Insulin resistance is characterized by the diminished ability of insulin to initiate intracellular signaling. It is a common manifestation of obesity and a prelude to type 2 diabetes. The primary targets of insulin are skeletal muscle, adipose, and the liver. Impaired insulin signaling in these tissues reduces glucose uptake and promotes a metabolic syndrome that is characterized by elevated levels of insulin, inappropriate synthesis of glucose, and dyslipidemia.1 However, insulin receptors and insulin signaling are not exclusively restricted to metabolically active tissue and can be observed in most cell types including vascular cells.
Individuals with insulin resistance have compromised endothelial cell function and increased frequency and severity of cardiovascular disease.2 Although it is clear that the metabolic consequences of insulin resistance are sufficient in themselves to induce cardiovascular dysfunction, the local actions of insulin on blood vessels are also thought to be of significance. Insulin directly stimulates nitric oxide (NO) release from the vascular endothelium in a phosphatidylinositol 3-kinase (PI3K)-dependent manner that involves the Akt-mediated phosphorylation of endothelial NO synthase (eNOS).3 Alternatively, insulin can stimulate the mitogen-activated protein kinase (MAPK) pathway to promote cellular proliferation.3 Selective or “pathway-specific” insulin resistance has also been described in blood vessels.4 This refers to the selective reduction in the ability of insulin to stimulate PI3K signaling while permitting or even enhancing MAPK activation (see the Figure). These effects are further magnified in insulin-resistant states where there is increased pancreatic secretion of insulin and by angiotensin II, which promotes MAPK signaling at the expense of the PI3K pathway. The reduction in PI3K signaling is proposed to attenuate eNOS activity and thus diminish the buffering and antiinflammatory actions of NO, and these events in conjunction with increased MAPK activity set the stage for increased vascular disease.5 Despite these observations, the local actions of insulin are not without controversy because insulin receptors are ubiquitous and insulin is a comparatively poor stimulus for both NO release and vasodilation.
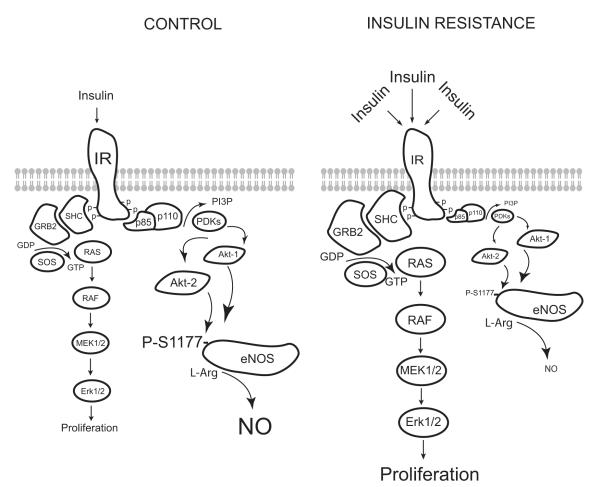
Figure.
Schematic diagram illustrating the major signaling pathways affected by insulin resistance in vascular cells. Hyperstimulation of the insulin receptor reduces the activation of the PI3K-Akt signaling axis, thus limiting the phosphorylation and activation of eNOS. Contemporaneously, insulin signaling to MAPK is unimpeded or enhanced.
Direct evidence for a vascular role of insulin comes from genetic studies in which the insulin receptor has been selectively deleted from the endothelium. These mice (VENIRKO) have normal blood pressure and glucose tolerance but diminished eNOS and endothelin-1 mRNA, which predisposes them to atherosclerosis.6,7 However, the vascular phenotype of these mice, in particular alterations in the regulation of eNOS, does not exactly replicate that seen in mice with metabolic insulin resistance.8-10 In addition, the phenotypes of the muscle-specific (MIRKO), liver-specific (LIRKO), or fat-specific (FIRKO) insulin receptor knockout mice indicates that the severity or type of insulin resistance is unlikely to be uniform in different tissues.11-13 Therefore, the extent to which local insulin resistance contributes to the vascular dysfunction and cardiovascular disease observed in states of metabolic insulin resistance remains unclear.
In this issue of Circulation Research, a study by Symons et al14 adds a new twist to what we know about roles of vascular insulin resistance and selective insulin resistance in cardio-vascular function. To test whether vascular insulin receptors can modify endothelial and vascular function, Symons et al used a relatively novel insulin receptor– deficient mouse. The TTr-IR is a global insulin receptor knockout that normally results in perinatal lethality. However, these mice have been rescued by transgenic reexpression of the insulin receptor in the brain, pancreas, and liver, which results in mild metabolic insulin resistance, as evidenced by normoglycemia and hy-perinsulinemia.15 Aside from the brain, pancreas, and liver, TTr-IR mice do not express the insulin receptor in other tissues including the endothelium and vascular smooth muscle. This is an important distinction from the VENIRKO mouse, which has intact insulin receptors in vascular smooth muscle and probably other vascular cells. Symons et al report that whereas the ability of insulin to induce vascular signaling and relaxation is impaired because of the loss of the insulin receptor, endothelial function in response to acetylcholine and mean blood pressure are unchanged in TTr-IR mice. The loss of insulin-dependent responses is not an unexpected finding, but the preservation of eNOS expression, endothelial function, and blood pressure is surprising. Although it can be argued that deletion of the insulin receptor would inhibit both arms of the opposing downstream PI3K and MAPK pathways to produce no net change in vascular function (see the Figure), results in mice with a more severe form of dietary-induced insulin resistance suggest otherwise.
In mice fed a high fat diet, Symons et al14 find that in contrast to the concept of pathway selective insulin resistance described above, both Erk1/2 and Akt phosphorylation are slightly diminished, whereas eNOS phosphorylation at S1177 is abolished. In addition, the high fat–fed mice have compromised endothelial function and elevated blood pressure. Why this model of insulin resistance produces a more severe phenotype compared to the TTr-IR mouse is not clear but may be because of a greater level of adiposity or a greater degree of insulin resistance. Also of significance in this model is the ability of insulin to robustly stimulate Akt phosphorylation in blood vessels, despite clear evidence for metabolic insulin resistance in other tissues. The mechanisms uncoupling Akt from eNOS phosphorylation are not known, and it would be interesting to see whether other Akt sub-strates are also hypophosphorylated.
To test the concept of pathway-selective vascular insulin resistance, the ability of insulin to stimulate eNOS phosphorylation in Akt-1 knockout mice was also studied. There are 3 isoforms of Akt (Akt-1, Akt-2, and Akt-3), and blood vessels express primarily Akt-1 and -2. Previously, it was reported that Akt-1, but not Akt-2, is important for basal and vascular endothelial growth factor–stimulated eNOS activity/NO release.16 However, the study by Symons et al14 identifies an important role for Akt-2 in insulin-stimulated eNOS phosphorylation that warrants further investigation. In Akt-1 knockout mice, both eNOS and Akt phosphorylation remain intact, and this was later shown to be attributable to the compensatory or perhaps primary actions of Akt-2. This finding is significant because the loss of Akt-1 does not impair the actions of insulin,17 whereas Akt-2 is enriched in insulin-responsive tissue and genetic deletion results in insulin resistance and diabetes.18 Along these lines, it would be interesting to see whether Akt-2 can phosphorylate eNOS and whether it is selectively affected by insulin resistance versus Akt-1. Blood pressure and endothelium-dependent relaxation are also unmodified in Akt-1 knockout mice, and this might be simply attributable to the fact that these animals do not have insulin resistance.17
The phosphorylation of S1177 is commonly reported as the major index of eNOS activation, but eNOS can also be regulated by the phosphorylation of other sites.19 In particular, Y657 was recently identified as a major insulin-sensitive phosphorylation site.20 The increased phosphorylation of Y657, which inhibits eNOS activity, may explain the reduced ability of insulin to stimulate NO release from blood vessels versus cultured endothelium. Although this may represent another mechanism of vascular insulin resistance, Symons et al14 did not investigate this site, and it is presently unknown whether Y657 phosphorylation is altered in models of insulin resistance. However, other sites of eNOS phosphorylation, S617 and T495, were unchanged in mice fed a high fat diet.
In summary, the mechanisms by which insulin resistance influences vascular function are many, complex, and poorly understood. The recent observations by Symons et al14 suggest that it is unlikely that vascular insulin receptors contribute directly to the vascular pathology of metabolic insulin resistance. However, this should also be confirmed in a more pronounced model of insulin resistance. It is also clear from these studies that the concept of selective insulin resistance is more complex and variable than previously thought, particularly as we become more aware that the participating signaling molecules are not always the ones we think they are. A good example of this is the putative role of Akt-2 in the regulation of eNOS function.
Acknowledgments
Sources of Funding The author is an Established Investigator of the American Heart Association and is also supported by NIH grants HL085827 and HL092446.
Footnotes
The opinions expressed in this editorial are not necessarily those of the editors or of the American Heart Association.
Disclosures None.
References
- 1.Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- 2.Semenkovich CF. Insulin resistance and atherosclerosis. J Clin Invest. 2006;116:1813–1822. doi: 10.1172/JCI29024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muniyappa R, Montagnani M, Koh KK, Quon MJ. Cardiovascular actions of insulin. Endocr Rev. 2007;28:463–491. doi: 10.1210/er.2007-0006. [DOI] [PubMed] [Google Scholar]
- 4.Jiang ZY, Lin YW, Clemont A, Feener EP, Hein KD, Igarashi M, Yamauchi T, White MF, King GL. Characterization of selective resistance to insulin signaling in the vasculature of obese Zucker (fa/fa) rats. J Clin Invest. 1999;104:447–457. doi: 10.1172/JCI5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Avogaro A, de Kreutzenberg SV, Fadini GP. Oxidative stress and vascular disease in diabetes: is the dichotomization of insulin signaling still valid? Free Radic Biol Med. 2008;44:1209–1215. doi: 10.1016/j.freeradbiomed.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 6.Vicent D, Ilany J, Kondo T, Naruse K, Fisher SJ, Kisanuki YY, Bursell S, Yanagisawa M, King GL, Kahn CR. The role of endothelial insulin signaling in the regulation of vascular tone and insulin resistance. J Clin Invest. 2003;111:1373–1380. doi: 10.1172/JCI15211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rask-Madsen C, Vecchia K Della, Wu I-H, Scott JA, Arikawa E, Matsumoto M, Sotiropoulos KB, Kahn CR, King GL. Loss of insulin signaling in vascular endothelium accelerates atherosclerosis in apoli-poprotein E knockout mice. Circulation. 2006;114(suppl II):II_24. Abstract. [Google Scholar]
- 8.Fulton D, Harris MB, Kemp BE, Venema RC, Marrero MB, Stepp DW. Insulin resistance does not diminish eNOS expression, phosphorylation, or binding to HSP-90. Am J Physiol Heart Circ Physiol. 2004;287:H2384–H2393. doi: 10.1152/ajpheart.00280.2004. [DOI] [PubMed] [Google Scholar]
- 9.Katakam PVG, Tulbert CD, Snipes JA, Erdos B, Miller AW, Busija DW. Impaired insulin-induced vasodilation in small coronary arteries of Zucker obese rats is mediated by reactive oxygen species. Am J Physiol Heart Circ Physiol. 2005;288:H854–H860. doi: 10.1152/ajpheart.00715.2004. [DOI] [PubMed] [Google Scholar]
- 10.Molnar J, Yu S, Mzhavia N, Pau C, Chereshnev I, Dansky HM. Diabetes induces endothelial dysfunction but does not increase neointimal formation in high-fat diet fed C57BL/6J mice. Circ Res. 2005;96:1178–1184. doi: 10.1161/01.RES.0000168634.74330.ed. [DOI] [PubMed] [Google Scholar]
- 11.Bluher M, Michael MD, Peroni OD, Ueki K, Carter N, Kahn BB, Kahn CR. Adipose tissue selective insulin receptor knockout protects against obesity and obesity-related glucose intolerance. Dev Cell. 2002;3:25–38. doi: 10.1016/s1534-5807(02)00199-5. [DOI] [PubMed] [Google Scholar]
- 12.Brüning JC, Michael MD, Winnay JN, Hayashi T, Höch D, Accili D, Goodyear LJ, Kahn CR. A muscle-specific insulin receptor knockout exhibits features of the metabolic syndrome of NIDDM without altering glucose tolerance. Mol Cell. 1998;2:559–569. doi: 10.1016/s1097-2765(00)80155-0. [DOI] [PubMed] [Google Scholar]
- 13.Michael MD, Kulkarni RN, Postic C, Previs SF, Shulman GI, Magnuson MA, Kahn CR. Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Mol Cell. 2000;6:87–97. [PubMed] [Google Scholar]
- 14.Symons JD, McMillin SL, Riehle C, Tanner J, Palionyte M, Hillas E, Jones D, Cooksey RC, Birnbaum MJ, McClain DA, Zhang Q-J, Gale D, Wilson LJ, Abel ED. Contribution of insulin and Akt1 signaling to endothelial nitric oxide synthase in the regulation of endothelial function and blood pressure. Circ Res. 2009;104:1085–1094. doi: 10.1161/CIRCRESAHA.108.189316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okamoto H, Nakae J, Kitamura T, Park BC, Dragatsis I, Accili D. Transgenic rescue of insulin receptor-deficient mice. J Clin Invest. 2004;114:214–223. doi: 10.1172/JCI21645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ackah E, Yu J, Zoellner S, Iwakiri Y, Skurk C, Shibata R, Ouchi N, Easton RM, Galasso G, Birnbaum MJ, Walsh K, Sessa WC. Akt1/protein kinase Balpha is critical for ischemic and VEGF-mediated angiogenesis. J Clin Invest. 2005;115:2119–2127. doi: 10.1172/JCI24726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cho H, Thorvaldsen JL, Chu Q, Feng F, Birnbaum MJ. Akt1/PKBalpha is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. J Biol Chem. 2001;276:38349–38352. doi: 10.1074/jbc.C100462200. [DOI] [PubMed] [Google Scholar]
- 18.Cho H, Mu J, Kim JK, Thorvaldsen JL, Chu Q, Crenshaw EB, III, Kaestner KH, Bartolomei MS, Shulman GI, Birnbaum MJ. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKBbeta) Science. 2001;292:1728–1731. doi: 10.1126/science.292.5522.1728. [DOI] [PubMed] [Google Scholar]
- 19.Church JE, Fulton D. Differences in eNOS activity because of subcellular localization are dictated by phosphorylation state rather than the local calcium environment. J Biol Chem. 2006;281:1477–1488. doi: 10.1074/jbc.M505968200. [DOI] [PubMed] [Google Scholar]
- 20.Fisslthaler B, Loot AE, Mohamed A, Busse R, Fleming I. Inhibition of endothelial nitric oxide synthase activity by proline-rich tyrosine kinase 2 in response to fluid shear stress and insulin. Circ Res. 2008;102:1520–1528. doi: 10.1161/CIRCRESAHA.108.172072. [DOI] [PubMed] [Google Scholar]