Abstract
BACKGROUND
Samarium-153 ethylenediaminetetramethylene phosphonic acid (153Sm-EDTMP) has been used to treat patients with high-risk osteosarcoma. The purpose of the current study was to determine the maximally tolerated dose of 153Sm-EDTMP that permits hematopoietic recovery within 6 weeks.
METHODS
Patients with recurrent or refractory osteosarcoma with bone metastases were enrolled in this study. Subjects were treated with increasing doses of 153Sm-EDTMP, beginning with 1.0 millicuries (mCi)/kg and followed initially with 40% increment dose level escalations, using a continual reassessment method for dose escalation and de-escalation with a target dose–limiting toxicity (DLT) rate of 30%. Complete blood counts were monitored weekly, and the primary DLT was defined as failure to achieve an absolute neutrophil count >750/mm3 and a platelet count >75,000/mm3 within 6 weeks of treatment. In addition to assessing toxicity, dosimetry measurements were made to estimate the radiation dose delivered to target lesions.
RESULTS
The maximally tolerated dose of 153Sm-EDTMP was 44.8 megabecquerel (MBq)/kg (1.21 mCi/kg). DLTs were confined to hematologic toxicities, particularly delayed platelet recovery in 2 patients treated at a dose of 51.8 MBq/kg (1.4 mCi/kg). Grade 2 and 3 pulmonary toxicity (graded according to the National Cancer Institute Common Toxicity Criteria [version 3.0]) as reported in 2 patients (at administered activities of 44.8 MBq/kg and 51.8 MBq/kg) was attributable to progressive pulmonary disease. No other significant nonhematologic toxicities were observed.
CONCLUSIONS
Patients with osteosarcoma who have previously been heavily treated with chemotherapy can be safely administered 153Sm-EDTMP with rapid hematologic recovery. The data from the current study support the development of a future trial to assess the efficacy of combining targeted radiotherapy with cytotoxic chemotherapy as a treatment option for patients with high-risk osteosarcoma.
Keywords: radiopharmaceuticals, targeted radiotherapy, osteosarcoma, bone neoplasm
Osteosarcoma is the most common primary malignant tumor of bone diagnosed in children and adolescents. The combination of chemotherapy and aggressive surgery can cure up to 75% of patients with localized disease, in contrast to patients with high-risk disease (defined as that found to be metastatic at presentation, especially to distant bony sites; refractory to chemotherapy; or unable to be completely resected), whose cure rate is reported to be <15%.1 Although there was a tremendous improvement in overall patient survival reported with the introduction of effective chemotherapy, subsequent dose intensification appears to have been of no benefit to patients with high-risk disease and therefore new treatment approaches are clearly needed.
Osteosarcoma is a relatively radioresistant tumor. Doses of <60 grays (Gy) are associated with only transient tumor control, and even when using doses of ≥80 Gy, viable tumor has been found in amputation specimens.2–4 This finding has limited the applicability of radiotherapy in this disease, because such doses are associated with significant normal tissue injury. Recent studies, however, have indicated that lower dose radiotherapy can assist in providing local control of osteosarcoma for patients with marginal resections.5 This finding supports the development of chemoradiotherapy treatment protocols for patients with high-risk disease, especially if treatment can be targeted directly to the tumor rather than to surrounding normal tissue.
Samarium-153 ethylenediaminetetramethylene phosphonic acid (153Sm-EDTMP) is a bone-seeking radiopharmaceutical designed to selectively deliver radiation to osteoblastic skeletal metastases.6 The radioisotope 153Sm emits an electron (beta particle) with an average energy of 224 kiloelectron volts (keV) (approximate range of 0.5 mm), which is appropriate for targeted cytotoxicity. Its photon emissions include a 103–keV photon appropriate for scintigraphic imaging, thereby allowing both confirmation of localization of the agent and quantification of the radiation delivered to target lesions. 153Sm has a half-life of 47 hours, allowing therapy to be delivered safely in the outpatient setting.
153Sm-EDTMP has been used for the treatment of high-risk osteosarcoma. A phase 1 study found that up to 11.1 gigabecquerels (GBq)/kg (30 millicuries [mCi]/kg) could be administered with autologous stem cell support; the only significant toxicity was myelosuppression.7 In an effort to improve efficacy, Anderson et al combined a dose of 11.1 GBq/kg 153Sm-EDTMP with gemcitabine as a radiation sensitizer.8 Although some patients achieved partial responses, none of the responses were durable. The approach of combining chemotherapy with a radiopharmaceutical requires a clear understanding of the kinetics of hematopoietic recovery after treatment with 153Sm-EDTMP.
The primary goal of the current study was to identify a dose of 153Sm-EDTMP that would allow hematopoietic recovery within 6 weeks without use of stem cell support. Six weeks was chosen so that myelosuppression related to 153Sm-EDTMP administration would not cause prolonged treatment delays when combined with chemotherapy. Secondary goals included the evaluation of the efficacy of low dose 153Sm-EDTMP for patients with high-risk osteosarcoma and quantification of the adsorbed dose delivered to target lesions by this treatment.
MATERIALS AND METHODS
Eligibility Requirements
Eligible patients were aged <40 years, with biopsy-proven high-risk osteogenic sarcoma (defined as recurrent after conventional therapy, unresectable, or newly diagnosed disease with bone metastases or >4 pulmonary metastases) that was detectable on a standard technetium 99m bone scan. Adequate renal and hematologic function were required (including an absolute neutrophil count [ANC] of ≥1000/mm3 and a platelet count≥100,000/mm3). Informed consent was obtained according to institutional policies. Patients were excluded if they had a Karnofsky performance status <50%, had clinically significant cardiac disease, were pregnant or breast-feeding, had received prior radiotherapy for osteosarcoma, or had prior radio-therapy for any other reason within the preceding 6 months. Patients with a prior malignancy must have been in remission for at least 5 years.
Treatment Plan and Dose Modifications
153Sm-EDTMP was administered intravenously in the Radiation Oncology outpatient clinic. The first dose level, 37 megabecquerel (MBq)/kg (1.0 mCi/kg), is the US Food and Drug Administration (FDA) -approved dose for the palliation of bone metastases. The second dose level was a 40% increase, 51.8 MBq/kg (1.4 mCi/kg). Subsequent dose levels were to be sequential 40% increases until toxicity was observed. When toxicity was first noted, a dose-response curve was estimated based on the observed data; further doses were then determined by the continuous reassessment method (CRM)9 using a target frequency of toxicity at the maximum tolerated dose (MTD) of 30%. Patients were enrolled in cohort sizes of 2, and the CRM algorithm was applied after each pair of patients was evaluated. The primary dose–limiting toxicity (DLT) was expected to be failure of hematopoietic recovery, defined as an ANC <750/mm3 and a platelet count <75,000/mm3 within 6 weeks. Grade 3 or 4 non-hematologic toxicities attributable to drug administration were also considered to be DLTs.
When the first DLT was encountered, the dose-response curve was estimated using a 1-parameter logistic model (ie, toxicity as a function of administered activity). Using the curve, we found the administered activity that is most consistent with 30% toxicity and defined this as the next dose level (Fig. 1). The specific form of the CRM model was suggested by Goodman et al.9
FIGURE 1.
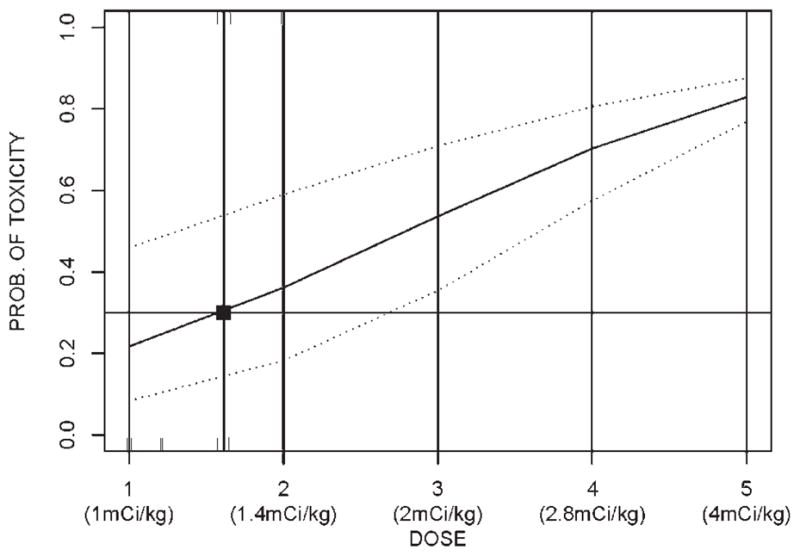
The continuous reassessment model. The heavy line indicates the relation between administered activity (x axis) and probability (PROB) of toxicity (y axis) as calculated by the formula given in the text. The dotted lines indicate the 90% confidence intervals. The square indicates the dose that corresponds to the predicted toxicity rate of 30%, which was the target of the model.
In the dose-toxicity model above, the model is estimated using a maximum likelihood approach.
Secondary outcomes were assessed by descriptive statistics, including the proportion of subjects with an objective response to therapy and the adsorbed dose to the target lesions. Other toxicities were assessed by the National Cancer Institute Common Toxicity Criteria (version 3.0). Adsorbed dose was compared using a 2-sample Student t test after taking a log-transform of the data to achieve a symmetric distribution. Mean and 95% confidence intervals were estimated. Kaplan-Meier estimation was used to describe time to tumor progression.
Radiation Dosimetry
153Sm-EDTMP scans were performed 4 hours after infusion, and again 24 to 48 hours later using a standard scintigraphic camera and an acquisition window centered around the 103 keV photopeak. Anterior/posterior whole–body sweeps were performed at a detector speed of 8 to 10 cm/minute with no contouring. The Medical Internal Radiation Dose (MIRD) Committee formalism was used, as implemented in the OLINDA software package.10 The measured lesion activity concentration was adjusted to account for detector count rate saturation (Siegel et al11 and unpublished data) to calculate the absorbed doses to target lesions and normal bone.
Response Evaluation
Before treatment, patients were evaluated with a computed tomography (CT) scan of the chest, CT or magnetic resonance imaging (MRI) of the primary tumor, and a bone scan. At the time of hematologic recovery, these imaging studies were repeated. Radiographic response was evaluated using Response Evaluation Criteria in Solid Tumors (RECIST) criteria: partial response indicated a >50% reduction in the sum of the products of perpendicular tumor dimensions; a minor response indicated a 25% to 50% reduction in the sum of the products of the tumor dimension without the emergence of new lesions, and no individual lesion progressing >25% in a single dimension; stable disease indicated no progressive disease; and progressive diseaseindicated the emergence of new lesions or the progression of any lesion by >25% in a single dimension. An objective response was defined as stable disease or better.
RESULTS
Patient Characteristics
Thirteen subjects were enrolled in this dose-finding study (Table 1). The median age was 14 years (range, 6–30 years). A total of 11 patients had high-grade osteoblastic osteosarcoma, 1 patient had chondroblastic osteosarcoma, and 1 patient had a giant-cell–rich grade 2 osteosarcoma. Five patients had primary refractory disease, with disease progression occurring during chemotherapy; 4 patients were treated at the time of first disease recurrence (2 of whom were refractory to rescue chemotherapy), and 4 patients were treated at the time of second or subsequent disease recurrence. All of the patients were heavily pre-treated with chemotherapy. All had received methotrexate, cisplatin, and doxorubicin; 11 had also received at least ifosfamide and etoposide. In addition, 3 patients had been treated with gemcitabine with or without taxotere, and 1 patient had received high-dose chemotherapy with autologous peripheral blood stem cell transplantation.
Table 1.
Patient Demographics
| Patient No. | Gender | Age | Pathology | Disease Status | Disease Sites | Previous Treatment | Other Medical Problems |
|---|---|---|---|---|---|---|---|
| 1 | F | 8 y, 11 mo | Chondroblastic | Recurrence 1 | Right lung, Right 8th rib | Cisplatin/dox/MTX/etoposide/ifos | — |
| 2 | M | 8 y, 5 mo | Low-grade osteosarcoma (2 of 3), giant cell rich | Refractory recurrence 1 | Clivus, bilateral taluses | Cisplatin/dox/MTX | — |
| 3 | M | 14 y, 2 mo | High-grade osteoblastic | Recurrence 1 | Bilateral pulmonary nodules | Cisplatin/dox/MTX/etoposide/ifos | Pleural effusion, renal Fanconi syndrome |
| 4 | F | 18 y, 7 mo | High-grade osteoblastic | Recurrence 2 | L2 vertebral body, left lung | Cisplatin/dox/MTX | — |
| 5 | M | 11 y, 6 mo | High-grade osteoblastic | Primary refractory | Pelvis, lungs, ribs, scapula | Cisplatin/dox/MTX/etoposide/ifos | — |
| 6 | M | 17 y, 6 mo | High-grade osteoblastic | Primary refractory | Lungs, inguinal lymph nodes | Cisplatin/dox/MTX/etoposide/ifos | — |
| 7 | F | 30 y, 9 mo | High-grade osteoblastic | Recurrence 4 | Chest wall | Cisplatin/doxo/MTX; gemcitabine/docetaxel; ifos | — |
| 8 | M | 15 y, 5 mo | High-grade osteoblastic | Primary refractory | Ribs, spine, right knee | Cisplatin/doxo/MTX/etoposide/ifos | — |
| 9 | F | 14 y, 3 mo | High-grade osteoblastic | Recurrence 3 | Left lung, skull, patella, pelvis | Cisplatin/doxo/MTX/etoposide/ifos | — |
| 10 | M | 21 y, 4 mo | High grade | Refractory recurrence 1 | Left chest wall, right lung, ribs, sternum, thoracic spine, iliac spine | Cisplatin/doxo/MTX/etoposide/ifos | Pleural effusion, cardiomyopathy, recent pulmonary embolus |
| 11 | M | 12 y, 7 mo | High-grade osteoblastic | Primary refractory | Skull, sternum, right humerus, right scapula, thoracic spine, pelvis, bilateral femurs, right fibula, left tibia | Cisplatin/doxo/MTX/etoposide/ifos; mel/thiotepa; bleo/cyclo/actino; WT1 immunotherapy; bleo/cyclo/actino; zoledronic acid | — |
| 12 | M | 21 y, 2 mo | High grade | Recurrence 2 | Right upper lobe of lung, L4 and S1 vertebral bodies, right ilium, retroperitoneal soft tissue | Cisplatin/doxo/MTX/etoposide/ifos; gemcitabine | Asthma |
| 13 | F | 6 y, 9 mo | High-grade osteoblastic | Primary refractory | Left iliac crest, T2, T10, left scapula, bilateral femurs | Cisplatin/doxo/MTX/etoposide/ifos; gemcitabine/docetaxel | — |
F indicates female; dox, doxorubicin; MTX, methotrexate; ifos, ifosfamide; M, male; Mel, melphalan; bleo, bleomycin; cyclo, cyclophosphamide; actino, actinomycin.
A total of 12 of the patients had bony lesions identifiable on standard bone scan; 7 also had pulmonary lesions that were not visible on bone scan. One patient had disease limited to the lungs, but these lesions were readily visible on bone scan. At the time of treatment, 2 patients had pleural effusions, 1 of whom also had cardiomyopathy and the other renal Fanconi syndrome.
Toxicities
The DLT was thrombocytopenia for 4 patients, 2 of whom also experienced dose–limiting neutropenia (Table 2). The MTD, as calculated by the CRM algorithm, was 44.8 MBq/kg (1.21 mCi/kg). This was 20% higher than the FDA-approved dose for palliation. Our CRM algorithm incorporated a target DLT rate at the MTD of 30%. The 90% confidence interval for the DLT rate at the dose of 44.8 MBq/kg was 15% to 54%.
Table 2.
Toxicities* and Response
| Patient No. | Dose Level | Dose Delivered, mCi/kg | Time to Recovery, Weeks | Hematologic Toxicity Grade | Treatment Response | Other Toxicities | |||
|---|---|---|---|---|---|---|---|---|---|
| ANC | Platelets | ANC | Hgb | Platelets | |||||
| 1 | 1 | 1.00 | NA | 6 | 0 | 1 | 2 | SD | — |
| 2 | 1 | 1.00 | NA | NA | 0 | 2 | 1 | SD | — |
| 3 | 2 | 1.40 | NE | NE | — | — | — | PD | — |
| 4 | 2 | 1.40 | NA | DLT | 3 | 2 | 3 | SD | — |
| 5 | 2a | 1.39 | NA | 6 | 2 | 2 | 3 | PD | Pleural effusion with pleuritic pain and dyspnea |
| 6 | 2a | 1.39 | DLT | DLT | 4 | 2 | 4 | SD | — |
| 7 | 2b | 1.20 | 6 | 6 | 3 | 2 | 3 | PD | — |
| 8 | 2b | 1.20 | DLT | DLT | 3 | 1 | 4 | SD | — |
| 9 | 2c | 1.065 | NA | 5 | 3 | 2 | 3 | PD | — |
| 10 | 2c | 1.065 | NA | 6 | 0 | 3 | 2 | SD | — |
| 11 | 2d | 1.24 | NA | 6 | 0 | 1 | 2 | PD | — |
| 12 | 2d | 1.24 | NA | 4 | 2 | 1 | 3 | PD | — |
| 13 | 2d | 1.24 | NA | DLT | 0 | 1 | 4 | PD | Grade 3 hyponatremia |
ANC, absolute neutrophil count; Hgb, hemoglobin; NA, not applicable (counts never fell below target for recovery); SD, stable disease; NE, not evaluable (patient taken off study prior to recovery); PD, progressive disease; DLT, dose-limiting toxicity (patient’s counts did not recover in time).
Toxicity was graded according to the National Cancer Institute Common Toxicity Criteria (version 3.0).
Toxicity attributable to treatment was limited to the hematopoietic system, and platelets were most profoundly affected (Table 2). Of the 12 evaluable patients, 8 experienced either grade 3 or grade 4 thrombocytopenia, with a nadir reached during Week 4 after treatment. Thrombocytopenia accounted for 4 DLTs. Platelet counts for these patients at Week 6 were 46,000/μL, 57,000/μL, 20,000/μL, and 7000/μL. For 3 of the patients, thrombocytopenia resolved by Week 8. The fourth patient (with a platelet count of 7000/μL) entered hospice care and had no further blood draws. Neutropenia was variable and usually resulted in a neutrophil nadir during Week 4. Three patients had grade 1 or 2 neutropenia and 5 patients had grade 3 or 4 neutropenia. There were 2 DLTs due to neutropenia, both in patients who also experienced a DLT due to thrombocytopenia. For these patients, absolute neutrophil counts were 36/μL and 260/μL at Week 6. Both patients had resolution of their neutropenia by Week 8. Anemia was generally mild, with 11 of 12 evaluable patients experiencing only grade 1 or 2 anemia. No red blood cell transfusions were required.
Only 2 patients experienced significant nonhematopoietic toxicity. One patient experienced an increase in the size of a pleural effusion, with increasing pleuritic chest pain and dyspnea (Patient 3, who was given other disease-directed therapy before count recovery and therefore was unevaluable for hematologic recovery). These symptoms were caused by progressive disease, and were not attributable to treatment. Another patient had asymptomatic grade 3 hyponatremia that resolved spontaneously. Most patients experienced a transient increase in pain at the site of bony lesions, beginning 24 to 48 hours after treatment, lasting 24 to 48 hours, and responding to oral pain relievers such as acetaminophen.
Dosimetry
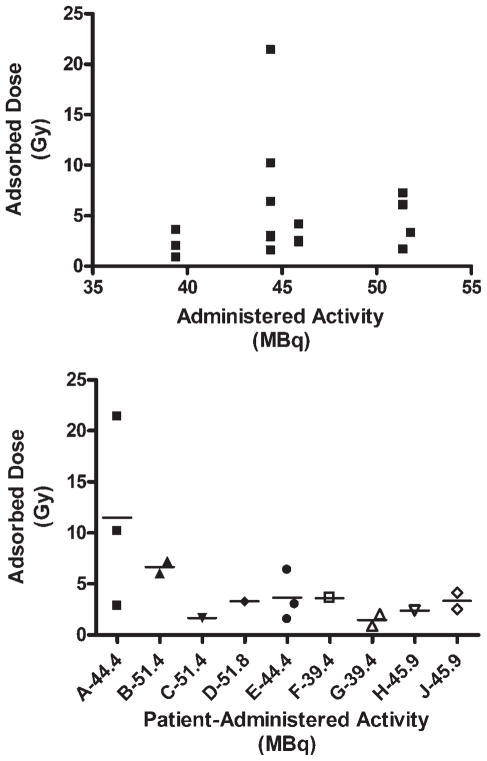
Visualization of 153Sm-EDTMP uptake provided confirmation of drug delivery to target lesions and allowed calculation of the tumor adsorbed dose. Evaluable dosimetry data were available for 16 distinct lesions in 9 of the patients. The tumor adsorbed dose varied from 0.9 to 21.4 Gy (Table 3). Despite this wide range, there was no significant correlation noted between administered activity and tumor adsorbed dose (Fig. 2 Top). The adsorbed dose also did not correlate with tumor size (data not shown). Surprisingly, we found that the tumor adsorbed dose appeared to vary more between patients than between tumors, with some patients having tumors that appeared to have much greater avidity for 153Sm-EDTMP than others (Fig. 2 Bottom).
Table 3.
Dosimetry*
| Patient No. | Lesion | Administered Activity, MBq/kg | Adsorbed Dose, Gy | Response |
|---|---|---|---|---|
| 4 | L2 vertebra | 51.8 | 3.31 | SD |
| 5 | Shoulder | 51.4 | 7.23 | SD |
| 5 | Hip | 51.4 | 6.05 | SD |
| 6 | Pelvic lymph node | 51.4 | 1.66 | SD |
| 7 | Sternum | 44.4 | 21.4 | PD |
| 7 | Chest wall | 44.4 | 10.2 | PD |
| 7 | Abdomen | 44.4 | 2.89 | PD |
| 8 | L1 vertebra | 44.4 | 3.01 | SD |
| 8 | Femur | 44.4 | 6.38 | SD |
| 8 | Femur | 44.4 | 1.55 | SD |
| 9 | Paraspinal | 39.4 | 3.61 | PD |
| 10 | Left chest | 39.4 | 0.89 | SD |
| 10 | Right chest | 39.4 | 2.03 | SD |
| 11 | Knee | 45.9 | 2.39 | PD |
| 12 | Spine | 45.9 | 4.14 | SD |
| 12 | Hip | 45.9 | 2.50 | SD |
MBq indicates megabecquerel; Gy, grays; SD, stable disease; PD, progressive disease.
For each listed patient, dosimetry was performed as described for 1 or more lesions.
FIGURE 2.
Relation between administered activity and adsorbed dose. (Top) The tumor adsorbed dose was measured for several lesions in each patient and compared with the dose of samarium-153 ethylenediaminetetramethylene phosphonic acid (153Sm-EDTMP) administered to the patient. Each data point represents an individual lesion. (Bottom) Tumor adsorbed dose compared with administered activity presented by patient. The letters A–I designate individual patients, and the activity administered to each patient is presented next to the patient’s identifying letter. Each symbol represents an individual lesion, and the small horizontal line indicates the mean tumor adsorbed dose for that patient. Gy indicates grays; MBq, megabecquerel.
Disease Response
Although it is used for palliation of painful bone metastases, the goal of treating osteosarcoma patients with 153Sm-EDTMP is to provide cytotoxic therapy. Therefore, we measured response to therapy radiographically. No patients achieved a radiographic complete or partial response as defined by RECIST criteria. However, radiographic response of osteosarcoma is extremely difficult to measure because the tumor is calcified. Newly diagnosed disease, even that with >95% necrosis, often demonstrates minimal radiographic change. Some even demonstrate increased tumor size despite excellent necrosis.
Of the 13 evaluable patients, 5 (38%) had stable disease at the end of the study (6 weeks after treatment), and the other 8 had disease progression. Assessment of durability of response was not possible, because the majority of patients went on to receive further therapy: 6 of the patients received a second, higher dose of 153Sm-EDTMP with autologous hematopoietic stem cell support and 2 received further chemotherapy. Nevertheless, as calculated using a Kaplan-Meier analysis, the median time to disease progression was 51 days (Fig. 3), and 3 patients survived for a significant period of time after treatment. One of these patients, with a low-grade, giant-cell–rich osteosar-coma of the clivus that was metastatic to the bilateral taluses, survived for 1 year after treatment. Two patients who were treated for widely metastatic, primary refractory disease remained alive (with active disease) at the time of last follow–up, 24 months and 41 months, respectively, after treatment. These 2 survivors were also treated with a second, higher samarium dose requiring autologous peripheral blood stem cell support as well as aggressive surgery.
FIGURE 3.
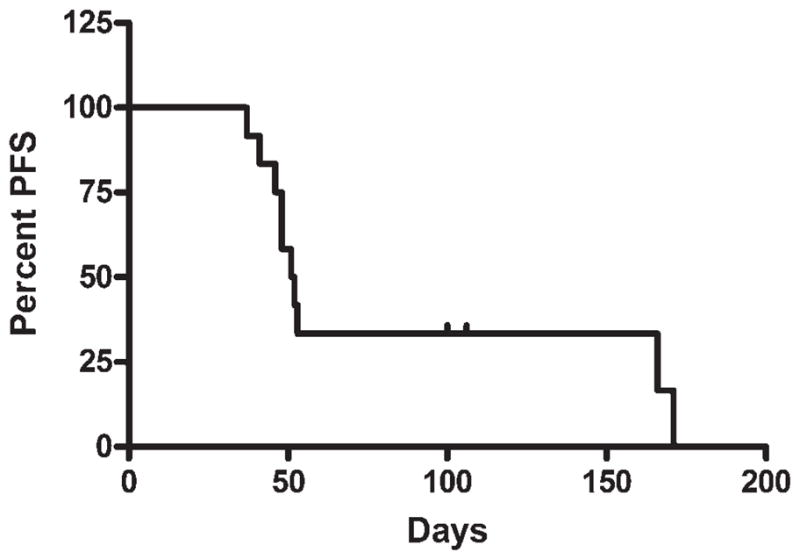
Time to disease progression. A Kaplan-Meier survival curve indicating the number of days from the pretreatment evaluation until the first evaluation that demonstrated radiographic evidence of progressive disease is shown. PFS indicates progression-free survival.
No correlation between tumor adsorbed dose and disease stabilization was noted (Fig. 4). The mean tumor adsorbed doses were not found to be significantly different between patients with stable disease (2.69 Gy) and patients with progressive disease (6.71 Gy), as judged by the Student t test for unpaired data (P = .115). To our knowledge, the factors that affect tumor avidity for and sensitivity to 153Sm-EDTMP remain to be identified. The 2 patients with nonosteoblastic morphology were among the 5 to achieve disease stabilization, but it is not clear if this finding reflects a particular sensitivity of these subtypes. Another related question is whether 153Sm-EDTMP is effective in patients with micrometastatic disease. Of the 8 patients with progressive disease in the current study, only 2 had distant failures, whereas 6 had their disease progression manifest as enlargement of existing lesions (local failure). This pattern of failure suggests the possibility that this treatment might have some effect on tumor micrometastases.
FIGURE 4.
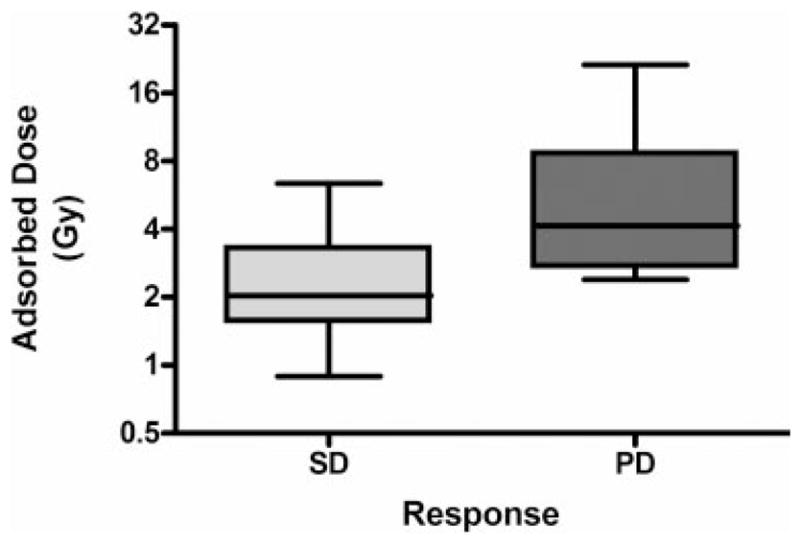
Correlation between adsorbed dose and tumor response. The median and range of tumor adsorbed doses for the patients who experienced stable disease (SD) are compared with the median and range of tumor adsorbed doses for the patients who experienced progressive disease (PD). Gy indicates grays.
DISCUSSION
In the current study, we report the results of a dose-finding study of 153Sm-EDTMP. Our dose-finding algorithm was designed to identify a dose of 153Sm-EDTMP that would have a DLT rate of 30%, with the DLT defined as failure of hematologic recovery within 6 weeks. We found this dose to be 44.8 MBq/kg (1.21 mCi/kg). Even in this heavily pretreated population, this level of radioactivity could be safely administered without undue hematologic toxicity, and, in keeping with previous reports, there were no significant nonhematologic drug-related toxicities attributable to treatment. Thus, it should be feasible to develop treatment regimens that combine chemotherapy with 153Sm-EDTMP for patients with bone metastases.
Our study design used the CRM to determine the MTD. The CRM was first introduced by O’Quigley et al in 1990.12 The version we implemented was described by Goodman et al.9 This method has been demonstrated by statistical simulation to be less biased and more efficient for estimating MTD than ad hoc dose ranging methods such as the Fibonacci scheme.13
Although not a primary objective of this dose-finding study, treatment response was evaluable in 13 patients. We noted an objective response rate of 38% (5 of 13 patients), despite treating a population that was comprised entirely of patients with refractory, progressive disease. Three of the patients survived for >1 year. We also determined the pattern of failure of the patients who did not respond to therapy, and found that only 2 of those 8 patients had distant failures. This finding suggests that 153Sm-EDTMP might have efficacy against micrometastatic disease, despite the inability to detect such disease on a standard bone scan. Given the low toxicity of this treatment and the ability to administer repeated doses, these features raise the possibility that 153Sm-EDTMP could be tested as a “maintenance” drug to prolong disease-free survival in high-risk patients.
There was no obvious correlation noted between the administered activity and the tumor adsorbed dose. However, the range of administered activities investigated in this study was relatively narrow. There was also no obvious correlation found between tumor adsorbed dose and response to treatment. We did, however, recognize 2 distinct populations of tumors: tumors from 3 of our patients (Patients C, G, and H from Fig. 2 Bottom) had almost no 153Sm uptake, whereas significant, although variable, uptake was noted in the other tumors. It is interesting to note that Patient C experienced disease stabilization and was alive and in disease remission at the time of last follow–up, 41 months after treatment.
In summary, we determined that a dose of 44.8 MBq/kg of 153Sm-EDTMP can be delivered to heavily pretreated patients with predictable hematopoietic toxicity. The results of the current study further demonstrated that this agent can be effective in the treatment of patients with high-risk osteosarcoma. Future work will focus on better understanding the factors that contribute to drug delivery and on improving efficacy by combination with chemotherapy, biologics, or external beam radiotherapy.
Footnotes
Conflict of Interest Disclosures
Financial support for this study was provided by EUSA Pharma (USA) Inc (Formerly Cytogen Corp.).
References
- 1.Link MP, Goorin AM, Miser AW, et al. The effect of adjuvant chemotherapy on relapse-free survival in patients with osteosarcoma of the extremity. N Engl J Med. 1986;314:1600–1606. doi: 10.1056/NEJM198606193142502. [DOI] [PubMed] [Google Scholar]
- 2.Francis KC, Phillips R, Nickson JJ, Woodard HQ, Higin-botham NL, Coley BL. Massive preoperative irradiation in the treatment of osteogenic sarcoma in children; a preliminary report. Am J Roentgenol Radium Ther Nucl Med. 1954;72:813–818. [PubMed] [Google Scholar]
- 3.Jenkin RD, Allt WE, Fitzpatrick PJ. Osteosarcoma. An assessment of management with particular reference to primary irradiation and selective delayed amputation. Cancer. 1972;30:393–400. doi: 10.1002/1097-0142(197208)30:2<393::aid-cncr2820300215>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 4.Lee ES, Mackenzie DH. Osteosarcoma. A study of the value of preoperative megavoltage radiotherapy. Br J Surg. 1964;51:252–274. doi: 10.1002/bjs.1800510405. [DOI] [PubMed] [Google Scholar]
- 5.Machak GN, Tkachev SI, Solovyev YN, et al. Neoadjuvant chemotherapy and local radiotherapy for high-grade osteosarcoma of the extremities. Mayo Clin Proc. 2003;78:147–155. doi: 10.4065/78.2.147. [DOI] [PubMed] [Google Scholar]
- 6.Anderson P, Nunez R. Samarium lexidronam (153Sm-EDTMP): skeletal radiation for osteoblastic bone metastases and osteosarcoma. Expert Rev Anticancer Ther. 2007;7:1517–1527. doi: 10.1586/14737140.7.11.1517. [DOI] [PubMed] [Google Scholar]
- 7.Anderson PM, Wiseman GA, Dispenzieri A, et al. High-dose samarium-153 ethylene diamine tetramethylene phosphonate: low toxicity of skeletal irradiation in patients with osteosarcoma and bone metastases. J Clin Oncol. 2002;20:189–196. doi: 10.1200/JCO.2002.20.1.189. [DOI] [PubMed] [Google Scholar]
- 8.Anderson PM, Wiseman GA, Erlandson L, et al. Gemcitabine radiosensitization after high-dose samarium for osteoblastic osteosarcoma. Clin Cancer Res. 2005;11(19 pt 1):6895–6900. doi: 10.1158/1078-0432.CCR-05-0628. [DOI] [PubMed] [Google Scholar]
- 9.Goodman SN, Zahurak ML, Piantadosi S. Some practical improvements in the continual reassessment method for phase I studies. Stat Med. 1995;14:1149–1161. doi: 10.1002/sim.4780141102. [DOI] [PubMed] [Google Scholar]
- 10.Stabin MG, Sparks RB, Crowe E. OLINDA/EXM: the second-generation personal computer software for internal dose assessment in nuclear medicine. J Nucl Med. 2005;46:1023–1027. [PubMed] [Google Scholar]
- 11.Siegel JA, Thomas SR, Stubbs JB, et al. MIRD pamphlet no. 16: techniques for quantitative radiopharmaceutical bio-distribution data acquisition and analysis for use in human radiation dose estimates. J Nucl Med. 1999;40:37S–61S. [PubMed] [Google Scholar]
- 12.O’Quigley J, Pepe M, Fisher L. Continual reassessment method: a practical design for phase 1 clinical trials in cancer. Biometrics. 1990;46:33–48. [PubMed] [Google Scholar]
- 13.Garrett-Mayer E. The continual reassessment method for dose-finding studies: a tutorial. Clin Trials. 2006;3:57–71. doi: 10.1191/1740774506cn134oa. [DOI] [PubMed] [Google Scholar]