Abstract
Receptor tyrosine kinases (RTKs) play important roles in the control of fundamental cellular processes, influencing the balance between cell proliferation and death. RTKs have emerged as molecular targets for the treatment of various cancers. Green tea and its polyphenolic compounds, the catechins, exhibit chemopreventive and chemotherapeutic properties in many human cancer cell types, as well as in various carcinogenicity models in vivo. Epidemiological studies are somewhat less convincing, but some positive correlations have been observed. The tea catechins, including (−)-epigallocatechin-3-gallate (EGCG), have pleiotropic effects on cellular proteins and signaling pathways. This review focuses on the ability of the tea constituents to suppress RTK signaling, and summarizes the mechanisms by which EGCG and other catechins might exert their protective effects towards dysregulated RTKs in cancer cells. The findings are discussed in the context of ongoing clinical trials with RTK inhibitors, and the possibility for drug/nutrient interactions enhancing therapeutic efficacy.
Keywords: Receptor tyrosine kinases, Green tea polyphenols, Hepatocyte growth factor, Met signaling
1. Introduction
The cultivation of the tea plant dates back to more than 5000 years, and originally its leaves were used medicinally. Today it is a popular beverage that is consumed by two-thirds of the world’s population. In recent years, green tea has received attention for its beneficial health effects, in particular the prevention of cancer. In 2009, Yang et al. reviewed the possible targets that could account for the chemopreventive effects of (−)-epigallocatechin-3-gallate (EGCG) [1]. The diverse mechanisms included inhibition of matrix metalloproteinases, cyclin dependant kinases, proteosomes, DNA methyltransferase, vitmentin, BCL-2, mitogen-activated protein kinase (MAPK) and receptor tyrosine kinase (RTK) pathways.
This review focuses specifically on the effects of tea and its constituents towards RTKs. First, the biochemical properties and bioavailability of the tea catechins will be discussed. Then, the ability of tea to inhibit tumorigenesis in animal models and human epidemiological data will be presented. RTKs and their downstream signaling pathways will be described. Finally, the interaction of tea catechins with these pathways and potential mechanisms of action will be covered.
2. Tea and cancer chemoprevention
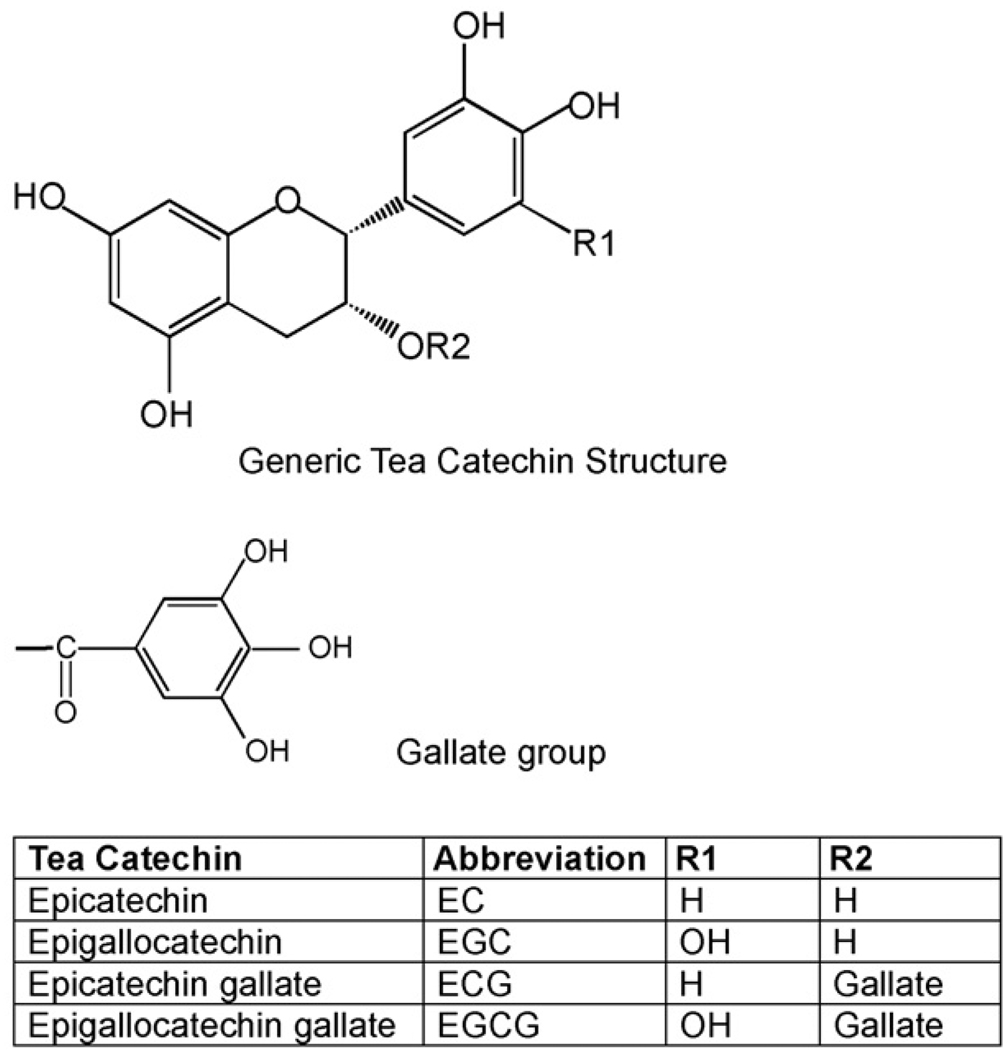
Aside from water, tea is the most widely consumed beverage worldwide [2]. This popular beverage has gained much attention for its purported health benefits, in particular for its possible role in preventing and treating cancer [3]. The chemistry of green tea, compared to other teas, is quite well characterized [4]. Green tea is produced by steaming or pan-frying the leaves of the Camellia sinensis plant. This process prevents the oxidation of the tea constituents. Among these constituents is a class of polyphenolic compounds known as the catechins (Fig. 1). Green tea catechins (GTCs) include (−)-epicatechin (EC), (−)-epigallocatechin (EGC), (−)-epicatechin gallate (ECG), and EGCG.
Fig. 1.
Structure of tea catechins.
A typical cup of brewed green tea has been defined as 2 g of tea leaves in 200 mL of hot water. The catechins make up 30–40% by dry weight of the water extractable material [5]. EGCG is the best studied and most abundant of the tea catechins, accounting for 50–80% of the total catechin content. This represents 200–300 mg per cup of brewed green tea [6].
Catechin pharmacokinetics has been studied by several groups. Tea catechins undergo methylation, glucuronidation, sulfation and ring fission metabolism [7–9]. The biotransformation of tea catechins was reviewed by Lambert et al. [10]. In one study, rats and mice were given 0.6% GTCs as drinking fluid [11]. EGCG accounted for 78% of the catechins present, but plasma concentrations were much lower for EGCG than EGC and EC. High levels of EGCG were found in the feces whereas high levels of EGC and EC were found in urine. In another study, examination of tissues showed that EGCG was distributed widely in the colon, small intestine, liver, lung, and other organs [8]. After intravenous administration of green tea to rats, EC was found mainly in the intestine, bladder and kidney, EGC was found in the intestine, bladder, kidney and lung, and EGCG was found mostly in the colon and liver [11,12].
In humans, high concentrations of individual catechins were administered orally. The plasma concentration for each catechin was observed to be as high as 1.53 µM for a dose of 1050 mg EC [13], 3.1 µM for a dose of 644 mg ECG [14], 5 µM for a dose of 459 mg EGC [14], and 6.35 µM for a dose of 1600 mg EGCG [15]. Recently, it was reported that in patients with an ileostomy, 70% of flavonols (which includes catechins) from orally consumed green tea was found in the small intestine. Plasma and urine contained comparatively low levels, suggesting that after oral ingestion the catechins accumulate in the intestines [16].
Tea extracts and tea constituents have gained much attention for their abilities to inhibit tumor formation in different animal models (reviewed in Refs. [6,17–20]). The inhibition of small intestine, colon, prostate, bladder, breast, stomach, liver, pancreas, esophagus, oral cavity, lung and skin cancers has been reported in animal models (Table 1). Although mostly positive (i.e. chemopreventive) results were reported, some equivocal findings were noted in which tea had no apparent protective effects. Many variables could explain inconsistencies in these studies. Differences in diets used, protocol for tumor initiation, the type and dose of tea polyphenols or extracts used may explain the variability in some of the animal study results. Another key issue may be the stability of tea polyphenols in solution and in the diet.
Table 1.
Summary of animal data in which tea or tea compounds were used as chemopreventive agents.
| Tea and inhibition of cancer in animal models | ||
|---|---|---|
| Positive results | Negative results | |
| Lung | 23 (2) | 3 |
| Bladder | 3 (1) | 0 |
| Oral Cavity | 6 | 0 |
| Esophagus | 5 | 0 |
| Stomach | 9 | 0 |
| Small intestine | 9 | 1 |
| Colon | 11 (3) | 6 |
| Liver | 8 (1) | 1 |
| Pancreas | 2 (2) | 0 |
| Prostrate | 7 (5) | 0 |
| Breast | 10 (10) | 0 |
| Thyroid | 1 | 0 |
| Skin | 29 (2) | 0 |
Updated from Ref. [1] with data current as of June 2010. The number of xenograft studies is shown in parentheses.
A clear-cut association between human cancer risk and tea consumption may be more difficult to establish from epidemiological studies. There are many reviews on this topic [21–27]. Some studies show a negative association, others report no association, and a positive association has been observed in other investigations. Most recent reviews conclude that the protective effects of tea depend on the various etiological factors involved in different cancer types, and even for the same cancer types in different geographical areas. Furthermore, the consumption of green and black tea differs significantly among the various populations examined. Due to the large differences between epidemiological studies, it is perhaps naïve to expect a simple conclusion concerning tea and cancer prevention in human populations.
Case–control studies and cohort studies have observed an association between green tea consumption and lowered risk for stomach cancer in Japan (reviewed in Refs. [26–28]). One of the most promising intervention studies was performed by Bettuzzi et al. [29]. Two hundred individuals with high-grade prostate intraepithelial neoplasia received either 600 mg GTC or placebo for 12 months. In the GTC group only 3% developed prostate cancer, whereas 30% developed prostate cancer in the placebo group. The latter findings demonstrate the importance of carefully controlled intervention trials with tea and tea compounds, and similarly conducted studies are warranted.
3. RTKs and their downstream signaling pathways
RTKs are key regulators of cell signaling pathways involved in proliferation, differentiation, migration, invasion and cell death. PubMed lists well over 5000 reviews on this topic, with the most recent article being by Lemmon and Schlessinger [30]. Activation of RTKs by specific ligands (growth factors and cytokines) and downstream effects are summarized in Fig. 2, which provides a framework for the following discussions.
Fig. 2.
RTK signaling map.
RTKs function in cell signaling by catalyzing the transfer of the γ-phosphate of ATP to tyrosine residues on protein substrates. This phosphorylation modulates enzymatic activity and creates binding sites for the recruitment of downstream signaling proteins. It is in this way that a signal cascade is established.
RTKs are activated by binding of a ligand to the extracellular domain. This induces dimerization of the RTK and cross-phosphorylation of tyrosine residues. This phosphorylation results in the stabilization of the dimer conformation, allows opening of the kinase domain for ATP binding, and access to docking sites for adapter proteins.
The MAPK pathway is activated by recruitment and activation of the adapter proteins (Grb2, Shc, and Sos) that in turn activate Ras. The serine/threonine kinase Raf-1 is activated by Ras which then phosphorylates and activates MEK 1/2 on two serine residues. MEK1/2 then activates extracellular signal-regulated kinase (ERK) member ERK1 (p44MAPK) and ERK2 (p42MAPK), which enables transcription factors, such as AP-1, to alter the expression of genes that are important for cell proliferation and migration [31].
The phosphoinositide-3-kinase (PI3K) pathway is another important signaling pathway that is activated by RTKs. PI3Ks are heterodimeric lipid kinases that are composed of a regulatory (p85) and catalytic (p110) subunit. When PI3K is activated by RTKs it induces the synthesis of a lipid second messenger, phosphatidylinositol(3,4,5)-triphosphate (PIP3). PIP3 is necessary for the phosphorylation of Akt, which is involved in cell survival. Akt phosphorylates and deactivates Bad, a proapoptotic protein [32]. Caspase-9 is also phosphorylated by Akt. This phosphorylation inhibits the catalytic activity of Caspase-9 thus enhancing cell survival [33]. The transcription factor nuclear factor-kappaB (NFκB) is also activated by Akt. NFκB regulates genes that promote survival in response to apoptotic stimuli [34].
In the cytoplasm,NFκB is kept in its inactive form through interaction with IκB. Ubiquitination and degradation of IκB is mediated by phosphorylation of IκB kinase (IKK). This releases NFκB such that it is able to translocate to the nucleus. Akt has an effect on NFκB by phosphorylating and activating IKK [34]. NFκB-inducing kinase (NIK) also controls the phosphorylation and activation of IKK [35,36]. The MAPK pathway has been shown to activate the NIK/IKK/NFκB pathway [37].
Activation of AP-1 and NFκB by ERK and Akt pathways occurs independently or coordinately to regulate specific targets, such as cyclin D1 and Cox-2. The promoters of the corresponding genes contain both AP-1 and NFκB binding sites [38–40]. The dysregulation of AP-1 and NFκB play important roles in the development of many types of cancer [35,41].
4. Tea catechins and RTKs
4.1. Insulin-like growth factor-1 receptor (IGF-1R)
IGF-1R is activated by its ligands IGF-1 and IGF-2. It has an essential role in cell growth and development [42,43]. IGF-1R is typically expressed at low levels in normal hepatocytes, but is overexpressed in hepatocellular carcinomas [44]. IGF-1R has also been found to be overexpressed in other types of cancer, including prostate [45] and colorectal cancer [46]. Activation of IGF-1R leads to the activation of PI3K and MAPK pathways [47]. The activity and function of the IGF ligands are controlled by insulin-like growth factor binding protein-3 (IGFBP-3) [44]. IGFBP-3 is found to be decreased in hepatocellular carcinoma samples compared to non-neoplastic liver tissues [48].
In one study, Shimizu et al. [49] treated HepG2 human hepatocellular carcinoma cells and Hc cells (normal hepatocytes) with 20 µg/mL EGCG. A preferential growth inhibition by EGCG in the HepG2 cells was observed. They found decreased phosphorylation of IGF-1R, ERK, Akt, Stat-2 and glycogen synthase kinase-3β with EGCG treatment. Decreases in protein and mRNA of IGF-1 and IGF-2 and an increase in IGFBP-3 protein were also observed.
Another study by Li et al. [50] demonstrated that EGCG binds competitively to the ATP binding site of IGF-1R, blocking downstream signaling of this receptor. They calculated the IC50 value to be 14 µM EGCG.
4.2. Epidermal growth factor receptor (EGFR) family
The EGFR signaling pathway is important for the regulation of cell growth, proliferation, differentiation and survival. The EGFR family of receptors includes EGFR (ErbB1), HER-2 (HER-2/neu, ErbB2), HER-3 (ErbB3) and HER-4 (ErbB4). Many different ligands have been identified for this family of receptors including epidermal growth factor (EGF), transforming growth factor (TGF)-α, β-cellulin and neoregulins.
Liang et al. [51] reported the inhibition of EGFR phosphorylation in A431, human epithelial carcinoma cells, by EGCG. In ligand binding assays, it was suggested that EGCG blocks the binding of EGF to its receptor. Others reported that not only does EGCG decrease receptor phosphorylation, the tea catechin also inhibits receptor expression by inhibiting the activity of ERK, which regulates the transcription factor Egr-1. Egr-1 controls the expression of EGFR [52].
Another study proposed that the oxidation of EGCG is necessary to reduce EGFR activation [53]. This was demonstrated by adding superoxide dismutase (SOD) to cell culture media to stabilize EGCG. This stabilized EGCG lost its ability to suppress receptor activation. The generation of hydrogen peroxide by EGCG also has been examined with respect to effects on signaling pathways in colon cancer [54]. In other studies, Adachi et al. [55] demonstrated that EGCG and polyphenon E (a mixture of GTCs), but not EC, disrupted lipid order and membrane organization. This caused the internalization of EGFR such that EGF could no longer bind. The addition of SOD had no effect on this process.
4.3. Vascular endothelial growth factor receptor (VEGF-R)
The VEGF-R family is involved in proliferation, migration and tube formation in endothelial cells. VEGF-R family members include VEGF-R1 (Flt-1), VEGF-R2b (KDR, Flk-1), and VEGF-R3 (Flt-4). Their ligands are vascular endothelial growth factor (VEGF), placental growth factor (PIGF) and VEGF-B, -C, -D. VEGF-R expression can be induced by many cytokines and growth factors including interleukin-1 (IL-1), IL-6, platelet-derived growth factor (PDGF), TGF, and hepatocyte growth factor (HGF) [56,57].
Lee et al. [58] showed that doses of EGCG as low as 7 µM in chronic lymphoid leukemia cells (CLL) could inhibit the phosphorylation of both VEGF-R1 and VEGF-R2 and induce apoptosis by down-regulating anti-apoptotic Bcl-1, Mcl-1 and XIAP proteins. The activation of Caspase-3 and cleavage of poly(ADP-ribose) polymerase was also observed. When Kondo et al. [59] investigated EC, ECG, EGC and EGCG, all four tea catechins were found to inhibit angiogenesis in an in vitro model using human umbilical vein endothelial cells (HUVECs) in concentrations ranging from 1.56 to 100 µM. EGCG was the most effective of the catechins, and was the only catechin that was able to inhibit VEGF binding to its receptor. In the HuH7 hepatoma cell line, EGCG reduced the expression of VEGF mRNA by inhibiting ERK and Akt phosphorylation, which regulates VEGF expression. Growth of the HuH7 cells was also inhibited by EGCG [60].
4.4. Platelet-derived growth factor receptor (PDGF-R)
The PDGF-R family members are expressed in connective tissue and glial cells, and play important roles in wound healing. The PDGF ligand is a dimeric protein that is composed of A- and B-chains. These chains combine to form three isoforms, PDGF-AA, PDGF-BB and PDGF-AB. The PDGF-Rα receptor can bind all three isoforms whereas the PDGF-Rβ only binds PDGF-BB with high affinity.
EGCG and ECG have been shown to suppress PDGF-BB-induced activation of PDGF-Rβ in rat and human vascular smooth muscle cells and in A172 human glioblastoma cells [61–63]. In these studies, EC had no effect.
In a study by Suzuki et al. [64] PDGF-BB was found to be bound to EGCG that had been immobilized on an agarose gel. Binding studies with [125I]-PDGF-BB was performed on EGCG-treated vascular smooth muscle cells (VSMC) [65]. This group found a 50% reduction of [125I]-PDGF-BB binding, suggesting that ECGC disrupts the ability of PDGF-BB to bind to its receptor.
4.5. Met
Met is important during embryonic development and wound healing [66]. It is dysregulated in many cancer types and is associated with poor prognosis [66]. Activation of Met by its ligand, HGF, results in increased proliferation, survival, angiogenesis, tissue regeneration, scattering, motility, and morphogenesis [67].
In the breast cell line MCF-10A, EGCG, ECG, EGC and EC were studied. The gallated catechins, EGCG and ECG, inhibited the activation of Met, Akt and ERK at 0.6 µM. Non-gallated catechin EGC did not inhibit Met activation, but nonetheless repressed Akt and ERK signaling at concentrations less than 2.5 µM. EC did not have an effect on Met, Akt or ERK phosphorylation [68]. This suggested a rational structure–activity relationship for tea catechins with respect to inhibition of Met and its downstream signaling pathways.
We reported similar findings with the Met receptor in HCT116 human colon cancer cells [69]. Using 5 µM of each tea catechin, EC was unable to suppress Met activation, EGC marginally suppressed Met activity, whereas ECG and EGCG had the greatest effect on suppressing receptor phosphorylation. In an in vitro kinase assay, ECG and EGCG were highly effective inhibitors [69]. Molecular docking and enzymatic kinetic analysis suggested a competitive mechanism of inhibition. Specifically, GTCs containing a 3-gallate ester group were capable of interacting with the hinge region of the ATP binding pocket of the Met receptor. Subsequent studies showed that EGCG inhibited HGF-induced invasion, proliferation, and downstream Met signaling in colon cancer cells [70].
5. Mechanisms of RTK inhibition by catechins
Most studies on the inhibition of RTK activity by tea constituents have been performed with EGCG. Among the various GTCs examined to date, it has been found that EC has little or no effect in the suppression of EGFR [54], VEGF-R [59], PDGF-R [62,63], or Met [68,69]. All receptors were inhibited most effectively by EGCG, followed by ECG and EGC. This suggests that the 3-gallate ester moiety is an important pharmacophore, with steric and electronic features necessary for optimal interactions with RTKs and the ability to target and block RTK activation.
Many possible mechanisms have been proposed and tested to account for the effect of EGCG on RTKs. These mechanisms include competitive inhibition, alterations of receptor transcription or translation, receptor internalization, disruption of lipid rafts in the vicinity of the receptor, and hydrogen peroxide generation.
EGCG can interfere with subcellular kinase activities of ERK and Akt directly [71]. Decreased activity of ERK leads to inhibition of Egr-1, a key transcription factor that regulates the production of EGF. Decreased ERK and Akt activities have been observed to attenuate VEGF expression [60].
Another factor contributing to EGCG-mediated inhibition of RTK activity is the stability of the catechin under various conditions. EGCG is known to be unstable in certain types of cell culture media [54,72], but when SOD is added EGCG is at least partially stabilized. The co-treatment of SOD and EGCG decreased the effectiveness of EGCG to attenuate EGFR activation [53]. This suggested that in some circumstances the effects on EGFR are due to the generation of reactive oxygen species, and not that of parent EGCG. However, Adachi et al. saw no difference on EGFR activity when EGCG was incubated in the presence or absence of SOD [55].
Hydrogen peroxide (H2O2) is one of the oxidation products of EGCG in cell culture media. High levels ofH2O2 (≥500 µM) have the ability to suppress Met receptor activation by inducing phosphorylation of serine 985 (a negative regulatory site) of Met [73]. We reported, however, that physiologically relevant concentrations of EGCG produce much less H2O2 in cell culture media, and that the suppressive effects of EGCG on Met were maintained in the presence or absence of catalase [74].
Lipid rafts are areas of the plasma membrane that contain a high level of cholesterol and sphingolipids [75,76]. EGFR [77] and IGF-R [78] are associated with lipid rafts. Ligand binding and tyrosine kinase activity of erbB family receptors is influenced by the lipid environment [79]. Adachi et al. demonstrated that EGCG disrupted the ordered lipid domains of the plasma membrane, and this was associated with inhibitory effects of EGCG on EGFR signaling [54].
Lipid organization is also thought to play a role in receptor internalization [80]. When cells are treated with EGF, its receptor is internalized into early endosomes [81]. Adachi et al. [55] showed that EGCG treatment induced internalization of EGFR into endosomes. The EGF-stimulated cells were able to produce a phosphorylation cascade, but EGCG-treated cells did not allow the signaling of EGFR by sequestering the receptor inside the cell, thereby making it inaccessible to EGF ligand.
EGCG has also been shown to bind directly to EGF [51], VEGF [59], and PDGF [64] ligands. This prevented the growth factor from interacting with its corresponding receptors and activating downstream signaling cascades. Further studies appear to be warranted in this avenue, for several other growth factors that affect cancer cell growth and survival, including HGF.
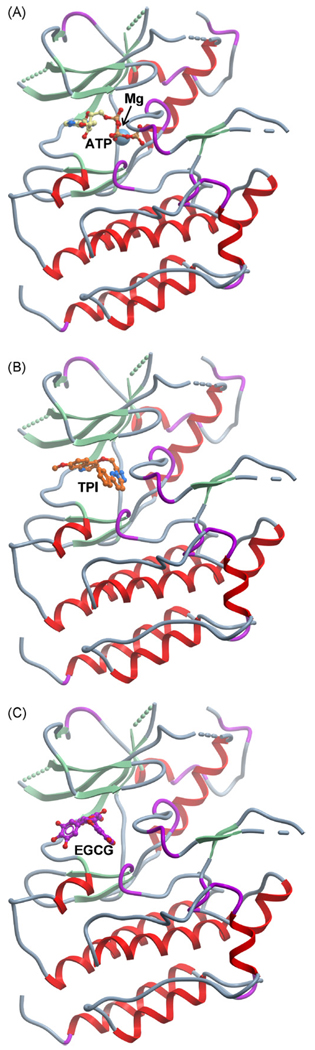
There is evidence that EGCG can compete with the ATP binding site of some proteins and act as an ATP mimetic of the adenine moiety [82]. We have shown by kinetic analysis and molecular docking that EGCG interacts with the ATP binding site of the Met receptor [69]. The γ-phosphate of ATP is transferred to tyrosine residues on protein substrates to initiate a cascade of signaling from the Met receptor (Fig. 3A). Comparative molecular docking simulations of a triazolopyridazine inhibitor and EGCG (Fig. 3B and C) revealed that both molecules interacted favorably with the ATP binding site of Met [69]. Recently, Hsp90 was shown to have a C-terminal nucleotide binding site that also has the ability to bind ATP or EGCG. Other ATP competitive inhibitors are reported in the literature [83,84], but tea catechins may have a unique conformational flexibility and the capacity of interacting with various types of molecule, allowing them to serve as chemical chaperones [85].
Fig. 3.
EGCG competes for the ATP binding site of the Met receptor. (A) ATP docked in the Met kinase domain. (B) Met kinase domain with bound triazolopyridazine inhibitor (TPI) [83]. (C) EGCG docked in the Met kinase domain [69].
6. Human relevance
Tea and its individual catechin constituents have gained much attention for their purported anticancer properties. There are numerous animal models of carcinogenesis in which tea compounds exhibited significant chemoprotective effects. However, human epidemiological data are somewhat less convincing [1,86]. Possible reasons for this discrepancy include human genetic variability, lifestyle, amount of tea and type consumed, and various cancer etiologies. It is therefore important to establish carefully conducted case–control studies to better assess the possible beneficial effects of tea in human populations. This would apply not only for cancer investigations, but also for other conditions in which the health benefits of tea have been attributed, including cardiovascular diseases, obesity, and neurological disorders [87–90].
When the data are summarized from the literature on the chemopreventive mechanisms of tea catechins, it is apparent that a wide range of concentrations has been used, some reaching into the mM level [1]. With specific reference to EGCG and RTKs, the concentrations in mechanistic studies typically fall within the range 1–100 µM (Table 2). In our own studies [70], 5 µM EGCG was the IC50 for inhibiting HGF-mediated activation of Met signaling in human colon cancer cells, but 0.5 and 1 µM EGCG also exhibited partial inhibition. How do these levels compare with the human situation? Due to extensive metabolism in humans, tea catechins typically reach plasma concentrations in the low µM range following normal tea consumption, but much higher levels can occur in the gastrointestinal tract [16,86]. Thus, some have argued that catechins may be more effective at targeting cancers of oral and gastrointestinal tissues, although as mentioned above, this is not apparent from the epidemiology studies conducted to date. An interesting question, and one for which there is need for greater research, is the extent to which tea catechins might complement ongoing cancer therapies that specifically target RTKs.
Table 2.
Summary of the suppressive effects of EGCG on different RTKs.
| RTK | Effective [EGCG] | Cell line | Ref |
|---|---|---|---|
| EGFR | 2.2 µM | SW480 | [55] |
| 10 µM | A431 | [91] | |
| 20 µM | KYSE150 | [53] | |
| 21.8 µM | YCH-H891, YCU-N861 | [92] | |
| 25 µM | HSC | [52] | |
| 30 µM | ECE16-1 | [71] | |
| 32.7 µM | A431 | [91] | |
| 43.6 µM | HT-29 | [54] | |
| Her-2/neu | 87.3 µM | NF639 | [93] |
| 87.3 µM | NF639, SMF | [94] | |
| IGF-R1 | 14 µM | MCF-7, HeLa, NIH3T3 | [50] |
| 43.6 µM | HEPG2 | [49] | |
| Met | 1 µM | FaDu | [95] |
| 5 µM | MDA-MB-231 | [68] | |
| 5 µM | HCT116 | [69] | |
| 10 µM | H2122, H358, H460 | [96] | |
| PDGF-R | 5 µM | LI90 | [97] |
| 10 µM | hVSMC | [98] | |
| 10 µM | NIH3T3 | [91] | |
| 12.5 µM | Fibroblasts | [64] | |
| 20 µM | VSMC | [63] | |
| 32.7 µM | NIH3T3 | [51] | |
| 50 µM | A172 | [62] | |
| 50 µM | VSMC | [65] | |
| 50 µM | HSC | [99] | |
| VEGF-R1 | 1.56 µM | HUVEC | [59] |
| 6.8 µM | CLL B | [58] | |
| 21.8 µM | HuH7 | [60] |
Indeed, several RTK inhibitors currently are being evaluated in the clinical setting [100–104]. One example is Herceptin (trastuzumad), a humanized monoclonal antibody against HER-2, used in the treatment of metastatic breast cancer [100]. Herceptin has demonstrated clinical promise, but not without some toxicity and drug resistance, which prompted the development of newer candidates such as lapatinib (Tykerb), HSP90 inhibitors, and T-DM1 [100]. A similar picture has emerged with RTK inhibitors against malignant glioma, gastrointestinal stromal tumors (GIST), malignant melanoma, pancreatic cancer, renal cell carcinoma, and thyroid cancers [101–104]. Sunitinib, for example, is currently approved as a first-line approach for renal cell carcinoma, and in combination treatment modalities for GIST [102]. Benefits of sunitinib therapy have been observed in cases of hepatocellular cancer, non-small cell lung carcinoma, and pancreatic neuroendocrine tumors. Sunitinib can be administered daily, but side effects include fatigue, diarrhea, nausea, vomiting, anorexia, and bleeding events [103]. Axitinib is a newer drug candidate undergoing phase II and III trials, with data accruing on benefits against melanoma, renal carcinoma, pancreatic cancer, and other malignancies [104].
As highlighted in this review, most of the studies to date focusing on tea catechins and RTK signaling have been performed in cell culture (Table 2). Tea compounds and their metabolites need to be evaluated more extensively in vivo, and also in human clinical trials, to ascertain the true chemopreventive relevance in the context of RTK inhibitory mechanisms. An important aspect of the mechanistic work is that tea catechins inhibit certain RTKs, such as Met, at low micromolar concentrations, considered to be within the physiologically relevant range for human exposure. Future research should further illuminate these issues, including identifying the major points along the pathway of dysregulated RTK signaling that tea catechins are most effective, and the wider context of drug/diet interactions that might impact cancer prevention and treatment in a beneficial manner.
Acknowledgements
Research in the authors’ laboratory which contributed to this review was supported in part by NIH grants CA90890, CA122959, and ES00210.
References
- 1.Yang CS, Wang X, Lu G, Picinich SC. Cancer prevention by tea: animal studies, molecular mechanisms and human relevance. Nat Rev Cancer. 2009;9:429–439. doi: 10.1038/nrc2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferruzzi MG. The influence of beverage composition on delivery of phenolic compounds from coffee and tea. Physiol Behav. 2010;100:33–41. doi: 10.1016/j.physbeh.2010.01.035. [DOI] [PubMed] [Google Scholar]
- 3.Higdon JV, Frei B. Tea catechins and polyphenols: health effects, metabolism, and antioxidant functions. Crit Rev Food Sci Nutr. 2003;43:89–143. doi: 10.1080/10408690390826464. [DOI] [PubMed] [Google Scholar]
- 4.Wang Y, Ho CT. Polyphenolic chemistry of tea and coffee: a century of progress. J Agric Food Chem. 2009;57:8109–8114. doi: 10.1021/jf804025c. [DOI] [PubMed] [Google Scholar]
- 5.Ju J, Lu G, Lambert JD, Yang CS. Inhibition of carcinogenesis by tea constituents. Semin Cancer Biol. 2007;17:395–402. doi: 10.1016/j.semcancer.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Santana-Rios G, Orner GA, Amantana A, Provost C, Wu SY, Dashwood RH. Potent antimutagenic activity of white tea in comparison with green tea in the Salmonella assay. Mutat Res. 2001;495:61–74. doi: 10.1016/s1383-5718(01)00200-5. [DOI] [PubMed] [Google Scholar]
- 7.Kohri T, Nanjo F, Suzuki M, Seto R, Matsumoto N, Yamakawa M, et al. Synthesis of (−)-[4-3H]epigallocatechin gallate and its metabolic fate in rats after intravenous administration. J Agric Food Chem. 2001;49:1042–1048. doi: 10.1021/jf0011236. [DOI] [PubMed] [Google Scholar]
- 8.Lambert JD, Lee MJ, Lu H, Meng X, Hong JJ, Seril DN, et al. Epigallocatechin-3-gallate is absorbed but extensively glucuronidated following oral administration to mice. J Nutr. 2003;133:4172–4177. doi: 10.1093/jn/133.12.4172. [DOI] [PubMed] [Google Scholar]
- 9.Li C, Meng X, Winnik B, Lee MJ, Lu H, Sheng S, et al. Analysis of urinary metabolites of tea catechins by liquid chromatography/electrospray ionization mass spectrometry. Chem Res Toxicol. 2001;14:702–707. doi: 10.1021/tx0002536. [DOI] [PubMed] [Google Scholar]
- 10.Lambert JD, Sang S, Yang CS. Biotransformation of green tea polyphenols and the biological activities of those metabolites. Mol Pharm. 2007;4:819–825. doi: 10.1021/mp700075m. [DOI] [PubMed] [Google Scholar]
- 11.Kim S, Lee MJ, Hong J, Li C, Smith TJ, Yang GY, et al. Plasma and tissue levels of tea catechins in rats and mice during chronic consumption of green tea polyphenols. Nutr Cancer. 2000;37:41–48. doi: 10.1207/S15327914NC3701_5. [DOI] [PubMed] [Google Scholar]
- 12.Chen L, Lee MJ, Li H, Yang CS. Absorption, distribution, elimination of tea polyphenols in rats. Drug Metab Dispos. 1997;25:1045–1050. [PubMed] [Google Scholar]
- 13.Natsume M, Osakabe N, Oyama M, Sasaki M, Baba S, Nakamura Y, et al. Structures of (−)-epicatechin glucuronide identified from plasma and urine after oral ingestion of (−)-epicatechin: differences between human and rat. Free Radic Biol Med. 2003;34:840–849. doi: 10.1016/s0891-5849(02)01434-x. [DOI] [PubMed] [Google Scholar]
- 14.Van Amelsvoort JM, Van Hof KH, Mathot JN, Mulder TP, Wiersma A, Tijburg LB. Plasma concentrations of individual tea catechins after a single oral dose in humans. Xenobiotica. 2001;31:891–901. doi: 10.1080/00498250110079149. [DOI] [PubMed] [Google Scholar]
- 15.Ullmann U, Haller J, Decourt JP, Girault N, Girault J, Richard-Caudron AS, et al. A single ascending dose study of epigallocatechin gallate in healthy volunteers. J Int Med Res. 2003;31:88–101. doi: 10.1177/147323000303100205. [DOI] [PubMed] [Google Scholar]
- 16.Stalmach A, Mullen W, Steiling H, Williamson G, Lean ME, Crozier A. Absorption, metabolism, and excretion of green tea flavan-3-ols in humans with an ileostomy. Mol Nutr Food Res. 2010;54:323–334. doi: 10.1002/mnfr.200900194. [DOI] [PubMed] [Google Scholar]
- 17.Hou Z, Lambert JD, Chin KV, Yang CS. Effects of tea polyphenols on signal transduction pathways related to cancer chemoprevention. Mutat Res. 2004;555:3–19. doi: 10.1016/j.mrfmmm.2004.06.040. [DOI] [PubMed] [Google Scholar]
- 18.Lambert JD, Hong J, Yang GY, Liao J, Yang CS. Inhibition of carcinogenesis by polyphenols: evidence from laboratory investigations. Am J Clin Nutr. 2005;81:284S–291S. doi: 10.1093/ajcn/81.1.284S. [DOI] [PubMed] [Google Scholar]
- 19.Yang CS, Lambert JD, Hou Z, Ju J, Lu G, Hao X. Molecular targets for the cancer preventive activity of tea polyphenols. Mol Carcinog. 2006;45:431–435. doi: 10.1002/mc.20228. [DOI] [PubMed] [Google Scholar]
- 20.Orner GA, Dashwood WM, Blum CA, Diaz GD, Li Q, Dashwood RH. Suppression of tumorigenesis in the Apc(min) mouse: down-regulation of beta-catenin signaling by a combination of tea plus sulindac. Carcinogenesis. 2003;24:263–267. doi: 10.1093/carcin/24.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown MD. Green tea (Camellia sinensis) extract and its possible role in the prevention of cancer. Altern Med Rev. 1999;4:360–370. [PubMed] [Google Scholar]
- 22.Katiyar SK, Mukhtar H. Tea consumption and cancer. World Rev Nutr Diet. 1996;79:154–184. [PubMed] [Google Scholar]
- 23.Kohlmeier L, Weterings KG, Steck S, Kok FJ. Tea and cancer prevention: an evaluation of the epidemiologic literature. Nutr Cancer. 1997;27:1–13. doi: 10.1080/01635589709514494. [DOI] [PubMed] [Google Scholar]
- 24.Yang CS, Wang ZY. Tea and cancer. J Natl Cancer Inst. 1993;85:1038–1049. doi: 10.1093/jnci/85.13.1038. [DOI] [PubMed] [Google Scholar]
- 25.Yang CS, Lambert JD, Ju J, Lu G, Sang S. Tea and cancer prevention: molecular mechanisms and human relevance. Toxicol Appl Pharmacol. 2007;224:265–273. doi: 10.1016/j.taap.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang CS, Maliakal P, Meng X. Inhibition of carcinogenesis by tea. Annu Rev Pharmacol Toxicol. 2002;42:25–54. doi: 10.1146/annurev.pharmtox.42.082101.154309. [DOI] [PubMed] [Google Scholar]
- 27.Chacko SM, Thambi PT, Kuttan R, Nishigaki I. Beneficial effects of green tea: a literature review. Chin Med. 2010;5 doi: 10.1186/1749-8546-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsubono Y, Nishino Y, Komatsu S, Hsieh CC, Kanemura S, Tsuji I, et al. Green tea and the risk of gastric cancer in Japan. N Engl J Med. 2001;344:632–636. doi: 10.1056/NEJM200103013440903. [DOI] [PubMed] [Google Scholar]
- 29.Bettuzzi S, Brausi M, Rizzi F, Castagnetti G, Peracchia G, Corti A. Chemoprevention of human prostate cancer by oral administration of green tea catechins in volunteers with high-grade prostate intraepithelial neoplasia: a preliminary report from a one-year proof-of-principle study. Cancer Res. 2006;66:1234–1240. doi: 10.1158/0008-5472.CAN-05-1145. [DOI] [PubMed] [Google Scholar]
- 30.Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2010;14:1117–1134. doi: 10.1016/j.cell.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim EK, Choi EJ. Pathological roles of MAPK signaling pathways in human diseases. Biochim Biophys Acta. 2010;1802:396–405. doi: 10.1016/j.bbadis.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 32.Kennedy SG, Wagner AJ, Conzen SD, Jordan J, Bellacosa A, Tsichlis PN, et al. The PI 3-kinase/Akt signaling pathway delivers an anti-apoptotic signal. Genes Dev. 1997;11:701–713. doi: 10.1101/gad.11.6.701. [DOI] [PubMed] [Google Scholar]
- 33.Cardone MH, Roy N, Stennicke HR, Salvesen GS, Franke TF, Stanbridge E, et al. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:1318–1321. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- 34.Romashkova JA, Makarov SS. NF-kappaB is a target of AKT in anti-apoptotic PDGF signalling. Nature. 1999;401:86–90. doi: 10.1038/43474. [DOI] [PubMed] [Google Scholar]
- 35.Karin M, Cao Y, Greten FR, Li ZW. NF-kappaB in cancer: from innocent bystander to major culprit. Nat Rev Cancer. 2002;2:301–310. doi: 10.1038/nrc780. [DOI] [PubMed] [Google Scholar]
- 36.Richmond A. NF-kappa B, chemokine gene transcription and tumour growth. Nat Rev Immunol. 2002;2:664–674. doi: 10.1038/nri887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dhawan P, Richmond A. A novel NF-kappa B-inducing kinase-MAPK signaling pathway up-regulates NF-kappa B activity in melanoma cells. J Biol Chem. 2002;277:7920–7928. doi: 10.1074/jbc.M112210200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Albanese C, Johnson J, Watanabe G, Eklund N, Vu D, Arnold A, et al. Transforming p21ras mutants and c-Ets-2 activate the cyclin D1 promoter through distinguishable regions. J Biol Chem. 1995;270:23589–23597. doi: 10.1074/jbc.270.40.23589. [DOI] [PubMed] [Google Scholar]
- 39.Guttridge DC, Albanese C, Reuther JY, Pestell RG, Baldwin AS., Jr NF-kappaB controls cell growth and differentiation through transcriptional regulation of cyclin D1. Mol Cell Biol. 1999;19:5785–5799. doi: 10.1128/mcb.19.8.5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tanabe T, Tohnai N. Cyclooxygenase isozymes and their gene structures and expression. Prostaglandins Other Lipid Mediat. 2002;68–69:95–114. doi: 10.1016/s0090-6980(02)00024-2. [DOI] [PubMed] [Google Scholar]
- 41.Shishodia A, Aggarwal BB. Nuclear factor-kappaB: a friend or a foe in cancer? Biochem Pharmacol. 2004;68:1071–1080. doi: 10.1016/j.bcp.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 42.Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev. 1995;16:3–34. doi: 10.1210/edrv-16-1-3. [DOI] [PubMed] [Google Scholar]
- 43.Liu JP, Baker J, Perkins AS, Robertson EJ, Efstratiadis A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r) Cell. 1993;75:59–72. [PubMed] [Google Scholar]
- 44.Pollak MN, Schernhammer ES, Hankinson SE. Insulin-like growth factors and neoplasia. Nat Rev Cancer. 2004;4:505–518. doi: 10.1038/nrc1387. [DOI] [PubMed] [Google Scholar]
- 45.Gennigens C, Menetrier-Caux C, Droz JP. Insulin-like growth factor (IGF) family and prostate cancer. Crit Rev Oncol Hematol. 2006;58:124–145. doi: 10.1016/j.critrevonc.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 46.Donovan EA, Kummar S. Role of insulin-like growth factor-1R system in colorectal carcinogenesis. Crit Rev Oncol Hematol. 2008;66:91–98. doi: 10.1016/j.critrevonc.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Larsson O, Girnita A, Girnita L. Role of insulin-like growth factor 1 receptor signalling in cancer. Br J Cancer. 2005;92:2097–2101. doi: 10.1038/sj.bjc.6602627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huynh H, Chow PK, Ooi LL, Soo KC. A possible role for insulin-like growth factor-binding protein-3 autocrine/paracrine loops in controlling hepatocellular carcinoma cell proliferation. Cell Growth Differ. 2002;13:115–122. [PubMed] [Google Scholar]
- 49.Shimizu M, Shirakami Y, Sakai H, Tatebe H, Nakagawa T, Hara Y, et al. EGCG inhibits activation of the insulin-like growth factor (IGF)/IGF-1 receptor axis in human hepatocellular carcinoma cells. Cancer Lett. 2007;262:10–18. doi: 10.1016/j.canlet.2007.11.026. [DOI] [PubMed] [Google Scholar]
- 50.Li M, He Z, Ermakova S, Zheng D, Tang F, Cho YY, et al. Direct inhibition of insulin-like growth factor-I receptor kinase activity by (−)-epigallocatechin-3-gallate regulates cell transformation. Cancer Epidemiol Biomarkers Prev. 2007;16:598–605. doi: 10.1158/1055-9965.EPI-06-0892. [DOI] [PubMed] [Google Scholar]
- 51.Liang YC, Lin-shiau SY, Chen CF, Lin JK. Suppression of extracellular signals and cell proliferation through EGF receptor binding by (−)-epigallocatechin gallate in human A431 epidermoid carcinoma cells. J Cell Biochem. 1997;67:55–65. doi: 10.1002/(sici)1097-4644(19971001)67:1<55::aid-jcb6>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 52.Fuand Y, Chen A. The phytochemical (−)-epigallocatechin gallate suppresses gene expression of epidermal growth factor receptor in rat hepatic stellate cells in vitro by reducing the activity of Egr-1. Biochem Pharmacol. 2006;72:227–238. doi: 10.1016/j.bcp.2006.04.026. [DOI] [PubMed] [Google Scholar]
- 53.Chin V, Yang CS. Mechanism of action of (−)-epigallocatechin-3-gallate: auto-oxidation-dependent inactivation of epidermal growth factor receptor and direct effects on growth inhibition in human esophageal cancer KYSE 150 cells. Cancer Res. 2005;65:8049–8056. doi: 10.1158/0008-5472.CAN-05-0480. [DOI] [PubMed] [Google Scholar]
- 54.Dashwood WM, Orner GA, Dashwood RH. Inhibition of beta-catenin/Tcf activity by white tea, green tea, and epigallocatechin-3-gallate (EGCG): minor contribution of H2O2 at physiologically relevant EGCG concentrations. Biochem Biophys Res Commun. 2002;296:584–588. doi: 10.1016/s0006-291x(02)00914-2. [DOI] [PubMed] [Google Scholar]
- 55.Adachi S, Nagao T, To S, Joe AK, Shimizu M, Matsushima-Nishiwaki R, et al. (−)-Epigallocatechin gallate causes internalization of the epidermal growth factor receptor in human colon cancer cells. Carcinogenesis. 2008;29:1986–1993. doi: 10.1093/carcin/bgn128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cross MJ, Dixelius J, Matsumoto T, Claesson-Welsh L. VEGF-receptor signal transduction. Trends Biochem Sci. 2003;28:488–494. doi: 10.1016/S0968-0004(03)00193-2. [DOI] [PubMed] [Google Scholar]
- 57.Ng YS, Krilleke D, Shima DT. VEGF function in vascular pathogenesis. Exp Cell Res. 2006;312:527–537. doi: 10.1016/j.yexcr.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 58.Lee YK, Bone ND, Strege AK, Shanafelt TD, Jelinek DF, Kay NE. VEGF receptor phosphorylation status and apoptosis is modulated by a green tea component, epigallocatechin-3-gallate (EGCG), in B-cell chronic lymphocytic leukemia. Blood. 2004;104:788–794. doi: 10.1182/blood-2003-08-2763. [DOI] [PubMed] [Google Scholar]
- 59.Kondo T, Ohta T, Igura K, Hara Y, Kaji K. Tea catechins inhibit angiogenesis in vitro, measured by human endothelial cell growth, migration and tube formation, through inhibition of VEGF receptor binding. Cancer Lett. 2002;180:139–144. doi: 10.1016/s0304-3835(02)00007-1. [DOI] [PubMed] [Google Scholar]
- 60.Shirakami Y, Shimizu M, Adachi S, Sakai H, Nakagawa T, Yasuda Y, et al. (−)-Epigallocatechin gallate suppresses the growth of human hepatocellular carcinoma cells by inhibiting activation of the vascular endothelial growth factor-vascular endothelial growth factor receptor axis. Cancer Sci. 2009;100:1957–1962. doi: 10.1111/j.1349-7006.2009.01241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ahn HY, Hadizadeh KR, Seul C, Yun YP, Vetter H, Sachinidis A. Epigallocathechin-3 gallate selectively inhibits the PDGF-BB-induced intracellular signaling transduction pathway in vascular smooth muscle cells and inhibits transformation of sis-transfected NIH 3T3 fibroblasts and human glioblastoma cells (A172) Mol Biol Cell. 1999;10:1093–1104. doi: 10.1091/mbc.10.4.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sachinidis A, Seul C, Seewald S, Ahn H, Ko Y, Vetter H. Green tea compounds inhibit tyrosine phosphorylation of PDGF beta-receptor and transformation of A172 human glioblastoma. FEBS Lett. 2000;471:51–55. doi: 10.1016/s0014-5793(00)01360-0. [DOI] [PubMed] [Google Scholar]
- 63.Sachinidis A, Skach RA, Seul C, Ko Y, Hescheler J, Ahn HY, et al. Inhibition of the PDGF beta-receptor tyrosine phosphorylation and its downstream intracellular signal transduction pathway in rat and human vascular smooth muscle cells by different catechins. FASEB J. 2002;16:893–895. doi: 10.1096/fj.01-0799fje. [DOI] [PubMed] [Google Scholar]
- 64.Suzuki Y, Hattori S, Isemura M. Epigallocatechin-3-O-gallate inhibits fibroblast contraction of floating collagen gel: interaction between epigallocatechin-3-O-gallate and platelet derived growth factor. Biosci Biotechnol Biochem. 2004;68:1817–1820. doi: 10.1271/bbb.68.1817. [DOI] [PubMed] [Google Scholar]
- 65.Weber AA, Neuhaus T, Skach RA, Hescheler J, Ahn HY, Schror K, et al. Mechanisms of the inhibitory effects of epigallocatechin-3 gallate on platelet-derived growth factor-BB-induced cell signaling and mitogenesis. FASEB J. 2004;18:128–130. doi: 10.1096/fj.03-0007fje. [DOI] [PubMed] [Google Scholar]
- 66.Naran S, Zhang X, Hughes SJ. Inhibition of HGF/MET as therapy for malignancy. Expert Opin Ther Targets. 2009;13:569–581. doi: 10.1517/14728220902853917. [DOI] [PubMed] [Google Scholar]
- 67.Ma PC, Maulik G, Christensen J, Salgia R. c-Met: structure, functions and potential for therapeutic inhibition. Cancer Metastasis Rev. 2003;22:309–325. doi: 10.1023/a:1023768811842. [DOI] [PubMed] [Google Scholar]
- 68.Bigelow RL, Cardelli JA. The green tea catechins, (−)-epigallocatechin-3-gallate (EGCG) and (−)-epicatechin-3-gallate (ECG), inhibit HGF/Met signaling in immortalized and tumorigenic breast epithelial cells. Oncogene. 2006;25:1922–1930. doi: 10.1038/sj.onc.1209227. [DOI] [PubMed] [Google Scholar]
- 69.Larsen CA, Bisson WH, Dashwood RH. Tea catechins inhibit hepatocyte growth factor receptor (MET kinase) activity in human colon cancer cells: kinetic and molecular docking studies. J Med Chem. 2009;52:6543–6545. doi: 10.1021/jm901330e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Larsen CA, Dashwood RH. (−)-Epigallocatechin-3-gallate inhibits Met signaling, proliferation, and invasiveness inhumancolon cancer cells. Arch Biochem Biophys. 2010 March 31; doi: 10.1016/j.abb.2010.03.017. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sah JF, Balasubramanian S, Eckert RL, Rorke EA. Epigallocatechin-3-gallate inhibits epidermal growth factor receptor signaling pathway. Evidence for direct inhibition of RK1/2 and AKT kinases. J Biol Chem. 2004;279:12755–12762. doi: 10.1074/jbc.M312333200. [DOI] [PubMed] [Google Scholar]
- 72.Long LH, Clement MV, Halliwell B. Artifacts in cell culture: rapid generation of hydrogen peroxide on addition of (−)-epigallocatechin, (−)-epigallocatechin gallate (+)-catechin, and quercetin to commonly used cell culture media. Biochem Biophys Res Commun. 2000;273:50–53. doi: 10.1006/bbrc.2000.2895. [DOI] [PubMed] [Google Scholar]
- 73.Hashigasako A, Machide M, Nakamura T, Matsumoto K, Nakamura T. Bidirectional regulation of Ser-985 phosphorylation of c-met via protein kinase C and protein phosphatase 2A involves c-Met activation and cellular responsiveness to hepatocyte growth factor. J Biol Chem. 2004;279:26445–26452. doi: 10.1074/jbc.M314254200. [DOI] [PubMed] [Google Scholar]
- 74.Larsen CA, Dashwood RH. Suppression of Met activation in human colon cancer cells treated with (−)-epigallocatechin-3-gallate: minor role of hydrogen peroxide. Biochem Biophys Res Commun. 2009;389:527–530. doi: 10.1016/j.bbrc.2009.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Brown DA, London E. Functions of lipid rafts in biological membranes. Annu Rev Cell Dev Biol. 1998;14:111–136. doi: 10.1146/annurev.cellbio.14.1.111. [DOI] [PubMed] [Google Scholar]
- 76.Prinetti A, Chigorno V, Tettamanti G, Sonnino S. Sphingolipid-enriched membrane domains from rat cerebellar granule cells differentiated in culture. A compositional study. J Biol Chem. 2000;275:11658–11665. doi: 10.1074/jbc.275.16.11658. [DOI] [PubMed] [Google Scholar]
- 77.Pike LJ, Han X, Gross RW. Epidermal growth factor receptors are localized to lipid rafts that contain a balance of inner and outer leaflet lipids: a shotgun lipidomics study. J Biol Chem. 2005;280:26796–26804. doi: 10.1074/jbc.M503805200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Remacle-Bonnet M, Garrouste F, Baillat G, Andre F, Marvaldi J, Pommier G. Membrane rafts segregate pro- from anti-apoptotic insulin-like growth factor-I receptor signaling in colon carcinoma cells stimulated by members of the tumor necrosis factor superfamily. Am J Pathol. 2005;167:761–773. doi: 10.1016/S0002-9440(10)62049-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pike LJ, Casey L. Cholesterol levels modulate EGF receptor-mediated signaling by altering receptor function and trafficking. Biochemistry. 2002;41:10315–10322. doi: 10.1021/bi025943i. [DOI] [PubMed] [Google Scholar]
- 80.Puri C, Tosoni D, Comai R, Rabellino A, Segat D, Caneva F, et al. Relationships between EGFR signaling-competent and endocytosis-competent membrane microdomains. Mol Biol Cell. 2005;16:2704–2718. doi: 10.1091/mbc.E04-07-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Massieand C, Mills IG. The developing role of receptors and adaptors. Nat Rev Cancer. 2006;6:403–409. doi: 10.1038/nrc1882. [DOI] [PubMed] [Google Scholar]
- 82.Teillet F, Boumendjel A, Boutonnat J, Ronot X. Flavonoids as RTK inhibitors and potential anticancer agents. Med Res Rev. 2008;28:715–745. doi: 10.1002/med.20122. [DOI] [PubMed] [Google Scholar]
- 83.Albrecht BK, Harmange JC, Bauer D, Berry L, Bode C, Boezio AA, et al. Discovery and optimization of triazolopyridazines as potent and selective inhibitors of the c-Met kinase. J Med Chem. 2008;51:2879–2882. doi: 10.1021/jm800043g. [DOI] [PubMed] [Google Scholar]
- 84.Timofeevski SL, McTigue MA, Ryan K, Cui J, Zou HY, Zhu JX, et al. Enzymatic characterization of c-Met receptor tyrosine kinase oncogenic mutants and kinetic studies with aminopyridine and triazolopyrazaine inhibitors. Biochemistry. 2009;48:5339–5349. doi: 10.1021/bi900438w. [DOI] [PubMed] [Google Scholar]
- 85.Kuzuhara T, Suganuma M, Fujiki H. Green tea catechin as a chemical chaperone in cancer prevention. Cancer Lett. 2008;261:12–20. doi: 10.1016/j.canlet.2007.10.037. [DOI] [PubMed] [Google Scholar]
- 86.Yang CS, Sang S, Lambert JD, Lee MJ. Bioavailability issues in studying the health effects of plant polyphenolic compounds. Mol Nutr Food Res. 2008;52 Suppl. 1:S139–S151. doi: 10.1002/mnfr.200700234. [DOI] [PubMed] [Google Scholar]
- 87.Sirtori CR, Galli C, Anderson JW, Sirtori E, Arnoldi A. Functional foods for dyslipidaemia and cardiovascular risk prevention. Nutr Res Rev. 2009;22:244–261. doi: 10.1017/S0954422409990187. [DOI] [PubMed] [Google Scholar]
- 88.Grove KA, Lambert JD. Laboratory, epidemiological, and human intervention studies show that tea (Camellia sinensis) may be useful in the prevention of obesity. J Nutr. 2010;140:446–453. doi: 10.3945/jn.109.115972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Richard D, Kefi K, Barbe U, Poli A, Bausero P, Visioli F. Weight and plasma lipid control by decaffeinated green tea. Pharmacol Res. 2009;59:351–354. doi: 10.1016/j.phrs.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 90.Kim J, Lee HJ, Lee KW. Naturally occurring phytochemicals for the prevention of Alzheimer’s disease. J Neurochem. 2010;112:1415–1430. doi: 10.1111/j.1471-4159.2009.06562.x. [DOI] [PubMed] [Google Scholar]
- 91.Liang YC, Chen YC, Lin YL, Lin-Shiau SY, Ho CT, Lin JK. Suppression of extracellular signals and cell proliferation by the black tea polyphenol, theaflavin-3,3′-digallate. Carcinogenesis. 1999;20:733–736. doi: 10.1093/carcin/20.4.733. [DOI] [PubMed] [Google Scholar]
- 92.Masuda M, Suzui M, Weinstein IB. Effects of epigallocatechin-3-gallate on growth, epidermal growth factor receptor signaling pathways, gene expression, and chemosensitivity in human head and neck squamous cell carcinoma cell lines. Clin Cancer Res. 2001;7:4220–4229. [PubMed] [Google Scholar]
- 93.Guo S, Lu J, Subramanian A, Sonenshein GE. Microarray-assisted pathway analysis identifies mitogen-activated protein kinase signaling as a mediator of resistance to the green tea polyphenol epigallocatechin 3-gallate in her-2/neu-overexpressing breast cancer cells. Cancer Res. 2006;66:5322–5329. doi: 10.1158/0008-5472.CAN-05-4287. [DOI] [PubMed] [Google Scholar]
- 94.Pianetti S, Guo S, Kavanagh KT, Sonenshein GE. Green tea polyphenol epigallocatechin-3 gallate inhibits Her-2/neu signaling, proliferation, and transformed phenotype of breast cancer cells. Cancer Res. 2002;62:652–655. [PubMed] [Google Scholar]
- 95.Lim YC, Park HY, Hwang SW, Kang SU, Pyun JH, Lee MH, et al. (−)-Epigallocatechin-3-gallate (EGCG) inhibits HGF-induced invasion and metastasis in hypopharyngeal carcinoma cells. Cancer Lett. 2008;271:140–152. doi: 10.1016/j.canlet.2008.05.048. [DOI] [PubMed] [Google Scholar]
- 96.Milligan SA, Burke P, Coleman DT, Bigelow RL, Steffan JJ, Carroll JL, et al. The green tea polyphenol EGCG potentiates the antiproliferative activity of c-Met and epidermal growth factor receptor inhibitors in non-small cell lung cancer cells. Clin Cancer Res. 2009;15:4885–4894. doi: 10.1158/1078-0432.CCR-09-0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sakata R, Ueno T, Nakamura T, Sakamoto M, Torimura T, Sata M. Green tea polyphenol epigallocatechin-3-gallate inhibits platelet-derived growth factor-induced proliferation of human hepatic stellate cell line LI90. J Hepatol. 2004;40:52–59. doi: 10.1016/s0168-8278(03)00477-x. [DOI] [PubMed] [Google Scholar]
- 98.Park JS, Kim MH, Chang HJ, Kim KM, Kim SM, Shin BA, et al. Epigallocatechin-3-gallate inhibits the PDGF-induced VEGF expression in human vascular smooth muscle cells via blocking PDGF receptor and Erk-1/2. Int J Oncol. 2006;29:1247–1252. [PubMed] [Google Scholar]
- 99.Chen A, Zhang L. The antioxidant (−)-epigallocatechin-3-gallate inhibits rat hepatic stellate cell proliferation in vitro by blocking the tyrosine phosphorylation and reducing the gene expression of platelet-derived growth factor-beta receptor. J Biol Chem. 2003;278:23381–23389. doi: 10.1074/jbc.M212042200. [DOI] [PubMed] [Google Scholar]
- 100.Murphy CC, Fornier M. HER2-positive breast cancer: beyond trastuzumab. Oncology. 2010;24:410–415. [PubMed] [Google Scholar]
- 101.Ren H, Yang BF, Rainov NG. Receptor tyrosine kinases as therapeutic targets in malignant glioma. Rev Recent Clin Trials. 2007;2:87–101. doi: 10.2174/157488707780599384. [DOI] [PubMed] [Google Scholar]
- 102.Wolter P, Schöffski P. Targeted therapies in the treatment of GIST: adverse events and maximising the benefits of sunitinib through proactive therapy management. Acta Oncol. 2010;49:13–23. doi: 10.3109/02841860903287205. [DOI] [PubMed] [Google Scholar]
- 103.Papaetis GS, Syrigo KN. Sunitinib: a multitargeted receptor tyrosine kinase inhibitor in the era of molecular cancer therapies. BioDrugs. 2009;23:377–389. doi: 10.2165/11318860-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 104.Kelly RJ, Rixe O. Axitinib (AG-013736) Recent Results Cancer Res. 2010;184:33–44. doi: 10.1007/978-3-642-01222-8_3. [DOI] [PubMed] [Google Scholar]