Abstract
In dosimetry-based treatment planning protocols, patients with rapid clearance of the radiopharmaceutical require a larger amount of initial activity than those with slow clearance to match the absorbed dose to the critical organ. As a result, the dose-rate to the critical organ is higher in patients with rapid clearance and may cause unexpected toxicity compared to patients with slow clearance. In order to account for the biological impact of different dose-rates, radiobiological modeling is beginning to be applied to the analysis of radionuclide therapy patient data. To date, the formalism used for these analyses is based on kinetics derived from activity in a single organ, the target. This does not include the influence of other source organs to the dose and dose-rate to the target organ. As a result, only self-dose irradiation in the target organ contributes to the dose-rate. In this work, the biological effective dose (BED) formalism has been extended to include the effect of multiple source organ contributions to the net dose-rate in a target organ. The generalized BED derivation has been based on the Medical Internal Radionuclide Dose Committee (MIRD) schema assuming multiple source organs following exponential effective clearance of the radionuclide. A BED-based approach to determine the largest safe dose to critical organs has also been developed. The extended BED formalism is applied to red marrow dosimetry, as well as kidney dosimetry considering the cortex and the medulla separately, since both those organs are commonly dose limiting in radionuclide therapy. The analysis shows that because the red marrow is an early responding tissue (high α/β), it is less susceptible to unexpected toxicity arising from rapid clearance of high levels of administered activity in the marrow or in the remainder of the body. In kidney dosimetry, the study demonstrates a complex interplay between clearance of activity in the cortex and the medulla, as well as the initial activity ratio and the S value ratio between the two. In some scenarios, projected BED based on both the cortex and the medulla is a more appropriate constraint on the administered activity than the BED based on the cortex only. Furthermore, different fractionated regimens were considered to reduce renal toxicity. The MIRD-based BED formalism is expected to be useful for patient-specific adjustments of activity and to facilitate the investigation of dose-toxicity correlations with respect to dose-rate and tissue repair mechanism.
Keywords: dosimetry, radionuclide therapy, biological effective dose
I. INTRODUCTION
The objective of dosimetry-based treatment-planning protocols in radionuclide therapy is to prescribe a clinically useful absorbed dose to the tumor while at the same time avoiding organ toxicity. If the clearance of the agent from critical organs is rapid, then the dose-rate must also be considered. Indeed, the increased dose-rate, arising from a large administered activity based on an “absorbed dose only” protocol, may cause unexpected toxicity. Even though the total absorbed dose to a dose-limiting tissue remains constant, the dose-rate can vary substantially among patients. Dose rate considerations have only recently been taken into account in radionuclide therapy dosimetry.1–10 Those effects may be examined through the biological effective dose (BED) that relates absorbed dose and dose-rate with radiosensitivity and repair of radiation damage using the standard linear-quadratic (LQ) model.11,12 The BED represents the dose required for a given biological effect when delivered by infinitely small doses per fraction or at very low dose-rates and is typically used to compare the response implications of total absorbed doses delivered at different dose-rates. To date, BED modeling for radionuclide therapy has accounted for the absorbed dose-rate from only a single source organ (the target).11 This is because the formalism is based on activity kinetics in the target. In this work, the BED formalism is extended to include the effect of multiple source organ contributions to the net dose-rate in a target organ. A generalized BED formulation is developed that follows the Medical Internal Radionuclide Dose Committee (MIRD) schema.13 The methodology is then applied to the red marrow and kidneys, which are the dose-limiting normal tissues in most radionuclide therapy. The issue of potential increased marrow toxicity due to the more rapid whole-body clearance associated with therapy using radiopeptides or engineered low molecular weight constructs as well as radioiodine therapy with protocols using recombinant human thyroid-stimulating hormone (rhTSH) is examined. The effect of cross irradiation of subkidney regions is also examined for small radiolabeled molecules. The application of BED in these examples is contrasted with its application in intact antibody mediated radioimmunotherapy.
II. MATERIALS AND METHODS
II.A. Standard BED formulation
The BED may be defined as the product of the total physical dose (D) and a modifying factor named the relative effectiveness per unit dose (RE) that quantifies dose-rate effects with respect to radiosensitivity and repair of radiation damage
| (1) |
It is important to note that repopulation of cells is not considered in this formulation (see Sec. IV). The model is usually applied for conventional fractionated high-dose-rate radiotherapy with N fractions of dose d, i.e., the total dose D = N · d. In this case, the RE function is given by
| (2) |
where α and β are the tissue specific coefficients for radiation damage with α proportional to dose (one single event is lethal) and β proportional to dose squared (two sublethal events required for lethal damage). The α/β ratio is also named the repair capacity and quantifies the sensitivity of a given tissue to changes in fractionation. Typical values for the α/β ratio are about 5–25 Gy for early-reacting normal tissues and tumors and about 2–5 Gy for late-responding normal tissues.14 For a single acute exposure, the fraction N is set to 1. Equation (2) assumes that acute dose fractions are spaced sufficiently to enable full recovery of sublethal damage remaining after each fraction. This is the usual situation of fractionated external beam radiation therapy (EBRT) where the regimen is based on daily fractions while the sub-lethal damage repair half-time ranges typically between 0.5 and 3 h.15 In continuous therapy such as radionuclide therapy, the repair process of sublethal damage takes place during the radiation dose delivery and, therefore, a more general formalism is required. Assuming an exponentially decreasing dose-rate and a complete decay of the source, Dale11 demonstrated that the RE function is given by the following expression:
| (3) |
where D is the absorbed dose, μ is the exponential repair rate constant that quantifies the rate of sublethal damage repair, and λ is the effective clearance rate constant given by the sum of the physical decay and the biological clearance rate constants. Note that the standard MIRD notation uses λ for the physical decay rate constant and λeff for the effective clearance rate constant.13 In the original equation, the product D · λ is replaced by the initial dose-rate R0. For a rapid clearance, i.e., λ ≫ μ, Eq. (3) is identical to the RE function of a single acute dose given by Eq. (2). Equation(3) is applicable only if the dose-rate, and accordingly the loss of activity in the organ, is well approximated by a monoexponential function. Howell et al. derived a more complex form of the RE using a dose-rate function that includes a time-dependent uptake contribution.7 The RE function can also be expressed using the Lea–Catcheside factor G (Ref. 8),
| (4) |
According to Eqs. (2) and (3), G is equal to 1/N for EBRT andλ/(λ + μ) for radionuclide therapy. The factor G ranges between 0 and 1 and expresses the reduction in cell kill due to sublethal damage repair during continuous irradiation and/or between fractions. Including the factor G, the standard LQ equation may be expressed as exp(−αD−GβD2). The generalized Lea–Catcheside factor G for a time-dependent dose-rate Ḋ(t) is given as follows:16,17
| (5) |
The second integration over the time parameter, w, refers to the exponential repair of first sublethal damage. The first integral term expresses the second event that can combine with the first event remaining after repair to produce a lethal lesion. As opposed to a single lethal event, sublethal damage is dependent on the rate of dose delivery. For a complete decay of the source, i.e., the radionuclide, the overall time of the treatment T goes to infinity.
II.B. BED formulation for multiple decaying sources
Assuming a temporal variation of activity in the source region h described by an instantaneous uptake and a multi-exponential clearance, the dose-rate to the target organ due to the single source organ h is given by the following functional form:
| (6) |
where Ḋ0,h is the initial dose-rate contribution from the source organ h to the target organ, i is multiexponential component index (i =1, …,n), λi,h is the effective clearance rate, and ai,h is the dose-rate fraction coefficient. For instance, an adequate description of blood clearance requires typically a biexponential function (n =2) or a triexponential function (n =3) in case of radiopeptides. In the case of multiple source organs, each source imparts a radiation dose-rate to the target organ with a multiexponential clearance pattern related to the time-activity function. The total dose-rate to the target organ exposed to s source organs is then given by the sum of the dose-rates,
| (7) |
The general expression for RE assuming a time-dependent dose-rate Ḋ(t) is given by Eq. (4) with the generalized Lea–Catcheside time factor G defined in Eq. (5). Substituting Eq. (7) in Eq. (5) and solving the double integration for a complete decay of the source gives the following expression:
| (8) |
Using the MIRD schema, the extrapolated “initial dose-rate” Ḋ0,h may be expressed by the following equation:
| (9) |
where A0 is the administered activity, Sh is the S value from the source organ h to the target organ, and fh is the “initial activity fraction” obtained by extrapolating to zero time an exponential expression fitted to the activity versus time curve. In clinical dosimetry, fh values are obtained during the biokinetic analysis based on a multiexponential fit of activity-time curve according to the function,
| (10) |
where Ah(t) is the activity in the organ h at time t. Substituting Eq. (9) in Eq. (8) gives the following expression:
| (11) |
In Eq. (11), the factor Gs,n is a function of both the total dose D and the administered activity A0. The total dose may be eliminated from the expression by inserting Eq. (9) into Eq. (7) and taking the time integral between zero and infinity; the following relation is obtained:
| (12) |
Substituting Eq. (12) in Eq. (11), the MIRD-based Lea–Catcheside factor is finally given by
| (13) |
As a test of the consistency, Eq. (13) is determined for a unique source with monoexponential clearance, i.e., s =n =1. In this special case, G1,1 = λ/(λ + μ) and the resulting RE function is identical to Eq. (3). As an example of using Eq. (13), the factor G for a unique biexponential decaying source is determined as follows:
| (14) |
II.C. BED formulation for two source organs
The special case with activities located in two source organs following monoexponential effective clearance is considered, i.e., s =2 and n =1. According to Eq. (12), the total absorbed dose to the target organ is described as follows:
| (15) |
In many situations, the first term corresponds to the self-absorbed dose in the target organ and the second term corresponds to the absorbed dose from activity in another organ or tissue. In this particular case of monoexponential clearance, the conventional MIRD formalism refers to the ratio f/λ as the residence time τ. Using Eq. (13), the factor G2,1 is expressed as a function of μ, f1, f2, λ1, λ2, S1, and S2,
| (16) |
Unlike the absorbed dose that needs only the knowledge of residence times τ, the factor G, requires the knowledge of both parameters f and λ independently. In this special case, the function G is driven by both ratios f1/f2 and S1/S2. As mentioned before, G is smaller than 1. In Eq. (16), each of the four terms in the numerator may be interpreted as a possible combination of generating two sublethal events to cause lethal damage. The variation of RE in case of two source organs will be discussed via the use of G2,1 for red marrow and kidneys in the next section.
II.D. BED formulation for fractionated radionuclide therapy
For treatments with a dose fractionated in multiple cycles, the BED is linearly additive16,18 and the following expression for the total BED may be derived using Eqs. (1) and (4):
| (17) |
where Di is the dose and Gi is the generalized Lea–Catcheside factor for the cycle i. For a total absorbed dose D given in N equal fractions, with a time lapse long enough to assume a complete decay of the radionuclide and full repair of sublethal damage between each cycle, Eq. (17) may be expressed as follows:
| (18) |
It was assumed that all biokinetic parameters remain constant during the overall treatment to consider the same factor Gs,n for each cycle. Note that Eq. (13) is independent of the fractionation scheme and thus Gs,n may be evaluated with cumulated initial activity fractions fh.
II.E. BED-based treatment planning
The conventional dosimetry-based approach in radionuclide therapy consists in determining the activity that would deliver the “largest safe absorbed dose” to the organ at risk. Considering a BED approach, the maximum safe absorbed dose D must satisfy the following equation:
| (19) |
where the BED is derived from clinical experience in EBRT. Indeed, limiting absorbed doses, such as D5,5 that results in a 5% probability of developing severe late damage within 5 years, are available for most normal tissues in EBRT and may be converted to BED5,5 using Eq. (2) with an appropriate dose per fraction (typically 2 Gy). The physical solution of Eq. (19) is given by
| (20) |
where Gs,n is determined using Eq. (13) with patient-specific biokinetic parameters and S values associated to the dose-limiting organ. Considering a fractionated regimen (N equal fractions), Gs,n is replaced by Gs,n/N in Eq. (20). Finally, the corresponding activity to be administered is easily calculated using Eq. (12).
III. RESULTS
III.A. Application to red marrow
For most nonmyeloablative radionuclide therapy, red marrow is the first dose-limiting organ. Since poor correlations between absorbed doses to red marrow and hematological toxicities have been reported in literature, it appears judicious to assess the use of BED for this purpose. The standard calculation of mean absorbed dose to red marrow is generally described as the sum of the self-absorbed dose in red marrow and the absorbed dose from activity in the remainder of the body
| (21) |
SRM←RB values are determined using the following expression:
| (22) |
SRM←RM and SRM←TB values are available from OLINDA/EXM.19 For the reference man, the following masses are used: mRM=1.12 kg, mTB=73.7 kg, and mblood=5.2 kg. S values for different radionuclides of interest are given in Table I. Supposing a monoexponential effective clearance, the residence times in the red marrow τRM and the remainder of the body τRB are expressed as fRM/λRM and fRB/λRB. It is assumed that (fRM+ fRB) corresponds to the initial activity in the whole body. In practice, fRM and fRB are extrapolated to the time of administration according to the best exponential fit and there is no explicit relationship between both parameters. Considering the blood-based approach,20 the extrapolated fraction in the red marrow may be expressed as
Table I.
S values for the red marrow as target tissue for the common radionuclides in radionuclide therapy (Ref. 19).
| Isotopes | SRM←RM (mGy/MBq s) | SRM←TB (mGy/MBq s) | SRM←RB (mGy/MBq s) | SRM←RM/SRM←RB |
|---|---|---|---|---|
| Y-90 | 5.87×10−5 | 1.41×10−6 | 5.26×10−7 | 112 |
| In-111 | 4.31×10−6 | 4.04×10−7 | 3.44×10−7 | 12.5 |
| I-131 | 1.55×10−5 | 6.29×10−7 | 4.00×10−7 | 38.8 |
| Lu-177 | 1.19×10−5 | 2.67×10−7 | 8.75×10−8 | 136 |
| (23) |
where RMBLR is the red marrow-to-blood activity concentration ratio that ranges between 0.19 and 0.63, up to 1.0 for fragments.21 Assuming a RMBLR of 0.5, fRM is about 0.1· fblood. According to this method, the effective clearance half-time in red marrow is equal to the effective clearance half-time in blood.
RE functions for the red marrow are then determined using Eq. (4) with G2,1 given in Eq. (16) (source 1=RM; source 2=RB). For the α/β ratios of red marrow cells, Fowler et al.12 indicate a range of 7–26 Gy. Dale11 assumed a value of 15 Gy for his calculations and Wilder et al.10 used 10 Gy in their study. Concerning the repair half-time, Dale11 and Howell7 assume 1.5 h while Wilder used 0.5 h.10 For the present study, an α/β of 10 Gy and a repair half-time Tμ of 1.5 h (corresponding to a repair rate μ of 0.46 h−1) are assumed. A safety threshold of 2 Gy for absorbed dose to red marrow is generally recommended to reduce the probability of severe marrow depression.22 Since G is smaller than 1 in any case, the highest value for RE is 1.20 according to Eq. (4). Figure 1 shows the RE isocurves of the red marrow for 90Y, 111In, 131I, and 177Lu as a function of effective clearance half-times T1/2,blood and T1/2,RB, assuming an extrapolated initial intake ratio fRM/fRB of 0.05. This ratio corresponds to a realistic extrapolated initial intake for blood of 0.5. Since effective clearance half-times are more intuitive than effective clearance rates, λblood and λRB have been replaced by ln(2)/T1/2,blood and ln(2)/T1/2,RB. In Fig. 2, RE functions for 90Y are plotted for a different α/β of 15 Gy and then a different repair half-time Tμ of 0.5 h.
Fig. 1.
Relative effectiveness isocurves of the red marrow for (a) 90Y, (b) 111In, (c) 131I, and (d) 177Lu as a function of effective clearance half-times (T1/2,b and T1/2,RB) assuming an initial intake ratio fRM/fRB of 0.05.
Fig. 2.
Relative effectiveness isocurves of the red marrow for 90Y as a function of effective clearance half-times (T1/2,b and T1/2,RB) assuming fRM/fRB =0.05 and (a) a different α/β =15 Gy and (b) a different Tμ =0.5 h.
III.B. Application to kidney dosimetry
Radiation dose to kidneys is of clinical significance for peptide receptor radionuclide therapy (PRRT) because of the high tubular uptake of the peptides after glomerular filtration and retention of the radionuclides in the proximal tubular cells. Large amounts of radiolabeled peptides, such as somatostatin analogs (e.g., 111In-DTPA-octreotide, 90Y-DOTATOC, and 177Lu-DOTATATE), retained in the renal cortex cause high radiation doses that may result in nephropathy.23,24 Animal studies indicated that the activity distribution in the cortex and medulla depends on the antibody or peptide size.25 According to the multiregion model of the MIRD Pamphlet No. 19 for kidneys,26 the mean dose to tissues of the renal cortex is given by
| (24) |
The renal pelvis and medullary papillae that represent a volume of 4.2% and 0.8%, respectively, of the total kidney tissue are neglected for the present study. Scort←cort and Scort←med values are available from MIRD19 (Ref. 26) and are given in Table II for the adult phantom. Since a monoexponential clearance is assumed for both subregions of the kidney, residence times τcort and τmed are expressed as fcort/λcort, and fmed/λmed. Note that f is the extrapolated initial fraction of activity within the subregion and not the fractional residence time as expressed in MIRD19. It is assumed that (fcort + fmed) corresponds to the extrapolated initial fraction of activity in the whole kidney.
Table II.
S values for the renal cortex as target tissue for the common radionuclides in radionuclide therapy (Ref. 26).
| Isotopes | Scort←cort (mGy/MBq s) | Scort←med (mGy/MBq s) | Scort←cort/Scort←med |
|---|---|---|---|
| Y-90 | 6.28×10−4 | 1.13×10−4 | 5.6 |
| In-111 | 4.70×10−5 | 1.59×10−5 | 3.0 |
| I-131 | 1.64×10−4 | 1.53×10−5 | 10.7 |
| Lu-177 | 1.17×10−4 | 2.62×10−6 | 44.7 |
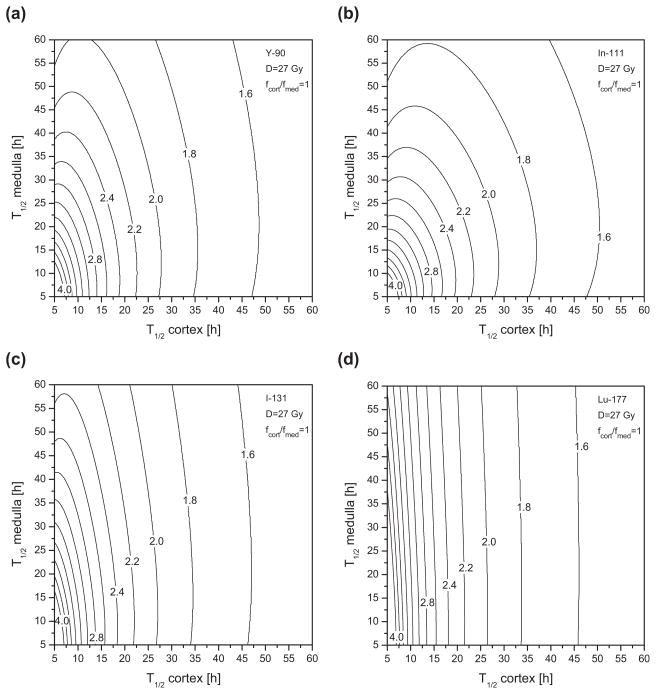
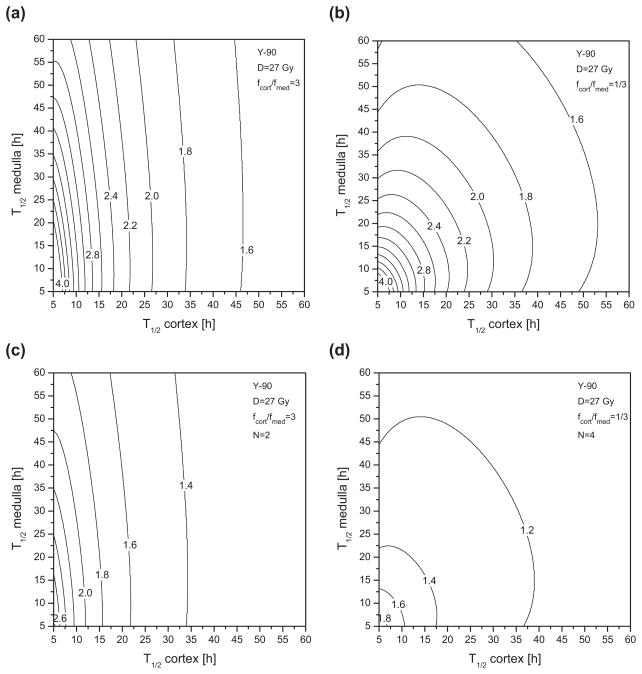
To evaluate the RE functions for the renal cortex using Eqs. (4) and (16) (source 1=cortex; source 2=medulla), the following parameters proposed by Barone et al.1 are used: repair half-time Tμ of 2.8 h, α/β ratio of 2.6 Gy, and maximal absorbed dose to kidneys D of 27 Gy. In fractionated EBRT, it is recommended that a kidney absorbed dose of 23 Gy (=D5,5) should not be exceeded.27 A higher critical dose has been tolerated for radionuclide therapy since much lower dose-rates are involved.8 In the present study, the limiting dose of 27 Gy is applied to the renal cortex, which is most likely to be responsible for observed radiation-induced toxicity, such as nephropathy, after radionuclide therapy. Using Eq. (4), the maximal RE is 11.4. This nonrealistic case would correspond to a single acute dose delivery (G =1) of 27 Gy. In other words, 27 Gy delivered as a single acute exposure would have a devastating biological effect equal to 308 Gy delivered in very small fractions over a very long time. Figure 3 provides the RE isocurves of the renal cortex for different radionuclides of interest, as a function of (T1/2,cort, T1/2,med) assuming that the initial intake of radioactivity is distributed equally in the cortex and the medulla (fmed/fcort =1). For conceivable effective clearance half-times, the RE ranges typically between 1.4 (shown as the region to the right of the 1.6 isodose curve) and 4.0, corresponding to a BED between 37.8 and 108 Gy. In Fig. 4, RE isocurves are shown for 90Y considering two different initial intake scenarios: (a) and (c) fcort/fmed=3; (b) and (d) fcort/fmed=1/3. In Figs. 4(c) and 4(d), RE isocurves are given taking into account fractionation effects using Eq. (18) for a two-cycle (N =2), and respectively, four-cycle (N =4), regimen. The range of RE values is then reduced to between 1.2 and 2.6, and between 1.1 and 1.8, respectively. Figure 5 shows the largest safe isodose, calculated with Eq. (20) and the G2,1 of the cortex, as a function of T1/2,cort and T1/2,med that corresponds to a BED5,5 of 41 Gy. The BED has been converted from the EBRT dose limit of 23 Gy using Eq. (2) with fractionation of 2 Gy. A ratio fcort/fmed of 1 was assumed. As an example, for both T1/2,cort and T1/2,med of 40 h, the largest safe absorbed dose to the renal cortex is of about 25 Gy with a single cycle [Fig. 5(a)]and 32 Gy with a three-dose fractionation regimen [Fig. 5(b)]. The corresponding activity to be administered A0 may be then calculated using Eq. (24). For this purpose, the total initial activity fraction in both kidneys, i.e., (fcort + fmed), is required in addition to the ratio fcort/fmed. Thus, assuming an initial uptake fraction of 10% in kidneys, a cumulated administered activity A0 of 12 GBq is required to deliver an absorbed dose to the cortex Dcort of 25 Gy, and 15 GBq for a Dcort of 32 Gy. For a smaller uptake fraction of 5%, maximal tolerable activities are exactly twice as high.
Fig. 3.
Relative effectiveness isocurves of the renal cortex for (a) 90Y, (b) 111In, (c) 131I, and (d) 177Lu as a function of effective clearance half-times (T1/2,cort and T1/2,med) assuming an initial intake ratio fcort/fmed of 1.
Fig. 4.
Relative effectiveness isocurves of the renal cortex for 90Y as a function of effective clearance half-times (T1/2,cort and T1/2,med) assuming an initial intake ratio fcort/fmed of respectively, 3 (a), (c) and 1/3 (b), (d). A fractionated regimen was also considered with (c) N =2 cycles and (d) N =4 cycles.
Fig. 5.
Isocurves of the largest safe absorbed dose to the renal cortex (in Gy) as a function of effective clearance half-times (T1/2,cort and T1/2,med)assuming a BED of 41 Gy and an initial intake ratio fcort/fmed=1 for 90Y. A fractionated regimen was considered in (b) with N=3 cycles.
IV. DISCUSSION
Figures 1–4 show that RE factors increase as effective clearance half-times decrease. Indeed, for short half-times, a higher activity has to be administered in order to reach the targeted absorbed dose. Consequently, the dose is delivered at a higher dose-rate and a reduction of cell repair is expected, resulting in an increased RE. As a result, the RE function is more sensitive to variation in the short half-time region. The maximal RE is obtained when both half-times tend to zero. This special situation corresponds to a single acute dose delivery, i.e., a factor G2,1 of 1. RE factors associated to red marrow are rather close to 1 as shown in Fig. 1. Considering effective half-times in blood above 20 h, the RE is less than 1.02 for the four isotopes of interest. Therefore, for radioimmunotherapy, since radiolabeled antibodies such as 131I-tositumomab and 90Y-ibritumomab tiuxetan tend to clear slowly, the BED is approximately equal to the absorbed dose. For PRRT, wash-out periods of peptides are usually short and effective clearance half-times in blood of about 1 h are appropriate. In this case, the RE factor ranges around 1.10. If an α/β ratio of 15 Gy is assumed instead of 10 Gy, the RE factors are smaller as illustrated in Fig. 2 by the shift of the isocurves in the direction of the shorter half-times. A similar pattern is observed when the repair half-time Tμ is shortened from 1.5 to 0.5 h [see Fig. 2(b)]. For radioiodine (131I) therapy with rhTSH, clearance half-times from blood may be of the order of a few hours and RE factors are thus similar to those obtained from PRRT. A more complete estimate of the RE could be achieved using a biexponential function to describe clearance kinetics in blood. Irrespective of LQ model parameters, biological processes involving dose-rate and repair of sublethal damage, quantified by the RE, do not yield a BED to red marrow that is significantly different from the physical absorbed dose, which is typically associated with uncertainties of more than 20%. This is in contrast to results in murine models suggesting a strong in-fluence of the dose-rate on the resulting myelotoxicity.21 Action involving stem cell repopulation and dose-rate may be responsible for these observations. Indeed, unlike normal organs with late response to radiation, such as kidneys, red marrow is an acute-responding tissue where significant cell repopulation occurs during irradiation. Based on the LQ model accounting for cell repopulation, the BED has been extended by Dale28 as follows: BED=D·RE–BRF. The BED related repopulation factor BRF is expressed in the form of a dose that reduces the biological effect due to concurrent cell repopulation. Using this model, Howell et al.29 examined the advantage of longer-lived radionuclides in radioimmunotherapy. Wilder et al.10 studied the correlation between the BED including cell repopulation and hematopoietic toxicity after 131I-Lym-1 infusions in patients. No clear association between bone marrow absorbed dose or BED was found. As expected, the RE had only a minor effect upon BED. They suggested that the weak association had been influenced by tumor involvement of the red marrow or previous myelotoxic therapy. A BED formula including cell repopulation dedicated to radionuclide therapy has been derived by Atthey et al. in case of a single exponentially decaying source.30 The formulation becomes more complex when the dose-rate temporal pattern depends on multiple irradiating source organs and no analytical solution has been found in this case. It should be noted that the bone marrow is a highly complex organ and other factors that may influence dose response, such as spatial distribution of active bone marrow and hematopoietic cells, remain outside the scope of the present study.31
In the case of kidneys, the RE factors take higher values than in the case of red marrow. For effective clearance half-times between 10 and 60 h, the RE to the renal cortex ranges between 1.5 and 4. Figure 3 shows that the medulla half-time contributes more significantly to the cortex RE as the ratio Scort←cort/Scort←med decreases across the different radionuclides examined. For a low-energy beta emitter such as 177Lu, the RE function is almost insensitive to T1/2,med variations and the resulting isocurves are nearly straight lines parallel to the T1/2,med axis. The highest medulla contribution is obtained for 111In due to the small Scort←cort/Scort←med ratio resulting from low electron dose deposition within the cortex from decays in the medulla. Figures 4(a) and 4(b) show that the RE isocurve pattern is strongly influenced by the initial intake fraction ratio. In fact, according to Eq. (9), initial intake factors contribute directly to the determination of the initial dose-rate that is critical for BED considerations. Figures 4(c) and 4(d) show that fractionation reduces the RE values and narrows the range of RE values as the number of cycles increases without affecting the shape of the functional dependence of T1/2,cort and T1/2,med. One to five cycles are typical fractionated regimens in PRRT.
The scenario with fcort/fmed=3, i.e., an initial radioactivity distribution of 3/4 in the cortex and 1/4 in the medulla, corresponds to a realistic situation in PRRT as shown by Konijnenberg et al.32 According to Barone et al.,1 the mean effective clearance half-time of 90Y-DOTATOC in kidneys is about 30 h. In this case, Fig. 4(a) yields a RE of about 1.9 and variations due to the medulla contribution are minor. Furthermore, with some retention in the cortex, the clearance in the medulla is certainly faster than in the cortex and fluctuations due to medulla are even smaller. As long as the renal activity is essentially located in the cortex of the kidney, the medulla contribution may be neglected. Then it is reasonable to use Eq. (3) with a single source organ. In their study, Barone et al.1 determined the BED to kidneys for each patient based on Eqs. (3) and (17). The results show a correlation between BED and renal toxicity, as opposed to the absorbed dose alone. This finding clearly demonstrates that BED is an appropriate quantity to improve prediction in dose-toxicity response. Those considerations suppose that the dose to the cortex is responsible for the radiation-induced renal diseases observed after radionuclide therapy. The irregular activity distribution within the cortex observed by de Jong et al.23 limits the appropriateness of MIRD19 and ideally imposes the use of three-dimensional (3D) voxel-based dosimetry. Indeed, for low-energy β-emitters (such as 111Lu) and Auger electron emitters (such as 111In), Konijnenberg et al.32 showed that heterogeneous activity distribution in the kidneys affects considerably the dose distribution, generating volumes with lower and higher doses than the average dose to the cortex. In contrast, for high-energy β- emitters (such as 90Y), dose distributions in the kidneys are essentially independent of the activity distribution pattern and, consequently, accurate kidney dosimetry can be based on MIRD19. Furthermore, using the Lyman model with the effective volume method, Konijnenberg et al. interpreted the nonuniform dose distributions over the kidney irrespective of any heterogeneity in activity distributions and suggested that the EBRT tolerance dose to kidneys, i.e., 23 Gy, may be augmented at least by a factor of 1.25 for PRRT. With respect to effects and limitations of dose nonuniformity, the MIRD19-based BED dosimetry enables a suitable intermediate level between the rough whole kidney dosimetry and the complex 3D voxel-based BED dosimetry. With current quantitative PET and SPECT imaging, activity uptake may be measured appropriately in the different renal regions.33
Figure 5 shows the largest safe dose to be delivered to the renal cortex according to effective clearance half-times in the cortex and medulla. As expected, fractionation of the total dose results in higher maximum tolerated doses, of about 30%–40% for a three-dose fractionation regimen, as compared to the unfractionated total dose. This type of approach is interesting for a first estimate of the maximal dose to an organ at risk in case of phase I/II trials of new therapeutic radiopharmaceuticals. At present, it is unclear which dose can be administered safely to kidneys in the context of radionuclide therapy.34 For instance, in skeletal targeted radiotherapy with 166Ho-DOTMP, nephropathy was reported for low absorbed dose to kidneys below 4 Gy.4 Since the radioisotope passes fast into the urine with minimal retention in the kidneys, the dose-rate effects have certainly accounted for the toxicity observed. However, the RE of 1.3 calculated by the authors using Eq. (3) does not provide a sufficient explanation and the renal toxicities observed in this study remain unclear. Recently, Chiesa et al.5 used the single organ BED concept to determine the maximal activity to be administered to patients in the ongoing phase I escalation study of myeloablative 90Y-ibritumomab tiuxetan therapy. BED toxicity thresholds were derived from EBRT for kidneys, but also heart, liver, lung, and testes. With myeloablative radionuclide therapy, accurate dosimetry of late-responding organs is essential for the design of the protocol in order to identify the second limiting organ(s) and then minimize or avoid toxicity.
Equations (1), (4), and (13) provide a generalized MIRD-based formalism to determine the BED in addition to absorbed dose. The implementation of the formulas in MIRD-based dosimetry software such as the OLINDA/EXM code19 require minor modifications. Biokinetic parameters are directly available from conventional multiexponential fits. Thus, radiobiological parameters α/β and repair rate constants μ are the only “new” quantities necessary for the computation. However, large uncertainties associated with α/β and μ necessitate caution, particularly when the method is applied to an individual patient. Indeed, radiosensitivity may differ among patients and is probably influenced by prior therapy. In addition, LQ model parameters currently available have been derived from EBRT that produces homogeneous dose distribution whereas, in radionuclide therapy, the dose pattern depends on the targeting of the therapeutic agents, e.g., stem cells for bone marrow in RIT or glomeruli for renal cortex in PRRT. Therefore, α/β and μ should be determined specifically for each new radiotherapeutic agent. The MIRD formalism is not limited to uniform activity distribution in organs or suborgans but is also appropriate for voxel-based dosimetry.35Equation (13) is then directly applicable at the voxel level and completes the simplified formula proposed by Prideaux et al.9 However, substantial computer time will be required for this task and appropriate algorithms for 3D imaging based BED dosimetry are still under investigation.
V. CONCLUSION
In addition to physical absorbed dose, the BED takes into account competing effects of dose-rate and tissue repair mechanism. In this work, a generic formula has been proposed to enlarge the BED formalism to the MIRD schema. The concern of dose-rate effect with cell repopulation that is relevant for acute-responding tissues such as red marrow and tumors requires more complex modeling and has not been considered in this study. It is generally admitted that BED may be a useful quantity to better predict dose-response and dose-toxicity relationships. Those relations are important in radionuclide therapy to optimize and evaluate treatment plans and modalities. Using radiation tolerance known from external radiation therapy, the BED formula may be used as a treatment-planning tool to incorporate dose-rate considerations when determining the activity to be administered. Although the LQ model is probably the best currently available model for radiation effect, large uncertainties are associated with radiobiological parameters, such as α/β or μ, that are crucial for BED calculation. Nevertheless, useful guidance could be obtained if those uncertainties are considered as an inherent part of treatment planning and clinical decision-making process.
The BED-based dosimetry was applied to the red marrow as well as the regional kidney model considering the cortex and the medulla. Red marrow dosimetry is of interest since hematopoietic suppression is the dose-limiting toxicity for most nonmyeolablative therapies. It has been shown that the dose-rate associated with repair mechanism does not play a major role for red marrow. With the increasing use of small radiolabeled molecules such as peptides, assessing the impact of the dose-rate in kidney dosimetry is of clinical significance due to the rapid excretion through the urinary tract and the retention of radioactivity in the renal cortex. In this case, the BED may differ largely from the absorbed dose and complex relations occur between biokinetic and dose parameters of both the cortex and the medulla.
Acknowledgments
The present study is supported by the Swiss National Science Foundation (Fellowship No. PBFR2-115886) and NIH/NCI Grant No. R01 CA116477.
Contributor Information
Sébastien Baechler, The Russell H. Morgan Department of Radiology and Radiological Sciences, School of Medicine, Johns Hopkins University, Baltimore, Maryland 21231 and University Institute of Radiation Physics (IRA-DUMSC), University of Lausanne, Lausanne, Switzerland.
Robert F. Hobbs, The Russell H. Morgan Department of Radiology and Radiological Sciences, School of Medicine, Johns Hopkins University, Baltimore, Maryland 21231
Andrew R. Prideaux, The Russell H. Morgan Department of Radiology and Radiological Sciences, School of Medicine, Johns Hopkins University, Baltimore, Maryland 21231
Richard L. Wahl, The Russell H. Morgan Department of Radiology and Radiological Sciences, School of Medicine, Johns Hopkins University, Baltimore, Maryland 21231
George Sgouros, The Russell H. Morgan Department of Radiology and Radiological Sciences, School of Medicine, Johns Hopkins University, Baltimore, Maryland 21231.
References
- 1.Barone R, Borson-Chazot FO, Valkerna R, Walrand S, Chauvin F, Gogou L, Kvols LK, Krenning EP, Jamar F, Pauwels S. Patient-specific dosimetry in predicting renal toxicity with Y-90-DOTATOC: Relevance of kidney volume and dose-rate in finding a dose-effect relationship. J Nucl Med. 2005;46:99S–106S. [PubMed] [Google Scholar]
- 2.Behr TM, Memtsoudis S, Sharkey RM, Blumenthal RD, Dunn RM, Gratz S, Wieland E, Nebendahl K, Schmidberger H, Goldenberg DM, Becker W. Experimental studies on the role of antibody fragments in cancer radioimmunotherapy: Influence of radiation dose and dose-rate on toxicity and anti-tumor efficacy. Int J Cancer. 1998;77:787–795. doi: 10.1002/(sici)1097-0215(19980831)77:5<787::aid-ijc19>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 3.Bodey RK, Evans PM, Flux GD. Application of the linear-quadratic model to combined modality radiotherapy. Int J Radiat Oncol Biol Phys. 2004;59:228–241. doi: 10.1016/j.ijrobp.2003.12.031. [DOI] [PubMed] [Google Scholar]
- 4.Breitz HB, Wendt RE, III, Stabin MS, Shen S, Erwin WD, Rajendran JG, Eary JF, Durack L, Delpassand E, Martin W, Meredith RF. 166Ho-DOTMP radiation-absorbed dose estimation for skeletal targeted radiotherapy. J Nucl Med. 2006;47:534–542. [PubMed] [Google Scholar]
- 5.Chiesa C, Botta F, Di Betta E, Coliva A, Maccauro M, Aliberti G, Bavusi S, Devizzi L, Guidetti A, Seregni E, Gianni AM, Bombardieri E. Dosimetry in myeloablative 90Y-labeled ibritumomab tiuxetan therapy: Possibility of increasing administered activity on the base of biological effective dose evaluation. Preliminary results. Cancer Biother Radiopharm. 2007;22:113–120. doi: 10.1089/cbr.2007.302. [DOI] [PubMed] [Google Scholar]
- 6.Dale RG. Dose-rate effects in targeted radiotherapy. Phys Med Biol. 1996;41:1871–1884. doi: 10.1088/0031-9155/41/10/001. [DOI] [PubMed] [Google Scholar]
- 7.Howell RW, Goddu SM, Rao DV. Application of the linear-quadratic model to radioimmunotherapy: Further support for the advantage of longer-lived radionuclides. J Nucl Med. 1994;35:1861–1869. [PubMed] [Google Scholar]
- 8.Konijnenberg MW. Is the renal dosimetry for [90Y-DOTA0,Tyr3]octreotide accurate enough to predict thresholds for individual patients? Cancer Biother Radiopharm. 2003;18:619–625. doi: 10.1089/108497803322287718. [DOI] [PubMed] [Google Scholar]
- 9.Prideaux AR, Song H, Hobbs RF, He B, Frey EC, Ladenson PW, Wahl RL, Sgouros G. Three-dimensional radiobiologic dosimetry: Application of radiobiologic modeling to patient-specific 3-dimensional imaging-based internal dosimetry. J Nucl Med. 2007;48:1008–1016. doi: 10.2967/jnumed.106.038000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilder RB, DeNardo GL, Sheri S, Fowler JF, Wessels BW, DeNardo SJ. Application of the linear-quadratic model to myelotoxicity associated with radioimmunotherapy. Eur J Nucl Med. 1996;23:953–957. doi: 10.1007/BF01084370. [DOI] [PubMed] [Google Scholar]
- 11.Dale RG. The application of the linear-quadratic dose-effect equation to fractionated and protracted radiotherapy. Br J Radiol. 1985;58:515–528. doi: 10.1259/0007-1285-58-690-515. [DOI] [PubMed] [Google Scholar]
- 12.Fowler JF. The linear-quadratic formula and progress in fractionated radiotherapy. Br J Radiol. 1989;62:679–694. doi: 10.1259/0007-1285-62-740-679. [DOI] [PubMed] [Google Scholar]
- 13.Loevinger R, Budinger TF, Watson EE. MIRD Primer for Absorbed Dose Calculations. The Society of Nuclear Medicine, Inc; New York: 1991. revised edition. [Google Scholar]
- 14.Joiner MC, van der Kogel AJ. In: Basic Clinical Radiobiology. 3. Steel GG, editor. Arnold; London: 2002. [Google Scholar]
- 15.Dale R, Carabe-Fernandez A. The radiobiology of conventional radiotherapy and its application to radionuclide therapy. Cancer Biother Radiopharm. 2005;20:47–51. doi: 10.1089/cbr.2005.20.47. [DOI] [PubMed] [Google Scholar]
- 16.Millar WT. Application of the linear-quadratic model with incomplete repair to radionuclide directed therapy. Br J Radiol. 1991;64:242–251. doi: 10.1259/0007-1285-64-759-242. [DOI] [PubMed] [Google Scholar]
- 17.Brenner DJ, Hlatky LR, Hahnfeldt PJ, Huang Y, Sachs RK. The linear-quadratic model and most other common radiobiological models result in similar predictions of time-dose relationships. Radiat Res. 1998;150:83–91. [PubMed] [Google Scholar]
- 18.Dale RG. A graphical method to simplify the application of the linear-quadratic dose-effect equation to fractionated radiotherapy. Br J Radiol. 1986;59:1111–1115. doi: 10.1259/0007-1285-59-707-1111. [DOI] [PubMed] [Google Scholar]
- 19.Stabin MG, Sparks RB, Crowe E. OLINDA/EXM: The second-generation personal computer software for internal dose assessment in nuclear medicine. J Nucl Med. 2005;46:1023–1027. [PubMed] [Google Scholar]
- 20.Sgouros G. Bone marrow dosimetry for radioimmunotherapy: Theoretical considerations. J Nucl Med. 1993;34:689–694. [PubMed] [Google Scholar]
- 21.Behr TM, Behe M, Sgouros G. Correlation of red marrow radiation dosimetry with myelotoxicity: Empirical factors influencing the radiation-induced myelotoxicity of radiolabeled antibodies, fragments and peptides in pre-clinical and clinical settings. Cancer Biother Radiopharm. 2002;17:445–464. doi: 10.1089/108497802760363231. [DOI] [PubMed] [Google Scholar]
- 22.O’Donoghue JA, Baidoo N, Deland D, Welt S, Divgi CR, Sgouros G. Hematologic toxicity in radioimmunotherapy: Dose-response relationships for I-131 labeled antibody therapy. Cancer Biother Radiopharm. 2002;17:435–443. doi: 10.1089/108497802760363222. [DOI] [PubMed] [Google Scholar]
- 23.De Jong M, Valkema R, van Gameren A, Van Boven H, Bex A, Van De Weyer EP, Burggraaf JD, Korner M, Reubi JC, Krenning EP. Inhomogeneous localization of radioactivity in the human kidney after injection of [(111)In-DTPA]octreotide. J Nucl Med. 2004;45:1168–1171. [PubMed] [Google Scholar]
- 24.Lambert B, Cybulla M, Weiner SM, Van De Wiele C, Ham H, Dierckx RA, Otte A. Renal toxicity after radionuclide therapy. Radiat Res. 2004;161:607–611. doi: 10.1667/rr3105. [DOI] [PubMed] [Google Scholar]
- 25.Flynn AA, Pedley RB, Green AJ, Dearling JL, El Emir E, Boxer GM, Boden R, Begent RH. The nonuniformity of antibody distribution in the kidney and its influence on dosimetry. Radiat Res. 2003;159:182–189. doi: 10.1667/0033-7587(2003)159[0182:tnoadi]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 26.Bouchet LG, Bolch WE, Blanco HP, Wessels BW, Siegel JA, Rajon DA, Clairand I, Sgouros G. MIRD Pamphlet No 19: Absorbed fractions and radionuclide S values for six age-dependent multi-region models of the kidney. J Nucl Med. 2003;44:1113–1147. [PubMed] [Google Scholar]
- 27.Emami B, Lyman J, Brown A, Coia L, Goitein M, Munzenrider JE, Shank B, Solin LJ, Wesson M. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991;21:109–122. doi: 10.1016/0360-3016(91)90171-y. [DOI] [PubMed] [Google Scholar]
- 28.Dale RG. Radiobiological assessment of permanent implants using tumour repopulation factors in the linear-quadratic model. Br J Radiol. 1989;62:241–244. doi: 10.1259/0007-1285-62-735-241. [DOI] [PubMed] [Google Scholar]
- 29.Howell RW, Goddu SM, Rao DV. Proliferation and the advantage of longer-lived radionuclides in radioimmunotherapy. Med Phys. 1998;25:37–42. doi: 10.1118/1.598171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Atthey M, Nahum AE, Flower MA, McCready VR. Effects of cellular repair and proliferation on targeted radionuclide therapy: A modelling study. Phys Med Biol. 2000;45:N15–N20. doi: 10.1088/0031-9155/45/4/402. [DOI] [PubMed] [Google Scholar]
- 31.Watchman CJ, Bourke VA, Lyon JR, Knowlton AE, Butler SL, Grier DD, Wingard JR, Braylan RC, Bolch WE. Spatial distribution of blood vessels and CD34+ hematopoietic stem and progenitor cells within the marrow cavities of human cancellous bone. J Nucl Med. 2007;48:645–654. doi: 10.2967/jnumed.106.035337. [DOI] [PubMed] [Google Scholar]
- 32.Konijnenberg M, Melis M, Valkema R, Krenning E, de Jong M. Radiation dose distribution in human kidneys by octreotides in peptide receptor radionuclide therapy. J Nucl Med. 2007;48:134–142. [PubMed] [Google Scholar]
- 33.Helisch A, Forster GJ, Reber H, Buchholz HG, Arnold R, Goke B, Weber MM, Wiedenmann B, Pauwels S, Haus U, Bouterfa H, Bartenstein P. Pre-therapeutic dosimetry and biodistribution of Y-86-DOTA-Phe(1)-Tyr(3)-octreotide versus In-111-pentetreotide in patients with advanced neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2004;31:1386–1392. doi: 10.1007/s00259-004-1561-6. [DOI] [PubMed] [Google Scholar]
- 34.O’Donoghue J. Relevance of external beam dose-response relationships to kidney toxicity associated with radionuclide therapy. Cancer Biother Radiopharm. 2004;19:378–387. doi: 10.1089/1084978041425025. [DOI] [PubMed] [Google Scholar]
- 35.Bolch WE, Bouchet LG, Robertson JS, Wessels BW, Siegel JA, Howell RW, Erdi AK, Aydogan B, Costes S, Watson EE, Brill AB, Charkes ND, Fisher DR, Hays MT, Thomas SR. MIRD Pamphlet No. 17: The dosimetry of nonuniform activity distributions–radionuclide S values at the voxel level. Medical Internal Radiation Dose Committee. J Nucl Med. 1999;40:11S–36S. [PubMed] [Google Scholar]