Abstract
Mutations in more than 15 genes are now known to cause severe congenital neutropenia (SCN); however, the pathologic mechanisms of most genetic defects are not fully defined. Deficiency of G6PC3, a glucose-6-phosphatase, causes a rare multisystem syndrome with SCN first described in 2009. We identified a family with 2 children with homozygous G6PC3 G260R mutations, a loss of enzymatic function, and typical syndrome features with the exception that their bone marrow biopsy pathology revealed abundant neutrophils consistent with myelokathexis. This pathologic finding is a hallmark of another type of SCN, WHIM syndrome, which is caused by gain-of-function mutations in CXCR4, a chemokine receptor and known neutrophil bone marrow retention factor. We found markedly increased CXCR4 expression on neutrophils from both our G6PC3-deficient patients and G6pc3−/− mice. In both patients, granulocyte colony-stimulating factor treatment normalized CXCR4 expression and neutrophil counts. In G6pc3−/− mice, the specific CXCR4 antagonist AMD3100 rapidly reversed neutropenia. Thus, myelokathexis associated with abnormally high neutrophil CXCR4 expression may contribute to neutropenia in G6PC3 deficiency and responds well to granulocyte colony-stimulating factor.
Introduction
Severe congenital neutropenia (SCN) results in severe, recurrent bacterial infections and is caused by mutations affecting the maturation, life span, and/or bone marrow egress of neutrophils.1–3 SCN was first reported more than 50 years ago4; however, causative genes are still being discovered, and all patterns of inheritance have been described. Mutations in the neutrophil elastase gene (ELANE) result in the majority of cases, but less commonly mutations in HAX1, GFI1, and CSF3R can also cause SCN.5–11 Multisystem congenital disorders, such as the Shwachman-Diamond, Wiscott-Aldrich, Barth, and Chediak-Higashi syndromes, and glycogen storage disease type Ib, among others, also feature severe neutropenia.12–15
In some forms of SCN, the disease gene normally regulates pathways important for neutrophil maturation and development (eg, CSF3R and GFI1). Other proposed mechanisms are less clear but include improper protein folding, disrupted granule formation, and enhanced apoptosis. Hyperfunctional chemokine receptor CXCR4 mutations, which cause WHIM syndrome (warts, hypogammaglobulinemia, infections, and myelokathexis syndrome), result in myelokathexis (defective neutrophil egress from bone marrow) resulting from increased CXCR4-dependent bone marrow retention of neutrophils.16
Recently, Boztug et al described a new SCN syndrome in 12 children from Europe and the Middle East that was associated with increased susceptibility to bacterial infections and cardiovascular abnormalities, including prominent ectactic superficial veins resulting from mutations in the gene G6PC3.17 Consistent with this, G6pc3 knockout mice had previously been reported to have neutropenia and increased susceptibility to Escherichia coli infection.18 G6PC3 encodes glucose-6-phosphatase-β, which hydrolyzes glucose-6-phosphate in the final step of gluconeogenesis and glycogenolysis. The enzyme is functionally coupled to a glucose transporter (G6PT) that moves glucose-6-phosphate from the cytoplasm to the lumen of the endoplasmic reticulum. Interestingly, mutations in the gene that encodes G6PT cause the SCN syndrome glycogen storage disease type Ib, which has variable neutropenia and infections and is associated with systemic complications, such as liver adenomas, growth retardation, osteoporosis, polycystic ovaries, and inflammatory bowel disease.19
A follow-up report has described a 13th G6PC3-deficient SCN patient, a Moroccan child, with a similar syndrome associated with a novel nonsense G6PC3 mutation designated 115X.20 A lack of bone marrow neutrophils was reported in 4 patients in the Boztug et al series (all homozygous for the mutation G6PC3 R253H) and in the patient with 115X.17,20 The bone marrow findings were not described for the other patients. Two of the original 12 patients reported, including one with R253H and one with G260R, were shown to have enhanced tumor necrosis factor-dependent neutrophil apoptosis.17 Thus, the proposed mechanism of neutropenia was a lack of neutrophil production, possibly with decreased neutrophil survival.
In the present work, we characterize 2 additional cases of G6PC3 deficiency and demonstrate a key clinical difference with the previously reported patients: on repeated bone marrow examination, there was evidence in both of our patients for full myeloid maturation in the bone marrow. In addition, we found that CXCR4 expression on peripheral blood neutrophils was markedly increased. Granulocyte colony-stimulating factor (G-CSF) treatment of both patients increased the absolute neutrophil count in the blood to normal and reduced neutrophil CXCR4 expression levels to normal.
Methods
Clinical materials
A white boy and his sister were referred for evaluation of SCN and recurrent bacterial infections. All research studies were conducted after obtaining written informed consent consistent with the Declaration of Helsinki under a National Institutes of Health Institutional Review Board–approved protocol.
Mouse experiments
An animal study proposal was approved at the National Institutes of Health. G6pc3 knockout mice were prepared as previously described,18 and C57BL/6J congenic mice were used in this study by backcrossing G6pc3−/− mice in a mixed background to C57BL/6J mice for 12 generations. Heterozygous mice were bred, and littermates were genotyped and analyzed. Ten- to 14-week-old littermates were used in the experiments. Bone marrow neutrophils were isolated by killing the mice, surgically removing femurs, and using phosphate-buffered saline to flush the marrow followed by immediate flow cytometry or positive magnetic bead selection (anti Gr-1+; Miltenyi Biotec) for use in the chemotaxis assay, which was performed as previously described.18 G-CSF levels were measured in serum using the manufacturer's instructions and the Quantikine ELISA kit (R&D Systems). A total of 200 μg of AMD3100 (Sigma Aldrich) dissolved in sterile phosphate-buffered saline was injected into each mouse subcutaneously. Tail vein blood samples (50 μL) were obtained before and 2.5 hours after injection, and complete blood count and manual differential were determined.
Genetic testing
Genomic DNA was isolated from peripheral blood of patients and healthy volunteers, and candidate genes were sequenced using standard capillary sequencing methods (ABI). Data were analyzed using Sequencher Version 4.9 (Gene Codes).
Microscopy
Microscopic images were taken using a Zeiss Axiovert 200M microscope equipped with a QImaging Retiga EXi camera using a 40× Zeiss oil immersion objective with or without a 1.6× optivar lens for a total magnification of 400× or 640×. Image acquisition was performed using iVision-Mac 4.0 (BioVision Technologies).
Flow cytometry
Leukocytes were obtained from heparinized blood using density gradient centrifugation to isolate peripheral blood mononuclear cells (PBMCs) with further steps of dextran sedimentation and red cell lysis with hypotonic saline to isolate neutrophils and rapidly analyzed (< 3 hours after phlebotomy). PBMCs were stained with combinations of the following mouse anti–human mAb conjugates: CD14-fluorescein isothiocyanate, CD3-fluorescein isothiocyanate, CD4-allophycocyanin (APC), CD8-APC, CD19-APC, CD56-APC, CD184-phycoerythrin (PE) and appropriate isotype control conjugates (BD Biosciences PharMingen). Neutrophils were stained with mouse anti–human phycoerythrin-labeled CD184 (BD Biosciences PharMingen) or CXCR7, FPR1, FPRL1, CXCR1, or CXCR2 (all from R&D Systems). Isotype control for these antibodies is mouse IgG2a-PE, except mouse IgG2b-PE (R&D Systems) for FPR1. Cells were stained with conjugated monoclonal antibodies and isotype controls using standard protocols, and at least 20 000 events were counted on a FACSCalibur flow cytometer. Data were then analyzed using the software program FlowJo (Version 9.0.1; TreeStar).
G6PC3 enzymatic function assay
Epstein-Barr virus-transformed lymphoblastoid cell lines were generated from PBMCs from each patient and 2 healthy anonymous blood donors. Microsomes isolated from these cells by centrifugation were disrupted in 0.2% deoxycholate for 20 minutes on ice. Disrupted microsomal membranes were then used in a phosphohydrolase assay as described previously.21 Briefly, a 50mM cacodylate buffer at pH 6.5 with 10mM glucose-6-phosphate was incubated at 37°C for 10 minutes before addition of 4 volumes of a stop solution (2.1mM ammonium molybdate, 0.33M sulfuric acid, 3.3% sodium dodecyl sulfate, and 0.07M ascorbic acid). Absorbance was then determined at 820 nm and the amount of released phosphate determined using a standard curve. Nonspecific phosphatase activity was estimated by preincubating the disrupted microsomal membranes at pH 5.0 for 10 minutes at 37°C to inactivate the acid labile glucose-6-phosphatase-β.
Apoptosis assays
Human neutrophils were cultured for 2 to 4 hours at 37°C in RPMI 1640 with 10% fetal bovine serum in a 5% carbon dioxide atmosphere. Some of the cells were stimulated with tumor necrosis factor-α (50 ng/mL) or thapsigargin (10μM) to induce apoptosis (Sigma-Aldrich). Cells were stained with annexin V and propidium iodide (BD Biosciences PharMingen), and flow cytometry was performed on a FACSCalibur and analyzed with FlowJo software (Version 9.0.1; TreeStar).
Neutrophil superoxide assays
The dihydrorhodamine (DHR) assay was performed as previously described.22 In brief, after red blood cell lysis, leukocytes were loaded with DHR (Invitrogen) for 5 minutes at 37°C in the presence of catalase (Sigma-Aldrich) followed by stimulation with 100 ng/mL phorbol myristate acetate (PMA; Sigma-Aldrich) for 14 minutes before flow cytometry. Real-time respiratory burst activity was detected by chemiluminescence using the LumiMax Superoxide Anion Detection kit (Stratagene) and Victor Light 1420 Luminescence counter (PerkinElmer Life and Analytical Sciences) using 200μM luminol, 250μM enhancer, and 200 000 neutrophils in 200 μL of Hanks balanced salt solution (HBSS) stimulated with 200 ng/mL PMA in HBSS or HBSS alone as a control. Endpoint superoxide production expressed as nanomoles of superoxide/106 cells was determined 15 minutes after the addition of buffer, 100 ng/mL PMA, or 100nM formyl-methionine-leucine-phenylalanine using a superoxide dismutase-inhibitable reduction of ferricytochrome c assay (Sigma-Aldrich). The reaction was stopped on ice, and supernatants collected after centrifugation at 500g for 15 minutes at 4°C. The reaction was read on a spectrophotometer (Beckman Coulter DU-450) using an analytical wavelength of 549.5 nm with background wavelengths of 541 and 556 nm. The optical density was converted to nanomoles of superoxide using ϵ550 nm = 0.0211 μM−1cm−1. Because cytochrome c does not traverse the plasma membrane, the assay only detects superoxide released extracellularly.
Bacterial killing assay
Staphylococcus aureus strain 502A was grown in trypticase soy broth (VWR Scientific) at 37°C to early log phase. The OD650 nm of the suspension was adjusted to 0.25 or 108 bacteria/mL. Viable bacteria were mixed with human neutrophils (5 × 106 cells/mL HBSS with Ca+2 and Mg+2 cations and 10% freshly frozen pooled type AB human sera) at a target/effector ratio of 8:1. During incubation at 37°C, aliquots were removed at 20, 45, and 90 minutes and added to distilled water (1:1000 dilution) to lyse the neutrophils. An aliquot of the bacterial suspension was transferred to a Petri plate, and warm trypticase soy agar (BD Biosciences) was added with mixing. The plates were incubated for 48 hours at 37°C. Bacterial colonies were counted using the Image-Pro Plus image analysis software Version 7.0.1 (Media Cybernetics).
Statistical analysis
Unless otherwise noted, the mean plus or minus SEM of the data is plotted. The unpaired t test was performed using Prism, Version 5 (GraphPad Software). Values were considered statistically significant at P less than .05.
Results
Clinical phenotypes
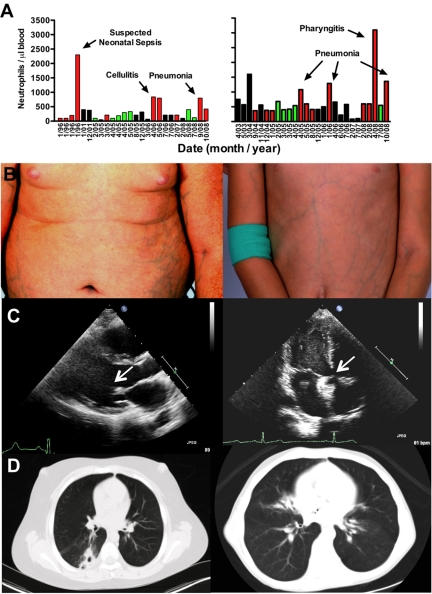
A 13-year-old white boy and his 9-year-old sister were referred in October 2008 for evaluation of SCN and myelokathexis. They both had a history of frequent infections and multiple clinical abnormalities as described in Table 1. Neutropenia was first documented in the boy during the perinatal period when he was hospitalized with suspected neonatal sepsis, and in the girl by age 2. Both patients had baseline absolute neutrophil count (ANC) of 50 to 900 cells/μL that was noncyclic and transiently reversible by severe infection, a pattern also observed in WHIM syndrome (Figure 1A). The girl had a history of a large atrial septal defect repaired via catheter implantation of an occluder. Vaccinations in both children were up-to-date and without any reported complications. Both children were at appropriate grade level in school, and a healthy 11-year-old sister and both parents are alive and well. A history of consanguinity could not be established.
Table 1.
Multisystem disease in two patients with G6PC3 deficiency resulting from G6PC3 G260R
| Patient no. | Sex | Age, y | PMNs, /μL | Infections | Other clinical features |
|---|---|---|---|---|---|
| 1 | M | 13 | 50-300 | Neonatal sepsis, frequent otitis media, frequent bronchitis, pneumonia, severe skin abscesses; frequent pharyngitis, cervical lymphadenitis, periodontal disease, severe dental caries, chronic oral ulcers | Cryptorchidism/testicular agenesis, inguinal hernia, prominent superficial veins, sensorineural hearing loss, heart valve abnormalities, asthma, bronchiectasis, iron deficiency anemia, giant platelets, muscle weakness, joint laxity, poor growth/malabsorption, intermittent abdominal pain |
| 2 | F | 9 | 50-900 | Frequent otitis media, frequent bronchitis, pneumonia, severe skin abscesses, frequent pharyngitis, cervical lymphadenitis, periodontal disease, severe dental caries, chronic oral ulcers, urosepsis | Prominent superficial veins, sensorineural hearing loss, atrial septal defect, bronchiectasis, iron deficiency anemia, giant platelets, muscle weakness, joint laxity, poor growth/malabsorption, intermittent abdominal pain |
PMNs indicates polymorphonuclear leukocytes.
Figure 1.
Patient phenotypes: brother (left panels); sister (right panels). (A) Neutropenia. All available absolute neutrophil counts (ANC) values were plotted by date (nonlinear time scale). Neither patient received G-CSF or other hematopoietic growth factors during the period of observation plotted. Green bars represent clinically uninfected; red bars, active clinical infection; and black bars, unknown infection status. (B) Ectatic superficial veins on chest, abdomen, and extremities. (C) Cardiac defects. Echocardiography revealed a thickened and stenotic mitral valve (arrow) with reduced excursion in the brother and a catheter-implanted atrial septal defect occlusion device (arrow) in the sister. (D) Pulmonary infiltrates and bronchiectasis on chest computed tomography.
On examination, both children had evidence for pneumonia, painful oral ulcers, cervical lymphadenopathy, microcephaly, digital clubbing, and prominent ectatic superficial veins on all 4 limbs and trunk (Figure 1B), and evidence for foot and knee ligamentous laxity and mild proximal muscle weakness with symptoms of pain with weight-bearing exercise. The brother, but not the sister, had a buffalo hump and central obesity, probably resulting from frequent short courses of oral corticosteroid therapy for asthma. The sister's height was at the 10th percentile, but weight was 5 pounds less than the third percentile. The brother had a history of slow growth as a child (weight 10th percentile at age 2.5), but this had appeared to resolve and at presentation his height and weight were in the 50th and more than 75th percentiles, respectively.
The main clinical laboratory abnormality was neutropenia in the setting of active infection (420 and 1360 polymorphonuclear leukocytes/μL in brother and sister, respectively). Circulating levels of CD3+, CD4+, CD8+, CD19+, and CD14+ cells were normal in both patients, whereas CD56+ NK cells were slightly decreased in frequency in both patients. Both patients were also anemic (hemoglobin 12.7 and 10.5 g/dL, respectively), resulting from iron deficiency and possibly anemia of chronic disease resulting from chronic inflammation from recurrent infections (serum iron 13 and 10 μg/dL, respectively, 3% transferrin saturation). Both had normal IgG and complement levels and positive titers to tetanus and diphtheria toxins.
Echocardiography revealed the occluder device in place in the atrial septum of the sister (Figure 1C right panel), and in the brother asymptomatic mitral valve thickening with reduced excursion thought to result from congenital abnormal valve development (Figure 1C left panel), a dilated right ventricle, mild pulmonary hypertension, and a small septal defect consistent with a patent foramen ovale. Computed tomography scan revealed bronchiectasis and pulmonary infiltrates in both patients (Figure 1D). Pulmonary function tests, performed 2 months after the first visit to the National Institutes of Health, demonstrated normal lung volumes and flow rates and a moderate diffusion defect (adjusted diffusing capacity for carbon monoxide = 54%) in the sister, and a moderate β-adrenergic receptor agonist-sensitive obstructive component in the brother (forced expiratory volume in 1 second: 51% before agonist treatment and 67% after agonist treatment). Audiometry revealed asymptomatic mild to moderate bilateral sensorineural hearing loss in both children. Renal and pelvic ultrasound was normal in both patients, with the exception that the boy's remaining testicle was noted to be small and have microlithiasis.
Pneumonia was treated empirically with oral levofloxacin for 2 months followed by oral trimethoprim-sulfamethoxazole prophylaxis. We initially began G-CSF 3 μg/kg by subcutaneous injection every other day and this was escalated to 5 μg/kg subcutaneously every day, to increase the ANC to greater than 1500 cells/μL. From the initial visit at the National Institutes of Health until the most recent follow-up (1.5 years), each patient has had only one documented infection requiring antibiotics (both early after starting G-CSF), and both patients have had improved quality of life. The brother has had one acute asthmatic exacerbation requiring hospitalization.
Identification of G6PC3 deficiency
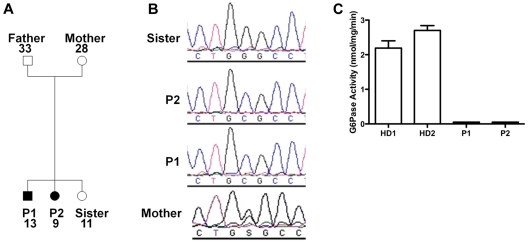
Because of the similarities of our patients' presentation to the patients described by Boztug et al,17 we sequenced G6PC3 and found that both patients were homozygous for a G→C missense mutation at nucleotide position +778 (using A in the translational start site as +1 in GenBank NM_138387) resulting in a glycine to arginine change at position 260 (G260R). The same mutation had been found in 1 patient previously.17 The family pedigree is shown in Figure 2A. The mother was heterozygous, and the unaffected sister lacked the mutation (Figure 2B). The father's genotype was not determined because he refused to provide DNA. Amino acid position 260 is in the predicted seventh transmembrane domain of G6PC3, and this site is highly conserved in the 2 other known glucose-6-phosphatase enzymes23; however, the enzymatic functional effect of this mutation had not been previously reported. Therefore, we tested the function in lymphoblastoid cell lines derived from the patients compared with healthy donors and found a complete loss of enzymatic activity in the patient-derived cells (Figure 2C).
Figure 2.
G6PC3 deficiency resulting from homozygous inheritance of G6PC3 mutation G260R in 2 siblings with a multisystem syndrome involving severe congenital neutropenia and recurrent infections. P1 indicates affected brother; and P2, affected sister. (A) Family pedigree. Circles represent female; squares, male; filled symbols, affected; and unfilled symbols, unaffected. Numbers indicate subject ages. (B) DNA sequencing. Chromatograms from family members (father refused). (C) G6PC3 G260R is a complete loss-of-function mutation. G6PC3 activity was measured in lymphoblastoid cell lines from 2 healthy blood donors (HD1 and HD2) and both patients.
Myelokathexis and other bone marrow abnormalities in patients with G6PC3 deficiency
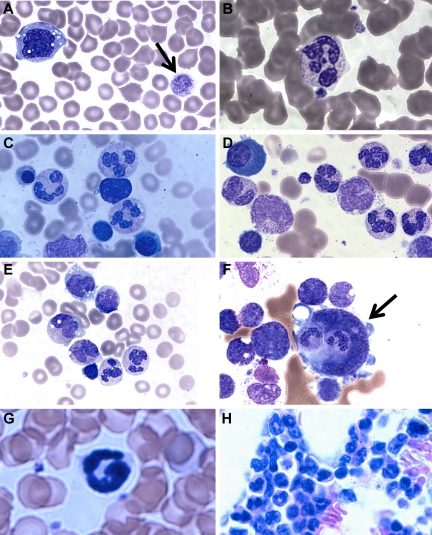
A major clinical difference in our patients compared with the G6PC3-deficient patients reported by Boztug et al was the presence of mature neutrophils in the bone marrow.17 In Boztug et al, an absence of marrow neutrophils was reported in all 4 patients who were examined.17 However, they were all in the same pedigree with a different mutation (R253H) and had bone marrow analysis performed as infants (age 1-2 months). We therefore examined the complete hematologic findings of our patients in greater detail. We found that, although the number of neutrophils in the blood was markedly decreased, the few that could be found appeared mature. Blood smear revealed a hypochromic microcytic anemia, with prominent monocyte cytoplasmic vacuolization and giant platelets (Figure 3A-B).
Figure 3.
Patient blood and bone marrow histopathology. (A-F) Human data for brother (A,C,E) and sister (B,D,F). Similar results were observed for both siblings. The patient samples shown were obtained before the patients had ever received hematopoietic growth factors and at a time when there was no evidence of active infection. (A-B) Patient peripheral blood smears. (A) Giant platelets (arrow) and vacuolization of monocytes. (B) Rare but morphologically normal neutrophils in the blood. (C-F) Patient bone marrow smears. (C-D) All stages of myeloid differentiation through an abundance of morphologically mature neutrophils are evident. (E) Vacuolization of 2 metamyelocytes. A majority of megakaryocytes were monocytoid (mean, 68%) and frequently (12%) demonstrated penetration by intact cells (emperipolesis) that appeared to be neutrophils (arrow). (G-H) Mouse data in the absence of G-CSF or AMD3100. (G) G6pc3−/− mouse peripheral blood. Morphologically mature neutrophils were evident. (H) G6pc3−/− mouse bone marrow. An abundance of mature neutrophils is evident.
Bone marrow biopsies had been performed on both of our patients twice in the past 3 years before any medications and at a time when they were not clinically infected. By reviewing the most recent pathology, we confirmed the original reports that all bone marrows were normocellular for age and exhibited multiple fully mature neutrophils (Figure 3C-E). This was further confirmed by flow cytometric analysis of bone marrow samples and microscopic enumeration of the cells (Table 2). Some bone marrow neutrophils appeared pyknotic, and there was evidence of both emperipolesis of neutrophils seen in 12% of megakarocytes (Figure 3F) and hemophagocytosis in rare macrophages. Ceroid-appearing cytoplasm was present in bone marrow macrophages from both patients, especially the sister. Marrows from both patients showed a predominance of atypical mononuclear megakaryocytes, myeloid hyperplasia, and vacuolization of the myeloid precursors was seen (especially the promyelocytes, metamyelocytes, and myelocytes). Taken together, the marrow pathology was consistent with myelokathexis and cytokine activation. Consistent with this, we confirmed that targeted deletion of the G6pc3 gene in mice resulted in reduced numbers of mature circulating neutrophils, as previously reported18 (Figure 3G), and we extended this by finding abundant fully mature neutrophils in the bone marrow (Figure 3H) with similar changes in the megakaryocytes but less vacuolization of the myeloid precursors (data not shown).
Table 2.
Bone marrow and corresponding blood cell counts
| Biopsy no. 1 |
Biopsy no. 2 |
Boztug et al117 | Normal values | |||
|---|---|---|---|---|---|---|
| Patient 1, male | Patient 2, female | Patient 1, male | Patient 2, female | |||
| Blood ANC, cells/μL | 190 | 540 | 120 | 550 | ND | ∼ 4000 |
| Age, y | 9 | 5 | 13 | 9 | 0.1 | (1-20) |
| Blasts, % | 1 | 1 | 4 | 4 | 0.9 | 1.2 (0-3) |
| Promyelocytes, % | 10 | 5 | 14 | 18 | 11.4 | 1.8 (0-5) |
| Myelocytes, % | 7 | 3 | 12 | 11 | 2.8 | 16 (9-25) |
| Metamyelocytes, % | 17 | 3 | 18 | 18 | 0.5 | 23 (14-34) |
| Bands + PMNs, % | 36 | 18 | 20 | 17 | 0 | 13 (5-29) |
| Eosinophilic granulocytes, % | 0 | 0 | 0 | 0 | 0.3 | 4 (1-9) |
| Basophilic granulocytes, % | 0 | 0 | 0 | 0 | 0 | 0.1 (0-0.8) |
| Monocytes, % | 8 | 7 | 5 | 4 | 9.4 | ND |
| Lymphocytes, % | 10 | 27 | 9 | 10 | 24 | 16 (5-36) |
| Plasma cells, % | 0 | 1 | 2 | 5 | 0 | ND |
| Erythroid progenitors, % | 11 | 35 | 16 | 13 | 50.6 | 23 (6-38) |
| M:E ratio | 7.2 | 1.1 | 4.6 | 5.5 | 0.5 | 3 (1-12) |
Data are ANC, age, and cell distribution in 2 different bone marrow samples obtained for each patient; the mean values reported by Boztug et al17 for 4 patients with G6PC3 mutation R253H,17 and normal mean values (range) for children (n = 92).24
PMNs indicates polymorphonuclear leukocytes; ND, not described; and M:E, myeloid:erythroid ratio.
Increased neutrophil expression of CXCR4 in patients and mice with G6PC3 deficiency
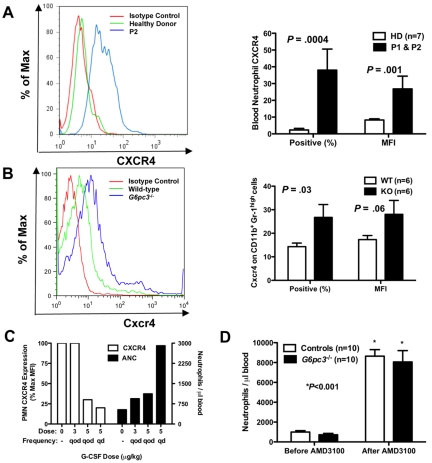
Because both patients have myelokathexis and because myelokathexis can be caused by gain-of-function mutations in the chemokine receptor CXCR4 as described for WHIM syndrome, we analyzed CXCR4 expression on peripheral blood neutrophils from both of our patients before they had received G-CSF and in neutrophils from G6pc3−/− mouse bone marrow (Figure 4A-B). We documented in both patients a dramatic increase in both CXCR4 expression per cell, measured as the mean fluorescence intensity (MFI), and the percentage of neutrophils expressing CXCR4. Similarly, the percentage of CD56+ NK cells in the peripheral blood was low in both patients, and the percentage of NK cells expressing CXCR4 was also increased in both patients (mean 41% vs 7% in 9 control donors, P < .001); the MFI of CXCR4 expression was also increased (mean 23% vs 9% in controls, P < .001). In contrast, expression of CXCR4 (percentage positive and MFI) was normal in all other leukocyte subsets tested (CD4+, CD8+, CD19+, and CD14+) and was absent in lymphoblastoid cell lines of both healthy persons and the patients (data not shown).
Figure 4.
Neutrophil CXCR4 expression in human and mouse G6PC3 deficiency. (A-B) Flow cytometry (FACS). FACS was performed on freshly isolated neutrophils from (A) P1 (brother) and P2 (sister) or (B) freshly isolated bone marrow cells gated for size and CD11b+Gr-1high expression from G6pc3−/− mice, and compared with patterns found in healthy human donors and wild-type mice. Results are shown as representative histograms for patient 2 and for a knockout mouse in the left panels and are summarized as the percentage of CXCR4+ cells and as the MFI in the right panels of A (human) and B (mouse). Human data are from a single experiment performed before the patients were administered G-CSF. Mouse data are the summary of 3 separate experiments with 2 knockout and 2 wild-type mice in each experiment. (C) Effect of G-CSF treatment on neutrophil CXCR4 and ANC. Freshly isolated peripheral blood neutrophils were analyzed by FACS before and after starting G-CSF. ANC and CXCR4 expression on each dose were measured once for each subject after approximately 2 months on that dose. Data are summarized as the average of both subjects for each parameter at each dose and are presented on the same axes as percentage of maximum MFI (where maximum is defined as the MFI of the pre–G-CSF sample; left axis) and as the average ANC (right axis). (D) Effect of acute CXCR4 blockade on mouse ANC. G6pc3 knockout and littermate control mice (G6pc3+/+, n = 4 and G6pc3+/−, n = 6) were injected subcutaneously with 200 μg of the specific CXCR4 antagonist AMD3100. Absolute blood neutrophil counts per microliter were determined immediately before and 2.5 hours after injection. Data are from 2 experiments and are presented as the mean ± SEM.
We observed that, during G-CSF therapy, the patients' neutrophils demonstrated a dose-dependent decrease in CXCR4 expression, as measured by MFI, whereas conversely the absolute neutrophil counts increased (Figure 4C). Other cell surface receptors tested, including the chemokine receptors CXCR7 and CX3CR1 and the chemoattractant receptor FPRL1, were not expressed at higher levels than normal and were not influenced by G-CSF therapy (data not shown). Endogenous levels of G-CSF in the mouse blood were not significantly different (G6pc3−/− 178 ± 21 vs 153 ± 12 pg/mL for wild-type, P = .31), indicating that the enzymatic defect did not affect the natural production of this cytokine.
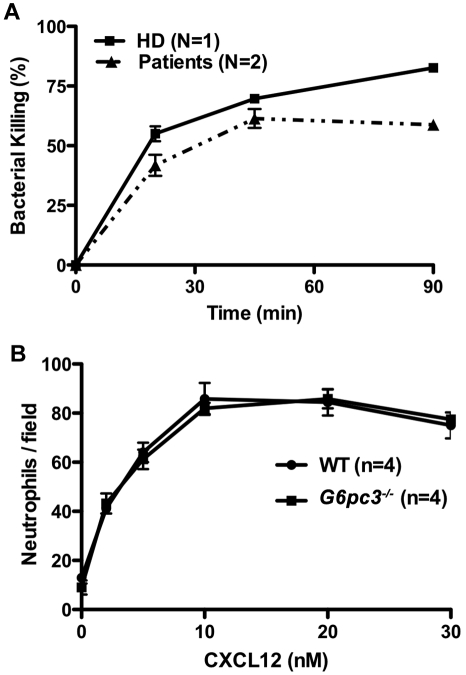
Because AMD3100 (plerixafor), a recently Food and Drug Administration-approved CXCR4 antagonist marketed as Mozobil, is known to mobilize neutrophils in humans,25 we tested whether this could create a similar neutrophil-mobilizing effect in G6pc3−/− mice. We injected mice subcutaneously with 200 μg of AMD3100 and measured the neutrophil response 2.5 hours later, the time point we had determined for optimal neutrophil mobilization to blood in wild-type mice (data not shown). The knockout mice that had approximately 25% fewer circulating neutrophils at baseline confirming our previous report18 had an 18-fold increase in peripheral blood neutrophils after injection, similar to controls (Figure 4D).
Neutrophil function and apoptosis in patients with G6PC3 deficiency
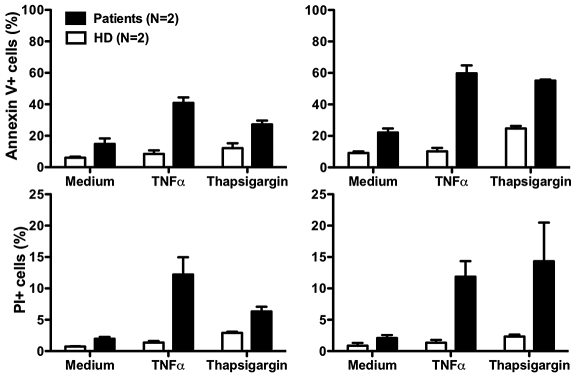
Although our data suggest that neutropenia in our patients results from impaired release of mature neutrophils from the bone marrow, there may also be other mechanisms. In this regard, Boztug et al reported that peripheral blood neutrophils from 2 patients tested, 1 with the R253H mutation (patient 5) and 1 with the G260R mutation (patient 10) had increased spontaneous apoptosis compared with a control person receiving G-CSF.17 The difference relative to the control was amplified when the cells were treated with tumor necrosis factor or the intracellular calcium ion-mobilizing agent thapsigargin.17 Due to the severity of the neutropenia off G-CSF, we were unable to obtain enough cells from our patients to perform most functional assays, including assays of CXCR4 function. After treatment with G-CSF, sufficient cells could be readily obtained as the ANC increased, however the results must be interpreted with caution because many neutrophil functions are known to be affected by G-CSF. Consistent with the report of Boztug et al17, we found that peripheral blood neutrophils from both of our patients had increased spontaneous apoptosis, and that the difference compared to healthy controls was amplified by both tumor necrosis factor and thapsigargin (Figure 5).
Figure 5.
Increased neutrophil apoptosis in patients with G6PC3 deficiency resulting from homozygous inheritance of G6PC3 G260R. Neutrophils from both patients and 2 healthy donors (HD) were cultured at 37°C for 2 hours (left panels) or 4 hours (right panels) in medium alone or medium with the addition of tumor necrosis factor (TNFα) or thapsigargin. Flow cytometry was then performed. Apoptotic cells are defined as annexin V+. Dead cells are defined as propidium iodide–positive (PI)+. Data are from a single experiment performed after the patients were treated for 7 months with G-CSF.
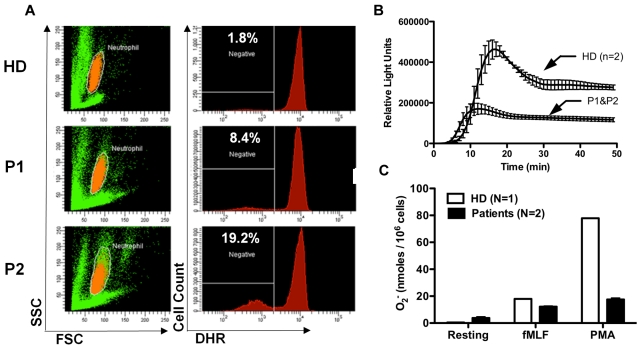
Although the increased susceptibility to infection in patients and mice with G6PC3 deficiency may be entirely the result of the severely reduced number of peripheral blood neutrophils, the possibility of functional defects must also be considered and is plausible given the important role of G6PC3 in metabolism. In this regard, Boztug et al reported results of the Oxyburst assay of superoxide production to be normal in 2 patients tested of the 5 patients with the R253H mutation.17 In contrast, we found decreased superoxide generation in response to PMA stimulation using 3 different assays in our patients (Figure 6). This was consistent with previous results reported for thioglycollate-elicited peritoneal neutrophils in G6pc3−/− mice.18 We also were able to demonstrate slightly reduced in vitro killing of Staphylococcus aureus by peripheral blood neutrophils from both patients incubated at an 8:1 target/effector cell ratio (Figure 7A). The patients killed approximately equal numbers of bacteria to a healthy control initially, but had diminished killing at the 90-minute time point (58.8% ± 3.0% vs 82.6% ± 1.2%). This 90-minute patient value was outside the normal range established in our laboratory for this assay (77.6% ± 7.6%). This result was also consistent with previously reported defects in E coli in vitro killing by thioglycollate-elicited peritoneal neutrophils from G6pc3−/− mice.18 In contrast, Boztug et al reported that 2 of their patients with the R253H mutation had no defect in vitro in killing E coli strain ML-35.17
Figure 6.
Defective neutrophil superoxide production in patients with G6PC3 deficiency resulting from homozygous inheritance of G6PC3 G260R. (A) DHR assay. (Left panels) Neutrophils in orange by side scatter (SSC) and forward scatter (FSC) characteristics. Fluorescence of DHR-loaded neutrophils after PMA stimulation for each subject is graphed in the corresponding panels to the right. Prestimulation histograms for each subject showed MFIs less than 200. HD indicates healthy donor; and P1 and P2, affected brother and sister, respectively. The number in the top left corner of each panel on the right indicates the percentage of gated cells that failed to respond to PMA. (B) Real-time assay of superoxide production. PMA was used as the stimulus. Data are from a single experiment with 2 replicates at each time point plotted as a single curve for healthy donors versus patients. (C) Endpoint superoxide production assay. Production of superoxide was measured 15 minutes after the addition of the indicated agonists. Data are shown for a healthy donor versus the average of the 2 patients together. Data are summarized from a single experiment in which each subject was tested in duplicate. (B-C) Data were obtained after the patients were treated for 5 months with G-CSF.
Figure 7.
Neutrophil functional capacity in G6PC3 deficiency. (A) Defective microbicidal activity in neutrophils from patients with G6PC3 deficiency resulting from homozygous inheritance of G6PC3 G260R. Staphylococcus aureus was incubated with polymorphonuclear leukocytes from a healthy donor (HD) and the 2 patients in an 8:1 target/effector ratio. Bacterial killing kinetics is plotted for each donor as a percentage of colony-forming units observed for control S aureus incubated in the absence of neutrophils. Data were obtained after the patients were treated for 5 months with G-CSF and are from a single experiment in which each condition was tested in duplicate. (B) Purified mouse bone marrow neutrophils from G6pc3 knockout and wild-type littermate control mice chemotax to CXCL12 equally well (P > .05 at all time points). Data are summarized from 2 experiments with 2 mice in each group.
We were unable to test whether CXCR4 was functional in the patients' neutrophils because we could not get enough to test off G-CSF, and there was almost no neutrophil CXCR4 expression on G-CSF. However, we were able to test the chemotaxis of freshly isolated and purified bone marrow-derived mouse neutrophils off G-CSF to various concentrations of CXCL12. This resulted in a typical bell-shaped chemotaxis curve that was nearly identical (P > .05 for all concentrations) between wild-type and G6pc3−/− mice (Figure 7B).
Discussion
In this paper, we describe 2 new sibling patients with G6PC3 deficiency, a syndrome first reported in 2009 by Boztug et al17 and characterized by SCN, increased susceptibility to infection, prominent cutaneous veins, and urogenital and cardiac malformations. Our patients were homozygous for the G6PC3 mutation G260R previously found in 1 patient in the original series.17 We have extended the original report by showing that the G260R mutation abolishes G6PC3 enzymatic function. This is the first test of G6PC3 function in cells derived from a patient with this syndrome, providing important additional evidence that lack of G6PC3 enzymatic function is causal. In addition, confirmation of the G260R missense mutation as causative of SCN when present in the homozygous but not heterozygous state suggests a fully penetrant, autosomal recessive genetic model of disease for this mutation, similar to that reported for the R253H mutation.17
A major conclusion of our work is that the homozygous G6PC3 G260R mutation may cause neutropenia without a failure of neutrophil production. We are confident in this result because it was found in both our patients on 2 separate occasions separated by 4 years. This was before their treatment with G-CSF or any other hematopoietic growth factor, and on both occasions they were not clinically infected. This finding in our patients was confirmed by examination of G6pc3 knockout mouse bone marrow and by the large mobilization of neutrophils to the circulation acutely in the knockout mouse after a single injection of AMD3100, which is known to cause the release of mature neutrophils acutely by blocking CXCR4-mediated retention mechanisms. The short time frame of the AMD3100 effect makes it unlikely to be resulting from increased production or diminished apoptosis and implicates the increased CXCR4 expression we observed with functional effects on abnormal neutrophil marrow retention (myelokathexis).
We do not have a direct explanation for the discrepancy in the bone marrow picture in our patients versus the 5 previously reported G6PC3-deficient patients whose bone marrows were analyzed17,20 but note that the precise mutation in our patients differs from the other patients and the ages of the patients when bone marrow was obtained are very different: 1-month average in the 4 previously reported patients versus 9 and 13 years in our female and male patients, respectively. The age at which the bone marrow was obtained for the Moroccan patient described by Arostegui et al was not reported.20 The possibility of genotypic heterogeneity accounting for this discrepancy has precedent in at least 2 other examples of SCN: cyclic neutropenia versus SCN depending on the precise mutation in ELANE, and variable severity of disease in cartilage hair dysplasia depending on the type of mutations.26,27 A take-home message from this work is that clinicians should be aware that impaired neutrophil production is not an invariant feature of G6PC3 deficiency.
A second major conclusion of our work is that G-CSF appears to be an effective therapy for these patients. Our patients had a large and sustained increase in neutrophil counts (Figure 4C), an apparent reduced risk of infection, and improved quality of life on G-CSF. Because G-CSF is known to have diverse mechanisms of action at correcting neutropenia, including enhancing marrow neutrophil production, decreasing neutrophil apoptosis,28 and enhancing marrow release of neutrophils by interfering with the CXCR4-CXCL12 receptor-ligand interaction,29–31 it is unclear which of these factors are principally responsible for the improvement in our patients. However, it is interesting that the neutrophil count increases seen with increasing doses of G-CSF were accompanied by a decline in surface expression levels of CXCR4 (Figure 4C) that were initially very elevated in our patients.
Although still controversial, long-term use of G-CSF may be associated with acquired mutations in the G-CSF receptor, diminished effectiveness, and the onset of acute myeloid leukemia and myelodysplasia in some patients.32,33 Thus, alternatives to G-CSF therapy should be sought. Testing AMD3100 further in the G6pc3−/− mice and G6PC3-deficient patients may be important in this regard, although this drug is short-acting and may have side effects because of CXCR4's important roles in the immune system. Patient management should also include proper nutrition, attention to oral hygiene, and appropriate vaccinations. The use of prophylactic antibiotics is justified by their demonstrated usefulness in other neutrophil disorders, such as chronic granulomatous disease.34
It is also important to note that we do not yet know how G6PC3 deficiency might lead to markedly increased CXCR4 expression selectively on neutrophil and NK cells. Normally, CXCR4 is expressed at exceedingly low levels on freshly isolated human neutrophils but increases as the cells age and become apoptotic. This has been proposed as a mechanism for return of senescent neutrophils to the bone marrow for elimination by macrophages.35,36 One attractive hypothesis is that G6PC3 deficiency resulting from G260R results in increased ER stress and apoptosis with increased CXCR4 expression driving neutrophil bone marrow retention and/or premature return for destruction. It is reasonable to speculate that neutropenia in our patients is multifactorial and may be the result of a positive feedback cycle in which neutrophils differentiate in a stressed state in the bone marrow, which increases CXCR4 levels and decreases egress, which in turn increases the risk of infection, which stresses mature neutrophils in the periphery, leading to further increases in CXCR4 levels and premature return to the marrow for destruction. Thus, neutropenia may be the result of decreased egress, accelerated senescence, and accelerated destruction driven by metabolic stress and infection.
Our research emphasizes the importance of precise genotype-phenotype correlations and mechanistic investigations, as heterogeneity may be found in the same syndrome and may affect treatment options. In this regard, it is also important to note that both of our patients have significant musculoskeletal abnormalities, including muscle weakness and joint laxity, which has not been previously noted. Consistent with this, G6PC3 is highly expressed in skeletal muscle cells.23 Similarly, G6PC3 is highly expressed in testis and 6 of the 8 male patients with G6PC3 deficiency syndrome have cryptorchidism. We also observed severe iron deficiency anemia in both our patients. This was not described in the Boztug et al series17 but was found in the patient described by Arostegui et al.20 The anemia in our patients was not responsive to oral iron supplementation but responded well to intravenous ferric gluconate, suggesting an absorption problem. Consistent with this, both patients had failure to thrive early in life, and the female was markedly below normal weight for her age at age 9, the time of presentation to the National Institutes of Health. In addition, both patients had been documented to have decreased serum levels of vitamins. Another interesting hematologic abnormality present in both of our patients but not reported in the Boztug et al series17 is abnormal megakaryocytes in the marrow (monocytoid nuclei, emperipolesis) and giant platelets in the peripheral blood. It is possible that disordered megakaryopoiesis is the pathogenesis of the intermittent thrombocytopenia seen in our patients and reported for 5 of the 12 patients in the Boztug et al series.17
In conclusion, we have identified 2 additional patients with a recently described SCN syndrome resulting from G6PC3 deficiency, and a novel contributing mechanism of neutropenia in this disease involving myelokathexis associated with increased neutrophil expression of the bone marrow homing receptor CXCR4. Myelokathexis points to a potential strategy for treating neutropenia in these patients, and we have provided proof of principle for the strategy in a mouse model of G6PC3 deficiency. Future work will be needed to test this strategy in clinical trials and to fully delineate the mechanisms responsible for neutropenia and the many other phenotypes we have identified in these patients. Finally, we have shown that G-CSF is safe and effective in restoring neutrophil counts to normal, reducing the incidence of infection, and improving quality of life in patients with this syndrome.
Acknowledgments
The authors thank the patients, members of their family, and healthy volunteers for participating in this study as well as the patient's primary care providers, Drs Anna Pak and Jay McDonald, for astute clinical observations and referring the patients.
This work was supported by the Division of Intramural Research of the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: D.H.M., S.S.D.R., S.M.P., D.H., M.M., J.U., H.L.M., and P.M.M. provided clinical care; D.H.M., Q.L., D.B.K., H.L.M., J.Y.C., and P.M.M. designed experiments; D.H.M., H.S.J., S.S.D.R., Q.L., D.A.L.P., and T.O. performed experiments; P.N., C.M.T., and K.P.D. provided hematologic and pathologic expertise; D.H.M. and P.M.M. primarily wrote the manuscript; and all authors contributed to writing the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: David H. McDermott, National Institutes of Health, Bldg 10, Rm 11N107, Bethesda, MD 20892-1886; e-mail: dmcdermott@niaid.nih.gov.
References
- 1.Bohn G, Welte K, Klein C. Severe congenital neutropenia: new genes explain an old disease. Curr Opin Rheumatol. 2007;19(6):644–650. doi: 10.1097/BOR.0b013e3282f05cc2. [DOI] [PubMed] [Google Scholar]
- 2.Dale DC, Link DC. The many causes of severe congenital neutropenia. N Engl J Med. 2009;360(1):3–5. doi: 10.1056/NEJMp0806821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ward AC, Dale DC. Genetic and molecular diagnosis of severe congenital neutropenia. Curr Opin Hematol. 2009;16(1):9–13. doi: 10.1097/MOH.0b013e32831952de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kostmann R. Infantile genetic agranulocytosis; agranulocytosis infantilis hereditaria. Acta Paediatr Suppl. 1956;45(suppl 105):1–78. [PubMed] [Google Scholar]
- 5.Ancliff PJ, Blundell MP, Cory GO, et al. Two novel activating mutations in the Wiskott-Aldrich syndrome protein result in congenital neutropenia. Blood. 2006;108(7):2182–2189. doi: 10.1182/blood-2006-01-010249. [DOI] [PubMed] [Google Scholar]
- 6.Devriendt K, Kim AS, Mathijs G, et al. Constitutively activating mutation in WASP causes X-linked severe congenital neutropenia. Nat Genet. 2001;27(3):313–317. doi: 10.1038/85886. [DOI] [PubMed] [Google Scholar]
- 7.Klein C, Grudzien M, Appaswamy G, et al. HAX1 deficiency causes autosomal recessive severe congenital neutropenia (Kostmann disease). Nat Genet. 2007;39(1):86–92. doi: 10.1038/ng1940. [DOI] [PubMed] [Google Scholar]
- 8.Person RE, Li FQ, Duan Z, et al. Mutations in proto-oncogene GFI1 cause human neutropenia and target ELA2. Nat Genet. 2003;34(3):308–312. doi: 10.1038/ng1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bohn G, Allroth A, Brandes G, et al. A novel human primary immunodeficiency syndrome caused by deficiency of the endosomal adaptor protein p14. Nat Med. 2007;13(1):38–45. doi: 10.1038/nm1528. [DOI] [PubMed] [Google Scholar]
- 10.Grenda DS, Murakami M, Ghatak J, et al. Mutations of the ELA2 gene found in patients with severe congenital neutropenia induce the unfolded protein response and cellular apoptosis. Blood. 2007;110(13):4179–4187. doi: 10.1182/blood-2006-11-057299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horwitz M, Benson KF, Person RE, Aprikyan AG, Dale DC. Mutations in ELA2, encoding neutrophil elastase, define a 21-day biological clock in cyclic haematopoiesis. Nat Genet. 1999;23(4):433–436. doi: 10.1038/70544. [DOI] [PubMed] [Google Scholar]
- 12.Introne W, Boissy RE, Gahl WA. Clinical, molecular, and cell biological aspects of Chediak-Higashi syndrome. Mol Genet Metab. 1999;68(2):283–303. doi: 10.1006/mgme.1999.2927. [DOI] [PubMed] [Google Scholar]
- 13.Orelio C, Kuijpers TW. Shwachman-Diamond syndrome neutrophils have altered chemoattractant-induced F-actin polymerization and polarization characteristics. Haematologica. 2009;94(3):409–413. doi: 10.3324/haematol.13733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Raam BJ, Kuijpers TW. Mitochondrial defects lie at the basis of neutropenia in Barth syndrome. Curr Opin Hematol. 2009;16(1):14–19. doi: 10.1097/MOH.0b013e32831c83f3. [DOI] [PubMed] [Google Scholar]
- 15.Visser G, Rake JP, Fernandes J, et al. Neutropenia, neutrophil dysfunction, and inflammatory bowel disease in glycogen storage disease type Ib: results of the European Study on Glycogen Storage Disease type I. J Pediatr. 2000;137(2):187–191. doi: 10.1067/mpd.2000.105232. [DOI] [PubMed] [Google Scholar]
- 16.Kawai T, Choi U, Cardwell L, et al. WHIM syndrome myelokathexis reproduced in the NOD/SCID mouse xenotransplant model engrafted with healthy human stem cells transduced with C-terminus-truncated CXCR4. Blood. 2007;109(1):78–84. doi: 10.1182/blood-2006-05-025296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boztug K, Appaswamy G, Ashikov A, et al. A syndrome with congenital neutropenia and mutations in G6PC3. N Engl J Med. 2009;360(1):32–43. doi: 10.1056/NEJMoa0805051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheung YY, Kim SY, Yiu WH, et al. Impaired neutrophil activity and increased susceptibility to bacterial infection in mice lacking glucose-6-phosphatase-beta. J Clin Invest. 2007;117(3):784–793. doi: 10.1172/JCI30443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Melis D, Fulceri R, Parenti G, et al. Genotype/phenotype correlation in glycogen storage disease type 1b: a multicentre study and review of the literature. Eur J Pediatr. 2005;164(8):501–508. doi: 10.1007/s00431-005-1657-4. [DOI] [PubMed] [Google Scholar]
- 20.Arostegui JI, de Toledo JS, Pascal M, Garcia C, Yague J, Diaz de Heredia C. A novel G6PC3 homozygous 1-bp deletion as a cause of severe congenital neutropenia. Blood. 2009;114(8):1718–1719. doi: 10.1182/blood-2009-04-219451. [DOI] [PubMed] [Google Scholar]
- 21.Lei KJ, Shelly LL, Pan CJ, Sidbury JB, Chou JY. Mutations in the glucose-6-phosphatase gene that cause glycogen storage disease type 1a. Science. 1993;262(5133):580–583. doi: 10.1126/science.8211187. [DOI] [PubMed] [Google Scholar]
- 22.Jirapongsananuruk O, Malech HL, Kuhns DB, et al. Diagnostic paradigm for evaluation of male patients with chronic granulomatous disease, based on the dihydrorhodamine 123 assay. J Allergy Clin Immunol. 2003;111(2):374–379. doi: 10.1067/mai.2003.58. [DOI] [PubMed] [Google Scholar]
- 23.Martin CC, Oeser JK, Svitek CA, Hunter SI, Hutton JC, O'Brien RM. Identification and characterization of a human cDNA and gene encoding a ubiquitously expressed glucose-6-phosphatase catalytic subunit-related protein. J Mol Endocrinol. 2002;29(2):205–222. doi: 10.1677/jme.0.0290205. [DOI] [PubMed] [Google Scholar]
- 24.Glaser K, Limarzi LR, Poncher HG. Cellular composition of the bone marrow in normal infants and children. Pediatrics. 1950;6(5):789–824. [PubMed] [Google Scholar]
- 25.Lack NA, Green B, Dale DC, et al. A pharmacokinetic-pharmacodynamic model for the mobilization of CD34+ hematopoietic progenitor cells by AMD3100. Clin Pharmacol Ther. 2005;77(5):427–436. doi: 10.1016/j.clpt.2004.12.268. [DOI] [PubMed] [Google Scholar]
- 26.Dale DC, Person RE, Bolyard AA, et al. Mutations in the gene encoding neutrophil elastase in congenital and cyclic neutropenia. Blood. 2000;96(7):2317–2322. [PubMed] [Google Scholar]
- 27.Grenda DS, Link DC. 5. Mechanisms of disordered granulopoiesis in congenital neutropenia. Curr Top Dev Biol. 2006;74:133–176. doi: 10.1016/S0070-2153(06)74005-4. [DOI] [PubMed] [Google Scholar]
- 28.Basu S, Hodgson G, Katz M, Dunn AR. Evaluation of role of G-CSF in the production, survival, and release of neutrophils from bone marrow into circulation. Blood. 2002;100(3):854–861. doi: 10.1182/blood.v100.3.854. [DOI] [PubMed] [Google Scholar]
- 29.Kim HK, De La Luz Sierra M, Williams CK, Gulino AV, Tosato G. G-CSF down-regulation of CXCR4 expression identified as a mechanism for mobilization of myeloid cells. Blood. 2006;108(3):812–820. doi: 10.1182/blood-2005-10-4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Semerad CL, Christopher MJ, Liu F, et al. G-CSF potently inhibits osteoblast activity and CXCL12 mRNA expression in the bone marrow. Blood. 2005;106(9):3020–3027. doi: 10.1182/blood-2004-01-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petit I, Szyper-Kravitz M, Nagler A, et al. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat Immunol. 2002;3(7):687–694. doi: 10.1038/ni813. [DOI] [PubMed] [Google Scholar]
- 32.Freedman MH, Alter BP. Risk of myelodysplastic syndrome and acute myeloid leukemia in congenital neutropenias. Semin Hematol. 2002;39(2):128–133. doi: 10.1053/shem.2002.31912. [DOI] [PubMed] [Google Scholar]
- 33.Rosenberg PS, Alter BP, Bolyard AA, et al. The incidence of leukemia and mortality from sepsis in patients with severe congenital neutropenia receiving long-term G-CSF therapy. Blood. 2006;107(12):4628–4635. doi: 10.1182/blood-2005-11-4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Margolis DM, Melnick DA, Alling DW, Gallin JI. Trimethoprim-sulfamethoxazole prophylaxis in the management of chronic granulomatous disease. J Infect Dis. 1990;162(3):723–726. doi: 10.1093/infdis/162.3.723. [DOI] [PubMed] [Google Scholar]
- 35.Martin C, Burdon PC, Bridger G, Gutierrez-Ramos JC, Williams TJ, Rankin SM. Chemokines acting via CXCR2 and CXCR4 control the release of neutrophils from the bone marrow and their return following senescence. Immunity. 2003;19(4):583–593. doi: 10.1016/s1074-7613(03)00263-2. [DOI] [PubMed] [Google Scholar]
- 36.Weisel KC, Bautz F, Seitz G, Yildirim S, Kanz L, Mohle R. Modulation of CXC chemokine receptor expression and function in human neutrophils during aging in vitro suggests a role in their clearance from circulation. Mediators Inflamm. 2009;2009:790174. doi: 10.1155/2009/790174. [DOI] [PMC free article] [PubMed] [Google Scholar]