Abstract
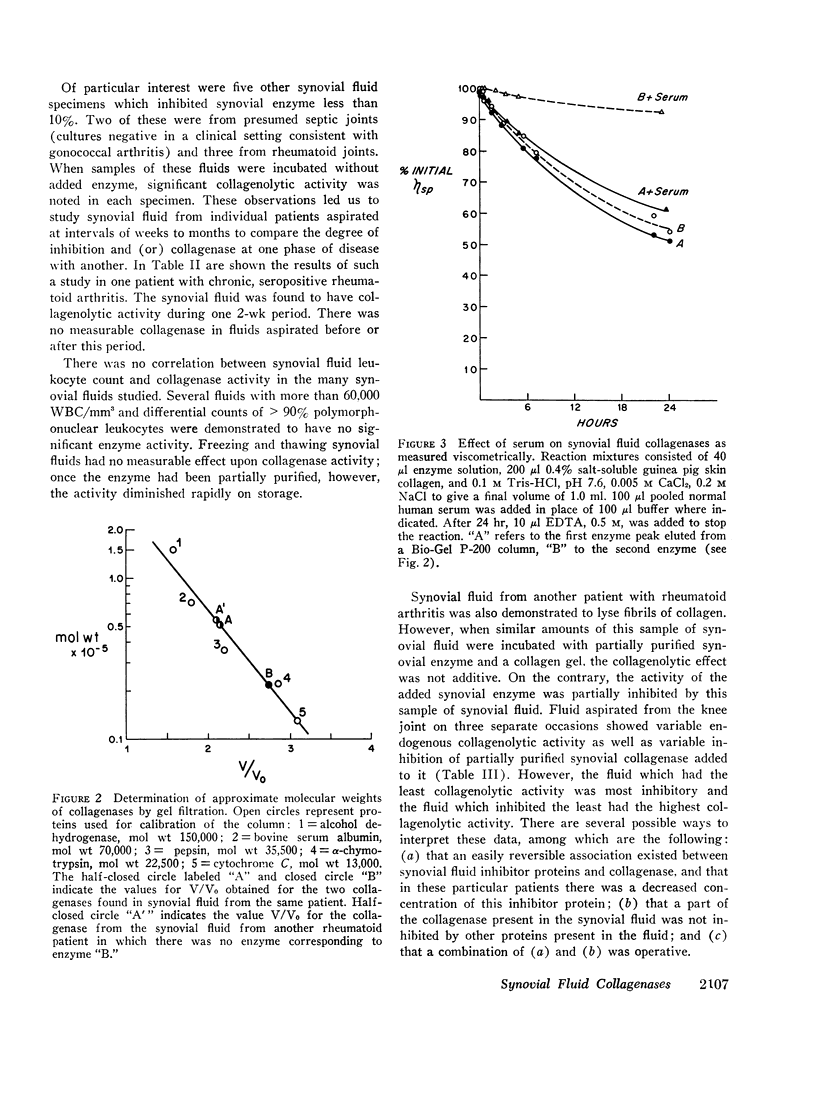
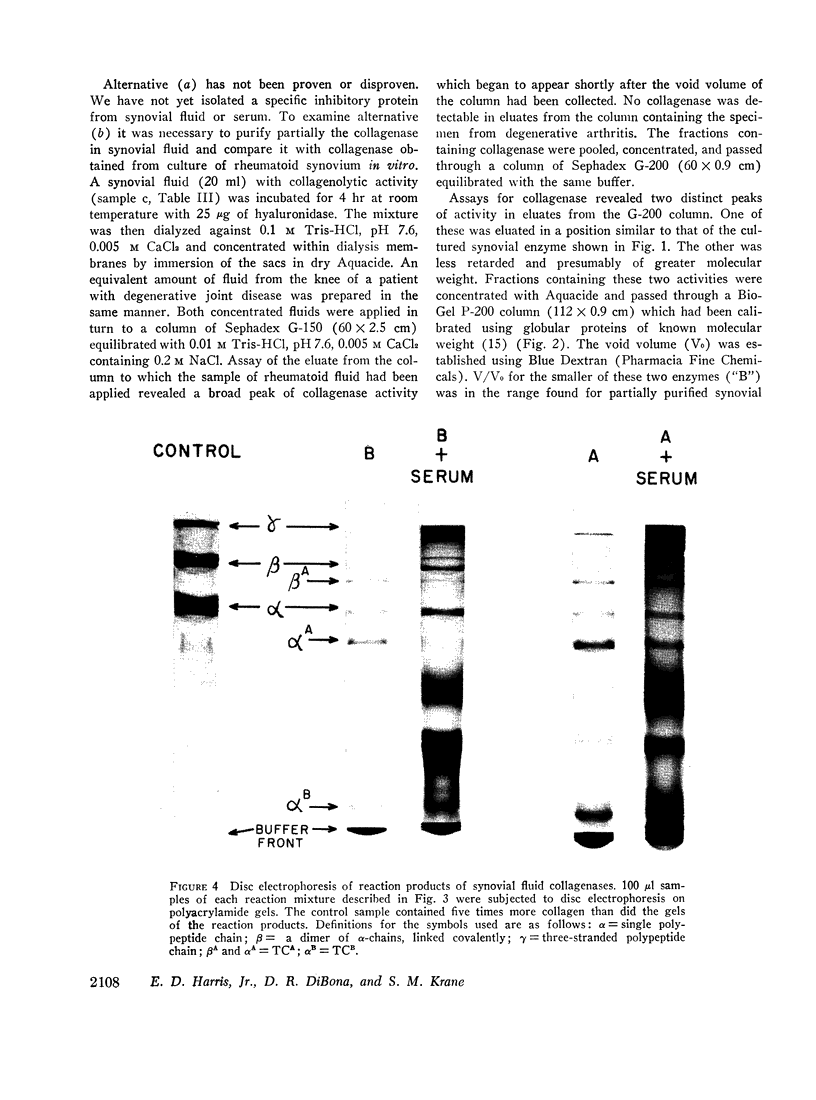
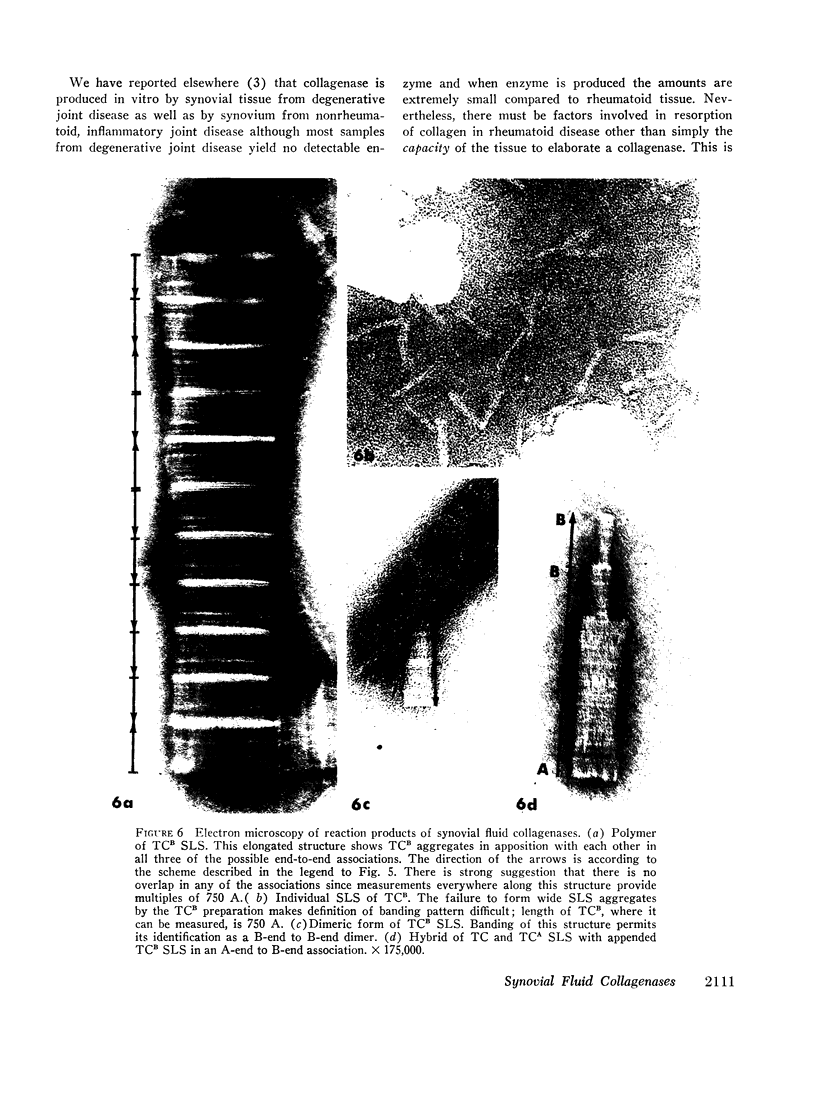
An enzyme which degrades native collagen at neutral pH has been isolated from cultures of rheumatoid synovium in vitro, but little or no collagenolytic activity has been found in homogenates of fresh rheumatoid synovium. Similar to most other mammalian collagenases this synovial enzyme is readily inhibited by serum proteins. Proteins of synovial fluid are derived largely from serum and synovial fluid from noninflamed joints was found to inhibit synovial collagenase; the inhibitor was destroyed by trypsin, but not by hyaluronidase. Inhibitory activity was reduced in approximately one-half of the fluids from patients with rheumatoid arthritis. In a total of nine synovial fluids, collagenolytic activity was detectable. This activity was not present in constant amounts in synovial fluids aspirated at different times from the same patient and tended to vary inversely with the titer of inhibitory proteins. The collagenolytic activity in the synovial fluids from different patients was variably inhibited by serum proteins. Two distinct collagenases were detected in some rheumatoid synovial fluids and separated by gel filtration. One, labeled “B” enzyme, with an estimated molecular weight 20,000-25,000 resembled the collagenase obtained from synovial cultures. The other, labeled “A” enzyme degraded collagen fibrils as well as collagen in solution. Disc electrophoresis on acrylamide gels and electron microscopy of segment long spacing (SLS) aggregates of reaction products of the enzymes at 27°C demonstrated that both “A” and “B” enzymes cleaved collagen molecules at a point three-quarters from the amino terminal end of the molecule. Thus collagen degradation in rheumatoid arthritis could result from the operation of these two collagenases.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CURTISS P. H., Jr, KLEIN L. Destruction of articular cartilage in septic arthritis. I. In vitro studies. J Bone Joint Surg Am. 1963 Jun;45-A:797–806. [PubMed] [Google Scholar]
- Eisen A. Z., Jeffrey J. J., Gross J. Human skin collagenase. Isolation and mechanism of attack on the collagen molecule. Biochim Biophys Acta. 1968 Mar 25;151(3):637–645. doi: 10.1016/0005-2744(68)90010-7. [DOI] [PubMed] [Google Scholar]
- Evanson J. M., Jeffrey J. J., Krane S. M. Human collagenase: identification and characterization of an enzyme from rheumatoid synovium in culture. Science. 1967 Oct 27;158(3800):499–502. doi: 10.1126/science.158.3800.499. [DOI] [PubMed] [Google Scholar]
- Evanson J. M., Jeffrey J. J., Krane S. M. Studies on collagenase from rheumatoid synovium in tissue culture. J Clin Invest. 1968 Dec;47(12):2639–2651. doi: 10.1172/JCI105947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GALLOP P. M., SEIFTER S., MEILMAN E. Studies on collagen. I. The partial purification, assay, and mode of activation of bacterial collagenase. J Biol Chem. 1957 Aug;227(2):891–906. [PubMed] [Google Scholar]
- Gross J., Nagai Y. Specific degradation of the collagen molecule by tadpole collagenolytic enzyme. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1197–1204. doi: 10.1073/pnas.54.4.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris E. D., Jr, Cohen G. L., Krane S. M. Synovial collagenase: its presence in culture from joint disease of diverse etiology. Arthritis Rheum. 1969 Apr;12(2):92–102. doi: 10.1002/art.1780120206. [DOI] [PubMed] [Google Scholar]
- Hodge A. J., Schmitt F. O. THE CHARGE PROFILE OF THE TROPOCOLLAGEN MACROMOLECULE AND THE PACKING ARRANGEMENT IN NATIVE-TYPE COLLAGEN FIBRILS. Proc Natl Acad Sci U S A. 1960 Feb;46(2):186–197. doi: 10.1073/pnas.46.2.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarus G. S., Brown R. S., Daniels J. R., Fullmer H. M. Human granulocyte collagenase. Science. 1968 Mar 29;159(3822):1483–1485. doi: 10.1126/science.159.3822.1483. [DOI] [PubMed] [Google Scholar]
- Lazarus G. S., Daniels J. R., Brown R. S., Bladen H. A., Fullmer H. M. Degradation of collagen by a human granulocyte collagenolytic system. J Clin Invest. 1968 Dec;47(12):2622–2629. doi: 10.1172/JCI105945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAGAI Y., GROSS J., PIEZ K. A. DISC ELECTROPHORESIS OF COLLAGEN COMPONENTS. Ann N Y Acad Sci. 1964 Dec 28;121:494–500. doi: 10.1111/j.1749-6632.1964.tb14221.x. [DOI] [PubMed] [Google Scholar]
- RACKER E. Crystalline alcohol dehydrogenase from baker's yeast. J Biol Chem. 1950 May;184(1):313–319. [PubMed] [Google Scholar]
- Sakai T., Gross J. Some properties of the products of reaction of tadpole collagenase with collagen. Biochemistry. 1967 Feb;6(2):518–528. doi: 10.1021/bi00854a021. [DOI] [PubMed] [Google Scholar]