Abstract
When cells in our body change their genome and develop into cancer, we blame it on genome instability. When novel species conquer inhospitable environments, we credit it to genome evolution. From a cellular perspective, however, both processes are outcomes of the same fundamental biological properties – genome and pathway plasticity and the natural selection of cells that escape death and acquire growth advantages. Unraveling the consequences of genome plasticity at a cellular level is not only central to the understanding of species evolution but also crucial to deciphering important cell biological problems, such as how cancer cells emerge and how pathogens develop drug resistance. Aside from the well-known role of DNA sequence mutations, recent evidence suggests that changes in DNA copy numbers in the form of segmental or whole-chromosome aneuploidy can bring about large phenotypic variation. Although usually detrimental under conditions suitable for normal proliferation of euploid cells, aneuploidization may be a frequently occurring genetic change that enables pathogens or cancer cells to escape physiological or pharmacological roadblocks.
Introduction
Genomic instability has been a central issue in several basic fields of cell biology for the past decades, spanning from growth and cell cycle regulation, mitosis and meiosis, to cancer progression. Among the main products of genomic instability, and especially of chromosome instability, are DNA copy number changes that often involve relatively large chromosomal regions and in some cases span entire chromosomes. For the scope of this review, we will hereafter refer to segmental and whole-chromosome copy number changes collectively as `aneuploidy'. Tremendous efforts have been devoted to the elucidation of the mechanisms leading to aneuploidy (comprehensively reviewed in [1,2]). We only emphasize here that there is a wide variety of roads that could lead to aneuploidy. Perturbations in components of the chromosome segregation machinery, in the spindle assembly checkpoint, in the S-phase checkpoint, as well as in homologous and non-homologous recombination pathways are some of the common causes leading to either segmental or whole-chromosome aneuploidy [1,2]. With so many different doors open to aneuploidy, frequent observation of this mutation comes with no surprise. In fact, aneuploidy has long been observed in many different organisms and contexts (Table 1). This article focuses on the consequences of aneuploidy at the cellular level, which has been debated in at least two different but related contexts.
Table 1.
Examples of spontaneous occurrence of aneuploidy in eukaryotic cells
| Organism | Context | Perturbation / selective force | Aneuploidy (a) | Consequence | Reference |
|---|---|---|---|---|---|
| H. sapiens | Cancer | Growth control mechanisms, microenvironment, chemotherapy, etc. | WCA/SA | Oncogenesis, metastasis, drug resistance, etc. | [4,11–13] |
| H. sapiens | Embryonic development | Unknown | WCA | Unknown | [40] |
| H. sapiens | Adult brain | Unknown | WCA | Unknown | [41] |
| M. musculus | Nervous system | Unknown | WCA | Unknown | [42] |
| C. glabrata | Fungal pathogenesis | Immune response | SA | Increased virulence | [15] |
| C. albicans | Fungal pathogenesis | Antifungal drug | SA | Drug resistance | [14] |
| C. albicans | Standard laboratory conditions | Transformation, counter-selection? | WCA/SA | Unclear | [43] |
| Lager yeast | Industrial setting | Selection for high ethanol production? | SA | Increased ethanol production? | [44] |
| S. cerevisiae | Natural habitat | Environmental | SA | Adaptation to environmental niches | [45] |
| S. cerevisiae | Standard laboratory conditions | Genetic disruption of non-essential genes | WCA/SA | Improved growth | [18] |
| S. cerevisiae | Standard laboratory conditions | Genetic disruption of TEL1 and MEC1 genes | WCA/SA | Improved growth | [20] |
| S. cerevisiae | Experimental evolution | Nutrient limitation | SA | Improved growth | [16,17] |
| S. cerevisiae | Experimental evolution | Genetic disruption of MYO1 gene | WCA | Restoration of cytokinesis | [19] |
Whole-chromosome aneuploidy (WCA) or segmental aneuploidy (SA)
Debates on aneuploidy
Since most cancer cells are aneuploid, one debate has centered on whether aneuploidy is a cause or a consequence of cancer [3,4]. As cancer cells are often defective in one or more cellular machineries that ensure genome integrity and stability, it is natural to think of aneuploidy as a, perhaps innocent, byproduct of the cellular transformation process itself [5]. The opposing argument is that, based on the view that cancer is a Darwinian process where cells are selected to overcome severe restrictive conditions, ranging from tissue-specific growth controls to clinical treatments [6–8], aneuploidy is a form of mutation that could contribute to oncogenesis, metastasis and the development of chemotherapy resistance [9–13]. Another debate revolves around whether aneuploidy is a beneficial or a detrimental mutation. Aneuploidy has been shown to facilitate pathogenic yeasts to escape antimicrobial drugs [14] or increase their virulence [15], as well as to help budding yeast cells to cope with either nutrient limitations [16,17] or strong genetic perturbations [18–20] (further discussed below). On the other hand, aneuploidy has long been known to have detrimental effects on organismal development and recently shown to reduce cellular fitness [21–23].
Below we offer an explanation that could reconcile the above debates. Based mostly on evidence accumulated from the study of pathogenic and budding yeast, we first argue that aneuploidy is a highly accessible mutation that can produce large effects on the cellular phenotype by changing the copy number of several to many genes simultaneously. While the euploid cells are optimized to thrive under `normal' conditions, aneuploids, by deviating from the normal physiological state, can become considerably less fit under such circumstances. However, under conditions severely adverse to euploid cells, it may be the aneuploid variants, much more than their euploid counterparts, that hold the potential to adapt by finding creative solutions as a result of the large phenotypic changes brought about by DNA copy number changes. Our analysis supports the view that genome instability and aneuploidy create a formidable positive feedback loop that may `switch on' aggressive cancer phenotypes and catalyze emergence of drug resistance [10,12].
Aneuploidy affects gene expression on multiple levels
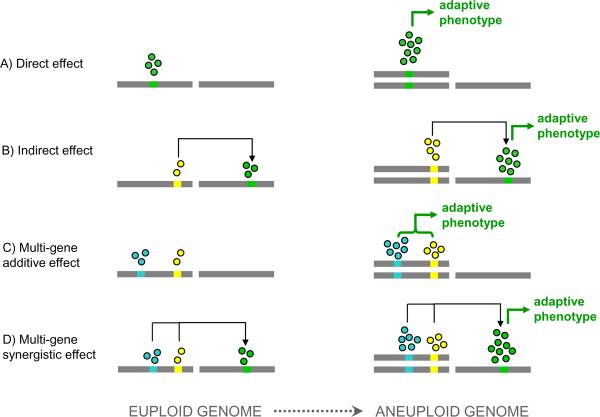
An important question to answer in order to resolve the above debated issues is whether aneuploidy has phenotypic consequences, i.e. whether it is `innocent' or `guilty' of causing phenotypic changes, and if the answer is yes, then what the consequences might be in respect to fitness or cancer progression. Evidence gathered in recent years across different organisms unequivocally suggests that changes in gene copy numbers caused by aneuploidy directly lead to changes in the level of mRNA expression [11,18,19,21,23–25]. These studies revealed that aneuploidy directly affects the expression of a large number of genes by a relatively small degree and a small number of genes by a relatively high degree (Figure 1). The vast majority of genes encoded on an aneuploid chromosome display an mRNA expression that is proportional to gene copy number (Figure 1A). For example, in a study characterizing aneuploid yeast strains able to overcome a severe defect in cytokinesis due to gene deletion of the Myo1 protein, diploid yeast strains that gained a single copy of a chromosome display a ~50% higher expression level compared to a control euploid strain for ~99% of the genes on the aneuploid chromosome [19]. In triploid strains that gained or lost a single copy of a chromosome, the average gene expression on the aneuploid chromosome was increased or decreased by ~33%, respectively [19]. In addition to this relatively moderate effect directly determined by gene dosage, these aneuploid yeast strains showed also a small fraction of outlier genes that were highly differentially expressed. Since these genes were not necessarily encoded on aneuploid chromosomes [19], their transcriptional change could not be explained by the gene copy number change. Instead, these outlier genes were highly enriched for direct or indirect targets of transcription factors encoded on the aneuploid chromosomes [19] (Figure 1B). Interestingly, such trans-acting effects of aneuploidy on gene expression had long been known in maize [26].
Figure 1. Effects of aneuploidy on gene expression and phenotype.
Schematic representation of several possible mechanisms by which aneuploidy can affect gene expression and give rise to adaptive phenotypes. (A) Direct effect of gene copy number change due to aneuploidy on gene expression level, which in some cases might be sufficient to bring about an adaptive phenotype. (B) Some of the genes directly affected by aneuploidy can have trans-acting effects on the expression of target genes not necessarily residing on the aneuploid chromosomes. In some cases, adaptive phenotypic changes are brought about by such indirect effects. (C) When aneuploidy increases the copy number of more than one gene at a time, changes in the expression of each of the genes can independently and additively converge to influence a cellular phenotype. (D) Changes in the expression of multiple genes on aneuploid chromosomes can synergistically cause large changes in the expression of target genes that are not necessarily carried on aneuploid chromosomes. In some cases, such synergistically-induced changes could give rise to adaptive phenotypes. Chromosomes are represented as grey bars, genes as colored rectangles, gene products as colored circles, trans-acting effects as black arrows and phenotypic effects as green arrows. The hypothetical two-chromosome haploid genome is depicted on the left; the corresponding aneuploid genome (carrying an extra copy of the first chromosome) is shown on the right.
While the ability of specific aneuploidy to confer adaptive phenotypes (further discussed below) suggests that the increased RNA expression level is likely to result in an increase in protein level in order to have functional consequences, this so far has not been consistently demonstrated by protein analysis. Immunoblot analysis of 16 different proteins encoded on aneuploid chromosomes in a systematic collection of aneuploid yeast strains found that only 3 exhibited the abundance increase predicted by the increased gene expression as a result of aneuploidy [21]. This finding led to the conclusion that either extra mRNA molecules were not translated or that the extra protein products were degraded shortly after translation, both ultimately leading to dosage compensation at the protein level. However, this finding may also be explained by the fact that 11 of the 13 proteins that did not show protein level increase were components of various protein complexes [21], and prior evidence suggests that proteins that are parts of larger complexes may be unstable until incorporated into complexes [27]. Also concluding against the presence of dosage compensation in yeast, a recent study found that for ~80% of 730 budding yeast proteins analyzed by flow cytometry, a 50% reduction in gene dosage leads to a ~50% reduction in protein level [28]; however, this study was conducted with diploid yeast strains bearing heterozygous deletion for individual genes rather than with aneuploid strains. A more systematic proteomics approach will be required to fully understand the extent to which aneuploidy-generated changes in mRNA level translate into changes in protein abundance.
Aneuploidy profoundly affects the cellular phenotype
Despite a lack of conclusive demonstration on how aneuploidy may affect gene expression at the proteome level, evidence abound that aneuploidy can bring about large changes in cellular phenotypes. Below we discuss several mechanisms by which aneuploidy affects phenotypic adaptation.
Single gene-based effects
Although an earlier study found that changing copy number of individual genes does not always produce a measurable phenotypic change in wild-type cells grown under optimal conditions [29], under severe genetic or environmental perturbations, aneuploidy has been shown to provide significant growth advantages through up-regulation of individual genes. For example, it was serendipitously found that ~8% of budding yeast strains deleted of non-essential genes spontaneously acquired aneuploidy [18]. In some cases the gained chromosomal element carried a gene that was highly similar to the deleted gene, suggesting that aneuploid karyotype might have been selected for due to an increased copy number of the paralog of the deleted gene [18]. More recently, it has been shown in experimental evolution under sulfate limitation, that amplification of the high-affinity sulfate transporter SUL1 through segmental aneuploidy conferred a ~50% growth advantage compared to wild-type budding yeast cells [17]. Furthermore, when aneuploidy simultaneously changes copy number of several different genes, the probability for it to produce significant phenotypic changes might become even larger due to either additive or synergistic effects (Figure 1C–D, and see below).
Multi-gene additive effects
Adaptive phenotypic changes, especially in response to severe stress, may require the modulation of several different cellular pathways at once. While such large effects may be more difficult to accomplish via point mutations, aneuploidy, which can simultaneously change the copy number of several to many different genes, drastically increases the probability of such phenotypic leaps (Figure 1C). A well-characterized example is represented by acquisition of resistance to the widely-employed anti-fungal drug fluconazole by the human pathogen Candida albicans. Clinical isolates of fluconazole-resistant C. albicans strains often carry two extra copies of the left arm of chromosome V [14]. The resistance mechanism was shown to be independently and additively mediated by increased expression of two genes harbored on the left arm of chromosome V: ERG11, an ergosterol biosynthesis gene encoding the drug target, and TAC1, a transcriptional regulator of drug efflux pumps [30]. Remarkably, this aneuploid genome can be rapidly reproduced in the laboratory by adaptively evolving C. albicans strains in the presence of fluconazole [31].
Multi-gene synergistic effects
In some cases single or multiple chromosome aneuploidy can simultaneously change the copy number of several genes involved in a same pathway (Figure 1D). Synergistic effects of these changes may produce phenotypic outcomes that can be much larger than the sum of the individual changes. Saccharomyces cerevisiae cells freshly deleted of the MYO1 gene die within a few mitotic cell cycles because of the repeated failure to complete cytokinesis [32]. When the few surviving myo1Δ cells were adaptively evolved for several generations they often gained extra copies of chromosome XVI [19]. These evolved strains restored cytokinesis by thickening of the cell wall at the cell division site, a mechanism morphologically distinct from the one observed in the wild type [19]. This phenotype correlated with up-regulation of a set of genes involved in cell wall biogenesis; however, few of these genes were themselves on chromosome XVI. Instead, two of their upstream regulators, a transcription factor and a component of the MAP kinase cascade, are encoded on chromosome XVI. It was demonstrated that increasing copy number of both but not either gene alone in freshly generated myo1Δ cells was sufficient to restore cytokinesis by cell wall thickening [19]. This example shows that synergistic effects of small changes in gene expression as a result of aneuploidy can create large effects on cellular pathways and bring about novel adaptive phenotypes.
Enhanced promiscuity in regulatory interactions
Another possible way by which aneuploidy could generate adaptive phenotypes is by increasing the promiscuity of protein-protein interactions. It was recently shown that over-expression alone is sufficient to increase the promiscuity with which proteins physically interact with their potential partners or targets [33]. Over-expression as a result of aneuploidy might therefore unleash the ability of promiscuous protein interactions to induce pleiotropic effects on a large number of cellular pathways. A possible example of this phenomenon is represented by the drug resistance mechanism developed by a subset of lung cancer patients towards `gefinitib' or `erlotinib', specific inhibitors of the EGF receptor. In these patients, the drug resistance is not mediated by mutation or amplification of genes directly involved in the EGF receptor pathway but by copy number amplification of the MET gene, encoding a kinase not previously recognized to have a role in the EGF signaling cascade [34,35]. At high levels of expression, MET kinase was shown to phosphorylate and activate proteins downstream of the EGF receptor that are not usually targets of MET when the kinase is expressed at normal levels [34,35]. Thus, over-expression of MET due to an increase in gene copy number appears to allow cancer cells to bypass the requirement of EGF receptor to activate downstream pathways.
Conclusion and resolving the debates on aneuploidy
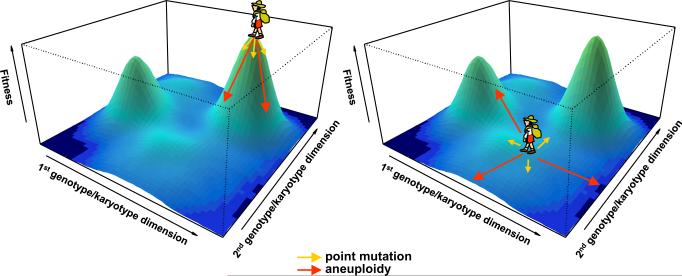
Based on the many ways by which aneuploidy can bring about phenotypic changes as discussed above, aneuploidy can be viewed as a large-effect mutation, through which large phenotypic leaps can be achieved in a single mutational step. In the classical theory of evolutionary adaptation, species are conceptualized as hikers on a fitness landscape that are trying to reach the nearest fitness peak through accumulation of a series of small-effect mutations [36] (Figure 2). In situations where cells are already atop a fitness peak, i.e. they are well adapted to a particular environment, large-effect mutations such as aneuploidy may be more likely to cause a fall from the peak, resulting in reduced fitness (Fig. 2A). However, if cells are situated in a fitness valley, far away from the nearest peaks, e.g. when they are under severe stress or growth inhibition, large-effect mutations such as aneuploidy may have a better chance to cause a large leap in the fitness landscape, enabling the cell to land on a higher fitness altitude, immediately gaining selective advantage [37] (Fig. 2B). By contrast, small-effect mutations, such as single nucleotide substitutions, would be less likely to result in significant fitness advantage in a single mutational step (Figure 2). This view resolves the debate over whether aneuploidy is beneficial or detrimental. We argue that aneuploidy is not intrinsically beneficial or detrimental to fitness; the outcome depends on the context, i.e. the shape of the fitness landscape and the initial position on the fitness landscape, as well as the nature of the specific aneuploidy under consideration, which could bring about specific changes in gene expression toward either higher or lower fitness.
Figure 2. Phenotypic leaps produced by aneuploidy during adaptive walks on a fitness landscape.
(A) When a cell (represented by a hiker) is atop or very close to a fitness peak, point mutations with small effects on fitness normally allow the cell to stay in the vicinity of the peak, while large-effect mutations such as aneuploidy typically push the cells towards regions of lower fitness. (B) When a cell is situated in a fitness valley far away from a peak (because of either a physiological growth control mechanism or a strong genetic or environmental perturbation), small-effect mutations rarely bring significant fitness gain in a single step, whereas the large phenotypic leaps brought about by aneuploidy can in some cases bring the cell much closer toward a nearby fitness peak, enabling immediate fitness advantage.
Although the above conclusions are drawn from work performed mostly in pathogenic and budding yeasts, the findings may lend insights into the question of whether aneuploidy is a cause or a consequence of cancer. In multi-cellular organisms, the presence of stringent and extensive growth control at the tissue or organ level suggests that most cells, from the perspective of their individual proliferative advantages, are not atop a fitness peak. Various types of checkpoints prevent genome plasticity and guard against the propensity of individual cells to undergo adaptive changes and escape growth control, thus ensuring fitness of the organism. In cancer, however, mutations impairing the chromosome segregation machinery, various checkpoints or normal growth control mechanisms enable the emergence of aneuploidy and other genetic changes [4]. Aneuploidy, in turn, has also been proposed to stimulate further genomic instability [12,38]. Consistent with this hypothesis, a recent study showed that even small dosage imbalances in the abundance of proteins involved in chromosome segregation increased chromosome instability in cultured human cells [39]. The emergence of aneuploidy could potentially provide large phenotypic leaps that enable cancer cells to gain proliferative advantages over euploid cells by overcoming tissue-specific growth control or differentiation signals, or even chemotherapy. Thus, oncogenesis and the rise of aneuploidy may constitute a formidable positive feedback loop driving the onset of highly aggressive tumor phenotypes. The implication of this idea is that effective cancer therapies must be able to break such viscous cycles by preventing the rise of aneuploidy and other adaptive mutations while imposing strong inhibition or rapidly killing of the highly proliferative tumor cell populations.
Acknowledgements
This work is supported by NIH grant RO1-059964 to RL.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
- 1.Aguilera A, Gomez-Gonzalez B. Genome instability: a mechanistic view of its causes and consequences. Nat Rev Genet. 2008;9:204–217. doi: 10.1038/nrg2268. [DOI] [PubMed] [Google Scholar]
- 2.Thompson SL, Bakhoum SF, Compton DA. Mechanisms of chromosomal instability. Curr Biol. 2010;20:R285–295. doi: 10.1016/j.cub.2010.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Webb T. When theories collide: experts develop different models for carcinogenesis. J Natl Cancer Inst. 2001;93:92–94. doi: 10.1093/jnci/93.2.92. [DOI] [PubMed] [Google Scholar]
- 4.Weaver BA, Cleveland DW. Does aneuploidy cause cancer? Curr Opin Cell Biol. 2006;18:658–667. doi: 10.1016/j.ceb.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 5.Zimonjic D, Brooks MW, Popescu N, Weinberg RA, Hahn WC. Derivation of human tumor cells in vitro without widespread genomic instability. Cancer Res. 2001;61:8838–8844. [PubMed] [Google Scholar]
- 6.Nowell PC. The clonal evolution of tumor cell populations. Science. 1976;194:23–28. doi: 10.1126/science.959840. [DOI] [PubMed] [Google Scholar]
- 7.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 8.Merlo LM, Pepper JW, Reid BJ, Maley CC. Cancer as an evolutionary and ecological process. Nat Rev Cancer. 2006;6:924–935. doi: 10.1038/nrc2013. [DOI] [PubMed] [Google Scholar]
- 9.Duesberg P, Stindl R, Hehlmann R. Origin of multidrug resistance in cells with and without multidrug resistance genes: chromosome reassortments catalyzed by aneuploidy. Proc Natl Acad Sci U S A. 2001;98:11283–11288. doi: 10.1073/pnas.201398998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duesberg P, Li R, Fabarius A, Hehlmann R. The chromosomal basis of cancer. Cell Oncol. 2005;27:293–318. doi: 10.1155/2005/951598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao C, Furge K, Koeman J, Dykema K, Su Y, Cutler ML, Werts A, Haak P, Vande Woude GF. Chromosome instability, chromosome transcriptome, and clonal evolution of tumor cell populations. Proc Natl Acad Sci U S A. 2007;104:8995–9000. doi: 10.1073/pnas.0700631104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nicholson JM, Duesberg P. On the karyotypic origin and evolution of cancer cells. Cancer Genet Cytogenet. 2009;194:96–110. doi: 10.1016/j.cancergencyto.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 13.Swanton C, Nicke B, Schuett M, Eklund AC, Ng C, Li Q, Hardcastle T, Lee A, Roy R, East P, et al. Chromosomal instability determines taxane response. Proc Natl Acad Sci U S A. 2009;106:8671–8676. doi: 10.1073/pnas.0811835106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Selmecki A, Forche A, Berman J. Aneuploidy and Isochromosome Formation in Drug-Resistant Candida albicans. Science. 2006;313:367–370. doi: 10.1126/science.1128242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Polakova S, Blume C, Zarate JA, Mentel M, Jorck-Ramberg D, Stenderup J, Piskur J. Formation of new chromosomes as a virulence mechanism in yeast Candida glabrata. Proc Natl Acad Sci U S A. 2009;106:2688–2693. doi: 10.1073/pnas.0809793106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dunham MJ, Badrane H, Ferea T, Adams J, Brown PO, Rosenzweig F, Botstein D. Characteristic genome rearrangements in experimental evolution of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 2002;99:16144–16149. doi: 10.1073/pnas.242624799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gresham D, Desai MM, Tucker CM, Jenq HT, Pai DA, Ward A, DeSevo CG, Botstein D, Dunham MJ. The repertoire and dynamics of evolutionary adaptations to controlled nutrient-limited environments in yeast. PLoS Genet. 2008;4:e1000303. doi: 10.1371/journal.pgen.1000303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hughes TR, Roberts CJ, Dai H, Jones AR, Meyer MR, Slade D, Burchard J, Dow S, Ward TR, Kidd MJ, et al. Widespread aneuploidy revealed by DNA microarray expression profiling. Nat Genet. 2000;25:333–337. doi: 10.1038/77116. [DOI] [PubMed] [Google Scholar]
- ** 19.Rancati G, Pavelka N, Fleharty B, Noll A, Trimble R, Walton K, Perera A, Staehling-Hampton K, Seidel CW, Li R. Aneuploidy underlies rapid adaptive evolution of yeast cells deprived of a conserved cytokinesis motor. Cell. 2008;135:879–893. doi: 10.1016/j.cell.2008.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study shows that when cells are highly impaired in their growth ability by a severe and irreversible disruption of a key cell division machinery, they are capable of finding creative workarounds by means of aneuploidy. Aneuploidy was shown to directly and indirectly cause a large number of gene expression changes, some of which synergistically modify the activity of specific pathways to accomplish cytokinesis through mechanisms not normally observed in wild-type yeast cells.
- 20.Vernon M, Lobachev K, Petes TD. High rates of “unselected” aneuploidy and chromosome rearrangements in tel1 mec1 haploid yeast strains. Genetics. 2008;179:237–247. doi: 10.1534/genetics.107.086603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Torres EM, Sokolsky T, Tucker CM, Chan LY, Boselli M, Dunham MJ, Amon A. Effects of aneuploidy on cellular physiology and cell division in haploid yeast. Science. 2007;317:916–924. doi: 10.1126/science.1142210. [DOI] [PubMed] [Google Scholar]
- 22.Torres EM, Williams BR, Amon A. Aneuploidy: cells losing their balance. Genetics. 2008;179:737–746. doi: 10.1534/genetics.108.090878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ** 23.Williams BR, Prabhu VR, Hunter KE, Glazier CM, Whittaker CA, Housman DE, Amon A. Aneuploidy affects proliferation and spontaneous immortalization in mammalian cells. Science. 2008;322:703–709. doi: 10.1126/science.1160058. [DOI] [PMC free article] [PubMed] [Google Scholar]; This represents the first attempt to systematically investigate the phenotypic effects of aneuploidy on development and cellular fitness in a mammalian system. First the authors found that all of the aneuploid mouse embryos examined showed a set of shared developmental defects. Secondly, primary cell lines derived from above aneuploid mice showed similar proliferation defects under standard tissue culture conditions.
- 24.Upender MB, Habermann JK, McShane LM, Korn EL, Barrett JC, Difilippantonio MJ, Ried T. Chromosome transfer induced aneuploidy results in complex dysregulation of the cellular transcriptome in immortalized and cancer cells. Cancer Res. 2004;64:6941–6949. doi: 10.1158/0008-5472.CAN-04-0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Birchler JA. Reflections on studies of gene expression in aneuploids. Biochem J. 2010;426:119–123. doi: 10.1042/BJ20091617. [DOI] [PubMed] [Google Scholar]
- 26.Guo M, Birchler JA. Trans-Acting Dosage Effects on the Expression of Model Gene Systems in Maize Aneuploids. Science. 1994;266:1999–2002. doi: 10.1126/science.266.5193.1999. [DOI] [PubMed] [Google Scholar]
- 27.Gorenstein C, Warner JR. Synthesis and turnover of ribosomal proteins in the absence of 60S subunit assembly in Saccharomyces cerevisiae. Mol Gen Genet. 1977;157:327–332. doi: 10.1007/BF00268670. [DOI] [PubMed] [Google Scholar]
- * 28.Springer M, Weissman JS, Kirschner MW. A general lack of compensation for gene dosage in yeast. Mol Syst Biol. 2010;6 doi: 10.1038/msb.2010.19. [DOI] [PMC free article] [PubMed] [Google Scholar]; By flow-cytometric measurement of GFP-tagged proteins in diploid yeast strains deleted of the second copy of the corresponding genes, authors were able to show that a 50% reduction in gene dosage generally leads to ~50% reduction in protein expression. This suggests that for most genes in yeast there is no dosage compensation at the protein level.
- 29.Moriya H, Shimizu-Yoshida Y, Kitano H. In vivo robustness analysis of cell division cycle genes in Saccharomyces cerevisiae. PLoS Genetics. 2006;2:e111. doi: 10.1371/journal.pgen.0020111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Selmecki A, Gerami-Nejad M, Paulson C, Forche A, Berman J. An isochromosome confers drug resistance in vivo by amplification of two genes, ERG11 and TAC1. Mol Microbiol. 2008;68:624–641. doi: 10.1111/j.1365-2958.2008.06176.x. [DOI] [PubMed] [Google Scholar]
- ** 31.Selmecki AM, Dulmage K, Cowen LE, Anderson JB, Berman J. Acquisition of Aneuploidy Provides Increased Fitness during the Evolution of Antifungal Drug Resistance. PLoS Genet. 2009;5:e1000705. doi: 10.1371/journal.pgen.1000705. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper reports the findings of an evolution experiment in which euploid C. albicans strains were adapted to grow in the presence of fluconazole, a widely employed antifungal drug. All drug-resistant clones recovered at the end of the experiment invariably carried specific aneuploidy that matched the ones previously reported to spontaneously occur in clinical isolates of fluconazole-resistant C. albicans strains. Intriguingly, some aneuploid isolates exhibited improved fitness not only in the presence but also in the absence of the drug.
- 32.Tolliday N, Pitcher M, Li R. Direct Evidence for a Critical Role of Myosin II in Budding Yeast Cytokinesis and the Evolvability of New Cytokinetic Mechanisms in the Absence of Myosin II. Mol Biol Cell. 2003;14:798–809. doi: 10.1091/mbc.E02-09-0558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ** 33.Vavouri T, Semple JI, Garcia-Verdugo R, Lehner B. Intrinsic protein disorder and interaction promiscuity are widely associated with dosage sensitivity. Cell. 2009;138:198–208. doi: 10.1016/j.cell.2009.04.029. [DOI] [PubMed] [Google Scholar]; This paper represents the first systematic attempt at dissecting the general mechanism by which over-expression of certain genes but not others causes large phenotypic effects. It was found that proteins containing intrinsically disordered linear motifs that mediate protein-protein interactions are intrinsically promiscous, meaning that they can bind at low affinity a large variety of proteins beyond their physiological targets. Under normal expression levels, such interactions rarely take place and thus do not have phenotypic consequences. When such intrinsically promiscous proteins are up-regulated, off-target interactions are more likely to occur and to cause measurable phenotypes.
- 34.Bean J, Brennan C, Shih JY, Riely G, Viale A, Wang L, Chitale D, Motoi N, Szoke J, Broderick S, et al. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proc Natl Acad Sci U S A. 2007;104:20932–20937. doi: 10.1073/pnas.0710370104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, Lindeman N, Gale CM, Zhao X, Christensen J, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–1043. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 36.Kauffman S, Levin S. Towards a general theory of adaptive walks on rugged landscapes. J Theor Biol. 1987;128:11–45. doi: 10.1016/s0022-5193(87)80029-2. [DOI] [PubMed] [Google Scholar]
- 37.MacLean RC, Buckling A. The distribution of fitness effects of beneficial mutations in Pseudomonas aeruginosa. PLoS Genet. 2009;5:e1000406. doi: 10.1371/journal.pgen.1000406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * 38.Thompson SL, Compton DA. Proliferation of aneuploid human cells is limited by a p53-dependent mechanism. J Cell Biol. 2010;188:369–381. doi: 10.1083/jcb.200905057. [DOI] [PMC free article] [PubMed] [Google Scholar]; Among several interesting findings, this study provides experimental support to a long-standing theory that aneuploidy generates chromosome instability. Using a p53-deficient human cell line as a starting point, the authors were able to show that induction of aneuploidy by a series of nocodazole treatment/wash-out cycles was able to significantly increase the chromosome instability of the cells.
- 39.Bakhoum SF, Thompson SL, Manning AL, Compton DA. Genome stability is ensured by temporal control of kinetochore-microtubule dynamics. Nat Cell Biol. 2009;11:27–35. doi: 10.1038/ncb1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vanneste E, Voet T, Le Caignec C, Ampe M, Konings P, Melotte C, Debrock S, Amyere M, Vikkula M, Schuit F, et al. Chromosome instability is common in human cleavage-stage embryos. Nat Med. 2009;15:577–583. doi: 10.1038/nm.1924. [DOI] [PubMed] [Google Scholar]
- 41.Rehen SK, Yung YC, McCreight MP, Kaushal D, Yang AH, Almeida BS, Kingsbury MA, Cabral KM, McConnell MJ, Anliker B, et al. Constitutional aneuploidy in the normal human brain. J Neurosci. 2005;25:2176–2180. doi: 10.1523/JNEUROSCI.4560-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rehen SK, McConnell MJ, Kaushal D, Kingsbury MA, Yang AH, Chun J. Chromosomal variation in neurons of the developing and adult mammalian nervous system. Proc Natl Acad Sci U S A. 2001;98:13361–13366. doi: 10.1073/pnas.231487398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Selmecki A, Bergmann S, Berman J. Comparative genome hybridization reveals widespread aneuploidy in Candida albicans laboratory strains. Mol Microbiol. 2005;55:1553–1565. doi: 10.1111/j.1365-2958.2005.04492.x. [DOI] [PubMed] [Google Scholar]
- 44.Bond U, Neal C, Donnelly D, James TC. Aneuploidy and copy number breakpoints in the genome of lager yeasts mapped by microarray hybridisation. Curr Genet. 2004;45:360–370. doi: 10.1007/s00294-004-0504-x. [DOI] [PubMed] [Google Scholar]
- 45.Infante JJ, Dombek KM, Rebordinos L, Cantoral JM, Young ET. Genome-wide amplifications caused by chromosomal rearrangements play a major role in the adaptive evolution of natural yeast. Genetics. 2003;165:1745–1759. doi: 10.1093/genetics/165.4.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]