AW, Bush JA, Plopper GE, et al. Osteogenic differentiation of mesenchymal stem cells in defined protein beads. J Biomed Mater Res B Appl Biomater. 2008;87(1):213–221. doi: 10.1002/jbm.b.31098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuigan AP, Sefton MV. Modular tissue engineering: fabrication of a gelatin-based construct. J Tissue Eng Regen Med. 2007;1(2):136–145. doi: 10.1002/term.14. [DOI] [PubMed] [Google Scholar]
- Nickerson MT, Patel J, Heyd DV, et al. Kinetic and mechanistic considerations in the gelation of genipin-crosslinked gelatin. Int J Biol Macromol. 2006;39:298–302. doi: 10.1016/j.ijbiomac.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Ofner CM, Bubnis WA. Chemical and swelling evaluations of amino group crosslinking in gelatin and modified gelatin matrices. Pharm Res. 1996;13:1821–1827. doi: 10.1023/a:1016029023910. [DOI] [PubMed] [Google Scholar]
- Patel ZS, Ueda H, Yamamoto M, et al. In vitro and in vivo release of vascular endothelial growth factor from gelatin microparticles and biodegradable composite scaffolds. Pharm Res. 2008a;25(10):2370–2378. doi: 10.1007/s11095-008-9685-1. [DOI] [PubMed] [Google Scholar]
- Patel ZS, Yamamoto M, Ueda H, et al. Biodegradable gelatin microparticles as delivery systems for the controlled release of bone morphogenetic protein-2. Acta Biomater. 2008b;4(5):1126–1138. doi: 10.1016/j.actbio.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider RK, Puellen A, Kramann R, et al. The osteogenic differentiation of adult bone marrow and perinatal umbilical mesenchymal stem cells and matrix remodelling in three-dimensional collagen scaffolds. Biomaterials. 2009 October 6; doi: 10.1016/j.biomaterials.2009.09.059. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Silva GA, Ducheyne P, Reis RL. Materials in particulate form for tissue engineering. 1. Basic concepts. J Tissue Eng Regen Med. 2007;1(1):4–24. doi: 10.1002/term.2. [DOI] [PubMed] [Google Scholar]
- Sisson K, Zhang C, Farach-Carson MC, et al. Evaluation of cross-linking methods for electrospun gelatin on cell growth and viability. Biomacromolecules. 2009 doi: 10.1021/bm900036s. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Sivadas N, O’Rourke D, Tobin A, et al. A comparative study of a range of polymeric microspheres as potential carriers for the inhalation of proteins. Int J Pharm. 2008;358(1–2):159–167. doi: 10.1016/j.ijpharm.2008.03.024. [DOI] [PubMed] [Google Scholar]
- Stegemann JP, Nerem RM. Altered response of vascular smooth muscle cells to exogenous biochemical stimulation in two-and three-dimensional culture. Exp Cell Res. 2003;283(2):146–155. doi: 10.1016/s0014-4827(02)00041-1. [DOI] [PubMed] [Google Scholar]
- Sung HW, Chang Y, Liang IL, et al. Fixation of a biological tissues with a naturally occurring crosslinking agent: fixation rate and effects of pH, temperature, and initial fixative concentration. J Biomed Mater Res. 2000;52:77–87. doi: 10.1002/1097-4636(200010)52:1<77::aid-jbm10>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Tabata Y, Ikada Y. Protein release from gelatin matrices. Adv Drug Deliv Rev. 1998;31:287–301. doi: 10.1016/s0169-409x(97)00125-7. [DOI] [PubMed] [Google Scholar]
- Tabata Y, Yamada K, Miyamoto S, et al. Bone regeneration by basic fibroblast growth factor complexed with biodegradable hydrogels. Biomaterials. 1998;19(7–9):807–815. doi: 10.1016/s0142-9612(98)00233-6. [DOI] [PubMed] [Google Scholar]
- Tessmar JK, Go¨pferich AM. Matrices and scaffolds for protein delivery in tissue engineering. Adv Drug Deliv Rev. 2007;59(4–5):274–291. doi: 10.1016/j.addr.2007.03.020. [DOI] [PubMed] [Google Scholar]
- Tsai CC, Huang RN, Sung HW, et al. In vitro evaluation of the genotoxicity of a naturally occurring crosslinking agent (genipin) for biologic tissue fixation. J Biomed Mater Res. 2000;52(1):58–65. doi: 10.1002/1097-4636(200010)52:1<58::aid-jbm8>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Tsai CC, Chang Y, Sung HW, et al. Effects of heparin immobilization on the surface characteristics of a biological tissue fixed with a naturally occurring crosslinking agent (genipin): an in vitro study. Biomaterials. 2001;22(6):523–533. doi: 10.1016/s0142-9612(00)00206-4. [DOI] [PubMed] [Google Scholar]
- Wei HJ, Yang HH, Chen CH, et al. Gelatin microspheres encapsulated with a nonpeptide angiogenic agent, ginsenoside Rg1, for intramyocardial injection in a rat model with infarcted myocardium. J Control Release. 2007;120(1–2):27–34. doi: 10.1016/j.jconrel.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Ikada Y, Tabata Y. Controlled release of growth factors based on biodegradation of gelatin hydrogel. J Biomater Sci Polym Ed. 2001;12(1):77–88. doi: 10.1163/156856201744461. [DOI] [PubMed] [Google Scholar]
- Young S, Wong M, Tabata Y, et al. Gelatin as a delivery vehicle for the controlled release of bioactive molecules. J Control Release. 2005;109:256–274. doi: 10.1016/j.jconrel.2005.09.023. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Chesnutt BM, Utturkar G, et al. The effect of crosslinking of chitosan microspheres with genipin on protein release. Carbohydrate Polymers. 2007;68:561–567. [Google Scholar]