Abstract
Objectives
This study examines C-reactive protein (CRP) levels predict cardiovascular events (CVE) among hormone therapy (HT) users and non-users.
Background
CRP levels are higher in women who use oral HT than in non-users; however, whether the same CRP cutpoints determine increased cardiovascular risk remains unanswered.
Methods
CRP was measured at baseline in 10,953 HT users and 15,838 non-users in the Women’s Health Study. In HT non-users and users, Cox-proportional hazard models adjusted for age, cardiovascular risk factors, BMI, and menopausal status were constructed using AHA/CDC-recommended CRP cut-points for low, average, and high cardiovascular risk as well as HT non-user-and user-derived quantiles of CRP to predict incident cardiovascular events(CVE).
Results
CVE risk increased with lnCRP for both HT users and non-users. After adjusting for cardiovascular risk factors including lipids, the multivariable relative risk (RR) per log unit of CRP was 1.27 (95% CI, 1.13 to 1.44) for HT non-users and 1.22 (95% CI, 1.07 to 1.40) for HT users. In order to compare the risk associated with AHA/CDC CRP categories across HT use groups, we fit a model in which non-users with CRP <1 mg/L were the reference group. After adjusting for other risk factors including lipids, HT users with CRP ≥3 had a RR of 1.93 (1.38–2.69) while non-users had a RR of 1.92 (1.35–2.72).
Conclusions
CRP predicts CVE in a linear relationship among both HT users and non-users. CRP levels ≥3 mg/L were associated with increased cardiovascular risk in both non-users and HT users.
Keywords: cardiovascular diseases, prevention, risk factors, hormones, C-reactive protein
Introduction
Prospective epidemiologic studies have consistently demonstrated that higher levels of high-sensitivity C-reactive protein (CRP) are independently associated with incident cardiac and vascular events in healthy men and women and add prognostic information to that conveyed by the Framingham risk score.1–10
CRP levels are higher in women taking oral postmenopausal hormone therapy (HT) as compared with HT non-users;11–13 however, whether or not the elevation in CRP that occurs with oral HT use is associated with increased risk in cardiovascular disease remains unanswered.14 A nested case-control study of women in the Women’s Health Initiative Observational Study (WHI-OS) suggested that CRP is associated with future coronary risk irrespective of HT status at baseline, although risk estimates for the prediction of cardiovascular events (CVE) were non-significant in HT users.15 Similarly, CRP has been shown to be a linear predictor of CVE in HT non-users in the Women’s Health Study (WHS),2, 16 although a detailed analysis of HT users has not yet been conducted in this population. Current AHA/CDC recommendations regarding use of CRP do not specify whether threshold cut-points for CRP are appropriate for HT users, but suggest that additional prospective studies are needed to more precisely define risk at various strata and to assure consistency in subgroup populations.14
This study sought to determine whether CRP is a linear predictor of cardiovascular outcomes among HT users in the prospective WHS. Additionally, we sought to evaluate whether existing AHA/CDC-recommended CRP threshold cut-points for low, average, and high cardiovascular risk (<1, 1–<3, and ≥3 mg/L) predict cardiovascular risk in HT users as appropriately as in non-users.14
Methods
Study Population and Design
The Women’s Health Study (WHS) was a randomized, double-blind, placebo-controlled, 2×2 factorial design trial of low-dose aspirin and vitamin E in the primary prevention of cardiovascular disease and cancer conducted among 39,876 apparently healthy women 45 years of age or older.17–21 Briefly, participants were enrolled between November 1992 and July 1995. For the purposes of our investigation, the study population was treated as an observational cohort of healthy adult women, for whom information regarding demographic, behavioral, medical, and lifestyle factors was collected via questionnaire at baseline; at 12-, 36-, 60-, and 96-month intervals; and at the conclusion of the follow-up period. Menopause status (premenopausal, postmenopausal, dubious, or uncertain) and history of postmenopausal HT use (dichotomized to represent never/past use vs. current use) were among the clinical variables reported. Women who used hormones that were not considered postmenopausal HT (such as oral progestogen or vaginal estrogen) were included in the never/past users category.
Participants were followed for a mean of 10 years for the occurrence of a first major cardiovascular event (CVE), defined as nonfatal myocardial infarction (MI), nonfatal ischemic stroke (CVA), coronary revascularization procedures [coronary artery bypass grafting (CABG) or percutaneous transluminal coronary angioplasty (PTCA)], or death from cardiovascular causes (CVD). A combined endpoint of cardiovascular disease was used, following recent recommendations and the use of this endpoint by major clinical trials as being most informative for clinical practice,22 and due to limited power for any single endpoint category by strata of hormone use. Myocardial infarction was confirmed if symptoms met World Health Organization criteria and if the event was associated with abnormal levels of cardiac enzymes or diagnostic electrocardiographic changes. A confirmed stroke was defined as a new neurologic deficit of sudden onset that persisted for at least 24 hours.23 The performance of either percutaneous coronary revascularization or CABG was confirmed by a review of hospital records. Deaths from cardiovascular causes were confirmed by review of autopsy reports, death certificates, medical records, and information obtained from family members.
Before randomization, blood samples were collected in tubes containing EDTA from 28,345 study participants (71% participation rate) and stored in liquid nitrogen until the time of analysis. These samples underwent analysis of lipids and CRP in a core laboratory facility certified by the National Heart, Lung, and Blood Institute/Centers for Disease Control and Prevention Lipid Standardization program. High- sensitivity CRP was measured using a validated immunoturbidometric method (Denka Seiken, Tokyo, Japan). Levels of total cholesterol and high-density lipoprotein cholesterol (HDL-C) were measured enzymatically on a Hitachi 911 autoanalyzer (Roche Diagnostics, Basel, Switzerland), and low-density lipoprotein cholesterol (LDL-C) was determined directly (Genzyme, Cambridge, Mass), as has been previously reported.24 Of the samples received, 27,939 could be evaluated. The 55 women for whom hormone status was not known were excluded from the analysis. The 1,093 transdermal HT users at baseline were also excluded, given the relative lack of change in CRP observed with transdermal use as compared with oral administration.25–28 Thus, 26,791 women [15,838 HT non-users (59.1%) and 10,953 HT users (40.9%)] were included in our analyses.
Statistical Analysis
Because oral hormone therapy increases levels of C-reactive protein, we first established population-based distributions for CRP among HT users and non-users at the time of study entry.29 Since CRP was not normally distributed, non-parametric univariate tests (Wilcoxon rank-sum test, two-sided t-approximation) were used to compare the distributions of CRP between HT groups.
The associations of hormone use category (current use vs. past/never use) with baseline demographic variables and cardiovascular risk factors were tested using univariable tests. The chi-square test was used for categorical predictors, and the Wilcoxon rank-sum test was used for continuous predictors, given the non-normality of their distribution.
To reduce misclassification error with respect to HT status, self-reported data regarding hormone status and hormone therapy regimens from the baseline questionnaire were compared with the 12-, 36-, 60-, and 96-month follow-up questionnaires, and women who changed hormone status during the course of study follow-up were censored at the first time point of self-reported change in hormone status. Censorship limited the number of CVE outcomes to 644 (278 in the 10,953 HT users and 366 in the 15,838 HT non-users); by contrast, there were 907 CVE (354 in HT users, 552 in non-users) in the uncensored cohort.2
The rate ratios (RRs) and 95% confidence intervals (CIs) of incident CVE were computed in both crude Cox proportional-hazards models and multivariable models adjusted for age, cardiovascular risk factors, body mass index (kg/m2), and menopausal status. Cardiovascular risk factors included in the model were blood pressure (history of hypertension, or baseline blood pressure ≥140/90 mm Hg), presence or absence of diabetes, current/past smoking status, family history of early coronary artery disease (<60 years of age), ratio of total cholesterol to HDL-C, and history of use of cholesterol-lowering medication. All analyses were controlled for randomized treatment to aspirin, vitamin E, and beta-carotene. Prediction of CV outcomes by CRP was initially established by the use of quintiles, similar to those used in the previous WHS analysis.2 Tertiles were then used for direct comparison to the established AHA/CDC (tertile) cut-points which were evaluated in this study. Tests for trends across quintiles and tertiles of CRP and across the AHA/CDC cut-points were assessed by entering a single ordinal term for each quantile or category based on the median CRP. In the evaluation of CRP as a continuous logarithm-transformed variable, the RR of incident CVE was compared between users and non-users, and the likelihood ratio X2 was compared for the models with and without the lnCRP variable. In addition, effect modification of the AHA/CDC cut-points (CRP <1, 1–<3, and ≥3 mg/L) by HT use in the prediction of CVE was evaluated by testing a formal interaction term [CRP (<1, 1–<3, and ≥3 mg/L)*HT use]. In order to compare risks between groups, a 6 level variable with AHA/CDC categories and HT use categories was created, using CRP <1 mg/L in non-users as the referent group.
All P values are two-tailed, and 95% CI were calculated. Data analysis was conducted using SAS statistical software version 9.1 (SAS Institute Inc., Cary, NC) and S-Plus software version 6.2 (Insightful Corp., Seattle, WA).
Results
The median age at baseline for the 26,791 women included in this study was 52.9 years, and the median body-mass index (BMI) was 24.9 kg/m2. At the time of study entry, 13,531 (50.5%) women had never used postmenopausal hormone replacement therapy, 2,307 (8.6%) were past users, and 10,953 (40.9%) were current users. A comparison of demographic and clinical variables in the analysis cohort, stratified by HT use, is provided (Table 1). HT users had higher CRP values, higher total and HDL cholesterol values, with lower LDL cholesterol and a lower ratio of total/HDL cholesterol. Of the 5331 current HT users who used estrogen alone, 83.3% (4442) used conjugated equine estrogen (CEE). Of the 4850 current HT users who used combination estrogen and progestogen, 83.8% (4063) used CEE.
Table 1.
Association between Postmenopausal Hormone Therapy Use and Demographic and Clinical Variables among 26,791 women* in the Women's Health Study.
| Demographic Variables |
Analysis Cohort* (N=26791) |
HT Never/Past Users (N=15838, 59.1%) |
HT Current Users (N=10953, 40.9%) |
|---|---|---|---|
| Age (years), median (25–75% interquartile range) | 52.9 (48.9–59.0) | 51.2 (48.0–58.8) | 54.8 (51.0–59.2) |
| Body mass index (kg/m2), median (25–75% interquartile range) | 24.9 (22.5–28.3) | 25.1 (22.6–29.1) | 24.4 (22.3–27.5) |
| Race, % | |||
| White | 95.3% | 94.9% | 95.8% |
| Black | 1.8% | 2.0% | 1.6% |
| Other Race | 2.9% | 3.0% | 2.6% |
| Cardiovascular Medical History and Behavioral Variables, % | |||
| Cardiovascular event during study† | 2.4% | 2.3% | 2.5% |
| History of HTN [BP>=140/90] | 25.2% | 24.7% | 25.9% |
| Family History of early CVD ≤age 60 | 12.8% | 13.0% | 12.5% |
| C-Reactive Protein Risk ≥3mg/L | 37.6% | 29.3% | 49.4% |
| Hypercholesterolemia (total cholesterol ≥240 mg/dL at baseline) | 22.0% | 21.3% | 23.0% |
| High LDL Cholesterol (≥160 mg/dL) | 13.8% | 15.7% | 11.0% |
| Low HDL (<40 mg/dL) | 17.2% | 21.6% | 11.0% |
| History of Diabetes | 2.8% | 3.4% | 2.0% |
| History of cholesterol-lowering medication | 3.1% | 2.8% | 3.7%% |
| History of blood pressure-controlling medication | 13.4% | 12.7% | 14.5% |
| Smoking | |||
| Never | 51.8% | 52.4% | 51.0% |
| Past | 36.5% | 35.4% | 38.1% |
| Current | 11.7% | 12.2% | 10.9% |
| Exercise Frequency | |||
| Rarely/Never | 37.3% | 38.5% | 35.7% |
| <1/Week | 19.6% | 19.9% | 19.2% |
| 1–3/Week | 31.6% | 30.8% | 32.8% |
| 4+/Week | 11.4% | 10.8% | 12.3% |
| Postmenopausal Status | |||
| Premenopausal | 28.4% | 44.2% | 5.6% |
| Postmenopausal | 53.8% | 40.2% | 73.5% |
| Biologically uncertain (hysterectomy without bilateral oophorectomy) | 13.7% | 10.9% | 17.7% |
| Unclear/subject uncertain | 4.1% | 4.7% | 3.2% |
| Clinical Parameters, median (25–75% interquartile range) | |||
| C-reactive protein (mg/L) | 2.04 (0.82–4.42) | 1.51 (0.61–3.47) | 2.95 (1.38–5.61) |
| Total cholesterol (mg/dL) | 208 (184–236) | 206 (181–234) | 211 (188–237) |
| Low-density lipoprotein cholesterol (mg/dL) | 121.2 (100.4–144.2) | 123.7 (102.3–147.4) | 117.8 (97.9–139.4) |
| High-density lipoprotein cholesterol (mg/dL) | 52.0 (43.2–62.4) | 49.1 (41.2–58.8) | 56.3 (47.0–66.8) |
| Ratio of total cholesterol to HDL-C | 3.96 (3.23–4.92) | 4.15 (3.36–5.16) | 3.72 (3.08–4.55) |
Analysis cohort includes total cohort, excluding 1,093 transdermal users and 55 women for whom hormone therapy status is missing.
Cardiovascular endpoints include myocardial infarction, stroke, coronary revascularization, and cardiovascular death.
Baseline distributions of CRP differ significantly between HT users and non-users (P<0.001) as demonstrated in Table 2. Baseline CRP was ≥3 mg/L in 29.3% of HT non-users and 49.4% of HT users. The cut-point for the highest CRP quintile was >4.18 mg/L for HT non-users compared with >6.44 mg/L for HT users.
Table 2.
Comparison* of C-reactive protein frequency distributions between 10,953 hormone therapy (HT) users and 15,838 HT non-users at the time of the baseline blood collection.
| Hormone Category |
Number of Women |
Mean (SD) | C-reactive protein (mg/L) | |||||
|---|---|---|---|---|---|---|---|---|
| Percentile | ||||||||
| 5th | 10th | 25th | 50th | 75th | 90th | |||
| HT Non- users† |
15,838 | 3.01 (5.22) | 0.19 | 0.29 | 0.61 | 1.51 | 3.47 | 6.59 |
| HT Users | 10,953 | 4.67 (6.25) | 0.39 | 0.60 | 1.38 | 2.95 | 5.61 | 9.14 |
P <0.001 for the comparison of C-reactive protein levels between hormone categories.
HT non-users include never users of HT and past users of HT.
When analyzed as a continuous logarithm-transformed variable, the multivariable relative risk for lnCRP was 1.27 (95% CI, 1.13 to 1.44) for HT non-users and 1.22 (95% CI, 1.07 to 1.40) for HT users, which demonstrates that CVE risk increases with lnCRP in a similar fashion for both HT users and non-users within their respective CRP distributions. A formal interaction term showed no difference in the two slopes (P=0.62).
The crude, age-adjusted, and risk factor-adjusted relative risks of a first CVE, stratified by HT use and according to increasing quintiles of baseline CRP and to the AHA/CDC risk cut-points for CRP, are presented in Tables 3 and 4, respectively. After adjustment for risk factors including age, postmenopausal status, body mass index, family history of myocardial infarction, hypertension, diabetes, smoking, hyperlipidemia treatment, total/ HDL cholesterol ratio, and randomized treatment assignment, significant linear trends for future CVE were observed across quintiles for both HT non-users and users, regardless of whether the CRP quintiles were based on HT users or non-users (Ptrend<0.02 for all quintiles) (Table 3). Using quintiles based on HT non-users, the highest quintile (>4.18 mg/L) significantly predicted CVE in HT non-users [RR: 2.85 (95% CI, 1.62 to 5.00)], but not in HT users [RR: 1.34 (95% CI, 0.69 to 2.62)], in the multivariable models. By contrast, HT users in the highest HT user-based quintile (>6.44 mg/L) had significantly increased CVE risk [RR: 1.88 (95% CI, 1.14 to 3.11)].
Table 3.
Crude, age-adjusted, and risk factor-adjusted relative risk of first cardiovascular event according to the quintile of C-reactive protein at baseline among 10,953 users and 15,838 non-users of hormone therapy at baseline, censored at time of change in hormone status.
| Relative Risk Models | ||||||
|---|---|---|---|---|---|---|
| Hormone Non-users | ||||||
| (N CVE/ N total) | ||||||
| Quintile of C-reactive protein based on hormone non-users | ||||||
| ≤0.49 mg/L |
>0.49–1.08 mg/L |
1.08–2.08 mg/L |
>2.08–4.18 mg/L |
>4.18 mg/L |
P value* for trend |
|
| (18/3208) | (47/3137) | (62/3166) | (91/3165) | (148/3162) | ||
| Age-adjusted RR (95% CI) | 1.0 | 2.22 (1.29, 3.83) | 2.53 (1.50, 4.29) | 3.53 (2.13, 5.86) | 6.04 (3.70, 9.86) | <0.001 |
| Risk factor-adjusted RR† (95% CI) | 1.0 | 1.70 (0.94, 3.08) | 1.72 (0.97, 3.06) | 2.19 (1.25, 3.82) | 2.85‡ (1.62, 5.00) | <0.001 |
| Hormone Users | ||||||
| (N CVE/N total) | ||||||
| Quintile of C-reactive protein based on hormone non-users | ||||||
| ≤0.49 mg/L |
>0.49–1.08 mg/L |
>1.08–2.08 mg/L |
>2.08–4.18 mg/L |
>4.18 mg/L |
P value* for trend |
|
| (12/812) | (15/1326) | (37/1963) | (74/2872) | (140/3980) | ||
| Age-adjusted RR (95% CI) | 1.0 | 0.68 (0.32, 1.46) | 1.07 (0.56, 2.06) | 1.38 (0.75, 2.54) | 1.87 (1.03, 3.37) | <0.001 |
| Risk factor-adjusted RR† (95% CI) | 1.0 | 0.67 (0.30, 1.54) | 0.98 (0.48, 2.01) | 1.11 (0.56, 2.19) | 1.34‡ (0.69, 2.62) | 0.02 |
| Hormone Users | ||||||
| (N CVE/N total) | ||||||
| Quintile of C-reactive protein based on hormone users | ||||||
| ≤1.11 mg/L |
>1.11–2.24 mg/L |
>2.24–3.81 mg/L |
>3.81–6.44 mg/L |
>6.44 mg/L |
P value* for trend |
|
| (27/2194) | (46/2193) | (56/2194) | (65/2187) | (84/2185) | ||
| Age-adjusted RR (95% CI) | 1.0 | 1.54 (0.95, 2.47) | 1.77 (1.12, 2.81) | 2.00 (1.28, 3.14) | 2.64 (1.71, 4.08) | <0.001 |
| Risk factor-adjusted RR† (95% CI) | 1.0 | 1.44 (0.86, 2.41) | 1.43 (0.86, 2.38) | 1.52 (0.91, 2.52) | 1.88‡ (1.14, 3.11) | 0.02 |
All models have been adjusted for treatment assignment (vitamin E, beta-carotene, aspirin).
Adjusted for age, postmenopausal status, body mass index, family history of myocardial infarction, hypertension, diabetes, smoking, hyperlipidemia treatment, and total l/HDL cholesterol ratio.
Table 4.
Crude, age-adjusted, and risk factor-adjusted relative risk of first cardiovascular event according to the AHA/CDC categories of C-reactive protein at baseline among 10,953 users and 15,838 non-users of hormone therapy at baseline, censored at time of change in hormone status.
| Relative Risk Models | ||||
|---|---|---|---|---|
| AHA/CDC Cut-points for C-reactive protein | ||||
| Hormone Non-users | ||||
| (N CVE/N total) | ||||
| <1.00 mg/L (60/5,971) |
≥1.00 – <3.00 mg/L (112/5,221) |
≥3.00 mg/L (194/4,646) |
P value* for trend |
|
| Age-adjusted RR* (95% CI) | 1.0 | 1.67 (1.22, 2.29) | 3.27 (2.44, 4.37) | <0.001 |
| Risk factor-adjusted RR*† (95% CI) | 1.0 | 1.27 (0.89, 1.83) | 1.96‡ (1.36, 2.82) | <0.001 |
| Hormone Users | ||||
| (N CVE/N total) | ||||
| <1.00 mg/L (26/1,928) |
≥1.00 – <3.00 mg/L (77/3,611) |
≥3.00 mg/L (175/5,414) |
P value* for trend |
|
| Age-adjusted RR (95% CI) | 1.0 | 1.42 (0.91, 2.22) | 1.99 (1.31, 3.00) | <0.001 |
| Risk factor-adjusted RR† (95% CI) | 1.0 | 1.25 (0.77, 2.03) | 1.42‡ (0.89, 2.28) | 0.12 |
All models have been adjusted for treatment assignment (vitamin E, beta-carotene, aspirin).
Adjusted for age, postmenopausal status, body mass index, family history of myocardial infarction, hypertension, diabetes, smoking, hyperlipidemia treatment, and total l/HDL cholesterol ratio.
P value is <0.001 for all model likelihood ratios. CI denotes confidence interval.
Table 4 demonstrates that a linear risk gradient was observed across the AHA/CDC clinical cut-points for CRP in HT non-users (Ptrend<0.001), but did not reach significance in HT users (Ptrend=0.12). After risk factor adjustment, the RR of a first CVE in the AHA/CDC category for high (≥3 mg/L) risk was 1.96 (95% CI, 1.36 to 2.82) for HT non-users, and 1.42 (95% CI, 0.89 to 2.28) for HT users. However, the formal interaction term [CRP (<1, 1–<3, ≥3 mg/L)*HT use] did not reach statistical significance (Pinteraction=0.16).
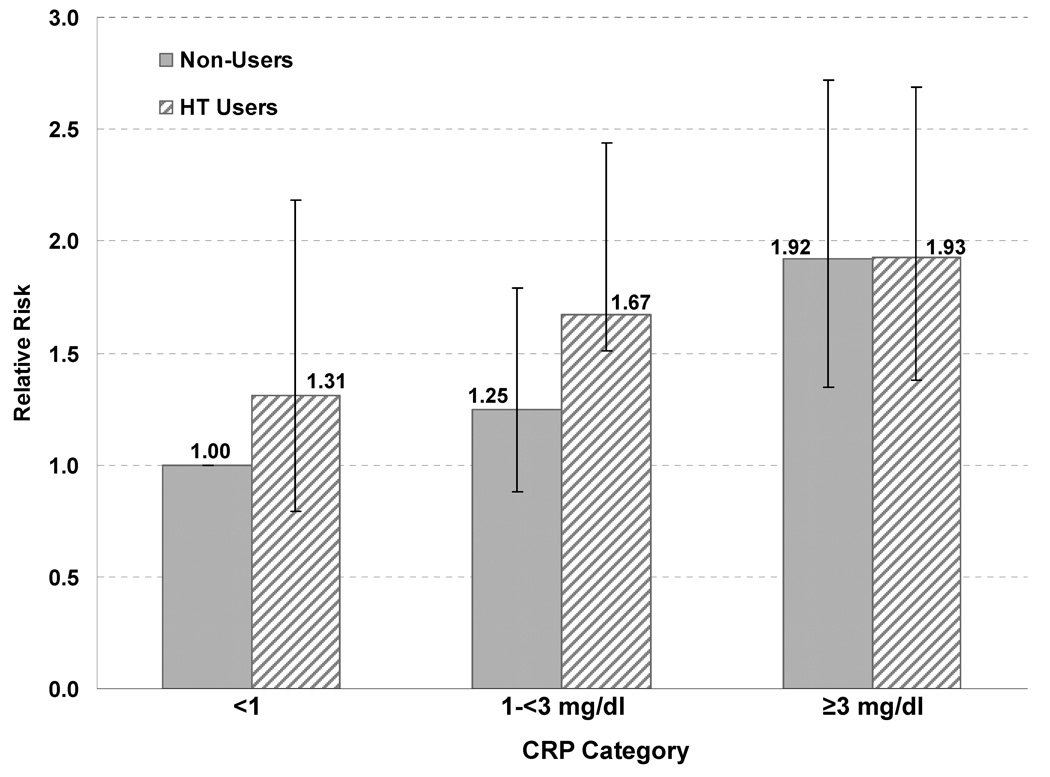
In order to compare the risk associated with AHA/CDC CRP categories across HT use groups, we fit a model in which non-users with CRP <1 mg/L were the reference group (Figure 1.). After adjusting for other risk factors including lipids, HT users with CRP ≥3 had a RR of 1.93 (1.38–2.69) while non-users had a RR of 1.92 (1.35–2.72).
Figure 1.
Relative risks* for cardiovascular events for AHA/CDC CRP categories in 10,953 HT users and 15,838 non-users, compared to a common referent group (non-users with CRP <1 mg/dl).
* Adjusted for age, postmenopausal status, body mass index, family history of myocardial infarction, hypertension, diabetes, smoking, hyperlipidemia treatment, total/HDL cholesterol ratio, and randomized treatment assignment.
Follow-up censored at change in HT use status.
Discussion
In this analysis of CRP among oral HT users and non-users derived from an observational cohort of apparently healthy women, we found two key findings. First, we observed a linear relationship between CRP and future CVE for both HT users and HT non-users in analyses using percentile cut-points defined separately for each group. There was generally a similar gradient of risk across AHA/CDC categories for non-users and HT users, although the trend was not significant when examined in HT users as a group. However, after adjusting for lipids and other risk factor differences, HT users with CRP >3 mg/L had a nearly 2-fold increased risk in CVE, when compared to non-users with low CRP (<1 mg/L). Taken together, this suggests that the current AHA/CDC cutpoints capture risk even for HT users.
Oral HT substantially raises CRP levels. For example, randomized treatment with oral conjugated equine estrogens (0.625 mg standard dose) led to a nearly two-fold median change from baseline in CRP levels,13 similarly to what we observed in our population of predominantly CEE users (1.51 nonusers versus 2.95mg/L in users). The current analyses confirm that CRP predicts cardiovascular risk in both HT users and nonusers. Prior analyses of participants in the Women’s Health Initiative Observational Study, Pradhan et al.,15 suggested that increasing concentrations of CRP were linearly associated with increasing odds of CVE across the full range of plasma values for both current HT users and non-users; however, the odds ratios were non-significant for HT users, in whom outcomes were limited to 124 cardiovascular events.15 Similarly, prior analyses from the WHS have shown that among HT non-users, there was a significant trend for risk of CVE across increasing quintiles of CRP (Ptrend<0.001), whereas for HT users, the relationship appeared linear but had lower risk estimates with a non-significant trend (Ptrend=0.08) when quintiles were based on HT non-users.2, 16 The current study design differed from the prior evaluation of the WHS population2 in that we censored women when they changed HT status and adjusted for measured lipids. This study provides additional evidence that a linear relationship of CRP to cardiovascular disease exists in both HT users and non-users.
The major limitations of this study include restriction to a single baseline measurement of CRP and lipids. In addition, HT use was self-selected, rather than randomized; thus, any comparison of HT users and non-users should be interpreted with caution. In addition, we had limited power to be able to examine either CHD or stroke separately. However, CRP has strongly predicted CHD and stroke in this cohort previously. We have, however, provided a large and detailed evaluation of HT with respect to CRP and cardiovascular outcomes and have strengthened this evaluation by censoring women upon change in HT status to ensure accurate HT use classification.
Conclusion
In conclusion, we found a linear gradient of cardiovascular risk with increasing CRP for both HT users and non-users. Clinically, our findings suggest that women with a CRP level of 3 mg/L or higher appear to be at higher cardiovascular risk even if they are HT users.
Acknowledgments
We thank all of the staff of the Women’s Health Study for their crucial contributions and we thank the 39,876 dedicated and committed participants of the Women’s Health Study.
Sources of Funding: The research for this article was supported by grants from the Donald W. Reynolds Foundation (Las Vegas, NV) and the Doris Duke Charitable Foundation (New York, NY). Dr. Kurtz is supported by NIH grants 2 T32 HL007411-26A1 and 9 K30 RR022298-07. The Women’s Health Study is supported by grants HL-43851 and CA-47988 from the National Heart, Lung, and Blood Institute and the National Cancer Institute.
Abbreviations List
- CRP
high-sensitivity C-reactive protein
- HT
postmenopausal hormone therapy
- CVE
cardiovascular events
- AHA
American Heart Association
- CDC
Centers for Disease Control and Prevention
- WHS
Women’s Health Study
- WHI-OS
Women’s Health Initiative Observational Study (WHI-OS)
- MI
myocardial infarction
- CABG
coronary artery bypass grafting
- CVA
cerebrovascular accident (ischemic stroke)
- PTCA
percutaneous transluminal coronary angioplasty
- CVD
cardiovascular death
- EDTA
ethylenediaminetetraacetic acid
- HDL-C
high-density lipoprotein cholesterol
- LDL-C
low-density lipoprotein cholesterol
- lnCRP
logarithm-transformed CRP
- CI
confidence intervals
- CEE
conjugated equine estrogen
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Clinical Trial Registration: NCT00000479 (http://clinicaltrials.gov/ct/show/NCT00000479)
Conflict of Interest: Dr. Ridker is listed as a co-inventor on patents held by the Brigham and Women’s Hospital that relate to the use of inflammatory biomarkers in cardiovascular disease. No other authors reported relevant financial disclosures, other than the institutional affiliation of all authors with Brigham and Women’s Hospital, which holds patents that relate to the use of inflammatory biomarkers in cardiovascular disease.
References
- 1.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997 Apr 3;336(14):973–979. doi: 10.1056/NEJM199704033361401. [DOI] [PubMed] [Google Scholar]
- 2.Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002 Nov 14;347(20):1557–1565. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 3.Koenig W, Lowel H, Baumert J, Meisinger C. C-reactive protein modulates risk prediction based on the Framingham Score: implications for future risk assessment: results from a large cohort study in southern Germany. Circulation. 2004 Mar 23;109(11):1349–1353. doi: 10.1161/01.CIR.0000120707.98922.E3. [DOI] [PubMed] [Google Scholar]
- 4.Pai JK, Pischon T, Ma J, et al. Inflammatory markers and the risk of coronary heart disease in men and women. N Engl J Med. 2004 Dec 16;351(25):2599–2610. doi: 10.1056/NEJMoa040967. [DOI] [PubMed] [Google Scholar]
- 5.Laaksonen DE, Niskanen L, Nyyssonen K, Punnonen K, Tuomainen TP, Salonen JT. C-reactive protein in the prediction of cardiovascular and overall mortality in middle-aged men: a population-based cohort study. Eur Heart J. 2005 Sep;26(17):1783–1789. doi: 10.1093/eurheartj/ehi237. [DOI] [PubMed] [Google Scholar]
- 6.Cushman M, Arnold AM, Psaty BM, et al. C-reactive protein and the 10-year incidence of coronary heart disease in older men and women: the cardiovascular health study. Circulation. 2005 Jul 5;112(1):25–31. doi: 10.1161/CIRCULATIONAHA.104.504159. [DOI] [PubMed] [Google Scholar]
- 7.Boekholdt SM, Hack CE, Sandhu MS, et al. C-reactive protein levels and coronary artery disease incidence and mortality in apparently healthy men and women: The EPIC-Norfolk prospective population study 1993–2003. Atherosclerosis. 2006 Aug;187(2):415–422. doi: 10.1016/j.atherosclerosis.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 8.Cook NR, Buring JE, Ridker PM. The effect of including C-reactive protein in cardiovascular risk prediction models for women. Ann Intern Med. 2006 Jul 4;145(1):21–29. doi: 10.7326/0003-4819-145-1-200607040-00128. [DOI] [PubMed] [Google Scholar]
- 9.Tzoulaki I, Murray GD, Lee AJ, Rumley A, Lowe GD, Fowkes FG. Relative value of inflammatory, hemostatic, and rheological factors for incident myocardial infarction and stroke: the Edinburgh Artery Study. Circulation. 2007 Apr 24;115(16):2119–2127. doi: 10.1161/CIRCULATIONAHA.106.635029. [DOI] [PubMed] [Google Scholar]
- 10.Buckley DI, Fu R, Freeman M, Rogers K, Helfand M. C-reactive protein as a risk factor for coronary heart disease: a systematic review and meta-analyses for the U.S. Preventive Services Task Force. Ann Intern Med. 2009 Oct 6;151(7):483–495. doi: 10.7326/0003-4819-151-7-200910060-00009. [DOI] [PubMed] [Google Scholar]
- 11.Cushman M, Legault C, Barrett-Connor E, et al. Effect of postmenopausal hormones on inflammation-sensitive proteins: the Postmenopausal Estrogen/Progestin Interventions (PEPI) Study. Circulation. 1999 Aug 17;100(7):717–722. doi: 10.1161/01.cir.100.7.717. [DOI] [PubMed] [Google Scholar]
- 12.Ridker PM, Hennekens CH, Rifai N, Buring JE, Manson JE. Hormone replacement therapy and increased plasma concentration of C-reactive protein. Circulation. 1999 Aug 17;100(7):713–716. doi: 10.1161/01.cir.100.7.713. [DOI] [PubMed] [Google Scholar]
- 13.Shifren JL, Rifai N, Desindes S, McIlwain M, Doros G, Mazer NA. A comparison of the short-term effects of oral conjugated equine estrogens versus transdermal estradiol on C-reactive protein, other serum markers of inflammation, and other hepatic proteins in naturally menopausal women. J Clin Endocrinol Metab. 2008 May;93(5):1702–1710. doi: 10.1210/jc.2007-2193. [DOI] [PubMed] [Google Scholar]
- 14.Pearson TA, Mensah GA, Hong Y, Smith SC., Jr CDC/AHA Workshop on Markers of Inflammation and Cardiovascular Disease: Application to Clinical and Public Health Practice: overview. Circulation. 2004 Dec 21;110(25):e543–e544. doi: 10.1161/01.CIR.0000148979.11121.6B. [DOI] [PubMed] [Google Scholar]
- 15.Pradhan AD, Manson JE, Rossouw JE, et al. Inflammatory biomarkers, hormone replacement therapy, and incident coronary heart disease: prospective analysis from the Women's Health Initiative observational study. JAMA. 2002 Aug 28;288(8):980–987. doi: 10.1001/jama.288.8.980. [DOI] [PubMed] [Google Scholar]
- 16.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000 Mar 23;342(12):836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 17.Buring J, Hennekens C. The Women's Health Study: summary of the study design. J Myocardial Ischemia. 1992;4:27–29. [Google Scholar]
- 18.Rexrode KM, Lee IM, Cook NR, Hennekens CH, Buring JE. Baseline characteristics of participants in the Women's Health Study. J Womens Health Gend Based Med. 2000 Jan–Feb;9(1):19–27. doi: 10.1089/152460900318911. [DOI] [PubMed] [Google Scholar]
- 19.Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005 Mar 31;352(13):1293–1304. doi: 10.1056/NEJMoa050613. [DOI] [PubMed] [Google Scholar]
- 20.Lee IM, Cook NR, Gaziano JM, et al. Vitamin E in the primary prevention of cardiovascular disease and cancer: the Women's Health Study: a randomized controlled trial. JAMA. 2005 Jul 6;294(1):56–65. doi: 10.1001/jama.294.1.56. [DOI] [PubMed] [Google Scholar]
- 21.Cook NR, Lee IM, Gaziano JM, et al. Low-dose aspirin in the primary prevention of cancer: the Women's Health Study: a randomized controlled trial. JAMA. 2005 Jul 6;294(1):47–55. doi: 10.1001/jama.294.1.47. [DOI] [PubMed] [Google Scholar]
- 22.Grundy SM. The changing face of cardiovascular risk. J Am Coll Cardiol. 2005 Jul 5;46(1):173–175. doi: 10.1016/j.jacc.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 23.Atiya M, Kurth T, Berger K, Buring JE, Kase CS. Interobserver agreement in the classification of stroke in the Women's Health Study. Stroke. 2003 Feb;34(2):565–567. doi: 10.1161/01.str.0000054159.21017.7c. [DOI] [PubMed] [Google Scholar]
- 24.Ridker PM, Rifai N, Cook NR, Bradwin G, Buring JE. Non-HDL cholesterol, apolipoproteins A-I and B100, standard lipid measures, lipid ratios, and CRP as risk factors for cardiovascular disease in women. JAMA. 2005 Jul 20;294(3):326–333. doi: 10.1001/jama.294.3.326. [DOI] [PubMed] [Google Scholar]
- 25.Modena MG, Bursi F, Fantini G, et al. Effects of hormone replacement therapy on C-reactive protein levels in healthy postmenopausal women: comparison between oral and transdermal administration of estrogen. Am J Med. 2002 Sep;113(4):331–334. doi: 10.1016/s0002-9343(02)01209-3. [DOI] [PubMed] [Google Scholar]
- 26.Modena MG, Sismondi P, Mueck AO, et al. New evidence regarding hormone replacement therapies is urgently required: Transdermal postmenopausal hormone therapy differs from oral hormone therapy in risks and benefits. Maturitas. 2005 Sep 16;52(1):1–10. doi: 10.1016/j.maturitas.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 27.Vehkavaara S, Silveira A, Hakala-Ala-Pietila T, et al. Effects of oral and transdermal estrogen replacement therapy on markers of coagulation, fibrinolysis, inflammation and serum lipids and lipoproteins in postmenopausal women. Thromb Haemost. 2001 Apr;85(4):619–625. [PubMed] [Google Scholar]
- 28.Decensi A, Omodei U, Robertson C, et al. Effect of transdermal estradiol and oral conjugated estrogen on C-reactive protein in retinoid-placebo trial in healthy women. Circulation. 2002 Sep 3;106(10):1224–1228. doi: 10.1161/01.cir.0000028463.74880.ea. [DOI] [PubMed] [Google Scholar]
- 29.Hainline A, Karon J, Lippel K. Manual of laboratory operations, lipid research clinics program, and lipid and lipoprotein analysis. 2nd ed. Bethesda, Md: Department of Health and Human Services; 1982. [Google Scholar]