Abstract
Background
Gastroesophageal reflux (GER) is common in asthma patients but often has mild or no symptoms. It is not known whether treatment of GER with proton pump inhibitors (PPIs) in poorly-controlled asthmatics without GER symptoms can substantially improve asthma control.
Methods
402 asthmatics with inadequate asthma control despite inhaled corticosteroids and absent or minimal GER symptoms were randomly assigned to either esomeprazole 40mg b.i.d. or matching placebo in a parallel-group double-masked clinical trial. Participants were followed for 24 weeks with daily asthma diaries, every 4-week spirometry, and asthma symptom questionnaires. Participants were classified with respect to GER status with ambulatory pH probe monitoring. The primary outcome was the rate of episodes of poor asthma control (EPACs) based on asthma diaries.
Results
Episodes of poor asthma control occurred with similar frequency in the placebo and esomeprazole treatment groups (2.3 vs 2.5 events/person-year, respectively, P=0.66). There was no treatment effect with respect to components of the EPACs, or secondary outcomes including pulmonary function, airways reactivity, asthma control, symptom scores, nocturnal awakenings, or quality of life. GER documented by pH probe studies in 40% of participants with absent or minimal symptoms did not identify a subgroup benefitting from PPI treatment.
Conclusion
Despite a high prevalence of asymptomatic GER in patients with poorly controlled asthma, treatment with proton pump inhibitors does not improve control. Silent GER is not a likely cause of poorly controlled asthma.
INTRODUCTION
Gastroesophageal reflux (GER) and asthma, both common conditions, often co-exist in the same patient. Persons with asthma are particularly prone to asymptomatic GER. Esophageal pH probe studies have documented that 32–84% of asthmatics have abnormal acid reflux.1, 2, 3, 4, 5 About half of asthma patients who have reflux have no symptoms. 3, 6, 7, 8 However, the extent that GER plays an important role in causing or maintaining asthma symptoms is not known. Symptoms of asthma – cough and chest discomfort – may overlap those of GER, making it difficult to distinguish between the two conditions.9 Moreover, the causal relationship between asthma and GER is complex. Acid reflux causes bronchoconstriction by microaspiration into the airways as well as by reflex-mediated effects of acid on the esophagus or upper airway.10,11,12, 13,14, 15, 16, 17, 18 Alternatively, asthmatic bronchoconstriction can induce acid reflux. Descent of the diaphragm with hyperinflation increases the pressure gradient between the abdomen and thorax and may cause the lower esophageal sphincter (LES) to herniate into the chest where its barrier function is diminished.19, 20 This may be exacerbated by the accentuated negative inspiratory pleural pressure in acute asthma which opposes the tone of the LES. Furthermore, beta-agonist and methylxanthine bronchodilators may decrease LES tone, but it has been difficult to demonstrate that these agents actually worsen reflux.21
Proton pump inhibitors (PPIs) are effective in suppressing gastric acid production and reducing GER symptoms whether or not asthma is present.22, 23 Previous trials have shown conflicting results regarding beneficial effects of PPI treatment in patients with asthma who have frequent symptoms of GERD. 23, 24 Whether PPIs are effective in improving asthma control in patients with minimal or absent GER symptoms is unknown, and whether objective measurement of acid reflux can personalize PPI treatment has not been established.25, 26 Current guidelines recommend consideration of evaluation for GERD in patients who have poorly controlled asthma, especially with nighttime symptoms, even in the absence of suggestive GERD symptoms; If GER is present, treatment recommendations include use of a PPI.27 Moreover, asthma patients treated for GER have substantially higher diagnostic and treatment costs than those of similar severity who do not have this diagnosis.28
Accordingly, we conducted a randomized placebo-controlled double-masked trial of esomeprazole (Nexium®, AstraZeneca, Wilmington, DE) in patients with inadequately controlled asthma despite inhaled corticosteroid therapy. We excluded patients with a history of GER symptoms who would already have an indication for PPIs on that basis. The primary objective of the trial was to determine whether acid suppression therapy improved asthma symptoms. A secondary objective was to determine whether ambulatory esophageal pH probe monitoring would distinguish individuals with absent or minimal GER symptoms who might respond to treatment.
METHODS
Participant Selection
Eligible participants had inadequately controlled asthma despite the use of moderate or higher doses of inhaled corticosteroids. Inclusion criteria were: age 18 years or older with a physician diagnosis of asthma supported by either a positive methacholine challenge test or a 12% increase in FEV1 with bronchodilators; 8 weeks of stable use of an inhaled corticosteroid equivalent to 400 ug/day or greater of fluticasone;27 poor asthma control defined by either: a Juniper Asthma Control Questionnaire score of 1.5 or greater29 or more than one acute episode of asthma requiring unscheduled medical care in the past year. Participants were excluded if they had smoked cigarettes within six months or had 10 or more pack-years of smoking; had an FEV1 less than 50% predicted 30; had anti-reflux or peptic ulcer surgery; or had clinical indications for acid suppression treatment (i.e. two or more episodes per week of heartburn requiring antacids). Participants were also ineligible if they had used anti-reflux medication within 1 month, or were taking drugs that could interact with PPIs such as theophylline, iron supplements, warfarin, anti-fungal drugs (“azoles”), or digitalis. Participants were also excluded if they were pregnant, intolerant of PPIs, or had any serious illness that would interfere with participation in the trial. Participants signed written consent statements approved by the local institutional review board.
Study Design
The study was conducted at 19 clinical centers between October, 2004 and May 2008. Data were analyzed by the Coordinating Center at Johns Hopkins University. The study was designed as a two-arm parallel design randomized clinical trial. Participants meeting eligibility criteria were enrolled in a 2–8 week run-in period during which time they completed daily baseline asthma diaries and had pH probe testing performed. Participants were randomly assigned in equal allocation to either esomeprazole 40mg twice daily or a similar-appearing placebo. Participants and study staff were masked to the treatment assignment. The randomization schedule was stratified by clinic using a permuted block design with concealed allocation. After randomization, participants returned to the clinic every 4 weeks for 24 weeks for assessment of outcome measures.
Outcome Measures
For the duration of the trial participants maintained diaries to record morning peak expiratory flow (PEF), asthma symptoms, nocturnal awakenings, and use of beta-agonists. The primary outcome measure was the rate of Episodes of Poor Asthma Control (Type I EPAC). These were defined by the occurrence of either: decrease in morning PEF 30% or more on two consecutive days compared to personal best during the run-in period; unscheduled health care visit for asthma symptoms; or addition of oral prednisone for treatment of asthma. Beta-agonist use was excluded from this definition because of the possibility that participants might use beta-agonists for treatment of GER-related symptoms. A secondary analysis added the use of beta-agonist (4 or more inhalations in one day above baseline) for asthma symptoms to the above definition (Type II EPAC). 31 Other asthma symptoms recorded on daily diaries were considered secondary outcomes.
Secondary outcomes recorded at each visit were: spirometry before and after 180 mcg of inhaled albuterol32, the Juniper Asthma Control Questionnaire (ACQ)29, the Asthma Symptom Utility Index (ASUI)33, the Asthma Quality of Life Questionnaire (AQLQ)34, and the MOS-SF36 generic quality of life questionnaire.35 Methacholine airways reactivity, expressed as the concentration causing a 20% fall in FEV1 (PC20), was measured at baseline and at 24 weeks for participants with an FEV1 > 70% predicted.36
The presence or absence of esophageal reflux was ascertained by ambulatory pH probe monitoring.37 Studies were reviewed at a central reading center for technical quality. A technically satisfactory study required the total recording time to be 16 or more hours, with at least one meal and two hours of recumbency. Reflux was considered present if the percent of time with pH less than 4.0 was more than 5.8% of the total time, or more than 8.2% of the upright time, or more than 3.5% of the supine time.38 Symptoms of GER were assessed with the Gastroesophageal Reflux Disease Symptom Assessment Scale (GSAS) – Distress Version, which measures both the number and severity of symptoms.39
Statistical Analysis
The target sample size of 400 participants was calculated to provide 77–97% power with 5% type I error (2-sided) and 10% data loss to detect a relative difference of 33% in the proportion of participants experiencing one or more EPACs assuming a rate of 40–60% in the control group. All analyses were performed based on treatment assignment and all available data were included in the evaluations regardless of treatment received (modified intention to treat analysis). Negative binomial regression models were used to evaluate the difference in EPAC rate and the individual component rates.40 Linear regression techniques were used to evaluate the mean differences from baseline during follow-up, robust variance estimates were calculated with GEE.41 Treatment effect modification for key covariates such as pH probe test results, age, race, self-reported GERD and asthma severity were performed by creating an interaction term and evaluating the term’s significance in a model with the main effects (treatment and covariate). Analyses presented are not adjusted for baseline covariates unless there was evidence of an imbalance across the treatment groups at baseline. Statistical significance was inferred when p< 0.05. P-values are not adjusted for multiple comparisons.
RESULTS
Participant Characteristics (Table 1, Figure 1)
Table 1.
Baseline Characteristics
| Treatment | ||
|---|---|---|
| Placebo | Esomeprazole | |
| Demographic Characteristics – N | 199 | 203 |
| Mean age at randomization, yr ± SD | 42 ± 13 | 42 ± 13 |
| Males (% of group) | 28 | 36 |
| Race or ethnic group (% of group) | ||
| White | 52 | 50 |
| Black | 37 | 39 |
| Hispanic | 9 | 9 |
| Other | 3 | 2 |
| Former smoker (% of group) | 20 | 15 |
| Mean BMI, kg/m2 ± SD | 32 ± 8 | 32 ± 9 |
| BMI >=30 kg/m2 (% of group) | 54 | 50 |
| Asthma Characteristics | ||
| Mean age of asthma onset yr ± SD | 17 ± 17 | 17 ± 16 |
| Use of inhaled short-acting beta-agonist (MDI/Neb) ≥ 2 times/week (% of group) | 83 | 79 |
| Unscheduled health care visit in past year (%of group) | 63 | 54 |
| Oral corticosteroids for asthma in past year (%of group) | 51 | 47 |
| Daily use of ICS (% of group) | 100 | 100 |
| Fluticasone/salmeterol (% of group) | 75 | 79 |
| Mean ACQ (↓) (score range: 0–6) ± SD | 1.9 ± 0.8 | 1.8 ± 0.8 |
| Mean ASUI (↑) (score range: 0–1) ± SD | 0.74 ± 0.18 | 0.76 ± 0.15 |
| Mean AQL (↑) (score range: 1–7) ± SD | 4.7 ± 1.2 | 4.7 ± 1.2 |
| SF-36 Quality of Life (↑) (score range: 0–100) - N | 199 | 202 |
| Mean physical score ± SD | 42 ± 10 | 43 ± 10 |
| Mean emotional score ± SD | 49 ± 10 | 50 ± 11 |
| Mean Pulmonary Function Measures - N | 198 | 203 |
| Pre-BD FEV1 (%predicted ± SD) * | 78 ± 15 | 76 ± 15 |
| Pre-BD FVC (%predicted ± SD) * | 87 ± 16 | 87 ± 14 |
| FEV1 %change post-BD ± SD | 10 ± 10 | 11 ± 16 |
| FVC %change post-BD ± SD | 5 ± 8 | 6 ± 11 |
| Peak flow rate (L/min ± SD) | 363 ± 97 | 357 ± 107 |
| PC20 mg/mL - N | 92 | 83 |
| mean ± SD | 2.8 ± 4.0 | 3.6 ± 4.4 |
| PC20 contraindicated n (% of group) | 103 (52) | 117 (59) |
| pH probe results – N | 151 | 152 |
| Positive (% of group) | 41 | 39 |
| GERD Symptom Assessment Scale - N | 199 | 203 |
| Mean number of symptoms (0–15) ) ± SD | 7 ± 3 | 6 ± 4 |
| Mean distress score (↓) (score range: 0–3) ± SD | 0.60 ± 0.46 | 0.51 ± 0.47 |
| Other conditions - N | 199 | 203 |
| Self-reported GERD (% of group) | 19 | 10 |
| Eczema (% of group) | 20 | 10 |
| Sinusitis (% of group) | 43 | 34 |
| Rhinitis (% of group) | 61 | 58 |
| Food allergies (% of group) | 24 | 15 |
| Allergies worsen asthma (% of group) | 78 | 78 |
KEY: BMI = Body Mass Index. ACQ = Asthma Control Questionnaire. ASUI = Asthma Symptom Utility Index. AQL = Asthma Quality of Life. ↑ = higher score is better, ↓ = lower score is better, FEV1 = Forced expiratory volume in 1 second, FVC = Forced vital capacity, BD = bronchodilator, SD = standard deviation, GERD = gastroesphogeal reflux disease, SF-36 = RAND 36 item health survey.
Predicted values for FEV1 and FVC are taken from: Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–87.
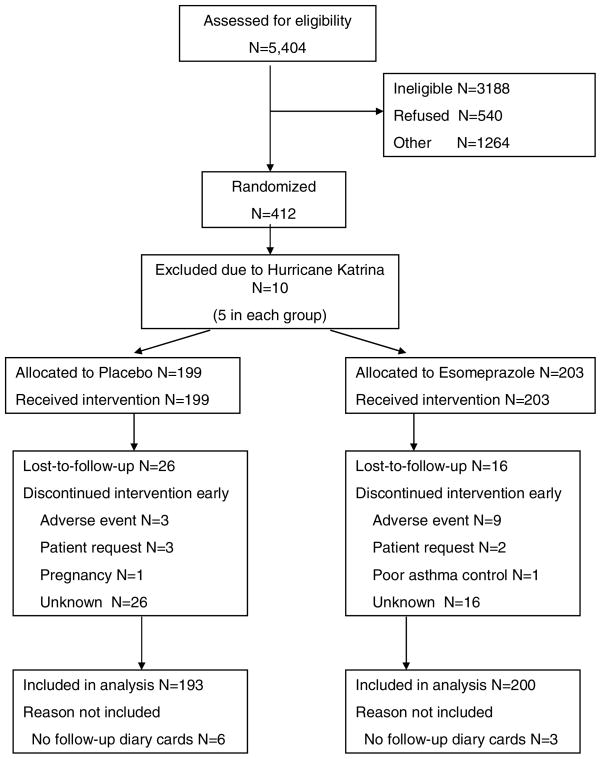
Figure 1.
CONSORT Diagram
412 patients were randomized to treatment in this study. Because of Hurricane Katrina, data from 10 patients in New Orleans were incomplete and these cases were not included in the analysis. As a group, the patients were predominantly female, had low normal lung function and very poor asthma control evidenced by an ACQ score of 1.9.29 Approximately 15% of the participants reported that they had a history of GER but the mean symptom scores were low. GER evidenced by ambulatory pH probe monitoring was present in 41% of the placebo group and 39% of the esomeprazole group. The asthma characteristics were similar between the two treatment groups.
Defining an adherent participant as one who takes both doses of drug on 80% or more of days on treatment, more participants were adherent with placebo (86%) than esomeprazole (84%) (P = 0.53). Participants took one or more doses of study drug on 80% or more days in 94% and 91% of the placebo and esomeprazole groups respectively. (P = 0.21) Esomeprazole was generally very well tolerated, but slightly more participants discontinued treatment in the esomeprazole group than the placebo group because of adverse effects, 9 vs 3, respectively. However, the esomeprazole group had fewer serious adverse events than the placebo group (11 vs 16, P = 0.29) including 3 asthma hospitalizations in the esomeprazole group and 4 in the placebo group. One patient in the esomeprazole group died following surgery for a bronchial carcinoid discovered during the study.
Episodes of Poor Asthma Control (Table 2)
Table 2.
Episodes of Poor Asthma Control
| N | Treatment Group |
IRR* (Eso:Plb) 95% CI | P-value* |
||
|---|---|---|---|---|---|
| Placebo 193 |
Esomeprazole 200 |
Eso vs Plb† | GER Interaction‡ | ||
| Asthma episodes - type 1 | |||||
| #Events | 201 | 224 | |||
| Rate (events/person-year) | 2.3 | 2.5 | 1.1 0.8, 1.5 |
0.66 | 0.93 |
| Patients with ≥1 event, N(%) | 42 | 42 | |||
| Exacerbation Components Peak flow, 30% drop | |||||
| #Events | 141 | 180 | |||
| Rate (events/person-year) | 1.7 | 2.1 | 1.2 0.8, 2.0 |
0.35 | 0.99 |
| Patients with ≥1 event, N (%) | 26 | 28 | |||
| Urgent Care | |||||
| #Events | 53 | 51 | |||
| Rate (events/person-year) | 0.7 | 0.6 | 0.9 0.6, 1.5 |
0.79 | 0.44 |
| Patients with ≥1 event, N (%) | 17 | 18 | |||
| New use of oral steroids | |||||
| #Events | 50 | 48 | |||
| Rate (events/person-year) | 0.6 | 0.5 | 0.9 0.6, 1.3 |
0.62 | 0.84 |
| Patients with ≥1 event, N (%) | 24 | 21 | |||
| Asthma episodes - type 2 | |||||
| #Events | 367 | 383 | |||
| Rate (events/person-year) | 4.4 | 4.3 | 1.0 0.8, 1.3 |
0.87 | 0.19 |
| Patients with ≥1 event, N (%) | 63 | 60 | |||
| Rescue medications | |||||
| #Events | 248 | 241 | |||
| Rate (events/person-year) | 3.0 | 2.8 | 0.9 0.7, 1.3 |
0.62 | 0.05 |
| Patients with ≥1 event, N (%) | 46 | 45 | |||
| Night awakening | |||||
| #Events | 2,518 | 2,241 | |||
| Rate (events/person-year) | 30 | 28 | 0.9 0.6, 1.4 |
0.71 | 0.38 |
| Patients with ≥1 event, N (%) | 55 | 52 | |||
Incidence rate ratio and P-value estimated from negative binomial regression with robust variance estimates.
P-value for treatment effect (Esomeprazole - Placebo)
P-value for modification of treatment effect by pH probe results estimated by linear regression;
Overall, the enrollees had persistent, poorly controlled asthma. Approximately 42% of the participants had a type I event and 61% had a type II event. Over the 24 weeks follow-up, about 18% of the patients required an urgent care visit or a course of prednisone. The annualized rates for EPACs, and the individual components (fall in PEF, urgent care visits, courses of steroids, or increased use of beta-agonists) did not differ between treatment groups. Night awakening due to asthma occurred on 1 or more occasions in about half of the participants, and this did not differ between treatment groups.
Secondary Outcomes (Table 3)
Table 3.
Secondary Outcomes – Mean change from Baseline
| P-values | |||||
|---|---|---|---|---|---|
| Placebo | Esomeprazole | Treatment effect | Plb vs Eso* | GER Interaction† | |
| N Mean Change Baseline to 24 weeks (95% confidence interval) | |||||
| Lung function | |||||
| pre-BD FEV1 (L) | 191 −0.02 −.06, 0.01 |
201 0.00 −0.04, 0.04 |
0.03 −0.03, 0.08 |
0.36 | 0.56 |
| pre-BD FVC (L) | 191 −0.03 −0.06, 0.01 |
201 0.00 −0.04, 0.05 |
0.03 −0.03, 0.09 |
0.30 | 0.77 |
| FEV1 change with BD (%) | 191 −0.2 −1.6, 0.1 |
201 −1.3 −3.4, 0.7 |
−1.0 −3.5, 1.4 |
0.41 | 0.45 |
| Peak flow rate (L/min) | 191 3.2 −3.5, 9.9 |
201 9.2 1.8, 16.6 |
6.0 −3.9, 16.0 |
0.24 | 0.03 |
| PC20 Methacholine (mg/mL)‡ | 40 1.5 0.2, 2.8 |
33 −0.3 −1.4, 1.6 |
−1.8 −3.6, −0.1 |
0.04 | 0.68 |
| PC20 not achieved at 24-weeks n (%) | 18 (31) | 20 (35) | 4% | 0.60 | |
| Asthma control scores and Mini-Asthma Quality of Life | |||||
| Asthma control score | 191 −0.3 −0.4, −0.2 |
201 −0.2 −0.3, −0.1 |
0.1 0.0,0.2 |
0.11 | 0.73 |
| ASUI | 191 0.05 0.03, 0.07 |
201 0.02 0.01, 0.04 |
−0.02 −0.05, 0.05 |
0.11 | 0.75 |
| Mini Asthma Quality of life score | 191 0.3 0.2, 0.4 |
201 0.3 0.2, 0.4 |
−0.1 −0.2, 0.1 |
0.33 | 0.81 |
| Quality of Life - SF36 score | |||||
| Mental | 191 0.0 −1.1, 1.1 |
201 0.4 −0.6, 1.4 |
0.5 −1.1, 2.0 |
0.56 | 0.46 |
| Physical | 191 2.0 1.1, 2.9 |
201 1.1 0.3, 1.9 |
−0.9 −2.1, 0.4 |
0.16 | 0.58 |
| Gastric Symptoms | |||||
| GSAS score | 191 −0.17 −0.21, −0.12 |
201 −0.16 −0.20, −0.11 |
0.01 −0.05, 0.07 |
0.76 | 0.99 |
| Number of symptoms | 191 −1.7 −2.1, −1.3 |
201 −1.9 −2.3, −1.6 |
−0.2 −0.8, 0.3 | 0.40 | 0.91 |
P-value for treatment effect (Esomeprazole - Placebo) estimated by linear regression
P-value for modification of treatment effect by pH probe results estimated by linear regression; the number of participants with pH probe data available was 302.
Adjusted for baseline value
Abbreviations: BD - bronchodilator; ASUI - Asthma Symptom Utility Index; GSAS -Gastroesophageal Reflux Disease Symptom Assessment Scale
Spirometry, bronchodilator response, PEF, and airways reactivity did not change during the study and was not different between treatment groups. Asthma symptoms, asthma control, and quality of life, assessed by questionnaires all improved slightly during the trial but did not differ by treatment assignment. GER symptom scores, by design, were low at baseline, showed small improvements during the study, but were not different by treatment group.
Subgroup Analyses
Pre-specified subgroup analyses were performed to determine whether we could identify a subgroup likely to benefit. For all outcomes, there was no statistical interaction between abnormal GER by pH probe and treatment assignment indicating that patients with documented GER did not respond differently to PPIs. Neither BMI nor the presence of night awakenings was indicative of a response to PPIs. Additionally there was no interaction of treatment effect with: age, race, gender, former smoking status, asthma control or severity scores, use of long-acting beta agonists, self-reported GER, or GSAS distress score.
DISCUSSION
The purpose of this trial was to determine whether the addition of a PPI, esomeprazole, in doses large enough to suppress gastric acid, would improve asthma control in patients with inadequately controlled asthma, who did not have frequent GER symptoms. Moreover, we performed ambulatory esophageal pH probe studies to establish whether individuals with documented acid reflux might benefit more from PPI therapy. After six months of treatment in 402 patients, we were not able to demonstrate any treatment benefit with respect to the primary outcome, the rate of episodes of poor asthma control, or secondary outcomes measuring asthma symptoms, nocturnal awakenings, quality of life, or lung function. Moreover, there was no difference in asthma outcomes between patients with documented reflux compared to those without.
A systematic review of twelve small trials concluded that most studies have shown improvement in asthma outcomes with PPIs, but the studies were marred by design flaws and did not show consistent improvement in the same asthma outcomes.25 More recently, Littner and colleagues reported a six-month placebo-controlled trial in 207 moderate-severe asthmatics who had definite GER symptoms. Lansoprazole 30 mg twice daily did not improve the primary outcome of daily asthma symptoms, but caused a reduction in exacerbations and improvement in asthma-related quality of life.23 The reduction in exacerbations was greatest in those taking more than one class of asthma controller. Kiljander and colleagues conducted a three-strata 24-week multicenter international trial in asthmatics with nocturnal or GER symptoms treated with esomeprazole 40 mg twice daily. Overall, there was no efficacy in terms of daily PEF, exacerbations, or asthma symptoms. However, the strata of 350 patients that had both GER symptoms and nocturnal asthma symptoms had an improvement in PEF, but no benefit for FEV1, rescue inhaler use, symptom scores, or nocturnal awakenings. The esomeprazole related improvement was most pronounced in patients taking long-acting beta-agonists. No prior studies have evaluated PPI treatment in poorly controlled asthmatics on ICS who had asymptomatic GER documented by ambulatory pH probe studies as suggested by current treatment guidelines.
This study differed from previous trials insofar as we excluded patients who had symptoms of GER two or more times per week. Our rationale was that these patients already have an indication for acid suppression treatment, regardless of their asthma. In our population, we found no benefit from PPIs in any primary or secondary asthma outcome measure. Moreover, ambulatory pH probe studies did not distinguish a subgroup likely to benefit. Further, we did not find that patients taking long-acting beta-agonists were more likely to respond to PPIs. Therefore, taken as a whole, the weight of evidence indicates that PPIs should not be routinely prescribed to improve asthma symptoms if the patient does not have symptoms of GER. In patients who have symptoms of GER, PPI therapy reduces GER symptoms, but likely has little impact on asthma. Because diagnostic tests and drug treatment of GER in asthma patients contribute substantially to the cost of asthma care, limited use of these measures seems warranted.28
This trial provides strong evidence that asymptomatic GER, which was present in nearly half of our participants with poorly controlled asthma and minimal or absent symptoms of heartburn, is not a frequent cause of poor asthma control, In addition, the failure of PPIs to improve methacholine reactivity suggests that airway inflammation from microaspiration or esophageal reflexes is not a common contributing mechanism of poor asthma control in patients who have persistent asthma symptoms despite inhaled corticosteroids.
Although the dose of esomeprazole used in this study is highly effective in suppressing gastric acid throughout the day, and is larger than the routinely administered dose for GER symptoms, it does not prevent alkaline reflux that may also trigger esophageal reflexes mediating neurogenic inflammation in the airways.42 On occasion, nocturnal gastric acid may occur with even high dose PPI, though it does not necessarily lead to reflux in asymptomatic individuals.43 Furthermore, asymptomatic GER may have other adverse health consequences such as Barrett’s esophagus and predisposition to esophageal cancer that are not related to asthma. Accordingly, the role for ambulatory esophageal pH probe testing in asthmatics ought to be guided by the need to diagnose and treat esophageal disease but not asthma.
In summary, we have found that there is no benefit from treating patients with poorly controlled asthma with a PPI if they have minimal or absent symptoms of GER. Ambulatory pH probe testing and clinical characteristics do not identify a subgroup that is likely to benefit.
Acknowledgments
Supported by: NIH-NHLBI 5U01HL072968 and the American Lung Association. Esomeprazole and placebo were provided under a grant from Astra-Zeneca.
The writing committee (John G. Mastronarde, Nicholas R. Anthonisen, Mario Castro, Janet T. Holbrook, Frank T. Leone, W. Gerald Teague, Robert A. Wise) takes full responsibility for the content and integrity of the manuscript. .
This study is supported by grants from the NIH-NHLBI and the American Lung Association. Study drug and placebo were generously supplied by Astra-Zeneca.
American Lung Association Asthma Clinical Research Centers
Baylor College of Medicine, Houston: N. A. Hanania (principal investigator), M. Sockrider (co-principal investigator), L. Giraldo (principal clinic coordinator), R. Valdez (coordinator);
Columbia University–New York University Consortium, New York: J. Reibman (principal investigator), E. DiMango (co-principal investigator), C CAmmarata and K Carapetyan (clinic coordinators at New York University), J. Sormillon and E Simpson (clinic coordinators at Columbia University);
Duke University Medical Center, Durham, N.C.: L. Williams (principal investigator), J. Sundy (co-principal investigator), G. Dudek (principal clinic coordinator), R. Newton and A Dugdale (coordinators);
Emory University School of Medicine, Atlanta: W.G. Teague (principal investigator), R Patel (principal clinic coordinator), J. Peabody, R. Patel, E Hunter;, D Whitlock (coordinators);
Illinois Consortium, Chicago: L. Smith (principal investigator), J. Moy, E Naureckas, C.S. Olopade (co-principal investigators), J. Hixon (principal clinic coordinator), A. Brees, G. Rivera, S. Sietsema, V. Zagaja (coordinators);
Indiana University, Asthma Clinical Research Center, Indianapolis: M. Busk (principal investigator), F. Leickly, C. Williams (co-principal investigators), P. Puntenney (coordinator);
Jefferson Medical College, Philadelphia: F. Leone (principal investigator), M. Hayes-Hampton (principal clinic coordinator);
Louisiana State University Health Sciences Center, Ernest N. Morial Asthma, Allergy, and Respiratory Disease Center, New Orleans: W.R. Summer (principal investigator), C. Glynn and G Meyaski (clinic coordinators);
National Jewish Medical and Research Center, Denver: S. Wenzel and R. Katial (principal investigators), P Silkoff (co-principal investigator), R. Gibbs (principal clinic coordinator), L. Lopez, C. Ruis, B. Schoen (coordinators);
Nemours Children’s Clinic–University of Florida Consortium, Jacksonville: J. Lima (principal investigator), K. Blake (co-principal investigator), A. Santos (principal clinic coordinator), L. Duckworth, D. Schaeffer, M McRae (coordinators);
North Shore–Long Island Jewish Health System, New Hyde Park, N.Y.: J. Karpel (principal investigator), R. Cohen (co-principal investigator), R. Ramdeo (principal clinic coordinator);
Northern New England Consortium formerly Vermont Lung Center at the University of Vermont), Colchester, Vt.: C.G. Irvin (principal investigator), A.E. Dixon, D.A. Kaminsky, E. Kent, T. Lahiri, P. Shapiro (co-principal investigators), S. Lang (principal clinic coordinator), J. Allen, A. Coote, L.M. Doucette, K. Girard, J. Lynn, L. Moon, T. Viola, S Burns (coordinators);
The Ohio State University Medical Center/Columbus Children’s Hospital, Columbus: J. Mastronarde (principal investigator), K. McCoy (co-principal investigator), J. Parsons (co-investigator), J. Drake (principal clinic coordinator), R. Compton, L. Raterman, D. Cosmar (coordinators);
University of Alabama at Birmingham, Birmingham: L.B. Gerald (principal investigator), W.C. Bailey (co-principal investigator), S. Erwin (principal clinic coordinator), H. Young, A. Kelley, D. Laken, B. Martin (coordinators);
University of Miami, Miami–University of South Florida, Tampa: A. Wanner (principal investigator, Miami), R. Lockey (principal investigator, Tampa), E. Mendes (principal clinic coordinator for University of Miami), S. McCullough (principal clinic coordinator for University of South Florida) B Fimbel, M Grandstaff (coordinators);
University of Minnesota, Minneapolis: M.N. Blumenthal (principal investigator), G. Brottman, J. Hagen (co-principal investigators), A. Decker, D. Lascewski, S. Kelleher (principal clinic coordinators), K. Bachman, M. Sneen (coordinators);
University of Missouri, Kansas City School of Medicine, Kansas City: G. Salzman (principal investigator), D. Pyszczynski (co-principal investigator), P. Haney (principal clinic coordinator);
St. Louis Asthma Clinical Research Center: Washington University, St. Louis University, and Clinical Research Center, St. Louis: M. Castro (principal investigator), L. Bacharier, K. Sumino (co-investigators), M.E. Scheipeter and J. Tarsi (coordinators);
University of California San Diego: S. Wasserman (principal investigator), J. Ramsdell (co-principal investigator). J Vitin and T Tucker (clinic coordinators)
Chairman’s Office, Respiratory Hospital, Winnipeg, Man., Canada: N. Anthonisen (research group chair);
Data Coordinating Center, Johns Hopkins University Center for Clinical Trials, Baltimore: R. Wise (center director), J. Holbrook (deputy director), E. Brown (principal coordinator), D. Amend-Libercci, K. Barry, M. Daniel, G. Leatherman, C. Levine, A. Lears, R. Masih, S. Modak, D. Nowakowski, N. Prusakowski, D. Shade, E. Sugar, C. Shiflett
Esophageal pH Probe Quality Control Center, Temple University School of Medicine: J. Richter (center director)
Data and Safety Monitoring Board: S. Lazarus(chair), W. Calhoun, P. Kahrilas, B. McWilliams, A. Rogatko, C. Sorkness
Project Office, American Lung Association, New York: E. Lancet, R. Vento (project officers), N. Edelman (scientific consultant), S. Rappaport, G. Pezza.
Project Office, National Heart Lung and Blood Institute: V. Taggart (project officer), G. Weinmann (DSMB secretary, airway branch chief)
ALA Scientific Advisory Committee: G. Snider (chair), N. Anthonisen, M. Castro, J. Fish, D. Ingbar, S. Jenkinson, D. Mannino, H. Perlstadt, L. Rosenwasser, J. Samet, T. Standiford, J. Smith, L. Smith, D. Schraufnagel, A. Wanner, T. Weaver.
Footnotes
Registered at ClinicalTrials.Gov: NCT00069823
Contributor Information
John G. Mastronarde, Email: john.mastronarde@osumc.edu.
Nicholas R. Anthonisen, Email: nanthonisen@exchange.hsc.mb.ca.
Mario Castro, Email: castrom@im.wustl.edu.
Janet T. Holbrook, Email: jholbroo@jhsph.edu.
Frank T. Leone, Email: frank.tleone@uphs.upenn.edu.
W. Gerald Teague, Email: wteague@emory.edu.
Robert A. Wise, Email: rwise@jhmi.edu.
References
- 1.Field SK, Underwood M, Brant R, Cowie RL. Prevalence of gastroesophageal reflux symptoms in asthma. Chest. 1996;109:316–22. doi: 10.1378/chest.109.2.316. [DOI] [PubMed] [Google Scholar]
- 2.Harding SM, Guzzo MR, Richter JE. 24-h esophageal pH testing in asthmatics: respiratory symptom correlation with esophageal acid events. Chest. 1999;115:654–9. doi: 10.1378/chest.115.3.654. [DOI] [PubMed] [Google Scholar]
- 3.Sontag SJ, O'Connell S, Khandelwal S, et al. Most asthmatics have gastroesophageal reflux with or without bronchodilator therapy. Gastroenterology. 1990;99:613–20. doi: 10.1016/0016-5085(90)90945-w. [DOI] [PubMed] [Google Scholar]
- 4.Vincent D, Cohen-Jonathan AM, Leport J, et al. Gastro-oesophageal reflux prevalence and relationship with bronchial reactivity in asthma. Eur Respir J. 1997;10:2255–9. doi: 10.1183/09031936.97.10102255. [DOI] [PubMed] [Google Scholar]
- 5.Simpson W. Gastroesophageal reflux disease and asthma. Arch Intern Med. 1995;155:798–803. [PubMed] [Google Scholar]
- 6.Harding SM, Guzzo MR, Richter JE. The prevalence of gastroesophageal reflux in asthma patients without reflux symptoms. Am J Respir Crit Care Med. 2000;162:34–9. doi: 10.1164/ajrccm.162.1.9907072. [DOI] [PubMed] [Google Scholar]
- 7.Irwin RS, Curley FJ, French CL. Difficult to control asthma: contributing factors and outcome of a systematic management protocol. Chest. 1993;103:1662–1669. doi: 10.1378/chest.103.6.1662. [DOI] [PubMed] [Google Scholar]
- 8.Kiljander TO, Laitinen JO. The prevalence of gastroesophageal reflux disease in adult asthmatics. Chest. 2004;126:1490–4. doi: 10.1378/chest.126.5.1490. [DOI] [PubMed] [Google Scholar]
- 9.Fish JE, Peters SP. Gastroesophageal reflux and upper airways disease in severe asthma. In: Holgate ST, Boushey HA, Fabbri LM, editors. Difficult Asthma. London: Martin Dunitz, Ltd; 1999. pp. 77–91. [Google Scholar]
- 10.Richter JE. Asthma and gastroesophageal reflux disease: the truth is difficult to define. Chest. 1999;116:1150–2. doi: 10.1378/chest.116.5.1150. [DOI] [PubMed] [Google Scholar]
- 11.Harding SM, Richter JE. The role of gastroesophageal reflux in chronic cough and asthma. Chest. 1997;111:1389–402. doi: 10.1378/chest.111.5.1389. [DOI] [PubMed] [Google Scholar]
- 12.Ekstrom T, Tibbling L. Esophageal acid perfusion, airway function, and symptoms in asthmatic patients with marked bronchial hyperreactivity. Chest. 1989;96:995–8. doi: 10.1378/chest.96.5.995. [DOI] [PubMed] [Google Scholar]
- 13.Herve P, Denjean A, Jian R, Simonneau G, Duroux P. Intraesophageal perfusion of acid increases the bronchomotor response to methacholine and to isocapnic hyperventilation in asthmatic subjects. Am Rev Respir Dis. 1986;134:986–9. doi: 10.1164/arrd.1986.134.5.986. [DOI] [PubMed] [Google Scholar]
- 14.Wu DN, Tanifuji Y, Kobayashi H, et al. Effects of esophageal acid perfusion on airway hyperresponsiveness in patients with bronchial asthma. Chest. 2000;118:1553–5. doi: 10.1378/chest.118.6.1553. [DOI] [PubMed] [Google Scholar]
- 15.Cuttitta G, Cibella F, Visconti A, Scichilone N, Bellia V, Bonsignore G. Spontaneous gastroesophageal reflux and airway patency during the night in adult asthmatics. Am J Respir Crit Care Med. 2000;161:177–81. doi: 10.1164/ajrccm.161.1.9808014. [DOI] [PubMed] [Google Scholar]
- 16.Jack CI, Calverley PM, Donnelly RJ, et al. Simultaneous tracheal and oesophageal pH measurements in asthmatic patients with gastro-oesophageal reflux. Thorax. 1995;50:201–4. doi: 10.1136/thx.50.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tuchman D, Boyle J, Pack I. Comparison of airway responses following tracheal or oesophageal acidification in the cat. Gastroenterology. 1984;87:872–881. [PubMed] [Google Scholar]
- 18.Harding SM, Schan CA, Guzzo MR, Alexander RW, Bradley LA, Richter JE. Gastroesophageal reflux-induced bronchoconstriction. Is microaspiration a factor? Chest. 1995;108:1220–7. doi: 10.1378/chest.108.5.1220. [DOI] [PubMed] [Google Scholar]
- 19.Choy D, Leung R. Gastro-oesophageal reflux disease and asthma. Respirology. 1997;2:163–8. doi: 10.1111/j.1440-1843.1997.tb00073.x. [DOI] [PubMed] [Google Scholar]
- 20.Zerbib F, Guisset O, Lamouliatte H, Quinton A, Galmiche JP, Tunon-De-Lara JM. Effects of bronchial obstruction on lower esophageal sphincter motility and gastroesophageal reflux in patients with asthma. Am J Respir Crit Care Med. 2002;166:1206–11. doi: 10.1164/rccm.200110-033OC. [DOI] [PubMed] [Google Scholar]
- 21.Lacy BE, Mathis C, Desbiens J, Liu MC. The Effects of Nebulized Albuterol on Esophageal Function in Asthmatic Patients. Dig Dis Sci. 2008 Feb 13; doi: 10.1007/s10620-007-0188-4. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 22.Donnellan C, Sharma N, Preston C, Moayyedi P. Medical treatments for the maintenance therapy of reflux oesophagitis and endoscopic negative reflux disease. Cochrane Database Syst Rev. 2005 Apr 18;(2):CD003245. doi: 10.1002/14651858.CD003245.pub2. [DOI] [PubMed] [Google Scholar]
- 23.Littner MR, Leung FW, Ballard ED, Huang B, Samra NK Lansoprazole Asthma Study Group. Effects of 24 Weeks of Lansoprazole Therapy on Asthma Symptoms, Exacerbations, Quality of Life, and Pulmonary Function in Adult Asthmatic Patients with Acid Reflux Symptoms. Chest. 2005;128:1128–1135. doi: 10.1378/chest.128.3.1128. [DOI] [PubMed] [Google Scholar]
- 24.Kiljander TO, Harding SM, Field SK, et al. Effects of esomeprazole 40 mg twice daily on asthma: a randomized placebo-controlled trial. Am J Respir Crit Care Med. 2006;173:1091–7. doi: 10.1164/rccm.200507-1167OC. [DOI] [PubMed] [Google Scholar]
- 25.Coughlan JL, Gibson PG, Henry RL. Medical treatment for reflux oesophagitis does not consistently improve asthma control: a systematic review. Thorax. 2001;56:198–204. doi: 10.1136/thorax.56.3.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gibson PG, Henry RL, Coughlan JL. Gastro-oesophageal reflux treatment for asthma in adults and children. Cochrane Database Syst Rev. 2003:CD001496. doi: 10.1002/14651858.CD001496. [DOI] [PubMed] [Google Scholar]
- 27.National Heart Lung and Blood Institute. National Asthma Education and Prevention Program Expert Panel Report 3 Guidelines for the Diagnosis and Management of Asthma. [last accessed March 16, 2008];Full Report 2007. available at: http://www.nhlbi.nih.gov/guidelines/asthma/asthgdln.htm.
- 28.Dal Negro RW, Turco P, Micheletto C, Tognella S, Bonadiman L, Guerriero M, Sandri M. Cost analysis of GER-induced asthma: a controlled study vs. atopic asthma of comparable severity. Respir Med. 2007;101:1814–20. doi: 10.1016/j.rmed.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 29.Juniper EF, Bousquet J, Abetz L, Bateman ED GOAL Committee. Identifying 'well-controlled' and 'not well-controlled' asthma using the Asthma Control Questionnaire. Respir Med. 2006;100:616–21. doi: 10.1016/j.rmed.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 30.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–87. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 31.American Lung Association Asthma Clinical Research Centers. Clinical trial of low-dose theophylline and montelukast in patients with poorly controlled asthma. Am J Respir Crit Care Med. 2007;175:235–42. doi: 10.1164/rccm.200603-416OC. [DOI] [PubMed] [Google Scholar]
- 32.Miller MR, Hankinson J, Brusasco V, et al. ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J. 2005;26:319–38. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 33.Revicki DA, Leidy NK, Brennan-Diemer F, Sorensen S, Togias A. Integrating patient preferences into health outcomes assessment. CHEST. 1998;114:998–1007. doi: 10.1378/chest.114.4.998. [DOI] [PubMed] [Google Scholar]
- 34.Juniper QOL, Juniper EF, Guyatt GH, Cox FM, Ferrie PJ, King DR. Development and validation of the Mini Asthma Quality of Life Questionnaire. Eur Respir J. 1999;14:32–8. doi: 10.1034/j.1399-3003.1999.14a08.x. [DOI] [PubMed] [Google Scholar]
- 35.Bousquet J, Knani J, Dhivert H, et al. Quality of life in asthma. I. Internal consistency and validity of the SF-36 questionnaire. Am J Respir Crit Care Med. 1994;149:371–5. doi: 10.1164/ajrccm.149.2.8306032. [DOI] [PubMed] [Google Scholar]
- 36.Crapo RO, Casaburi R, Coates AL, et al. Guidelines for methacholine and exercise challenge testing-1999. Am J Respir Crit Care Med. 2000;161:309–29. doi: 10.1164/ajrccm.161.1.ats11-99. [DOI] [PubMed] [Google Scholar]
- 37.DeVault KR, Castell DO American College of Gastroenterology. Updated guidelines for the diagnosis and treatment of gastroesophageal reflux disease. Am J Gastroenterol. 2005;100:190–200. doi: 10.1111/j.1572-0241.2005.41217.x. [DOI] [PubMed] [Google Scholar]
- 38.Richter JE, Bradley LA, DeMeester TR, Wu WC. Normal 24-h ambulatory esophageal pH values: influence of study center, pH electrode, age and gender. Dig Dis Sci. 1992;37:849–856. doi: 10.1007/BF01300382. [DOI] [PubMed] [Google Scholar]
- 39.Damiano A, Handley K, Adler E, Siddique R, Bhattacharyja A. Measuring symptom distress and health-related quality of life in clinical trials of gastroesophageal reflux disease treatment: further validation of the Gastroesophageal Reflux Disease Symptom Assessment Scale (GSAS) Dig Dis Sci. 2002;47:1530–7. doi: 10.1023/a:1015815102175. [DOI] [PubMed] [Google Scholar]
- 40.Keene ON, Jones MR, Lane PW, Anderson J. Analysis of exacerbation rates in asthma and chronic obstructive pulmonary disease: example from the TRISTAN study. Pharm Stat. 2007;6:89–97. doi: 10.1002/pst.250. [DOI] [PubMed] [Google Scholar]
- 41.Liang K, Zeger S. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13. [Google Scholar]
- 42.Canning BJ, Mazzone SB. Reflex mechanisms in gastroesophageal reflux disease and asthma. Am J Med. 2003;115(Suppl 3A):45S–48S. doi: 10.1016/s0002-9343(03)00192-x. [DOI] [PubMed] [Google Scholar]
- 43.Ours TM, Fackler WK, Richter JE, Vaezi MF. Nocturnal acid breakthrough: clinical significance and correlation with esophageal acid exposure. Am J Gastroenterol. 2003;98:545–50. doi: 10.1111/j.1572-0241.2003.07304.x. [DOI] [PubMed] [Google Scholar]