Human cytomegalovirus (HCMV) encodes the seven transmembrane (7TM)/G-protein coupled receptor (GPCR) US28, which signals and endocytoses in a constitutive, ligand-independent manner. Here we show that, following endocytosis, US28 is targeted to the lysosomes for degradation as a consequence of its interaction with the GPCR-associated sorting protein-1 (GASP-1). We find that GASP-1 binds to US28 in vitro and that disruption of the GASP-1/US28 interaction by either (i) overexpression of dominant negative cGASP-1 or by (ii) shRNA knock-down of endogenous GASP-1 is sufficient to inhibit the lysosomal targeting of US28 and slow its post-endocytic degradation. Furthermore, we found that GASP-1 affects US28-mediated signalling. The knockdown of endogenous GASP-1 impairs the US28-mediated Gαq/PLC/inositol phosphate (IP) accumulation as well as the activation of the transcription factors Nuclear Factor –κB (NF-κB) and cyclic AMP responsive element binding protein (CREB). Overexpression of GASP-1 enhances both IP accumulation and transcription factor activity. Thus, GASP-1 is an important cellular determinant that not only regulates the post-endocytic trafficking of US28, but also regulates the signalling capacities of US28.
Cytomegaloviruses are widespread opportunistic pathogens that cause acute, latent and chronic infections. Although primary infection is asymptomatic in immunocompetent individuals, these viruses are implicated in a great variety of diseases in immunocompromised hosts (1). Human cytomegalovirus (HCMV) encodes four putative seven transmembrane (7TM) spanning/G-protein coupled receptors (GPCRs), namely US27, US28, UL33 and UL78 (2), all of which have structural homology to chemokine receptors. US28—by far the best characterized of these four receptors—has been shown to bind a wide range of endogenous chemokines. In addition, US28 is able to endocytose and signal in a constitutive, i.e. ligand-independent, manner (3–5). Although US28 may utilize arrestin for internalization (6), this receptor can be targeted to intracellular compartments in arrestin-deficient cells (7) and moreover is able to use both clathrin-dependent and independent endocytic pathways (8).
In most cell types studied so far, US28 is typically located in the membranes of intracellular organelles, especially late endosomes/lysosomes and multi-vesicular bodies (MVBs) (9), whereas only a minor fraction is found on the cell surface (5,9). The majority of receptors, however, reaches these intracellular compartments after first travelling to the cell surface (5). It has been suggested that the virions of HCMV may be assembled in these MVBs, whereby the viral receptors are incorporated into the viral membranes during the final stages of virus assembly (10,11). US28 has further been implicated to be important for the viral life cycle as it might enhance cell–cell fusion, thus promoting viral spread (12,13).
Several proteins have been identified that specifically target 7TM/GPCRs to either recycling or lysosomal pathways. One such protein is the GPCR-associated sorting protein-1 (GASP-1) (14,15). For a variety of 7TM/GPCRs it has been shown that the interaction with GASP-1 favours their targeting to the lysosomal pathway, whereas receptors that do not interact with GASP-1 recycle to the cell surface both in vitro(14,16–18)and in vivo(19,20). Moreover, GASP-1 regulates dopamine and cannabinoid (16,19,20)receptor signalling and it was recently reported that acute and sensitized cocaine locomotor effects were attenuated in GASP-1 knock-out mice (21). One study suggested the existence of nine additional GASP paralogues (15). However, except for GASP-2 (~ 150 kDa protein, 50% identity with the amino-terminal-and 68% identity with the carboxy-terminal sequence of GASP-1), none of the other eight family members has a more than 20% homology to GASP-1, and whether any of these paralogues are involved in 7TM/GPCR sorting has not been determined.
The post-endocytic sorting of 7TM/GPCRs is a fundamental process that can also control the signalling capacity of these receptors. US28 has been reported to activate downstream transcription factors such as NF-κB, cyclic AMP responsive element binding (CREB) (3,4), the nuclear factor of activated T-cells (NFAT) (22)and the serum response factor (SRF) (23). This ‘pirating’ of signalling networks and modulation of cellular functions may be an effective strategy of HCMV, and viruses in general, to evade the host immune system. Here, we show that the sorting protein GASP-1 is not only a key regulator in the post-endocytic sorting of US28, but is also crucially involved in the signalling capacities of this viral receptor.
Results
GASP-1 interacts with the cytoplasmatic portions of many 7TM/GPCRs (24). For members of the opioid (14), dopamine (18,20), bradykinin (17) and cannabinoid (16,19)receptor families, GASP-1 has been shown to not only bind but also regulate the trafficking of these receptors, suggesting that interaction with GASP-1 could facilitate degradation of diverse 7TM/GPCRs. The GASP-2 homologue displays similar binding patterns for receptors as GASP-1 (18); however, to date no functionalconsequences of this interaction have been reported.
As US28 has been reported to constitutively endocytose and sort to vesicles of the classical degradation pathway, i.e. late endosomes and lysosomes (5,9), we examined whether degradation of the virally encoded receptor US28 was modulated by GASPs.
First, we examined whether GASP-1 and GASP-2 interacted with a glutathione S-transferase (GST)-fusion protein containing the 59 amino acid carboxyterminus (C-terminus) of US28. In vitro, both GASP-1 (Figure 1A) and its homologue GASP-2 (Figure 1B) bind to the cytoplasmic tail of US28 as did the ‘degrading’ delta opioid receptor (DOP) (14), which served as positive control. In contrast, a ‘recycling’ receptor, the mu opioid receptor (MOP), did not bind to either GASP-1 or GASP-2 (14). Consistent with its ability to interact with GASP-1 and GASP-2 in vitro, US28 co-immunoprecipitated endogenous GASP-1 and GASP-2 in HEK293 cells (Figure 1C, upper panel, 1Dfor quantification). Indeed, US28 bound more GASP-1 and GASP-2 than DOP, which served as positive control (Figure 1C,D). Neither GASP-1 nor GASP-2 co-immunoprecipitated with the recycling β2-adrenergic receptor (β2AR) (18)(for graphic representation see Figure 1D), which together with HEK293 cells transfected with empty vector (pcDNA) served as the negative controls.
Figure 1.
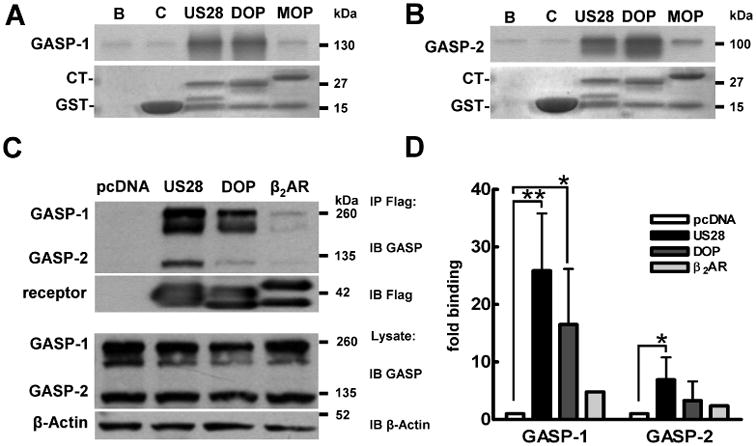
US28 interacts with GASP-1 and GASP-2 in vitro. A,B) In vitro translation assay of GASP binding. 35S-methionine-labelled recombinant GASP-1 (A) and GASP-2 (B) bind to GST-fusion proteins containing the CT of US28 and the DOP, but not the GST-protein (C), beads (B) alone or the MOP. The corresponding inputprotein levels are shown below the radiographs. Blots are representative of at least three independent experiments. C) Co-immunoprecipitation of US28 with GASP-1 and GASP-2 in HEK293 cells. HEK293 transiently transfected with pcDNA3.1 (pcDNA), Flag-US28 (US28), Flag-DOP (DOP) or Flag-β2AR (β2AR) were lysed and receptors immunoprecipitated with Flag-antibody sepharose beads (IP). Precipitates were resolved by SDS/PAGE, transferred to PVDF membranes and immunoblotted for GASP-1 and GASP-2 (first panel) and receptor levels (second panel). Lysates were probed for GASP (third panel) and β-actin (fourth panel) levels. As observed before (14), native GASP proteins (C) run at higher molecular weight levels than recombinant proteins (A and B), likely because of post-translational modifications. D) Quantification of multiple experiments performed in C. Data are means of two to four experiments ± SD, *p < 0.05, **p < 0.01.
Summarized, these data suggest that US28 directly interacts with GASP-1 and GASP-2 via its C-terminus.
GASP-1 mediates lysosomal targeting of US28
We next sought to elucidate the role of GASP-1 in the post-endocytic trafficking of US28. We have previously shown that US28 is constitutively internalized, and that the majority of the receptor accumulates in late endosomes and lysosomes (5,9). As GASP-1 has been reported to be a sorting protein that targets various 7TM/GPCRs to the lysosomal pathway, we tested whether preventing the interaction of US28 and GASP-1 could alter the post-endocytic localization of US28. We approached this question using two independent techniques. We examined the effects of (i) dominant negative cGASP-1 and of (ii) shRNA knock-down of GASP-1 on the localization of US28.
In the previous studies (14,16), the C-terminal fragment of GASP-1, corresponding to the COOH-terminal 497 residues of GASP-1 (cGASP-1), has been used as a dominant negative to compete with GASP-1 for receptor binding, thus changing thesorting of receptors targeted for degradation by GASP-1. We found that dominant negative cGASP-1 binds to the c-tail of US28 (Figure 2A) andcompetes for binding to the US28 c-tail with full-length GASP-1 (Figure 2B). To examine whether overexpression of cGASP-1 altered the trafficking of US28, we generated a cell line that stably expressed GFP-cGASP at ~ 100-fold over endogenous GASP-1 (Figures S1 and 4A). Cells overexpressing GFP-cGASP-1 and untransfected HEK293 cells were transiently transfected with Flag-US28 and ‘fed’ antibody to the extracellular Flag-tag of US28 for 45 min to monitor the constitutive endocytosis and trafficking of US28 in live cells (14,16). As has been shown previously (5), and in Figure 2C, US28 is predominantly found in lysosomes after 45 min in HEK293 cells, as evidenced by its colocalization with the lysosomal markers LAMP1 and LAMP2. In contrast, in the presence of GFP-cGASP-1, US28 undergoes a dramatic redistribution to vesicles close to the cell membrane that do not colocalize with the lysosomal markers (Figure 2D, see arrows).
Figure 2.

Post-endocytic sorting of US28 is mediated by GASP-1. A) The dominant negative cGASP-1 binds to GST-fusion proteins containing the CT of US28, the DOP, but not the GST-protein (C), beads (B) alone or the MOP. The corresponding input protein levels are shown below the radiographs. Blots are representative of at least three independent experiments. B) cGASP-1 competes with GASP-1 for the binding site on the US28-CT. MBP-US28 was incubated with increasing concentrations of purified cGASP-1 in the presence of 35S-Met-labelled recombinant GASP-1. MBP-protein was used as control (C). Molecular weight marker (S). The corresponding input protein level is shown below the radiograph. C) US28 is sorted to lysosomes. HEK293 cells transiently transfected with Flag-US28 were ‘fed’ antibody to the extracellular Flag-tag for 45 min, then fixed and permeabilized. Receptors (green) were analysed for colocalization with the lysosomal markers LAMP1 and LAMP2 (red), merge: yellow. D) cGASP-1 alters the localization of US28. Antibody feeding experiments in HEK293 cells stably expressing GFP-cGASP-1 (see insert) and transiently expressing Flag-US28 revealed a redistribution of the receptor to vesicles close to the cell surface (see arrows). Green: Flag-US28, red: LAMP1/2, merge: yellow. E) Knock-down of endogenous GASP-1 in HEK293 cells using a shRNA-lentivirus. HEK293 cells were infected with either shGASP-1 or shScr virus. Forty-eight hours post-infection, cells were lysed, separated by SDS/PAGE and immunoblotted for GASP-1. β-actin staining served as loading control (lower panel). GASP-2 levels remained unchanged in both shScr and shGASP-1 infected cells (see 130-kDa band). F) EGF receptor degradation is not impaired by shGASP-1 knock-down. HEK293 cells infected with shScr or shGASP-1 virus were transiently transfected with Flag-US28 and either left untreated or incubated with EGF (5 μm) for 3 h. Lysates were analysed by SDS/PAGE and immunoblotted for endogenously expressed EGF receptor (first panel, EGFR), GASP-1 and GASP-2, US28 and β-actin (lower panels). G,H) shRNA knock-down of GASP-1 prevents lysosomal targeting of US28. HEK293 cells were infected with either shScr virus (G) or shGASP-1 virus (H) and transfected with Flag-US28. Antibody feeding experiments showed that US28 colocalizes with LAMP1 and LAMP2 in cells infected with scrambled virus (G), whereas the receptor is redistributed to vesicles close to the cell surface in cells infected with shGASP-1 (H, see arrows). Green: Flag-US28, red: LAMP1/2, merge: yellow, insert: EGFP-shRNA-virus. Scale bars = 10 μm.
Figure 4.
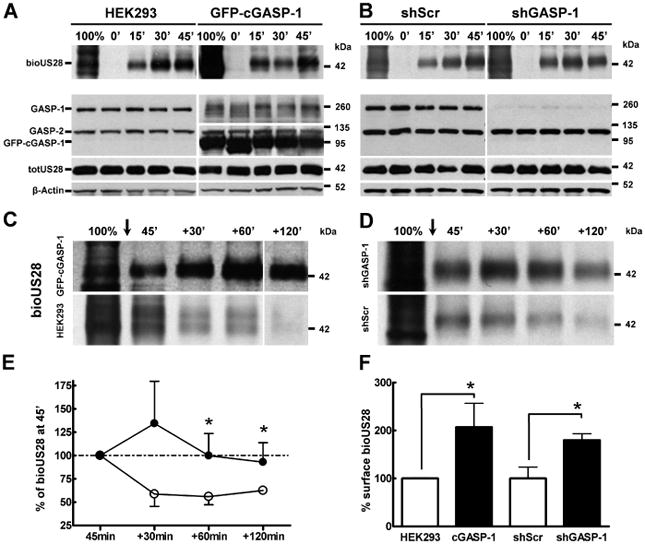
Disrupting the GASP-1/US28 interaction does not affect constitutive internalization of US28, but delays receptor degradation. A) Overexpression of GFP-cGASP-1 does not affect receptor internalization. HEK293 cells (upper left panel) or cells stably expressing GFP-cGASP-1 (upper right panel) were transiently transfected with Flag-US28. Internalization of biotinylated Flag-US28 (bioUS28) was monitored at the time-points indicated, before cells were stripped of surface-biotin. The total amount of biotinylated proteins before the strip is shown by 100%. The lower panels show the corresponding lysate samples immunoblotted for GASP, total receptor (totUS28) and β-actin. B) Disrupting the GASP-1/US28 interaction by shRNA does not affect receptor internalization. HEK293 cells were infected with shScr (upper left panel) or shGASP-1 virus (upper right panel) for 48 h and transiently transfected with Flag-US28. Internalization of biotinylated Flag-US28 (bioUS28) was monitored as described in A. Disrupting the GASP-1/US28 interaction delays receptor degradation. C) Overexpression of GFP-cGASP-1 delays US28 degradation. HEK293 cells stably expressing GFP-cGASP-1 (upper panel) or control cells (lower panel) were transiently transfected with Flag-US28. Biotinylated Flag-US28 (bioUS28) was allowed to constitutively internalize for 45 min (see A and B), then cells where stripped of surface-biotin (arrow). Thus, 45′ indicates the internalized US28 receptor pool at 45 min. Subsequently, the degradation of US28 was monitored for additional 120 min. D) shRNA knock-down of GASP-1 delays US28 degradation. HEK293 cells were infected with shGASP-1 (upper panel) or shScr virus (lower panel) for 48 h and transiently transfected with Flag-US28. Biotinylation assays were performed as described in C. E) Quantification of biotinylation assay corresponding to D. Biotinylated receptor bands in shScr (○) or shGASP-1 (●) cells were normalized to the total US28 receptor pool and β-actin and the 45′ value was set to 100%. Data are means of five experiments ± SD, *p < 0.05. F) Quantification of surface-biotinylated US28. Biotinylated surface receptor bands (100% lanes) were normalized to the total US28 (totUS28) receptor pool and quantified with ImageJ software. Control cells (white bars) were set to 100%. In both cGASP-1 and shGASP-1 expressing cells (black bars), surface bioUS28 was significantly higher than in control cells (white bars). Data are means of two to three experiments ± SD, *p < 0.05.
To further address the role of GASP-1 in the lysosomal targeting of US28, we developed shRNAs that knock down the expression of GASP-1. Lentiviral infection of HEK293 cells with shRNA targeting GASP-1 (Figure 2E, shGASP-1) for 48 h showed an efficient knock-down of GASP-1 protein levels, while scrambled shRNA virus had no effect on GASP-1 levels (Figure 2E, shScr). In both, shScr and shGASP-1 infected cells, GASP-2 levels remained unchanged (Figure 2E, see 130-kDa band). US28 colocalized with LAMP positive endosomes in cells infected with shScr virus (Figure 2G). In contrast, in cells infected with the shGASP-1 virus, US28 showed a prominent redistribution to vesicles close to the cell membrane (Figure 2H, white arrows), with a close resemblance to the receptor localization observed in cells overexpressing cGASP-1 (compare Figure 2D and 2H, respectively), and no colocalization with lysosomes (Figure 2H, red).
To rule out the possibility that shRNA knock-down of GASP-1 exerts off-target effects on lysosomal function per se, we examined whether ligand-induced proteolysis of the epidermal growth factor receptor (EGFR) was intact in cells infected with shScr or shGASP-1 virus. EGFR is a receptor tyrosine kinase endogenously expressed in HEK293 cells and traffics to lysosomes after prolonged agonist treatment (25). Moreover, we have previously shown that the expression of the dominant negative cGASP-1 did not interfere with EGFR degradation in HEK293 cells (14), suggesting that its degradation is not GASP-1 dependent. Indeed, the degradation of EGFR was not impaired in cells depleted of GASP-1 by shRNA (Figure 2F, top panel). Furthermore, the knock-down of GASP-1 does not alter the expression levels of US28 (Figure 2F, 42-kDa bands).
In order to identify the vesicles to which US28 is redistributed in cells overexpressing cGASP-1 (Figure 2D) and in shGASP-1 infected cells (Figure 2H) we tested whether these vesicles belonged to the early endosomes, defined by colocalization with the early endosomal antigen 1 (EEA.1) or to the recycling endsomal pathway defined as colocalization with transferrin.
US28 partially colocalizes with EEA.1 in HEK293 cells (Figure 3A, see insert). However, in cells overexpressing GFP-cGASP-1 (Figure 3B, insert), no colocalization with EEA.1 was detectable in the US28-containing vesicles (Figure 3B, arrows). Similarly, US28 colocalizes with EEA.1 in shScr infected cells (Figure 3C, insert), whereas US28 vesicles did not overlap with EEA.1 in shGASP-1 infected cells (Figure 3D, arrows). Hence, the small US28 receptor pool detectable in early endosomes (Figure 3A,C) seems to be dependent on the presence of GASP-1.
Figure 3.
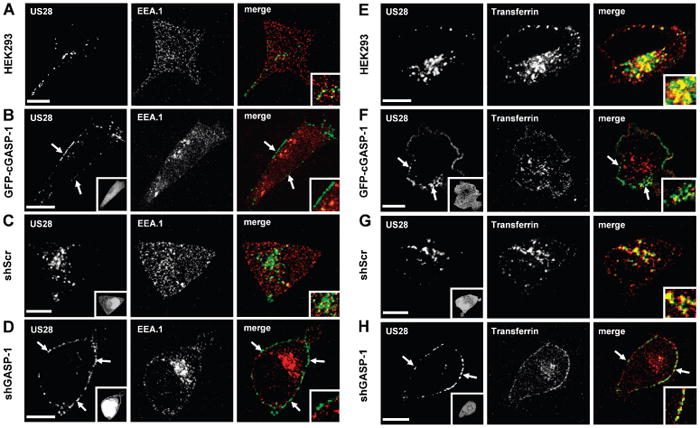
Colocalization of US28 with the endosomal markers EEA.1 and transferrin. A) Colocalization of US28 with EEA.1. HEK293 cells transiently transfected with Flag-US28 were ‘fed’ antibody to the extracellular Flag-tag for 45 min, then fixed and permeabilized. Receptors (green) were analysed for colocalization with the early endosomal marker EEA.1 (red), merge: yellow. B) US28 does not colocalize with EEA.1 in cGASP-1-overexpressing cells. Antibody feeding experiments in HEK293 cells stably expressing GFP-cGASP-1 and transiently expressing Flag-US28 revealed no colocalization of the receptors with EEA.1. Green: Flag-US28, red: EEA.1, insert: GFP-cGASP-1. C,D) Colocalization of US28 with EEA.1 in shScr and shGASP-1 infected cells. HEK293 cells were infected with either shScr virus (C) or the shGASP-1 virus (D) and transfected with Flag-US28. Antibody feeding experiments showed that US28 partially colocalizes with EEA.1 in cellsinfected with scrambled virus (C), whereas there was no colocalization detectable in cells infected with shGASP-1 (D). Green: Flag-US28, red: EEA.1, merge: yellow, insert: EGFP-shRNA-virus. E) US28 colocalizes with the endosomal marker transferrin. Antibody feeding experiments in HEK293 cells transiently transfected with Flag-US28 were conducted in the presence of AlexaFluor 594-conjugated transferrin (for details see Methods). Receptors (green) were analysed for colocalization with the endosomal marker transferrin (red), merge: yellow. F) US28 colocalizes with transferrin in GFP-cGASP-1-overexpressing cells. Antibody feeding experiments in HEK293 cells stably expressing GFP-cGASP-1 and transiently expressing Flag-US28. Green: Flag-US28, red: transferrin, merge: yellow, insert: GFP-cGASP-1. G,H) Colocalization of US28 with transferrin in shScr and shGASP-1 infected cells. HEK293 cells were transduced with either shScr virus (G) or the shGASP-1 virus (H) and transfected with Flag-US28. Antibody feeding experiments showed that US28 partially colocalizes with transferrin in cells infected with scrambled virus (G) as well as in cells infected with shGASP-1 (H). Green: Flag-US28, red: transferrin, merge: yellow, insert: EGFP-shRNA-virus. Scale bars = 10 μm.
It was previously reported that a fraction of the US28 receptor pool is found in early recycling endosomes ((5,9)and Figure 3E,G). Hence, we tested whether the US28-containing vesicles in cells overexpressing GFP-cGASP-1 or devoid of GASP-1 colocalize with the recycling endosomal marker transferrin. Indeed, we observe a colocalization with transferrin in both, GFP-cGASP1 (Figure 3F, arrows and insert) and shGASP-1 (Figure 3H, arrows and insert) infected cells.
Together, these results suggest that GASP-1 is a key mediator in targeting US28 to lysosomes, because the disruption of GASP-1/US28 interaction prevents the sorting of US28 tolysosomes.
GASP-1 mediates degradation, but not the constitutive internalization of US28
In order to quantitatively monitor the endocytic and post-endocytic fate of US28 in the absence or presence of GASP-1, we next performed biotin protection and degradation assays. This assay allows selective monitoring of the endocytic-and post-endocytic fate of a pool of receptors that reaches the cell surface and is subsequently endocytosed and either recycled or degraded [see also (14,16,20)].
Although our antibody feeding experiments suggest that US28 constitutively endocytoses in the absence of GASP-1, we wanted to test whether GASP-1alters the kinetics of US28 internalization in either GFP-cGASP-1 overexpressing (Figure 4A) or in shGASP-1 (Figure 4B) infected cells. To this end, receptors that reached the cell surface of intact cells were labelled with thio-cleavable disulfide-linked biotin for 30 min in the cold to prevent internalization (corresponding to 100% in Figures 4A,B). Cells were then returned to warm media, and the biotinylated receptor pool was allowed to constitutively internalize for up to 45 min. Following this incubation, biotin on cell surface receptors was cleaved with a membrane impermeable reducing agent. The internalized ‘protected’ pool of receptors was then immunoprecipitated and the remaining biotinylated receptor was visualized by streptavidin overlay (see Methods for details). Figure 4A,B(top panels) shows the increasing amount of internalized receptor (bioUS28) during a time–course from 0 to 45 min in HEK293 cells, cells stably overexpressing GFP-cGASP-1 (Figure 4A) and in cells infected with shScr versus shGASP-1 (Figure 4B). US28 internalizes with similar kinetics in all conditions tested. Lysate samples were blotted for the expression of GASP-1, GASP-2, GFP-cGASP-1, Flag-US28 and β-actin (Figure 4A,B, lower panels). These data show that GASP-1 levels do not interfere with the constitutive internalization kinetics of US28. However, we consistently observed a higher amount of biotinylated surface receptor in cells overexpressing GFP-cGASP-1 or in shGASP-1 cells (compare 100% lanes in Figure 4A,B, for quantification see Figure 4F).
Next, if GASP-1 were mediating the degradation of US28, one would expect that either the overexpression of cGASP-1 or the knock-down of endogenous GASP-1 levels could delay or even inhibit receptor degradation. Hence, we next monitored the stability of the biotinylated and constitutively endocytosed US28 receptor pool. After biotinylation and 45 min in prewarmed media to allow a ‘pulse’ of receptor to be endocytosed, surface-biotin was stripped and cells were incubated or ‘chased’ for an additional 30, 60 or 120 min (see +30, +60 and +120 min lanes in Figure 4C,D) to monitor the stability of the protected pool. At the end of the experiment, US28 receptors were immunoprecipitated and remaining biotinylated receptor was visualized by streptavidin overlay.
In cells overexpressing GFP-cGASP-1 ~ 100-fold (see Figure S1), the degradation of biotinylated and internalized US28 was delayed (Figure 4C, upper panel) when compared to control cells (Figure 4C, lower panel). Likewise, in HEK293 cells infected with shGASP-1 lentivirus (Figure 4D, upper panel), internalized and biotinylated US28 receptor was also significantly more stable than in cells infected with shScr (Figure 4D, lower panel, compare also shGASP-1 (●) and shScr (○) in Figure 4E).
Taken together, these results indicate that the interaction between US28 and GASP-1 is crucial for the sorting of the receptor to the degradative pathway.
US28 recycles in the absence of GASP-1
We next examined whether disruption of the US28 GASP-1 interaction not only blocked receptor degradation but also facilitated recycling. Because US28 has not only been reported to constitutively internalize, but also constitutively recycle in some circumstances [(9)and Figure 3E–H], we anticipated this might be the case.
To assess recycling, cells transiently transfected with Flag-US28 alone (Figure 5A) and overexpressing GFP-cGASP-1 (Figure 5B) were fed M1-antibody for 45 min to allow for receptor internalization. Then cells were stripped of the M1-antibody (Figure 5A,B, strip) and resurfacing (recycling) of the receptors was monitored for 15 and 30 min. Cells were then fixed and the recycled receptor was detected by staining with a fluorescent antibody targeting the M1-antibody under non-permeabilizing conditions. Control cells show weak surface staining of US28 (Figure 5A,C, 45 min), which is consistent with ~ 20% of surface expression of the total US28 receptor pool reported previously (5,9). Nevertheless, recycling US28 was detected as early as 15 min after stripping (Figure 5A,C, +15 min panel). However, in cells overexpressing GFP-cGASP-1 (Figure 5B, 45 min) or shGASP-1 cells (Figure 5D, 45 min), significantly more US28 surface staining was visible than in control cells. Similarly to the control cells, recycling US28 was detectable in cells as early as 15 min after stripping (Figure 5B,D, +15 min panels) and US28 surface staining was detectable up to +30 min. These data are also consistent with the increased surface expression of US28 in shGASP-1 and GFP-cGASP-1 overexpressing cells detected in the biotinylation assays (see Figure 4F).
Figure 5.
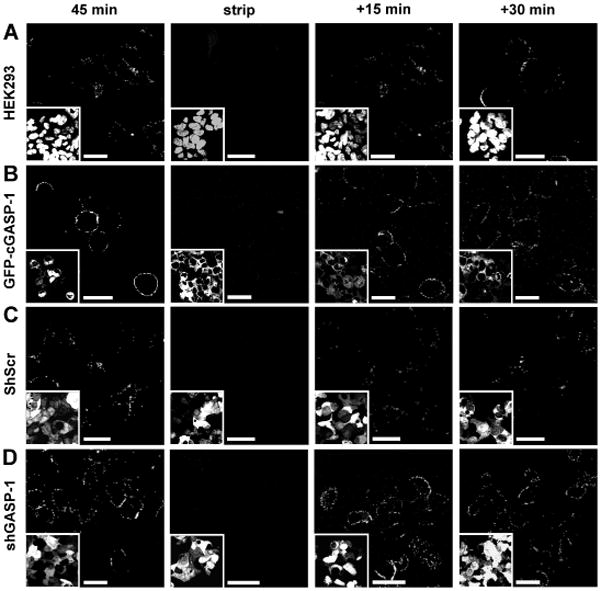
Constitutive recycling of US28 is not dependent on GASP-1. A,B) Recycling of US28 is not impaired in cells overexpressing GFP-cGASP-1. HEK293 cells (A) or cells stably expressing GFP-cGASP-1 (B) transiently transfected with Flag-US28 were ‘fed’ a Ca2+ –sensitive antibody to the extracellular Flag-tag for 45 min. Then the cells were stripped with PBS–EDTA (strip) and warm growth medium was added. After 15 or 30 min the cells were fixed and the recycled receptor was stained under non-permeabilizing conditions. Inserts: DAPI (A) and GFP-cGASP-1 (B). C,D) Recycling of US28 is not impaired in shScr or shGASP-1 infected cells. HEK293 cells infected with shScr virus (C) or shGASP-1 virus (D) were transiently transfected with Flag-US28. Recycling experiments were performed as in A and B. Inserts: GFP-shRNA lentivirus. Scale bars = 20 μm.
GASP-1 alters the constitutiveGαq-mediated signalling capacity of US28
The sorting of individual receptors between recycling and degradative pathways is a fundamental process that can regulate the signalling capacity of a receptor. Hence, we set out to elucidate the implications of the GASP-1-mediated post-endocytic sorting on the signalling capacity of US28.
US28 has been shown to regulate the Gαq/phospholipase C (PLC) pathway both constitutively and in a ligand-dependent manner (3,4,26,27). Here we set out to examine the ability of US28 to mediate the Gαq/PLC-dependent accumulation of inositol phosphate (IP) in cells where the interaction with GASP-1 had been altered. As we found that GASP-1 facilitates the degradation of US28 after endocytosis, we anticipated that disruption of the US28/GASP-1 interaction might enhance constitutive signalling of US28. Surprisingly, an ~ 0.5-fold overexpression of GASP-1 (Figure 6B, upper panel) significantly increased the IP turnover by US28 (Figure 6A; compare black versus striped bars). In contrast, overexpression of cGASP-1 (Figure 6B, lower panel, and Figure S2B) significantly reduced the IP turnover (Figure 6A; compare black versus grey bars). These effects were not attributable to different total expression levels of US28 in the respective assays, as verified by enzyme-linked immunosorbent assay (ELISA) (Figure 6C). Next, we examined the IP turnover in cells in which GASP-1 has been knocked down with the shGASP-1 virus. In agreement with our previous findings, the IP turnover was significantly reduced in shGASP-1 infected cells (Figure 6D, compare black versus striped bar).
Figure 6.
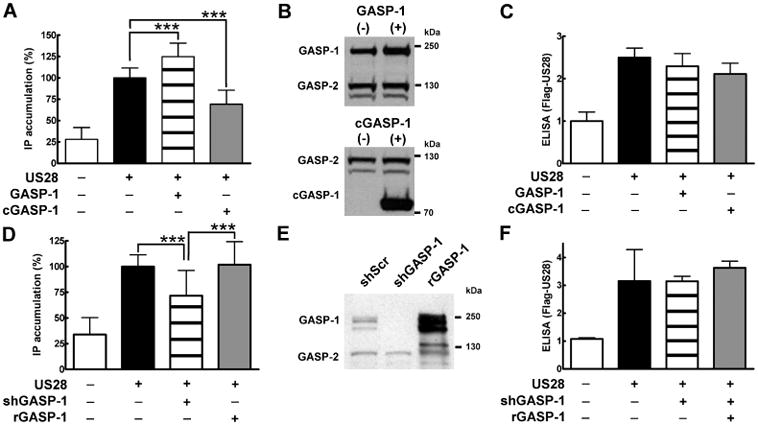
GASP-1 modulates US28-mediated IP accumulation. A) Overexpression of GASP-1 and cGASP-1 alters US28-mediated IP turnover. HEK293 cells were transiently transfected with Flag-US28 (75 ng/well) and 100 ng/well of either pcDNA3.1 (black bar), HA-GASP-1 (striped bar) or HA-cGASP-1 (grey bar). Controls were transfected with pcDNA3.1 (175 ng/well, white bar). IP production mediated by US28 was allowed to accumulate for 45 min. Values were normalized to the cell number, and 100% corresponds to the basal activity of US28 (black bar). Data are means of six experiments ± SD carried out in quadruplicates, ***p < 0.001. B) Expression levels of HA-GASP-1 and HA-cGASP-1 in HEK293-US28 cells. HEK293 cells were transiently transfected with Flag-US28 in combination with pcDNA3.1 and HA-GASP-1 (upper panel) or with HA-cGASP-1 (lower panel). The cells were lysed, separated by SDS/PAGE and blotted with GASP antibody after 24 h. Overexpression of HA-GASP-1 or HA-cGASP-1 does not alter GASP-2 levels. C) US28 levels in HEK293 cells overexpressing GASP-1 and cGASP-1. HEK293 cells were transfected as in A. The expression levels of US28 were assayed by ELISA against the Flag-epitope tag of US28. Values were normalized to the cell number. Data are means ± SD of one representative experiment carried out in quadruplicates. The control (white bar) was set to one and the other values were normalized accordingly. D) Reduced IP accumulation in shGASP-1 infectedcells is restored by overexpression of rGASP-1. HEK293 cells were infected with shScr (black bar) or the shGASP-1 virus (striped bar and grey bar). Then cells were transiently transfected with Flag-US28 (75 ng/well) and either 100 ng/well pcDNA3.1 (black and striped bars) or 100 ng/well HA-rGASP-1 (grey bar). Controls were transfected with pcDNA3.1 (175 ng/well, white bar). The IP accumulation assay was conducted as described in A. Values were normalized to the cell number, and 100% corresponds to the basal activity of US28 in shScr infected cells (black bar). Data are means of four experiments ± SD carried out in quadruplicates, ***p < 0.001. E) GASP-1 levels in shGASP-1 infected cells can be restored with HA-rGASP-1. ShScr infected cells were transfected with Flag-US28 and pcDNA3.1. shGASP-1 infected cells were transfected with either pcDNA3.1 or HA-rGASP-1. The cells were lysed, separated by SDS/PAGE and blotted with the GASP antibody after 24 h. Blot shows one representative of at least three independentexperiments. F) US28 levels in shScr and shGASP-1 infected cells. HEK293 cells were infected and transfected (see D) and ELISA was performed as described in C.
To confirm that the decreased signalling in shGASP-1 infected cells was because of the knock-down of GASP-1, we conducted rescue experiments with a shRNA-insensitive r(escue)GASP-1 (for details see Methods). Transfection of shGASP-1 infected cells with rGASP-1 resulted in a replenished GASP-1 pool (Figures 6E and S2B, compare grey versus black bars). Furthermore, we confirmed that rGASP-1 bound to the C-terminus of US28 in vitro (Figure S3). Indeed, as Figure 6D (compare striped versus grey bars) shows, the decreased IP turnover in shGASP-1 infected cells could be restored by the re-expression of rGASP-1. US28 receptor levels were comparable in all assays (Figure 6F).
In summary, these data show that GASP-1 modulates the constitutive Gαq/PLC/IP turnover mediated by US28.
The activation of transcription factors by US28 is impaired in cells deprived of GASP-1
US28 has been reported to activate downstream transcription factors such as NF-κB, CREB (3,4,28), NFAT (22) and SRF (23). Following our findings that GASP-1 levels alter the ability of US28 to activate the Gαq/PLC/IP pathway, we examined whether this effect would also translate to an altered downstream signal transduction profile. Therefore, we tested the impact of altered GASP-1 levels on the US28-mediated activation of the transcription factors NF-κB and CREB. Indeed, transient expression of US28 and overexpression of GASP-1 in HEK293 cells resulted in a significant increase of both NF-κB and CREB-luciferase activity (Figure 7A,B, respectively, compare black and striped bars). Next, we disrupted the interaction of US28 and endogenous GASP-1 by overexpression of cGASP-1. cGASP-1 blocked the downstream activation of both NF-κB and CREB-luciferase activity (Figure 7A,B, compare black versus grey bars). Receptor expression levels were similar in all experiments (Figure 7C,D).
Figure 7.
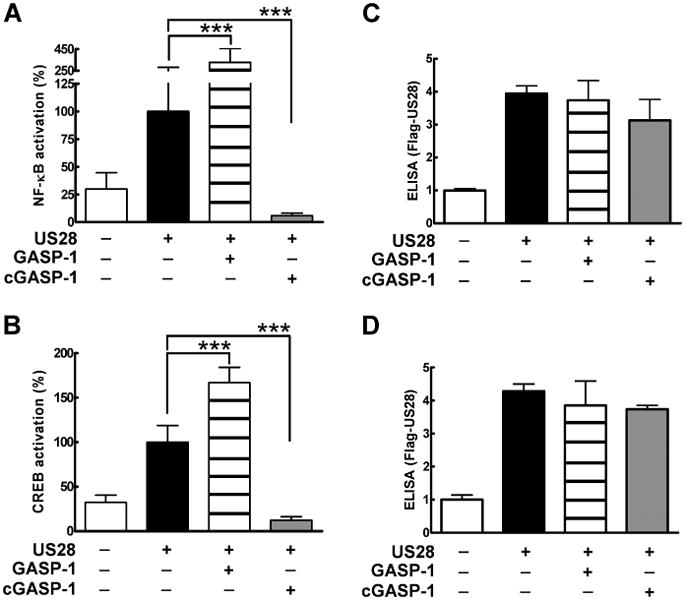
US28-mediated NF-κB and CREB transcription is modulated by GASP-1. A,B) US28-mediated transcription factor activation is altered upon overexpression of GASP-1 and cGASP-1. HEK293 cells were transiently transfected with Flag-US28 (50 ng/well) in combination with 100 ng/well pcDNA3.1 (black bar), HA-GASP-1 (striped bars) or HA-cGASP-1 (grey bars) and the respective cis-reporter luciferase plasmid for either NF-κB (50 ng/well, A) or CREB (200 ng/well, B). Controls were transfected with pcDNA3.1 (white bars). A luciferase reporter assay was conducted 24 h post-transfection. Values were normalized to the cell number, whereby 100% corresponds to the basal activity of US28 (black bar). Data are means of four experiments ± SD carried out in quadruplicates, ***p < 0.001. C, D) US28 levels in HEK293 cells overexpressing GASP-1 and cGASP-1. HEK293 cells were transiently transfected as described in A and B. The expression levels of US28 were assayed by ELISA as described before.
To further test the hypothesis that GASP-1 is involved in the regulation of the downstream signal transduction pathways of US28, we performed the NF-κB and CREB-luciferase assays in cells infected with the shGASP-1 and shScr virus. Indeed, the activation of transcription factors is significantly decreased in shGASP-1 infected cells in comparison to shScr infected cells (Figure 8A,B, compare black bars versus grey bars). To confirm that the reduced NF-κB and CREB activation in shGASP-1 infected cells was because of the knock-down of GASP-1, we reintroduced GASP-1 into shGASP-1 cells by transfection with rGASP-1. Again, rGASP-1 ‘rescued’ the activation of transcription factors via US28 in shGASP-1 infected cells (Figure 8A,B, compare grey versus striped bars). Receptor expression levels were similar in all experiments (Figure 8C,D).
Figure 8.

Reduced NF-κB and CREB activation can be restored by overexpression of rGASP-1. A,B) US28-mediated transcription factor activation is rescued upon overexpression of rGASP-1. ShScr infected cells (black bars) and shGASP-1 infected cells (grey and striped bars) were transfected with Flag-US28 (50 ng/well) in combination with 100 ng/well of pcDNA3.1 (black bar and grey bars) or HA-rGASP-1 (striped bars) as well as the respective cis-reporter plasmids for either NF-κB (50 ng/well, A) or CREB (200 ng/well, B). Controls were transfected with pcDNA3.1 (white bars). A luciferase reporter assay was conducted 24 h post-transfection. Values were normalized to the cell number, whereby 100% corresponds to the basal activity of US28 (black bar). Data are means of four experiments ± SD carried out in quadruplicates, ***p < 0.001. C,D) US28 levels in shGASP-1 infected and rGASP-1 transfected HEK 293 cells. HEK293 cells were infected and transiently transfected as described in A and B. The expression levels of US28 were assayed by ELISA as described before.
In summary, our data suggest that GASP-1 not only modulates the ability of US28 to activate Gαq/PLC/IP-dependent signalling, but is also a crucial component in activating further downstream signalling events, such as the transcription factors NF-κB and CREB.
Discussion
Here we show that the sorting protein GASP-1 is an important cellular determinant in regulating the post-endocytic trafficking and constitutive signalling capacity of the viral receptor US28. Our data suggest that US28 is targeted for degradation via a direct protein–protein interaction with the sorting protein GASP-1 (Figure 1). Disruption of this process results in a vesicular redistribution of US28 close to the plasma membrane (Figure 2D,H). These vesicles colocalize with transferrin (Figure 3F,H) but do not co-stain for the early endosomal marker EEA.1 (Figure 3B,D). Importantly, the constitutive internalization kinetics of US28 are not altered when the interaction of US28 and GASP-1 is disrupted (Figure 4A,B). The degradation of US28 is significantly delayed in cells knocked down for GASP-1 expression (Figure 4D,E) and also slowed in cells overexpressing the dominant negative cGASP-1 (Figure 4C). In addition, recycling of US28 is enhanced when the US28–GASP-1 interaction is disrupted (Figure 5) and results in a higher surface expression of US28 in these cells (Figure 4F).
In addition, we show that GASP-1 is an important regulator of signalling pathways activated by US28. US28 has previously been reported to constitutively activate the Gαq/PLC/IP pathway and the transcription factors NF-κB and CREB (3,4,28). Here we show that GASP-1 is crucial for US28-mediated IP production, i.e. overexpression of GASP-1 enhances IP turnover, whereas competition with cGASP-1 (Figure 6A) or knock-down of GASP-1 expression (Figure 6D) decreases IP accumulation. In addition, the activation of the transcription factors NF-κB and CREB via US28 is also dependent on GASP-1 levels (see Figures 7 and 8). Hence, GASP-1 is a key component in the regulation of both, the post-endocytic sorting and signalling properties of US28.
As mentioned before, GASP-2 is the closest homologue to GASP-1, and although the C-terminus of US28 binds GASP-2 in vitro (Figure 1B), we do not observe any regulatory and/or compensatory effects of GASP-2 in any of our functional assays. GASP-2 levels remain unchanged in cells either infected with shGASP-1 lentiviruses (see Figure 2E) or when co-transfected with cGASP-1 (Figure 4A, 95-kDa marker, or Figure 6B, lower panel).
The sorting of individual receptors to either the recycling or degradation pathways is a tightly regulated process that is of fundamental importance for the regulation of 7TM/GPCR signalling. It is well established that 7TM/GPCRs mainly transmit their signals through G-proteins from the cell surface. Following their activation, GPCRs are often desensitized and then endocytosed. After endocytosis, receptors can be either recycled to the plasma membrane or targeted to lysosomes for degradation. However, there is accumulating evidence that the relationship between receptor trafficking and signalling is more complex than this. In particular, endosomes have been implicated as important sites for receptor-initiated signalling, as they contain a wide range of signalling molecules in their membranes and are able to recruit scaffolding proteins and signalling mediators. Furthermore, recent findings indicate that, in some cases, endocytosis might even be required as it can enhance receptor-mediated signalling for some pathways [for reviews see (29–31)].
Our data suggest that the sorting protein GASP-1 is crucial for the constitutive signalling activity of US28. Hence, GASP-1 may be one such ‘signal mediator’ for US28 once it is present in early sorting endosomes. We can only speculate on the reasons for the decreased ability of US28 to activate Gαq/PLC-mediated signalling in cells where the US28–GASP-1 interaction is disrupted (see Figure 6). One possible explanation would be the changed localization of the receptor in the absence of GASP-1 (see Figures 2 and 3). If US28 transmits its signals from the cell surface, this process might be disturbed when the receptor is localized in vesicles close to the cell membrane, such as that observed in cells where GASP-1 expression has been knocked down or in cells overexpressing the dominant negative cGASP-1. For instance, US28 cannot be found in EEA.1 positive early endosomes in cells devoid of GASP-1 or in the presence of dominant negative cGASP-1 (Figure 3); hence, one might speculate that these vesicles represent the ‘signalosome’ for US28. On the other hand, overexpression of GASP-1 appears to upregulate signalling from US28, suggesting that GASP-1 plays a direct role in signal transduction. Thus, one could speculate that GASP-1 enhances signalling because of a conformational change of the receptor to a more active state upon interaction with GASP-1. But likewise, it could be possible that GASP-1 is necessary for targeting the receptor to a particular compartment for signal transduction, especially because only a minor fraction of this receptor is typically found on the cell surface (5,9). Importantly, a recent report shows that HCMV acquires its final envelopment on cytoplasmic membranes containing, among others, endosomal markers such as EEA.1, transferrin and the closely related UL33 receptor (11). Hence, GASP-1 may target the viral receptors to endosomes necessary for virion formation. Further experiments will be necessary to determine this.
Such interplay between the sorting of US28 and its constitutive signalling capacity might also be of clinical relevance. Human cytomegalovirus is a widespread pathogen that has been shown to be present in various malignancies, including breast cancer (32), colon cancer (33)or malignant glioblastoma (34), and US28 was recently shown to induce tumours in an NIH3T3 nude mouse model (35). Furthermore, US28 is able to upregulate several proteins involved in oncogenesis, especially cyclooxygenase-2 (COX-2) a transcriptional target of NF-κB, suggesting a possible role for US28 in tumorigenesis (36). By identifying the sorting protein GASP-1 as a key regulator of not only the trafficking, but also, importantly, the signalling of US28, we may be one step closer to gaining a better understanding of this viral receptor and its significance in the pathogenesis implicated by HCMV.
Materials and Methods
Mouse M1 and M2 monoclonal antibody, ANTI-Flag M2 Affinity Gel, Albumin from bovine serum, l-Glutathione, Iodoacetamide, gelatine from bovine skin Type B, Triton X-100, Kodak BioMax light films, 3, 3′, 5, 5′-Tetramethylbenzidine Liquid substrate system and Tween-20 were purchased from Sigma Aldrich. Tris–Glycine gradientgels (4–20%), cell culture media (DMEM), foetal bovine serum (FBS), Dulbeccos phosphate-buffered saline (DPBS), ViraPowerTM Lentiviral Expression Systems and Lipofectamine 2000, Alexa Fluor 647 nm conjugated IgG2b, 594 nm conjugated IgG1a, 594 nm conjugated IgG2b, 594 nm conjugated donkey anti-rabbit IgG and 594 nm conjugated transferrin were purchased from Invitrogen. QuickTiterTM Lentivirus Quantification Kit (HIV p24 ELISA) was purchased from Cell Biolabs (THP). Horseradishperoxidase (HRP)-conjugated anti-rabbit antibody and HRP-conjugated anti-mouse antibody were from Jackson Immuno Research (Dianova). Generation of anti-GASP1 antibodies has been described previously (14). Mouse monoclonal LAMP1 and LAMP2 antibodies were from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by the University of Iowa, Department of Biological Sciences, Iowa City, IA 52242. Complete Protease inhibitor cocktail tablets were from Roche. Immobilon-P Transfer Membrane was purchased from Millipore. Enhanced chemiluminescence (ECL) Western Blotting Substrate was from Pierce (THP). Vectashield mounting medium and Vectastain ABC Kit were purchased from Vector Laboratories (Szabo-Scandic). The white 96-well culture plates and the Steadylite Plus Assay Kit, high-sensitivity luminescence reporter gene assay system, [3H]myo-inositol and EasyTag 35S-methionine were purchased from PerkinElmer. Yttrium silicate (YSi) SPA beads were from GE Healthcare, EZ-Link Sulfo-NHS-SS-Biotin was from Thermo Scientific (Histocom). NaOH, KCl and CaCl2* 2H2O were obtained from Merck, NaCl, Tris andFormaldehyde were from Roth (Lactan). TNT T7 Coupled Reticulocyte Lysate System were purchased from Promega and PAGEBlue from Fermentas. Goat antibodies to EGFR and HRP-conjugated secondary anti-goat antibody were from Santa Cruz Biotechnology (Szabo-Scandic).
Polyclonal rabbit anti-EEA.1 was purchased from Calbiochem (Merck). Human EGF was from Peprotech.
G418 sulphate solution was purchased from PAA Laboratories.
DNA constructs
A Flag-tagged version of US28 (Flag-US28) was cloned by using the BamHI and XbaI restriction sites in the SS-Flag pcDNA3.1 zeo (+) vector (16). The cloning of HA-tagged GASP-1 (HA-GASP-1 ), cGASP-1 (HA-cGASP-1), GFP-tagged cGASP-1 (GFP-cGASP-1), the Flag-DOP-R and Flag-β2AR were described previously (14,18). Oligonucleotides for shRNA were constructed using AAG(N18)TT criteria to select an appropriate sequence. The oligonucleotides were annealed and cloned into the lentilox vector pLL3.7 (ATCC) using the XhoI and HpaI restriction sites. The target sequence for GASP-1 (GTACCTGTCTGAGG ATAGA) and the scrambled shRNA (GTTAAAGGTTCGGCACGAA) were synthesized by Operon. Lentiviruses encoding for either shRNA against GASP-1 (shGASP-1) or scrambled shRNA (shScr) were produced in HEK293FT cells. The generation of the GST-fusion proteinconstructs containing the 59 carboxy-terminal amino acids of US28 (GST-US28) (24), the carboxy-terminal amino acids of the delta opioid receptor (GST-DOP), the mu opioid receptor (GST-MOP) and the carboxy-terminal part of GASP-1 (GST-cGASP-1) were described elsewhere (14). The maltose binding (MBP) fusion protein MBP-US28 was generated by subcloning the 59 C-terminal amino acids of US28 into the pMALc2 expression vector (New England Biolabs) using the BamHI and Xho I restriction sites. For rescue experiments we designed a rescue full-length GASP-1 (rGASP-1). To inactivate the shRNA recognition site we performed targeted mutagenesis (QuikChange Site-Directed Mutagenesis Kit, Stratagene) according to the instruction manual. Three point mutations were introduced without altering the protein sequence by the following oligonucleotide primers (mutations are bold and underlined): F-ctgtcttggttgctaaaacaaagtatctgtccgag gacagagaactggttaatac, R-gtattaaccagttctctgtcctcgg acagatactttgttttagcaaccaagacag. All DNA constructs were verified by sequencing.
GST-fusion protein-binding assay
GST-fusion protein-binding assays were performed essentially as described in (14,24). In brief, GST-fusion proteins were expressed in XL BLUE Top 10 Escherichia coli and bound to glutathione-agarose. Fusion proteins on beads (~ 3 μg) were incubated in blocking buffer (20 mm Hepes, pH 7.4, 100 mm NaCl, 2 mmMgCl2, 0.1% Triton X-100, 5% BSA) overnight at 4°C. Subsequently, fusion proteins were incubated with 2 μL of in vitro translated 35S-methionine-labelled HA-GASP-1, HA-cGASP-2 or HA-rGASP-1 (TNT T7 Coupled Reticulocyte Lysate System) in wash buffer (20 mmHEPES, pH 7.4, 100 mm NaCl, 2 mm MgCl2, 0.1% Triton X-100) under vigorous shaking for 1 h at 4°C. Probes were then washed five times in ice-cold wash buffer, eluted in sample buffer and resolved by SDS–polyacrylamide gel electrophoresis (SDS/PAGE). Gels were stained with PAGEBlue, dried for 3 h in a gel drier and exposed to X-ray films.
MBP competition binding assay
MBP-US28 and MBP alone (control) were expressed and purified on amylose resin. 35S-methionine-labelled HA-GASP-1 was generated as described above and incubated with 15 μg MBP-US28 or MBP. For competition assays, GST-cGASP-1 was bacterially expressed and purified on glutathione-agarose, and the purified protein was eluted with 10 mm glutathione. Increasing concentrations of eluted GST-cGASP-1 were added to the MBP-US28 or MBP (control) slurry just before addition of 35S-methionine-labelled HA-GASP-1. Probes were further processed and analysed as described above.
Cell culture
HEK293 cells were maintained at 5% CO2 at 37°C in DMEM supplemented with 10% FBS. The construction of HEK293 cells stably expressing GFP-cGASP-1 was described in (14), cells were maintained as described above plus 0.2 mg/mL G418.
Co-immunoprecipitation
HEK293 cells were transfected with 20 μg/10 cm dish of pcDNA3.1, Flag-US28, Flag-DOP or Flag-β2AR using Lipofectamine 2000. Forty-eight hours post-transfection, co-immoprecipitation of tagged receptors and endogenous GASP-1 and GASP-2 was performed essentially as described in (14,16). In brief, cells were washed twice with PBS and lysed in 0.1% Triton X-100, 150 mm NaCl, 25 mm KCl, 10 mm Tris–HCl pH 7.4, 1 mm CaCl2 supplemented with complete protease inhibitors. Cleared lysates were incubated with 20 μL of M2 monoclonal antibody affinity matrix overnight at 4°C. After extensive washing, precipitates were resolved on a 4–20% Tris–Glycine gradient gel and then transferred to Polyvinylidene difluoride (PVDF) membrane. The blots were cut below the 70-kDa marker band and separately immunoblotted for either receptor or β-actin (lower panels) or GASP-1 and GASP-2 (upper panels). GASP blots were incubated with the rabbit anti-GASP serum recognizing both GASP-isoforms (1:1000) and visualized with a HRP-conjugated anti-rabbit antibody (1:4000). Receptor blots were incubated for 2 h with M2 monoclonal antibody (1:500) followed by 2 h incubation with HRP-conjugated anti-mouse antibody (1:4000). β-actin blots were incubated for 2 h with β-actin monoclonal antibody (1:4000) followed by 2 h incubation with HRP-conjugated anti-mouse antibody(1:4000). Blots were visualized with ECL western blotting substrate.
Lentivirus production and shRNA knock-down of endogenous GASP-1
The Lentiviruses with scrambled or GASP-1 shRNA were produced in HEK293FT cells. 2 × 107 HEK293FT cells were grown in DMEM supplemented with 10% FBS and transfected with 7 μg of scrambled shRNA LentiLox or GASP-1 shRNA LentiLox vectors and 20 μg of Virapower packaging mix. Forty-eight hours post-transfection, viruses were harvested and purified by ultracentrifugation (40000 g, 90 min, 4°C). The virus pellets were resuspended in 25 μL salt-free PBS, frozen in liquid nitrogen and stored at −80°C. Virus titre was estimated by ELISA using the QuickTiterTM Lentivirus Quantification Kit. For the knock-down of endogenous GASP-1, HEK293 cells were infected with 10 MOI (multiplicity of infection) of Lenti-shGASP-1 (shGASP-1) or Lenti-shScrambled (shScr) virus and incubated at 37°C and 5% CO2 for 48–72 h. Successful Lenti-shRNA infection was monitored by immunofluorescence of EGFP, which is under the control of a CMV promoter following the LoxP site of the LentiLox 3.7 vector. GASP-1 knock-down was verified by western blot analysis of GASP-1 protein levels.
EGFR western blot
HEK293 cells infected with shScr or shGASP-1 virus were transiently transfected with Flag-US28 and treated with 5 μm EGF in OptiMeM or OptiMeM alone for 3 h. Cells were washed with PBS and lysates were separated by SDS/PAGE. Finally, samples were immunoblotted with goat antibodies to EGFR (1:500), followed by HRP-conjugated secondary anti-goat (1:4000) antibody and development with ECL substrate.
Immunocytochemistry
Antibody feeding experiments
Wild-type HEK293 cells or HEK293 cells stably expressing GFP-cGASP-1 were grown on gelatine-coated cover slips and transfected with Flag-US28 (4 μg/coverslip). Twenty-four hours post-transfection, antibody feeding experiments were conducted essentially as described before (16). In brief, living cells were incubated with 1:500 anti-Flag M1 antibody for 45 min, fixed with 3.7% formaldehyde in PBS and permeabilized in blotto (50 mm Tris–HCl, pH 7.5, 1 mm CaCl2, 0.1% Triton X-100 and 3% milk). After colocalization staining (see below), all cells were mounted with Vecta shield mounting medium.
Colocalization experiments with LAMP1 and LAMP2
Following the feeding experiments described above, cells were incubated with a monoclonal antibody directed against LAMP 1/2 (IgG1 1:500) for 60 min. Subsequently, the cells were stained with the subtype-selective antibodies AlexaFluor 647-conjugated IgG2b against the Flag-tag (1:1000, 20 min) and AlexaFluor 594-conjugated IgG1a against LAMP 1/2 (1:500, 20 min).
Colocalization experiments with EEA.1
Following the feeding experiments described above, cells were incubated with a polyclonal antibody directed against EEA.1 (IgG, 1:500) for 60 min. Cells were stained with the subtype-selective antibodies AlexaFluor 647-conjugated IgG2b against the Flag-tag (1:1000) and the AlexaFluor 594-conjugated goat-anti-rabbit IgG (1:1000) for 20 min.
Colocalization experiments with transferrin
For colocalization studies with transferrin, cells were serum-starved for 60 min prior to incubation with M1 antibody directed against the Flag-epitope (1:1000, 45 min). Then cells were labelled with AlexaFluor 594-nm-conjugated transferrin (1:500) for the last 30 min. After fixation and permeabilization, the cells were stained with the subtype-selective antibody AlexaFluor 647 nm conjugated IgG2b against the Flag-tag (1:1000, 20 min).
Antibody feeding experiments in shScr and shGASP-1 infected cells
Cells infected with either shScr or shGASP-1 virus for 48 h were transiently transfected with 4 μg/coverslip Flag-US28. Twenty-four hours post-transfection, antibody feeding and colocalization experiments were performed as described above.
Microscopy
Cells were analysed with a Zeiss LSM 510 META confocal microscope. To determine the exact localization of the receptor, Z-stacks of representative cells were taken and the pictures of the middle of the cells are shown.
Biotin internalization and biotin protection/degradation assays
HEK293 cells, HEK293 cells stably expressing GFP-cGASP-1 or HEK293 cells infected with either shScr or shGASP-1 virus for 48 h were seeded on gelatine-coated 10-cm dishes and transiently transfected with 20 μg of Flag-US28. Biotin assays were performed 24 h post-transfection essentially as previously described (14,16). In brief, cells were washed in PBS, treated with 3 μg/mL disulfide cleavable biotin for 30 min at 4°C and washed in TBS (25 mm Tris base, 135 mm NaCl, 2.5 mm KCl, 1 mm CaCl2* 2H2O, pH 7.4). Cells labelled 100% biotinylated were left on ice in cold medium. For internalization assays cells were placed in prewarmed medium for 0–45 min to allow constitutive receptor endocytosis. The cells were then washed in PBS, and the remaining cell surface-biotinylated receptors were stripped in stripbuffer (50 mm glutathione, 75 mm NaCl, 10% fetal bovine serum) at 4°C for 30 min (except 100% plates) and quenched with PBS containing 50 mm iodoacetamide and 1% bovine serum albumin. The cells were then washed in PBS and cell lysates were prepared with 0.1% Triton X-100, 150 mm NaCl, 25 mm KCl,10 mm Tris–HCl, 1 mm CaCl2 and 5 mm iodoacetamide, pH 7.4, with protease inhibitors. For protection/degradation assays biotinylated cells were placed in prewarmed medium for 45 min to allow constitutive receptor endocytosis. The cells were then washed in PBS, and the remaining cell surface-biotinylated receptors were stripped in stripbuffer at 4°C for 30 min (except 100% plates) and quenched with PBS containing 50 mm iodoacetamide and 1% bovine serum albumin. The cells were then washed in PBS and placed inprewarmed medium for the indicated time-points to allow receptor degradation, whereby the 100% plates remained at 4°C. Finally, the plates were washed in PBS and cell lysates were prepared. Samples of cleared lysates were taken for control western blots (anti-GASP 1:1000; anti β-actin 1:4000, anti-Flag M2 1:500), and the remaining lysates were immunoprecipitated with anti-Flag affinity matrix overnight at 4°C. Precipitates were resolved by SDS/PAGE, and visualized by incubating with Vectastain ABC immunoperoxidase reagent. Blots were scanned and quantified using ImageJ Software.
Recycling experiments
Wild-type HEK293 cells, cells stably expressing GFP-cGASP-1 or cells infected with either shScr or shGASP-1 were grown on gelatine-coated coverslips. Subsequently, the cells were transiently transfected with Flag-US28 (4 μg/coverslip). Twenty-four hours post–transfection, recycling experiments were performed essentially as described in (16). In brief, living cells were fed with the Ca2+ sensitive M1 antibody directed against the Flag-epitope (1:500, 45 min). Then residual surface receptors were stripped of antibody by washing twice with cold 0.04 mm PBS–EDTA solution. To determine the constitutive recycling of the receptor, warm medium was readded to the cells. After 15 or 30 min the medium was aspirated and the cells were fixed with 3.7% formaldehyde in PBS. Cells were stained with the subtype-selective antibody AlexaFluor 594-conjugated IgG2b against the Flag-tag (1:1000, 20 min) under non-permeabilizing (minus Triton-X) conditions. Finally, the cells were mounted with Vecta shield mounting medium.
Reporter gene assays
Transcription factor luciferase assays were performed essentially as described in (4). In brief, HEK293 cells (40 000 cells/well) were seeded on gelatine-coated 96-well plates. For GASP-1 knock-down experiments, HEK293 cells were infected with either shScr or shGASP-1 virus 48 h before transfection. Cells were transiently transfected with 50 ng/well Flag-US28 and the cis-reporter plasmid for CREB (200 ng/well) or NF-κB (50 ng/well). Depending on the experiment (indicated at the figure legends) US28 was co-transfected with 100 ng/well pcDNA3.1, HA-GASP-1, HA-cGASP-1 or HA-rGASP-1. The amount of DNA was kept constant for all samples. In parallelto each assay, an additional plate was prepared for ELISA (see below) to control for receptor expression levels. The luciferase reporter gene assay was conducted 24 h post-transfection according to the manufacturer’s guidelines. In brief, cells were washed twice with PBS and the cell number was determined by optical density in a FlexStation II Device (Molecular Probes, Endpoint Measurement). Then 100 μL/well Steadylight luciferase assay reagent were added to 100 μL/well PBS. Following a 10-min incubation period, luminescence was measured using a TopCounter Device (TopCount NXT, PerkinElmer).
Enzyme-linked immunosorbent assay
On the same day as the respective signalling assay (see above), cells were fixed with 3.7% formaldehyde in PBS and permeabilized in blotto (50 mm Tris–HCl, pH 7.5, 1 nm CaCl2, 0.1% Triton X-100 and 3% milk) for 1 h. Next, the cells were incubated with 1:500 anti-Flag M1 antibody overnight at 4°C. On the next day, the cells were washed with TBS (25 mm Tris base, 135 mm NaCl, 2.5 mm KCl, 1mm CaCl2* 2H2O, pH 7.4) and incubated with HRP-conjugated anti-mouse antibody (1:2500) in blotto (50 mm Tris–HCl, pH 7.5, 1 mm CaCl2 and 1.5% milk) for 2 h at room temperature. After three washes with TBS, the cell number was determined by optical density in a FlexStation II Device. Then 100 μL/well TMB (3, 3′, 5, 5′-Tetramethylbenzidine) substrate was added, and the colouring reaction was stopped by the addition of 50 μL/well 0.5 m sulfuric acid after 2 min at room temperature. Receptor levels were measured at the optical density of 450 nm in a BioRad xMark Microplate Spectrophotometer.
Inositol phosphate accumulation assay
HEK293 cells were seeded on gelatine-coated 96-well plates (40 000 cells/well). For GASP-1 knock-down experiments, HEK293 cells were infected with either shScr or shGASP-1 virus 48 h before transfection. Cells were transfected with 75 ng/well Flag-US28 and depending on the experimental setting indicated in the figure legends, co-transfected with 100 ng/well of either pcDNA3.1, HA-GASP-1, HA-cGASP-1 or HA-rGASP-1 (rescue experiments). Controls were transfected with 175 ng/well pcDNA3.1 in the absence of US28. Twenty-four hours post-transfection, the cells were loaded with 1 μCi/well [3H]myo-inositol in 100 μl/well Optimem and incubated overnight at 37°C and 5% CO2. The next day, the labelling medium was aspirated, 100 μl/well Hanks’ balanced salt solution (HBSS) buffer (including CaCl2 and MgCl2) containing 10 mm LiCl was added and the cells were incubated for 45 min at 37°C and 5% CO2. The reaction was terminated by aspiration and the cell number was determined by optical density in a FlexStation II Device. Subsequently, cells were lysed with 50 μl/well 10 mm formic acid for 90 min on ice. The resulting cell extract of about 40 μL was transferred to 160 μL of YSi-SPA beads (12.5 mg/mL) and shaken for 90 min at 4°C. The plates were then stored at 4°C and the accumulation of IP was counted in a TopCount microplate scintillation counter (TopCount NXT, PerkinElmer) 24 h later.
Statistical analysis
Statistical analyses were performed using anova analysis of variance for comparisons between multiple groups, followed by a Bonferroni’s post hoc analysis and t-tests using GraphPad Prism software.
Supplementary Material
Figure S1: Overexpression of GFP-cGASP-1 in HEK293 cells. Serial dilutions of lysates from a HEK293 cell line stably expressing GFP-cGASP-1 (14) on immunoblot show that cGASP-1 is overexpressed by at least 100-fold compared to endogenous GASP-1.
Figure S2: Quantification of western blots. A) Quantification of the GASP-1 levels in cells overexpressing HA-GASP-1. HEK293 cells transfected with Flag-US28 in combination with either pcDNA3.1 (white bar) or HA-GASP-1 (black bar) were analysed by western blot. The GASP-1 levels corresponding to western blots in Figure 6A, upper panel, were compared and quantified with ImageJ software. Finally, the GASP-1 level in the control (white bar) was set to 100% and the overexpression of GASP-1 was normalized accordingly. B) Quantification of the restored GASP-1 levels in shGASP-1 infected cells. HEK293 cells infected with shScr virus were transfected with Flag-US28 and pcDNA3.1 (white bar). shGASP-1 infected cells were transfected with Flag-US28 and either pcDNA3.1 (grey bar) or HA-rGASP-1 (black bar). The GASP-1 levels corresponding to western blots in Figure 6E were analysed by western blot and quantified with ImageJ software. Finally, the GASP-1 level in shScr infected cells (white bar) was set to 100% and the other values were normalized accordingly.
Figure S3: US28 interacts with rGASP-1 in vitro. In vitro translation assay of GASP-1 binding. 35S-methionine labelled recombinant rGASP-1 binds to GST-fusion proteins containing the CT of US28 and the DOP, but not the GST-protein (C), beads (B) alone or the MOP. The corresponding input protein levels are shown below the radiograph. Blots are representative of at least three independent experiments.
Acknowledgments
We would like to thank Tatjana Kueznik for her excellent support using the ZEISS confocal microscope at the ZMF at the Medical University of Graz, and Rufina Schuligoi and Gunther Marsche for critically reading this manuscript. This work was supported by grants from the Austrian Science Fund (P18723 to MW, P15591 and P18630 to HS), the Jubil umsfonds of the Austrian National Bank and the Lanyar Stiftung Graz (all to MW), the ‘Molecular Medicine Ph.D. program’ from the Medical University of Graz, Austria (to PMT and EM) and the BaCaVisiting Scientists program (to PMT). M. J. S. and H. F. V. were supported by the Dutch Organization for Scientific Research NW (Vidi grants 700.54.425 to MJS and 700.55.403 to HFV). D. T., L. M. and J. L. W. were supported by funds provided by the State of California for medical research through the University of California San Francisco (to J. L. W.), and by the National Institute of Mental Health Grant R01 MH68442 (to J. L. W.). L. M. was supported by a grant from the Lundbeck Foundation, Denmark.
References
- 1.Michelson S. Consequences of human cytomegalovirus mimicry. Hum Immunol. 2004;65:465–475. doi: 10.1016/j.humimm.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 2.Rosenkilde MM, Smit MJ, Waldhoer M. Structure, function and physiological consequences of virally encoded chemokine seven transmembrane receptors. Br J Pharmacol. 2008;153(Suppl 1):S154–S166. doi: 10.1038/sj.bjp.0707660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Casarosa P, Bakker RA, Verzijl D, Navis M, Timmerman H, Leurs R, Smit MJ. Constitutive signaling of the human cytomegalovirus-encoded chemokine receptor US28. J Biol Chem. 2001;276:1133–1137. doi: 10.1074/jbc.M008965200. [DOI] [PubMed] [Google Scholar]
- 4.Waldhoer M, Kledal TN, Farrell H, Schwartz TW. Murine cytomegalovirus (CMV) M33 and human CMV US28 receptors exhibit similar constitutive signaling activities. J Virol. 2002;76:8161–8168. doi: 10.1128/JVI.76.16.8161-8168.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Waldhoer M, Casarosa P, Rosenkilde MM, Smit MJ, Leurs R, Whistler JL, Schwartz TW. The carboxyl terminus of human cytomegalovirus-encoded 7 transmembrane receptor US28 camouflages agonism by mediating constitutive endocytosis. J Biol Chem. 2003;278:19473–19482. doi: 10.1074/jbc.M213179200. [DOI] [PubMed] [Google Scholar]
- 6.Miller WE, Houtz DA, Nelson CD, Kolattukudy PE, Lefkowitz RJ. G-protein-coupled receptor (GPCR) kinase phosphorylation and beta-arrestin recruitment regulate the constitutive signaling activity of the human cytomegalovirus US28 GPCR. J Biol Chem. 2003;278:21663–21671. doi: 10.1074/jbc.M303219200. [DOI] [PubMed] [Google Scholar]
- 7.Fraile-Ramos A, Kohout TA, Waldhoer M, Marsh M. Endocytosis of the viral chemokine receptor US28 does not require beta-arrestins but is dependent on the clathrin-mediated pathway. Traffic. 2003;4:243–253. doi: 10.1034/j.1600-0854.2003.00079.x. [DOI] [PubMed] [Google Scholar]
- 8.Droese J, Mokros T, Hermosilla R, Schulein R, Lipp M, Hopken UE, Rehm A. HCMV-encoded chemokine receptor US28 employs multiple routes for internalization. Biochem Biophys Res Commun. 2004;322:42–49. doi: 10.1016/j.bbrc.2004.07.076. [DOI] [PubMed] [Google Scholar]
- 9.Fraile-Ramos A, Kledal TN, Pelchen-Matthews A, Bowers K, Schwartz TW, Marsh M. The human cytomegalovirus US28 protein is located in endocytic vesicles and undergoes constitutive endocytosis and recycling. Mol Biol Cell. 2001;12:1737–1749. doi: 10.1091/mbc.12.6.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fraile-Ramos A, Pelchen-Matthews A, Kledal TN, Browne H, Schwartz TW, Marsh M. Localization of HCMV UL33 and US27 in endocytic compartments and viral membranes. Traffic. 2002;3:218–232. doi: 10.1034/j.1600-0854.2002.030307.x. [DOI] [PubMed] [Google Scholar]
- 11.Cepeda V, Esteban M, Fraile-Ramos A. Human cytomegalovirus final envelopment on membranes containing both trans-Golgi network and endosomal markers. Cell Microbiol. 2009 doi: 10.1111/j.1462-5822.2009.01405.x. [DOI] [PubMed] [Google Scholar]
- 12.Kledal TN, Rosenkilde MM, Schwartz TW. Selective recognition of the membrane-bound CX3C chemokine, fractalkine, by the human cytomegalovirus-encoded broad-spectrum receptor US28. FEBS Lett. 1998;441:209–214. doi: 10.1016/s0014-5793(98)01551-8. [DOI] [PubMed] [Google Scholar]
- 13.Pleskoff O, Tréboute C, Alizon M. The cytomegalovirus-encoded chemokine receptor US28 can enhance cell-cell fusion mediated by different viral proteins. J Virol. 1998;72:6389–6397. doi: 10.1128/jvi.72.8.6389-6397.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whistler JL, Enquist J, Marley A, Fong J, Gladher F, Tsuruda P, Murray SR, Von Zastrow M. Modulation of postendocytic sorting of G protein-coupled receptors. Science. 2002;297:615–620. doi: 10.1126/science.1073308. [DOI] [PubMed] [Google Scholar]
- 15.Simonin F, Karcher P, Boeuf JJ, Matifas A, Kieffer BL. Identification of a novel family of G protein-coupled receptor associated sorting proteins. J Neurochem. 2004;89:766–775. doi: 10.1111/j.1471-4159.2004.02411.x. [DOI] [PubMed] [Google Scholar]
- 16.Martini L, Waldhoer M, Pusch M, Kharazia V, Fong J, Lee JH, Freissmuth C, Whistler JL. Ligand-induced down-regulation of the cannabinoid 1 receptor is mediated by the G-protein-coupled receptor-associated sorting protein GASP1. FASEB J. 2007;21:802–811. doi: 10.1096/fj.06-7132com. [DOI] [PubMed] [Google Scholar]
- 17.Enquist J, Skroder C, Whistler JL, Leeb-Lundberg LM. Kinins promote B2 receptor endocytosis and delay constitutive B1 receptor endocytosis. Mol Pharmacol. 2007;71:494–507. doi: 10.1124/mol.106.030858. [DOI] [PubMed] [Google Scholar]
- 18.Thompson D, Pusch M, Whistler JL. Changes in G protein coupled receptor sorting protein affinity regulate post-endocytic targeting of G protein coupled receptors. J Biol Chem. 2007;282(40):29178–85. doi: 10.1074/jbc.M704014200. [DOI] [PubMed] [Google Scholar]
- 19.Tappe-Theodor A, Agarwal N, Katona I, Rubino T, Martini L, Swiercz J, Mackie K, Monyer H, Parolaro D, Whistler J, Kuner T, Kuner R. A molecular basis of analgesic tolerance to cannabinoids. J Neurosci. 2007;27:4165–4177. doi: 10.1523/JNEUROSCI.5648-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bartlett SE, Enquist J, Hopf FW, Lee JH, Gladher F, Kharazia V, Waldhoer M, Mailliard WS, Armstrong R, Bonci A, Whistler JL. Dopamine responsiveness is regulated by targeted sorting of D2 receptors. Proc Natl Acad Sci U S A. 2005;102:11521–11526. doi: 10.1073/pnas.0502418102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boeuf J, Trigo JM, Moreau PH, Lecourtier L, Vogel E, Cassel JC, Mathis C, Klosen P, Maldonado R, Simonin F. Attenuated behavioural responses to acute and chronic cocaine in GASP-1-deficient mice. Eur JNeurosci. 2009;30:860–868. doi: 10.1111/j.1460-9568.2009.06865.x. [DOI] [PubMed] [Google Scholar]
- 22.McLean KA, Holst PJ, Martini L, Schwartz TW, Rosenkilde MM. Similar activation of signal transduction pathways by the herpesvirus-encoded chemokine receptors US28 and ORF74. Virology. 2004;325:241–251. doi: 10.1016/j.virol.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 23.Moepps B, Tulone C, Kern C, Minisini R, Michels G, Vatter P, Wieland T, Gierschik P. Constitutive serum response factor activation by the viral chemokine receptor homologue pUS28 is differentially regulated by Galpha(q/11) and Galpha(16) Cell Signal. 2008;20:1528–1537. doi: 10.1016/j.cellsig.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 24.Heydorn A, Sondergaard BP, Ersboll B, Holst B, Nielsen FC, Haft CR, Whistler J, Schwartz TW. A library of 7TM receptor C-terminal tails. Interactions with the proposed post-endocytic sorting proteins ERM-binding phosphoprotein 50 (EBP50), N-ethylmaleimide-sensitive factor (NSF), sorting nexin 1 (SNX1), and G protein-coupled receptor-associated sorting protein (GASP) J Biol Chem. 2004;279:54291–54303. doi: 10.1074/jbc.M406169200. [DOI] [PubMed] [Google Scholar]
- 25.Longva KE, Blystad FD, Stang E, Larsen AM, Johannessen LE, Madshus IH. Ubiquitination and proteasomal activity is required for transport of the EGF receptor to inner membranes of multivesicular bodies. J Cell Biol. 2002;156:843–854. doi: 10.1083/jcb.200106056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Minisini R, Tulone C, Luske A, Michel D, Mertens T, Gierschik P, Moepps B. Constitutive inositol phosphate formation incytomegalovirus-infected human fibroblasts is due to expression of the chemokine receptor homologue pUS28. J Virol. 2003;77:4489–4501. doi: 10.1128/JVI.77.8.4489-4501.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stropes MP, Schneider OD, Zagorski WA, Miller JL, Miller WE. The carboxy-terminal tail of human cytomegalovirus (HCMV) US28 regulates both chemokine-independent and chemokine-dependent signaling in HCMV-infected cells. J Virol. 2009;83:10016–10027. doi: 10.1128/JVI.00354-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wen DQ, Zhang YY, Lv LP, Zhou XP, Yan F, Ma P, Xu JB. Human cytomegalovirus-encoded chemokine receptor homolog US28 stimulates the major immediate early gene promoter/enhancer via the induction of CREB. J Recept Signal Transduct Res. 2009;29:266–273. doi: 10.1080/10799890903178141. [DOI] [PubMed] [Google Scholar]
- 29.Sadowski L, Pilecka I, Miaczynska M. Signaling from endosomes: location makes a difference. Exp Cell Res. 2008;315(9):1601–9. doi: 10.1016/j.yexcr.2008.09.021. CrossRef, PubMed, ChemPort EBSCO LinkSource. [DOI] [PubMed] [Google Scholar]
- 30.Sorkin A, Von Zastrow M. Signal transductionand endocytosis: close encounters of many kinds. Nat Rev Mol Cell Biol. 2002;3:600–614. doi: 10.1038/nrm883. [DOI] [PubMed] [Google Scholar]
- 31.Von Zastrow M, Sorkin A. Signaling on the endocytic pathway. Curr Opin Cell Biol. 2007;19:436–445. doi: 10.1016/j.ceb.2007.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Soderberg-Naucler C. HCMV microinfections in inflammatory diseases and cancer. J Clin Virol. 2008;41:218–223. doi: 10.1016/j.jcv.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 33.Harkins L, Volk AL, Samanta M, Mikolaenko I, Britt WJ, Bland KI, Cobbs CS. Specific localisation of human cytomegalovirus nucleic acids and proteins in human colorectal cancer. Lancet. 2002;360:1557–1563. doi: 10.1016/S0140-6736(02)11524-8. [DOI] [PubMed] [Google Scholar]
- 34.Sabatier J, Uro-Coste E, Pommepuy I, Labrousse F, Allart S, Tremoulet M, Delisle MB, Brousset P. Detection of human cytomegalovirus genome and gene products in central nervous system tumours. Br J Cancer. 2005;92:747–750. doi: 10.1038/sj.bjc.6602339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maussang D, Verzijl D, Van WM, Leurs R, Holl J, Pleskoff O, Michel D, Van Dongen GA, Smit MJ. Human cytomegalovirus-encoded chemokine receptor US28 promotes tumorigenesis. Proc Natl Acad Sci U S A. 2006;103:13068–13073. doi: 10.1073/pnas.0604433103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maussang D, Langemeijer E, Fitzsimons CP, Stigter-van WM, Dijkman R, Borg MK, Slinger E, Schreiber A, Michel D, Tensen CP, Van Dongen GA, Leurs R, Smit MJ. The human cytomegalovirus-encoded chemokine receptor US28 promotes angiogenesis and tumor formation via cyclooxygenase-2. Cancer Res. 2009;69:2861–2869. doi: 10.1158/0008-5472.CAN-08-2487. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Overexpression of GFP-cGASP-1 in HEK293 cells. Serial dilutions of lysates from a HEK293 cell line stably expressing GFP-cGASP-1 (14) on immunoblot show that cGASP-1 is overexpressed by at least 100-fold compared to endogenous GASP-1.
Figure S2: Quantification of western blots. A) Quantification of the GASP-1 levels in cells overexpressing HA-GASP-1. HEK293 cells transfected with Flag-US28 in combination with either pcDNA3.1 (white bar) or HA-GASP-1 (black bar) were analysed by western blot. The GASP-1 levels corresponding to western blots in Figure 6A, upper panel, were compared and quantified with ImageJ software. Finally, the GASP-1 level in the control (white bar) was set to 100% and the overexpression of GASP-1 was normalized accordingly. B) Quantification of the restored GASP-1 levels in shGASP-1 infected cells. HEK293 cells infected with shScr virus were transfected with Flag-US28 and pcDNA3.1 (white bar). shGASP-1 infected cells were transfected with Flag-US28 and either pcDNA3.1 (grey bar) or HA-rGASP-1 (black bar). The GASP-1 levels corresponding to western blots in Figure 6E were analysed by western blot and quantified with ImageJ software. Finally, the GASP-1 level in shScr infected cells (white bar) was set to 100% and the other values were normalized accordingly.
Figure S3: US28 interacts with rGASP-1 in vitro. In vitro translation assay of GASP-1 binding. 35S-methionine labelled recombinant rGASP-1 binds to GST-fusion proteins containing the CT of US28 and the DOP, but not the GST-protein (C), beads (B) alone or the MOP. The corresponding input protein levels are shown below the radiograph. Blots are representative of at least three independent experiments.


