Abstract
Multiple cellular signaling pathways have been involved in the processes of cancer cell invasion and metastasis. Among many signaling pathways, Wnt and Hedgehog (Hh) signaling pathways are critically involved in embryonic development, in the biology of cancer stem cells (CSCs) and in the acquisition of epithelial to mesenchymal transition (EMT), and thus this article will remain focused on Wnt and Hh signaling. Since CSCs and EMT are also known to be responsible for cancer cell invasion and metastasis, the Wnt and Hedgehog signaling pathways are also intimately associated with cancer invasion and metastasis. Emerging evidence suggests the beneficial role of chemopreventive agents commonly known as nutraceutical in cancer. Among many such agents, soy isoflavones, curcumin, green tea polyphenols, 3,3′-diindolylmethane, resveratrol, lycopene, vitamin D, etc. have been found to prevent, reverse, or delay the carcinogenic process. Interestingly, these agents have also shown to prevent or delay the progression of cancer, which could in part be due to their ability to attack CSCs or EMT-type cells by attenuating the Wnt and Hedgehog signaling pathways. In this review, we summarize the current state of our knowledge on the role of Wnt and Hedgehog signaling pathways, and their targeted inactivation by chemopreventive agents (nutraceuticals) for the prevention of tumor progression and/or treatment of human malignancies.
Keywords: Wnt, Hedgehog, Nutraceutical
1 Introduction
Although significant effort has been made in the fight against cancers for several decades, cancer is still the second most common cause of death in the USA, exceeded only by heart disease, with an estimated 1,479,350 new cases and 562,340 deaths was expected in 2009 [1]. The 5-year relative survival rate for all cancers diagnosed is 66% [1], and this high morality is primarily due to cancer cell invasion and metastasis.
It is known that multiple cellular signaling pathways are critically involved in the processes of tumor progression including invasion and metastasis of cancer. Among these pathways, Wnt and Hedgehog signaling pathways are the two most important signaling pathways that appears to play key roles in embryonic development, in the biology of cancer stem cells (CSCs), and in the acquisition of the epithelial to mesenchymal transition (EMT) phenotypic cells [2–7], and thus we focused our discussion on the role of Wnt and Hedgehog (Hh) signaling in this article. Since CSCs and EMT are known to be responsible for cancer cell invasion and metastasis, the Wnt and Hedgehog signaling pathways are also intimately associated with cancer invasion and metastasis.
Recently, the dietary chemopreventive agents have received much attention in the area of cancer research. It is estimated that one third of all cancers are preventable simply by modification of diet, maintenance of optimum body weight, and regular physical activity [8]. Several dietary chemopreventive agents such as soy isoflavones, curcumin, green tea polyphenols, resveratrol, 3,3′-diindolylmethane, lycopene, etc. have been found to prevent, reverse, or delay the carcinogenic process [9–15]. Interestingly, these agents have also shown to prevent or delay the progression of cancer, which could in part be due to their ability to attack CSCs or EMT-type cells by attenuating the Wnt and Hedgehog signaling pathways. Therefore, it is necessary to reveal the molecular determinants involved in the processes of cancer progression and the molecular effects of these nutraceuticals on Wnt and Hedgehog signaling, CSCs, and EMT in order to better design novel therapeutic strategies for the treatment of cancers using nutraceuticals combined with conventional chemotherapeutics. In this review, we summarize the current state of our knowledge on the role of Wnt and Hedgehog signaling pathways, and their targeted inactivation by chemopreventive agents (nutraceuticals) for the prevention of tumor progression and/or treatment of human malignancies.
2 Wnt and Hedgehog signaling pathways
It is well known that cancer cells display malfunction because of multiple molecular alterations in cellular signaling network. In cancer cells, the altered proteins produced from the mutations, defects, and amplifications of genes impact the way how they could communicate with each other. The cellular signaling pathways that are known to be important in cancer development and progression include Akt, nuclear factor-kappa B (NF-κB), mitogen-activated protein kinase (MAPK), Wnt, Hedgehog, Notch, androgen receptor (AR), etc. Since both Wnt and Hedgehog signaling pathways play key roles in the embryonic and stem cell development, Wnt and Hedgehog signaling and their crosstalk are critically involved in the processes of CSCs and EMT formation, which are believed to be responsible for cancer recurrence, invasion and metastasis.
2.1 Wnt signaling
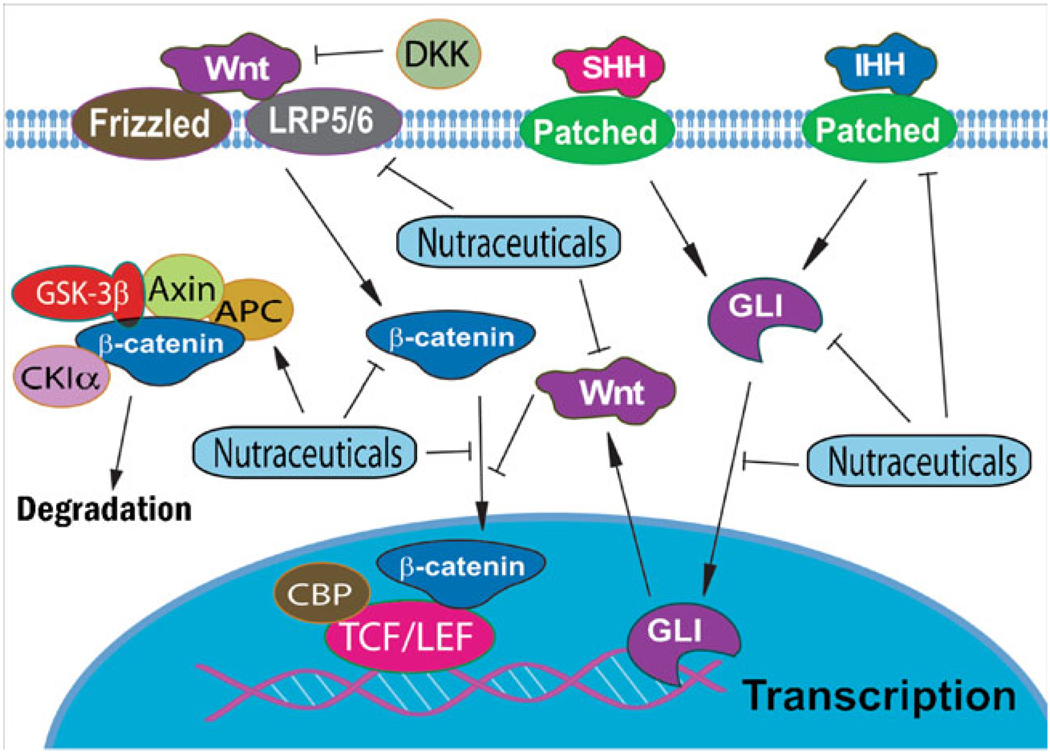
It is well known that many molecules in the Wnt signaling pathways contribute to control the processes of embryonic development including cell proliferation, differentiation, and epithelial-mesenchymal interactions [2, 16]. Canonical Wnt signals are transduced through membrane Frizzled (FZD) receptors and low density lipoprotein receptor-related protein (LRP) to the β-catenin signaling cascade (Fig. 1). In the absence of active Wnt ligands, β-catenin is complexed with scaffold proteins axis inhibition protein (Axin) and adenomatosis polyposis coli (APC), and phosphorylated by glycogen synthase kinase (GSK)-3β and casein kinase Iα (CKIα) at N-terminal residues. Phosphorylated β-catenin is then ubiquitinated and undergoes proteasome-mediated degradation. In the presence of active Wnt signaling, Wnt ligands bind to FZD and LRP, resulting in the phosphorylation of LRP6 by GSK-3β in its cytoplasmic region, leading to the recruitment of the cytosolic proteins Dishevelled (Dvl) and Axin. Because of the formation of FZD-DVL complex and LRP-Axin-FRAT complex, β-catenin is released from phosphorylation by GSK-3β and degradation by proteosome. Then, the accumulated β-catenin translocates to the nucleus and regulates the expression of target genes (Fig. 1). In the absence of nuclear β-catenin, T cell factor (TCF) and Groucho form a constitutive repressor complex. β-catenin translocated into the nucleus disrupts this interaction between TCF and Groucho. The nuclear β-catenin is then complexed with T cell factor/lymphoid enhancer factor (TCF/LEF) transcription factors and Legless family docking proteins (BCL9 and BCL9L) associated with PYGO family coactivators. The TCF/LEF-β-catenin-Legless-PYGO nuclear complex then activates the transcription of Wnt signaling target genes such as FGF20, DKK1, Myc, cyclin D1, etc. There are several secreted or intracellular proteins, which regulate the activation of Wnt signaling. Among them, secreted frizzled receptor proteins (sFRPs) and Dickkopf proteins (DKKs) inhibit the activation of Wnt signaling by inhibiting active Wnt ligands binding to FZD and LRP, respectively. APC, Axin, CKIα, and GSK-3β are intracellular proteins which down-regulate Wnt signaling. In noncanonical Wnt pathway, active signals are transduced through FZD receptors and co-receptors including ROR2 and RYK. DVL, c-jun NH2-terminal kinase, and small G proteins such as RHOA, RHOU, RAC, and CDC42 are also involved in the noncanonical Wnt pathway. It is known that small G proteins are implicated in the cytoskeletal reorganization during invasion and metastasis, suggesting the importance of noncanonical Wnt signaling in cancer invasion and metastasis.
Fig. 1.
Wnt and Hedgehog signaling pathways and their crosstalk are implicated in the cancer development and progression. The effects of nutraceuticals on Wnt and Hedgehog signaling pathways are also shown
The aberrant activation of the canonical Wnt/β-catenin signaling is one of the most frequent signaling abnormalities known in human cancers. During the development of human cancer, activated Wnt signal promotes β-catenin accumulation in the nucleus, resulting in the consequent transcriptional activation of specific target genes (Fig. 1) [17, 18]. The inappropriate expression of the Wnt ligand and Wnt binding proteins and the inappropriate activation of the Wnt signaling have been found in a variety of human cancers [19–21]. In colorectal cancer, mutations in APC and other components of the β-catenin destruction complex promote the activation of Wnt signaling [22, 23]. A recent study has also shown that Wnt activity could define colon CSCs and could be regulated by the microenvironment [23]. However, the activation of the Wnt signaling may occur in a different manner in other cancers such as prostate or breast cancers because the mutations in APC are rare. Therefore, other regulatory mechanisms could play dominant roles in the activation of β-catenin in other cancers. In prostate cells, Wnt-3a was found to stimulate proliferation selectively in the AR-positive 22Rv1 and LNCaP cells; however, TCF-dependent reporter gene transcription was not induced in LNCaP cells, suggesting that the activation of Wnt signaling in AR-positive prostate cancer cells may be through AR-dependent mechanisms rather than classical TCF-dependent mechanisms [24]. It is also found that β-catenin could enhance the function of AR and that nuclear translocation of β-catenin was observed in prostate cancer tissues, indicating that Wnt signaling is required for disease progression in prostate cancer [25]. In addition, loss or reduction of E-cadherin together with abnormal expression of Wnt ligands, receptors, inhibitors, and other co-regulators could also contribute to the activation of Wnt signaling in cancers with the acquisition of EMT phenotype of cancer cells [19, 21]. Therefore, inhibition of aberrant Wnt activity in cancer cells could provide an opportunity for the prevention of tumor progression and/or treatment of cancers [20, 26, 27].
2.2 Hedgehog signaling
Another important signaling pathway involved in cell development and proliferation is the Hh signaling pathway. The Hedgehog signaling pathway is a major regulator of cell differentiation, tissue polarity and cell proliferation [28, 29]. The Hh family consists of three secreted proteins including Sonic Hedgehog (Shh), Desert Hedgehog, and Indian Hedgehog. The Hh proteins undergo multiple processing steps to become activated. First, the protein is cleaved. Then, the C-terminal domain of the Hh polypeptide catalyzes an intramolecular cholesteroyl transfer reaction, forming a Hedgehog ligand with a C-terminal cholesterol moiety. The cholesterol modification leads to the association of Hh with membranes, facilitating the final modification step in which a palmitoyl moiety is added to the N terminus of Hh by the transmembrane acyltransferase. Thus, the fully active Hedgehog is double lipid-modified. The receptor of Hh ligands is Patched (Ptc), which is located on membrane (Fig. 1). In the absence of Hh, Ptc catalytically inhibits the activity of Smoothened (Smo) which is the seven-transmembrane-span receptor-like protein. Binding of Hh to Ptc leads to the loss of Ptc activity, and consequent activation of Smo, which transduces the Hh signal to the cytoplasm. Subsequently, the activated Smo causes the activation of Gli (glioma-associated oncogene family zinc finger) family of transcriptional effectors through complex interactions with Costal2 (Cos2), Fused (Fu) and Suppressor of fu (Sufu), leading to the up-regulation of Ptc, Wnt, BMP, etc. Therefore, the Hh ligands, Sonic Hedgehog, Desert Hedgehog, and Indian Hedgehog stimulate Gli transcription factors which constitute the final effectors of the Hh signaling pathway (Fig. 1).
It has been found that germline mutations that subtly affect Hh pathway activity are associated with developmental disorders [30]. Moreover, somatic mutations which activate Hh pathway have been linked to a variety of human cancers [30]. Emerging evidence clearly suggesting the activation of Hh signaling in various human cancers, including basal cell carcinomas, medulloblastomas, leukemia, gastrointestinal, lung, ovarian, breast and prostate cancers [29]. Furthermore, because Hh plays a central role in the control of proliferation and differentiation of both embryonic stem cells and adult stem cells, the aberrant activation of Hh signaling could lead to the generation of CSCs and the development of cancer [31]. It has been known that epithelial expression of hedgehog ligand during prostate development exerts autocrine and paracrine signaling activities that regulates the growth and differentiation of the prostate gland. Increased Hh signaling has been associated with prostate cancer progression and has also been shown to accelerate prostate cancer growth [32]. Experimental studies have revealed the critical role of Hh signaling in prostate cancer, and further demonstrated that autocrine Hh signaling by tumor cells is required for proliferation, viability and invasive behavior of prostate cancer [33]. Recent studies also showed that activation of Hedgehog signaling is critically related to CSCs and EMT features in many types of cancers including colon, gastric, esophagus, hepatic, and other cancers [5, 34–37]. Therefore, the development of Hedgehog inhibitors is coming through the drug pipeline, which holds a great promise for the prevention of tumor progression and/or treatment of human malignancies.
2.3 The crosstalk between Wnt and Hedgehog signaling
The most molecules in both Wnt and Hh signaling are evolutionarily conserved proteins that are critically important for many developmental processes in human and animals. It is known that abnormal regulation of these signaling pathways in human and animals could result in tumor formation and other diseases. Since both Wnt and Hedgehog signaling play key roles in the physiological and pathological development of embryonic and stem cells, it is believed that the two signaling have crosstalk between each other. Some progress has been made in understanding how Wnt and Hh signals are transduced into one response. Moreover, several studies have suggested that there are fundamental similarities between Wnt and Hh signaling pathways [38]. Both signaling are activated by G-protein-coupled receptors (Frizzled or Smoothened) [39, 40]. In both pathways, signaling prevents phosphorylation-dependent proteolysis of a key effector (Cubitus interruptus or β-catenin), which converts a DNA-binding protein from a repressor to an activator of transcription [41]. Furthermore, Wnt signaling has been found to be downstream of hedgehog signaling, participating in the bone development [42]. It has also been found that activation of GLI stimulates the transcription of Wnt ligands, suggesting that Wnt signaling is downstream of hedgehog [43]. However, it is important to note that the molecules in Wnt signaling such as GSK-3β also regulate the molecules in Hedgehog signaling [38] and that the pathological response to oncogenic Hedgehog signaling is also dependent on canonical Wnt/beta3-catenin signaling [44], suggesting the crosstalk between Wnt and Hedgehog signaling. These available literature suggests that Wnt and Hh signaling is a vicious cycle for the maintenance of cancer cells, and driving cancer cells toward aggressiveness, which contributes to cancer deaths. Therefore, the development and identification of selective inhibitors of Wnt and Hh signaling is an attractive approach for the prevention of tumor progression and/or treatment of cancers.
3 Nutraceuticals in the regulation of Wnt and Hedgehog signaling
3.1 Soy isoflavone
Isoflavones are found primarily in members of the Leguminosae family. Foods such as soy, lentil, bean, and chickpea are sources of isoflavones; however, soybean is the food that contains abundant amounts of isoflavones. Genistein, daidzein, and glycitein are the three main isoflavones found in soybeans and most soy-protein products. Several epidemiological studies have shown that soy could have protective effects against prostate and other cancers [45–48]. A prospective study of 12,395 California Seventh-Day Adventist men who often drank soy milk showed that frequent consumption of soy milk was associated with 70% reduction of the risk of prostate cancer [48], suggesting the possible association between high intake of soy isoflavones and reduced risk of prostate cancer. Experimental studies have also revealed that isoflavones, particularly genistein and daidzein, exert their anti-oxidant effects on human cells. It has been known that genistein protects cells against ROS by scavenging free radicals and reducing the expression of stress-response related genes. Isoflavone genistein is also well known as a tyrosine kinase inhibitor, and thus far isoflavones are considered pleitropic affecting multiple signaling pathways [49].
Our laboratory has investigated the effects of isoflavone genistein on Wnt signaling pathways. We have found that isoflavone mixture significantly inhibited the activation of Wnt signaling [50]. Through mechanistic studies, we found that isoflavone up-regulated the expression of GSK-3β, enhanced GSK-3β binding to β-catenin, and increased the phosphorylation of β-catenin, suggesting that isoflavone could inactivate Wnt signaling to inhibit the growth of prostate cancer cells [50]. Other investigators also reported that genistein up-regulated epithelial adhesion molecule E-cadherin expression, diminished basal and Wnt-1-induced cell proliferation, and attenuated the expression of Wnt-1 targets such as c-Myc and Cyclin D1 [51]. Isoflavone genistein also inhibited the expression of Wnt-5a [52], suggesting the inhibitory effects of isoflavone genistein on Wnt signaling. Moreover, an animal study with microarray analysis showed that isoflavone genistein down-regulated Wnt signaling in the tumor tissues of animals treated with genistein [52]. The gene expression profiles also showed reduced expression of Wnt target genes such as cyclin D1 in mammary ductal epithelium of the animals treated with isoflavone genistein compared to control [52]. In addition, a decrease in the gene expression of Wnt-7a was also observed in endometrial adenocarcinoma cells treated with isoflavone genistein, suggesting the inhibitory effects of isoflavone on Wnt signaling [53]. A recent report showed that HEK293 cells transfected with Dkk-1, an antagonist of Wnt/β-catenin signaling pathway, have significantly decreased accumulation of both β-catenin and E-cadherin at the cell membrane, which could increase migration of cells [54]. More importantly, treatment with isoflavone genistein could inhibit the migration effect of Dkk-1 and significantly increase the membrane localization of β-catenin and E-cadherin in HEK293 cells transfected with Dkk-1, suggesting the inhibitory effects of isoflavone genistein on Wnt signaling and metastasis [54].
The in vitro and in vivo effects of isoflavone genistein on the Hedgehog signaling have been recently reported [55]. It was found that isoflavone genistein could decrease Gli1 mRNA concentration and down-regulate Gli reporter activity with significant inhibition of prostate cancer cell growth. Importantly, isoflavone genistein could reduce or delay prostate cancer growth in vivo in TRAMP mice [55]. These results suggest that the Hedgehog signaling could be a direct or indirect target of isoflavone genistein, leading to the inhibition of prostate cancer cell growth in vitro and in vivo. These limited results suggest that a non-toxic component of the human diet could be useful inhibitor of Wnt and Hh signaling although further in-depth mechanistic pre-clinical and clinical studies are required for assessing the health benefit of isoflavones in cancer patients.
3.2 Curcumin
Curcumin is a bioactive compound found in Curcuma longa (tumeric). Turmeric extract from the rhizomes, commonly called curcuminoids, is mainly composed of curcumin. The research on curcumin has received considerable attention due to its pronounced anti-inflammatory, anti-oxidative, immunomodulating, anti-atherogenic, and anti-carcinogenic activities [56, 57]. It has been known that curcumin inhibited IKK, suppressed both constitutive and inducible NF-κB activation, and potentiated TNF-induced apoptosis. Curcumin also showed strong anti-oxidant and anti-cancer properties through regulating the expression of genes that are critically related to the oxidant stress.
We and other investigators have found that curcumin could inhibit several important signaling pathways including Notch and Wnt signaling [58, 59]. It is believed that cancer arises from stem cells and/or progenitor cells through the dysregulation or acquisition of self-renewal. Interestingly, it was found that curcumin could inhibit mammosphere formation and could also decrease the amount of aldehyde dehydrogenase-positive cells in normal and malignant breast cells through the inhibition of Wnt signaling [60], suggesting the inhibitory effects of curcumin on breast CSCs [60]. Curcumin has also been found to inhibit the expression of several molecules in Wnt/β-catenin pathway including disheveled, β-catenin, cyclin D1, and slug in both MCF-7 and MDA-MB-231 breast cancer cells [61]. Immunofluorescence staining showed that curcumin significantly reduced the nuclear expression of disheveled and β-catenin proteins. The expression levels of GSK-3β and E-cadherin were also altered by curcumin treatment [61]. These findings suggest that the curcumin could inhibit cell proliferation and induce apoptosis through regulation of β-catenin signaling in human breast cancer cells.
In colon cancer, curcumin treatment caused p53- and p21-independent G2/M phase arrest and apoptosis in HCT-116, HCT-116, and HCT-116 cells [62]. Furthermore, caspase-3-mediated cleavage of β-catenin, E-cadherin, and APC, decreased transactivation of β-catenin/TCF/LEF, decreased promoter DNA-binding activity of the β-catenin/TCF/LEF complex, and decreased levels of c-Myc protein were also observed after curcumin treatment [62]. These results suggest that curcumin treatment could impair both Wnt signaling and cell–cell adhesion pathways, leading to the G2/M phase arrest and apoptosis in colon cancer cells. Aberrant activation of the Wnt/β-catenin signaling has been observed in osteosarcoma tumorigenesis and metastasis. A study has been conducted to determine whether osteosarcoma progression could be delayed by disrupting the Wnt/β-catenin signaling using natural agent curcumin [63]. It was found that curcumin suppressed intrinsic and activated β-catenin/TCF transcriptional activities, and that nuclear β-catenin was significantly reduced by treatment with curcumin. Overexpression of the wild-type β-catenin in osteosarcoma cells led to enhance cell invasiveness while curcumin significantly inhibited cancer cell invasion resulting from the down-regulation of Wnt/β-catenin, matrix metalloproteinase (MMP)-9, cyclin D1, c-Myc, and survivin [63], suggesting that curcumin has therapeutic potential for the treatment of osteosarcoma.
By microarray profiling analysis, the expression of molecules in apoptosis and Wnt signaling pathway have been found to be altered. The Wnt signaling pathway components, AXIN2 and FRA1 (FOS-like antigen 1), showed a decreasing expression after curcumin treatment [64], providing additional evidence in support of the inhibitory effects of curcumin on Wnt signaling. Other mechanisms may also be involved in the curcumin regulated down-regulation of Wnt signaling. Ryu et al. reported that novel curcumin analogs, demethoxycurcumin and bisdemethoxycurcumin, could attenuate the Wnt/β-catenin pathway through down-regulation of the transcriptional coactivator p300, which is a positive regulator of the Wnt/beta-catenin pathway [58]. In addition, curcumin also inhibited inflammation-induced obesity through the suppression of Wnt signaling [65]. It is known that curcumin could improve obesity-associated inflammation and diabetes in obese mice [66]. It is interesting to note that curcumin also inhibited adipocyte differentiation. During differentiation, curcumin restored nuclear translocation of the integral Wnt signaling component β-catenin. Curcumin also reduced differentiation-stimulated expression of CK1α, GSK-3β, and Axin, which forms the destruction complex targeting β-catenin, suggesting that Wnt signaling pathway is involved in curcumin-induced suppression of adipogenesis [66].
Medulloblastoma is an aggressive primary brain tumor that arises in the cerebellum of the children and young adults. It is believed that Sonic Hedgehog signaling plays important roles in the pathology of this aggressive disease. Studies have shown that curcumin suppressed medulloblastoma cell proliferation and triggered cell-cycle arrest at G2/ M phase [67]. Moreover, curcumin was shown to inhibit the Shh-Gli1 signaling by the down-regulation of Shh protein and its most important downstream targets Gli1 and PTCH1. Importantly, curcumin also reduced the levels of β-catenin and its downstream targets, c-Myc and cyclin D1 [67]. These results suggest that there could be a crosstalk between Hedgehog and Wnt signaling and that curcumin could inhibit both Hedgehog and Wnt signaling pathways. More importantly, curcumin could enhance the efficiency of non-toxic doses of cisplatin and γ-rays through the down-regulation of Bcl-2 [67], which is also a downstream gene of Hedgehog signaling. These results indicate that curcumin, a natural non-toxic compound, could be a promising agent in Shh-targeted therapy for the treatment of medul-loblastomas. The in vitro and in vivo effects of curcumin on the Hedgehog signaling in prostate cancer have been recently reported [55]. It was found that curcumin inhibited Gli1 mRNA expression and down-regulated Gli reporter activity with a significant inhibition of prostate cancer cell growth. More importantly, curcumin could reduce or delay prostate cancer growth in vivo in TRAMP mice [55]. These results indicate that the Hedgehog signaling could be a target of curcumin, leading to the inhibition of prostate cancer cell growth in vitro and in vivo. However, the beneficial effects of curcumin in humans have been limited due to its poor bioavailability and rapid inactivation, which prompted the development of novel analogs of curcumin as detailed in our recent review article [68]. These newer agents could be useful in targeted inactivation of Wnt and Hh signaling for the prevention of tumor progression and/or treatment of human malignancies in the future.
3.3 Tea polyphenols
Consumption of green tea has been associated with human health including the prevention of cancers and heart disease. Epidemiological studies showed lower incidence of prostate cancer among Asian men with a high dietary intake of green tea, suggesting that green tea might be a preventive agent against cancers [69]. The result from Japan's Public Health Center-based Prospective Study showed that the high consumption of green tea was associated with a decreased risk of advanced prostate cancer [70]. Green tea and its constituents have been studied both in vitro and in vivo. Green tea contains several catechins including epicatechin (EC), epigallocatechin, epicatechin-3-gallate, and epigallocatechin-3-gallate (EGCG). However, EGCG has been shown to be the most potent for the inhibition of carcinogenesis and the reduction of oxidative stress among these catechins [71].
The inhibitory effects of EGCG on Wnt and Hedgehog signaling have been reported in various cancers. It was found that 2 to 25 µM EGCG inhibited β-catenin expression and β-catenin/TCF-4 reporter activity in a concentration-dependent manner, suggesting the inhibitory effects of EGCG on Wnt signaling [72]. EGCG treatment also decreased cytosolic β-catenin protein level and inhibited TCF/LEF reporter activity in lung cancer H460 and A549 cell lines [73]. In colon cancer, treatment of HT29 cells with EGCG led to a potent inhibition of GSK3-α and GSK-3β activity [74]. The amount of phosphorylated β-catenin was diminished and the overall amount of β-catenin and the TCF/LEF-mediated luciferase expressions were decreased by EGCG treatment [74]. It has also been reported that the canonical Wnt signaling could be inhibited at the level of TCF/LEF by EGCG in antler progenitor cells [75]. Moreover, the effect of a combination of EGCG with fish oil on intestinal tumorigenesis in ApcMin/+ mice fed a high-fat diet was investigated [76]. The combination treatment for 9 weeks significantly reduced the tumor number through the inhibition of Wnt signaling. β-catenin nuclear positivity in adenomas from the combination group was lower than control mice, indicating the inhibition of Wnt signaling by the combination treatment [76].
By microarray gene expression profiling analysis, investigators also identified two signaling pathways, Wnt and Id signaling, involved in cell proliferation, were inhibited by EGCG treatment, indicating the negative regulation of EGCG on cell proliferation [77]. Other mechanisms may be also involved in the EGCG-mediated inhibition of Wnt signaling. In breast cancer cells, Wnt signaling has been found to be inhibited by EGCG in a dose-dependent manner. This effect could be mediated by HBP1 transcriptional repressor, which is a suppressor of Wnt signaling. EGCG treatment induced HBP1 transcriptional repressor levels through an increase in HBP1 mRNA stability [78]. However, knockdown of HBP1 reduced sensitivity to EGCG in the suppression of Wnt signaling and its target gene c-Myc expression [78]. Moreover, EGCG also reduced both breast cancer cell proliferation and invasiveness in an HBP1-dependent manner, suggesting that EGCG blocks Wnt signaling and inhibits invasion of breast cancer through the induction of HBP1 transcriptional repressor [78].
The in vitro and in vivo effects of EGCG on the Hedgehog signaling have been reported [55]. It has been found that EGCG could inhibit Gli1 mRNA expression and down-regulated Gli reporter activity with a significant inhibition of prostate cancer cell growth. More importantly, EGCG could reduce or delay prostate cancer growth in vivo in TRAMP mice [55] as stated earlier. These results indicate that the Hedgehog signaling could be a target of EGCG, leading to the inhibition of prostate cancer cell growth in vitro and in vivo. Recently, another report also showed that EGCG could effectively inhibit cellular proliferation and induce apoptosis of SW1353 and CRL-7891 human chondrosarcoma cells with inhibition of Indian Hedgehog pathway, and down-regulation of PTCH and Gli-1 expression, suggesting that EGCG could be a new therapeutic agent for patients with chondrosarcoma [79].
3.4 Resveratrol
Resveratrol is a stilbenoid found in the skin of red grapes and peanuts. It suppresses many types of cancers by regulating cell proliferation and apoptosis through a variety of mechanisms. The effects of resveratrol on obesity-promoted colon cancer have been investigated. It was found that resveratrol exhibited anti-proliferative properties in HT-29 colon cancer cells by arresting G0/G1-S phase cell-cycle progression [80]. Treatment with resveratrol suppressed insulin-like growth factor 1 (IGF-1)R protein levels and inhibited the downstream Akt and Wnt signaling pathways [80]. It was also found that low concentrations of resveratrol (<10 µM) significantly decreased the amount and proportion of β-catenin in the nucleus and reduced the expression of lgs and pygoI, two regulators of β-catenin localization, in colon-derived cells, suggesting the implications of resveratrol for colon cancer prevention [81]. It has also been found that resveratrol inhibited proliferation and induced apoptosis in Waldenstrom's macroglobulinemia (WM) and IgM-secreting cells through the down-regulation of Akt, MAPK, and Wnt signaling followed by the activation of caspase signaling [82]. Importantly, resveratrol showed synergistic inhibitory effects on WM cells when combined with dexamethasone, fludarabine, and bortezomib [82], which are commonly used for the treatment of WM, suggesting the therapeutic effects of resveratrol.
However, the effect of resveratrol on the differentiation of osteoblast is different from that on cancer cells. During osteoblast differentiation, Wnt signaling could be up-regulated. This phenomena is supported by the observations showing that treatment of pre-osteoblast with sFRP-2 and sFRP-4 significantly increased Wnt-3A-induced alkaline phosphatase activities [83] and that Wnt inhibitory factor 1 could inhibit osteoblastic differentiation in mouse embryonic mesenchymal cells [84]. Experimental study showed that resveratrol treatment of mesenchymal cells led to an increase in stabilization and nuclear accumulation of β-catenin. Following the increased nuclear accumulation of β-catenin, the ability to activate transcription of β-catenin-TCF/LEF target genes that are required for osteoblastic differentiation was up-regulated [85].
The in vitro and in vivo effects of resveratrol on the Hedgehog signaling have also been reported as stated earlier [55]. Resveratrol could inhibit Gli1 mRNA expression and down-regulate Gli reporter activity with a significant inhibition of prostate cancer cell growth. More importantly, resveratrol could reduce or delay prostate cancer growth in vivo in TRAMP mice [55]. These results demonstrated that the Hedgehog signaling could be a target of resveratrol and that resveratrol treatment could inhibit prostate cancer cell growth in vitro and in vivo.
3.5 Indole-3-carbinol and 3,3′-diindolylmethane
3,3′-diindolylmethane (DIM) is the dimeric product of indole-3-carbinol (I3C) which is produced from naturally occurring glucosinolates contained in a wide variety of plants including members of the family Cruciferae such as broccoli. Under the acidic conditions of the stomach, I3C undergoes extensive and rapid self-condensation reactions to form several derivatives. DIM is the major derivative and condensation product of I3C. Epidemiological studies indicated that human exposure to indoles through consumption of cruciferous vegetable could decrease cancer risk [86]. DIM has been shown to reduce oxidative stress and stimulate the expression of anti-oxidant response element-driven gene, suggesting the anti-oxidant function of indole compounds [87, 88]. Animal study showed that DIM was not toxic and had an in vivo preventive effect against the development of prostate cancer in a mouse model [89]. Furthermore, several experimental studies have shown that DIM inhibited oncogenesis and cancer cell growth, and induced apoptosis in cancer cells in vitro and in vivo, suggesting that DIM could serve as a potent agent for the prevention of tumor progression and/or treatment of cancers.
We and other investigators have investigated the molecular targets of DIM. It has been found that DIM could regulate Wnt signaling through the regulation of Akt/GSK-3β signaling. We found that DIM could inhibit the phosphorylation of GSK-3β and increase the phosphorylation of β-catenin in prostate cancer cells, leading to the inhibition of cell growth and the induction of apoptosis [90]. These results suggest the inhibitory effects of DIM on Wnt signaling. Sulforaphane is another natural compound derived from broccoli/broccoli sprouts. It was found that sulforaphane decreased aldehyde dehydrogenase-positive cell population in human breast cancer cells and reduced the size and number of primary mammospheres through the down-regulation of Wnt/β-catenin self-renewal pathway, suggesting the effect of sulforaphane against breast cancer stem cells [91], which indeed could become useful for the prevention and cancer invasion and metastasis.
3.6 Lycopene
Tomatoes are rich in lycopene, which is the pigment principally responsible for the deep-red color of tomato and its products. Tomato products including ketchup, tomato juice, and pizza sauce, are the richest sources of lycopene in the US diet. Lycopene is a potent anti-oxidant. It has been known that lycopene is a biologically active carotenoid exhibiting high physical quenching rate constant with singlet oxygen, suggesting its high activity as anti-oxidant. Giovannucci et al. have reported that frequent consumption of tomato products is associated with a lower risk of prostate cancer [92]. The inverse associations between plasma lycopene and prostate cancer have also been reported [93, 94]. Experimental studies also showed that lycopene inhibited cell growth in breast, prostate and endometrial cancer cells with regulation of cell-cycle-related genes. An in vivo animal study showed that lycopene had anti-tumor effects that could be potentiated by vitamin E, an anti-oxidant that is also present in tomatoes [95], confirming the anti-cancer activity of lycopene. A phase II clinical trial from our group has shown that lycopene supplements reduced tumor size and PSA level in localized prostate cancer [96, 97], suggesting its promising effects on prostate cancer prevention and/or treatment. Experimental studies have shown that lycopene reduced inflammatory signals, prevented oxidative DNA damage, and regulated the expression or activity of IGF/ Akt, Wnt/β-catenin, and AR signaling [98]. Lycopene reduced AR and β-catenin nuclear localization and inhibited IGF-1-stimulated prostate cancer growth, perhaps by attenuating the effects of IGF-1 on phosphorylation of Akt and GSK-3β. It is believed that the effects of lycopene on these pathways contribute to the observed cell growth inhibition and apoptosis induction [98].
3.7 Vitamin D
There are two major forms of vitamin D, vitamin D2 and D3 that are important for humans. The active form of vitamin D in the body is 1,25-dihydroxyvitamin D, which can be made from either vitamin D2 or vitamin D3. Epidemiologic and experimental studies suggest that higher intakes of vitamin D from food and/or supplements and higher levels of vitamin D in the blood are associated with reduced risks of cancer [99]. Epidemiologic studies have also suggested that vitamin D could be a preventive agent for cancers. It has been reported that reduced levels of active vitamin D resulted in a higher incidence and mortality of prostate cancer [99]. Native Japanese men, whose diet is rich in vitamin D, have a low incidence of prostate cancer, supporting the protective role of vitamin D. Another study also showed that dietary supplementation with >600 IU of vitamin D reduced the risk of prostate cancer [100]. The precise mechanisms of Vitamin D action on the prevention of cancers are not clear although it is known that vitamin D regulates the expression of genes such as LRP5, VDR, etc. [101], which are related to Wnt signaling.
Experimental studies showed that vitamin D could suppress Wnt, Notch, NF-κB, and IGF-1 signaling [102]. In addition, vitamin D receptor could also inhibit β-catenin-mediated transcription [103]. Moreover, the inhibition of β-catenin activity by vitamin D and its receptor was significantly enhanced by wild-type APC [103], suggesting the importance of vitamin D status in Wnt signaling. Recent report showed that macrophage-derived soluble factors could induce canonical Wnt signaling in colon cancer cells and promote their growth through the crosstalk between STAT1 and IL-1β [104]. However, Vitamin D, an effective chemopreventive agent, could interrupt this crosstalk in macrophages and inhibit the activation of Wnt signaling in colon carcinoma cells [104]. DKK-1 is expressed at high level in colon cancer cell lines with a differentiated phenotype such as Caco-2 or HT-29. It has been found that vitamin D activated the transcription of the DKK-1 gene, which could be the mechanism by which vitamin D inhibited Wnt signaling and tumor growth [105]. It was found that Snail1 could abrogate the inhibitory effect of vitamin D on Wnt signaling and, therefore, it is a positive regulator of the Wnt/β-catenin signaling [106]. These results suggest the beneficial effects of vitamin D on the prevention and treatment of cancers.
As mentioned above, the Hedgehog signaling plays a key role in directing growth and patterning during embryonic development and is required for the normal development of many structures including the neural tube, axial skeleton, skin, and hair. Aberrant activation of the Hedgehog signaling in adult tissue is associated with the development of basal cell carcinoma, medulloblastoma, and a subset of pancreatic, gastrointestinal, and other cancers. Vitamin D could serve as treatment option for human cancers that possess high activity of Hedgehog signaling because of the ability of vitamin D on the inhibition of Hedgehog signaling [107, 108]. The Hedgehog signaling has also been found to be highly activated in the proximal gastrointestinal tract; however, vitamin D could inhibit the growth of cells in the tract specifically through inactivation of Smo and the downstream Hh signaling [109].
3.8 Other agents
Other nutraceuticals could also regulate Wnt and Hedgehog signaling. Selenium is an essential micronutrient found in grains, fish, meat, poultry, or eggs. Now, selenium is available in over-the-counter supplements and multivitamins. It has been known that selenium is distributed in body tissues and has anti-oxidant effect. Epidemiological studies showed that selenium could be a protective agent against the development of prostate and colorectal cancers. A study was conducted to test the level of selenium in serum and prostate of 52 men after selenium supplementation. It was found that selenium supplementation resulted in a significantly higher levels of selenium in the prostatic tissue [110], suggesting the high bioavailability of selenium. Importantly, it is known that the targets of selenium include Wnt, AR, Notch, etc. [111], suggesting that the effects of selenium could be due to attenuation of many signaling pathways including Wnt signaling.
The plant flavonoid fisetin can induce apoptosis and suppress the growth of colon cancer cells by inhibition of Wnt and COX-2 signaling. It was found that the treatment of colon cancer cells with fisetin inhibited the activity of Wnt signaling through down-regulation of β-catenin and TCF- 4, resulting in decreased expression of Wnt signaling target genes such as cyclin D1 and MMP-7 [112]. Another novel natural product dammarane-type triterpene sapogenin (PPD25) isolated from the leaves of Panax notoginseng showed anti-cancer activity in colon and lung cancer cells [113], which was found to be mediated through reduced expression of β-catenin and its transcriptional targets including c-Myc, cyclin D1, cdk4 and TCF-4, further suggesting that the anti-cancer effects of PPD25 is mediated by Wnt signaling [113].
In addition, the effects of the naturally derived agent deguelin on the prevention of mammary tumorigenesis have been investigated. It was found that deguelin inhibited Wnt/β-catenin signaling in breast cancer cell lines [114]. It was also reported that dietary fish oil could play a protective role against colorectal cancer through the down-regulation of COX and Wnt/β-catenin pathways [115], suggesting the added beneficial effects of fish oil.
4 Conclusions and perspectives
In conclusion, cancer cells are known to have alterations in multiple cellular signaling pathways among which Wnt and Hedgehog signaling pathways appear to play prominent roles especially because these two developmental pathways are also important in the biology of CSCs and EMT phenotypic cells. Moreover, the molecules in both Wnt and Hedgehog signaling pathways appear to be critical for the processes of cancer cell invasion and metastasis. Therefore, novel strategies targeting these important pathways and their upstream and downstream signaling could be very promising for the prevention of cancer and its metastases. It is important to note that natural dietary agents known as nutraceuticals such as soy isoflavone, curcumin, EGCG, resveratrol, DIM, lycopene, and others that target Wnt and Hedgehog signaling could be useful for the prevention of tumor progression and/or could also be useful for the treatment of human malignancies in combination with conventional therapeutics. However, further in vitro mechanistic studies and in vivo animal studies together with clinical trials are needed to fully appreciate the value of the nutraceuticals for the prevention of tumor progression and/ or treatment of human cancers.
Acknowledgements
The authors’ work cited in this review article was funded by grants from the National Cancer Institute, NIH (5R01CA083695, 2R01CA1085 35, 5R01CA131151, 3R01CA131151-02S109, and 1R01CA132794 awarded to FHS), and a sub-contract award to FHS from the University of Texas MD Anderson Cancer Center through SPORE grant (5P20-CA101936, 3P20CA101936-05S109) on pancreatic cancer awarded to James Abbruzzese. We also thank Puschelberg and Guido foundations for their generous contribution to support our research.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA: A Cancer Journal for Clinicians. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Woll PS, Morris JK, Painschab MS, et al. Wnt signaling promotes hematoendothelial cell development from human embryonic stem cells. Blood. 2008;111:122–131. doi: 10.1182/blood-2007-04-084186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takahashi-Yanaga F, Kahn M. Targeting Wnt signaling: can we safely eradicate cancer stem cells? Clinical Cancer Research. 2010;16:3153–3162. doi: 10.1158/1078-0432.CCR-09-2943. [DOI] [PubMed] [Google Scholar]
- 4.Merchant A, Joseph G, Wang Q, et al. Gli1 regulates the proliferation and differentiation of HSCs and myeloid progenitors. Blood. 2010;115:2391–2396. doi: 10.1182/blood-2009-09-241703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Varnat F, Duquet A, Malerba M, et al. Human colon cancer epithelial cells harbour active HEDGEHOG-GLI signalling that is essential for tumour growth, recurrence, metastasis and stem cell survival and expansion. EMBO Molecular Medicine. 2009;1:338–351. doi: 10.1002/emmm.200900039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Syn WK, Jung Y, Omenetti A, et al. Hedgehog-mediated epithelial-to-mesenchymal transition and fibrogenic repair in nonalcoholic fatty liver disease. Gastroenterology. 2009;137:1478–1488. doi: 10.1053/j.gastro.2009.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malizia AP, Lacey N, Walls D, et al. CUX1/Wnt signaling regulates epithelial mesenchymal transition in EBV infected epithelial cells. Experimental Cell Research. 2009;315:1819–1831. doi: 10.1016/j.yexcr.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 8.American Cancer Society. Cancer facts & figures 2009. Atlanta: American Cancer Society Inc; 2009. [Google Scholar]
- 9.Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nature Reviews. Cancer. 2003;3:768–780. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- 10.Khan N, Afaq F, Mukhtar H. Apoptosis by dietary factors: the suicide solution for delaying cancer growth. Carcinogenesis. 2007;28:233–239. doi: 10.1093/carcin/bgl243. [DOI] [PubMed] [Google Scholar]
- 11.Lamartiniere CA, Cotroneo MS, Fritz WA, et al. Genistein chemoprevention: timing and mechanisms of action in murine mammary and prostate. The Journal of Nutrition. 2002;132:552S–558S. doi: 10.1093/jn/132.3.552S. [DOI] [PubMed] [Google Scholar]
- 12.Li Y, Sarkar FH. Gene expression profiles of genistein-treated PC3 prostate cancer cells. The Journal of Nutrition. 2002;132:3623–3631. doi: 10.1093/jn/132.12.3623. [DOI] [PubMed] [Google Scholar]
- 13.Li Y, Li X, Sarkar FH. Gene expression profiles of I3C-and DIM-treated PC3 human prostate cancer cells determined by cDNA microarray analysis. The Journal of Nutrition. 2003;133:1011–1019. doi: 10.1093/jn/133.4.1011. [DOI] [PubMed] [Google Scholar]
- 14.Mukhopadhyay A, Bueso-Ramos C, Chatterjee D, et al. Curcumin downregulates cell survival mechanisms in human prostate cancer cell lines. Oncogene. 2001;20:7597–7609. doi: 10.1038/sj.onc.1204997. [DOI] [PubMed] [Google Scholar]
- 15.Gupta S, Hussain T, Mukhtar H. Molecular pathway for (−)-epigallocatechin-3-gallate-induced cell cycle arrest and apoptosis of human prostate carcinoma cells. Archives of Biochemistry and Biophysics. 2003;410:177–185. doi: 10.1016/s0003-9861(02)00668-9. [DOI] [PubMed] [Google Scholar]
- 16.Angers S, Moon RT. Proximal events in Wnt signal transduction. Nature Reviews Molecular Cell Biology. 2009;10:468–477. doi: 10.1038/nrm2717. [DOI] [PubMed] [Google Scholar]
- 17.Behrens J. Control of beta-catenin signaling in tumor development. Annals of the New York Academy of Sciences. 2000;910:21–33. doi: 10.1111/j.1749-6632.2000.tb06698.x. [DOI] [PubMed] [Google Scholar]
- 18.Peifer M, Polakis P. Wnt signaling in oncogenesis and embryogenesis—a look outside the nucleus. Science. 2000;287:1606–1609. doi: 10.1126/science.287.5458.1606. [DOI] [PubMed] [Google Scholar]
- 19.Taipale J, Beachy PA. The Hedgehog and Wnt signalling pathways in cancer. Nature. 2001;411:349–354. doi: 10.1038/35077219. [DOI] [PubMed] [Google Scholar]
- 20.Verras M, Sun Z. Roles and regulation of Wnt signaling and beta-catenin in prostate cancer. Cancer Letters. 2006;237:22–32. doi: 10.1016/j.canlet.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 21.Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 22.Clevers H. Wnt breakers in colon cancer. Cancer Cell. 2004;5:5–6. doi: 10.1016/s1535-6108(03)00339-8. [DOI] [PubMed] [Google Scholar]
- 23.Vermeulen L, De Sousa EMF, van der Heijden M, et al. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nature Cell Biology. 2010;12:468–476. doi: 10.1038/ncb2048. [DOI] [PubMed] [Google Scholar]
- 24.Cronauer MV, Schulz WA, Ackermann R, et al. Effects of WNT/beta-catenin pathway activation on signaling through T-cell factor and androgen receptor in prostate cancer cell lines. International Journal of Oncology. 2005;26:1033–1040. doi: 10.3892/ijo.26.4.1033. [DOI] [PubMed] [Google Scholar]
- 25.Chesire DR, Ewing CM, Gage WR, et al. In vitro evidence for complex modes of nuclear beta-catenin signaling during prostate growth and tumorigenesis. Oncogene. 2002;21:2679–2694. doi: 10.1038/sj.onc.1205352. [DOI] [PubMed] [Google Scholar]
- 26.Barker N, Clevers H. Mining the Wnt pathway for cancer therapeutics. Nature Reviews Drug Discovery. 2006;5:997–1014. doi: 10.1038/nrd2154. [DOI] [PubMed] [Google Scholar]
- 27.Dihlmann S, von Knebel DM. Wnt/beta-catenin-pathway as a molecular target for future anti-cancer therapeutics. International Journal of Cancer. 2005;113:515–524. doi: 10.1002/ijc.20609. [DOI] [PubMed] [Google Scholar]
- 28.Gritli-Linde A, Bei M, Maas R, et al. Shh signaling within the dental epithelium is necessary for cell proliferation, growth and polarization. Development. 2002;129:5323–5337. doi: 10.1242/dev.00100. [DOI] [PubMed] [Google Scholar]
- 29.Yang L, Xie G, Fan Q, et al. Activation of the hedgehog-signaling pathway in human cancer and the clinical implications. Oncogene. 2010;29:469–481. doi: 10.1038/onc.2009.392. [DOI] [PubMed] [Google Scholar]
- 30.Varjosalo M, Taipale J. Hedgehog: functions and mechanisms. Genes & Development. 2008;22:2454–2472. doi: 10.1101/gad.1693608. [DOI] [PubMed] [Google Scholar]
- 31.Medina V, Calvo MB, Diaz-Prado S, et al. Hedgehog signalling as a target in cancer stem cells. Clinical & Translational Oncology. 2009;11:199–207. doi: 10.1007/s12094-009-0341-y. [DOI] [PubMed] [Google Scholar]
- 32.Vezina CM, Bushman AW. Hedgehog signaling in prostate growth and benign prostate hyperplasia. Current Urology Reports. 2007;8:275–280. doi: 10.1007/s11934-007-0073-x. [DOI] [PubMed] [Google Scholar]
- 33.Anton Aparicio LM, Garcia CR, Cassinello EJ, et al. Prostate cancer and Hedgehog signalling pathway. Clinical & Translational Oncology. 2007;9:420–428. doi: 10.1007/s12094-007-0080-x. [DOI] [PubMed] [Google Scholar]
- 34.Choi SS, Omenetti A, Witek RP, et al. Hedgehog pathway activation and epithelial-to-mesenchymal transitions during myofibroblastic transformation of rat hepatic cells in culture and cirrhosis. American Journal of Physiology. Gastrointestinal and Liver Physiology. 2009;297:G1093–G1106. doi: 10.1152/ajpgi.00292.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Isohata N, Aoyagi K, Mabuchi T, et al. Hedgehog and epithelial-mesenchymal transition signaling in normal and malignant epithelial cells of the esophagus. International Journal of Cancer. 2009;125:1212–1221. doi: 10.1002/ijc.24400. [DOI] [PubMed] [Google Scholar]
- 36.Ohta H, Aoyagi K, Fukaya M, et al. Cross talk between hedgehog and epithelial-mesenchymal transition pathways in gastric pit cells and in diffuse-type gastric cancers. British Journal of Cancer. 2009;100:389–398. doi: 10.1038/sj.bjc.6604846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Omenetti A, Porrello A, Jung Y, et al. Hedgehog signaling regulates epithelial-mesenchymal transition during biliary fibrosis in rodents and humans. The Journal of Clinical Investigation. 2008;118:3331–3342. doi: 10.1172/JCI35875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kalderon D. Similarities between the Hedgehog and Wnt signaling pathways. Trends in Cell Biology. 2002;12:523–531. doi: 10.1016/s0962-8924(02)02388-7. [DOI] [PubMed] [Google Scholar]
- 39.Huelsken J, Birchmeier W. New aspects of Wnt signaling pathways in higher vertebrates. Current Opinion in Genetics & Development. 2001;11:547–553. doi: 10.1016/s0959-437x(00)00231-8. [DOI] [PubMed] [Google Scholar]
- 40.Ingham PW, McMahon AP. Hedgehog signaling in animal development: paradigms and principles. Genes & Development. 2001;15:3059–3087. doi: 10.1101/gad.938601. [DOI] [PubMed] [Google Scholar]
- 41.Price MA, Kalderon D. Proteolysis of the Hedgehog signaling effector Cubitus interruptus requires phosphorylation by Glycogen Synthase Kinase 3 and Casein Kinase 1. Cell. 2002;108:823–835. doi: 10.1016/s0092-8674(02)00664-5. [DOI] [PubMed] [Google Scholar]
- 42.Day TF, Yang Y. Wnt and hedgehog signaling pathways in bone development. The Journal of Bone and Joint Surgery. American Volume. 2008;90 Suppl 1:19–24. doi: 10.2106/JBJS.G.01174. [DOI] [PubMed] [Google Scholar]
- 43.Cohen MM., Jr The hedgehog signaling network. American Journal of Medical Genetics. Part A. 2003;123A:5–28. doi: 10.1002/ajmg.a.20495. [DOI] [PubMed] [Google Scholar]
- 44.Yang SH, Andl T, Grachtchouk V, et al. Pathological responses to oncogenic Hedgehog signaling in skin are dependent on canonical Wnt/beta3-catenin signaling. Nature Genetics. 2008;40:1130–1135. doi: 10.1038/ng.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Adlercreutz H, Honjo H, Higashi A, et al. Urinary excretion of lignans and isoflavonoid phytoestrogens in Japanese men and women consuming a traditional Japanese diet. The American Journal of Clinical Nutrition. 1991;54:1093–1100. doi: 10.1093/ajcn/54.6.1093. [DOI] [PubMed] [Google Scholar]
- 46.Adlercreutz H, Markkanen H, Watanabe S. Plasma concentrations of phyto-oestrogens in Japanese men. Lancet. 1993;342:1209–1210. doi: 10.1016/0140-6736(93)92188-y. [DOI] [PubMed] [Google Scholar]
- 47.Hebert JR, Hurley TG, Olendzki BC, et al. Nutritional and socioeconomic factors in relation to prostate cancer mortality: a cross-national study. Journal of the National Cancer Institute. 1998;90:1637–1647. doi: 10.1093/jnci/90.21.1637. [DOI] [PubMed] [Google Scholar]
- 48.Jacobsen BK, Knutsen SF, Fraser GE. Does high soy milk intake reduce prostate cancer incidence? The Adventist Health Study (United States) Cancer Causes & Control. 1998;9:553–557. doi: 10.1023/a:1008819500080. [DOI] [PubMed] [Google Scholar]
- 49.Sarkar FH, Li Y, Wang Z, et al. Lesson learned from nature for the development of novel anti-cancer agents: implication of isoflavone, curcumin, and their synthetic analogs. Current Pharmaceutical Design. 2010;16(16):1801–1812. doi: 10.2174/138161210791208956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li Y, Wang Z, Kong D, et al. Regulation of Akt/ FOXO3a/GSK-3beta/AR signaling network by isoflavone in prostate cancer cells. The Journal of Biological Chemistry. 2008;283:27707–27716. doi: 10.1074/jbc.M802759200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Su Y, Simmen RC. Soy isoflavone genistein upregulates epithelial adhesion molecule E-cadherin expression and attenuates beta-catenin signaling in mammary epithelial cells. Carcinogenesis. 2009;30:331–339. doi: 10.1093/carcin/bgn279. [DOI] [PubMed] [Google Scholar]
- 52.Su Y, Simmen FA, Xiao R, et al. Expression profiling of rat mammary epithelial cells reveals candidate signaling pathways in dietary protection from mammary tumors. Physiological Genomics. 2007;30:8–16. doi: 10.1152/physiolgenomics.00023.2007. [DOI] [PubMed] [Google Scholar]
- 53.Wagner J, Lehmann L. Estrogens modulate the gene expression of Wnt-7a in cultured endometrial adenocarcinoma cells. Molecular Nutrition & Food Research. 2006;50:368–372. doi: 10.1002/mnfr.200500215. [DOI] [PubMed] [Google Scholar]
- 54.Kuang HB, Miao CL, Guo WX, et al. Dickkopf-1 enhances migration of HEK293 cell by beta-catenin/E-cadherin degradation. Frontiers in Bioscience. 2009;14:2212–2220. doi: 10.2741/3373. [DOI] [PubMed] [Google Scholar]
- 55.Slusarz A, Shenouda NS, Sakla MS, et al. Common botanical compounds inhibit the hedgehog signaling pathway in prostate cancer. Cancer Research. 2010;70:3382–3390. doi: 10.1158/0008-5472.CAN-09-3012. [DOI] [PubMed] [Google Scholar]
- 56.Miquel J, Bernd A, Sempere JM, et al. The curcuma antioxidants: pharmacological effects and prospects for future clinical use. A review. Archives of Gerontology and Geriatrics. 2002;34:37–46. doi: 10.1016/s0167-4943(01)00194-7. [DOI] [PubMed] [Google Scholar]
- 57.Banerjee M, Tripathi LM, Srivastava VM, et al. Modulation of inflammatory mediators by ibuprofen and curcumin treatment during chronic inflammation in rat. Immunopharmacology and Immunotoxicology. 2003;25:213–224. doi: 10.1081/iph-120020471. [DOI] [PubMed] [Google Scholar]
- 58.Ryu MJ, Cho M, Song JY, et al. Natural derivatives of curcumin attenuate the Wnt/beta-catenin pathway through down-regulation of the transcriptional coactivator p300. Biochemical and Biophysical Research Communications. 2008;377:1304–1308. doi: 10.1016/j.bbrc.2008.10.171. [DOI] [PubMed] [Google Scholar]
- 59.Wang Z, Desmoulin S, Banerjee S, et al. Synergistic effects of multiple natural products in pancreatic cancer cells. Life Sciences. 2008;83:293–300. doi: 10.1016/j.lfs.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kakarala M, Brenner DE, Korkaya H, et al. Targeting breast stem cells with the cancer preventive compounds curcumin and piperine. Breast Cancer Research and Treatment. 2009;122:777–785. doi: 10.1007/s10549-009-0612-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Prasad CP, Rath G, Mathur S, et al. Potent growth suppressive activity of curcumin in human breast cancer cells: Modulation of Wnt/beta-catenin signaling 14. Chem-Biol Interact. 2009;181:263–271. doi: 10.1016/j.cbi.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 62.Jaiswal AS, Marlow BP, Gupta N, et al. Beta-catenin-mediated transactivation and cell-cell adhesion pathways are important in curcumin (diferuylmethane)-induced growth arrest and apoptosis in colon cancer cells. Oncogene. 2002;21:8414–8427. doi: 10.1038/sj.onc.1205947. [DOI] [PubMed] [Google Scholar]
- 63.Leow PC, Tian Q, Ong ZY, et al. Antitumor activity of natural compounds, curcumin and PKF118-310, as Wnt/beta-catenin antagonists against human osteosarcoma cells 9. Investigational New Drugs. 2009 doi: 10.1007/s10637-009-9311-z. doi:10.1007/s10637-009-9311-z. [DOI] [PubMed] [Google Scholar]
- 64.Shin HW, Park SY, Lee KB, et al. Down-regulation of Wnt signaling during apoptosis of human hepatic stellate cells 16. Hepatogastroenterology. 2009;56:208–212. [PubMed] [Google Scholar]
- 65.Aggarwal BB. Targeting inflammation-induced obesity and metabolic diseases by curcumin and other nutraceuticals 2. Annual Review of Nutrition. 2010;30:173–199. doi: 10.1146/annurev.nutr.012809.104755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ahn J, Lee H, Kim S, et al. Curcumin-induced suppression of adipogenic differentiation is accompanied by activation of Wnt/beta-catenin signaling 3. American Journal of Physiology. Cell Physiology. 2010;298:C1510–C1516. doi: 10.1152/ajpcell.00369.2009. [DOI] [PubMed] [Google Scholar]
- 67.Elamin MH, Shinwari Z, Hendrayani SF, et al. Curcumin inhibits the Sonic Hedgehog signaling pathway and triggers apoptosis in medulloblastoma cells. Molecular Carcinogenesis. 2010;49:302–314. doi: 10.1002/mc.20604. [DOI] [PubMed] [Google Scholar]
- 68.Padhye S, Chavan D, Pandey S, et al. Perspectives on chemopreventive and therapeutic potential of curcumin analogs in medicinal chemistry. Mini Reviews in Medicinal Chemistry. 2010;10:372–387. doi: 10.2174/138955710791330891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jian L, Lee AH, Binns CW. Tea and lycopene protect against prostate cancer. Asia Pacific Journal of Clinical Nutrition. 2007;16 Suppl 1:453–457. [PubMed] [Google Scholar]
- 70.Kurahashi N, Sasazuki S, Iwasaki M, et al. Green tea consumption and prostate cancer risk in Japanese men: a prospective study. American Journal of Epidemiology. 2008;167:71–77. doi: 10.1093/aje/kwm249. [DOI] [PubMed] [Google Scholar]
- 71.Khan N, Adhami VM, Mukhtar H. Review: green tea polyphenols in chemoprevention of prostate cancer: preclinical and clinical studies. Nutrition and Cancer. 2009;61:836–841. doi: 10.1080/01635580903285056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dashwood WM, Orner GA, Dashwood RH. Inhibition of beta-catenin/Tcf activity by white tea, green tea, and epigallocatechin-3-gallate (EGCG): minor contribution of H(2)O (2) at physiologically relevant EGCG concentrations. Biochemical and Biophysical Research Communications. 2002;296:584–588. doi: 10.1016/s0006-291x(02)00914-2. [DOI] [PubMed] [Google Scholar]
- 73.Gao Z, Xu Z, Hung MS, et al. Promoter demethylation of WIF-1 by epigallocatechin-3-gallate in lung cancer cells 15. Anticancer Research. 2009;29:2025–2030. [PubMed] [Google Scholar]
- 74.Pahlke G, Ngiewih Y, Kern M, et al. Impact of quercetin and EGCG on key elements of the Wnt pathway in human colon carcinoma cells. Journal of Agricultural and Food Chemistry. 2006;54:7075–7082. doi: 10.1021/jf0612530. [DOI] [PubMed] [Google Scholar]
- 75.Mount JG, Muzylak M, Allen S, et al. Evidence that the canonical Wnt signalling pathway regulates deer antler regeneration. Developmental Dynamics. 2006;235:1390–1399. doi: 10.1002/dvdy.20742. [DOI] [PubMed] [Google Scholar]
- 76.Bose M, Hao X, Ju J, et al. Inhibition of tumorigenesis in ApcMin/+ mice by a combination of (−)-epigallocatechin-3-gallate and fish oil. Journal of Agricultural and Food Chemistry. 2007;55:7695–7700. doi: 10.1021/jf071004r. [DOI] [PubMed] [Google Scholar]
- 77.Liu L, Lai CQ, Nie L, et al. The modulation of endothelial cell gene expression by green tea polyphenol-EGCG. Molecular Nutrition & Food Research. 2008;52:1182–1192. doi: 10.1002/mnfr.200700499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kim J, Zhang X, Rieger-Christ KM, et al. Suppression of Wnt signaling by the green tea compound (−)-epigallocatechin 3-gallate (EGCG) in invasive breast cancer cells. Requirement of the transcriptional repressor HBP1. The Journal of Biological Chemistry. 2006;281:10865–10875. doi: 10.1074/jbc.M513378200. [DOI] [PubMed] [Google Scholar]
- 79.Tang GQ, Yan TQ, Guo W, et al. (−)-Epigallocatechin-3-gallate induces apoptosis and suppresses proliferation by inhibiting the human Indian Hedgehog pathway in human chondrosarcoma cells. Journal of Cancer Research and Clinical Oncology. 2010;136:1179–1185. doi: 10.1007/s00432-010-0765-3. [DOI] [PubMed] [Google Scholar]
- 80.Vanamala J, Reddivari L, Radhakrishnan S, et al. Resveratrol suppresses IGF-1 induced human colon cancer cell proliferation and elevates apoptosis via suppression of IGF-1R/ Wnt and activation of p53 signaling pathways 1. BMC Cancer. 2010;10:238. doi: 10.1186/1471-2407-10-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hope C, Planutis K, Planutiene M, et al. Low concentrations of resveratrol inhibit Wnt signal throughput in colon-derived cells: implications for colon cancer prevention. Molecular Nutrition & Food Research. 2008;52 Suppl 1:S52–S61. doi: 10.1002/mnfr.200700448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Roccaro AM, Leleu X, Sacco A, et al. Resveratrol exerts antiproliferative activity and induces apoptosis in Walden-strom's macroglobulinemia. Clinical Cancer Research. 2008;14:1849–1858. doi: 10.1158/1078-0432.CCR-07-1750. [DOI] [PubMed] [Google Scholar]
- 83.Cho SW, Her SJ, Sun HJ, et al. Differential effects of secreted frizzled-related proteins (sFRPs) on osteoblastic differentiation of mouse mesenchymal cells and apoptosis of osteoblasts. Biochemical and Biophysical Research Communications. 2008;367:399–405. doi: 10.1016/j.bbrc.2007.12.128. [DOI] [PubMed] [Google Scholar]
- 84.Cho SW, Yang JY, Sun HJ, et al. Wnt inhibitory factor (WIF)-1 inhibits osteoblastic differentiation in mouse embryonic mesenchymal cells. Bone. 2009;44:1069–1077. doi: 10.1016/j.bone.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 85.Zhou H, Shang L, Li X, et al. Resveratrol augments the canonical Wnt signaling pathway in promoting osteoblastic differentiation of multipotent mesenchymal cells 12. Experimental Cell Research. 2009;315:2953–2962. doi: 10.1016/j.yexcr.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 86.Higdon JV, Delage B, Williams DE, et al. Cruciferous vegetables and human cancer risk: epidemiologic evidence and mechanistic basis. Pharmacological Research. 2007;55:224–236. doi: 10.1016/j.phrs.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nho CW, Jeffery E. Crambene, a bioactive nitrile derived from glucosinolate hydrolysis, acts via the antioxidant response element to upregulate quinone reductase alone or synergistically with indole-3-carbinol. Toxicology and Applied Pharmacology. 2004;198:40–48. doi: 10.1016/j.taap.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 88.Benabadji SH, Wen R, Zheng JB, et al. Anticarcinogenic and antioxidant activity of diindolylmethane derivatives. Acta Pharmacologica Sinica. 2004;25:666–671. [PubMed] [Google Scholar]
- 89.Fares F, Azzam N, Appel B, et al. The potential efficacy of 3, 3'-diindolylmethane in prevention of prostate cancer development. European Journal of Cancer Prevention. 2010;19:199–203. doi: 10.1097/CEJ.0b013e328333fbce. [DOI] [PubMed] [Google Scholar]
- 90.Li Y, Wang Z, Kong D, et al. Regulation of FOXO3a/beta-catenin/GSK-3beta signaling by 3, 3'-diindolylmethane contributes to inhibition of cell proliferation and induction of apoptosis in prostate cancer cells. The Journal of Biological Chemistry. 2007;282:21542–21550. doi: 10.1074/jbc.M701978200. [DOI] [PubMed] [Google Scholar]
- 91.Li Y, Zhang T, Korkaya H, et al. Sulforaphane, a dietary component of broccoli/broccoli sprouts, inhibits breast cancer stem cells. Clinical Cancer Research. 2010;16:2580–2590. doi: 10.1158/1078-0432.CCR-09-2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Giovannucci E, Rimm EB, Liu Y, et al. A prospective study of tomato products, lycopene, and prostate cancer risk. Journal of the National Cancer Institute. 2002;94:391–398. doi: 10.1093/jnci/94.5.391. [DOI] [PubMed] [Google Scholar]
- 93.Lu QY, Hung JC, Heber D, et al. Inverse associations between plasma lycopene and other carotenoids and prostate cancer. Cancer Epidemiology, Biomarkers & Prevention. 2001;10:749–756. [PubMed] [Google Scholar]
- 94.Gann PH, Ma J, Giovannucci E, et al. Lower prostate cancer risk in men with elevated plasma lycopene levels: results of a prospective analysis. Cancer Research. 1999;59:1225–1230. [PubMed] [Google Scholar]
- 95.Limpens J, van Weerden WM, Kramer K, et al. Re: Prostate carcinogenesis in N-methyl-N-nitrosourea (NMU)-testosterone-treated rats fed tomato powder, lycopene, or energy-restricted diets. Journal of the National Cancer Institute. 2004;96:554–555. doi: 10.1093/jnci/djh089. [DOI] [PubMed] [Google Scholar]
- 96.Kucuk O, Sarkar FH, Sakr W, et al. Phase II randomized clinical trial of lycopene supplementation before radical prostatectomy. Cancer Epidemiology, Biomarkers & Prevention. 2001;10:861–868. [PubMed] [Google Scholar]
- 97.Kucuk O, Sarkar FH, Djuric Z, et al. Effects of lycopene supplementation in patients with localized prostate cancer. Experimental Biology Medicine (Maywood) 2002;227:881–885. doi: 10.1177/153537020222701007. [DOI] [PubMed] [Google Scholar]
- 98.Wertz K. Lycopene effects contributing to prostate health 4. Nutrition and Cancer. 2009;61:775–783. doi: 10.1080/01635580903285023. [DOI] [PubMed] [Google Scholar]
- 99.Garland CF, Garland FC, Gorham ED, et al. The role of vitamin D in cancer prevention. American Journal of Public Health. 2006;96:252–261. doi: 10.2105/AJPH.2004.045260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ahn J, Albanes D, Peters U, et al. Dairy products, calcium intake, and risk of prostate cancer in the prostate, lung, colorectal, and ovarian cancer screening trial. Cancer Epidemiology, Biomarkers & Prevention. 2007;16:2623–2630. doi: 10.1158/1055-9965.EPI-07-0601. [DOI] [PubMed] [Google Scholar]
- 101.Pike JW, Meyer MB, Martowicz ML, et al. Emerging regulatory paradigms for control of gene expression by 1, 25-dihydroxyvitamin D(3) Journal of Steroid Biochemistry and Molecular Biology. 2010;121(1–2):130–135. doi: 10.1016/j.jsbmb.2010.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kovalenko PL, Zhang Z, Cui M, et al. 1, 25 dihydroxyvitamin D-mediated orchestration of anticancer, transcript-level effects in the immortalized, non-transformed prostate epithelial cell line, RWPE1. BMC Genomics. 2010;11:26. doi: 10.1186/1471-2164-11-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Egan JB, Thompson PA, Vitanov MV, et al. Vitamin D receptor ligands, adenomatous polyposis coli, and the vitamin D receptor FokI polymorphism collectively modulate beta-catenin activity in colon cancer cells. Molecular Carcinogenesis. 2010;49:337–352. doi: 10.1002/mc.20603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kaler P, Augenlicht L, Klampfer L. Macrophage-derived IL-1beta stimulates Wnt signaling and growth of colon cancer cells: a crosstalk interrupted by vitamin D3. Oncogene. 2009;28:3892–3902. doi: 10.1038/onc.2009.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Aguilera O, Pena C, Garcia JM, et al. The Wnt antagonist DICKKOPF-1 gene is induced by 1alpha, 25-dihydroxyvitamin D3 associated to the differentiation of human colon cancer cells. Carcinogenesis. 2007;28:1877–1884. doi: 10.1093/carcin/bgm094. [DOI] [PubMed] [Google Scholar]
- 106.Larriba MJ, Valle N, Palmer HG, et al. The inhibition of Wnt/beta-catenin signalling by 1alpha, 25-dihydroxyvitamin D3 is abrogated by Snail1 in human colon cancer cells. Endocrine-Related Cancer. 2007;14:141–151. doi: 10.1677/ERC-06-0028. [DOI] [PubMed] [Google Scholar]
- 107.Bijlsma MF, Peppelenbosch MP, Spek CA. (Pro-)vitamin D as treatment option for hedgehog-related malignancies. Medical Hypotheses. 2008;70:202–203. doi: 10.1016/j.mehy.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 108.Tang JY, So PL, Epstein EH., Jr Novel Hedgehog pathway targets against basal cell carcinoma. Toxicology and Applied Pharmacology. 2007;224:257–264. doi: 10.1016/j.taap.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bruggemann LW, Queiroz KC, Zamani K, et al. Assessing the efficacy of the hedgehog pathway inhibitor vitamin D3 in a murine xenograft model for pancreatic cancer. Cancer Biology and Therapy. 2010;10:78–88. doi: 10.4161/cbt.10.1.12165. [DOI] [PubMed] [Google Scholar]
- 110.Gianduzzo TR, Holmes EG, Tinggi U, et al. Prostatic and peripheral blood selenium levels after oral supplementation. Journal d'Urologie. 2003;170:870–873. doi: 10.1097/01.ju.0000081052.51707.cf. [DOI] [PubMed] [Google Scholar]
- 111.Kipp A, Banning A, van Schothorst EM, et al. Four selenoproteins, protein biosynthesis, and Wnt signalling are particularly sensitive to limited selenium intake in mouse colon. Molecular Nutrition & Food Research. 2009;53:1561–1572. doi: 10.1002/mnfr.200900105. [DOI] [PubMed] [Google Scholar]
- 112.Suh Y, Afaq F, Johnson JJ, et al. A plant flavonoid fisetin induces apoptosis in colon cancer cells by inhibition of COX2 and Wnt/EGFR/NF-kappaB-signaling pathways. Carcinogenesis. 2009;30:300–307. doi: 10.1093/carcin/bgn269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bi X, Zhao Y, Fang W, et al. Anticancer activity of Panax notoginseng extract 20(S)-25-OCH3-PPD: Targetting beta-catenin signalling. Clinical and Experimental Pharmacology & Physiology. 2009;36:1074–1078. doi: 10.1111/j.1440-1681.2009.05203.x. [DOI] [PubMed] [Google Scholar]
- 114.Murillo G, Peng X, Torres KE, et al. Deguelin inhibits growth of breast cancer cells by modulating the expression of key members of the Wnt signaling pathway 7. Cancer Prevention Research (Phila Pa) 2009;2:942–950. doi: 10.1158/1940-6207.CAPR-08-0232. [DOI] [PubMed] [Google Scholar]
- 115.Vanamala J, Glagolenko A, Yang P, et al. Dietary fish oil and pectin enhance colonocyte apoptosis in part through suppression of PPARdelta/PGE2 and elevation of PGE3. Carcinogenesis. 2008;29:790–796. doi: 10.1093/carcin/bgm256. [DOI] [PMC free article] [PubMed] [Google Scholar]