Abstract
Hydrophobic UV-activatable compounds have been shown to partition into the hydrophobic region of biological membranes to selectively label transmembrane proteins, and to inactivate enveloped viruses. Here, we analyze various UV-activatable azido- and iodo- based hydrophobic compounds for their ability to inactivate a model enveloped virus, human immunodeficiency virus (HIV-1 MN). Treatment of HIV-1 with 1,5-diazidonapthalene (DAN), 1-iodo, 5-azidonaphthalene (INA), 1-azidonaphthalene (AzNAP) or 4,4’-diazidobiphenyl (DABIPH) followed by UVA irradiation for 2 minutes, resulted in complete viral inactivation, whereas treatment using analogous non-azido containing controls had no effect. Incorporation of an azido moiety within these hydrophobic compounds to promote photoinduced covalent reactions with proteins was found to be the primary mechanism of viral inactivation for this class of compounds. Prolonged UVA irradiation of the virus in the presence of these azido compounds resulted in further modifications of viral proteins, due to the generation of reactive oxygen species, leading to aggregation as visualized via western blot analysis, providing additional viral modifications that may inhibit viral infectivity. Furthermore, inactivation using these compounds resulted in the preservation of surface antigenic structures (recognized by neutralizing antibodies b12, 2g12 and 4e10), which is favorable for the creation of vaccines from these inactivated virus preparations.
INTRODUCTION
There are a variety of strategies available for the preparation of vaccines against a large number of viruses, such as virus-like particles (VLP), live-attenuated virus, sub-unit, inactivated virus, and split virus vaccines. In particular, inactivated viruses have been used successfully and are currently licensed in the USA in vaccines against influenza, hepatitis A and poliovirus. Inactivated virus vaccines are derived from infectious material and therefore contain viruses as close to their native configuration as possible, while still being non-infectious. These inactivated virus vaccines can, therefore, potentially elicit an immune response comparable to that of the live virus. With the appearance of new pandemic viruses, such as SARS and H1N1 influenza, the need for rapid, efficient and safe methods of inactivation for the preparation of vaccines became essential. The ideal inactivated virus vaccine should be free of residual infectious material, while still maintaining the necessary antigens and epitopes from the virion structure to produce an effective immune response. The ideal method for this inactivation should not only be rapid, efficient and reproducible, but should also be broadly applicable to a wide variety of viruses.
The most common approach for the preparation of inactivated virus vaccines is to use chemical inactivation methods such as formaldehyde (formalin), glutaraldehyde, and beta-propiolactone treatment. Some of these methods were shown to damage immunogenic epitopes, which could adversely effect the efficacy of vaccines prepared using these methods (1–7). There is also concern over the toxicity of residual chemical inactivators such as glutaraldehyde, formaldehyde and beta-propiolactone because these are reactive until either allowed enough time to fully react, removed from the preparation, or diluted to permissible levels. Photoactivatable compounds, used for viral inactivation, have an advantage from this perspective since their chemical reactivity can be controlled by light. Psoralens, a group of UV activatable compounds that selectively bind and crosslink DNA were used for inactivation of viruses with preservation of viral surface epitopes.(8) However, there were some concerns that repair and recombination of DNA could lead to the resurrection of infectious virus (“multiplicity reactivation”).(9)
Hydrophobic membrane probes containing a UV-activatable labeling group (such as an aryl azide or aryl diazirine) partition into the hydrophobic regions of biological membranes and have been used to selectively label the hydrophobic domains of transmembrane proteins.(10, 11) The UV-activatable groups produce either a nitrene or a carbene (respectively) upon irradiation with UV light. When these compounds are based on azidonaphthalene, they can be photoactivated to generate the nitrene at wavelengths above 300 nm thus preventing UV-irradiation induced protein or nucleic acid damage.(12) For example, 1-Iodo, 5-azidonaphthalene (INA)(12) proved particularly useful for the study of membrane structure and dynamics of enveloped viruses.(13–15) We have recently shown that INA effectively inactivated enveloped viruses when photoactivated by UV light. We have demonstrated for a variety of enveloped viruses that, by this approach, the inactivation is complete with preservation of viral antigenicity.(16–19) This breadth of inactivation for a wide-variety of enveloped viruses, makes this class of photoactivatable hydrophobic alkylating compounds ideal candidates for use in chemical inactivation for whole virus vaccine preparations.
Herein, a study of various hydrophobic compounds with azido-functionality was carried out and analyzed for their ability to inactivate HIV-1 (as a model enveloped virus) while maintaining the integrity of hydrophilic surface antigens. The mechanism of inactivation was studied, with particular attention to the formation of reactive oxygen species with prolonged UV irradiation times.
MATERIALS AND METHODS
Safety
All handling of infectious HIV-1 isolates were done under Biosafety Level 2 conditions, following Biosafety level 3 procedures, with the proper personal protective equipment. Synthesis and handling of the azido compounds was performed using the proper precautions due to the potentially explosive nature of these compounds (including handling in a chemical fume hood, no metal utensils, minimal use of chlorinated solvents, small scale <1 g).
Reagents and Cells
The chemical precursors to the azido compounds, 1-aminonaphthalene (98%, Aldrich), 1,5-diaminonaphthalene (97%, Acros), 4,4’-diaminoazobenzene (95%, Acros), and 4,4’-diaminobiphenyl (benzidine, 95%, Sigma), were purchased from Sigma-Aldrich. 1-iodonaphthalene (INAP, 97%, Aldrich), DMSO (≥99.7%, Sigma, Hybri-Max ), sodium azide (SigmaUltra), sodium nitrite (ACS grade, Fisher), were used without further purification. 1-iodo,5-azidonaphthalene (INA) was custom-synthesized by Biotium (Hayward, CA). Glutaraldehyde (Tousimis, Rockville, MD), Sodium cacodylate, Osmium, and Embed-812 epoxy resin (Electron Microscope Sciences, Fort Washington, PA) were used in the TEM study. HIV-1 MN cl. 4 concentrated virus stock (lots #P3592 and #P3602) were prepared by the AIDS Vaccine Program, SAIC.(20) The following reagents were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: TZM-bl indicator cell line from J.C. Kappes; anti-HIV-1 broadly neutralizing antibodies 2G12 and 4E10 were from Herman Katinger; B12 antibody was obtained from Dennis Burton and Carlos Barbas; anti-gp41 monoclonal antibody was purified from the hybridoma Chessie 8 from Dr. George Lewis; anti-gp120 (ID6) monoclonal antibody used for Western blots was obtained from Dr. Kenneth Ugen and Dr. David Weiner; anti-p24 (183-H12-5C) monoclonal antibody from Dr. Bruce Chesebro and Kathy Wehrly. Secondary antibody used for Western was Alexafluor680 goat anti-mouse IgG (Invitrogen). Catalase and glucose oxidase were purchased from Sigma-Aldrich.
Synthesis of compounds
General procedure: The synthesis of the diazido compounds from their diamine precursors, was adapted from previously published procedures.(21, 22) In brief, aqueous sodium nitrite (5 mmol) was added to a cooled solution of the amino precursor (2.4 mmol, for a 1.1: 1 ratio of nitrite to amino group) in dilute hydrochloric acid to create the diazonium salt. After stirring for two hours, an aqueous solution of sodium azide (5 mmol) was added dropwise, while cooling in an ice bath, to the diazonium salt. The reaction was stirred for another 15 minutes and the precipitate was recovered by filtration.
1-Azidonaphthalene (AzNAP) was synthesized according to the general procedure above, with the exception of using half the amount of sodium nitrate and sodium azide, and reaction was allowed to warm to room temperature overnight after the addition of sodium azide. Extracted with ethyl acetate, dried, and purified by column chromatography in hexanes. Yellow oil, 1H NMR (CDCl3, 300 MHz): 7.21–7.26 ppm (m, 1H), 7.40–7.53 ppm (m, 3H), 7.58–7.63 ppm (d, 1H), 7.76–7.83 ppm (m, 1H), 8.04–8.12 ppm (m, 1H).
1,5-diazidonaphthalene (DAN) was synthesized according to the general procedure above. The crude solid was dissolved in methylene chloride, decolored by the addition of activated carbon and recrystallized from methylene chloride, resulting in peach-colored needles. 1H NMR (CDCl3, 300 MHz): 7.27–7.32 ppm (d, 1H), 7.45–7.52 ppm (t, 1H), 7.88–7.92 ppm (d, 1H).
4,4’-diazidobiphenyl (DABIPH) was synthesized according to the general procedure above, with the exception that the reaction solution was allowed to warm to room temperature overnight after the addition of sodium azide. The product was extracted using ethyl acetate, dried and purified by column chromatography using hexanes/dichloromethane. Yellow solid. 1H NMR (CDCl3, 300 MHz): 7.07–7.12 ppm (m, 4H), 7.52–7.57 ppm (m, 4H).
1,5-Diiodonaphthalene (DINAP) was synthesized according to published procedures(23), extracted using dichloromethane, dried, and purified by column chromatography with hexanes/dichloromethane. Off-white solid. 1H NMR (CDCl3, 300 MHz): 7.23–7.29 ppm (t, 2H), 8.11–8.17 ppm (m, 4H).
UV Spectroscopy
Analysis of the UV absorbance spectra for the compounds studied was performed using a SpectraMax M2 spectrometer (Molecular Devices), with DMSO as a reference/blank.
Treatment of Virus with hydrophobic naphthalene compounds
The indicated HIV-1 MN isolate was diluted with phosphate-buffered saline (PBS) to 0.5 mg/mL total protein. Stock solutions, 8 mM in DMSO, of each naphthalene or biphenyl compound were added to the diluted HIV-1 isolate with vortexing in a biosafety hood, for a final concentration of 100 μM, (unless otherwise noted). For samples that contained glutathione (a subset of the UV = 15 minutes treated samples, as noted), a stock solution of glutathione (1.0 M in PBS, PH = 7.5) was prepared and added to the viral suspension for a final glutathione concentration of 20 mM. The viral suspension was incubated at room temperature for 5 minutes then exposed to UV light for 2 or 15 minutes, as specified. The light source was a 100-W ozone-free mercury arc lamp placed in a lamp-house with collector lens (Olympus). Samples were irradiated at a distance of 5 cm from the light source where the dose was 10 mW/cm2, consistent with our previously published procedure.(18) A water filter (a polystyrene culture flask filled with water) was placed between the sample and the lamp to prevent sample heating. Sample volumes used for irradiation varied from 0.10 mL to 1.0 mL in clear polypropylene microfuge tubes. The UV emission from the light source was measured using a Stellarnet Spectroradiometer and was found to have the mercury emission lines where λ>335 nm (data not shown). The mercury emission lines below this wavelength were absent due to the absorption by the lamp housing. Additionally, there was negligible absorption by the water filter and polypropylene microfuge tubes at these wavelengths.
Western Blot Analysis for Protein Aggregation/Crosslinking
HIV-1 samples treated with the azido compounds plus UV irradiation, and controls were lysed in SDS sample buffer for 15–20 minutes at 55–60°C. Aliquots of these were added to a 4–20% Tris-glycine gel. Gel electrophoresis was done under reducing conditions. After electrophoresis, the proteins were transferred to a nitrocellulose membrane. The proteins were detected using various monoclonal antibodies to HIV-1 MN; anti-gp41, and anti-gp120. Secondary antibodies containing AlexaFluor680 were used for readout on an Odyssey Infrared Imaging System (LiCOR Biosciences). Blots were not reprobed; a new blot was done for each antibody probe used.
Virus Infectivity Assay by luciferase reporter
The infectivity of HIV-1 MN samples were determined using the TZM-bl cell line as previously reported.(24) Cells were added (2 × 104 cells/well) to a 96-well clear-bottomed microtiter plate with 100 uL of complete Dulbecco’s modified eagle medium (DMEM) per well. Plates were incubated to allow the cells to adhere for 18–24 hours at 37°C. The media was aspirated from each well and was replaced with 100 uL of fresh DMEM containing 40 micrograms/mL DEAE-dextran per well. To each well was added either the crosslinked or untreated HIV samples in serial dilutions, diluted with DMEM/dextran. The plate was again incubated at 37°C for 18–24 hours. Luciferase activity was analyzed using the Steady-Glo Luciferase System (Promega). Using infectious controls of the same viral lot in serial dilution, the sensitivity of this assay was determined to be at least 2 logs of inactivation.
HIV virus pellet preparation for electron microscope (EM) analysis
HIV virus pellet preparation for the EM analysis was described before.(25) Briefly, HIV virus pellet was made by an ultracentrifuge at 100,000xg for 60min with 2% glutaraldehyde (in 0.1M cacodylate buffer, pH 7.2) solution. The pellet was post fixed in 1% osmium tetroxide in the same buffer for 1hr. The pellet was dehydrated in ethanol (e.g., 35%, 50%, 75%, 95%, 100%) and 100% propylene oxide. The pellet was infiltrated overnight in 1:1 mixture of propylene oxide and epoxy resin, and embedded in a pure resin and cured for 48hrs in 55°C. The 70nm thin sections were made and mounted on naked copper grids. The thin sections were double stained (uranyl acetate and lead citrate), examined in a Hitachi (Tokyo, Japan) H-7600 transmission electron microscope, and the images were taken using an AMT (Danvers, MA) digital camera.
Virus Capture Assay of treated virus
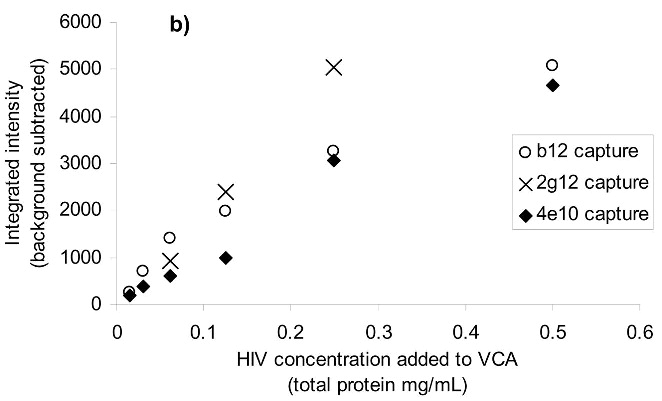
Neutralizing antibodies against gp120 (b12 and 2g12) or gp41(4e10) were plated on Immulon 4HBX 96-well plates (Thermo Scientific) according to previously published procedures.(26) In brief, 100 microliters of 10 ug/mL (b12 or 2g12) or 20 ug/mL (4e10) of each antibody in carbonate buffer was added per well. Wells to assess background binding were also prepared using only carbonate buffer with no antibody added. After incubation at 4°C overnight, the wells were washed with PBS and blocked with 3% BSA for 1 hour. Wells were again washed with RPMI. Treated virus samples were prepared (0.5 mg/mL total protein in PBS with 100 micromolar of each hydrophobic compound, plus UV irradiation for 2 minutes) and BSA was added to these samples for a final concentration of 1%. Samples were diluted by an equal volume of RPMI containing 1% BSA, and 100 uL of each was added to the specified wells. Incubation was carried out at 37°C for 1 hour, after which wells were washed with RPMI. To each well, 50 microliters of Laemmli SDS reducing sample buffer (1x) were added. Plate was sealed and incubated at 37°C for 10 minutes. Sample buffer containing the lysed sample was transferred to 200 μL PCR tubes with lids for storage at −20°C until analysis via SDS-PAGE/Western blot.
Western blot analysis of virus captured on the virus capture assay
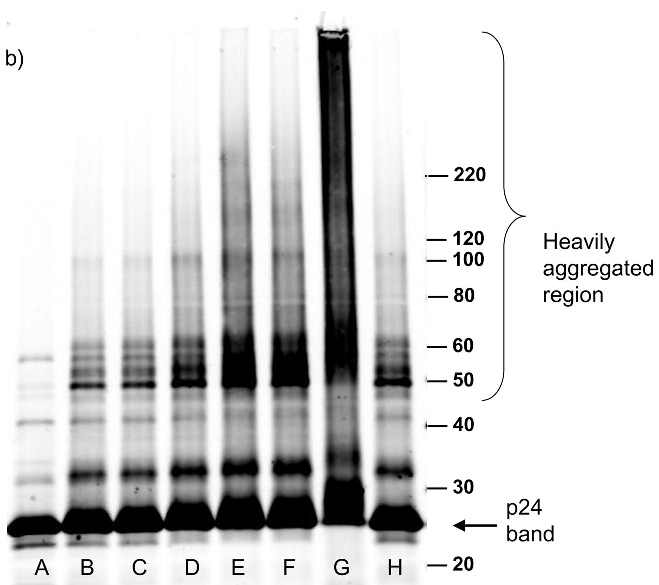
Samples that were isolated via the virus capture assay were stored at −20°C in SDS Laemmli reducing sample buffer, and heated at 60°C for 15 minutes just prior to loading on the gel. 20 microliters of each sample was added to each lane of a Novex 4–20% Tris-glycine 15-well gel (Invitrogen). Proteins were transferred to nitrocellulose and probed using MAb for p24. Secondary antibody, goat anti-mouse Alexafluor680 was used and the resulting blot was imaged using an Odyssey IR imaging system. Individual bands were integrated using the Odyssey software, to determine the overall raw integrated intensity. Background samples were run as separate lanes on the same gel that contained mock samples (that used no antibody in the virus capture assay) to account for any signal due to non-specific binding of the virus to the well plate. Also run on the gel, in the remaining lanes, were control samples of HIV-1 and INA-treated (UV= 2 minutes) HIV-1, diluted from the treated suspensions and not subjected to the virus capture assay. These were run at the same concentration on each gel to normalize between gels. For the samples that were treated at prolonged UV irradiation times (15 minutes) the entire lane of the gel was integrated in order to account for the proteins that were aggregated, but were still recognized by the antibody.
Oxygen depletion studies during viral inactivation
Samples of virus were prepared that contained glucose oxidase, catalase and glucose to remove oxygen from the system(27) prior to prolonged UV irradiation. To HIV-1 in PBS (0.5 mg/mL total protein) was added: the azido compound studied (from 8 mM stock in DMSO), glucose (1.0 M stock in PBS), Catalase (4 mg/mL in PBS), and glucose oxidase (4 mg/mL in PBS) for final concentrations of 0.45 mg/mL total viral protein, 100 micromolar azido compound, 50 millimolar glucose, and 0.2 mg/mL of each enzyme in a final volume of 200 microliters. Additional mock-treated samples were prepared where the enzyme solutions were replaced by PBS. Each mixture was allowed to sit at room temperature for 1 hour to facilitate the removal of oxygen from the solution. After 1 hour, each microfuge tube was carefully irradiated with UVA for 15 minutes. Aliquots were either frozen at −80°C for infectivity assays or placed into sample buffer for Western analysis of viral proteins for the extent of aggregation.
RESULTS
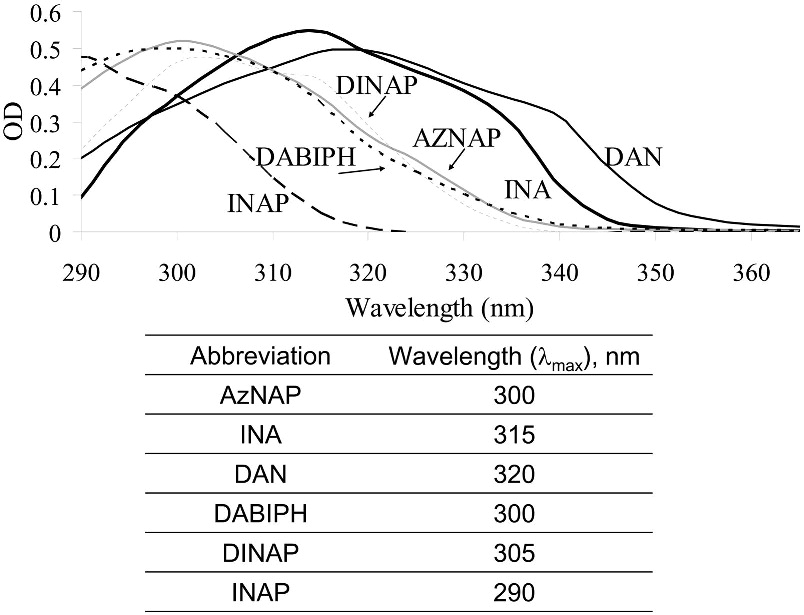
Several compounds, mostly naphthalene derivatives (see Scheme 1), were analyzed in this study for their effect on the structure and function of an enveloped virus, HIV-1, following photoactivation. Four of these compounds contained a photoactivatable azido group whereas the remainder (INAP and DINAP) were used as controls. The compounds had absorption maxima ranging between ~290 nm – 320 nm with similar molar extinction coefficients (Figure 1). The mercury light source was found to emit at wavelengths above 335 nm, therefore the activation of these naphthalenic compounds did not occur at their λmax, but rather within the higher wavelength tail in their absorption spectra. Three different parameters were used to evaluate the effect of these compounds on HIV-1: (1) Electrophoretic mobility and state of aggregation of viral proteins as revealed by SDS-PAGE and by western blot analysis of specific viral proteins of interest, (2) The infectivity of the virus as measured by a luciferase reporter gene assay, (3) The integrity of the immunogenic epitopes on the surface of the virus as measured by a virus capture assay.
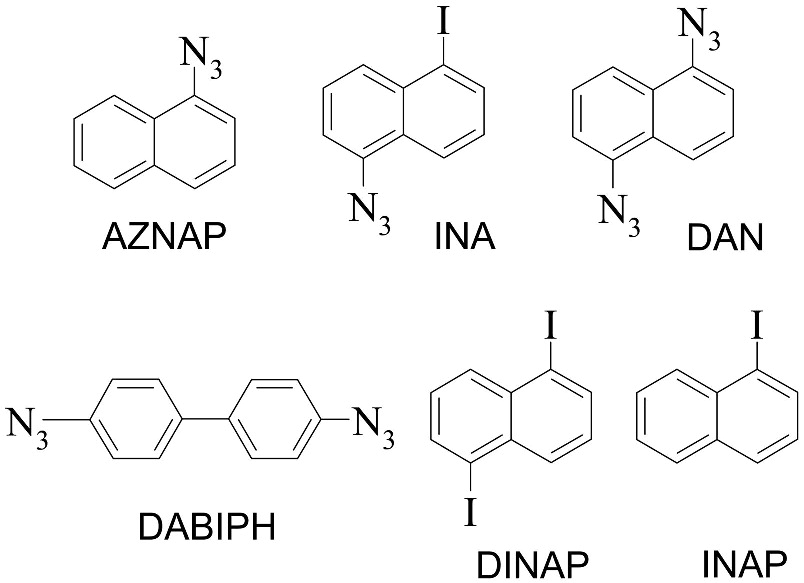
Scheme 1.
Various hydrophobic aromatic compounds studied. Azidonaphthalene (AZNAP), 1-iodo, 5-azidonaphthalene (INA), 1,5-diazidonaphthalene (DAN), 4,4’-diazidobiphenyl (DABIPH), 1,5-diiodonaphthalene (DINAP), 1-iodonaphthalene (INAP). The UV-activatable compounds contain azides, whereas DINAP and INAP are the control compounds.
Figure 1.
UV absorbance of the various synthesized compounds from Scheme 1.
Photoinduced aggregation of HIV-1 proteins by azide containing compounds
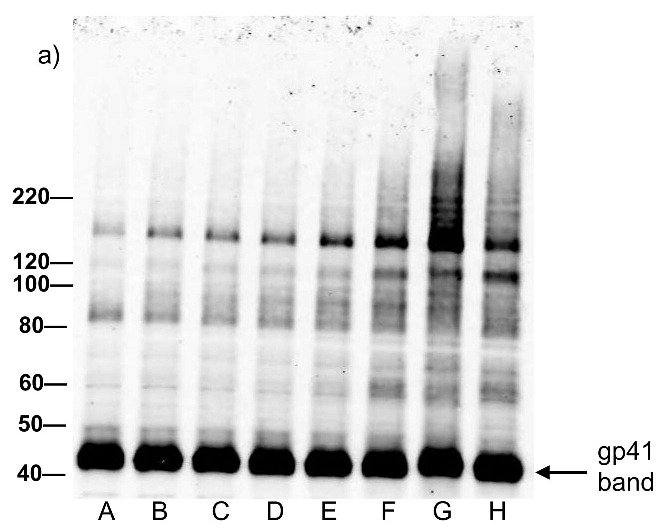
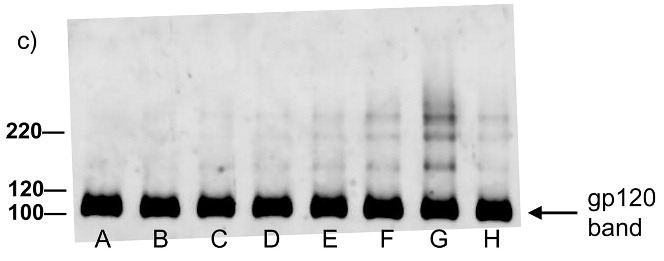
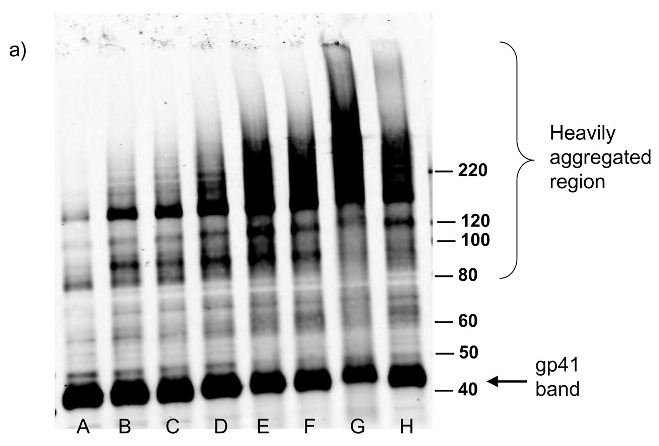
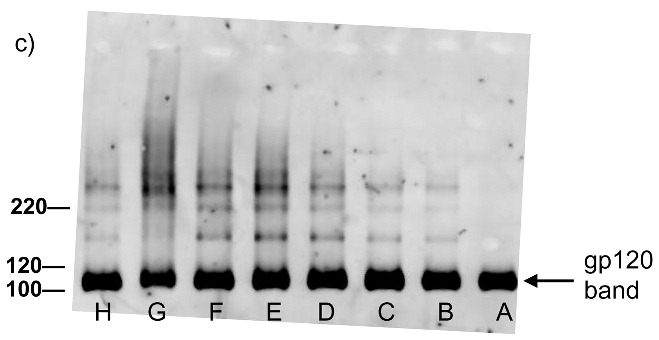
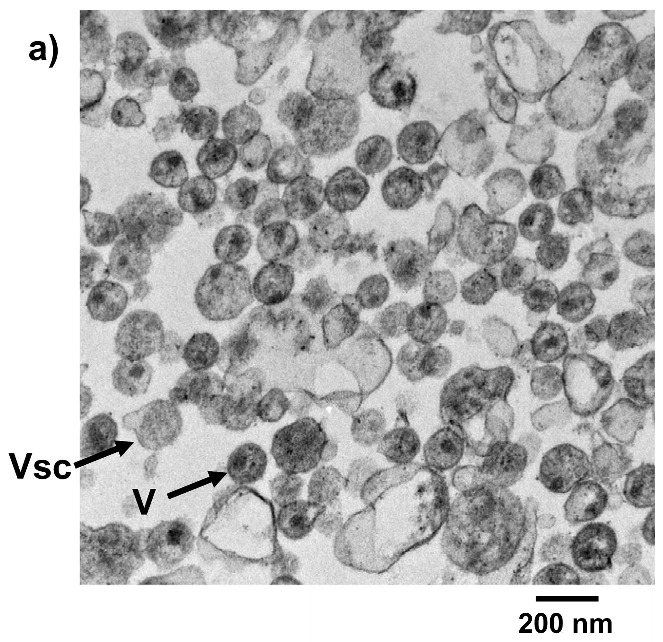
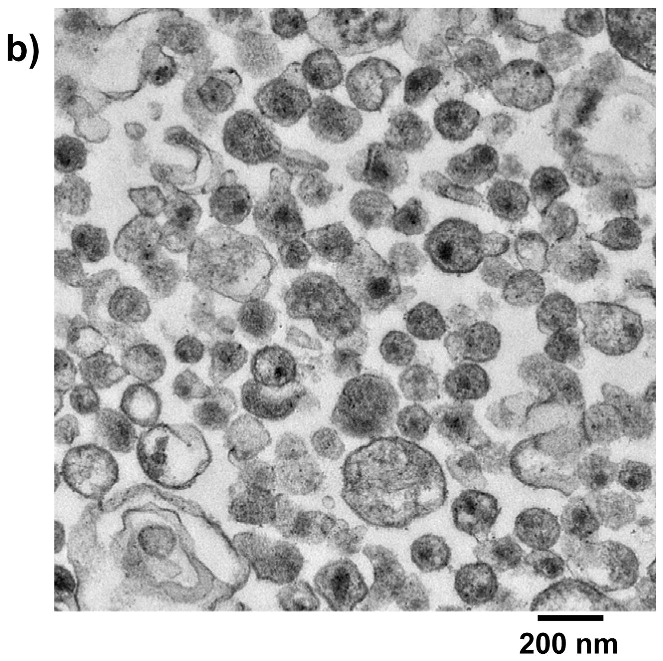
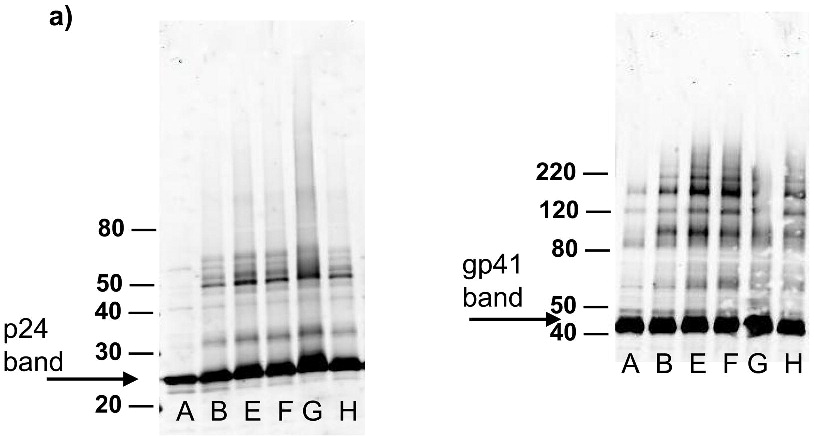
Each of the compounds was added to a purified HIV-1 suspension for a final concentration of 100 μM from a stock solution in DMSO, followed by irradiation with UV light (λ > 335 nm) for 2 minutes. Following this treatment, specific viral proteins of interest were examined by electrophoretic mobility using SDS-PAGE followed by western blot analysis (see Figure 2). The envelope glycoprotein of the HIV-1 that mediates fusion between viral and cell membranes consists of two noncovalently associated subunits, gp120 and gp41. The outer surface subunit, gp120, contains sites necessary for specific binding to target cells whereas the transmembrane gp41 is responsible for the fusion. Since we are interested in the effects of our treatments on the structure and function of surface-exposed proteins, we examined those subunits. In addition we examined effects on the capsid protein, p24. We found that the compounds had only a minimal effect on the migration or aggregation of the probed proteins when 2 minutes of UV irradiation was applied. Compounds that were expected to behave as crosslinkers, DAN and DABIPH, also did not show a pronounced increase in aggregation or oligomerization under these conditions. However, when the HIV-1 samples were irradiated for longer periods of time (15 minutes), the proteins that were treated by the azido-functionalized naphthalene derivatives exhibited a higher extent of molecular aggregation as visualized by western blot analysis for the specific proteins of interest (Figure 3). The appearance of high molecular weight oligomers was evident for HIV-1 samples treated with the azido-functionalized naphthalenes and diazidobiphenyl (AZNAP, INA, DAN, DABIPH), but was not evident in the controls (DINAP, INAP). The higher molecular weight aggregates were more pronounced in the transmembrane (gp41) and capsid (p24) proteins, but not within the external surface proteins (gp120). Transmission electron microscopy images of the treated HIV-1 virions and the untreated controls show that the viral structure was preserved in all viruses regardless of irradiation time (Figure 4). The untreated HIV-1 shows intact virions with dark cores (capsid), as well as microvesicles(28), and appears indistinguishable from the HIV-1 preparation that was treated with an azido compound followed by UV irradiation for 15 minutes (DAN was chosen as a representative sample).
Figure 2.
Western blot analysis showing minimal effect of the compounds on the electrophoretic migration of HIV-1 viral proteins after treatment with compounds plus UV for 2 minutes. Blots were probed using (a) anti-gp41, (b) anti-p24, (c) anti-gp120. Lane A is the HIV control (No UV), the remaining lanes are HIV + compound listed + UV irradiation for 2 minutes. B: DMSO, C: INAP, D: DINAP, E: AZNAP, F:DAN, G: INA, H: DABIPH.
Figure 3.
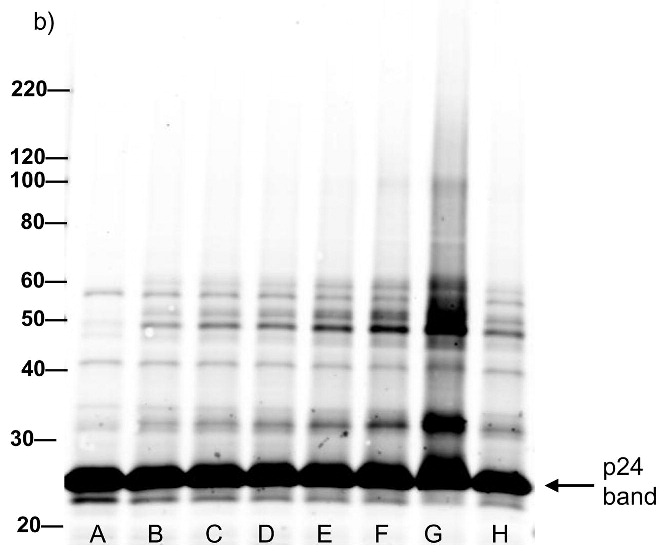
Western blot analysis showing increased effects of compounds on proteins within the virus with increased UV irradiation (15 minutes). Blots were probed using (a) anti-gp41, (b) anti-p24, (c) anti-gp120. Lane A is the HIV control (No UV), the remaining lanes are HIV + compound listed + UV irradiation for 15 minutes. B: DMSO, C: INAP, D: DINAP, E: AZNAP, F:DAN, G: INA, H: DABIPH.
Figure 4.
Treatment of HIV virus with hydrophobic compounds does not disrupt viral integrity as assessed by TEM. a) HIV-1 MN, b) HIV-1 MN treated with 100 μM DAN + UV irradiation for 15 minutes. Vsc = microvesicles, V = HIV viron.
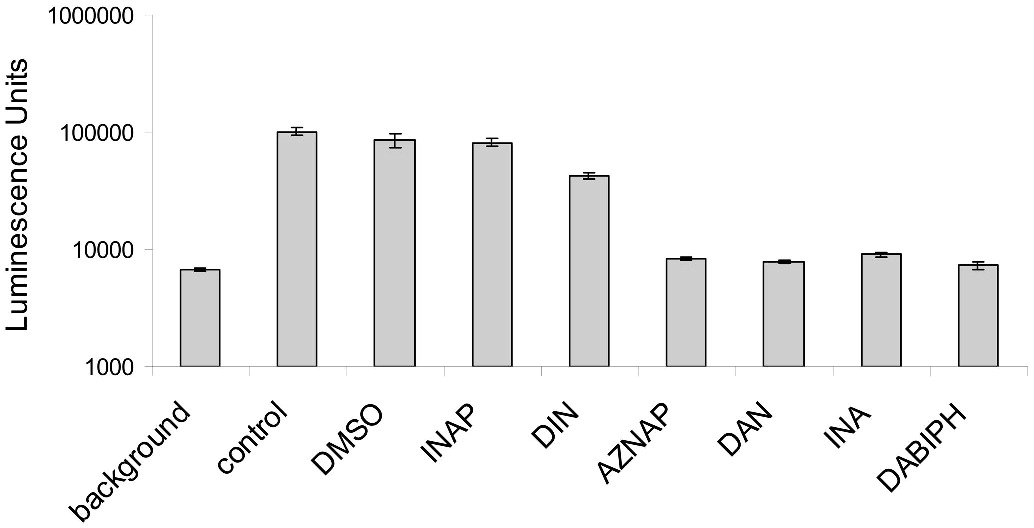
Treatment of HIV-1 with the azido-containing hydrophobic compounds plus UV irradiation results in loss of infectivity of the virus
When HIV-1 was treated with 100 μM of the azido-based compounds, the virus was inactivated after 2 minutes of UV irradiation when analyzed using a 24-hour cell-based luciferase reporter assay (see Figure 5). Similar results were obtained for concentrations down to 12.5 μM for the azido-containing compounds when tested in the same assay (data not shown). Treatment of HIV-1 with 100 μM of each of the control compounds, without azido-functionality, showed no change in the infectivity of the virus when compared to the UV irradiated control, with the exception of the diiodonaphthalene (DINAP). Although DINAP shows a partial inactivation, the residual infectivity is still significantly above the background limits.
Figure 5.
Hydrophobic azido-containing compounds and analogs induce viral inactivation when added to HIV-1 followed by UV irradiation for 2 minutes. The concentration of HIV-1 used in this assay was 3.1 × 103 ng/mL of total protein added per well. Readout using a TZM luciferase reporter assay. Data is the average of two separate experiments and is normalized to 100,000 luminescence units. The error bars represent the difference in the values obtained from two independent experiments.
Pretreatment of HIV-1 with glutathione or enzymatic depletion of oxygen eliminates photoinduced aggregation while maintaining viral inactivation
To elucidate the mechanism for the aggregation, samples were tested with and without the addition of glutathione. Glutathione is typically used as a radical scavenger and reacts with any hydrophilic reactive radical intermediates that may be produced as a result of the UV irradiation. When glutathione was added (20 mM) prior to irradiation with UV light, the previous aggregation that was seen was significantly reduced (see Figure 3 and Figure 6a). Additionally, enzymatic depletion of oxygen was studied, using a catalase/glucose/glucose oxidase system for the removal of oxygen prior to UV irradiation of virus in the presence of the azido compounds. When oxygen was removed from the system, the photoinduced aggregation of viral proteins was significantly reduced (for both p24 and gp41) (see Figure 6b). Although the aggregation of the proteins was reduced under these conditions, the viral preparations were still inactivated, and the controls were still infectious, as seen using a 24-hour cell-based luciferase reporter assay (data not shown).
Figure 6.
Aggregation of viral proteins is induced by reactive oxygen species. (a) The addition of glutathione (20 mM) prior to UV irradiation significantly reduced the higher molecular weight aggregates, as can be seen in western blots probed for p24 and gp41. (b) Oxygen depletion prior to UV irradiation for 15 minutes in the presence of the azido compounds, significantly reduced the aggregation at higher molecular weights, while maintaining viral inactivation. Lane A is the HIV control (No UV), the remaining lanes are HIV + compound listed + UV irradiation for 15 minutes. B: DMSO, E: AZNAP, F:DAN, G: INA, H: DABIPH.
Structure of surface viral immunogens are preserved after treatment with the azide containing compounds
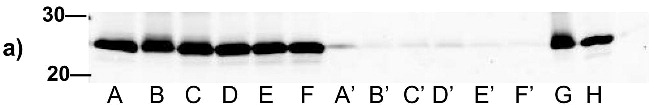
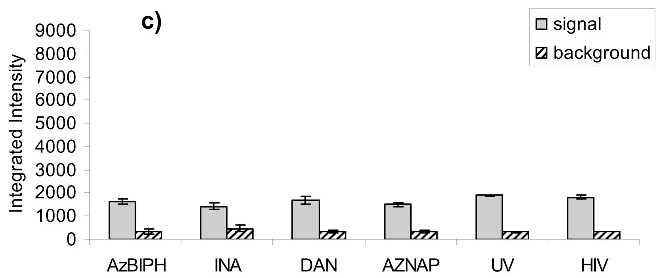
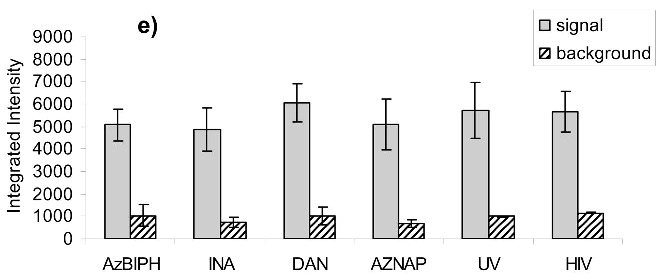
After treatment of HIV-1 with the azido-containing hydrophobic compounds and UV irradiation for two minutes, the resulting non-infectious virus preparation was studied for the preservation of known neutralizing epitopes on the viral surface. A neutralizing antibody is one that when added to the virus, causes the virus to be non-infectious by binding to specific epitopes on the viral surface. Three known neutralizing antibodies 4E10 (anti-gp41), 2g12 (anti-gp120) and b12 (anti-gp120) were studied for their ability to recognize and capture the treated HIV-1. A virus capture assay, using these antibodies to capture the whole inactivated virus, was performed in a 96-well format. The readout for the assay was done using quantitative Western analysis for p24 (capsid protein) with Alexafluor IR dyes. Detection and quantitation of the p24 bands provided an indication of how much HIV was recognized by the neutralizing antibodies. The resulting bands were integrated and compared to mock samples run at the same time (same protocol without capturing antibodies) to assess any non-specific background binding of HIV to the plate (see Figure 7a). Control lanes comprising of lysed HIV-1 were also run for normalization between gels for the various antibodies studied. A working range of the assay was established (see Figure 7b) in which the capture of the HIV-1 did not saturate the plate and resulted in a linear response with decreasing HIV-1 added for binding. For the three antibodies tested (b12, 2g12 and 4E10), the various treatments do not have a significant effect on the recognition of these viruses by these antibodies (see Figure 7c-e), and the variations in intensity shown are all within the deviation between the separate experiments (as shown by the error bars). There is minimal apparent damage to the epitope for b12 when the virus is treated with INA plus UV irradiation for 2 minutes, although the majority of the b12 binding sites appear unaffected (Figure 7d) when the overall intensity is compared to that of the control versus the background. Similar results were obtained upon treatment with UV for 15 minutes, via integration of the main band plus aggregated area, indicating the majority of the epitopes 2g12 and b12 were maintained (Figure 7f-g). The one exception was INA treated HIV-1 with UV for 15 minutes, which lost b12 recognition. This however, was regained after the addition of glutathione, indicating the loss of b12 recognition was due to secondary side reactions and not the reaction of the INA itself (data not shown).
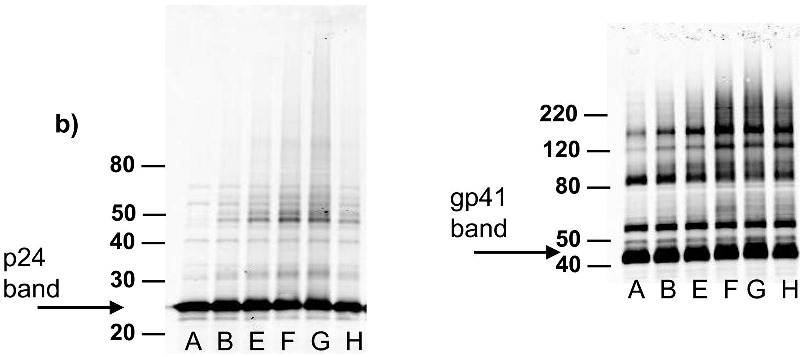
Figure 7.
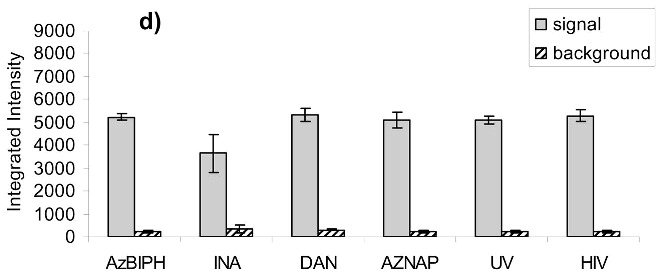
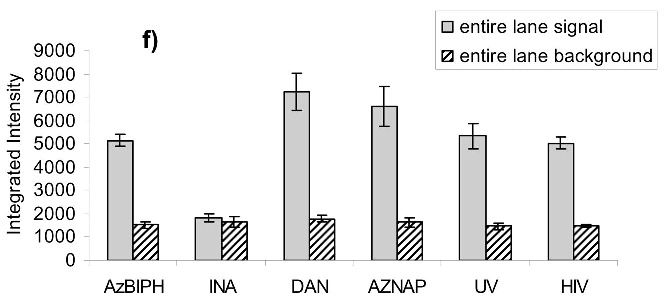
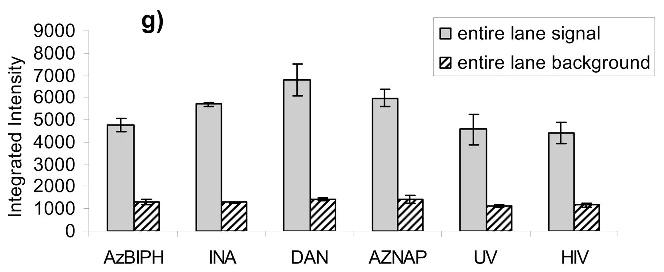
Virus capture assay (VCA) using neutralization epitopes indicates preservation of surface epitopes on HIV-1 MN after treatment with compounds plus UV for 2 minutes. (a) Representative blot showing p24 amounts after capture with b12 (2g12, and 4e10 not shown), where A: DAN, B: INA, C: AzNAP, D: DABIPH, E: UV, F: control captured by b12, and A’-F’ are mock samples added to the virus capture assay without b12, G and H are controls not subjected to the virus capture assay run on the gel to normalize between gels, G: INA control, H: HIV control; (b) Capture of untreated virus with varying concentrations showing functional range of assay (data not normalized between data sets); (c) 4e10 capture of 0.25 mg/mL virus after UV for 2 minutes; (d) b12 capture of 0.25 mg/mL virus after UV for 2 minutes; (e) 2g12 capture of 0.25 mg/mL virus after UV for 2 minutes, (f) b12 capture of 0.25 mg/mL virus after UV for 15 minutes; (g) 2g12 capture of 0.25 mg/mL virus after UV for 15 minutes. Readout using quantitative Western analysis for p24, where raw integrated intensity is the integration of the main p24 band (for UV 2 min) or integration of entire lane (for UV 15 min., to include protein aggregates that contain the desired protein in the integration). Error bars for (c)-(g) are the difference in the values obtained between two independent experiments which were normalized to an HIV control lane, containing HIV not subjected to the virus capture assay, run on each gel.
DISCUSSION
We have recently described the use of the hydrophobic photoactivatable compound iodonaphthylazide (INA) as a general approach for the inactivation of enveloped viruses for vaccine application.(18, 19) In this work, we were interested in systematically evaluating the structural features of hydrophobic aromatic derivatives, based off naphthyl or biphenyl that could make them efficient agents for the preparation of whole virus vaccines. The various azido-based compounds studied herein (see Scheme 1) all showed the ability to inactivate a model enveloped virus, HIV-1, when used in conjunction with UVA irradiation at short irradiation times (2 minutes). Similar non-azido based controls that absorb at similar wavelengths (Figure 1) did not show this ability, indicating that the inactivation of the HIV-1 was due to the covalent binding of the azido-moiety upon UV irradiation. Previous work using radiolabeled INA had shown that the INA forms a covalent bond with HIV-1 proteins through the reaction of the nitrene formed through photoactivation.(15, 18) We surmised that viral inactivation is due to this covalent reaction. Indeed, several studies have shown that the transmembrane segment of fusion proteins was involved in the fusion process. Mutational studies of the membrane spanning domain of HIV have indicated that its amino acid composition influenced the ability of gp41 to undergo proper fusion.(29) In the case of influenza virus, the replacement of the transmembrane segment of HA2 by a gpi anchor yielded a protein that was able to induce hemifusion but no longer full fusion of the bilayers.(30) Due to their hydrophobicity, the azido compounds we use will primarily target the transmembrane portion of fusion proteins thereby presumably preventing them from performing their critical role in the overall fusion process.
Analysis of various HIV-1 proteins via western blot indicated that these compounds, along with their non-azido analogs, did not effect the electrophoretic migration of these proteins after treatment with UV for 2 minutes, which is consistent for modifications of proteins with small molecules. However, for the diazido compounds (DAN and DABIPH), it may be expected that covalent crosslinking could occur if both azides were activated. For short UV irradiation times, this was not evident for these compounds, as can be seen in Figure 2, where there was no significant aggregation at higher molecular weights. This is most likely due to the short length of these molecules and the potential side reactions of azides.(31, 32) Once covalent binding of one azide has occurred, it is likely that the second azide could undergo another reaction that does not result in a crosslink, such as proton abstraction or ring expansion, should another protein not be in close-enough proximity for a crosslinking reaction to occur. Clustering of proteins by photoactivation of DAN and DABIPH had been previously shown in erythrocyte membranes although in that study it could not be conclusively established whether it was a result of aggregation or crosslinking.(33)
In an effort to assess the effect of prolonged UV irradiation on this system, and to see if longer UV irradiation times would promote crosslinking of proteins using the diazido compounds, the UV irradiation times were increased to 15 minutes. At this longer irradiation time there was an increase in the amount of higher molecular weight aggregates observed by western blot (see Figure 3). However, this aggregation was seen for all the azido-containing compounds with prolonged UV irradiation time, including those that would not be expected to form crosslinks, specifically, those with only one azido functionality. These higher molecular weight aggregates are only seen for the transmembrane (gp41) and capsid (p24) proteins in HIV-1 and not for the more hydrophilic external glycoprotein, (gp120) indicating a preference for the aggregation on internal and hydrophobic proteins (see Figure 3a,b). This mechanism appears to be due to secondary reactions that are promoted by the azide-containing compounds, since the UV control and non-azido containing compounds do not indicate such an increase in aggregation. These secondary reactions may involve the formation of chromophores created by prolonged irradiation of naphthyl-azides, such as from oligomerization of the reactive naphthyl species, or from coupling to form diazo compounds. The products of these secondary reactions or the covalently bound compounds themselves can then act as photosensitizers to produce reactive oxygen species (ROS). ROS have been previously shown to promote aggregation of proteins due to the formation of disulfide links from cysteine oxidation as well as other covalent modifications by oxygen radicals.(34) Support for this hypothesis comes from the finding that the addition of glutathione, or the enzymatic depletion of oxygen completely blocked the formation of protein aggregates at prolonged irradiation times (Figure 6). The addition of glutathione results in the scavenging of radicals as they are formed therefore preventing the viral protein aggregation seen for long UV irradiation times. The glutathione does not inhibit the covalent binding of the azido compounds and therefore, the virus is still inactivated even in the presence of glutathione (see Figure 6a). Additionally, the removal of oxygen from the system removed the source of the reactive oxygen species thereby preventing aggregate formation, but still resulted in viral inactivation when the compounds were used with UV light. (see Figure 6b) The observation that the diiodo naphthyl derivative (DINAP) can also induce protein aggregates, although to a lesser extent than the azido derivatives, is also consistent with the generation of reactive oxygen species (Figure 3a,b). The two iodines in this molecule can induce, upon irradiation, a high yield of triplet exited states with lower excitation energy more suitable for triplet sensitization of molecular oxygen. Previous studies with INA used for hydrophobic protein labeling and viral inactivation were not done for such long irradiation times, therefore this phenomenon was not observed. These aggregates are seen for internal viral proteins such as capsid (p24) and matrix protein (p17, data not shown), as well as the hydrophobic transmembrane protein (gp41). Aggregates are not seen for the hydrophilic external glycoprotein (gp120) which is heavily glycosylated as the sugars that hinder the amino acid side chains do not readily react with ROS. Remarkably, this longer UV irradiation time (15 minutes) does not effect the overall structure of the virus, as can be seen by TEM, where the virions are still intact and are indistinguishable from the untreated control (see Figure 4). Further studies will be necessary to evaluate the inactivated virus after prolonged irradiation as an effective vaccine.
While treatment of HIV-1 with the azido-based compounds at low irradiation times (UV for 2 minutes) did not affect the mobility of HIV-1 proteins in SDS-PAGE, it did result in the complete loss of infectivity of the virus when monitored by a cell-based luciferase reporter assay (see Figure 5). The non-azido containing controls did not have an effect on the infectivity of the virus, with the exception of diiodonaphthalene that had a small effect, indicating that the azide is needed for this efficient and rapid inactivation. For the samples that were treated with UV for 15 minutes, it is important to note that the higher molecular weight aggregates are not needed for viral inactivation, since inactivation occurs after 2 minutes of irradiation. Also, when the samples are treated with glutathione or enzymatically depleted of oxygen prior to UV irradiation for 15 minutes, the aggregation is eliminated, but the viral inactivation remains (Figure 6, inactivation data not shown). This supports the hypothesis that the mechanism for viral inactivation is due to the covalent binding of the azido molecules to the viral proteins and not the protein aggregation from the formation of reactive oxygen species. It is important to note, as well, for these preparations that the virus was shown to be inactivated by 2 logs using a 24 hour cell-based luciferase reporter assay, which is less than the 10 logs of inactivation that has been proposed as acceptable for use as a killed virus vaccine.(35) Our previous studies using solely 1-iodo, 5-azidonaphthalene have shown 6 logs of inactivation in a more involved 24 day assay.(18) For the studies within this manuscript the cell-based assay was sufficient to compare and contrast the inactivation of the HIV under various conditions. More stringent, involved assays, however, would need to be tested on the conditions as outlined herein to be certain of greater than 2 logs of viral inactivation, especially if these conditions were used to make a killed virus vaccine.
In addition to viral inactivation, the preservation of viral antigens and epitopes is necessary for efficient vaccine candidates. After treatment of HIV-1 with the azido compounds plus UV irradiation for 2 minutes, the epitopes for b12, 2g12 and 4e10 were all preserved (see Figure 7). Similar results were obtained with irradiation for 15 minutes except for the b12 epitope on viruses treated with INA, which showed some damage (Figure 7f-g). The loss of b12 recognition with INA treatment was recovered when glutathione was added to the suspension, indicating that the epitope damage was due to secondary reactions and not from the primary reaction of the azide with the viral proteins (data not shown). Therefore, the treatment of HIV-1 with the azido-based compounds, and UV irradiated either for 2 or 15 minutes produced an inactivated virus that remained intact with preservation of key immunogenic epitopes.
The increased aggregation of viral proteins as seen for prolonged irradiation times, although not necessary for viral inactivation, allows for the additional modification of proteins within the virus similar to what is seen for treatments using hydrophilic chemicals such as formalin. Formalin creates covalent modifications and crosslinks proteins throughout the virus, including the viral DNA.(36) Thus, treatment with UV for 15 minutes with the hydrophobic compounds allow for a concurrent method of viral modification/inactivation that can be applied to vaccines resulting in additional levels of vaccine safety. However, unlike formalin treatment, when lethal amounts of formalin are used(1, 3–7), surface epitopes are maintained, even with the ROS-induced modifications throughout the virus. Additionally, treatment of live virus with the UV-activatable hydrophobic compounds allows for precise control over the chemical species that is inactivating (ie the formation of nitrenes), and the time for viral inactivation. Chemical inactivating agents such as formaldehyde can take hours or days, requiring extensive optimization for inactivation conditions. For the hydrophobic compounds herein, when the UV irradiation is stopped, any remaining hydrophobic compound is non-toxic, unlike formalin, where any remaining formaldehyde remains toxic and needs to be removed prior to vaccine administration. Thus, the use of the azido compounds, as described, provides a rapid, effective and reproducible general approach for inactivation of enveloped viruses with the preservation of viral epitopes for vaccine applications.
CONCLUSION
Azido-containing hydrophobic compounds based on naphthalenic or biphenyl structures can be used to rapidly and efficiently inactivate HIV-1 when irradiated by UVA light with short irradiation times. Longer UV irradiation times resulted in further protein modifications, due to the generation of ROS that could allow for concurrent inactivation methods for safer inactivated vaccines. These further modifications via ROS may provide an additional inactivation step for the safety of vaccine preparations created using these compounds since the production of the ROS modifies proteins throughout the virus and is not limited to the viral membrane. The cell-based luciferase reporter assay used herein has shown reproducibly 2 logs of inactivation for the viral lot tested under these various conditions. Further studies are needed and are ongoing on a selected subset of these conditions using more sensitive, involved tests to illustrate additional logs of inactivation before this technique can be used to safely generate killed virus vaccines. For the majority of the conditions tested, there was no significant damage to the epitopes that are recognized by the neutralizing antibodies 2g12 (gp120), b12 (gp120), and 4e10 (gp41) indicating that epitopes known to be targets for neutralizing antibodies were still intact even under conditions where ROS were created. These results indicate that all of the azido-containing compounds studied herein, could be potentially used for the generation of inactivated-virus vaccines, with minimal differences in the chemical structures (mono- or di-azido) having insignificant effects on either changes in viral inactivation, or epitope preservation.
Acknowledgments
The authors would like to thank Julian W. Bess, Jr. of the AIDS and Cancer Virus Program, SAIC-Frederick, for many helpful conversations and his critical reading of this manuscript. We thank ARRRP for reagents, and Jeff Lifson and Julian W. Bess, Jr. for generously providing purified virus. This research was supported [in part] by federal funds from the Intramural AIDS Targeted Antiviral Program and the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract HHSN26120080001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
References
- 1.Duque H, Marshall RL, Israel BA, Letchworth GJ. Effects of formalin inactivation on bovine herpes virus-1 glycoprotein and antibody response elicited by formalin-inactivated vaccines in rabbits. Vaccine. 1989;7:513–520. doi: 10.1016/0264-410X(89)90275-2. [DOI] [PubMed] [Google Scholar]
- 2.Adler-Storthz K, Sehulster LM, Dreesman GR, Hollinger FB, Melnick JL. Effect of alkaline glutaraldehyde on hepatitis B virus antigens. Eur J Clin Microbiol. 1983;2(4):316–320. doi: 10.1007/BF02019460. [DOI] [PubMed] [Google Scholar]
- 3.Grovit-Ferbas K, Hsu JF, Ferbas J, Gudeman V, Chen ISY. Enhanced binding of antibodies to neutralization epitopes following thermal and chemical inactivation of human immunodeficiency virus type 1. J Virol. 2000;74(13):5802–5809. doi: 10.1128/jvi.74.13.5802-5809.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tano Y, Shimizu H, Martin J, Nishimura Y, Simizu B, Miyamura T. Antigenic characterization of a formalin-inactivated poliovirus vaccine derived from live-attenuated sabin strains. Vaccine. 2007;25:7041–7046. doi: 10.1016/j.vaccine.2007.07.060. [DOI] [PubMed] [Google Scholar]
- 5.Sattentau OJ. Conservation of HIV-1 gp120 neutralizing epitopes after formalin inactivation. AIDS. 1995;9:1383–1385. doi: 10.1097/00002030-199512000-00017. [DOI] [PubMed] [Google Scholar]
- 6.Bachmann MF, Bast C, Hengartner H, Zinkernagel RM. Immunogenicity of a viral model vaccine after different inactivation procedures. Med Microbiol Immunol. 1994;183:95–104. doi: 10.1007/BF00277160. [DOI] [PubMed] [Google Scholar]
- 7.Poon B, Safrit JT, McClure H, Kitchen C, Hsu JF, Gudeman V, Petropolous C, Wrin T, Chen ISY, Grovit-Ferbas K. Induction of humoral immune response following vaccination with envelope-containing, formaldehyde-treated, thermally inactivated human immunodeficiency virus type 1. J Virol. 2005;79(8):4927–4935. doi: 10.1128/JVI.79.8.4927-4935.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanson CV. Photochemical inactivation of viruses with psoralens: an overview. Blood Cells. 1992;18:7–25. [PubMed] [Google Scholar]
- 9.Hall JD. Repair of psoralen-induced crosslinks in cells multiply infected with SV40. Mol Gen Genet. 1982;188:135–138. doi: 10.1007/BF00333007. [DOI] [PubMed] [Google Scholar]
- 10.Brunner J. New photolabeling and crosslinking methods. Annu Rev Biochem. 1993;62:483–514. doi: 10.1146/annurev.bi.62.070193.002411. [DOI] [PubMed] [Google Scholar]
- 11.Bayley H, Knowles JR. Photogenerated reagents for membranes: selective labeling of intrinsic membrane proteins in the human erythrocyte membrane. Biochemistry. 1980;19(17):3883–3892. doi: 10.1021/bi00558a001. [DOI] [PubMed] [Google Scholar]
- 12.Bercovici T, Gitler C. 5-[125I]Iodonaphthyl azide, a reagent to determine the penetration of proteins into the lipid bilayer of biological membranes. Biochemistry. 1978;17(8):1484–1489. doi: 10.1021/bi00601a020. [DOI] [PubMed] [Google Scholar]
- 13.Pak CC, Krumbiegel M, Blumenthal R, Raviv Y. Detection of influenza hemagglutinin interaction with biological membranes by photosensitized activation of [125I]iodonaphthylazide. J Biol Chem. 1994;269:14614–14619. [PubMed] [Google Scholar]
- 14.Raviv Y, Viard M, Bess JW, Jr, Blumenthal R. Quantitative measurement of fusion of HIV-1 and SIV with cultured cells using photosensitized labeling. Virology. 2002;293(2):243–251. doi: 10.1006/viro.2001.1237. [DOI] [PubMed] [Google Scholar]
- 15.Viard M, Ablan SD, Zhou M, Veenstra T, Freed EO, Raviv Y, Blumenthal R. Photoinduced reactivity of the HIV-1 envelope glycoprotein with a membrane-embedded probe reveals insertion of portions of the HIV-1 gp41 cytoplasmic tail into the viral membrane. Biochemistry. 2008;47(7):1977–1983. doi: 10.1021/bi701920f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharma A, Raviv Y, Puri A, Viard M, Blumenthal R, Maheshwari RK. Complete inactivation of venezuelan equine encephalitis virus by 1,5-iodonaphthylazide. Biochem Biophys Res Commun. 2007;358:392–398. doi: 10.1016/j.bbrc.2007.04.115. [DOI] [PubMed] [Google Scholar]
- 17.Warfield KL, Swenson DL, Olinger GG, Kalina WV, Viard M, Aitichou M, Chi X, Ibrahim S, Blumenthal R, Raviv Y, Bavari S, Aman MJ. Ebola virus inactivation with preservation of antigenic and structural integrity by a photoinducible alkylating agent. J Infect Dis. 2007;196(2):S276–S283. doi: 10.1086/520605. [DOI] [PubMed] [Google Scholar]
- 18.Raviv Y, Viard M, Bess JW, Jr, Chertova E, Blumenthal R. Inactivation of retroviruses with preservation of structural integrity by targeting the hydrophobic domain of the viral envelope. J Virol. 2005;79(19):12394–12400. doi: 10.1128/JVI.79.19.12394-12400.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raviv Y, Blumenthal R, Tompkins SM, Humberd J, Hogan RJ, Viard M. Hydrophobic inactivation of influenza viruses confers preservation of viral structure with enhanced immunogenicity. J Virol. 2008;82(9):4612–4619. doi: 10.1128/JVI.02233-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chertova E, Bess JW, Jr, Crise BJ, Sowder RC, II, Schaden TM, Hilburn JM, Hoxie JA, Benveniste RE, Lifson JD, Henderson LE, Arthur LO. Envelope glycoprotein incorporation, not shedding of surface envelope glycoprotein (gp120/SU), is the primary determinant of SU content of purified human immunodeficiency virus type I and simian immunodeficiency virus. J Virol. 2002;76(11):5315–5325. doi: 10.1128/JVI.76.11.5315-5325.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ling C, Minato M, Lahti PM, Van Willigen H. Models for intramolecular exchange in organic pi-conjugated open-shell systems. A comparison of 1,1-ethenediyl and carbonyl linked bis(arylnitrenes) J Am Chem Soc. 1992;114(25):9959–9969. doi: 10.1021/ja00064a015. [DOI] [Google Scholar]
- 22.Smith PAS, Brown BB. The reaction of aryl azides with hydrogen halides. J Am Chem Soc. 1951;73(6):2438–2441. [Google Scholar]
- 23.Rodriguez JG, Tejedor JL. Carbon networks based on 1,5-naphthalene units. Synthesis of 1,5-naphthalene nanostructures with extended pi-conjugation. J Org Chem. 2002;67(22):7631–7640. doi: 10.1021/jo0203589. [DOI] [PubMed] [Google Scholar]
- 24.Roos JW, Maughan MF, Liao Z, Hildreth JEK, Clements JE. LuSIV cells: A reporter cell line for the detection and quantitation of a single cycle of HIV and SIV replication. Virology. 2000;273:307–315. doi: 10.1006/viro.2000.0431. [DOI] [PubMed] [Google Scholar]
- 25.Mariner JM, McMahon JB, O’Keefe BR, Nagashima K, Boyd MR. The HIV-inactivating protein, Cyanovirin-N, does not block gp120-mediated virus-to-cell binding. Biochem Biophys Res Commun. 1998;248:841–845. doi: 10.1006/bbrc.1998.9060. [DOI] [PubMed] [Google Scholar]
- 26.Nyambi PN, Burda S, Bastiani L, Williams C. A virus binding assay for studying the antigenic landscape on intact, native, primary human immunodeficiency virus-type 1. J Immunol Methods. 2001;253:253–262. doi: 10.1016/S0022-1759(01)00384-2. [DOI] [PubMed] [Google Scholar]
- 27.Meiklejohn BI, Rahman NA, Roess DA, Barisas BG. 5-Iodonaphthyl-1-azide labeling of plasma membrane proteins adjacent to specific sites via energy transfer. Biochim Biophys Acta. 1997;1324:320–332. doi: 10.1016/S0005-2736(96)00237-4. [DOI] [PubMed] [Google Scholar]
- 28.Bess JW, Jr, Gorelick RJ, Bosche WJ, Henderson LE, Arthur LO. Microvesicles are a source of contaminating cellular proteins found in purified HIV-1 preparations. Virology. 1997;230(1):134–144. doi: 10.1006/viro.1997.8499. [DOI] [PubMed] [Google Scholar]
- 29.Yue L, Shang L, Hunter E. Truncation of the membrane-spanning domain of human immunodeficiency virus type 1 envelope glycoprotein defines elements required for fusion, incorporation, and infectivity. J Virol. 2009;83(22):11588–11598. doi: 10.1128/JVI.00914-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kemble GW, Danieli T, White JM. Lipid-anchored influenza hemagglutinin promotes hemifusion, not complete fusion. Cell. 1994;76(2):383–391. doi: 10.1016/0092-8674(94)90344-1. [DOI] [PubMed] [Google Scholar]
- 31.Maltsev A, Bally T, Tsao ML, Platz MS, Kuhn A, Vosswinkel M, Wentrup C. The rearrangements of naphthylnitrenes: UV/Vis and IR spectra of azirines, cyclic ketenimines, and cyclic nitrile ylides. J Am Chem Soc. 2004;126:237–249. doi: 10.1021/ja038458z. [DOI] [PubMed] [Google Scholar]
- 32.Gritsan NP, Platz MS. Kinetics and spectroscopy of substituted phenylnitrenes. Adv Phys Org Chem. 2001;36:255–304. doi: 10.1002/chin.200416272. [DOI] [Google Scholar]
- 33.Mikkelson RB, Wallach DFH. Photoactivated cross-linking of proteins within the erythrocyte membrane core. J Biol Chem. 1976;251(23):7413–7416. [PubMed] [Google Scholar]
- 34.Xiong YL, Park D, Ooizumi T. Variation in the cross-linking pattern of porcine myofibrillar protein exposed to three oxidative environments. J Agric Food Chem. 2009;57:153–159. doi: 10.1021/jf8024453. [DOI] [PubMed] [Google Scholar]
- 35.Schultz AM, Koff CW, Lawrence DN. Conference Reports: Workshop on HIV inactivated vaccines. Vaccine. 1990;8:516–517. [Google Scholar]
- 36.Ritchey JW, Black DH, Rogers KM, Eberle R. In vivo experimentation with Simian Herpesviruses: Assessment of biosafety and molecular contamination. J Am Assoc Lab Anim Sci. 2006;45(2):7–12. [PubMed] [Google Scholar]