Abstract
Purpose
Conjunctival membranes are differentiated from pseudomembranes by bleeding on removal. We sought to study the cellular constituents and extracellular structure of formed conjunctival exudates in epidemic keratoconjunctivitis (EKC), and to determine whether these represent true conjunctival membranes.
Methods
Formed conjunctival exudates harvested from two patients with EKC were studied by immunohistochemistry. Adenoviral infection was evaluated by PCR.
Results
Each patient’s tears were PCR positive for adenovirus, but not HSV-I. Both conjunctival specimens contained a fibrinous stroma richly populated with cells positive for CD4, CD8, CD20, CD68, myeloperoxidase, and fascin. Both specimens also contained cells positive for CD31 and Tie-2, and demonstrated expression of transforming growth factor-β and vascular endothelial growth factor.
Conclusions
Conjunctival exudates in EKC contain a range of leukocytes representing both innate and acquired immune responses, along with angiogenic factors and proliferating endothelial cells. Therefore, true conjunctival membranes can and do form in EKC.
Keywords: Adenovirus, Conjunctiva, Epidemic Keratoconjunctivitis, Membrane, Pseudomembrane
Introduction
Adenoviruses are icosahedral, non-enveloped, double stranded DNA viruses.1 Human adenoviruses (HAdV) are significant pathogens, and cause infections of ocular, gastrointestinal, and respiratory mucosa.2 They are perhaps the most common infectious agents on the ocular surface, and can cause up to 75% of conjunctivitis cases.3
HAdV are responsible for three principal ocular syndromes: epidemic keratoconjunctivitis (EKC), pharyngoconjunctival fever, and follicular conjunctivitis.4 EKC is distinguished from other ocular adenovirus infection syndromes by infection of the entire ocular surface, including the corneal epithelium.5–9 In addition, EKC characteristically causes delayed onset, multifocal, subepithelial corneal infiltrates that can persist or recur in up to one-third of infected patients.4
Membranes that develop on mucosal surfaces during inflammation can be divided into pseudomembranes and true membranes. Pseudomembranes are characterized by a fibrin rich exudate lacking blood or lymphatic vessels, and removal does not induce bleeding. Formation of pseudomembranes is characteristic in a variety of disorders, including diphtheria and pseudomembranous colitis.10, 11 True membranes are formed by coagulum of exudate over a mucosal substantia propria, and when removed leave behind a denuded bleeding surface.12, 13 Conjunctival membranes are distinguished from conjunctival pseudomembranes by the occurrence of bleeding upon removal.12 Formed conjunctival exudates in EKC were first described by Hogan and Crawford in 1942,14 and they occur in up to sixty percent of affected patients.4, 6, 15
The published literature is inconsistent, but it is commonly taught that EKC is associated with conjunctival pseudomembranes rather than true membranes.14–17 This teaching persists despite the clinical observation that removal of formed conjunctival exudates in EKC commonly results in bleeding, 6, 13–15, 18 and that common sequelae of formed conjunctival exudates in EKC include subconjunctival fibrosis and symblepharon,19 suggesting the activation of fibrotic and angiogenic mechanisms in their formation.
Laibson and Green examined a formed conjunctival exudate from a female patient afflicted with EKC, and described a fibrinous matrix infiltrated by polymorphonuclear neutrophils.18 However, not much else is known about their formation, structure, or cellular phenotype. The goal of this study was to examine formed conjunctival exudates in EKC to determine their architecture and cellular constituents, and to determine if they contain nascent vasculature or show evidence for expression of angiogenic factors.
Materials and Methods
Patients, Reagents and Antibodies
The study was approved by the institutional review board at the University of Oklahoma Health Sciences Center, and informed consent was obtained. Tears were collected by glass capillary tube. Conjunctival exudates were stripped with forceps from the ocular surface of two patients with EKC, and the specimens were fixed in 10% neutral buffered formalin.
Antibodies against CD4, myeloperoxidase, vascular endothelial growth factor (VEGF), fascin, and transforming growth factor-β (TGF-β) were purchased from Lab Vision Corporation (Fremont, CA). Antibodies against CD8, CD20, CD68, and S100 were purchased from Cell Marque Corporation (Rocklin, CA). Antibody against lymphatic vessel endothelial receptor-1 (LYVE-1) was purchased from R&D Systems (Minneapolis, MN) and against Tie-2 from Santa Cruz Biotechnology (Santa Cruz, CA). The anti-rabbit and anti-mouse secondary antibodies were purchased from Dako (Carpinteria, CA). PCR master mix and 10mM dNTP were purchased from Promega (Madison, WI).
PCR for Viral DNA
A tear sample from each patient was placed on A549 cells in culture to confirm virus induced cytopathic effect. Culture supernatants were frozen at −80° C, and DNA isolation was performed on thawed samples via phenol-chloroform extraction. DNA was dissolved in nuclease-free water, and the purity of DNA confirmed by absorbance at 260/280 nm. PCR for adenovirus hexon gene and herpes simplex virus-1 (HSV-1) VP16 gene was performed on both samples. HAdV-19, HAdV-37, and HSV-1 DNAs were used for positive controls. PCR conditions were 95° C for 10 minutes; 95° C, 30 seconds; 58° C, 30 seconds; 72° C, 1 minute; for 35 cycles, followed by 72° C for 10 minutes. PCR products were visualized on a 0.8 % agarose gel. Primer pairs utilized were as follows: HAdV hexon: sense 5′-GCCGCAGTGGTCTTACATGCACATC- 3′; antisense 5′-CAGCACGCCGCGGATGTCAAAGT- 3′. HSV-1 VP16: sense 5′-GGACTCGTATTCCAGCTTCAC- 3′; antisense 5′-CGTCCTCGCCGTCTAAGTG- 3′.
Immunohistochemistry
Membranes were fixed in 10 % normal buffered formalin for 24 hours and embedded in paraffin. Five micron thick tissue sections were mounted on positively charged slides and air dried overnight. After deparaffinization and rehydration, slides were stained with hematoxylin and eosin or treated with EDTA buffer (Lab Vision Corporation) for epitope retrieval. Non-specific binding was blocked using Protein Block (Dako) supplemented with 5 % rat serum (Jackson ImmunoResearch Laboratories, West Grove, PA). The slides were incubated with primary antibody for 1 hour at room temperature, followed by incubation in secondary antibody labeled with horse radish peroxidase. Slides were counterstained with hematoxylin, and coverslipped using a synthetic resin. The slides were photographed with an inverted microscope (Zeiss Axiovert, Thornwood, NY), using a 20× objective.
Confocal Microscopy
Deparaffinized tissue sections were stained with primary antibody against Tie-2. Slides were viewed with an Olympus IX81-FV500 confocal laser scanning microscope (Olympus, Melville, NY) using the 40× water immersion objective lens. Tissue sections were scanned in the z axis with a step size of 1–2 μm. FluoView software (Olympus) was used for analysis.
Results
PCR for Adenovirus DNA
Both patients presented to the clinic with signs of acute bilateral EKC, including tender, palpable, preauricular lymph nodes, conjunctival follicular hyperplasia, petechial subconjunctival hemorrhages, and punctuate epithelial keratitis. Both patients had formed conjunctival exudates covering the tarsal conjunctiva of both upper and lower eyelids. Cultured tear samples from both patients induced cytopathic effect on A549 cells typical of adenovirus infection (data not shown). PCR for adenovirus hexon gene was performed as described previously.20 PCR confirmed the presence of adenovirus DNA in both patients (Fig. 1, top panel). HSV-1 infection was ruled out by the absence of HSV-1 VP16 DNA (Fig. 1, lower panel). DNA from HAdV and HSV-1 used as positive controls showed appropriate amplification of hexon and VP16 genes, respectively. These data confirmed that both patients were infected with adenovirus and not HSV-1.
Figure 1.
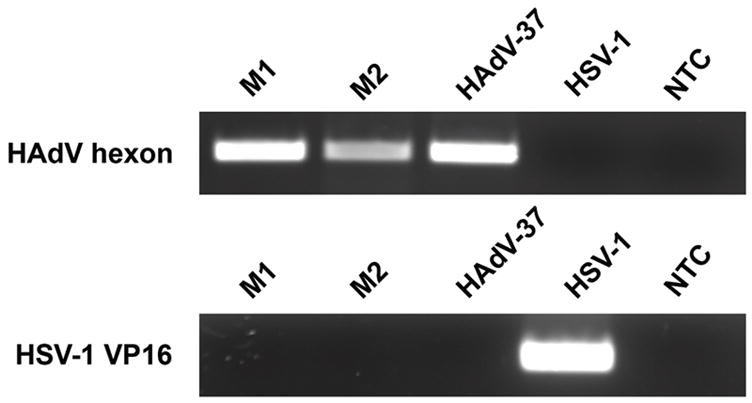
Identification of HAdV DNA in patient samples by PCR. Samples from both patients (M1 and M2) showed presence of HAdV (top panel). HAdV-37 DNA was used as a positive control, and HSV-1 VP16 gene (bottom panel) as a negative control. Neither membrane contained HSV-1. NTC: no template control.
Structure of Conjunctival Exudates in EKC
The general architecture of the formed conjunctival exudates was analyzed by routine histological stains. Hematoxylin and eosin staining (Fig. 2A) demonstrated a loose eosinophilic matrix highly infiltrated with polymorphonuclear cells and lymphocytes. Masson’s trichrome staining (Fig. 2B) showed strongly eosinophilic bands overlying and surrounding numerous foci of basophilic material. A reticulum silver stain revealed no reticular fibers (data not shown). Staining for high and low molecular weight cytokeratins with AE1/AE3 antibody showed numerous discrete circular and rod shaped foci (Fig. 2C), consistent with degenerating epithelial cells. Use of a pan-cytokeratin antibody revealed similar staining, which appeared to be concentrated toward one surface of the specimens (data not shown). Both specimens showed a similar pattern of staining with all histochemical methods.
Figure 2.
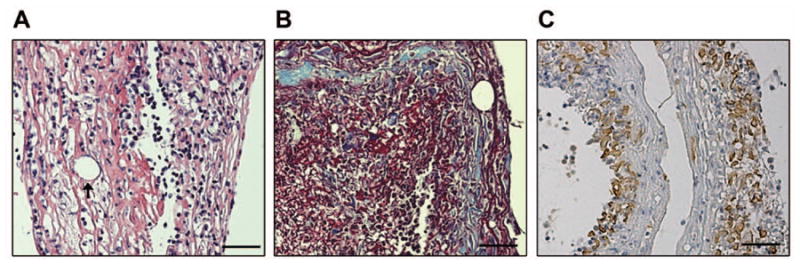
Representative photomicrographs demonstrate the general architecture of conjunctival membranes in EKC. (A) H&E staining of one membrane shows a highly eosinophilic stroma with abundant leukocytes and circular channels lined with a single cell layer (arrow), suggesting the possible presence of vascular channels. (B) Masson’s trichrome stain is consistent with the presence of fibrin (red staining), in addition to less prominent regions with collagen (blue staining). (C) Staining for cytokeratin (AE1/AE3) showed numerous discrete foci mainly located on one side of the membranes. Scale bar, 50 μM.
Immunohistochemistry for Cellular Phenotypes
The cellular phenotype in formed conjunctival exudates in EKC has not been previously studied with immunohistochemistry. Strong staining of cells in each specimen was observed for CD68 (Fig. 3A), consistent with an abundance of macrophages; myeloperoxidase (Fig. 3B), representative of the presence of neutrophils; and CD4 (Fig. 3C) and CD8 (Fig. 3D), each markers for T lymphocyte subpopulations. Positive staining for CD20, a marker for B lymphocytes, was uncommon (Fig. 3E), as was staining for S-100, a marker for Langerhans cells (data not shown). However, fascin-positive cells were frequent (Fig. 3F), consistent with the presence of activated dendritic cells. By immunohistochemistry, both specimens appeared to contain similar types and proportions of cells.
Figure 3.
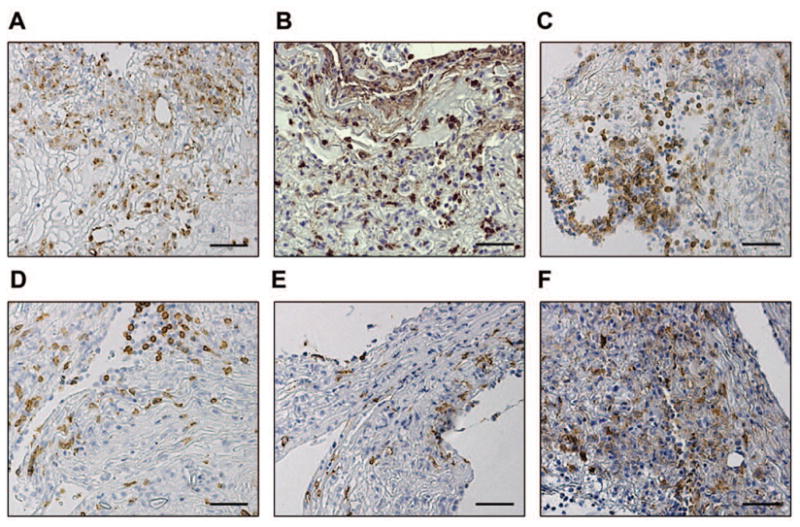
Leukocyte phenotype of conjunctival membranes in EKC. Representative photomicrographs of (A) CD68, (B) myeloperoxidase, (C) CD4, (D) CD8, (E) CD20, and (F) fascin. Scale bar, 50 μM.
Detection of Angiogenesis
Formed conjunctival exudates in EKC often bleed when stripped.6, 13–15, 18 This suggests the possible presence of vascular channels within the removed tissue. In our specimens, hematoxylin and eosin stained sections showed numerous circular spaces lined with a single layer of cells (Fig. 2A, arrow), suggesting the presence of incipient capillaries, but red blood cells were not seen in either of our two samples. In order to test the possibility that these channels represent nascent blood or lymphatic vessels within conjunctival membranes, we performed immunohistochemistry and confocal microscopy for angiogenic markers. Both membranes contained cells positive for Tie-2 (Fig 4A) and CD31 (Fig. 4B), each a marker for proliferating endothelial cells.21, 22 Circular staining for Tie-2 was also observed (Fig. 4A, arrow). The presence of lymphatic vessels was assessed by staining for LYVE-1 and D240,23, 24 with archived tonsil as a positive control, but neither membrane was positive for these markers (data not shown). We also tested for the presence of pro-angiogenic factors. Both TGF-β (Fig. 4C) and VEGF (Fig. 4D) were detected within stromal cells within the putative membranes and throughout their extracellular matrix.
Figure 4.
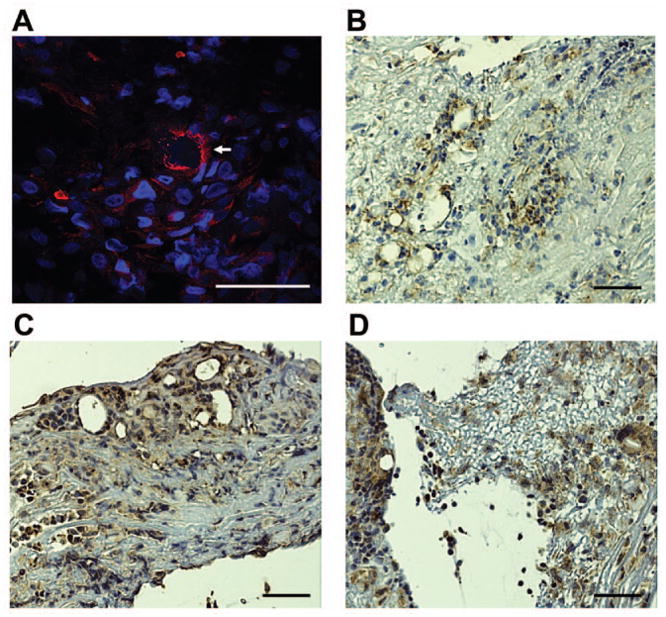
Angiogenesis in conjunctival membranes in EKC. (A) Representative confocal microscopic image showing Tie-2 staining. Numerous foci of cells positive for Tie-2 are evident, along with a less common circular pattern of staining (arrow). Immunohistochemistry for (B )CD31, (C) TGF-β, and (D) VEGF. Scale bar, 50 μM.
Discussion
EKC is a common ocular infection, and can result in significant morbidity to the patient.4 Formed conjunctival exudates in patients with EKC have been variably referred to as pseudomembranes 15–17 or membranes.4, 14, 18 Their removal often results in bleeding, and persistence may lead to symblepharon formation. In specimens from two patients with EKC, we demonstrate an array of leukocytes, including neutrophils, macrophages, T cells, and activated dendritic cells, enmeshed in an eosinophilic extracellular matrix. Trichrome staining of our samples was consistent with past studies indicating fibrin as the major component of conjunctival membranes,10, 18 but also indicated the presence of collagen. True conjunctival membranes have been defined by formation of a coagulum behind an epithelial cell layer, 12, 13 An asymmetrically located cytokeratin positive cell layer suggestive of a degenerated conjunctival epithelium, further supports our hypothesis that these structures represent true membranes. In both specimens, circular channels lined with a lining cell layer were observed. We identified Tie-2 positive cells in a circular arrangement in both specimens, although most of the cells with endothelial cell markers were clumped together, suggesting an early stage in angiogenesis. We also found evidence for expression of the angiogenic factors, VEGF and TGF- β,25 the latter also a promoter of fibrosis.26 The description of conjunctival exudates in EKC as pseudomembranes might be appropriate early in their formation when the exudates tend to be poorly adherent, but not later in severe infections when removal induces bleeding, and persistence leads to subepithelial fibrosis and symblepharon. We propose, as did Dawson and colleagues previously,6 that the difference between pseudomembranes and membranes of the conjunctiva in EKC may be a matter of degree and intensity of inflammation. Our analysis confirms that true conjunctival membranes can and do form in patients with EKC, and suggests the potential benefit of topical corticosteroids at this stage of the infection.
Based on our study, conjunctival membranes in EKC appear to be highly complex and immunologically active structures, consistent with the intense inflammation typical of the infection. The single previous report of histopathology of a conjunctival membrane in EKC described it as fibrinous and containing polymorphonuclear neutorphils.18 We show that macrophages, T lymphocytes, and activated dendritic cells are also present, suggesting activation of an acquired immune response. Further studies will be necessary to determine if conjunctival membranes in EKC are a site of antigen presentation and to what degree they contribute to the immune response to adenovirus infection of the ocular surface.
Acknowledgments
This work was supported by U.S. Public Health Service grant R01 EY13124 and Research to Prevent Blindness, New York, NY. The authors wish to thank Roger Astley for technical assistance.
References
- 1.Shenk T. Adenoviridae: the viruses and their replication. In: Fields BN, Knipe DM, Howley PM, editors. Fields Virology. Philadelphia: Lippincott-Raven; 1996. pp. 2111–2148. [Google Scholar]
- 2.Horwitz MS. Adenoviruses. In: Fields BN, Knipe DM, Howley PM, editors. Fields Virology. Philadelphia: Lippincott-Raven; 1996. pp. 2149–2171. [Google Scholar]
- 3.Woodland RM, Darougar S, Thaker U, et al. Causes of conjunctivitis and keratoconjunctivitis in Karachi, Pakistan. Trans R Soc Trop Med Hyg. 1992;86:317–320. doi: 10.1016/0035-9203(92)90328-a. [DOI] [PubMed] [Google Scholar]
- 4.Butt AL, Chodosh J. Adenoviral keratoconjunctivitis in a tertiary care eye clinic. Cornea. 2006;25:199–202. doi: 10.1097/01.ico.0000170693.13326.fb. [DOI] [PubMed] [Google Scholar]
- 5.Chodosh J, Miller D, Stroop WG, Pflugfelder SC. Adenovirus epithelial keratitis. Cornea. 1995;14:167–174. [PubMed] [Google Scholar]
- 6.Dawson CR, Hanna L, Togni B. Adenovirus type 8 infections in the United States. IV. Observations on the pathogenesis of lesions in severe eye disease. Arch Ophthalmol. 1972;87:258–268. doi: 10.1001/archopht.1972.01000020260005. [DOI] [PubMed] [Google Scholar]
- 7.Imre G, Korchmaros I, Opauszki A. on Epidemic Keratoconjunctivitis. Klin Monbl Augenheilkd. 1963;143:362–375. [PubMed] [Google Scholar]
- 8.Maudgal PC. Cytopathology of adenovirus keratitis by replica technique. Br J Ophthalmol. 1990;74:670–675. doi: 10.1136/bjo.74.11.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiao J, Natarajan K, Rajala MS, Astley RA, Ramadan RT, Chodosh J. Vitronectin: a possible determinant of adenovirus type 19 tropism for human corneal epithelium. Am J Ophthalmol. 2005;140:363–369. doi: 10.1016/j.ajo.2005.03.077. [DOI] [PubMed] [Google Scholar]
- 10.Hadfield TL, McEvoy P, Polotsky Y, Tzinserling VA, Yakovlev AA. The pathology of diphtheria. J Infect Dis. 2000;181 (Suppl 1):S116–120. doi: 10.1086/315551. [DOI] [PubMed] [Google Scholar]
- 11.Lemos LB, Baba N, de Araujo OJ. Clindamycin-induced pseudomembranous colitis. Report of three cases. Am J Clin Pathol. 1976;65:455–461. doi: 10.1093/ajcp/65.4.455. [DOI] [PubMed] [Google Scholar]
- 12.Duke-Elder S. Inflammations of the conjunctiva and associated inflammations of the cornea. In: Duke-Elder S, editor. Diseases of the outer eye. St. Louis: C. V. Mosby company; 1965. pp. 55–56. [Google Scholar]
- 13.Hogan MJ. Conjunctivitis with membranes formation. American Journal of Ophthalmology. 1947;30:1495–1512. doi: 10.1016/s0002-9394(47)91855-2. [DOI] [PubMed] [Google Scholar]
- 14.Hogan MJ, Crawford JW. Epidemic keratoconjunctivitis: superficial punctate keratitis, keratitis subepithelialis, keratitis maculosa, keratitis nummularis. American Journal of Ophthalmology. 1942;25:1059–1078. doi: 10.1016/j.ajo.2018.03.041. [DOI] [PubMed] [Google Scholar]
- 15.Darougar S, Quinlan MP, Gibson JA, Jones BR. Epidemic keratoconjunctivitis and chronic papillary conjunctivitis in London due to adenovirus type 19. Br J Ophthalmol. 1977;61:76–85. doi: 10.1136/bjo.61.2.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duke-Elder S. Inflammations of the conjunctiva and associated inflammations of the cornea. In: Duke-Elder S, editor. Diseases of the outer eye. St. Louis: C. V. Mosby company; 1965. pp. 348–358. [Google Scholar]
- 17.Gordon YJ, Aoki K, Kinchington PR. Adenovirus keratoconjunctivitis. In: Pepose JS, Holland GN, Wilhelmus KR, editors. Ocular Infection and Immunity. St. Louis: Mosby; 1996. pp. 877–894. [Google Scholar]
- 18.Laibson PR, Green WR. Conjunctival membranes in epidemic keratoconjunctivitis. Arch Ophthalmol. 1970;83:100–102. doi: 10.1001/archopht.1970.00990030102019. [DOI] [PubMed] [Google Scholar]
- 19.Hammer LH, Perry HD, Donnenfeld ED, Rahn EK. Symblepharon formation in epidemic keratoconjunctivitis. Cornea. 1990;9:338–340. [PubMed] [Google Scholar]
- 20.Echavarria M, Forman M, Ticehurst J, Dumler JS, Charache P. PCR method for detection of adenovirus in urine of healthy and human immunodeficiency virus-infected individuals. J Clin Microbiol. 1998;36:3323–3326. doi: 10.1128/jcm.36.11.3323-3326.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sato TN, Tozawa Y, Deutsch U, et al. Distinct roles of the receptor tyrosine kinases Tie-1 and Tie-2 in blood vessel formation. Nature. 1995;376:70–74. doi: 10.1038/376070a0. [DOI] [PubMed] [Google Scholar]
- 22.DeLisser HM, Newman PJ, Albelda SM. Platelet endothelial cell adhesion molecule (CD31) Curr Top Microbiol Immunol. 1993;184:37–45. doi: 10.1007/978-3-642-78253-4_3. [DOI] [PubMed] [Google Scholar]
- 23.Banerji S, Ni J, Wang SX, et al. LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J Cell Biol. 1999;144:789–801. doi: 10.1083/jcb.144.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kahn HJ, Bailey D, Marks A. Monoclonal antibody D2-40, a new marker of lymphatic endothelium, reacts with Kaposi’s sarcoma and a subset of angiosarcomas. Mod Pathol. 2002;15:434–440. doi: 10.1038/modpathol.3880543. [DOI] [PubMed] [Google Scholar]
- 25.Pardali E, ten Dijke P. Transforming growth factor-beta signaling and tumor angiogenesis. Front Biosci. 2009;14:4848–4861. doi: 10.2741/3573. [DOI] [PubMed] [Google Scholar]
- 26.Pohlers D, Brenmoehl J, Loffler I, et al. TGF-beta and fibrosis in different organs - molecular pathway imprints. Biochim Biophys Acta. 2009;1792:746–756. doi: 10.1016/j.bbadis.2009.06.004. [DOI] [PubMed] [Google Scholar]


