Abstract
Patients with acute leukemia were given repeated cycles consisting of infusions of methotrexate followed by “rescue” with folinic acid. Peripheral blood leukemic cells were harvested from patients before cyclical treatment, and the rates of incorporation of thymidine and of deoxyuridine into deoxyribonucleic acid (DNA) were measuared in vitro. There was no relationship between the pretreatment incorporation of either deoxynucleoside into DNA and the clinical response to therapy. Methotrexate suppressed deoxyuridine incorporation into DNA by the leukemic blasts in vitro, but the patients whose cells were most sensitive to this effect did not necessarily go into remission when treated.
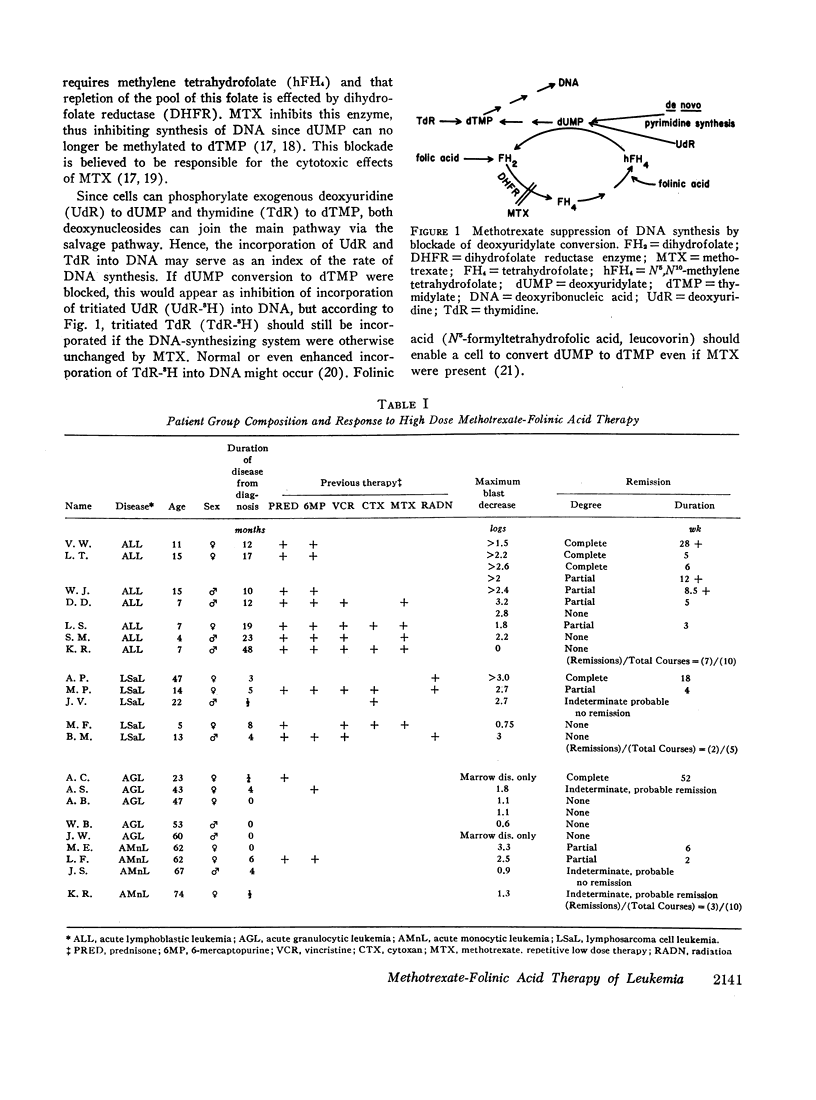
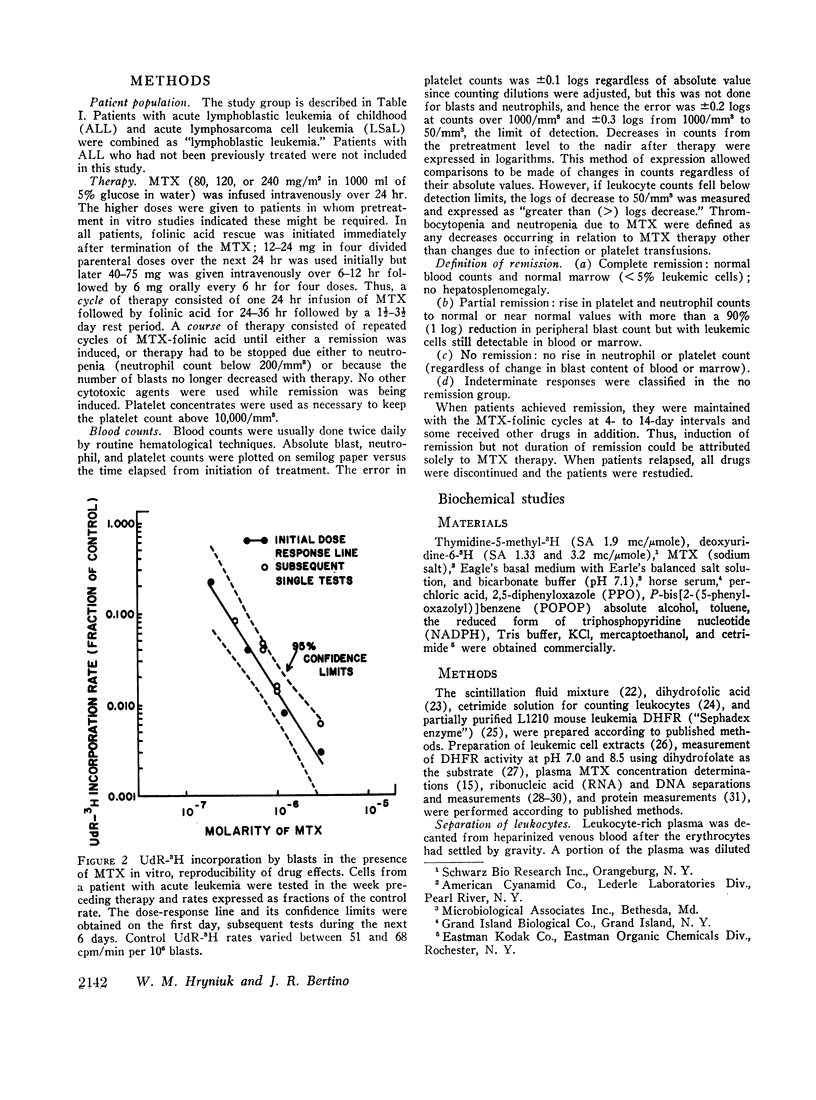
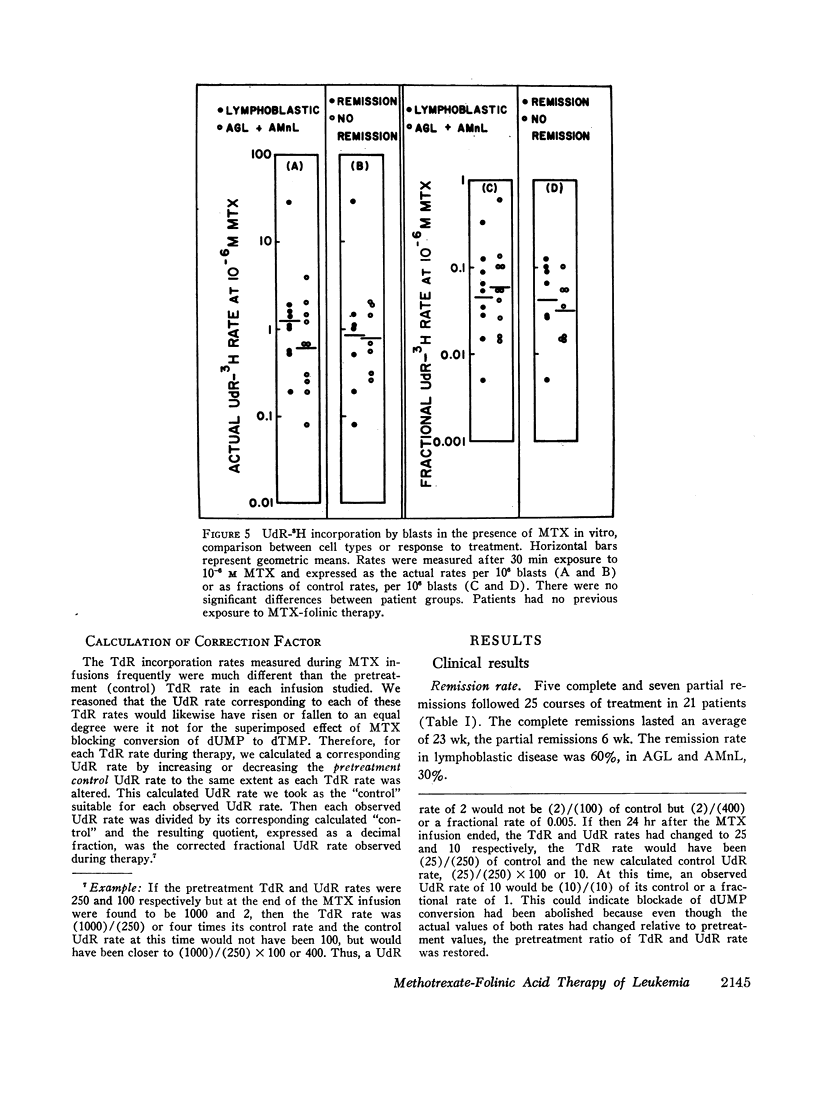
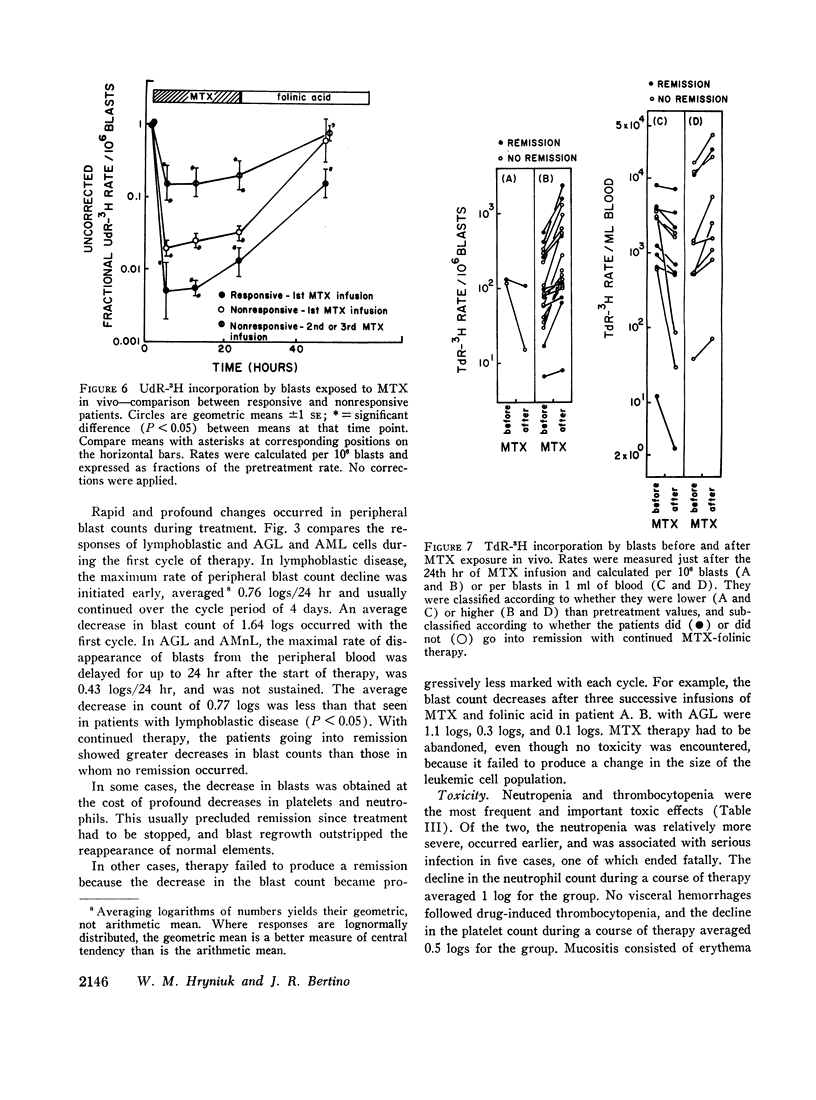
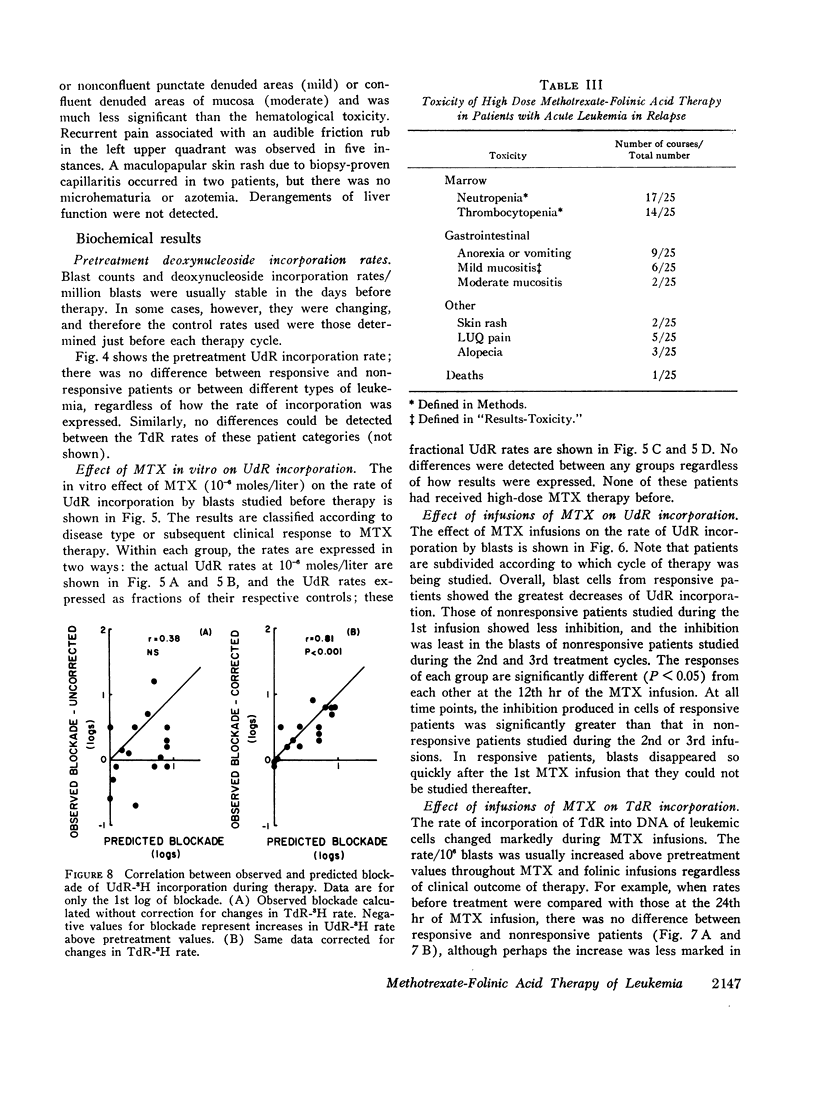
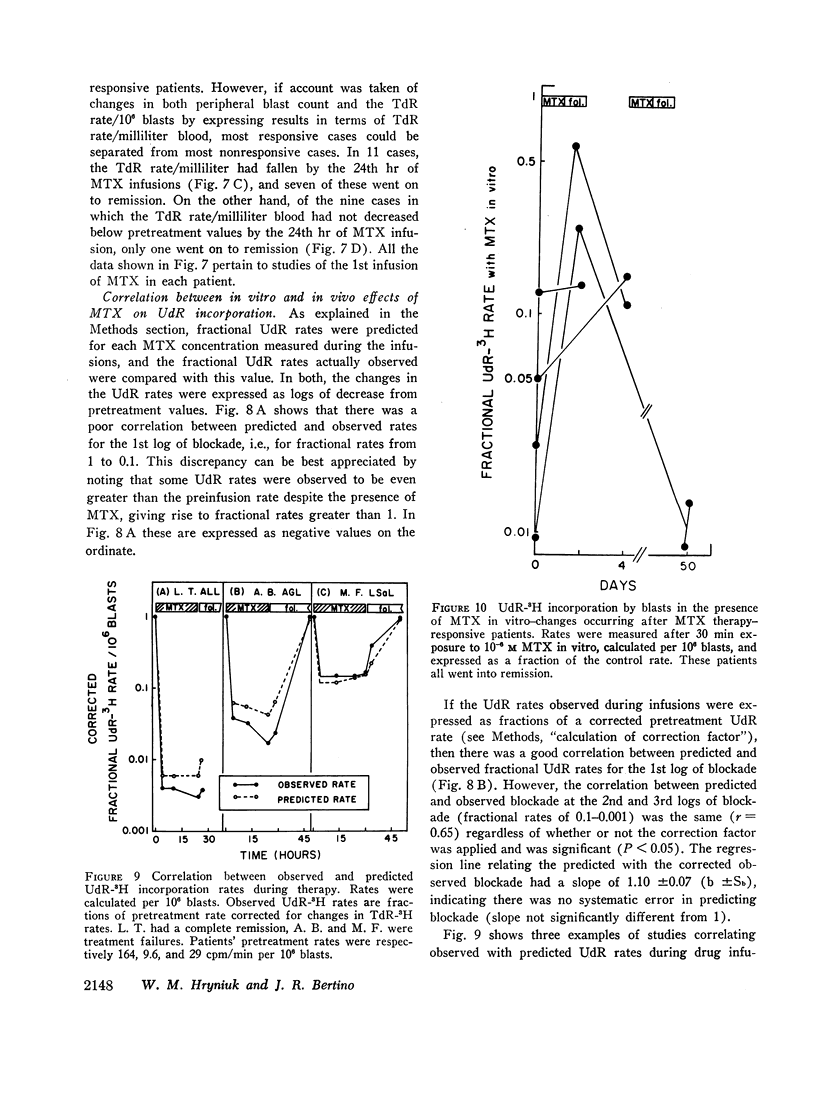
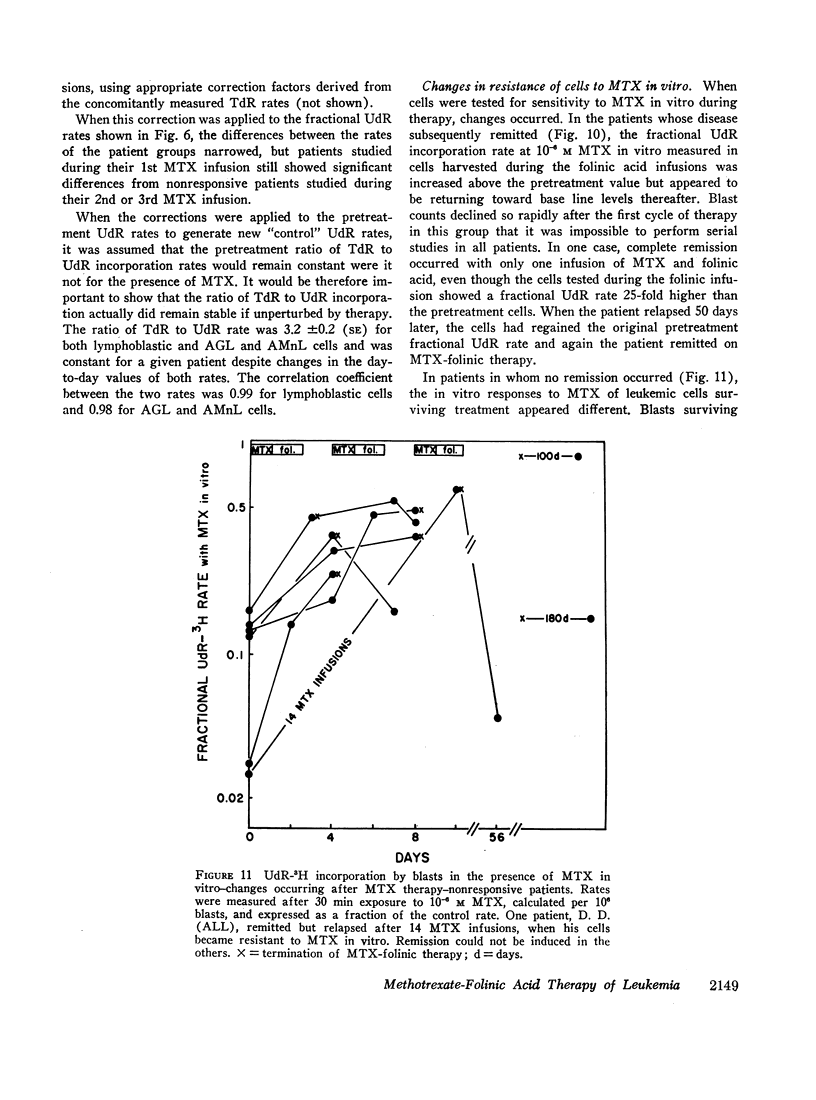
Leukemic cells were sampled during methotrexate infusions and the deoxynucleoside incorporation rates were determined. Thymidine incorporation into DNA was variably affected. If, by the end of the first infusion, it remained elevated, remission rarely followed, whereas if it was below the pretreatment value, remission was much more likely. In all cases, deoxyuridine incorporation was suppressed during the infusion. The greatest suppression occurred in patients who went on to remission, but the suppression did not correlate with that expected from pretreatment in vitro tests unless due weight was given to the concomitant effects of the methotrexate therapy on thymidine incorporation.
Leukemic blasts surviving successive cycles of therapy became progressively more resistant to the suppressing effects of methotrexate in vitro. This resistance became especially marked in the blasts of patients who did not go into remission.
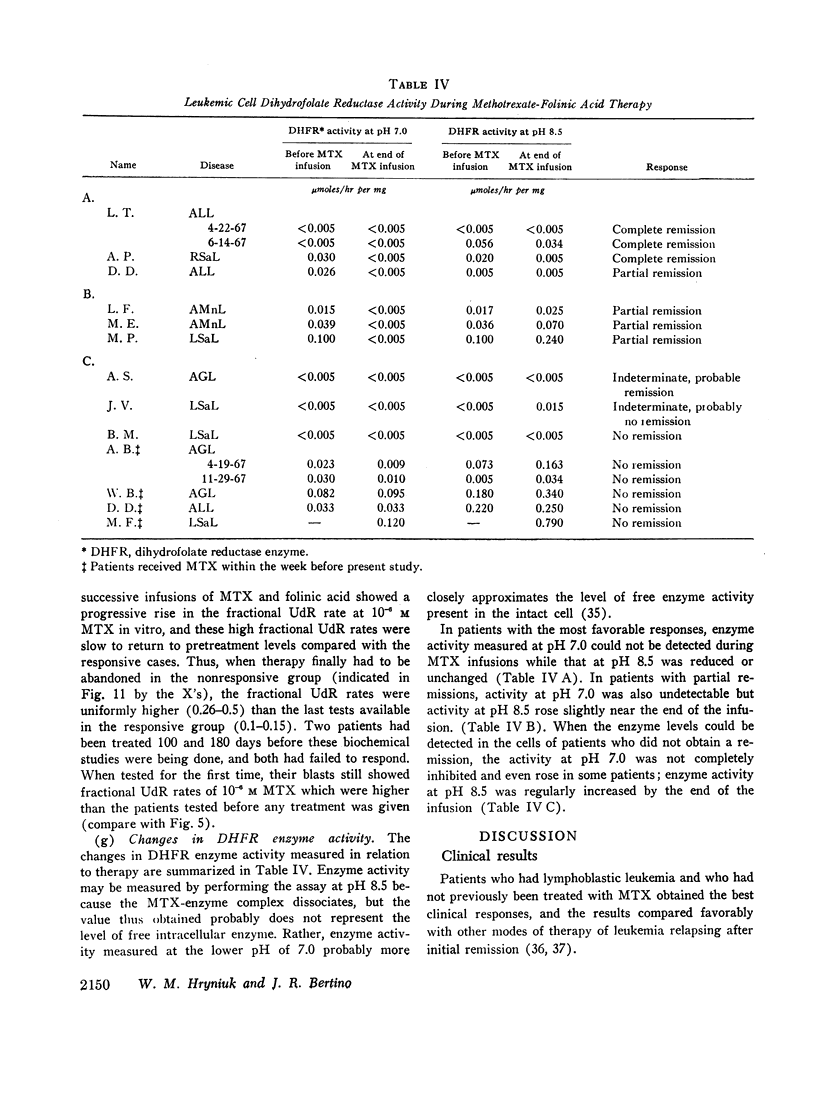
During methotrexate infusions, inhibition of leukemic cell dihydrofolate reductase activity was greatest in blasts of patients whose disease subsequently remitted.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERTINO J. R., BOOTH B. A., BIEBER A. L., CASHMORE A., SARTORELLI A. C. STUDIES ON THE INHIBITION OF DIHYDROFOLATE REDUCTASE BY THE FOLATE ANTAGONISTS. J Biol Chem. 1964 Feb;239:479–485. [PubMed] [Google Scholar]
- BERTINO J. R. PREPARATION OF NORMAL AND MALIGNANT HUMAN CELLS FOR STUDY OF RESISTANCE. Methods Med Res. 1964;10:263–268. [PubMed] [Google Scholar]
- BERTINO J. R. PREPARATION OF NORMAL AND MALIGNANT HUMAN CELLS FOR STUDY OF RESISTANCE. Methods Med Res. 1964;10:263–268. [PubMed] [Google Scholar]
- BERTINO J. R., SILBER R., FREEMAN M., ALENTY A., ALBRECHT M., GABRIO B. W., HUENNEKENS F. M. STUDIES ON NORMAL AND LEUKEMIC LEUKOCYTES. IV. TETRAHYDROFOLATE-DEPENDENT ENZYME SYSTEMS AND DIHYDROFOLIC REDUCTASE. J Clin Invest. 1963 Dec;42:1899–1907. doi: 10.1172/JCI104875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERTINO J. R. THE MECHANISM OF ACTION OF THE FOLATE ANTAGONISTS IN MAN. Cancer Res. 1963 Sep;23:1286–1306. [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertino J. R., Donohue D. M., Simmons B., Gabrio B. W., Silber R., Huennekens F. M. THE "INDUCTION" OF DIHYDROFOLIC REDUCTASE ACTIVITY IN LEUKOCYTES AND ERYTHROCYTES OF PATIENTS TREATED WITH AMETHOPTERIN. J Clin Invest. 1963 Apr;42(4):466–475. doi: 10.1172/JCI104735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickis I. J., Henderson I. W., Quastel J. H. Biochemical studies of human tumors. II. In vitro estimation of individual tumor sensitivity to anticancer agnets. Cancer. 1966 Jan;19(1):103–113. doi: 10.1002/1097-0142(196601)19:1<103::aid-cncr2820190111>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Bruce W. R., Meeker B. E., Valeriote F. A. Comparison of the sensitivity of normal hematopoietic and transplanted lymphoma colony-forming cells to chemotherapeutic agents administered in vivo. J Natl Cancer Inst. 1966 Aug;37(2):233–245. [PubMed] [Google Scholar]
- CONDIT P. T., SHNIDER B. I., OWENS A. H., Jr Studies on the folic acid vitamins. VII. The effects of large doses of amethopterin in patients with cancer. Cancer Res. 1962 Jul;22:706–712. [PubMed] [Google Scholar]
- Chu M. Y., Fischer G. A. Effects of cytosine arabinoside on the cell viability and uptake of deoxypyrimidine nucleosides in L5178Y cells. Biochem Pharmacol. 1968 May;17(5):741–751. doi: 10.1016/0006-2952(68)90011-7. [DOI] [PubMed] [Google Scholar]
- Cline M. J. Prediction of in vivo cytotoxicity of chemotherapeutic agents by their effect on malignant leukocytes in vitro. Blood. 1967 Aug;30(2):176–188. [PubMed] [Google Scholar]
- Djerassi I. Methotrexate infusions and intensive supportive care in the management of children with acute lymphocytic leukemia: follow-up report. Cancer Res. 1967 Dec;27(12):2561–2564. [PubMed] [Google Scholar]
- FISCHER G. A. Detective transport of amethopterin (methotrexate) as a mechanism of resistance to the antimetabolite in L5178Y leukemic cells. Biochem Pharmacol. 1962 Dec;11:1233–1234. doi: 10.1016/0006-2952(62)90200-9. [DOI] [PubMed] [Google Scholar]
- FLECK A., MUNRO H. N. The precision of ultraviolet absorption measurements in the Schmidt-Thannhauser procedure for nucleic acid estimation. Biochim Biophys Acta. 1962 May 14;55:571–583. doi: 10.1016/0006-3002(62)90836-3. [DOI] [PubMed] [Google Scholar]
- GOLDIN A., VENDITTI J. M., HUMPHREYS S. R., DENNIS D., MANTEL N. Studies on the management of mouse leukemia (L1210) with antagonists of folic acid. Cancer Res. 1955 Dec;15(11):742–747. [PubMed] [Google Scholar]
- Gallo R. C., Perry S., Breitman T. R. The enzymatic mechanisms for deoxythymidine synthesis in human leukocytes. I. Substrate inhibition by thymine and activation by phosphate or arsenate. J Biol Chem. 1967 Nov 10;242(21):5059–5068. [PubMed] [Google Scholar]
- HERTZ R., BERGENSTAL D. M., LIPSETT M. B., PRICE E. B., HILBISH T. F. Chemotherapy of choriocarcinoma and related trophoblastic tumors in women. Ann N Y Acad Sci. 1959 Aug 28;80:262–284. doi: 10.1111/j.1749-6632.1959.tb49206.x. [DOI] [PubMed] [Google Scholar]
- Hillcoat B. L., Swett V., Bertino J. R. Increase of dihydrofolate reductase activity in cultured mammalian cells after exposure to methotrexate. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1632–1637. doi: 10.1073/pnas.58.4.1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNS D. G., HOLLINGSWORTH J. W., CASHMORE A. R., PLENDERLEITH I. H., BERTINO J. R. METHOTREXATE DISPLACEMENT IN MAN. J Clin Invest. 1964 Apr;43:621–629. doi: 10.1172/JCI104947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessel D., Hall T. C., Roberts D. Modes of uptake of methotrexate by normal and leukemic human leukocytes in vitro and their relation to drug response. Cancer Res. 1968 Mar;28(3):564–570. [PubMed] [Google Scholar]
- Kessel D., Hall T. C., Roberts D., Wodinsky I. Uptake as a determinant of methotrexate response in mouse leukemias. Science. 1965 Nov 5;150(3697):752–754. doi: 10.1126/science.150.3697.752. [DOI] [PubMed] [Google Scholar]
- Killmann S. A. Proliferative activity of blast cells in leukemia and myelofibrosis. Morphological differences between proliferating and non-proliferating blast cells. Acta Med Scand. 1965 Sep;178(3):263–280. doi: 10.1111/j.0954-6820.1965.tb04271.x. [DOI] [PubMed] [Google Scholar]
- LASZLO J., STENGLE J., WIGHT K., BURK D. Effects of chemotherapeutic agents on metabolism of human acute leukemia cells in vitro. Proc Soc Exp Biol Med. 1958 Jan;97(1):127–131. doi: 10.3181/00379727-97-23666. [DOI] [PubMed] [Google Scholar]
- Lefkowitz E., Papac R. J., Bertino J. R. Head and neck cancer. 3. Toxicity of 24-hour infusions of methotrexate (NSC-740) and protection by leucovorin (NSC-3590) in patients with epidermoid carcinomas. Cancer Chemother Rep. 1967 Sep;51(5):305–311. [PubMed] [Google Scholar]
- MAUER A. M., FISHER V. Comparison of the proliferative capacity of acute leukaemia cells in bone marrow and blood. Nature. 1962 Mar 17;193:1085–1086. doi: 10.1038/1931085a0. [DOI] [PubMed] [Google Scholar]
- Madoc-Jones H., Bruce W. R. Sensitivity of L cells in exponential and stationary phase to 5-fluorouracil. Nature. 1967 Jul 15;215(5098):302–303. doi: 10.1038/215302a0. [DOI] [PubMed] [Google Scholar]
- O'BRIEN J. S. The role of the folate coenzymes in cellular division. A review. Cancer Res. 1962 Apr;22:267–281. [PubMed] [Google Scholar]
- OETTGEN H. F., BURKITT D., BURCHENAL J. H. Malignant lymphoma involving the jaw in African children: treatment with Methotrexate. Cancer. 1963 May;16:616–623. doi: 10.1002/1097-0142(196305)16:5<616::aid-cncr2820160512>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- OSBORN M. J., FREEMAN M., HUENNEKENS F. M. Inhibition of dihydrofolic reductase by aminopterin and amethopterin. Proc Soc Exp Biol Med. 1958 Feb;97(2):429–431. doi: 10.3181/00379727-97-23764. [DOI] [PubMed] [Google Scholar]
- OSBORN M. J., HUENNEKENS F. M. Enzymatic reduction of dihydrofolic acid. J Biol Chem. 1958 Oct;233(4):969–974. [PubMed] [Google Scholar]
- Oettgen H. F., Old L. J., Boyse E. A., Campbell H. A., Philips F. S., Clarkson B. D., Tallal L., Leeper R. D., Schwartz M. K., Kim J. H. Inhibition of leukemias in man by L-asparaginase. Cancer Res. 1967 Dec;27(12):2619–2631. [PubMed] [Google Scholar]
- PERRIN J. C., MAUER A. M., STERLING T. D. Intravenous methotrexate (amethopterin) therapy in the treatment of acute leukemia. Pediatrics. 1963 May;31:833–839. [PubMed] [Google Scholar]
- Perkins J. P., Hillcoat B. L., Bertino J. R. Dihydrofolate reductase from a resistant subline of the L1210 lymphoma. Purification and properties. J Biol Chem. 1967 Oct 25;242(20):4771–4776. [PubMed] [Google Scholar]
- RUECKERT R. R., MUELLER G. C. Studies on unbalanced growth in tissue culture. I. Induction and consequences of thymidine deficiency. Cancer Res. 1960 Dec;20:1584–1591. [PubMed] [Google Scholar]
- Roberts D., Hall T. C. The reduction of folate and of dihydrofolate by homogenates of leukocytes from patients with leukemia or with myeloid metaplasia. Cancer Res. 1967 May;27(5):994–999. [PubMed] [Google Scholar]
- SARTORELLI A. C., UPCHURCH H. F., BOOTH B. A. Effects of folinic acid on amethopterin-induced inhibition of the Ehrlich ascites carcinoma. Cancer Res. 1962 Jan;22:102–106. [PubMed] [Google Scholar]
- SMITH C. G., LUMMIS W. L., GRADY J. E. An improved tissue culture assay. II. Cytotoxicity studies with antibiotics, chemicals, and solvents. Cancer Res. 1959 Sep;19:847–852. [PubMed] [Google Scholar]
- SULLIVAN R. D., MILLER E., SIKES M. P. Antimetabolite-metabolite combination cancer chemotherapy. Effects of intraarterial methotrexate-intramuscular Citrovorum factor therapy in human cancer. Cancer. 1959 Nov-Dec;12:1248–1262. doi: 10.1002/1097-0142(195911/12)12:6<1248::aid-cncr2820120619>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Scott J. L., Marino J. V., Gabor E. P. Human leukocyte metabolism in vitro. II. The effect of 6-mercaptopurine on formate-C-14 incorporation into the nucleic acids of acute leukemic leukocytes. Blood. 1966 Nov;28(5):683–691. [PubMed] [Google Scholar]
- Thompson I., Hall T. C., Moloney W. C. Combination therapy of adult acute myelogenous leukemia: experience with the simultaneous use of vincristine, amethopterin, 6-mercaptopurine and prednisone. N Engl J Med. 1965 Dec 9;273(24):1302–1307. doi: 10.1056/NEJM196512092732403. [DOI] [PubMed] [Google Scholar]
- WATNE A. L., DI PAOLO J. A. A correlation of clinical observations to in vitro sensitivity test for cancer chemotherapy. Cancer Chemother Rep. 1962 Dec;25:109–112. [PubMed] [Google Scholar]
- WINZLER R. J., WILLIAMS A. D., BEST W. R. Metabolism of human leukocytes in vitro. I. Effects of A-methopterin on formate-C14 incorporation. Cancer Res. 1957 Feb;17(2):108–116. [PubMed] [Google Scholar]
- WOLBERG W. H. STUDIES ON THE MECHANISM OF HUMAN TUMOR RESISTANCE TO THE FLUORINATED PYRIMIDINES. Cancer Res. 1964 Sep;24:1437–1447. [PubMed] [Google Scholar]
- WRIGHT J. C., COBB J. P., GUMPORT S. L., SAFADI D., WALKER D. G., GOLOMB F. M. Further investigation of the relation between the clinical and tissue culture response to chemotherapeutic agents on human cancer. Cancer. 1962 Mar-Apr;15:284–293. doi: 10.1002/1097-0142(196203/04)15:2<284::aid-cncr2820150212>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]


