Abstract
The Vif protein of primate lentiviruses interacts with APOBEC3 proteins, which results in shunting of the APOBEC3-Vif complex to the proteosome for degradation. Using the simian-human immunodeficiency virus (SHIV)/macaque model, we compared the replication and pathogenicity of SHIVs that express a Vif protein in which the entire SLQ(Y/F)LA (SHIVVif5A) or HCCH (SHIVVifHCCH(−)) domains were substituted with alanine residues. Each virus was inoculated into three macaques and various viral and immunological parameters followed for six months. All macaques maintained stable circulating CD4+ T cells, developed low viral loads, maintained the engineered mutations, yielded no histological lesions, and developed immunoprecipitating antibodies early post-inoculation. Sequence analysis of nef and vpu from three lymphoid tissues revealed a high percentage of G-to-A-substitutions. Our results show that while the presence of HCCH and SLQ(Y/F)LA domains are critical in vivo, there may exist APOBEC3 negative reservoirs that allow for low levels of viral replication and persistence but not disease.
INTRODUCTION
Human immunodeficiency virus type 1 (HIV-1) and other lentiviruses encode for a Vif (virion infectivity factor) protein, which has been shown to be essential for HIV-1 replication in primary CD4+ T cells and macrophages (Fan et al., 1992; Gabuzda et al., 1992; Blanc et al., 1993; Sakai et al., 1993; von Schwedler et al., 1993; Borman et al., 1995). The Vif protein interacts with apolipoprotein B mRNA editing enzyme catalytic peptide-like 3G (APOBEC3G; hA3G) promoting its accelerated degradation by the proteosome (Sheehy et al., 2003). APOBEC3G is a cytidine deaminase that, if packaged into HIV-1Δvif virions, induces cytidine deamination of newly synthesized minus-strand viral DNA from cytosines to uracils, leading to G to A transitions in plus strand synthesis (Jarmuz et al., 2002; Harris et al., 2003; Mariani et al., 2003; Mangeat et al., 2003; Sheehy et al., 2003; Yu et al., 2004a). The RNA editing activity of the APOBEC3 family of proteins involve an active site characterized by a conserved zinc-binding motif, (Cys/His)-Xaa-Glu-Xaa23-28-Pro-Cys-Xaa2-4-Cys, containing a glutamate involved in proton shuttling during deamination (Jarmuz et al., 2002). In addition to A3G, humans have six other APOBEC3 genes; hA3A, hA3B, hA3C, hA3DE, hA3F, and hA3H (Jarmuz et al., 2002). Of those APOBEC3 genes, hA3B, hA3DE, hA3G, and hA3F, have been shown to inhibit the replication of HIV-1Δvif (Dang et al., 2006; Dang et al., 2008; Doehle et al., 2005; Wiegnad et al., 2004; Yang et al., 2007; Yu et al., 2004b; Zheng et al., 2004). SIVmac239Δvif has been shown to be restricted by hA3G, hA3F, hA3H and to a lesser extent hA3B, hA3C, hA3DE (Dang et al., 2006; Dang et al., 2008; Mariani etal., 2003; Yu et al., 2004b; Zennou et al., 2006). The HIV-1 Vif has limited activity against rhesus and African green monkey A3 proteins while Vif from SIVmac239 and SIVagm have broader specificities. While less is presently known about the rhesus A3 proteins, it is known that HIV-1Δvif can be inhibited by rhA3G, rhA3F, rhA3B, and to a lesser extent rhA3H and rhA3DE (Virgen et al., 2007). SIVmac239Δvif has been shown to be restricted by rhA3G, rhA3F, rhA3C, rhA3B and rhA3DE, and to a lesser extent rhA3H (Virgen et al., 2007; Zennou et al., 2006).
Sequence analysis of Vif proteins from different lentiviruses reveals that there are two highly conserved domains in the carboxyl terminus of Vif, the SLQ(Y/F)LA and Zn++ binding (HCCH) motifs. Previous studies showed that introduction of amino acid substitutions in the viral BC box (SLQ(Y/F)LA) resulted in decreased binding of Vif to Elongin C while substitutions in the HCCH domain prevent interactions with Cullin 5 of the Cul5/Elongin B/C/Rbx E3 ligase complex (Luo et al., 2005; Mehle et al., 2004a; Mehle et al., 2004 b; Mehle et al., 2006; Stopak et al., 2003; Yu et al., 2003; Yu et al., 2004c). This results in increased A3G incorporation into virions and G-to-A hypermutation (Mangeat et al., 2003; Shindo et al., 2003; Zhang et al., 2003). Our laboratory has been using the chimeric simian-human immunodeficiency (SHIV)/macaque model to study the role of Vpu and its various domains in CD4+ T cell loss, virus release and pathogenesis (Stephens et al., 2002; Singh et al., 2003; Hout et al., 2005; 2006; Hill et al., 2008). In this study, we constructed simian-human immunodeficiency viruses (SHIVs) in which five amino acids in the SLQYLA motif (SHIVVif5A) and four amino acids of the HCCH (SHIVVifHCCH(−)) domain were changed to alanine residues. Our results indicate that rhA3F is stable in the presence of wild type and mutant vif viruses and that rhA3G is more effective at cytidine deamination than rhA3F. Our results show that both the HCCH and SLQYLA domains were critical to Vif function in vivo but that production of viral RNA only persisted in macaques inoculated with the SHIVVifHCCH(−).
RESULTS
Replication of SHIVVif5A and SHIVVifHCCH(−) in APOBEC3 positive and negative cell lines
The sequence of the Vif mutants that were analyzed in this study are shown in Figure 1. We performed assays to examine the replication of parental SHIVKU-2MC4, SHIVVif5A, SHIVVifHCCH(−), SHIVVifAAQYLA and SHIVVifSTOP in hA3G/F positive (C8166) and negative (SupT1) cell lines as well as rhesus PBMC (rhA3G/F+). We included SHIVVifAAQYLA in these growth curves for comparison as we previously reported on the replication of this mutant in tissue culture and in macaques (Schmitt et al., 2009). Cells were inoculated with equivalent amounts (25 ng of p27) each of the virus and the levels of p27 Gag released into the culture medium were quantified using a commercial antigen capture assay. All four mutant viruses (SHIVVif5A, SHIVVifHCCH(−), SHIVVifAAQYLA and SHIVVifSTOP) replicated in SupT1 cells to similar levels as parental SHIVKU-2MC4 by day 15 post-inoculation, although the kinetics of replication were slower (Figure 2A). Inoculation of equivalent amounts (25 ng p27) of SHIVVif5A, SHIVVifHCCH(−), SHIVVifAAQYLA, and SHIVVifSTOP into hA3G/F+ C8166 cell cultures resulted in less than 0.01% of the p27 released compared to parental SHIVKU-2MC4 (Figure 2B). As shown in Figure 2C, in rhesus PBMC SHIVKU-2MC4 replicated to high levels (6232 pg/ml) while SHIVVifHCCH(−) (119 pg/ml) and SHIVVif5A (197 pg/ml), and SHIVVifAAQYLA (110 pg/ml) replicated to low but detectable levels. Replication was undetectable for SHIVVifSTOP in rhesus PBMC.
Figure 1.
Sequence of the wild type SIVmac239 Vif protein and the two mutants analyzed in this study.
Figure 2.
Replication of SHIVVif5A, SHIVVifHCCH(−), SHIVVifAAQYLA, SHIVVifSTOP, and SHIVKU-2MC4 in C8166 cells (hA3G/F positive), SupT1 cells (hA3G/F negative) and rhesus macaque PBMC. Cells were inoculated with equal amounts of each virus (25ng of p27) and the levels of p27 in the culture supernatants was assessed at various times post-inoculation. Panel A. Replication of viruses in SupT1 cells. Panel B. Replication of viruses in C8166 cells. Panel C. Replication of viruses in rhesus PBMC. (▲) SHIVVif5A, (□) SHIVVifAAQYLA(●) SHIVVifHCCH(−), (◆) SHIVVifSTOP and (■)SHIVKU-2MC4.
Both SHIVVif5A and SHIVVifHCCH(−) incorporate rhA3G and rhA3F into virus particles
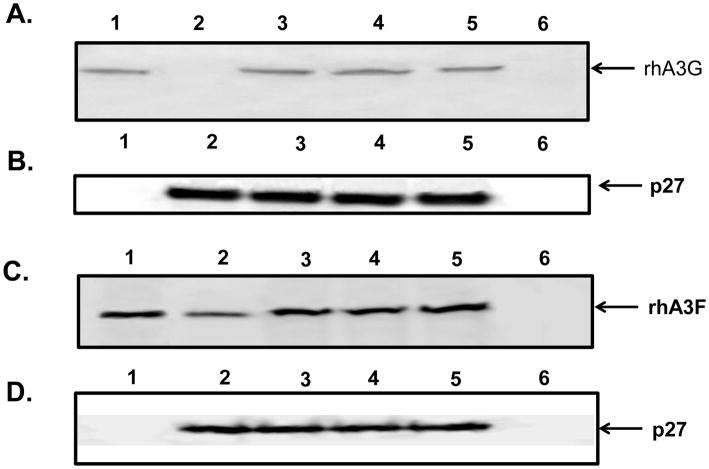
The above results suggested that hA3G and hA3F or rhA3G and rhA3F might be incorporated into maturing virus particles resulting in restriction of replication. As we are most interested in an rhA3G and rhA3F, we transfected 293 cells with plasmids expressing an HA-tagged rhA3G or a V5-tagged rhA3F and the complete genomes of either SHIVVif5A, SHIVVifHCCH(−), SHIVVifSTOP, or parental SHIVKU-2MC4. At 48 hours post-transfection, the culture medium was collected, clarified, and the virus partially purified and concentrated by ultracentrifugation. The amount of p27 was determined followed by Western blot analysis to detect for the presence or absence of rhA3G or rhA3F. The results shown in Figure 3A indicate that rhA3G was incorporated into SHIVVif5A, SHIVVifHCCH(−), and SHIVVifSTOP virus particles but was excluded from SHIVKU-2MC4 particles. However, we found that the rhA3F protein was incorporated into all four viruses (Figure 3B). These results indicate that rhA3G was selectively incorporated only into the Vif mutants while rhA3F was incorporated into all viruses. The inability of SIVmac239 Vif to degrade and prevent the incorporation of rhA3F has been previously reported (Virgen et al., 2007).
Figure 3.
The incorporation of rhA3G and rhA3F into virus particles. 293 cells were transfected with a plasmid that expresses the genome of either SHIVKU-2MC4, SHIVVif5A, SHIVVifHCCH(−), or SHIVVifSTOP and a vector expressing HA-rhA3G or V5-rhA3F. At 48 hours, the culture medium was collected, clarified by low speed centrifugation, and concentrated by ultracentrifugation through a 20/60 (w/v) step-gradient. Equivalent levels of p27 from each sample were resuspended in sample reducing buffer, boiled, and the proteins were separated by SDS -PAGE. The presence of either rhA3G or rhA3F was detected by Western blot using an antibody directed against the HA-tag or V5 tag, respectively. Panel A. Results of rhA3G incorporation. Lane 1. rhA3G proteins detected in a lysate from cells transfected with vector expressing rhA3G alone. Lane 2–5. rhA3G proteins detected in virus from cells transfected with vector expressing rhA3G and SHIVKU-2MC4 (lane 2), SHIVVif5A (lane 3), and SHIVVifHCCH(−) (Lane 4); and SHIVVifSTOP (lane 5). Lane 6. rhA3G proteins in pelleted supernatant from cells transfected with empty vectors.
Panel B. Blot in Panel A stripped and reprobed with an antibody against p27 protein.
Panel C. Results of incorporation of rhA3F. Lane 1. rhA3F proteins detected in a lysate from cells transfected with vector expressing rhA3F alone. Lane 2–5. rhA3F proteins detected in virus from cells transfected with vector expressing rhA3F and SHIVKU-2MC4 (lane 2), SHIVVif5A (lane 3), and SHIVVifHCCH(−) (Lane 4); and SHIVVifSTOP (lane 5). Lane 6. rhA3F proteins in pelleted supernatant from cells transfected with empty vectors.
Panel D. Blot in Panel C stripped and reprobed with an antibody against p27 protein.
Rhesus A3F is stable in the presence of SIVmac Vif
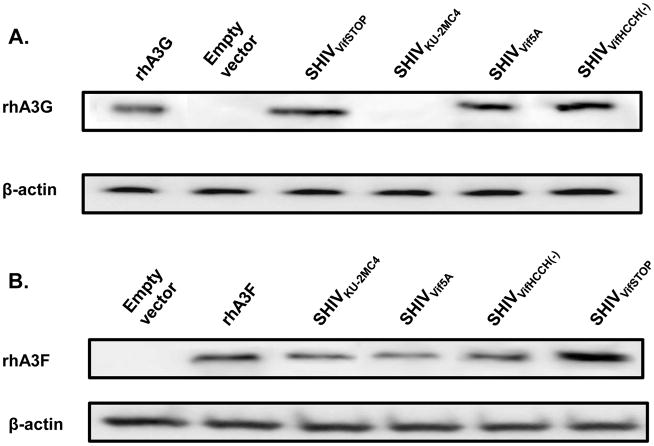
Since we observed that rhA3F was incorporated into virus particles, we determined the stability of rhA3F and rhA3G in the presence of the viral genome. 293 cells were co-transfected with vectors expressing rhA3G or rhA3F and the genomes of SHIVKU-2MC4, SHIVVif5A, SHIVVifHCCH(−), or SHIVVifSTOP. Our results indicate that in the presence of the SHIVKU-2MC4 genome, rhA3G was not stable whereas it was detected in the presence of the SHIVVif5A, SHIVVifHCCH(−) or SHIVVifSTOP (Figure 4). We also found that rhA3F appeared to be stable in the presence of both SHIVKU-2MC4 and also SHIVVif5A, SHIVVifHCCH(−), or SHIVVifSTOP genomes. However, it should be noted that we consistently found higher levels of rhA3F in the presence of the SHIVVifSTOP genome, indicating fundamental differences between the targeting site-directed Vif mutants and the absence of the Vif protein. The results obtained with rhA3F correlate well with the above experiment showing that rhA3F was incorporated into virus particles.
Figure 4.
Stability of the rhA3G and rhA3F in the presence of wild type and vif mutant viral genomes. Full-length mutated SHIVs were co-transfected into 293 cells with a vector expressing either HA-rhA3G or V5-rhA3F. At 24 hours post-transfection, cells were lysed and proteins were precipitated using methanol. Proteins were separated on a 10% SDS-PAGE gel and probed using an antibody directed against either the HA or V5-tag. All samples were normalized to the amount of β-actin protein. Panel A. Stability of rhesus APOBEC3G in the presence of SHIVVifSTOP (lane 3), SHIVKU-2MC4 (lane 4), SHIVVif5A (lane 5), or SHIVVifHCCH(−). Lanes 1 and 2 were used as a positive (rhA3G only; lane 1) or negative (pcDNA3.1(+) only; lane 2) control. Panel B. Stability of rhesus APOBEC3F in the presence of SHIVKU-2MC4 (lane 3), SHIVVif5A (lane 4), SHIVVifHCCH(−) (lane 5), or SHIVVifSTOP (lane 6). Lanes 1 and 2 were used as a negative (pcDNA3.1(+) only; lane 1) or positive (rhA3F only; lane 2) control.
Rhesus A3G but not rhA3F causes significant G-to-A mutations in the nef gene of the SHIV genome
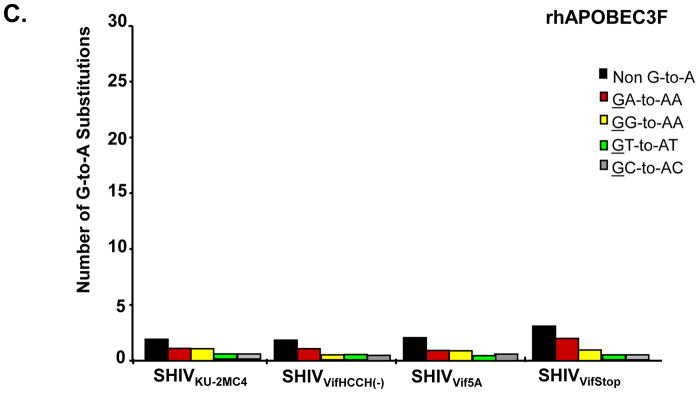
The results above indicated that significant levels of rhA3F were incorporated into virions and rhA3F was stable in cells expressing the wild type genome. We compared the level of G-to-A mutations of the SHIV genome in the presence of either rhA3G or rhA3F. The results are shown in Figure 5 and Table 1. Using viral genomes to express the Vif protein, we observed minimal G-to-A changes in SHIVKU-2MC4 nef in the presence of either rhA3G or rhA3F. In the presence of rhA3G, we found that SHIVVif5A, SHIVVifHCCH(−) or SHIVVifSTOP nef had an increase in the number of G-to-A changes (from 2 to 24–29) or approximately 0.5% of the bases sequenced (Table 1). However, in the presence of rhA3F, we observed that SHIVVif5A, SHIVVifHCCH(−), and SHIVVifSTOP nef had a similar level of G-to-A changes compared to SHIVKU-2MC4 (Table 1). The results with the rhA3F suggest that incorporation of rhA3F does not result in significant G-to-A substitutions in nef.
Figure 5.
Rhesus A3G but not rhA3F induces G-to-A mutations in single colony sequences obtained from SIVmac239 nef. Full length genomes of the wild type and vif mutant viruses were co-transfected with vectors expressing either rhA3G or rhA3F into 293 cells. At 48 hours post-transfection, the culture medium was collected, clarified by low speed centrifugation and used to infect TZM-bl cells. A 325 base pair fragment of SIVmac239 nef was amplified from DNA at 24 hours post-infection and assessed for G-to-A substitutions. Panel A. G-to-A mutations obtained from bulk (top line) and 15 independent clone sequences are shown. Each mutation is denoted by a vertical line that is color coded with respect to the dinucleotide context: GA (red), GG (yellow), GT (cyan), GC (gray) or non-G-to-A (black). B. Graph depicting the cumulative number of mutations from the 15 clones per virus in the presence of rhA3G. Each bar is shaded according to the proportion of G-to-A-substitutions that occurred in the context of GA (red), GG (yellow), GT (Cyan), GC (gray) or non-G-to-A (black). Panel C. Graph depicting the cumulative number of mutations from the 15 clones per virus in the presence of rhA3F.
Table 1.
Results of the hypermutation assay of nef amplified by rhA3G and rhA3F in the presence of wild type and mutant Vif proteins.
| APOBEC3 protein | Virus | a Total number of bases sequenced | Total # of G-to-A mutations/percent G-to-A mutations of bases sequenced | Number and context of cytidine deamination |
||
|---|---|---|---|---|---|---|
| 5′-TC | 5′-CC | 5′-GC | ||||
| rhA3G | SHIVKU-2MC4 | 4875 | 2 (0.04) | 1 | 1 | 0 |
| rhA3G | SHIVVif5A | 4875 | 24 (0.49) | 23 | 0 | 1 |
| rhA3G | SHIVVifHCCH(−) | 4875 | 26 (0.53) | 21 | 1 | 4 |
| rhA3G | SHIVVifSTOP | 4875 | 29 (0.59) | 27 | 2 | 0 |
| rhA3F | SHIVKU-2MC4 | 4875 | 2 (0.04) | 1 | 1 | 0 |
| rhA3F | SHIVVif5A | 4875 | 1 (0.02) | 1 | 0 | 0 |
| rhA3F | SHIVVifHCCH(−) | 4875 | 2 (0.04) | 1 | 1 | 0 |
| rhA3F | SHIVVifSTOP | 4875 | 3 (0.06) | 2 | 1 | 0 |
A 325 base fragment corresponding to the 5′ end of nef was amplified, cloned and 15 individual clones were selected for sequence analysis.
Incorporation of rhA3G into virions results in restriction of virus replication
We examined whether the growth of SHIVVif5A, SHIVVifSTOP, and SHIVVifHCCH(−) in the presence rhA3G or rhA3F restricted the production of infectious virus (Bishop et al., 2008; Russell et al., 2009). 293 cells were transfected with the genomes of SHIVVif5A, SHIVVifHCCH(−), SHIVVifSTOP, or SHIVKU-2MC4 and a plasmid expressing either an HA-tagged rhA3G, V5-tagged rhA3F or the empty vector. At 48 hours, the virus containing supernatant was collected, clarified by centrifugation, and the p27 levels quantified. Equivalent levels of p27 from each virus stock were used to titrate infectious virus by a series of 10-fold dilutions onto TZM.bl cells. At 48 hours, the cells were stained for β-galactosidase activity (β-galactosidase is under the control of the LTR and expression of Tat from the incoming virus) and the number of TCID50 determined. If rhA3G or rhA3F was packaged into the virions, the virus should be hypermutated resulting in the expression of a non-functional Tat. As shown in Figure 6A, SHIVKU-2MC4 replicated to approximately the same levels in the presence or absence (empty vector) of rhA3G while the titers of SHIVVifHCCH(−), SHIVVif5A and SHIVVifSTOP were 100 to 1000 fold less than the unmodified virus (SHIVKU-2MC4). With rhA3F, our results indicate that SHIVKU-2MC4, SHIVVifHCCH(−) and SHIVVif5A had an approximate 10-fold decrease in titers and SHIVVifSTOP had greater than a 1000-fold decrease in titer (Figure 6B). The results with rhA3F also emphasize the differences in site-directed mutations in SHIVVifHCCH(−), SHIVVif5A and the severely truncated Vif in SHIVVifSTOP.
Figure 6.
Incorporation of rhA3G results in less infectious virus. 293 cells were co-transfected with the SHIVKU-2MC4, SHIVVif5A, and SHIVVifHCCH(−) and SHIVVifSTOP genomes and plasmids expressing either rhA3G or rhA3F. Controls included co-transfection of each viral genome with empty plasmids (pcDNA3.1(+)). At 48 hours, the culture medium was harvested, assessed for p27, and the amount of infectious virus titrated on the TZM.bl cells as described in the Material and Methods section. Shown are infectious titers in the absence or presence of either rhA3G (Panel A) or rhA3F (Panel B).
Assessment of SHIVVifHCCH(−) replication in macaques
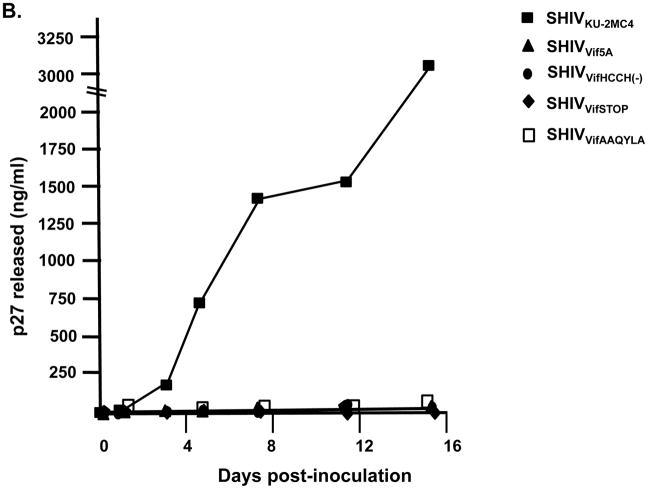
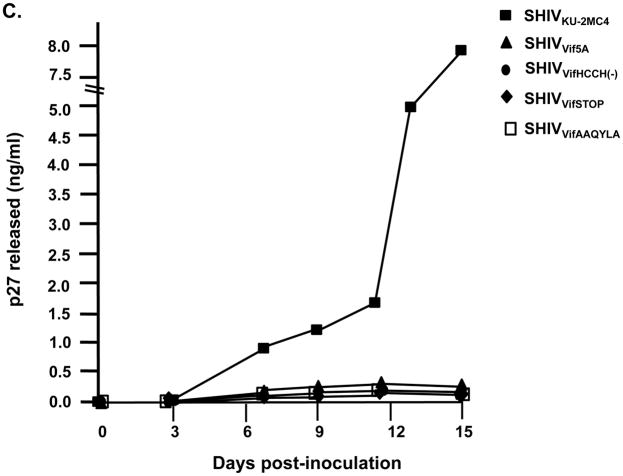
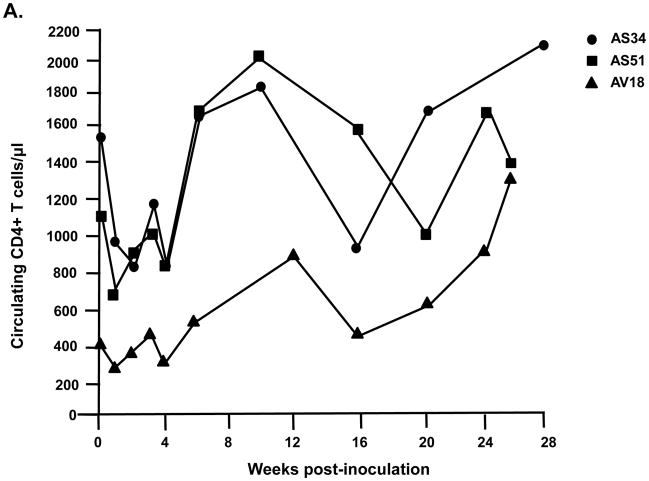
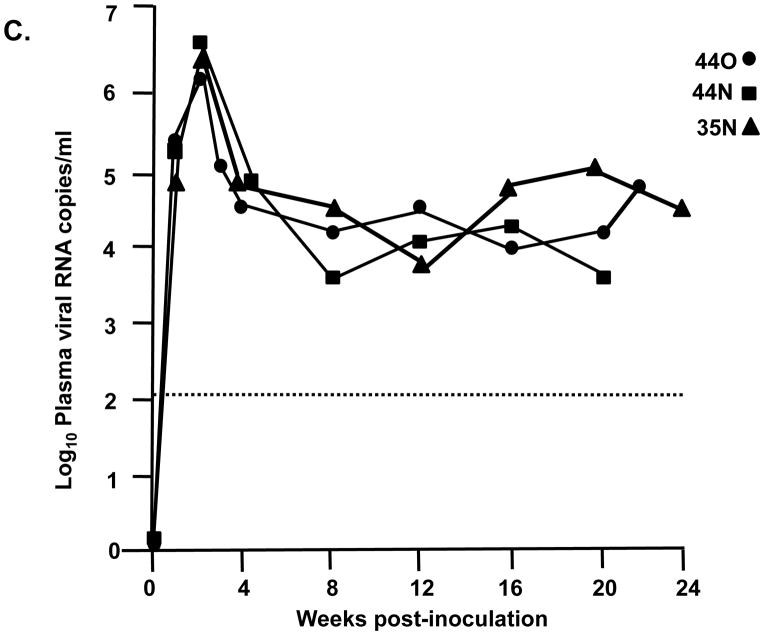
We inoculated three macaques with 104 TCID50 of SHIVVifHCCH(−). Prior to inoculation, macaques AS34, AS51, and AV18 had circulating CD4+ cell levels of 1,509, 1,109, and 400 cells per μl, respectively. These three macaques maintained levels of circulating CD4+ T cells at pre-inoculation levels or higher throughout the course of the 6 month infection (Figure 7A). All three macaques were euthanized at 26–28 weeks post-inoculation in excellent condition. At necropsy, AS34, AS51, and AV18 had circulating CD4+ T cell levels of 2,124, 1,338, and 1,298 cells/μl, respectively. This contrasts with macaques inoculated with parental SHIVKU-2MC4, which developed a severe loss of circulating CD4+ T cells within 3–4 weeks post-inoculation (Figure 7C). Analysis of the plasma viral loads revealed a mean viral load of 4.15 x103 copies per ml at one week post-inoculation (Figure 8A), which is approximately 10,000-fold less than the macaques inoculated with parental SHIVKU-2MC4 (Figure 8C). Following the first week of infection, the plasma viral loads in macaques AS34, AS51, and AV18 were at or just above the limits of detection. At necropsy, viral loads in all three macaques were undetectable. These results indicate that SHIVVifHCCH(−) transiently replicated in these macaques prior to control of the infection.
Figure 7.
Circulating CD4+ T cell levels in macaques inoculated with SHIVVif5A, SHIVVifHCCH(−), and SHIVKU-2MC4. Panel A. The levels of circulating CD4+ T cells in three macaques (AS34, ●; AS51, ■; and AV18, ▲) following inoculation with SHIVVifHCCH(−). Panel B. The levels of circulating CD4+T cells in three macaques (CX54, ●; ER65, ■; and I92, ▲) following inoculation with SHIVVif5A. Panel C. The levels of circulating CD4+ T cells in three macaques (44O, ●; 44N, ■; 35N, ▲) following inoculation with SHIVKU-2MC4.
Figure 8.
Plasma viral loads in macaques inoculated with SHIVVif5A, SHIVVifHCCH(−), and SHIVKU-2MC4 as determined by real time quantitative PCR. Panel A. Plasma viral loads in three macaques (AS34, ●; AS51, ■; and AV18, ▲) following inoculation with SHIVVifHCCH(−). Panel B. Plasma viral loads in three macaques (CX54, ●; ER65, ■; and I92, ▲) following inoculation with SHIVVif5A. Panel C. The plasma viral loads in three macaques (44O, ●; 44N, ■;35N, ▲) following inoculation with SHIVKU-2MC4.
Assessment of SHIVVif5A replication in macaques
We also assessed the ability of SHIVVif5A to replicate in three macaques (CX54, ER65, and I92). Prior to inoculation, macaques CX54, ER65, and I92 had circulating CD4+ T cell levels of 777, 844, and 1,451 cells per μl, respectively (Figure 7B). These macaques, similar to those inoculated with SHIVVifHCCH(−), maintained circulating CD4+ T cell levels near the pre-inoculation levels throughout the course of the 6 month infection. All three macaques were euthanized at 26 weeks post-inoculation in excellent condition. At necropsy, CX54, ER65, and I92 had circulating CD4+ T cell levels of 513, 853, and 1192 cells per μl, respectively (Figure 7B). This also contrasts with macaques inoculated with parental SHIVKU-2MC4 (Figure 7C). Analysis of the plasma viral loads of the macaques inoculated with SHIVVif5A revealed a mean viral load of 1.3 x103 copies per ml at one week post-inoculation (Figure 8B). Following the first week of infection, the plasma viral loads in macaques CX54, ER65, and I92 fell to undetectable levels.
Mutations in vif amplified from isolated DNA of PBMC during the course of infection
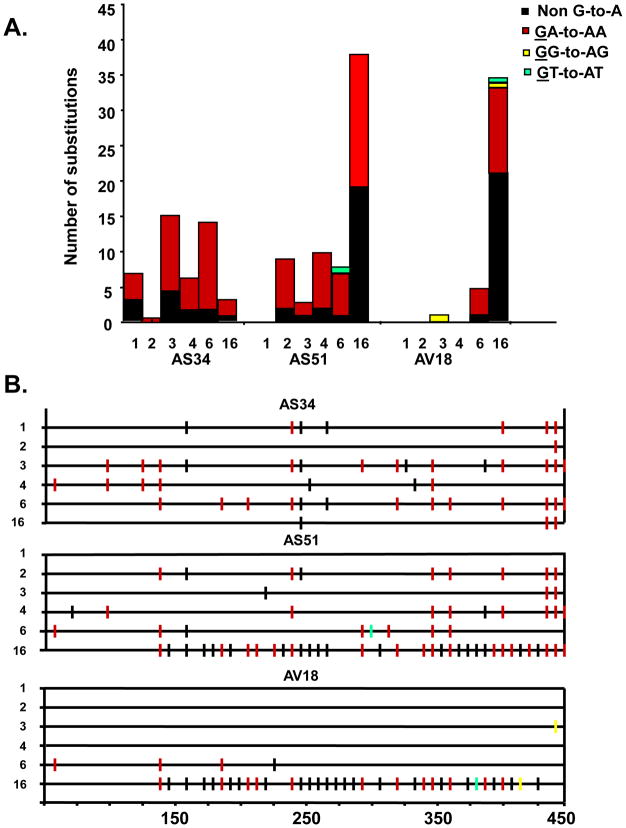
We assessed the stability of the engineered mutations in the vif gene from DNA isolated PBMC throughout the course of infection. The vif sequence was amplified, directly sequenced and compared to the input vif sequence of SHIVVif5A or SHIVVifHCCH(−). We observed that the engineered mutations were stable throughout the course of the six month infection for macaques inoculated with SHIVVifHCCH(−). We performed bulk sequencing of the vif amplified at 1, 2, 3, 4, 6, and 16 weeks. As shown in Figure 9, the number of G-to-A mutations in the amplified vif gene increased with time in two (AS51, AV18) of the three macaques while the third macaque (AS34) appeared to have the largest number of G-to-A mutations at 3 weeks post-inoculation. As we had reported earlier, the majority of the G-to-A mutations were in the context of the 5′-TC rather than 5′-CC (Schmitt et al., 2009). We were able to amplify part of the vif sequence at 1 week from PBMC DNA isolated from macaques inoculated with SHIVVif5A (CX54,ER65, I92). The sequence analysis also revealed that these vif mutations were stable at one week post-inoculation (data not shown). However, from 4 weeks until necropsy, we were unable to amplify the vif gene, which correlates with the undetectable plasma viral loads in these macaques after week 1 post-inoculation.
Figure 9.
Analysis of vif sequences amplified from DNA isolated from PBMC at various times post-inoculation. Panel A. Quantitative representation of mutations induced in bulk sequences obtained from a 450 base pair fragment of SHIVVifHCCH(−) Vif. Each vertical bar represents the total number of G-to-A substitutions. Each bar is shaded according to the proportion of G-to-A-substitutions the occurred in the context of GA (red), GG (yellow), GT (cyan), or non-G-to-A (black). Panel B. A 450 base pair fragment of the SIV Vif was amplified using DNA isolated from macaque PBMC inoculated with SHIVVifHCCH(−). Mutations from bulk sequence analysis from each macaque are shown. Each mutation is denoted by a vertical line that is color coded with respect to the dinucleotide context: GA-to-AA (red), GG-to-AG (yellow), GT-to-AT (cyan), and non-G-to-A mutations (black).
Macaques inoculated with SHIVVif5A and SHIVVifHCCH(−) developed anti-viral antibody responses early after inoculation
We analyzed the plasma from infected macaques at 12 weeks and at necropsy (26–28 weeks) for the presence of immunoprecipitating antibody responses. At 12 weeks post-inoculation, all three macaques inoculated with SHIVVif5A had developed antibody responses to p27 but only macaque ER65 developed antibody responses to the Env glycoprotein (Figure 10A). For macaques inoculated with SHIVVifHCCH(−), all three macaques developed antibodies to p27 and the Env glycoprotein (Figure 10A). At necropsy, we could not detect the presence of immunoprecipitating antibodies from macaques inoculated with either SHIVVif5A or SHIVVifHCCH(−) (Figure 10B). A macaque inoculated with parental SHIVKU-2MC4 (44O) did not develop antibodies to the virus at either time point, which is common for macaques that develop severe CD4+ T cell loss during the acute phase (<4 weeks) following inoculation with a pathogenic X4 SHIV (data not shown).
Figure 10.
Macaques inoculated with SHIVVif5A and SHIVVifHCCH(−) developed antibody responses against each virus early after inoculation. C8166 cells were inoculated with SHIVKU-2MC4 for 5 days, starved for methionine/cysteine and then radiolabeled overnight with 35S-methionine/cysteine. The culture medium was harvested and used in immunoprecipitation reactions with plasma from AS34, AS51, AV18, CX54, ER65, and I92 as described in the Materials and Methods section. The immunopreciptates were washed with 1X RIPA buffer, boiled in 2X sample reducing buffer, proteins were separated on 12% SDS-PAGE gels, and visualized using autoradiographic techniques. Panel A. SHIV proteins immunoprecipitated with plasma obtained at 12 weeks post-inoculation. The molecular weight standards are shown on the left. Panel B. SHIV proteins immunoprecipitated with plasma obtained at necropsy.
Histological examination of tissues and viral loads in visceral organs of macaques inoculated with SHIVVif5A and SHIVVifHCCH(−)
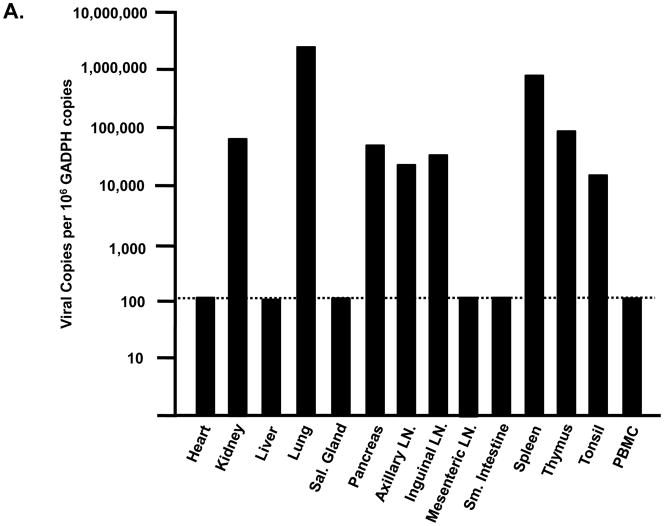
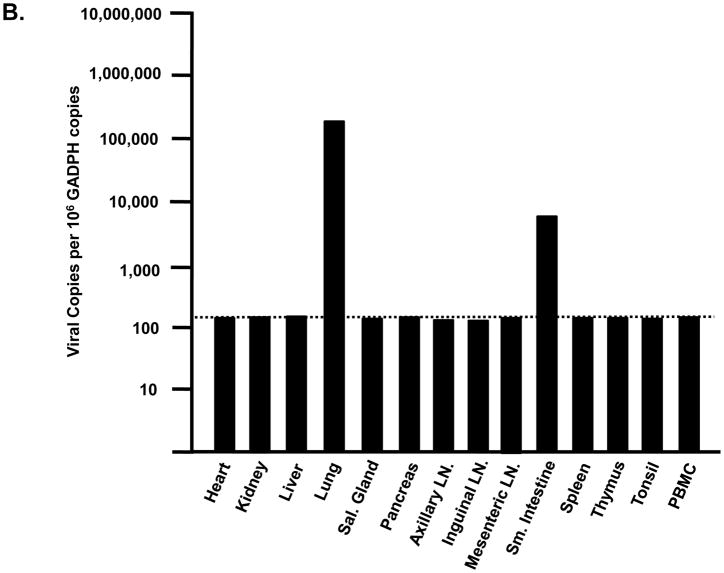
Tissues from infected macaques were examined for the presence of lesions consistent with this pathogenic X4 SHIV (Joag et al., 1998). Macaques inoculated with either SHIVVif5A or SHIVVifHCCH(−) did not exhibit histological lesions in any of the 13 visceral organs examined or in the CNS (data not shown). As the plasma viral loads were significantly less than macaques inoculated with SHIVKU-2MC4, we assessed the tissue distribution of these two viruses in macaques. RNA was isolated from 13 visceral organs from macaques inoculated with SHIVVif5A and SHIVVifHCCH(−), DNase I treated, and copy numbers determined by real time quantitative RT-PCR. The number of copies is expressed per 106 copies of GADPH. The results obtained from macaques inoculated with SHIVVifHCCH(−) are shown in Figure 11A-C. We found that macaque AS34 had eight tissues (kidney, lung, pancreas, axillary lymph node, inguinal lymph node, spleen, thymus and tonsil) greater than 1,000 copies per 106 copies of GAPDH (Figure 11A). Macaque AS51 was found to have two tissues (lung and small intestine) with greater than 1,000 copies per 106 copies of GAPDH, and macaque AV18 had six tissues (lung, pancreas, axillary lymph node, inguinal lymph node, mesenteric lymph node and spleen) with greater than 1,000 copies per 106 copies of GAPDH (Figure 11B-C). Interestingly, the lung had the highest viral copy number in each macaque. In contrast, we were unable to quantify viral RNA from tissues of macaques that were inoculated with SHIVVif5A (data not shown). Macaques inoculated with parental SHIVKU-2MC4, 44O and 44N, had high copy numbers of virus in various tissues (Figure 11D). Taken together, these results indicate that both SHIVVifHCCH(−) and SHIVVif5A infections were significantly less widespread compared to macaques inoculated with parental SHIVKU-2MC4.
Figure 11.
Comparison of viral copy numbers in the visceral tissues of macaques inoculated with SHIVVifHCCH(−) and SHIVKU-2MC4. RNA was isolated from different visceral organs of macaques inoculated with SHIVVifHCCH(−) or SHIVKU-2MC4, DNase I treated to remove residual DNA and used in real time quantitative RT-PCR using oligonucleotide primers specific for gag as described in the Materials and Methods. The levels of viral RNA are shown per 106 copies of GADPH RNA. Panel A-C. The results of the real time quantitative RT-PCR using RNA isolated from tissues of macaques AS34 (Panel A), AS51 (Panel B) and AV18 (Panel C), which were inoculated with SHIVVifHCCH(−). Panel D. The results of the real time quantitative RT-PCR using RNA isolated from tissues of macaque 44O, which was inoculated with SHIVKU-2MC4. The dotted line represents the limits of detection of the assay.
G-to-A mutations in nef and vpu amplified from DNA of primary and secondary lymphoid organs
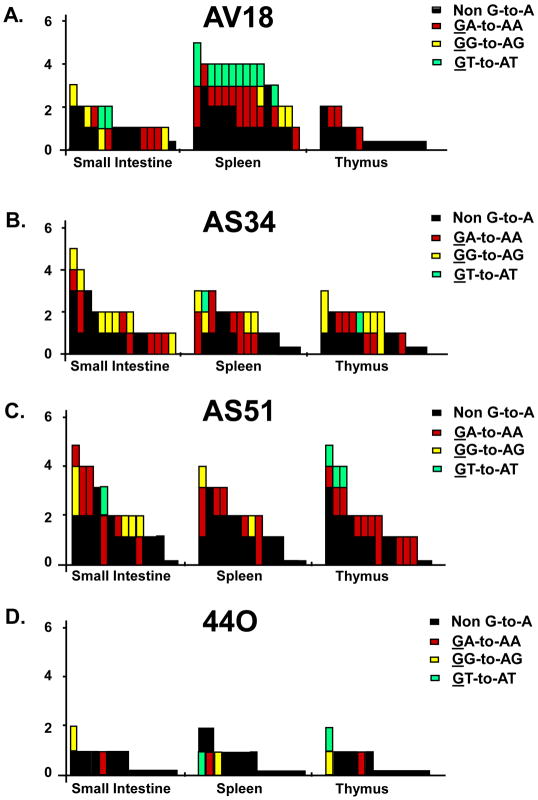
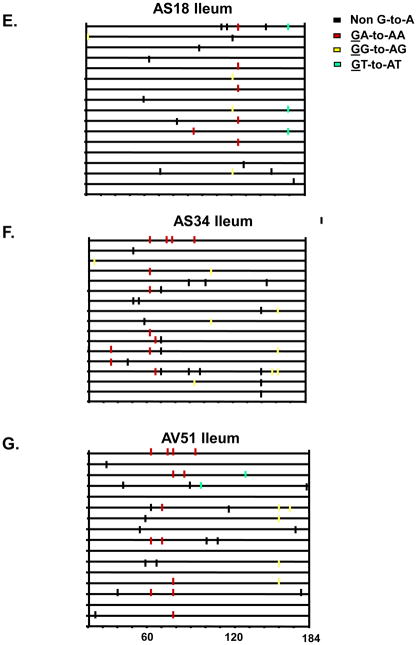
A well documented feature of HIV-1Δvif infection is the incorporation of select APOBEC3 proteins into viral particles leading to cytosine deamination during minus strand DNA synthesis (Yu et al., 2003). This results in hypermutation (guanosine to adenosine transitions) of the viral genome and inhibition of virus replication. We examined the number of G-to-A mutations in the nef and vpu genes amplified from DNA isolated from one primary lymphoid organ (thymus) and two secondary lymphoid organs (ileum of the small intestine and spleen) from the three macaques inoculated with SHIVVifHCCH(−) and one macaque inoculated with SHIVKU-2MC4. We first PCR amplified nef and vpu sequences from tissue DNA and directly sequenced the amplified products. The results showed that there were multiple G-to-A mutations in the nef and vpu regions amplified from three tissues. The number of G-to-A mutations ranged from 54 to 88 or 1.2 to 1.9 percent of the total base pairs sequenced (Figure 12; Table 2). While the percentage of mutations observed in the vpu was lower (perhaps reflecting the gradient of mutations from nef to vpu), the overall number of G-to-A mutations in the context of 5′-TC to 5′-CC was approximately 2:1 (75 versus 31 mutations) (Figure 13; Table 3). We confirmed the presence of these G-to-A mutations by sequencing 15 individual clones of the nef and vpu genes. We plotted the G-to-A mutations obtained from 15 single colony sequences of nef and vpu amplified from DNA isolated from the ileum of macaques inoculated SHIVVifHCCH(−) (Figures 12 and 13). These results showed that many of the G-to-A mutations found in the direct bulk sequencing products were also found in the individually cloned both genes (Figures 12 and 13). Similar to what we reported previously, the majority of the changes were in the context of 5′-TC (minus strand) (Table 2 and 3). Macaque 44O, (inoculated with SHIVKU2-MC4) had very few G-to-A substitutions in vpu and nef amplified from the same three lymphoid organs, which is similar to other macaques inoculated with this parental virus (unpublished observations). Macaque AV18 yielded the highest number of G-to-A substitutions (88) in the ileum of the small intestine. Taken together these findings suggest that one or more rhA3 proteins that have target sequence preference for 5′-TC is the major cytidine deaminase at work in vivo.
Figure 12.
Quantitative assessment of mutations in the nef gene.
Panels A-D. A 300 base pair fragment of nef was amplified using DNA isolated from the small intestine (ileum), spleen and thymus of macaques inoculated with SHIVVifHCCH(−). Mutations obtained using sequence analysis of fifteen independent clones from each macaque are shown. Each vertical bar represents an individual clone, whose height represents the total number substitutions. Each bar is shaded according to the proportion of G-to-A-substitutions the occurred in the dinucleotide context of GA (red), GG (yellow), GT (cyan), or non-G-to-A (black).
Panels E-G. Hypermutation plot illustrating the G-to-A and non-G-to-A substitutions in the clones from the ileum amplified DNA. Each mutation is denoted by a vertical line that is color coded with respect to the dinucleotide context: GG to AG (yellow), GA to AA (red), GT to AT (cyan), and non G to A (black).
Table 2.
Results of sequence analysis of nef amplified from the thymus, ileum, and spleen.
| Animal tissue | a Total number of bases sequenced | Total # of G-to-A mutations/percent G-to-A mutations of bases sequenced | Number and context of cytidine deamination |
||
|---|---|---|---|---|---|
| 5′-TC | 5′-CC | % 5′-TC to CC | |||
| AS34 | |||||
| Thymus | 4500 | 71/1.5 | 67 | 4 | 94.3 |
| Spleen | 4500 | 61/1.3 | 59 | 2 | 96.7 |
| Ileum | 4500 | 83/1.8 | 82 | 1 | 98.7 |
| AS51 | |||||
| Thymus | 4500 | 75/1.5 | 69 | 6 | 92.0 |
| Spleen | 4500 | 62/1.3 | 62 | 0 | 100 |
| Ileum | 4500 | 66/1.4 | 65 | 1 | 98.4 |
| AV18 | |||||
| Thymus | 4500 | 54/1.2 | 54 | 0 | 100 |
| Spleen | 4500 | 58/1.2 | 54 | 4 | 93.1 |
| Ileum | 4500 | 88/1.9 | 86 | 2 | 97.7 |
A 300 base fragment corresponding to the 5′ end of nef was amplified, cloned and 15 separate clones sequenced.
Figure 13.
Quantitative assessment of mutations in the vpu gene.
Panels A-D. A 184 base pair fragment of nef was amplified using DNA isolated from the ileum, spleen and thymus of macaques inoculated with SHIVVifHCCH(−) and one macaque inoculated with SHIVKU-2MC4. Substitutions were determined using sequence analysis of fifteen independent clones from each macaque are shown. Each mutation is denoted by a vertical line that is color coded with respect to the dinucleotide context: GG to AG (yellow), GA to AA (red), GT to AT (cyan), and non G to A (black).
Panels E-G. Hypermutation plot illustrating the G-to-A and non-G-to-A substitutions in the clones from the ileum amplified DNA. Each mutation is denoted by a vertical line that is color coded with respect to the dinucleotide context: GG to AG (yellow), GA to AA (red), GT to AT (cyan), and non G to A (black).
Table 3.
Results of sequence analysis of vpu amplified from the thymus, ileum, and spleen.
| Animal tissue | a Total number of bases sequenced | Total # of G-to-A mutations/percent of G- to-A mutations of bases sequenced | Number and context of cytidine deamination |
|||
|---|---|---|---|---|---|---|
| 5′-TC | 5′-CC | %5′-TC to CC | ||||
| AS34 | ||||||
| Thymus | 2760 | 13/0.47 | 6 | 6 | 50.0 | |
| Spleen | 2760 | 15/0.54 | 10 | 4 | 71.4 | |
| Ileum | 2760 | 15/0.54 | 8 | 7 | 53.3 | |
| AS51 | ||||||
| Thymus | 2760 | 14/0.51 | 11 | 0 | 100 | |
| Spleen | 2760 | 9/0.33 | 7 | 2 | 77.8 | |
| Ileum | 2760 | 15/0.54 | 9 | 5 | 64.2 | |
| AV18 | ||||||
| Thymus | 2760 | 3/0.12 | 3 | 0 | 100 | |
| Spleen | 2760 | 30/0.98 | 16 | 3 | 84.2 | |
| Ileum | 2760 | 11/.326 | 5 | 4 | 55.5 | |
A 184 base fragment corresponding to vpu was amplified, sub-cloned and 15 independent clones sequenced
DISCUSSION
There are only a limited number of studies that have analyzed the role of the Vif protein in the SIV or SHIV/macaque models of HIV-1 disease. In the first study, the role of various accessory proteins expressed from SIVmac239 were evaluated in terms of potential to cause disease (Desrosiers et al., 1998). These investigators found that an SIV with deletion of vif had only transient viral loads, could only be detected in PBMC for a short period of time and the macaques did not produce antibodies against SIV proteins. In a second study, investigators that showed that a vif-deleted virus would generate antibody and cellular immune responses if the virus was administered 2–3 times but was not protective against challenge (Sparger et al., 2008). In both studies, the lymphoid and non-lymphoid tissues were not examined for the presence of viral RNA from these vif-deleted viruses. Finally, we previously showed that the introduction of amino acid substitutions into the SLQYLA domain of Vif (to AAQYLA) resulted in a virus (SHIVVifAAQYLA) that did not replicate well in hA3G/F positive cells. However, following inoculation into macaques, the plasma viral loads were lowered from approximately 107 copies per ml for wild type virus to approximately 105 copies per ml for the SHIVVifAAQYLA during the acute phase of infection (Schmitt et al., 2009). In these macaques, the levels of circulating CD4+ T cells were maintained near pre-inoculation levels and they did not develop histological lesions consistent with a pathogenic X4 SHIV. Sequence analysis of the vif gene amplified from the PBMC revealed that the engineered AAQYLA motif had mutated to TAQYLA in two of three macaques. Since serine and threonine are hydroxylated amino acids that differ by a methyl group, it was possible that these Vif proteins may have been “partially” functional in vivo to permit limited spread within macaques. Examination of the tissues from these macaques revealed that viral RNA was present in select tissues, and the viral sequences contained low levels of G-to-A substitutions. We also showed that the inoculation of plasma from two of these macaques into a naive macaque did not result in detectable viral loads up to five months post-inoculation (Schmitt et al., 2009). Thus, despite the low level of viral replication in cell culture systems, SHIVVifAAQYLA was still capable of significant in vivo replication, thus validating the need to examine targeted Vif mutants in macaques.
In the present study, we have extended our in vivo studies using SHIV mutants that express either a Vif protein with the first five amino acids of the SLQYLA domain substituted with alanines (SHIVVif5A) or a Vif with the HCCH domain substituted with alanines (SHIVVifHCCH(−)). Interestingly, our restriction assays showed that incorporation of rhA3G but not rhA3F resulted in the greatest reduction of replication, which is somewhat different from the results previously reported (Zennou and Bieniasz, 2006). Our results indicate that unlike the parental virus, both viruses incorporated rhA3G while both the parental and mutant viruses incorporated rhA3F into virions. Our analysis of rhA3F and rhA3G stability in the presence of viral genome indicated that rhA3F is stable while rhA3G is degraded. The incorporation of rhA3F into wild type virions is in agreement with a previous study (Zennou and Bieniasz, 2006; Virgen and Hatzioannou, 2007). These paradoxical findings suggest that rhA3F can be incorporated in the presence of SIVmac Vif and that the virus can seemly replicate without the accumulation of lethal mutations. In the former study, these investigators found that rhA3F was resistant to the SIVmac Vif protein. They suggested that rhA3F may not exert a strong negative presence on SIVmac in vivo and that the SIVmac Vif may actually permit some degree of mutagenesis by rhA3F. This was supported by analysis of HIV-1 gag, pol, and env for potential editing sites (GG or GA) that would yield a termination codon if mutated. Their analysis revealed that the percentage of GA editing sites leading to termination codons was lower than GG sites in the gag, pol, and env. Analysis of the SIV gag, pol, vif, vpr, vpx, and nef (the SIV genes of our SHIV) also revealed this trend (unpublished observations) yet the mutations observed in the SIVmac nef were predominantly in the context of GA and not GG. Whether this was due to lethal GG-to-AG mutations that were subsequently cleared (or not detectable) or whether this was due to rhA3F or another rhA3 protein is unknown at this time. However, it should be noted that there were differences in the our study and the previous study. First, in the previous study the investigators used a HIV-1Δvif genome and we used an SIV-based viral genome with targeted mutations in the vif. We showed that replication of SHIVVifSTOP was much lower compared to SHIVVif5A and SHIVVifHCCH(−) in the presence of rhA3F. Thus, the results of SHIVVifSTOP in our study and HIV-1Δvif in their study is comparable. Our study also shows that our viruses with targeted mutations (SHIVVif5A and SHIVVifHCCH(−) ) were restricted less efficiently in the presence of rhA3F compared to SHIVVifSTOP, suggesting that the presence of an intact, “crippled” Vif protein can impose some restriction compared to having a Δvif virus. This may relate to the ability of the VifHCCH(−) and the Vif5A proteins to still bind to rhA3F. Previous studies have defined regions of the HIV-1 Vif that are required for binding various hA3 proteins. Many of these regions are in the amino terminal region of the protein. Previous studies demonstrated that HIV-1 Vif residues 40YRHHY44 and 12QVDRMR17 are important for the interaction with hA3G and hA3F/C, respectively (Russell and Pathak, 2007; Mehle et al., 2007). In a more recent study, a conserved YXXL domain at residues 69–72 of the HIV-1 Vif was found to be important for the interaction of Vif with hA3G, hA3F and hA3C (Pery et al., 2009). While the 12QVDRMR17 and 40YRHHY44 are not conserved in the SIVmac239 Vif, there is a lysine at position 27 of the SIVmac Vif that is required for interaction with rhA3G (Chen et al., 2009; Dang et al., 2009). The YWGL domain of the HIV-1 Vif is also conserved in the SIV Vif (as YWHL). Thus, it is possible that the Vif proteins with targeted amino acid substitutions may still bind and incorporate rhA3Finto virus particles but may be inefficient in deamination because of its association with Vif. A second difference in the two studies is the hypermutation assay used to analyze the percentage of G-to-A changes in the genome. While the previous study showed that rhA3F caused G-to-A hypermutation at levels higher than rhA3G, we found that rhA3F produced little hypermutation in the viral genome. In the previous study, these investigators used a VSV pseudotyped HIV-1 vector with the vif deleted as well as other genes and analyzed hrGFP sequences. In our system, we used complete SIV viral genomes expressing the Vif mutant.
One goal of these studies was to determine if SHIVVif5A was effectively controlled by macaques during the primary phase of infection or if viral variants would emerge that would permit limited replication in macaque tissues. Our results indicate that following inoculation of SHIVVif5A into macaques, there was a further reduction of plasma viral loads compared to SHIVVifAAQYLA (Schmitt et al., 2009). The mean peak plasma virus load (week 1) was 1.3 × 103 viral copies per ml or approximately 4,000-fold lower than parental SHIVKU-2MC4. However, following this initial burst of replication, viral RNA from macaques inoculated with SHIVVif5A was near or below the limits in this assay (~180 copies). It should be noted that the virus was not completely eliminated because viral RNA was occasionally detected in the plasma throughout the 6-month infection using nested RT-PCR (data not shown). At necropsy, the number of copies of viral RNA could not be quantified from various organs of these macaques (detection limit 180 copies) but could be detected by nested RT-PCR. We were unable to detect the virus in PBMC by 3 weeks post-inoculation and were unable to amplify the nef gene at necropsy for sequence analysis. While the results presented here may be predicted based on studies in cell culture systems, these results demonstrate for the first time that a primate lentivirus expressing a Vif protein with only targeted mutations in a critical domain can be completely controlled by the host. Taken together, these results indicate that the SHIVVif5A replicated similarly to a virus that does not express a functional Vif protein (Desrosiers et al., 1998).
No studies have analyzed the role of the Zn++ binding domain of Vif in vivo. Previous studies have shown that this domain is critical to Vif function, interacting with Cul 5 of the Cul 5/ElonginB/C/Rbx E3 ligase (Luo et al., 2005; Xiao et al., 2007). Our results showed that these macaques had slightly higher initial viral loads at one week post-inoculation than macaques inoculated with SHIVVif5A but that the virus was effectively controlled by the macaques by 3 weeks post-inoculation. Similar to our previous study, the majority of the G-to-A mutations were in the context of 5′-TC (minus strand) and not 5′-CC. In addition, we observed that the majority of the G-to-A changes found in the vif gene amplified from PBMC DNA occurred during the first weeks of infection. This correlated well with the plasma viral loads which indicated the viral replication primarily occurred during this time period.
Taken together, our results show that abolishing the SLQYLA domain of Vif may be more detrimental to the virus in vivo than the HCCH domain. These data bring up the question, “Why were we able to quantify viral RNA from tissues of macaques inoculated with SHIVVifHCCH(−) but not SHIVVif5A?” While the answer is unknown, several possibilities exist. First, the virus may have initially replicated in cells expressing active rhA3 proteins and incorporated one or more rhA3 proteins into the viral progeny. In the next round of replication the genome was mutated but not to the extent that RNA polymerase II could not transcribe viral RNA. Second, there may be a tissue/cellular reservoir for virus replication that do not express the rhA3 proteins that would restrict the crippled SIVmac239 Vif protein. We previously showed using immunohistochemistry that rhA3G was not expressed in all cell types in the brain and kidneys (Hill et al., 2006;2007). Finally, during the initial rounds of replication and potential deamination, G-to-A mutations may have led to compensating mutations that made the viral Vif partially functional. The later scenario is not likely as our sequence analysis did not identify consensus mutations in either the SHIVVif5A or SHIVVifHCCH(−)-inoculated macaques.
As previous studies showed that antibody responses were not generated with a single inoculation of vif-deleted viruses (Desrosiers et al., 1998; Sparger et al., 2008), we determined if a single inoculation of either virus would result in an antibody response against viral proteins. Comparison of the antibody responses from macaques inoculated with either SHIVVifHCCH(−) or SHIVVif5A shows that at 12 weeks post-inoculation macaques had developed antibody responses against the virus but these were undetectable at necropsy. This would argue that the viral RNA detected in tissues at necropsy may not have been translated into viral proteins. This would also suggest that infectious virus was cleared by the macaque and that antigenic stimulation through viral proteins did not occur. However, this study provides evidence that such viruses containing site-directed mutations in vif could be used to “prime” the immune system and suggests that such a virus could be useful in a prime-boost strategy. It will be of interest to determine if multiple inoculations will result in stronger immune responses (both humoral and cellular) and a further reduction of viral loads with increasing inoculations. If successful, it will be of interest to determine if the immune responses are protective against challenge. While the use of live attenuated lentiviruses has led to useful information concerning the role of accessory genes in replication of the virus in vivo, the use of such vaccines has several underlying risks (Koff et al., 2006). First, these vaccines have relied on the deletion/disruption of accessory genes (nef, vpu, vpx/vpr) that enhance replication but are not absolutely required for replication in vivo. For this reason these viruses can select for compensating mutations that ultimately result in the virus becoming pathogenic (Baba et al., 1995; 1999). The second problem is the risk of recombination of the viral genome to yield a pathogenic virus (Kim et al., 2005). A vaccine based on a “crippled Vif” that allows for limited replication resulting in G-to-A substitutions and inactivation of the virus should prevent the scenarios discussed above. It would be of interest to determine if inoculation of macaques with two viruses, one harboring a mutation in vif and another having a mutation in another accessory gene such as nef can recombine in vivo to generate a wild type virus causing CD4+ T cell loss and disease prior to the virus accumulating G-to-A mutations.
MATERIALS AND METHODS
Cells, plasmids, and viruses
The C8166 and SupT1 lymphocyte cell lines were used for transfections of full-length SHIVs and as indicator cells to measure infectivity and cytopathicity of the viruses used in this study. Both cell lines were maintained in RPMI-1640, supplemented with 10 mM Hepes buffer pH 7.3, 2 mM glutamine, 5 μg per ml gentamicin, 100 units/μg penicillin-streptomycin and 10% fetal bovine serum (R10FBS). Rhesus macaque PBMCs were obtained from uninfected animals and isolated on Ficoll/Hypaque gradients for p27 growth curves. The 293 cell line was maintained in Dulbecco’s minimal essential medium (DMEM) with 10% fetal bovine serum and the antibiotics described above. The derivation of SHIVKU-2MC4 has been previously described (Joag et al., 1998). The pcDNA3.1(+)-rhesus APOBEC3G-HA and pcDNA3.1(+)-rhesus APOBEC3F-V5 were kindly provided by Nathaniel Landau (New York University School of Medicine, New York, New York).
Construction of SHIVVif5A, SHIVVifHCCH(−) and SHIVVifSTOP
For the construction of SHIVVifHCCH(−), the PacI/SphI fragment (nucleotides 5132 to 6452) from the p5′-SHIV4 was subcloned into the pGEM3Zf (+) vector. The histidine to alanine substitution at position 110 of Vif was introduced using oligonucleotides (only sense strand shown) 5′-GCAGACATTTTACTGGCTAGCACTTATTTCC- 3′. The cytidine to alanine substitution at position 116 of Vif was introduced using oligonucleotides (only sense strand shown) 5′-GCACTTATTTCCCTGCCTTTACAGCGGGAG- 3′. The cysteines at positions 134 and 135 of Vif were substituted for alanines using the oligonucleotides (only sense strand shown) 5′-CAACTGCTGTCTGCCGCCAGGTTCCCG-3′. The histidine to alanine substitution at position 144 of Vif was introduced using oligonucleotides (only sense strand shown) 5′-GGTTCCCGAGAGCTGCTAAGTACCAGGTACC -3′. All substitutions were made using the Quick-Change Mutagenesis Kit (Stratagene) following the manufacturer’s instructions. The resulting PacI/SphI fragment was digested, isolated, and subcloned into full length SHIVKU-2MC4. The resulting plasmid was sequenced to ensure that the desired mutations were introduced as expected.
For the construction of SHIVVif5A, the PacI/SphI fragment (nucleotides 5132 to 6452) from full-length SHIVKU-2MC4 was subcloned into the pGEM3zf (+) vector. The serine and leucine at positions 147 and 148 were substituted for alanines using the oligonucleotides (sense strand shown) 5′-CCAGGTACCAGCCGCACAGTACTTAGCAC-3′. The glutamine and tyrosine at positions 149 and 150 were substituted for alanines using the oligonucleotides (sense strand shown) 5′-CCAGGTACCAGCCGCAGCGGCCTTAGCACTGAAAGTAGTAAGC-3′. The leucine at position 151 was substituted for an alanine using the oligonucleotide (sense strand shown) 5′-GGTACCAGCCGCAGCGGCCGCAGCACTGAAGTAGTAAGCG-3′. All substitutions were made using the Quick-Change Mutagenesis Kit (Stratagene) following the manufacturer’s instructions and virus constructed and prepared as described above.
For the construction of SHIVVifSTOP, the tyrosine and leucine at amino acid positions 28 and 29 were engineered to have stop codons using site-directed mutagenesis in order to produce a full-length SHIV that does not express a functional Vif protein. The oligonucleotide used to introduce these mutations was 5′-GCCTCATTAAAATAGTAGAAATATAAAACTAAAG-3′ (only sense strand shown). The substitutions were made using the Quick-Change Mutagenesis Kit (Stratagene) following the manufacturer’s instructions. The resulting PacI/SphI fragment was digested, isolated, and subcloned into full length SHIVKU-2MC4. The resulting plasmid was sequenced to ensure that the desired mutations were introduced as expected. For all three viruses, the plasmids containing the full-length genomes were transfected into SupT1 cells as previously described (Stephens et al., 2002; Hout et al., 2005). Stocks of SHIVVifHCCH(−), SHIVVif5A, and SHIVVifSTOP were prepared, tittered on SupT1 cells, and stored at −86°C.
Analysis of the replication of SHIVVif5A and SHIVVifHCCH(−) in APOBEC3G/F positive and negative cells
C8166 (A3G/F positive) and SupT1 (A3G/F negative) cells were inoculated with equivalent levels (25 ng) of parental SHIVKU-2MC4, SHIVVif5A, SHIVVifHCCH(−), SHIVVifAAQYLA, or SHIVVifSTOP for 4 hours at 37C. For rhesus PBMC, cells were isolated on Ficoll-Hypaque gradients, stimulated for 48 hours in R10FBS supplemented with concanvalin A (10 μg/ml) and interleukin-2 (IL-2; 50 ng/ml). The cells were washed, and inoculated with virus (25 ng) incubated in R10FBS containing interleukin-2 (50 ng/ml) for 4 hours. At 4 hours, cells were centrifuged, washed 3 times to remove the inoculum and incubated in fresh medium (medium for the rhesus PBMC also contained 50 ng/ml IL-2) at 37C for up to 15 days. Aliquots of culture supernatants were obtained at 0, 1, 3, 5, 7, 9, 11, 13, and 15 days post-inoculation and the levels of p27 assessed using commercial p27 antigen capture kits (Zeptometrix).
APOBEC3G/F incorporation assays
Plasmids containing the genomes viruses (derived from SHIVKU-2MC4) expressing each of the mutant Vif proteins described above were co-transfected into 293 cells using PEI Transfection reagent (Ex-Gen 500) along with plasmids expressing either HA-rhA3G or V5-rhA3F. At 48 hours, virus containing supernatants were harvested and clarified by low speed centrifugation. The clarified supernatant was then subjected to ultracentrifugation to pellet the virus (SW41 rotor, 247,000xg, 1 hour). The pellet was resuspended in PBS (pH 7.4) and layered on a 20/60% sucrose step gradient and again subject to ultracentrifugation (SW55Ti, 247,000xg, 1 hour). The virus (at the interface) was harvested, pelleted again by ultracentrifugation described above, and resuspended in 200μl of 1x PBS (pH 7.4). An aliquot was saved to determine the p27 content by antigen capture assay (Zeptometrix). The remaining sample was boiled in sample reducing buffer. Equivalent amounts of p27 were loaded on a 12% SDS-PAGE gel and transferred to PVDF membranes. APOBEC3 proteins were detected by Western blotting using an antibody directed against either the HA tag (HA-probe; Santa Cruz) or V5 tag (Clone V5-10; Sigma). Blots were stripped in 1X stripping buffer (62.5 mM Tris-HCl, pH 6.8 and 2% SDS) and reprobed using a rabbit polyclonal antibody specific for p27. As a control, plasmids expressing either rhA3G or rhA3F were transfected into 293 cells using PEI transfection reagent (ExGen500, Fermentas). At 48 hours post-transfection, cells were lysed in 1x RIPA and the nuclei were removed. Whole protein was precipitated with methanol and resuspended in 2x sample buffer. Normalized to β-actin, the samples were run on a 12%-SDS-PAGE gel, and probed with the antibodies stated above.
Stability of rhA3G and rhA3F in the presence of SHIV genomes
We determined if rhA3G or rhA3F were stable in the presence of SHIVKU-2MC4, SHIVVifHCCH(−), SHIVVif5A, or SHIVVifSTOP. Full-length mutated SHIVs were co-transfected in a 2:1 ratio with either APOBEC3G-HA or APOBEC3F-V5 using polyethylenimine transfection reagent (PEI, Fermentas). Twenty-four hours post-transfection, the supernatant was removed and the cells were harvested and lysed using 1 × RIPA (50 mM Tris-HCl, pH 7.5; 50 mM NaCl; 0.5% deoxycholate; 0.2% SDS; 10 mM EDTA). Following lysis, the nuclei were removed by microcentrifugation at 1400 rpm for 15 minutes. The protein was prepared using methanol, resuspended in 2X sample reducing buffer, and boiled for 5 minutes. Proteins were separated on a 10% SDS-PAGE gel and probed using commercially available rabbit polyclonal HA antibody (HA-probe, Santa Cruz) for rhA3G-HA or mouse monoclonal V5 antibody (Clone V5-10, Sigma) for rhA3F. All samples were normalized to the amount of β-actin protein using a mouse monoclonal antibody (Novous Biologicals) specific for β-actin.
Hypermutation assays in the presence of rhA3G or rhA3F
Hypermutation of various SHIVs in the presence of rhesus APOBEC3G/F 293 cells were transfected with SHIVKU-2MC4, SHIVVifHCCH(−), SHIVVif5A, or SHIVVifSTOP in the presence of either rhA3G or rhA3F using polyethylenimine transfected reagent (PEI, Fermentas). Twenty-four hours post-transfection, cells were washed and fresh DMEM was added. After forty-eight hours, the supernatant containing virus was subjected to low speed centrifugation. The resulting supernatant was DNase-I—treated (Fermentas) at 37C for 30 minutes to eliminate any trace of plasmid carry-over from the initial transfection. The DNase-I-treated supernatant was titrated on TZM-bl cells to both assess infectivity and hypermutation. Twenty-four hours post-infection, total DNA cellular DNA was harvested and extracted using the DNeasy kit and the manufacturer’s instructions (Qiagen). The DNA was used in a nested DNA polymerase chain reaction to amplify a 300 base pair fragment of nef. The PCR reaction was carried out using rTaq, the manufacturer’s instructions (Takara), and the oligonucleotides listed below. 1 μl of the first PCR product was added to a nested reaction. The PCR reactions were perfomed using an Applied Systems 2720 Thermal Cycler with the following thermal profile: 95C for 2 minutes, 1 cycle; 95C for 30 seconds, 48C for 30 seconds, 65C for 2 minutes, 35 cycles; 65C for 7 minutes. The PCR products were separated by electrophoresis, isolated, purified, sequenced and sub-cloned into pGEM-TEasy (Promega) as described below. Fifteen independent clones were sequenced and assessed for each mutant SHIV as described above.
Restriction of Vif Mutants on rhA3G or rhA3F
We determined the effect of the Vif mutants on the suppression of rhA3G and rhA3F. Full-length mutated SHIVs were co-transfected with either rhA3G or rhA3F using PEI transfection reagent (ExGen500, Fermentas). Forty-eight hours post-transfection, the supernatant was harvested and purified by low speed centrifugation. Equivalent amounts of p27 were serially diluted using 10-fold dilutions from 101 to 106 and used to inoculate TZM-bl cells. Forty-eight hours later, the media was removed, cells washed with 1X PBS and the monolayer fixed using 1% formaldehyde-0.2% glutaraldehyde in phosphate-buffered saline (1 × PBS). The cells were washed and incubated in a solution for 2 hours at 37C in 4 mM potassium ferrocyanide, 4 mM potassium ferricyanide, 4 mM magnesium chloride, and 0.4 mg X-gal per ml. The reaction was stopped and the number of TCID50 were calculated (Derdeyn et al., 2000; Wei et al., 2002).
Macaques analyzed in this study
Three rhesus macaques (Macaca mulatta: CX54, ER65, and I92) were intravenously inoculated with 1 ml of undiluted culture supernatant from SupT1 grown virus stocks (containing 104 TCID50 per ml). Three additional rhesus macaques (AS34, AS51, and AV18) were inoculated with 104 TCID50 SHIVVifHCCH(−) (titered in SupT1 cells). The animals were housed in the AAALAC-approved animal facility at the University of Kansas Medical Center. All aspects of the animal studies were performed according to the institutional guidelines for animal care and use at the University of Kansas Medical Center. Heparinized blood was collected weekly for the first 4 weeks, then at 2 week intervals for the next month and at monthly intervals thereafter.
Assays for circulating CD4+ T cells
Changes in the levels of CD4+ T cells after viral inoculations were monitored sequentially by flow cytometric analysis (BD Biosciences). T cell subsets were labeled with a commercially available anti-CD3/CD4/CD8 mixture. T cell subsets from a normal uninfected macaque were always performed at the same time to serve as a control for the flow cytometry analysis.
Processing of tissue samples at necropsy
At the time of euthanasia (26 or 28 weeks), all macaques in this study were anesthetized by administration of 10 mg/kg ketamine (IM) followed by intravenous administration of sodium phenobarbital at 20–30 mg/kg. At the time of necropsy, all macaques were in a healthy condition. A laparotomy was performed. The animal was exsanguinated by aortic canulation and perfused with one liter of cold Ringer’s saline. Lymphoid and non lymphoid tissues (heart, kidneys, liver, lungs, mesenteric, inguinal and axillary lymph nodes, pancreas, salivary gland, small intestine, spleen, thymus, and tonsils) were obtained and aliquots of tissue snap frozen for DNA and RNA assays. Aliquots of lymphoid tissues were immersed in HBSS to quantify levels of infectious virus in tissues using infectious centers assays with SupT1 cells.
Sequence analysis of the vif, nef, and vpu genes
To determine the stability of the mutations that were introduced into vif and assess whether these macaques aquired G-to-A mutations, the vif, nef, and vpu genes were amplified from either PBMCs at different time points during infection (vif) or from several tissue DNA samples taken at necropsy (nef and vpu) that were positive for viral RNA. One hundred nanograms of extracted genomic DNA was used in a nested DNA polymerase chain reaction (Takara, Madison, WI) following the manufacturer’s instructions. The oligonucleotides employed during the first round to amplify vif were 5′-GGCTAAAATTATCAAAGATTATGGAGG-3′ (sense) and 5′-GGTGACATCCCTTGTTCATCATGCC-3′ (antisense), which corresponds to bases 5326–5352 and 5984–6008, respectively. The nested oligonucleotides were 5′-GGAGGAGGAAAAGAGGTGGATAGCAGTTCCC-3′ (sense) and 5′-CCAGTATTCCCAAGACCTTTGCC-3′ (antisense), which corresponds to bases 5348–5378 and 5963–5985, respectively. The oligonucleotides used during the first round to amplify the nef gene were 5′-GGTGGAGCTATTTCCATGAGG-3′ (sense) and 5′-GTCTTCTTGGACTGTAATAAATCCC-3′ (antisense), which corresponds to bases 9445–9465 and 9832–9856, respectively. The nested oligonucleotides were 5′-CCATGAGGCGGTCCAGGCAGTCTAGAG-3′ (sense) and 5′-CCTCCCAGTCCCCCCTTTTC-3′ (antisense), which corresponds to bases 9458–9484 and 9814–9833, respectively. The oligonucleotides used during the first round to amplify the vpu gene were 5′-CCTAGACTAGAGCCCTGGAAGCATCC-3′ (sense) and 5′-GTACCTCTGTATCATATGCTTTAGCAT-3′ (antisense), which corresponds to bases 6486–6511 and 7034–7061, respectively. The nested oligonucleotides used were 5′-TTAGGCATCTCCTATGGCAGGAAGAAG-3′ (sense) and 5′-CACAAAATAGAGTCCTGGTTGCTTCCT-3′ (antisense), which corresponds to bases 6597–6623 and 7001–7027, respectively.
For sequence analysis, the PCR products from three separate PCR reactions were pooled and separated by electrophoresis in a 1.5% agarose gel, isolated, and each PCR reaction directly sequenced. Cycle sequencing reactions using the BigDye Terminator Cycle Sequencing Ready Reaction Kit with AmpliTaq DNA polymerase, FS (PE Applied Biosystems, Foster City, CA) sequence detection was conducted with an Applied Biosystems 377 Prism XL automated DNA sequencer and visualized using the ABI Editview program. Sequences were compared to the sequence of SHIVKU-2MC4. A total of 615 nucleotides were analyzed from vif, 300 nucleotides analyzed from nef, and 184 nucleotides from vpu using the SE Central Software package. In order to isolate and analyze single sequences, bulk PCR reactions were subcloned into the pGEM-TEasy (Promega) cloning vector. Fifteen clones were selected, sequenced as described above, and analyzed to assess the number of G-to-A substitutions that occurred.
Plasma virus loads
Plasma viral RNA loads were determined on RNA extracted from 1 ml of EDTA-treated plasma. Virus was pelleted using ultracentrifugcation (Beckman SW55Ti, 250,000xg, 2 hours) and RNA extracted using the Qiagen viral RNA kit (Qiagen). RNA samples were analyzed by real-time RT-PCR using gag primers and a 5′FAM and 3′TAMRA labeled Taqman probe that is homologous to the SIV gag gene as previously described (Hofmann-Lehmann et al., 2000). Standard curves were prepared using a series of six ten-fold dilutions of viral SIV gag RNA of known concentration. The sensitivity of the assay was 100 RNA equivalents per ml. Samples were analyzed in triplicate and the number of RNA equivalents per ml of plasma were calculated. Viral RNA was also quantified from in visceral tissues using this primer/probe reaction. In order to quantify the amount of viral RNA, GAPDH was used as a control and data is presented as copies per 106 GAPDH molecules (Marcario et al., 2008).
Immunoprecipitation assays
To determine if the macaques developed antibodies to SHIV proteins following inoculation, the plasma at 12 weeks and at necropsy was used in immunoprecipitation assays. C8166 cells were inoculated with approximately 104 TCID50 of SHIVKU-2MC4 for 5 days. The cells were then incubated in methionine/cysteine-free media for 2 hours and radiolabeled labeled with 500 μCi of 35Smethionine/cysteine for 15 hours. The cells were lysed in 1ml of 1X RIPA buffer and nuclei were removed by centrifugation. The cell lysates were incubated overnight at 4°C with 10 μl of plasma from each macaque and protein A Sepharose beads. The immunoprecipitates bound to the beads were washed three times in 1X RIPA, resuspended in 75 μl of 2X sample reducing buffer, and boiled for 5 minutes. Proteins were separated on a 10% SDS-PAGE gel and visualized by autoradiography. Controls included pooled prebleed plasma from macaques (negative control) and plasma from macaques that had been previously inoculated with a non-pathogenic SHIV (SHIVTM, positive control).
Acknowledgments
The work reported here is supported by grants NIH grants AI64019 and AI51981 to E.B.S. The pcDNA3.1(+)-rhesus A3G-HA and pcDNA3.1(+)-rhesus A3F-V5 were kindly provided by Nathaniel Landau (New York University School of Medicine, New York, New York). The TZM-bl cells were obtained through the NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH and was from Drs. John C. Kappes and Xiaoyun Wu and Transzyme, Inc. We thank members of the KUMC Biotechnology Support Facility for their assistance with the sequence analysis and oligonucleotide synthesis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baba TW, Jeong YS, Pennick D, Bronson R, Greene MF, Ruprecht RM. Pathogenicity of live, attenuated SIV after mucosal infection of neonatal macaques. Science. 1995;267:1820–1825. doi: 10.1126/science.7892606. [DOI] [PubMed] [Google Scholar]
- Baba TW, Liska V, Khimani AH, Ray NB, Dailey PJ, Penninck D, Bronson R, Greene MF, McClure HM, Martin LN, Ruprecht RM. Live attenuated, multiply deleted simian immunodeficiency virus causes AIDS in infant and adult macaques. Nat Med. 1999;5:194–203. doi: 10.1038/5557. [DOI] [PubMed] [Google Scholar]
- Bishop KN, Verma M, Kim EY, Wolinsky SM, Malim MH. APOBEC3G inhibits elongation of HIV-1 reverse transcripts. PLoS Pathog. 2008;4:e1000231. doi: 10.1371/journal.ppat.1000231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borman AM, Quillent C, Charneau P, Dauguet C, Clavel F. Human immunodeficiency virus type 1 Vif-mutant particles from restrictive cells: role of Vif in correct particle assembly and infectivity. J Virol. 1995;69:2058–2067. doi: 10.1128/jvi.69.4.2058-2067.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanc D, Patience C, Schulz TF, Weiss R, Spire B. Transcomplementation of VIF-HIV-1 mutants in CEM cells suggests that VIF affects late steps of the viral life cycle. Virology. 1993;193:186–192. doi: 10.1006/viro.1993.1114. [DOI] [PubMed] [Google Scholar]
- Chen G, He Z, Wang T, Xu R, Yu XF. A patch of positively charged amino acids surrounding the human immunodeficiency virus type 1 Vif SLVx4Yx9Y motif influences its interaction with APOBEC3G. J Virol. 2009;83:8674–8682. doi: 10.1128/JVI.00653-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang Y, Wang X, Zhou T, York IA, Zheng YH. Identification of a novel WxSLVK motif in the N terminus of human immunodeficiency virus and simian immunodeficiency virus Vif that is critical for APOBEC3G and APOBEC3F neutralization. J Virol. 2009;83:8544–8552. doi: 10.1128/JVI.00651-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang Y, Wang X, Esselman WJ, Zheng YH. Identification of APOBEC3DE as another antiretroviral factor from the human APOBEC family. J Virol. 2006;80:10522–10533. doi: 10.1128/JVI.01123-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang Y, Siew LM, Wang X, Han Y, Lampen R, Zheng YH. Human cytidine deaminase APOBEC3H restricts HIV-1 replication. J Biol Chem. 2008;283:11606–11614. doi: 10.1074/jbc.M707586200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derdeyn CA, Decker JM, Sfakianos JN, Wu X, O’Brien WA, Ratner L, Kappes JC, Shaw GM, Hunter E. Sensitivity of human immunodeficiency virus type 1 to the fusion inhibitor T-20 is modulated by coreceptor specificity defined by the V3 loop of gp120. J Virol. 2000;74:8358–8367. doi: 10.1128/jvi.74.18.8358-8367.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrosiers RC, Lifson JD, Gibbs JS, Czajak SC, Howe AY, Arthur LO, Johnson RP. Identification of highly attenuated mutants of simian immunodeficiency virus. J Virol. 1998;72:1431–1437. doi: 10.1128/jvi.72.2.1431-1437.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doehle BP, Schafer A, Cullen BR. Human APOBEC3B is a potent inhibitor of HIV-1 infectivity and is resistant to Vif. Virology. 2005;339:281–288. doi: 10.1016/j.virol.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Fan L, Peden K. Cell-free transmission of Vif mutants of HIV-1. Virology. 1992;190:19–29. doi: 10.1016/0042-6822(92)91188-z. [DOI] [PubMed] [Google Scholar]
- Gabuzda DH, Lawrence K, Langhoff E, Terwilliger E, Dorfman T, Haseltine WA, Sodroski J. Role of vif in replication of human immunodeficiency virus type 1 in CD4+ T lymphocytes. J Virol. 1992;66:6489–6495. doi: 10.1128/jvi.66.11.6489-6495.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris RS, Bishop KN, Sheehy AM, Craig HM, Petersen-Mahrt SK, Watt IN, Neuberger MS, Malim MH. DNA deamination mediates innate immunity to retroviral infection. Cell. 2003;113:803–809. doi: 10.1016/s0092-8674(03)00423-9. [DOI] [PubMed] [Google Scholar]
- Hill MS, Ruiz A, Pacyniak E, Pinson DM, Culley N, Yen B, Wong SW, Stephens EB. Modulation of the severe CD4+ T-cell los caused by a pathogenic simian-human immunodeficiency virus by replacement of the subtype B vpu from a subtype C HIV-1 clinical isolate. Virology. 2008;371(1):86–97. doi: 10.1016/j.virol.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann-Lehmann R, Swenerton RK, Liska V, Leutenegger CM, Lutz H, McClure HM, Ruprecht RM. Sensitive and robust one-tube real-time reverse transcriptase-polymerase chain reaction to quantify SIV RNA load: comparison of one- versus two-enzyme systems. AIDS Res Hum Retro. 2000;16:1247–1257. doi: 10.1089/08892220050117014. [DOI] [PubMed] [Google Scholar]
- Hout DR, Gomez ML, Pacyniak E, Gomez LM, Inbody SH, Mulcahy ER, Culley N, Pinson DM, Powers MF, Wong SW, Stephens EB. Scrambling of the amino acids within the transmembrane domain of Vpu results in a simian-human immunodeficiency virus (SHIVTM) that is less pathogenic for pig-tailed macaques. Virology. 2005;339:56–69. doi: 10.1016/j.virol.2005.04.038. [DOI] [PubMed] [Google Scholar]
- Hout DR, Gomez ML, Pacyniak E, Gomez LM, Fegley B, Mulcahy ER, Hill MS, Culley N, Pinson DM, Nothnick W, Powers MF, Wong SW, Stephens EB. Substitution of the transmembrane domain of Vpu in simian-human immunodeficiency virus (SHIVKU1bMC33) with that of M2 of influenza A results in a virus that is sensitive to inhibitors of the M2 ion channel and is pathogenic for pig-tailed macaques. Virology. 2006;344:541–559. doi: 10.1016/j.virol.2005.08.022. [DOI] [PubMed] [Google Scholar]
- Jarmuz A, Chester A, Bayliss J, Gisbourne J, Dunham I, Scott J, Navaratnam N. An anthropoid-specific locus of orphan C to U RNA-editing enzymes on chromosome 22. Genomics. 2002;79:285–296. doi: 10.1006/geno.2002.6718. [DOI] [PubMed] [Google Scholar]
- Joag SV, Li Z, Wang C, Jia F, Foresman L, Adany I, Pinson DM, Stephens EB, Narayan O. Chimeric SHIV that causes CD4+ T cell loss and AIDS in rhesus macaques. J Med Primatol. 1998;27(2–3):59–64. doi: 10.1111/j.1600-0684.1998.tb00227.x. [DOI] [PubMed] [Google Scholar]
- Kim EY, Busch M, Abel K, Fritts L, Bustamante P, Stanton J, Lu D, Wu S, Glowczwskie J, Rourke T, Bogdan D, Piatak M, Jr, Lifson JD, Desrosiers RC, Wolinsky S, Miller CJ. Retroviral recombination in vivo: viral replication patterns and genetic structure of simian immunodeficiency virus (SIV) populations in rhesus macaques after simultaneous or sequential intravaginal inoculation with SIVmac239Deltavpx/Deltavpr and SIVmac239Deltanef. J Virol. 2005;79:4886–4895. doi: 10.1128/JVI.79.8.4886-4895.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koff WC, Johnson PR, Watkins DI, Burton DR, Lifson JD, Hasenkrug KJ, McDermott AB, Schultz A, Zamb TJ, Boyle R, Desrosiers RC. HIV vaccine design: insights from live attenuated SIV vaccines. Nat Immunol. 2006;7:19–23. doi: 10.1038/ni1296. [DOI] [PubMed] [Google Scholar]
- Luo K, Xiao Z, Ehrlich E, Yu Y, Liu B, Zheng S, Yu XF. Primate lentiviral virion infectivity factors are substrate receptors that assemble with cullin 5-E3 ligase through a HCCH motif to suppress APOBEC3G. Proc Natl Acad Sci U S A. 2005;102:11444–11449. doi: 10.1073/pnas.0502440102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangeat B, Turelli P, Caron G, Friedli M, Perrin L, Trono D. Broad antiretroviral defence by human APOBEC3G through lethal editing of nascent reverse transcripts. Nature. 2003;424:99–103. doi: 10.1038/nature01709. [DOI] [PubMed] [Google Scholar]
- Marcario JK, Riazi M, Adany RI, Kenjale H, Fleming K, Marquis J, Nemon O, Mayo S, Yankee T, Narayan O, Cheney PD. Effect of Morphine on the Neuropathogenesis of SIVmac Infection in Indian Rhesus Macaques. J Neuroimmune Pharmacol. 2008;3(12):12–25. doi: 10.1007/s11481-007-9085-z. [DOI] [PubMed] [Google Scholar]
- Mariani R, Chen D, Schrofelbauer B, Navarro F, Konig R, Bollman B, Munk C, Nymark-McMahon H, Landau NR. Species-specific exclusion of APOBEC3G from HIV-1 virions by Vif. Cell. 2003;114:21–31. doi: 10.1016/s0092-8674(03)00515-4. [DOI] [PubMed] [Google Scholar]
- Mehle A, Goncalves J, Santa-Marta M, McPike M, Gabuzda D. Phosphorylation of a novel SOCS-box and upstream cysteines. Genes Dev. 2004a;18:2867–2872. doi: 10.1101/gad.1249904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehle A, Strack B, Ancuta P, Zhang C, McPike M, Gabuzda D. Vif overcomes the innate antiviral activity of APOBEC3G by promoting its degradation in the ubiquitin-proteasome pathway. J Biol Chem. 2004b;279:7792–7798. doi: 10.1074/jbc.M313093200. [DOI] [PubMed] [Google Scholar]
- Mehle A, Thomas ER, Rajendran KS, Gabuzda D. A zinc-binding region in Vif binds Cul5 and determines cullin selection. J Biol Chem. 2006;281:17259–17265. doi: 10.1074/jbc.M602413200. [DOI] [PubMed] [Google Scholar]
- Mehle A, Wilson H, Zhang C, Brazier AJ, McPike M, Pery E, Gabuzda D. Identification of an APOBEC3G binding site in human immunodeficiency virus type1 Vif and inhibitors of Vif-APOBEC3G binding. J Virol. 2007;81:13235–13241. doi: 10.1128/JVI.00204-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pery E, Rajendran KS, Brazier AJ, Gabuzda D. Regulation of APOBEC3 proteins by a novel YXXL motif in human immunodeficiency virus type 1 Vif and simian immunodeficiency virus SIVagm Vif. 2009 doi: 10.1128/JVI.01898-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell RA, Pathak VK. Identification of two distinct human immunodeficiency virus type 1 Vif determinants critical for interactions with human APOBEC3G and APOBEC3F. J Virol. 2007;81:8201–8210. doi: 10.1128/JVI.00395-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell RA, Morre MD, Pathak VK. APOBEC3G induces a hypermutation gradient: purifying selection at multiple steps during HIV-1 replication results in levels of G-to-A mutations that are high in DNA, intermediate in cellular viral RNA, and low in virion RNA. Retrovirology. 2009;6:16. doi: 10.1186/1742-4690-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai H, Shibata R, Sakuragi J, Sakuragi S, Kawamura M, Adachi A. Cell-dependent requirement of human immunodeficiency virus type 1 Vif protein for maturation of virus particles. J Virol. 1993;67:1663–1666. doi: 10.1128/jvi.67.3.1663-1666.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt K, Hill MS, Ruiz A, Culley N, Pinson DM, Wong SW, Stephens EB. Mutations in the highly conserved SLQYLA motif of Vif in a simian-human immunodeficiency virus result in a less pathogenic virus and are associated with G-to-A mutations in the viral genome. Virology. 2009;383:362–372. doi: 10.1016/j.virol.2008.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehy AM, Gaddis NC, Malim MH. The antiretroviral enzyme APOBEC3G is degraded by the proteasome in response to HIV-1 Vif. Nat Med. 2003;9:1404–1407. doi: 10.1038/nm945. [DOI] [PubMed] [Google Scholar]
- Shindo K, Takaori-Kondo A, Kobayashi M, Abudu A, Fukunaga K, Uchiyama T. The enzymatic activity of CEM15/Apobec-3G is essential for the regulation of the infectivity of HIV-1 virion but not a sole determinant of its antiviral acitivity. J Biol Chem. 2003;278:44412–44416. doi: 10.1074/jbc.C300376200. [DOI] [PubMed] [Google Scholar]
- Singh DK, Griffin DM, Pacyniak E, Jackson M, Werle MJ, Wisdom B, Sun F, Hout DR, Pinson DM, Gunderson RS, Powers MF, Wong SW, Stephens EB. The presence of the casein kinase II phosphorylation sites of Vpu enhances the CD4+ T cell loss caused by the simian-human immunodeficiency virus SHIVKU-lbMC33 in pig-tailed macaques. Virology. 2003;313:435–451. doi: 10.1016/s0042-6822(03)00339-8. [DOI] [PubMed] [Google Scholar]
- Stephens EB, McCormick C, Pacyniak E, Griffin D, Pinson DM, Sun F, Nothnick W, Wong SW, Gunderson R, Berman NE, Singh DK. Deletion of the vpu sequences prior to the env in a simian-human immunodeficiency virus results in enhanced Env precursor synthesis but is less pathogenic for pig-tailed macaques. Virology. 2002;293:252–261. doi: 10.1006/viro.2001.1244. [DOI] [PubMed] [Google Scholar]
- Stopak K, de Noronha C, Yonemoto W, Greene WC. HIV-1 Vif blocks the antiviral activity of APOBEC3G by impairing both its translation and intracellular stability. Mol Cell. 2003;12:591–601. doi: 10.1016/s1097-2765(03)00353-8. [DOI] [PubMed] [Google Scholar]
- von Schwedler U, Song J, Aiken C, Trono D. Vif is crucial for human immunodeficiency virus type 1 proviral DNA synthesis in infected cells. J Virol. 1993:67-4945–4955. doi: 10.1128/jvi.67.8.4945-4955.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virgen CA, Hatziioannou T. Antiretroviral activity and Vif sensitivity of rhesus macaque APOBEC3 proteins. J Virol. 2007;81:13932–13937. doi: 10.1128/JVI.01760-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X, Decker JM, Liu H, Zhang Z, Arani RB, Kilby JM, Saag MS, Wu X, Shaw GM, Kappes JC. Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrob Agents Chemother. 2000;46:1896–1905. doi: 10.1128/AAC.46.6.1896-1905.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegand HL, Doehle BP, Bogerd HP, Cullen BR. A second human antiretroviral factor, APOBEC3F, is suppressed by the HIV-1 and HIV-2 Vif proteins. EMBO J. 2004;23:2451–2488. doi: 10.1038/sj.emboj.7600246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Z, Xiong Y, Zhang W, Tan L, Ehrlich E, Guo D, Yu XF. Characterization of a novel Cullin5 binding domain in HIV-1 Vif. J Mol Biol. 2007;373:541–550. doi: 10.1016/j.jmb.2007.07.029. [DOI] [PubMed] [Google Scholar]
- Yang Y, Guo F, Cen S, Kleiman L. Inhibition of initiation of reverse transcription in HIV-1 by human APOBEC3F. Virology. 2007;365:92–100. doi: 10.1016/j.virol.2007.03.022. [DOI] [PubMed] [Google Scholar]
- Yu Q, Konig R, Pillai S, Chiles K, Kearney M, Palmer S, Richman D, Coffin JM, Landau NR. Single-strand specificity of APOBEC3G accounts for minus-strand deamination of the HIV genome. Nat Struct Mol Biol. 2004a;11:435–442. doi: 10.1038/nsmb758. [DOI] [PubMed] [Google Scholar]
- Yu Q, Chen D, Konig R, Mariani R, Unutmaz D, Landau NR. APOBEC3B and APOBEC3C are potent inhibitors of simian immunodeficiency virus replication. J Biol Chem. 2004b;279:53379–53386. doi: 10.1074/jbc.M408802200. [DOI] [PubMed] [Google Scholar]
- Yu X, Yu Y, Liu B, Luo K, Kong W, Mao P, Yu XF. Induction of APOBEC3G ubiquitination and degradation by an HIV-1 Vif-Cul5-SCF complex. Science. 2003;302:1056–1060. doi: 10.1126/science.1089591. [DOI] [PubMed] [Google Scholar]
- Yu Y, Xiao Z, Ehrlich ES, Yu X, Yu XF. Selective assembly of HIV-1 Vif-Cul5-ElonginB-ElonginC E3 ubiquitin ligase complex through a novel SOCS box and upstream cysteines. Genes Dev. 2004c;18:2867–2872. doi: 10.1101/gad.1250204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zennou V, Bieniasz PD. Comparative analysis of the antiretroviral activity of APOBEC3G and APOBEC3F from primates. Virology. 2006;349:31–40. doi: 10.1016/j.virol.2005.12.035. [DOI] [PubMed] [Google Scholar]
- Zhang H, Yang B, Pomerantz RJ, Zhang C, Arunachalam SC, Gao L. The cytidine deaminase CEM15 induces hypermutation in newly synthesized HIV-1 DNA. Nature. 2003;424:94–98. doi: 10.1038/nature01707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng YH, Irwin D, Kurosu T, Tokunaga K, Sata T, Peterlin BM. Human APOBEC3F is another host factor that blocks human immunodeficiency virus type 1 replication. J Virol. 2004;78:6072–6076. doi: 10.1128/JVI.78.11.6073-6076.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]