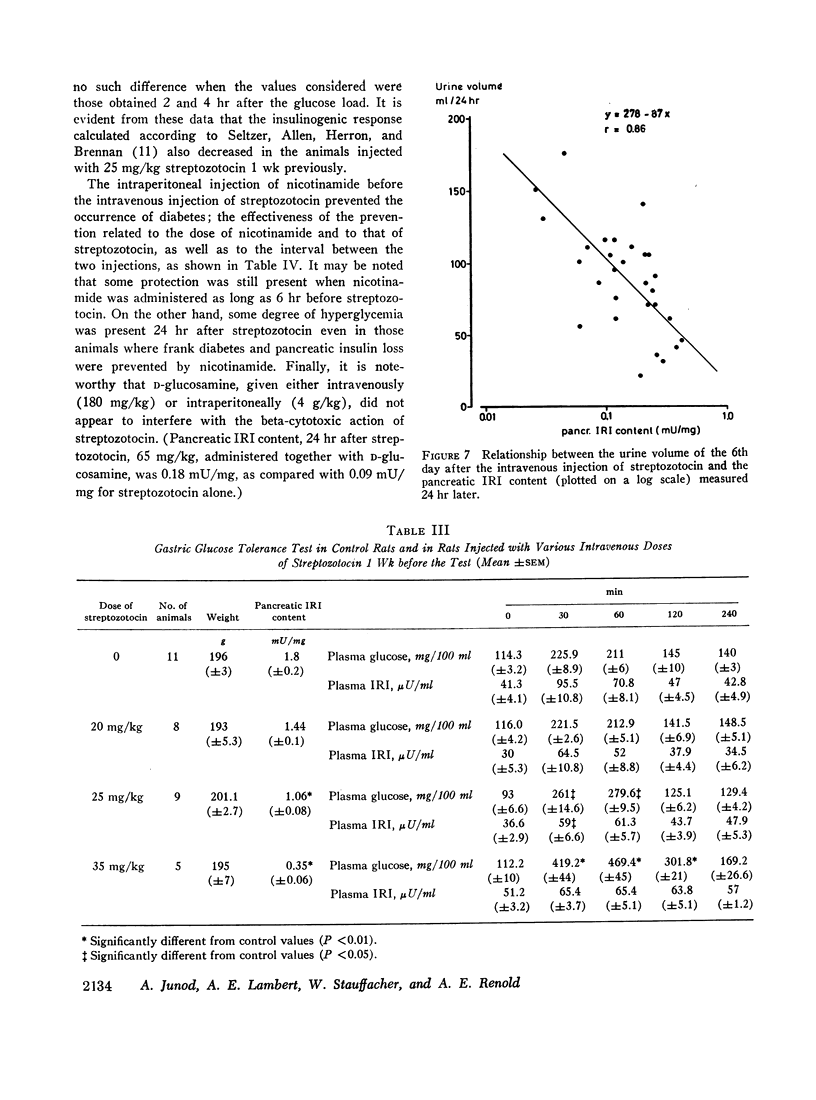
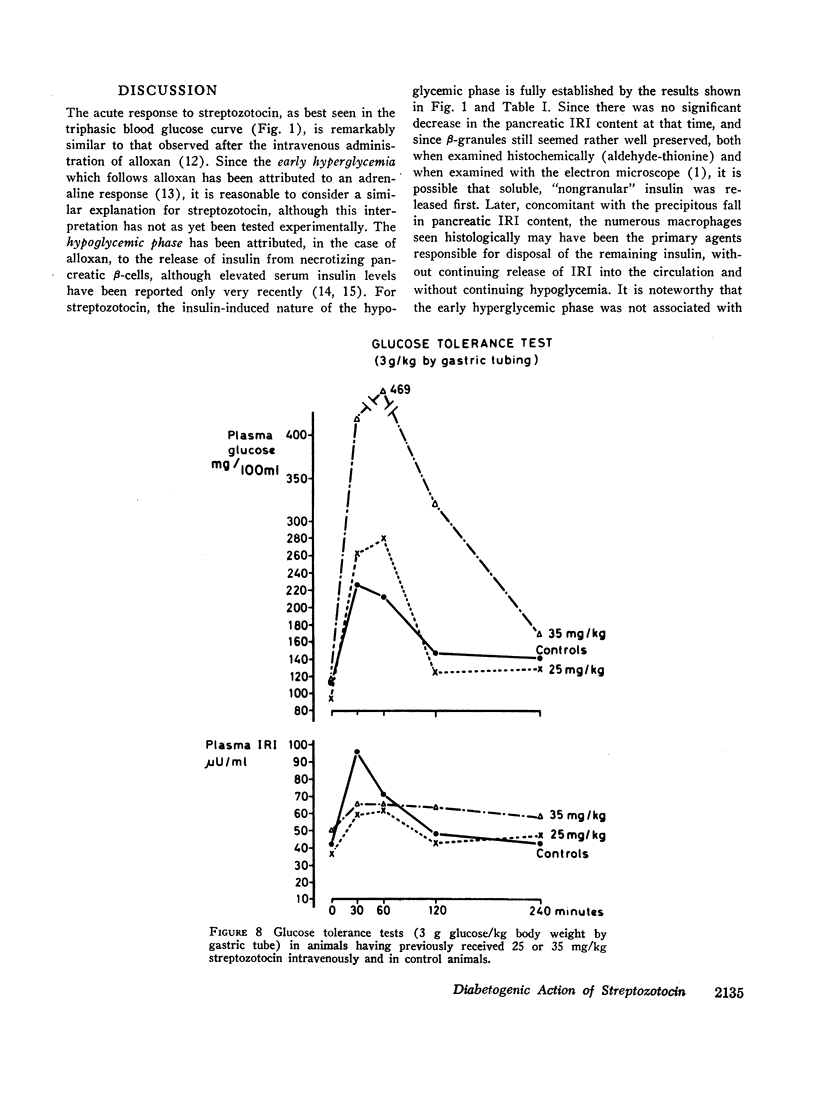
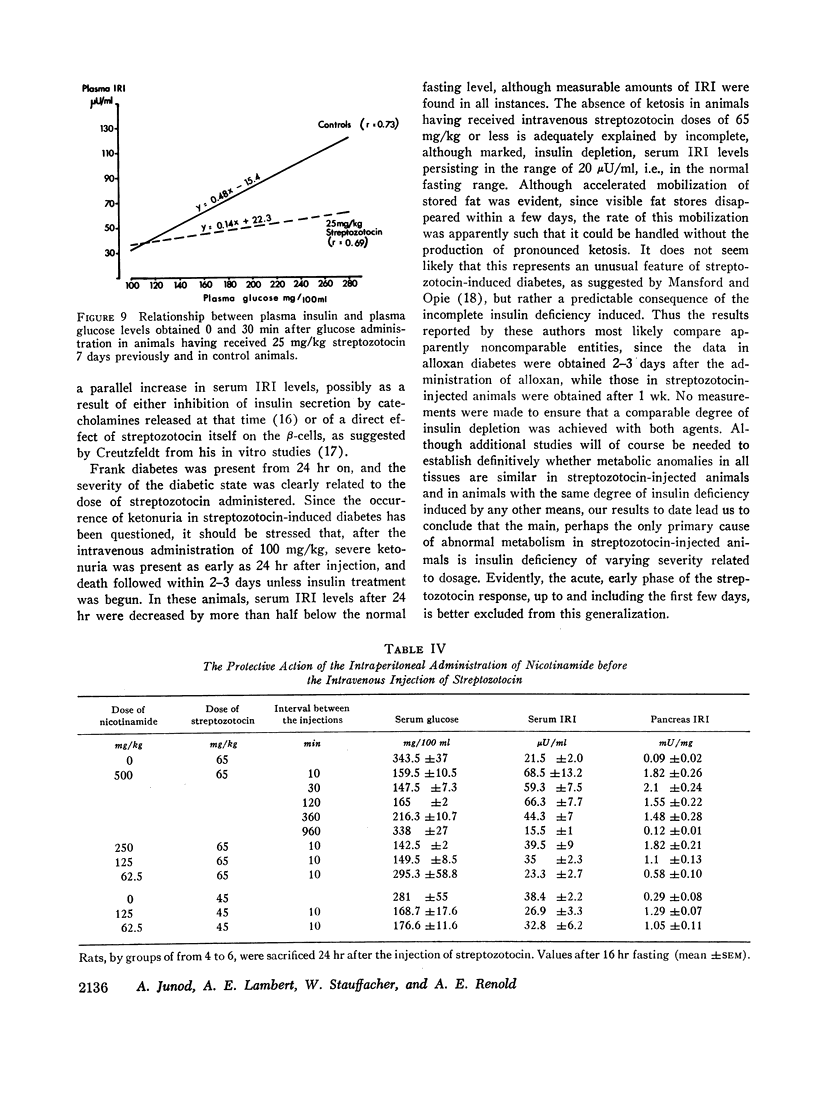
Abstract
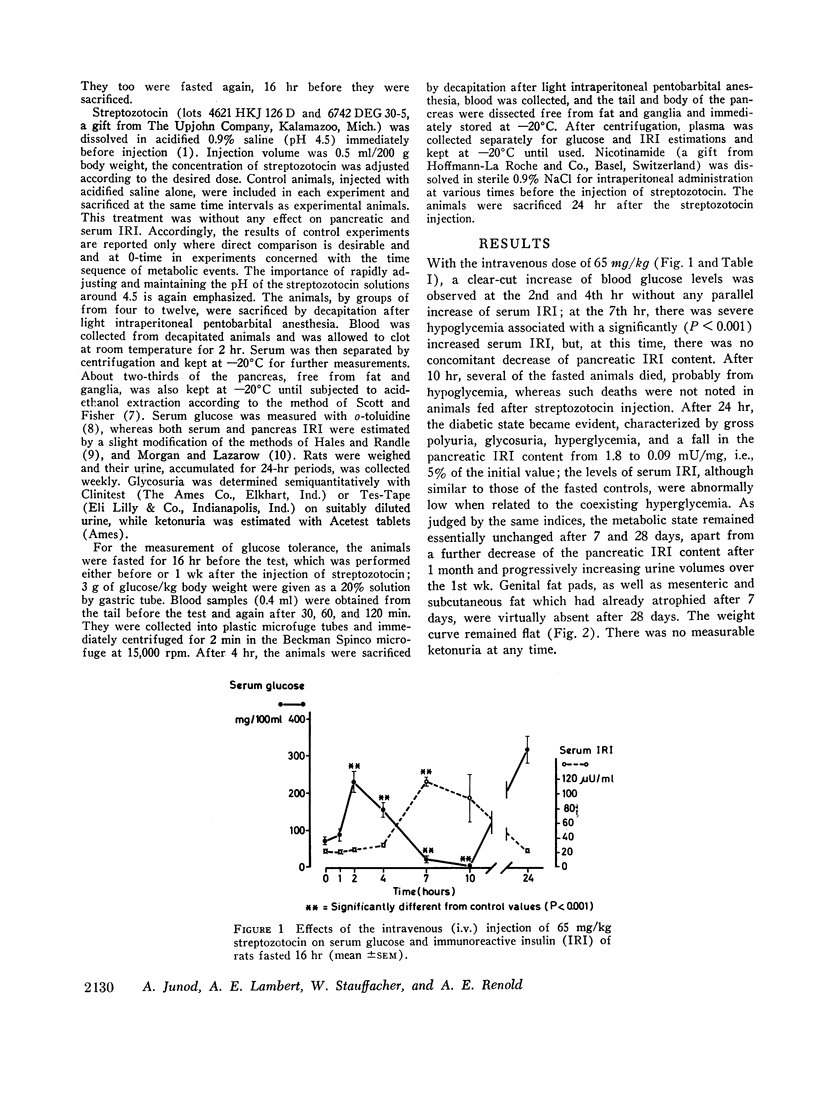
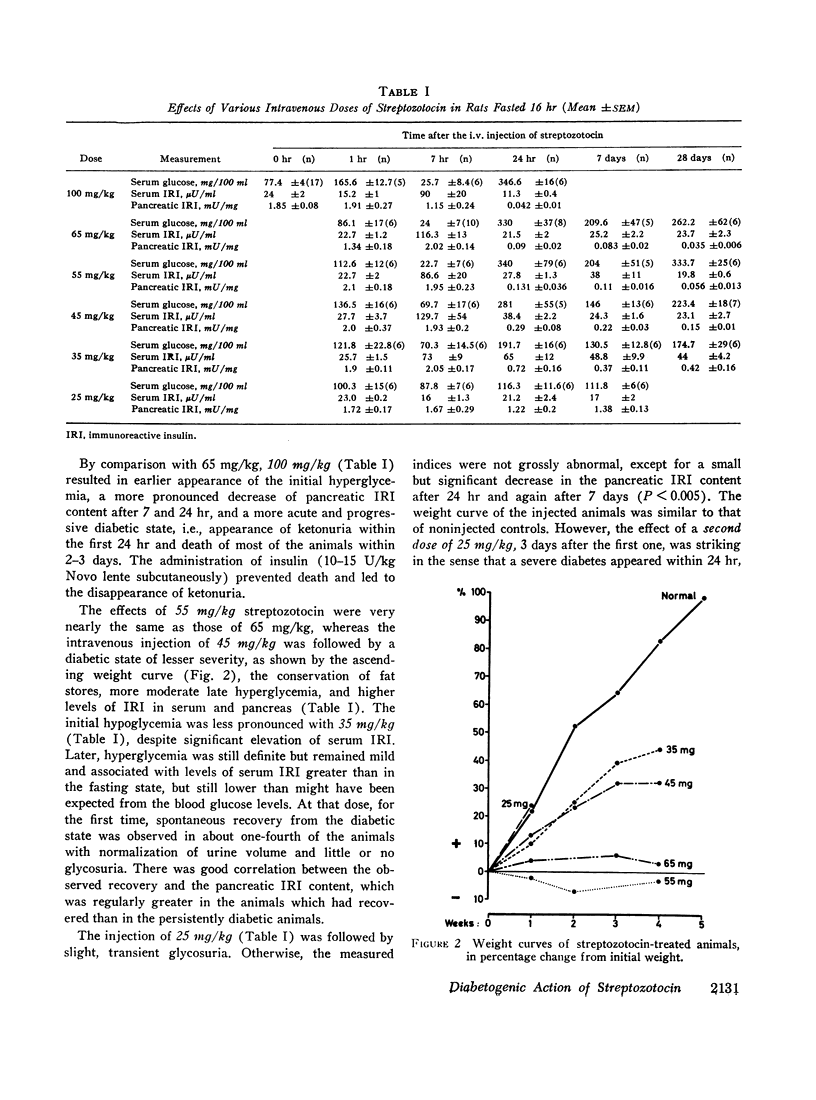
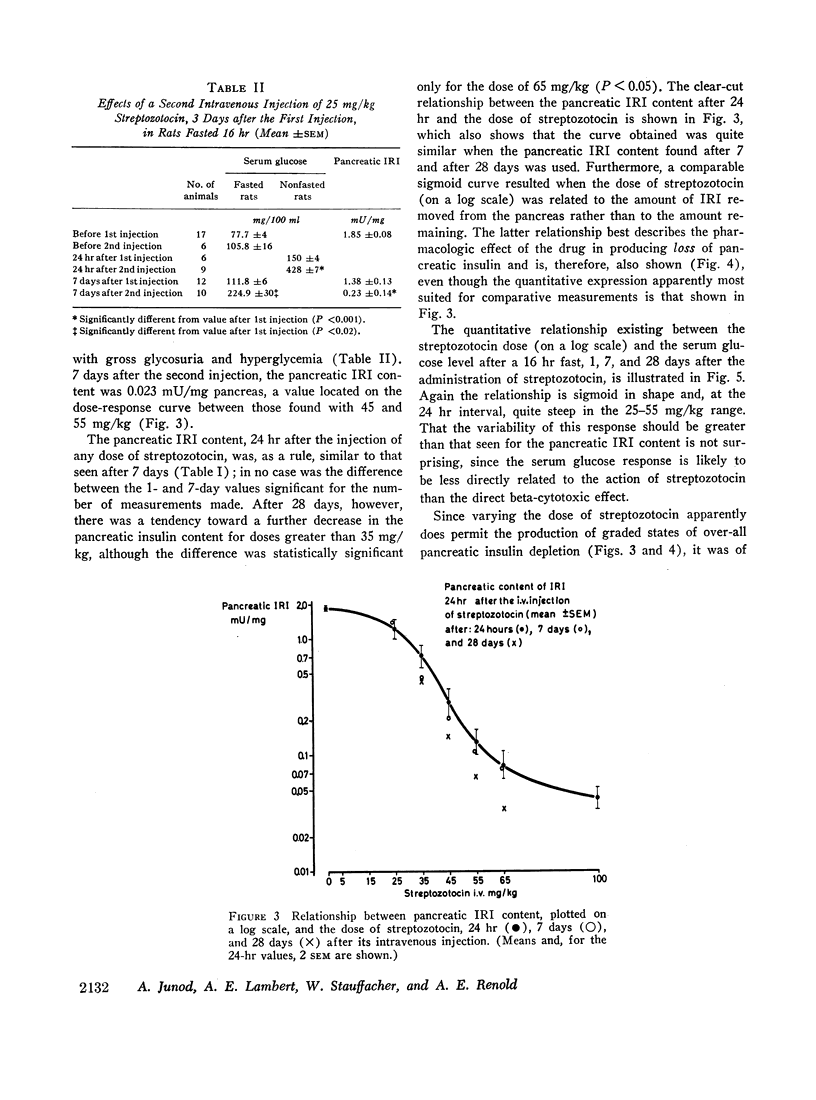
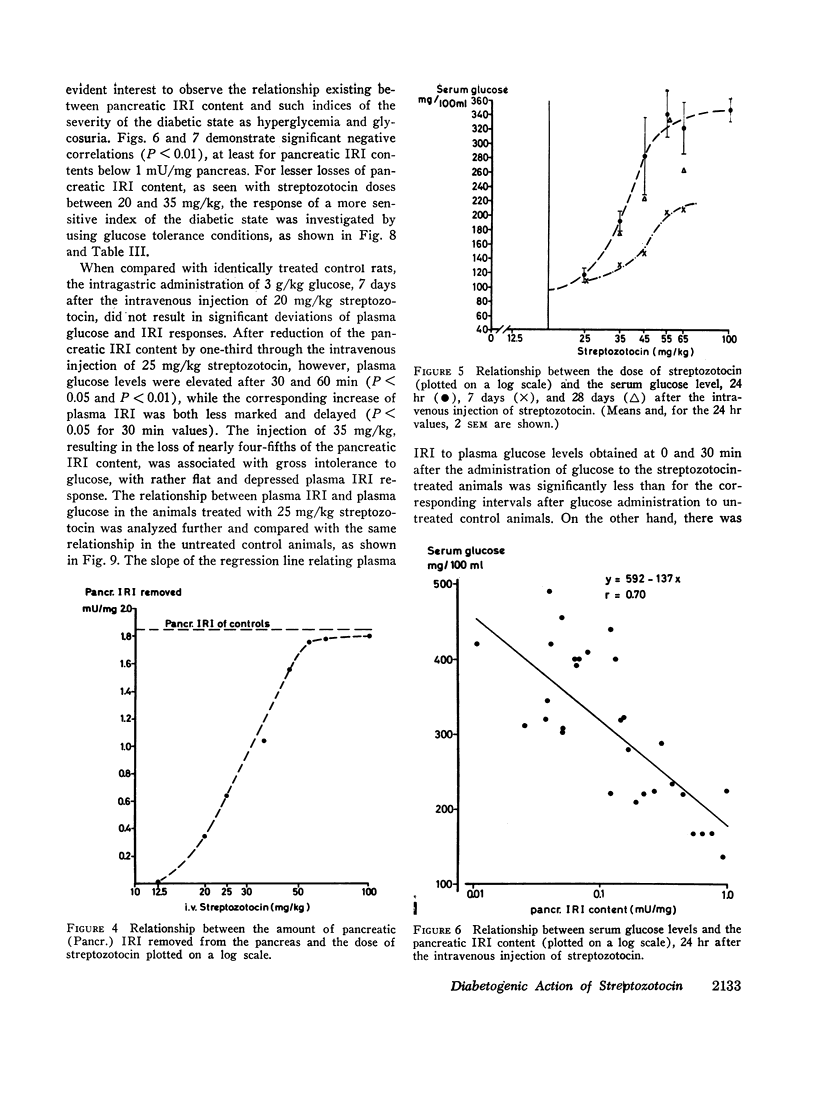
The relationship between the dose of intravenously administered streptozotocin (a N-nitroso derivative of glucosamine) and the diabetogenic response has been explored by use of the following indices of diabetogenic action: serum glucose, urine volume, and glycosuria, ketonuria, serum immunoreactive insulin (IRI), and pancreatic IRI content. Diabetogenic activity could be demonstrated between the doses of 25 and 100 mg/kg, all indices used showing some degree of correlation with the dose administered. Ketonuria was only seen with the largest dose, 100 mg/kg. The most striking and precise correlation was that between the dose and the pancreatic IRI content 24 hr after administration of the drug, and it is suggested that this represents a convenient test system either for both related and unrelated beta cytotoxic compounds or for screening for modifying agents or antidiabetic substances of a novel type. Ability to produce graded depletion of pancreatic IRI storage capacity led to an analysis of the relationship between pancreatic IRI content and deranged carbohydrate metabolism. Abnormal glucose tolerance and insulin response were seen when pancreatic IRI was depleted by about one-third, while fasting hyperglycemia and gross glycosuria occurred when the depletion had reached two-thirds and three-quarters, respectively. The mild yet persistent anomaly produced by the lowest effective streptozotocin dose, 25 mg/kg, exhibits characteristics resembling the state of chemical diabetes in humans and might thus warrant further study as a possible model. Finally, the loss of the diabetogenic action of streptozotocin by pretreatment with nicotinamide was confirmed and was shown to be a function of the relative doses of nicotinamide and streptozotocin and of the interval between injections.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arison R. N., Ciaccio E. I., Glitzer M. S., Cassaro J. A., Pruss M. P. Light and electron microscopy of lesions in rats rendered diabetic with streptozotocin. Diabetes. 1967 Jan;16(1):51–56. doi: 10.2337/diab.16.1.51. [DOI] [PubMed] [Google Scholar]
- Cerasi E., Luft R. The plasma insulin response to glucose infusion in healthy subjects and in diabetes mellitus. Acta Endocrinol (Copenh) 1967 Jun;55(2):278–304. doi: 10.1530/acta.0.0550278. [DOI] [PubMed] [Google Scholar]
- Colwell J. A., Lein A. Diminished insulin response to hyperglycemia in prediabetes and diabetes. Diabetes. 1967 Aug;16(8):560–565. doi: 10.2337/diab.16.8.560. [DOI] [PubMed] [Google Scholar]
- Dulin W. E., Wyse B. M. Studies on the ability of compounds to block the diabetogenic activity of streptozotocin. Diabetes. 1969 Jul;18(7):459–466. doi: 10.2337/diab.18.7.459. [DOI] [PubMed] [Google Scholar]
- Evans J. S., Gerritsen G. C., Mann K. M., Owen S. P. Antitumor and hyperglycemic activity of streptozotocin (NSC-37917) and its cofactor, U-15,774. Cancer Chemother Rep. 1965 Oct;48:1–6. [PubMed] [Google Scholar]
- HALES C. N., RANDLE P. J. Immunoassay of insulin with insulin-antibody precipitate. Biochem J. 1963 Jul;88:137–146. doi: 10.1042/bj0880137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr R. R., Jahnke J. K., Argoudelis A. D. The structure of streptozotocin. J Am Chem Soc. 1967 Aug 30;89(18):4808–4809. doi: 10.1021/ja00994a053. [DOI] [PubMed] [Google Scholar]
- Howell S. L., Taylor K. W. The acute pancreatic effect of alloxan in the rabbit. J Endocrinol. 1967 Apr;37(4):421–427. doi: 10.1677/joe.0.0370421. [DOI] [PubMed] [Google Scholar]
- Junod A., Lambert A. E., Orci L., Pictet R., Gonet A. E., Renold A. E. Studies of the diabetogenic action of streptozotocin. Proc Soc Exp Biol Med. 1967 Oct;126(1):201–205. doi: 10.3181/00379727-126-32401. [DOI] [PubMed] [Google Scholar]
- KAPLAN N. O., GOLDIN A., HUMPHREYS S. R., CIOTTI M. M., STOLZENBACH F. E. Pyridine nucleotide synthesis in the mouse. J Biol Chem. 1956 Mar;219(1):287–298. [PubMed] [Google Scholar]
- LAZAROW A., LIAMBIES J., TAUSCH A. J. Protection against diabetes with nicotinamide. J Lab Clin Med. 1950 Aug;36(2):249–258. [PubMed] [Google Scholar]
- Lundquist I., Rerup C. On the development of alloxan diabetes in mice. Eur J Pharmacol. 1967 Oct;2(1):35–41. doi: 10.1016/0014-2999(67)90020-9. [DOI] [PubMed] [Google Scholar]
- Mansford K. R., Opie L. Comparison of metabolic abnormalities in diabetes mellitus induced by streptozotocin or by alloxan. Lancet. 1968 Mar 30;1(7544):670–671. doi: 10.1016/s0140-6736(68)92103-x. [DOI] [PubMed] [Google Scholar]
- Perley M. J., Kipnis D. M. Plasma insulin responses to oral and intravenous glucose: studies in normal and diabetic sujbjects. J Clin Invest. 1967 Dec;46(12):1954–1962. doi: 10.1172/JCI105685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porte D., Jr, Graber A. L., Kuzuya T., Williams R. H. The effect of epinephrine on immunoreactive insulin levels in man. J Clin Invest. 1966 Feb;45(2):228–236. doi: 10.1172/JCI105335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAKIETEN N., RAKIETEN M. L., NADKARNI M. V. Studies on the diabetogenic action of streptozotocin (NSC-37917). Cancer Chemother Rep. 1963 May;29:91–98. [PubMed] [Google Scholar]
- Ricketts H. T., Cherry R. A., Kirsteins L. Biochemical studies of "prediabetes". Diabetes. 1966 Dec;15(12):880–888. doi: 10.2337/diab.15.12.880. [DOI] [PubMed] [Google Scholar]
- Schein P. S., Bates R. W. Plasma glucose levels in normal and adrenalectomized mice treated with streptozotocin and nicotinamide. Diabetes. 1968 Dec;17(12):760–765. doi: 10.2337/diab.17.12.760. [DOI] [PubMed] [Google Scholar]
- Schein P. S., Cooney D. A., Vernon M. L. The use of nicotinamide to modify the toxicity of streptozotocin diabetes without loss of antitumor activity. Cancer Res. 1967 Dec;27(12):2324–2332. [PubMed] [Google Scholar]
- Seltzer H. S., Allen E. W., Herron A. L., Jr, Brennan M. T. Insulin secretion in response to glycemic stimulus: relation of delayed initial release to carbohydrate intolerance in mild diabetes mellitus. J Clin Invest. 1967 Mar;46(3):323–335. doi: 10.1172/JCI105534. [DOI] [PMC free article] [PubMed] [Google Scholar]



