Abstract
There are concerns about the health effects of formaldehyde exposure, including carcinogenicity, in light of elevated indoor air levels in new homes and occupational exposures experienced by workers in health care, embalming, manufacturing and other industries. Epidemiological studies suggest that formaldehyde exposure is associated with an increased risk of leukemia. However, the biological plausibility of these findings has been questioned because limited information is available on formaldehyde’s ability to disrupt hematopoietic function. Our objective was to determine if formaldehyde exposure disrupts hematopoietic function and produces leukemia-related chromosome changes in exposed humans. We examined the ability of formaldehyde to disrupt hematopoiesis in a study of 94 workers in China (43 exposed to formaldehyde and 51 frequency-matched controls) by measuring complete blood counts and peripheral stem/progenitor cell colony formation. Further, myeloid progenitor cells, the target for leukemogenesis, were cultured from the workers to quantify the level of leukemia-specific chromosome changes, including monosomy 7 and trisomy 8, in metaphase spreads of these cells. Among exposed workers, peripheral blood cell counts were significantly lowered in a manner consistent with toxic effects on the bone marrow and leukemia-specific chromosome changes were significantly elevated in myeloid blood progenitor cells. These findings suggest that formaldehyde exposure can have an adverse impact on the hematopoietic system and that leukemia induction by formaldehyde is biologically plausible, which heightens concerns about its leukemogenic potential from occupational and environmental exposures.
INTRODUCTION
Formaldehyde and goods containing this chemical reportedly account for more than 5% of the yearly US Gross National Product (GNP), which is about $500 billion. There are longstanding concerns about the adverse health effects of formaldehyde exposure, including carcinogenicity, for professionals exposed to formalin-based fixatives such as pathologists, anatomy students, nurses and embalmers, and for workers exposed to formaldehyde in manufacturing. Recently, public awareness of this issue has been raised in the wake of Hurricane Katrina, as high levels of formaldehyde have been found in the temporary housing trailers provided by the Federal Emergency Management Agency (FEMA). At the same time, a large number of workers are exposed to formaldehyde in their workplaces (1). A significantly greater number of people are exposed to lower levels of formaldehyde in the environment, as it is generated by automobile engines (2), is present in tobacco smoke, and is released from various household products such as plywood, particleboard, furniture and carpeting (3–5).
Recently, the International Agency for Research on Cancer (IARC) classified formaldehyde as a known human carcinogen (Group 1) based on “sufficient epidemiological evidence that formaldehyde causes nasopharyngeal cancer in humans” (3, 5). IARC also concluded that there was “strong but not sufficient evidence for a causal association between leukemia and occupational exposure to formaldehyde” (3, 5). The known impact of formaldehyde on the human cancer burden would increase markedly if it was found to cause leukemia in addition to nasopharyngeal cancer, which is relatively rare (6). The evidence for an association with leukemia came primarily from a number of epidemiological studies. Several studies of pathologists, embalmers and other professionals exposed to formaldehyde (7–12) have observed an increased risk of leukemia, with myeloid leukemia being most prominent in some studies, as well as modest associations with lymphoma. Further, two major industrial cohort studies of formaldehyde-exposed workers have also shown elevated risks of lympho-hematopoietic cancers, especially myeloid leukemia (13, 14). The most recent update of one of these studies with an additional 10 years of follow-up continues to suggest a possible link between formaldehyde exposure and mortality due to lymphohematopoetic malignancies, particularly myeloid leukemia (15). However, results from a British cohort study did not show the same association (16).
Although the epidemiological data are generally consistent with a causal association between leukemia and occupational exposure to formaldehyde and chromosome damage has been observed in the blood cells of exposed workers (17–21), questions have been raised over whether or not formaldehyde reaches the bone marrow, due to its highly reactive nature, and whether it damages hematopoietic stem or progenitor cells, which are the targets for leukemogenesis (22–26). One of the clinical consequences of damage to hematopoietic stem or progenitor cells is a decrease in circulating red blood cell, white blood cell and platelet counts. Human leukemogens cause a marked decrease in white blood cell, platelet and red blood cell counts at high doses and lower the ability of progenitor cells to replicate in colony-forming cell culture assays (27, 28). For example, the number of granulocyte-macrophage colonies in culture (CFU-GM) is used as an indicator of stem cell changes caused by ionizing radiation and cancer chemotherapy (29, 30). Thus, if formaldehyde were a human leukemogen, one would expect to see a lowering of peripheral blood counts in exposed workers and an effect on the ability of progenitor cells to form granulocyte-macrophage colonies in culture (CFU-GM).
The published data on formaldehyde hematotoxicity are limited and inconsistent. Several previous studies have shown that formaldehyde alters the counts of different types of blood cells. One study reported that exposure to formaldehyde in humans reduced white blood cell counts (31). Another recent study concluded that formaldehyde increased B cells, but decreased total T cells (CD3) and T-suppressor cells (CD8) in the blood of exposed workers, while T-helper cells (CD4) cells remained unchanged (19). However, a study of people environmentally exposed to formaldehyde during an accidental spill showed no difference in white blood cells, lymphocytes, or T-cells (CD4 and CD8) (32). In male rats exposed to a high dose of formaldehyde, increased monocytes, red blood cells and hemoglobin were detected, but lymphocyte counts were decreased (33).
On the other hand, several studies in experimental animals have shown increased levels of cytogenetic damage in the bone marrow of formaldehyde-exposed mice and rats (17, 34), and in Syrian hamster embryo cells exposed to formaldehyde in vitro (35). Formaldehyde clearly damages chromosomes and may potentially cause the specific cytogenetic changes found in hematological malignancies if it reaches the target cells of importance. Early blood stem and progenitor cells are the target cells involved in leukemogenesis (36). Mutations arising in these cells through gene mutation or chromosome breaks and aneuploidy may give rise to leukemic stem cells (37). To date, however, formaldehyde has not been reported to induce leukemia-specific chromosomal alterations in human blood stem or progenitor cells.
In the present study we have examined this issue both in exposed humans and in cell cultures to evaluate the capacity of formaldehyde to produce hematotoxicity and cause damage to hematopoietic progenitor cells. To achieve this goal a molecular epidemiology study of workplaces in Guangdong, China was performed in which we measured the complete blood counts (CBC), CFU-GM and leukemia-specific chromosome changes among formaldehyde-exposed and unexposed workers.
MATERIALS AND METHODS
Identification of study factories
We identified one factory that produced formaldehyde-melamine resins and one factory that used formaldehyde-melamine resins to manufacture plastic utensils. Monitoring of formaldehyde levels was performed in these factories during an initial screening and it was established that there were no other exposures to known or suspected leukemogens or hematotoxicants (e.g., benzene, phenol, chlorinated solvents). We selected a control population from three workplaces in the same geographic region as factories with formaldehyde exposure and enrolled workers who had comparable demographic and socioeconomic characteristics and who were engaged primarily in manufacturing. Detailed inspection of the control workplaces did not identify potential for occupational exposure to formaldehyde or any other hematotoxic or genotoxic chemicals in excess of exposure levels in the general population.
Characteristics of study subjects
The study was approved by Institutional Review Boards at the U.S. National Cancer Institute and the Guangdong Poisoning Control Center, participation was voluntary, and written informed consent was obtained. All exposed workers had to meet two inclusion criteria: 1) they had to have had formaldehyde exposure levels of about 1 to 2 ppm on most days during the initial screening; and 2) they held the same job for at least the previous 3 months in the same factory. Exclusion criteria for both formaldehyde-exposed and control workers were history of cancer, chemotherapy, and radiotherapy, as well as previous occupations with notable exposure to benzene, butadiene, styrene and/or ionizing radiation. We enrolled 43 study subjects exposed to relatively high levels of formaldehyde (mostly between 0.6 and 2.5 ppm). Forty-one of the 43 workers (95%) worked in the study factories for at least one year. We enrolled 51 controls who were frequency-matched by age (±5 years) and gender to the exposed workers. The participation rates for formaldehyde-exposed workers and controls were 92% and 95%, respectively. Subjects were administered a questionnaire by trained interviewers requesting information on occupational history, environmental exposures, medical history and current medications, and past and current tobacco and alcohol use (Table 1) on the same day that biologic samples were collected.
Table 1.
Demographic and exposure characteristics of study subjects from Guangdong, China.
| Formaldehyde | Study Subjects | Formaldehyde Air Level | Age | Gender | Current Smoking | Current Alcohol Drinking | Recent Respiratory Infections | Body Mass Index | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exposure | (n) | (ppm) a | (Year) | Male | Female | Yes | No | Yes | No | Yes | No | |
| Controls | 51 | 0.032 (0.018, 0.032)b | 30±7c | 44(86)d | 7(14) | 23(45) | 28(55) | 21 (41) | 30 (59) | 15 (29) | 36 (71) | 22.16±3.18 |
| Exposed | 43 | 1.28 (0.63, 2.51)b | 31±6 | 37(86) | 6(14) | 18(42) | 25(58) | 11 (26) | 32 (74) | 17 (40) | 26 (60) | 21.46±2.54 |
An 8-hour time-weighted average based on arithmetic mean of individual exposure measurements;
Median exposure (10th, 90th %tile). A value of the limit of detection divided by square root of 2 was assigned to individuals for non-detectable formaldehyde exposure
Mean ± SD; and
Subject # (%).
Exposure assessment
Formaldehyde exposure was monitored with UMEx 100 diffusion samplers. Samplers were worn by the workers in the exposed workplaces for a full-shift (> 240 min) on ~3 working days over a three-week period. Study subjects were interviewed and biologic samples were collected towards the end of this period. Each formaldehyde-exposed subject had a minimum of two diffusion samplers collected. The average formaldehyde exposure level was calculated by taking the arithmetic mean of each subject’s measurements. A subgroup of workers in the unexposed workplaces was monitored for formaldehyde exposure on a single day. The limit of detection was 0.012 ppm. Personal exposure to other organic compounds was measured at least twice for each formaldehyde-exposed worker by 3MTM organic vapor monitors (OVM). OVMs were analyzed for chloroform, methylene chloride, tetrachloroethylene, trichloroethylene, and benzene, and no hydrocarbons were detected in any of the selected samples. The analysis laboratory was blinded with regard to the source of the diffusion samplers. Urinary benzene was measured in a subset of enrolled subjects (20 controls and 21 workers from both exposed factories) using GC-MS methods as described by Kim et al. (38). Although it was an original exclusion criteria, the potential for exposure to benzene, radiation and other potential hematotoxic agents in previous jobs was assessed systematically by review of each worker’s detailed occupational history.
Biological sampling
In June and July 2006, we obtained biological samples from the 94 study subjects at their workplaces after informed consent was obtained. Biological samples were collected after the formaldehyde-exposed workers had been monitored at least twice for personal formaldehyde air exposure in their workplace. Chinese physicians from the Guangdong Poisoning Control Center conducted a standard physical examination that included evaluation for signs of current upper or lower respiratory infection and measurements of temperature, blood pressure, height and weight, CBC with differential, and lymphocyte subsets, as well as routine biochemical tests (standard liver and renal chemistries) in blood and urine. Peripheral blood and urine samples (post-shift and overnight) were collected from each study subject and delivered to the processing laboratories within ~4h of collection. Blood from each study subject was drawn into different vacutainer tubes by specially trained nurses. This was used for the culture of peripheral blood myeloid progenitor cells (CFU-GM) using a methylcellulose based colony assay (described in detail below), whole blood cultures to prepare metaphase spreads, and the measurement of the CBC and differential (which was analyzed using a Sysmex XT-1800i automated hematology analyzer (Kobe, Japan) with coefficients of variation< 5% for all endpoints). Field collection and laboratory processing personnel differed and the biological samples were coded so that laboratory personnel on-site were blinded with respect to exposure status.
A portion of each type of biological sample, including prepared slides, was shipped to Bioreliance Inc. (Rockville, MD) under contract to NCI, where the samples were recoded and sent to the U.C. Berkeley laboratory for analysis. Thus, all laboratory analyses carried out after completion of the field phase of the study were performed blinded to exposure status.
Culturing of Myeloid Progenitor Cells
It is extremely difficult to obtain bone marrow from around 100 healthy workers under field conditions in occupational studies to directly evaluate the effect of formaldehyde exposure on bone marrow cells. However, a fraction of hematopoietic stem and progenitor cells circulate in the bloodstream in dynamic equilibrium with the stem cell pools in the bone marrow. These cells can be cultured in colony-forming assays to measure their proliferative potential in semi-solid media containing appropriate growth factors (28). The individual colonies can be classified microscopically according to the progenitor cell type. Colonies arising from the most primitive, early progenitor cells are called colony-forming-unit–granulocyte, erythroid, macrophage, megakaryocyte (CFU-GEMM) because the progenitors can give rise to any of these mature cells. Colonies derived from more committed progenitor cells that give rise to reticulocytes and erythrocytes are called burst-forming unit–erythroid (BFU-E), whereas those that give rise to granulocytes and macrophages are called colony-forming unit–granulocyte-macrophage (CFU-GM).
We applied the CFU-GM colony-forming assay to peripheral blood mononuclear cells from 43 exposed workers and 51 frequency-matched controls. Hematopoietic progenitor cells from the peripheral blood were cultured in growth factor-containing methylcellulose media (MethoCult GF H4534, according to the protocol provided by StemCell Technologies Inc) without erythropoietin (EPO) and the number of CFU-GM colonies formed was scored in 6 petri dishes after 14 days. Limitations of the field setting and personnel available only allowed for cultures without EPO to be prepared, therefore only CFU-GM colonies could be examined in vivo and not BFU-E or CFU-GEMM.
For laboratory studies performed in vitro, formaldehyde-treated mononuclear cells from a volunteer of Chinese origin were cultured in the same media in the absence or presence of EPO, such that colonies of BFU-E, CFU-GM and CFU-GEMM were formed after 14 days of culture. Formaldehyde, diluted from a 37% solution, was added on day 1 to final concentrations of 0, 100, 150 and 200 μM, which is in the dose-range found in human blood (39, 40) and utilized in many in vitro studies including in cultured human blood cells (41, 42). A young male volunteer was used as the blood donor for these cell culture experiments to reduce variation.
Metaphase Preparation from Cultured CFU-GM Cells
Metaphases from CFU-GM cells were prepared after 14 days of culture by adding colcemid (0.05 μg/ml) to 6 petri dishes overnight prior to harvest. CFU-GM colony cells were harvested, washed, and dispersed into a hypotonic solution (0.075 M KCI). After 30 min, the cells were fixed in methanol-acetic acid (3:1) twice and then dropped onto several slides, air dried, and stored in slide boxes at −20°C.
Fluorescence in situ Hybridization (FISH)
The loss of chromosome 7 and gain of chromosome 8 are among the most frequent cytogenetic changes observed in myeloid leukemia and myelodysplastic syndromes (43) and can be readily analyzed by FISH. These leukemia-specific chromosome changes in CFU-GM cells were examined in a subset of study subjects selected from the most highly exposed workers (n = 10) and in 12 unexposed controls frequency-matched to the 10 exposed workers by age and sex (characteristics of the two groups and exposure levels shown in Table 2). Specifically, the loss (monosomy) of chromosome 7 and gain (trisomy) of chromosome 8 was examined using FISH staining of metaphase spreads as previously described (44–46). Briefly, fixed metaphase spreads were prepared from cultured CFU-GM progenitor cells of exposed or control workers. Chromosomes 7 and 8 were painted with FISH probes directly labeled with FITC (green) and Texas Red (red). The probes and target DNA were simultaneously denatured, washed rapidly using a formamide-free stringency wash-solution, and each metaphase spread was evaluated microscopically. For efficiency, all scorable metaphase spreads on each slide were analyzed, and a minimum of 150 cells per subject were scored.
Table 2.
Characteristics of a subset of study subjects in the study of leukemia-specific chromosome aneuploidy by FISH
| Formaldehyde | Study Subjects | Formaldehyde Air Level | Age | Gender | Current Smoking | Current Alcohol Drinking | Recent Respiratory Infections | Body Mass Index | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exposure | (n) | (ppm) a | (Year) | Male | Female | Yes | No | Yes | No | Yes | No | |
| Controls | 12 | 0.032 (0.018, 0.032)b | 31.85±5.77c | 11(92)d | 1(8) | 5(42) | 7(58) | 2(17) | 10(83) | 3(25) | 9(75) | 22.35±2.80 |
| Exposed | 10 | 2.14 (1.38, 4.14)b | 30.59±5.35 | 9(90) | 1(10) | 4(40) | 6(60) | 2(20) | 8(80) | 3(30) | 7(70) | 21.65±2.15 |
Based on arithmetic mean of individual exposure measurements;
Median exposure (10th, 90th %tile). A value of the limit of detection divided by square root of 2 was assigned to individuals for non-detectable formaldehyde exposure
Mean ± SD; and
Subject # (%).
Statistical Analysis
Unadjusted summary measures are presented for all endpoints. Linear regression using the natural logarithm (ln) of data derived from the CBC was used to test for differences between workers exposed to formaldehyde and controls. Negative binomial regression was used to analyze CFU-GM, monosomy 7, and trisomy 8 data. All analyses were carried out using SAS version 8.0 software (SAS Institute, Cary, North Carolina, USA). The frequency-matching variables, age and sex were included in all models. Additional covariates that have been variably reported to influence these endpoints were included in the final models (i.e., current cigarette smoking status (yes/no), current alcohol consumption (yes/no), recent infections (flu or respiratory infections in the previous month), and BMI (Body Mass Index)) if they were significant at p < 0.05 or if there was evidence of confounding (i.e., greater than a 15% change in the regression coefficient). Smoking was the only covariate, in addition to age and sex, included in any regression model (i.e., for monocyte and red blood cell counts).
RESULTS
Exposure assessment
Exposed subjects were young (mean±SD: 31±6 years), male (86%), and similar to the unexposed controls (Table 1). The median (10th, 90th percentile) formaldehyde exposure level in the formaldehyde-melamine resin producing factory was 1.13 (0.94, 1.38) ppm and in the utensil factory 1.32 (0.51, 2.60) ppm, respectively. For all 43 exposed subjects the median was 1.28 (0.63, 2.51) ppm as an 8 h time-weighted average (Table 1).
Blood cell counts in formaldehyde-exposed and unexposed workers
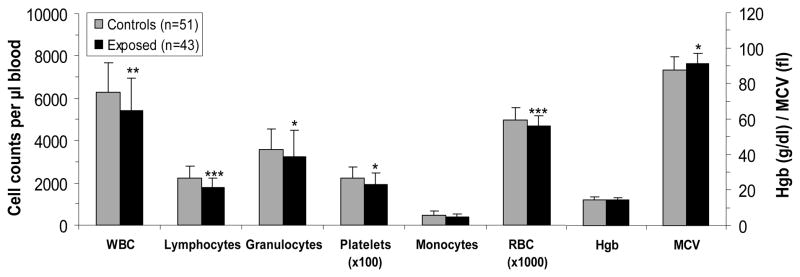
Total white blood cell (WBC) counts were significantly lower in workers exposed to formaldehyde compared to controls [mean (SD): 5,422 (1,529) cells per μl blood vs. 6,269 (1,422), respectively, p = 0.0016, Figure 1]. Lower levels were also observed for all the major myeloid cell types, including granulocytes, platelets, and red blood cells (RBC), and the mean corpuscular volume (MCV) of RBC was elevated (Figure 1). In addition, the lymphocyte count was significantly lower (p = 0.0002) in workers exposed to formaldehyde compared with controls (Figure 1). The observed effects were unlikely due to the presence of other hematotoxic agents, such as benzene, because lowered blood counts were found at both workplaces that used formaldehyde, and no co-exposures with known hematotoxic or genotoxic properties were detected. We also measured urinary benzene in a subset of workers exposed to formaldehyde (n = 21) and unexposed subjects (n = 20) and found essentially the same low background levels in both groups (mean±SD, 0.027±0.035 μg/L in exposed workers and 0.042±0.087 μg/L in controls). Detailed review of previous occupations identified 10 workers (4 exposed to formaldehyde, 6 controls) who had the potential for previous exposure to solvents. Adjusting for these potential past exposures, or excluding these subjects from the analysis, had a negligible impact on the results (data not shown).
Fig. 1. Myeloid, erythroid and lymphocyte blood cell counts in formaldehyde-exposed and unexposed workers.
A total of 43 workers exposed to formaldehyde and 51 controls were studied. Differences in cell counts were tested by linear regression, adjusting for relevant covariates as indicated in Methods. The p values are indicated as: * p < 0.05; ** p < 0.01; and *** p < 0.001. (WBC, white blood cells; RBC, red blood cells; Hgb, hemoglobin in g/deciliter; and, MCV, mean corpuscular volume in femtoliters as fl).
Colony formation from myeloid progenitor cells of formaldehyde-exposed and unexposed workers
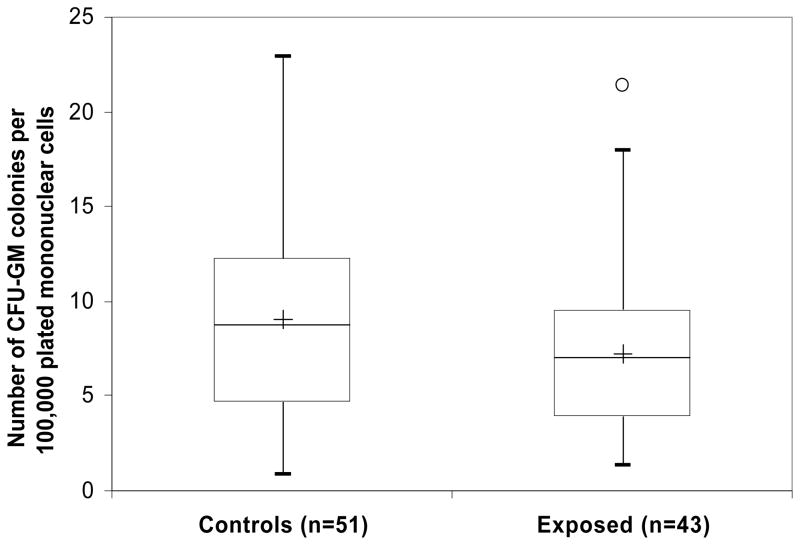
As formaldehyde exposure was associated with abnormalities in the myeloid, erythroid and lymphoid lineages of exposed workers, it seemed likely that formaldehyde produced an inhibitory effect on stem or progenitor cell differentiation in the bone marrow. Since a fraction of hematopoietic stem and progenitor cells circulate in the bloodstream in dynamic equilibrium with the stem cell pools in the bone marrow, we were able to examine this possibility by measuring colony formation from circulating CFU-GM progenitor cells using peripheral blood from the study subjects. A 20% decrease in colony formation from progenitor cells was observed in the formaldehyde-exposed workers (which is slightly greater than the 14% reduction observed in peripheral white blood cell counts), but this was not statistically significant (p = 0.10; Figure 2). This suggests a possible toxic and/or inhibitory effect of formaldehyde on the myeloid progenitor cells in the exposed workers.
Fig. 2. Colony formation from the colony-forming unit–granulocyte/macrophage (CFU- GM) hematopoietic progenitors in formaldehyde-exposed and unexposed workers.
Hematopoietic progenitor cells from the peripheral blood of 43 exposed workers and 51 frequency-matched controls were cultured in methylcellulose-based media without erythropoietin. Differences in cell counts were tested by negative binomial regression, adjusting for relevant covariates as indicated in Methods. The lower and higher edges of box are the 25 and 75 percentiles of the data, respectively. The lower and higher whiskers are 10 and 90 percentiles, respectively. The central line in the box presents the median and the cross is the mean. One outlier is indicated as a circle in the exposed group.
Effect of formaldehyde on human myeloid progenitor cells in vitro
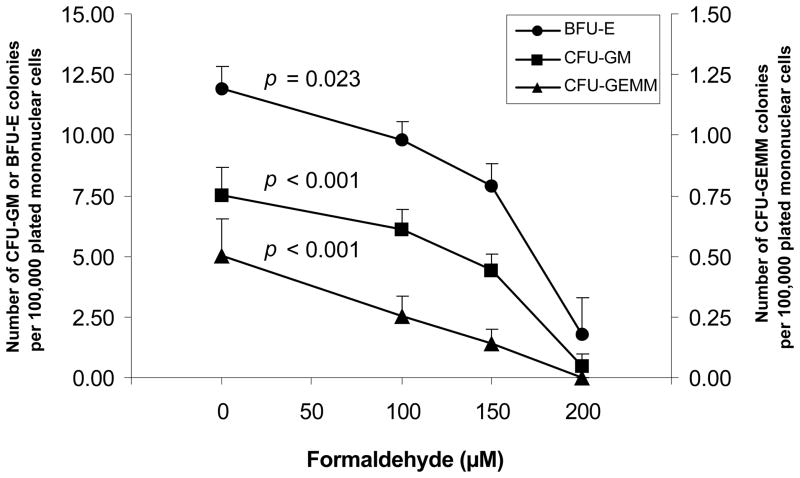
We expanded upon the studies in exposed workers by performing cell culture studies to investigate the impact of formaldehyde on human blood progenitor cells in culture. Hematopoietic myeloid progenitor cells were cultured in the absence and presence of EPO, so that CFU-GM, BFU-E and CFU-GEMM colonies were formed. Figure 3 shows that the colony forming number of all progenitor cell types was significantly decreased with increasing concentrations of formaldehyde, with the most primitive CFU-GEMM progenitors showing a linear negative dose-response relationship. These in vitro data (Figure 3) are consistent with the decreased CFU-GM colony formation found in workers exposed to formaldehyde (Figure 2) and suggest that formaldehyde inhibits the proliferation of myeloid progenitor cells.
Fig. 3. Colony formation from human myeloid progenitor cells following formaldehyde exposure in cell culture.
Hematopoietic myeloid progenitor cells were cultured from the peripheral blood of a volunteer of Chinese origin in methylcellulose-based media in the absence and presence of erythropoietin (EPO), after treatment with formaldehyde. The number of BFU-E (filled circles,–●–), CFU-GM (squares, –■–) and CFU-GEMM (triangles, –▲–) colonies were scored in 6 petri dishes after 14 days of culture. CFU-GM colony counts in the absence of EPO are presented for consistency with the in vivo data. The results shown are the means and standard errors of 6 separate experiments. The p values are indicated as p trend calculated using negative binomial regression and robust standard errors adjusting for possible residual correlation due to being on the same dish using a sandwich-type estimate (GEE approach).
Detection of leukemia-specific chromosome aneuploidy in the progenitor cells of formaldehyde-exposed and unexposed workers
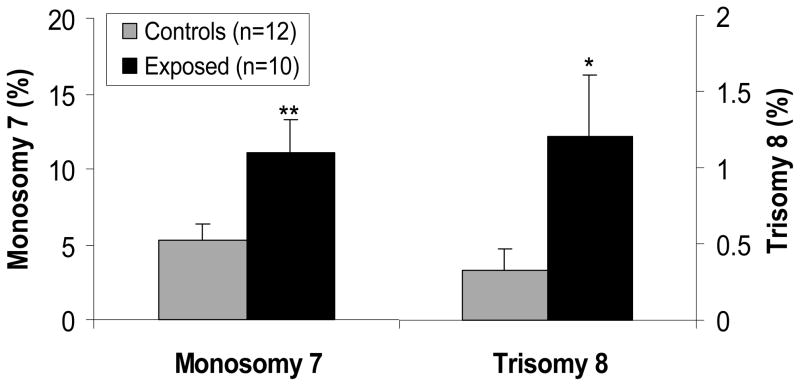
In a subset of highly exposed subjects (n=10) and matched controls (n=12) (Table 2), we examined aneuploidy of chromosomes 7 and 8 in metaphase spreads prepared from the cultured CFU-GM colony cells. The frequency of monosomy (loss) of chromosome 7 in formaldehyde-exposed workers was significantly elevated (p = 0.0039) compared with their matched controls, while the frequency of trisomy 8 (gain) had a 4-fold significant increase (p = 0.040) (Figure 4). Formaldehyde exposure was, therefore, associated with an increase in leukemia-specific chromosomal aneuploidy in the hematopoietic progenitor cells of the exposed workers. Three of these study subjects (1 exposed to formaldehyde, 2 controls) had the potential for previous exposure to solvents. Adjusting for these potential past exposures or excluding these subjects from the analysis had a negligible impact on the results (data not shown).
Fig. 4. Levels of monosomy of chromosome 7 and trisomy of chromosome 8 in the hematopoietic progenitor cells of formaldehyde-exposed and unexposed workers.
Leukemia-specific chromosome changes in CFU-GM cells, such as loss (monosomy) of chromosome 7 and gain (trisomy) of chromosome 8, were examined in metaphase spreads of 10 formaldehyde-exposed workers and 12 unexposed matched controls. The data represent the percentage of the metaphases in which each abnormality is found. Rates of monosomy are considerably higher in the controls than trisomy because of artifactual chromosome loss during metaphase spread preparation. Differences in aneuploidy were tested by negative binomial regression, adjusting for relevant covariates as indicated in Methods. The p values are indicated as: * p < 0.05 and ** p < 0.01.
DISCUSSION
The findings described here add biologic support to traditional epidemiological studies that have shown an association between formaldehyde exposure and increased risk of myeloid leukemia (8, 10, 13, 14). In the present study, we found that formaldehyde-exposed workers had lower blood counts in vivo consistent with toxic effects on the bone marrow and that formaldehyde exposure in vitro affected human hematopoietic stem or myeloid progenitor cells at toxicologically relevant concentrations. We also showed in a subset of the most highly exposed subjects that monosomy (loss) of chromosome 7 and trisomy (gain) of 8 were significantly elevated in the myeloid progenitor cells of formaldehyde-exposed workers compared with unexposed controls. We examined the loss of chromosome 7 and gain of chromosome 8 because they are among the most frequent cytogenetic changes observed in myeloid leukemia and myelodysplastic syndromes (43) and have been shown to be affected by exposure to the established human leukemogen, benzene (44, 46). Thus, formaldehyde exposure was associated with increased levels of specific chromosome aberrations related to myeloid leukemia in the stem/progenitor cells that are the targets for leukemogenesis. Future studies should aim to replicate these findings, and examine additional chromosomes, such as chromosome 5 which is also commonly altered in myeloid leukemias (47, 48), and to detect leukemia-related structural changes such as translocations and deletions.
Only a few previous studies of hematological parameters have been reported in formaldehyde-exposed humans in the English literature. A study of nurses in Taiwan showed that exposure to formaldehyde was correlated with reduced white blood cell counts (31). A recent study in China showed that formaldehyde was associated with lowered T-lymphocytes in the blood of exposed workers, but data on myeloid cells was not provided (19). Several studies in the Chinese literature also reported that occupational formaldehyde exposure was associated with a decrease in white blood cell counts and possibly other cell counts such as platelets (49), which is consistent with our findings.
Particular strengths of the hematological evaluation in our study include a comprehensive evaluation of formaldehyde’s possible effects on hematological parameters, assessment of potential confounding factors, and extensive exposure assessment. Our sample size was relatively small, but large enough to observe statistically significant associations. With an average exposure of 1.28 ppm as an 8 h time-weighted average, which is somewhat higher than the US Permissible Exposure Limit of 0.75 ppm, the subjects in our study were highly exposed and may explain why we were able to see hematotoxic effects in a relatively small population. Additional studies are needed to replicate these findings, preferably with a larger sample size and a broader range of exposures including relatively lower exposed individuals. Future studies could perhaps also be enhanced by using biomarkers of cumulative internal dose. However, no such biomarkers exist at present although some are in development and show promise. These include formaldehyde-DNA adducts (50) and adducts between glutathione and DNA induced by formaldehyde (51).
Our study is also unique in that we studied the effect of formaldehyde exposure both in vivo and in vitro on colony formation from the hematopoietic progenitor cells that circulate in the bloodstream in dynamic equilibrium with those in the bone marrow. A 20% decrease in colony formation from CFU-GM progenitor cells was observed in the exposed workers, which approached statistical significance. We were able to show that CFU-GM progenitor cells are sensitive to formaldehyde exposure in cell culture at toxicologically relevant concentrations, and the more primitive CFU-GEMM progenitors, that give rise to all myeloid cells, showed a linear negative dose-response relationship. These effects were observed at concentrations between 100 and 200 μM, which are toxicologically relevant because background levels of formaldehyde in human blood have been reported to be 50–100 μM (39, 40). Since CFU-GEMM multipotential myeloid progenitor cells and the earlier pluripotential stem cells are the target cells for leukemogenesis and are converted to leukemic stem cells in acute myeloid leukemia, the finding that formaldehyde damages these cells adds weight to the notion that it may be associated with myeloid leukemia.
It seems unlikely that the effects we observed on hematological parameters and chromosomal changes were due to confounding factors, such as the presence of other hematotoxic agents apart from formaldehyde, because lowered blood counts were found at both workplaces that used formaldehyde, and no co-exposures with known hematotoxic or genotoxic properties were detected. Further, adjustment for potential exposure to hematotoxicants in previous occupations had no impact on the results and the control population from 3 different factories had no occupational exposure to formaldehyde or any other hematotoxic or genotoxic chemicals in excess of levels in the general population. It is theoretically possible that other confounding factors, such as a lower dietary vitamin B12, could explain the elevated MCV and lowered blood counts in the exposed workers compared to their matched controls. However, all the workplaces studied were in the same geographic region and the enrolled workers had comparable demographic and socioeconomic characteristics and were engaged primarily in manufacturing. Thus, significantly different dietary, genetic and environmental factors, other than formaldehyde are unlikely to explain the results.
Given the fact that formaldehyde is a highly reactive gas, the question arises as to how it reaches the blood and bone marrow to elicit toxic effects. Several studies have reported increased chromosomal damage in the form of aberrations and micronuclei in circulating peripheral blood lymphocytes of workers exposed to formaldehyde (17–21). Increased levels of cytogenetic damage have also been reported in the bone marrow of exposed mice and rats, suggesting that formaldehyde reaches the bone marrow in experimental animals (17, 34). In aqueous solution, formaldehyde is converted mostly to oligomers of its diol form, methanediol (formaldehyde hydrate, CH2(OH)2, or methylene glycol), and a dynamic equilibrium with formaldehyde is formed. The concentration of the diol oligomers versus that of formaldehyde depends on the precise conditions (temperature, pH, formaldehyde concentration) under which the reaction occurs (52). Thus, methanediol, with a molecular weight of only 48, which can readily penetrate into tissues, may travel to the marrow through the blood where it is in equilibrium with reactive formaldehyde. The formaldehyde, once generated, can react with cellular macromolecules producing toxic injury (53).
It is possible, therefore, that formaldehyde promotes leukemogenesis through direct induction of DNA damage and chromosome aneuploidy in hematopoietic stem or early progenitor cells in the bone marrow. This hypothesis clearly requires additional testing and there are at least two alternate mechanisms. As suggested by Zhang et al formaldehyde may induce leukemia by damaging hematopoietic stem/progenitor cells circulating in the peripheral blood; or by damaging the primitive pluripotent stem cells present within the nasal turbinates and/or olfactory mucosa (54). In either of these two alternate models, damaged stem/progenitor cells would then travel to the bone marrow and become initiated leukemic stem cells (54). However, the data described herein and the earlier findings of cytogenetic damage in the blood and marrow of humans and mice suggests that formaldehyde damages hematopoietic stem or early progenitor cells in the bone marrow and/or peripheral blood. Overall, the data presented here heighten concern about the leukemogenic potential of formaldehyde and suggest that the carcinogenic risk presented by this ubiquitous compound should be evaluated further.
Acknowledgments
This work was supported by intramural funds from the National Cancer Institute, and grants from the National Institute of Environmental Health Sciences (P42ES004705 to M.T.S. and R01ES017452 to L.Z.), the Northern California Center for Occupational and Environmental Health, and the Department of Science and Technology of Guangdong Province, China (2007A050100004). We thank Jackie King, MD (BioReliance Corp., Rockville) for logistical support. We also thank the participants and members of the Guangdong field team: Cishan Chen, Xiaojun Yang, Jin Wu, Huiqing Chen, Guoqiang Xie, Huifang Sun, Zongjun Zhang, Qiaoyu Pan, Ziqun Zhang, Zuokan Lin, Yongshun Huang, Guoyong Xu, Jinghui Guo, Ruiyan Huang, Yibing Hu, Neng Li, Yun Huang, Xiaojie Li, Zhiyong Shuai, Zhuliang Mo, Danxia Fang, Yiyu Yu, Meipiao Feng, Pin Cai, Saiju Zhang, Min Liu, Jianxun Huang, Jiaobing Chen, Banghua Wu, Aichu Yang, Shijie Hu, Xianzhong Wen, Junyi Ma, Funan Liu, Jun Li, Kaiqian Liu, Chuanan Wu, Xuepiao Zhong, Jian He, Zheng Ma, JianhuiHu, Zhaoxiang Huang,Yuping Wu, Shengmin Luo, Shunjing Ruan, Yongzhuang Cen, Xianzhao Xia, Mushun Mo. The names in Chinese of all members of the Guangdong field team being acknowledged are:

References
- 1.OSHA. Occupational Safety and Health Administration: Occupational Exposure to Formaldehyde Fact Sheet. Department of Labor; 1995. p. 95. [Google Scholar]
- 2.Turrio-Baldassarri L, Battistelli CL, Conti L, Crebelli R, De Berardis B, Iamiceli AL, et al. Emission comparison of urban bus engine fueled with diesel oil and 'biodiesel' blend. Sci Total Environ. 2004;327:147–62. doi: 10.1016/j.scitotenv.2003.10.033. [DOI] [PubMed] [Google Scholar]
- 3.Cogliano VJ, Grosse Y, Baan RA, Straif K, Secretan MB, El Ghissassi F. Meeting report: summary of IARC monographs on formaldehyde, 2-butoxyethanol, and 1-tert-butoxy-2-propanol. Environ Health Perspect. 2005;113:1205–8. doi: 10.1289/ehp.7542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Formaldehyde Council Inc. Formaldehyde: A Brief History and Its Contributions to Society and the US Economy. Arlington, VA: 2005. [Google Scholar]
- 5.IARC. International Agency for Research on Cancer: Formaldehyde, 2-butoxyethanol, and 1-tert-butoxy-2propanol. Monogr Eval Carcinog Risks Hum. 2006:88. [PMC free article] [PubMed] [Google Scholar]
- 6.SEER. Surveillance Epidemiology and End Results: Cancer Statistics Review, 1975 - 2004: Overview. Bethesda, MD: National Cancer Institute; 2006. p. 31. [Google Scholar]
- 7.Hall A, Harrington JM, Aw TC. Mortality study of British pathologists. Am J Ind Med. 1991;20:83–9. doi: 10.1002/ajim.4700200108. [DOI] [PubMed] [Google Scholar]
- 8.Hayes RB, Blair A, Stewart PA, Herrick RF, Mahar H. Mortality of U.S. embalmers and funeral directors. Am J Ind Med. 1990;18:641–52. doi: 10.1002/ajim.4700180603. [DOI] [PubMed] [Google Scholar]
- 9.Levine RJ, Andjelkovich DA, Shaw LK. The mortality of Ontario undertakers and a review of formaldehyde-related mortality studies. J Occup Med. 1984;26:740–6. doi: 10.1097/00043764-198410000-00014. [DOI] [PubMed] [Google Scholar]
- 10.Stroup NE, Blair A, Erikson GE. Brain cancer and other causes of death in anatomists. J Natl Cancer Inst. 1986;77:1217–24. [PubMed] [Google Scholar]
- 11.Walrath J, Fraumeni JFJ. Mortality patterns among embalmers. Int J Cancer. 1983;31:407–11. doi: 10.1002/ijc.2910310403. [DOI] [PubMed] [Google Scholar]
- 12.Walrath J, Fraumeni JFJ. Cancer and other causes of death among embalmers. Cancer Res. 1984;44:4638–41. [PubMed] [Google Scholar]
- 13.Hauptmann M, Lubin JH, Stewart PA, Hayes RB, Blair A. Mortality from lymphohematopoietic malignancies among workers in formaldehyde industries. J Natl Cancer Inst. 2003;95:1615–23. doi: 10.1093/jnci/djg083. [DOI] [PubMed] [Google Scholar]
- 14.Pinkerton LE, Hein MJ, Stayner LT. Mortality among a cohort of garment workers exposed to formaldehyde: an update. Occup Environ Med. 2004;61:193–200. doi: 10.1136/oem.2003.007476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beane Freeman LE, Blair A, Lubin JH, Stewart PA, Hayes RB, Hoover RN, et al. Mortality from lymphohematopoietic malignancies among workers in formaldehyde industries: the National Cancer Institute Cohort. J Natl Cancer Inst. 2009;101:751–61. doi: 10.1093/jnci/djp096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coggon D, Harris EC, Poole J, Palmer KT. Extended follow-up of a cohort of british chemical workers exposed to formaldehyde. J Natl Cancer Inst. 2003;95:1608–15. doi: 10.1093/jnci/djg046. [DOI] [PubMed] [Google Scholar]
- 17.Kitaeva LV, Kitaev EM, Pimenova MN. The cytopathic and cytogenetic sequelae of chronic inhalational exposure to formaldehyde on female germ cells and bone marrow cells in rats. Tsitologiia. 1990;32:1212–6. [PubMed] [Google Scholar]
- 18.Orsiere T, Sari-Minodier I, Iarmarcovai G, Botta A. Genotoxic risk assessment of pathology and anatomy laboratory workers exposed to formaldehyde by use of personal air sampling and analysis of DNA damage in peripheral lymphocytes. Mutat Res. 2006;605:30–41. doi: 10.1016/j.mrgentox.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 19.Ye X, Yan W, Xie H, Zhao M, Ying C. Cytogenetic analysis of nasal mucosa cells and lymphocytes from high-level long-term formaldehyde exposed workers and low-level short-term exposed waiters. Mutat Res. 2005;588:22–7. doi: 10.1016/j.mrgentox.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 20.Yu LQ, Jiang SF, Leng SG, He FS, Zheng YX. Early genetic effects on workers occupationally exposed to formaldehyde. Zhonghua Yu Fang Yi Xue Za Zhi. 2005;39:392–5. [PubMed] [Google Scholar]
- 21.Suruda A, Schulte P, Boeniger M, Hayes RB, Livingston GK, Steenland K, et al. Cytogenetic effects of formaldehyde exposure in students of mortuary science. Cancer Epidemiol Biomarkers Prev. 1993;2:453–60. [PubMed] [Google Scholar]
- 22.Cole P, Axten C. Formaldehyde and leukemia: an improbable causal relationship. Regul Toxicol Pharmacol. 2004;40:107–12. doi: 10.1016/j.yrtph.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 23.Collins JJ, Lineker GA. A review and meta-analysis of formaldehyde exposure and leukemia. Regul Toxicol Pharmacol. 2004;40:81–91. doi: 10.1016/j.yrtph.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 24.Golden R, Pyatt D, Shields PG. Formaldehyde as a potential human leukemogen: an assessment of biological plausibility. Crit Rev Toxicol. 2006;36:135–53. doi: 10.1080/10408440500533208. [DOI] [PubMed] [Google Scholar]
- 25.Heck H, Casanova M. The implausibility of leukemia induction by formaldehyde: a critical review of the biological evidence on distant-site toxicity. Regul Toxicol Pharmacol. 2004;40:92–106. doi: 10.1016/j.yrtph.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 26.Pyatt D, Natelson E, Golden R. Is inhalation exposure to formaldehyde a biologically plausible cause of lymphohematopoietic malignancies? Regul Toxicol Pharmacol. 2008;51:119–33. doi: 10.1016/j.yrtph.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 27.Kreja L, Greulich KM, Fliedner TM, Heinze B. Stable chromosomal aberrations in haemopoietic stem cells in the blood of radiation accident victims. Int J Radiat Biol. 1999;75:1241–50. doi: 10.1080/095530099139395. [DOI] [PubMed] [Google Scholar]
- 28.Lan Q, Zhang L, Li G, Vermeulen R, Weinberg RS, Dosemeci M, et al. Hematotoxicity in workers exposed to low levels of benzene. Science. 2004;306:1774–6. doi: 10.1126/science.1102443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eaves AC, Bruce WR. Altered sensitivity of hematopoietic stem cells to 5-fluorouracil (NSC-19893) following endotoxin (NSC-189681), cyclophosphamide (NSC-26271), or irradiation. Cancer Chemother Rep. 1974;58:813–20. [PubMed] [Google Scholar]
- 30.Pessina A, Malerba I, Gribaldo L. Hematotoxicity testing by cell clonogenic assay in drug development and preclinical trials. Curr Pharm Des. 2005;11:1055–65. doi: 10.2174/1381612053381648. [DOI] [PubMed] [Google Scholar]
- 31.Kuo H, Jian G, Chen C, Liu C, Lai J. White blood cell count as an indicator of formaldehyde exposure. Bull Environ Contam Toxicol. 1997;59:261–7. doi: 10.1007/s001289900473. [DOI] [PubMed] [Google Scholar]
- 32.Madison RE, Broughton A, Thrasher JD. Immunologic biomarkers associated with an acute exposure to exothermic byproducts of a ureaformaldehyde spill. Environ Health Perspect. 1991;94:219–23. doi: 10.1289/ehp.94-1567939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vargova M, Wagnerova J, Liskova A, Jakubovsky J, Gajdova M, Stolcova E, et al. Subacute immunotoxicity study of formaldehyde in male rats. Drug Chem Toxicol. 1993;16:255–75. doi: 10.3109/01480549309081819. [DOI] [PubMed] [Google Scholar]
- 34.Tao XY, Yu SY, Kang L, Huang HX, Wei AY. Study on the genetic damage in mice induced by the volatile organic compounds of decoration materials. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2004;22:194–6. [PubMed] [Google Scholar]
- 35.Hagiwara M, Watanabe E, Barrett JC, Tsutsui T. Assessment of genotoxicity of 14 chemical agents used in dental practice: ability to induce chromosome aberrations in Syrian hamster embryo cells. Mutat Res. 2006;603:111–20. doi: 10.1016/j.mrgentox.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 36.Passegue E, Jamieson CH, Ailles LE, Weissman IL. Normal and leukemic hematopoiesis: are leukemias a stem cell disorder or a reacquisition of stem cell characteristics? Proc Natl Acad Sci U S A. 2003;100(Suppl 1):11842–9. doi: 10.1073/pnas.2034201100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pedersen-Bjergaard J, Andersen MK, Andersen MT, Christiansen DH. Genetics of therapy-related myelodysplasia and acute myeloid leukemia. Leukemia. 2008;22:240–8. doi: 10.1038/sj.leu.2405078. [DOI] [PubMed] [Google Scholar]
- 38.Kim S, Vermeulen R, Waidyanatha S, Johnson BA, Lan Q, Rothman N, et al. Using urinary biomarkers to elucidate dose-related patterns of human benzene metabolism. Carcinogenesis. 2006;27:772–81. doi: 10.1093/carcin/bgi297. [DOI] [PubMed] [Google Scholar]
- 39.Casanova M, Heck HD, Everitt JI, Harrington WWJ, Popp JA. Formaldehyde concentrations in the blood of rhesus monkeys after inhalation exposure. Food Chem Toxicol. 1988;26:715–6. doi: 10.1016/0278-6915(88)90071-3. [DOI] [PubMed] [Google Scholar]
- 40.Heck HD, Casanova-Schmitz M, Dodd PB, Schachter EN, Witek TJ, Tosun T. Formaldehyde (CH2O) concentrations in the blood of humans and Fischer-344 rats exposed to CH2O under controlled conditions. Am Ind Hyg Assoc J. 1985;46:1–3. doi: 10.1080/15298668591394275. [DOI] [PubMed] [Google Scholar]
- 41.Neuss S, Speit G. Further characterization of the genotoxicity of formaldehyde in vitro by the sister chromatid exchange test and co-cultivation experiments. Mutagenesis. 2008;23:355–7. doi: 10.1093/mutage/gen025. [DOI] [PubMed] [Google Scholar]
- 42.Schmid O, Speit G. Genotoxic effects induced by formaldehyde in human blood and implications for the interpretation of biomonitoring studies. Mutagenesis. 2007;22:69–74. doi: 10.1093/mutage/gel053. [DOI] [PubMed] [Google Scholar]
- 43.Rowley JD. Molecular genetics in acute leukemia. Leukemia. 2000;14:513–7. doi: 10.1038/sj.leu.2401600. [DOI] [PubMed] [Google Scholar]
- 44.Smith MT, Zhang L, Wang Y, Hayes RB, Li G, Wiemels J, et al. Increased translocations and aneusomy in chromosomes 8 and 21 among workers exposed to benzene. Cancer Res. 1998;58:2176–81. [PubMed] [Google Scholar]
- 45.Zhang L, Lan Q, Guo W, Li G, Yang W, Hubbard AE, et al. Use of OctoChrome fluorescence in situ hybridization to detect specific aneuploidy among all 24 chromosomes in benzene-exposed workers. Chem Biol Interact. 2005;153–154:117–22. doi: 10.1016/j.cbi.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 46.Zhang L, Rothman N, Wang Y, Hayes RB, Li G, Dosemeci M, et al. Increased aneusomy and long arm deletion of chromosomes 5 and 7 in the lymphocytes of Chinese workers exposed to benzene. Carcinogenesis. 1998;19:1955–61. doi: 10.1093/carcin/19.11.1955. [DOI] [PubMed] [Google Scholar]
- 47.Jadersten M, Hellstrom-Lindberg E. Myelodysplastic syndromes: biology and treatment. J Intern Med. 2009;265:307–28. doi: 10.1111/j.1365-2796.2008.02052.x. [DOI] [PubMed] [Google Scholar]
- 48.Mohamedali A, Mufti GJ. Van-den Berghe's 5q- syndrome in 2008. Br J Haematol. 2009;144:157–68. doi: 10.1111/j.1365-2141.2008.07447.x. [DOI] [PubMed] [Google Scholar]
- 49.Tang X, Bai Y, Duong A, Smith MT, Li L, Zhang L. Formaldehyde in China: production, consumption, exposure levels, and health effects. Environ Int. 2009;35:1210–24. doi: 10.1016/j.envint.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 50.Wang M, Cheng G, Balbo S, Carmella SG, Villalta PW, Hecht SS. Clear differences in levels of a formaldehyde-DNA adduct in leukocytes of smokers and nonsmokers. Cancer Res. 2009;69:7170–4. doi: 10.1158/0008-5472.CAN-09-1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lu K, Ye W, Gold A, Ball LM, Swenberg JA. Formation of S-[1-(N2-deoxyguanosinyl)methyl]glutathione between glutathione and DNA induced by formaldehyde. J Am Chem Soc. 2009;131:3414–5. doi: 10.1021/ja808048c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Walker FJ. American Chemical Society Monographs. New York: Reinhold; 1964. Formaldehyde. [Google Scholar]
- 53.Fox CH, Johnson FB, Whiting J, Roller RP. Formaldehyde Fixation. Journal of Histochemistry and Cytochemistry. 1985;33:845–53. doi: 10.1177/33.8.3894502. [DOI] [PubMed] [Google Scholar]
- 54.Zhang L, Steinmaus C, Eastmond DA, Xin XK, Smith MT. Formaldehyde exposure and leukemia: a new meta-analysis and potential mechanisms. Mutat Res. 2009;681:150–68. doi: 10.1016/j.mrrev.2008.07.002. [DOI] [PubMed] [Google Scholar]