Abstract
Methamphetamine (METH) is a widely abused drug. However, little is known about the effects of chronic METH consumption on HPA axis function and psychiatric symptomatology in adolescent METH users. The current study evaluated psychiatric symptoms and changes in the stress response of adolescent METH users. Forty-one adolescent METH users and 75 comparison subjects in the same age range (ages 12–23 years) were recruited. Each subject completed the Symptom Checklist-90R (SCL-90) and was evaluated using the Brief Psychiatric Rating Scale. In addition, the subjects completed the Trier Social Stress Test (TSST) and had salivary cortisol levels measured 30 min before, immediately after, and 60 min after the TSST. Adolescent METH users showed greater severity of symptoms across all measures of the SCL-90. Younger female METH users had the most symptoms. Furthermore, the METH users exhibited significantly enhanced cortisol levels immediately following the TSST (+31%, p=0.03). Adolescent METH use is associated with greater psychiatric symptoms and enhanced cortisol secretion following a social stressor, particularly in younger female METH users. The psychiatric symptoms may reflect altered prefrontal cortical function resulting from chronic stress/drug use and the resulting glucocorticoid exposure. The results further suggest that treatment approaches should focus on stress-coping strategies to decrease the probability of relapse.
Keywords: adolescence, methamphetamine, psychiatric symptoms, stress, HPA axis function
Introduction
Methamphetamine (METH) is a psychostimulant that has an extensive history of widespread abuse (King and Ellinwood 2004). For example, the 2007 National Survey on Drug Use and Health report from Substance Abuse and Mental Health Services Administration indicated that roughly 640,000 Americans, 12 years or older, initiated stimulant (excluding cocaine) use in the past year, with the highest rates found in the western half of the USA. Methamphetamine use thus represents a major public health concern in part because of the reported neurotoxic effects associated with chronic administration to animals and abuse by human adult METH users. For example, chronic METH exposure is clearly neurotoxic in non-human subjects (Davidson et al. 2001), especially to striatal dopaminergic neurons (Kieburtz et al. 1996). A similar pattern of neurotoxicity has also been confirmed in post-mortem brain tissue obtained from verified METH users (Mirecki et al. 2004). Furthermore, neuroimaging studies consistently demonstrated evidence of lower density of neuronal markers, including N-acetylaspartate, dopamine, and serotonin transporters, particularly in striatal areas (Sekine et al. 2008; Ernst et al. 2000; McCann et al. 2008).
These neurotoxic effects are thought to contribute to the development of both psychiatric disorders (Cadet et al. 2003) and neurocognitive deficits seen in adult METH users (Meredith et al. 2005; Moon et al. 2007). For example, high-dose METH use can induce stimulant psychosis (see King and Ellinwood 2004; Scott et al. 2007 for reviews), as well as violent and aggressive behaviors (Freese et al. 2002). Other common symptoms of METH use include greater impulsivity (Semple et al. 2004) and depression, which appear to be more prevalent in female METH users than male METH users (Semple et al. 2007). Depressive symptoms in METH users are especially common during early abstinence and are associated with craving for the drug, which might exacerbate relapse to METH use (Nakama et al. 2008).
The basic neural circuitry of the stress response is reasonably well understood. Briefly, stressful stimuli induce corticotropin-releasing release from the paraventricular nucleus in the hypothalamus and the subsequent release of cortisol (Millan 2003). Hypothalamic-pituitary-adrenal (HPA) axis function is regulated by neurons from several different cortical and subcortical structures. For example, neurons from hippocampus and prefrontal cortex inhibit, while those from amygdala stimulate, the HPA axis (Diorio et al. 1993). Stress and METH abuse may exhibit bidirectional effects on each other. Specifically, stress can affect drug consumption by contributing to drug abuse and relapse, as well as amphetamine-mediated neurotoxicity (Soderpalm et al. 2003; Marinelli and Piazza 2002). In addition, METH consumption affects HPA axis function. For example, METH raised corticosterone levels more than other stressors such as the forced swim test in mice (Grace et al. 2008). Furthermore, rhesus monkeys exposed to METH exhibited significantly increased 24-h cortisol levels (Madden et al. 2005). Lastly, both d-amphetamine and 3,4-methylenedioxy-methamphetamine (MDMA) have been reported to increased cortisol levels in healthy human volunteers (Gouzoulis-Mayfrank et al. 1999).
The literature regarding changes in the stress response in human adult stimulant abusers is conflicting. Some reported no effect of psychological stress on salivary cortisol levels in long-term METH and cocaine users (Harris et al. 2005), while others reported decreased salivary cortisol levels after a social stressor in ecstasy (Gerra et al. 2003) and cocaine users (Sinha et al. 2003) or increased salivary cortisol levels in cocaine users following stressful imagery (Sinha et al. 2000). These reports suggest that the stress response in stimulant users may be dependent on the drug abused, as well as the type of stressor used to induce cortisol response.
Despite the high prevalence of METH use among adolescents, little is known about the neuropsychiatric and neurobiological effects of METH abuse in adolescents. The paucity of data is surprising for two reasons: first, METH users often initiate their use of the drug during adolescence; second, the adolescent brain exhibits gender- and region-specific developmental trajectories (Lenroot et al. 2007). Therefore, METH abuse may affect the adolescent brain in unique ways and may lead to age-and gender-specific effects on brain development. These potential METH-induced effects may also produce differential neuropsychiatric symptoms.
The current study aims to (1) evaluate and compare the psychiatric symptoms in adolescent METH users and adolescent nondrug users using the SCL-90 and the Brief Psychiatric Rating Scale (BPRS) and (2) to evaluate any alterations in HPA axis function in adolescent METH users, both at baseline and in response to a social stress test, the TSST. Based on the literature discussed above, we predicted that adolescent METH users would have an elevated basal salivary cortisol level and a blunted cortisol response to the TSST. Salivary cortisol is measured to be consistent with previous research. We also hypothesized that the stress response would be significantly correlated with psychotic symptoms. Lastly, we explored whether these abnormalities in psychiatric symptoms and the cortisol responses are gender-specific.
Materials and methods
One hundred sixteen subjects, including 41 METH subjects and 75 healthy comparison nondrug users, were screened and enrolled in the study. METH subjects were included in the study only if they fulfilled the following inclusion criteria: (1) had a diagnosis of METH abuse or dependence as determined by the National Institute of Mental Health Diagnostic Interview Schedule for Children Version-IV, (2) male or female ages 12–23 years; this age range was selected since the brain is still developing through the early portion of the third decade of life (Shaw et al. 2008), (3) residing in Hawaii, (4) willing and able to comply with study procedures, (5) able to verbalize understanding of the consent/assent form, and (6) had parental or a guardian consent (if under 18 years old). Healthy nondrug users were included if they also fulfilled the above study criteria 2 through 6.
For subjects 18 years or older, a written consent was obtained after the entire experiment was explained to the subject. For subjects 12–17 years of age, parental consent and child assent were obtained after the entire experiment was explained to them. The University of Hawaii Cooperative Institutional Review Board approved the protocol and consent/assent forms.
All subjects were evaluated with detailed drug use history and psychiatric evaluations during face-to-face interviews by trained research staff and verified by a board-certified adolescent psychiatrist (DA). Cumulative METH use was determined by detailed interviews regarding the quantity of METH use per day, number of days METH used per week, and the duration of METH use. The influence of METH use on the subject’s life was assessed with the Teen Addiction Severity Index (T-ASI). The structured assessments included the SCL-90 and the BPRS to evaluate psychiatric symptoms. Any subject, who reported a psychiatric diagnosis currently or in the past, was receiving or had received psychotropic medication, or had significant responses on the SCL-90 or the Psychiatric Status on the T-ASI, was further evaluated (including interviews with the subject’s parent or legal custodian) with an abbreviated psychiatric assessment by the clinician, in order to clarify the diagnosis and to assess for stability. All subjects received additional assessments including physical and neurological examinations and urine toxicology and urine pregnancy tests (in female subjects). The urine toxicology screened for cocaine, tetrahydrocannabinol, opiates, amphetamine, methamphetamine, and benzodiazepenes. The urine toxicology screen had to be negative on the test day, and if a subject tested positive, further assessments were performed to ensure they were not dependent on drugs other than METH in the METH user group and not dependent on any drugs in the healthy control group. Subjects who had positive urine toxicology were rescheduled for the study at a later date. Although the subjects were not tested for HIV-1, none had a diagnosis or symptoms suggestive of HIV infection.
Psychiatric symptom assessments
The Brief Psychiatric Rating Scale (Overall and Gorham 1962)
This scale contains 18 items designed to assess changes in psychotic disorders over time and as a function of treatment. The subjects were administered the BPRS by the same board-certified adolescent psychiatrist (DA). The BPRS was scored and divided into four subcategories: manic excitement (motor hyperactivity, elated mood, excitement, distractibility, hostility, and grandiosity), negative symptoms (blunted affect, motor retardation, emotional withdrawal, and self-neglect), positive symptoms (bizarre behavior, unusual thought content, disorientation, hallucinations, and suspiciousness), and depression anxiety (depression, anxiety, suicidality, and guilt; Ventura et al. 2000).
Symptom Check List-90-revised®
The SCL-90 is a self-report tool designed to assess the symptoms of a broad range of psychological problems and psychiatric disorders. The subjects answered the 90 questions on a five-point scale, which assessed the severity of their symptoms within the past week. The SCL-90 scores also yielded nine primary dimensions and three global scales. T scores were sex- and age-specific and used for further analysis.
Trier social stress test
During the preparatory phase, the subjects waited approximately 5 min prior to the test. At that time, a brief description of the TSST was given to the subjects. The subjects then were told that they would be read a six-sentence story and that they would then have 5 min to complete the story in front of two investigators who would record their responses. The subjects also were told that after completing the story, they would have to perform some mental arithmetic for 5 min. Twenty minutes later, the subject’s blood pressure was taken, and a saliva sample was collected. The subject was then read the story, which took approximately 2 min. Five minutes after the story was read, the subject was told to complete the story in any way, while the investigators recorded their responses (the content was later analyzed as part of a psychological profile of the subject). After the subject finished the story, they were instructed to count backwards by 13 starting at 1,023. If they made a mistake, they had to start from the beginning and that the investigators would be counting the number of mistakes they made. At the end of the mental arithmetic, blood pressure, pulse rate, and a saliva sample were taken. The subjects were then escorted to the waiting room, where they could read, listen to music, or watch a movie of their choice for 60 min. After which, their blood pressures were taken, as were the final saliva samples.
Salivary cortisol measurement
Saliva for cortisol analysis was collected using a Salivette (Sarstedt, Inc., Newton, NC, USA). Cortisol levels (microgram/deciliter) were assayed using a 98-well plate enzyme immunoassay method (High Sensitivity Salivary Cortisol Enzyme Immunoassay Kit: Salimetrics LLC, State College, PA, USA).
Statistical analyses
Data were analyzed using Systat version 10 (SAS Institute Inc., Cary, NC, USA). Statistical significance was defined as P values ≤0.05. The t test and Fisher’s exact test were used to compare demographic characteristics of METH users and non-METH users. An age factor was constructed by dividing the subjects, separately for each sex and treatment group into “Younger” and “Older” subgroups based on a median split. For females, both control and METH, the younger subjects were defined as those ≤17 years, while for the males, both control and METH, the younger subjects were defined as those ≤18 years old. The SCL-90 data were analyzed by ANCOVA, with group (METH user vs. control subject), age, and sex as the main factors and years of education as the covariate. Variables that showed significant main effects were analyzed further by Bonferroni’s post hoc tests. Variables that showed group differences were correlated with METH usage parameters (e.g., cumulative amount used and length of abstinence). The BPRS data were analyzed by non-parametric Mann-Whitney U tests. The cortisol data were analyzed by a mixed-model ANOVA with group status (METH user vs. control subject), age, and sex as the between-subjects factors and time of the test (baseline, immediately after the TSST, and recovery) as the within-subjects factor.
Results
Subject demographics
All subject characteristics and the METH subjects’ drug use histories are shown in Table 1. Age in years was not different between male and female subjects or between METH and control subjects (two-way ANOVA: sex: F(1, 72)=1.82; METH status: F(1, 72)= 0.22). The years of education were also not different between male and female subjects or between METH and control subjects (two-way ANOVA: sex: F(1, 72)=0.15; METH status: F(1, 72)=0.11). Lastly, none of the variables for METH usage was significantly different between male and female METH users. The apparently higher mean amount of METH used per day in the female compared to male subjects was due to the large amount of METH used (3 and 7 g day−1) by two female subjects. The difference in mean amount consumed between the male and female METH users not significant (t (39)=1.68, p=0.1). The median usage was more similar between the two groups, where the female METH users consumed a median of 0.76 g/day−1 and the male METH users consumed a median of 0.50 g day−1 (Table 1).
Table 1.
Demographic and METH use characteristics of the subjects (means and standard errors)
| Controls (n=75) |
Methamphetamine users (n=41) |
|||
|---|---|---|---|---|
| Males (n=40) | Females (n=35) | Males (n=17) | Females (n=24) | |
| Age (years) | 17.23 (0.64) | 17.14 (0.55) | 18.40 (0.67) | 18.09 (0.49) |
| Number of younger vs. older* subjects | 20/20 | 17/18 | 8/9 | 12/12 |
| Education (years) | 11.16 (0.39) | 10.46 (0.45) | 11.18 (0.34) | 10.83 (0.36) |
| Methamphetamine use characteristics | ||||
| Age of first use (years) | 15.0 (0.50) | 14.88 (0.36) | ||
| Frequency of use (days/week) | 5.94 (0.42) | 5.93 (0.33) | ||
| Mean daily use (grams/day) | 0.57 (0.09) | 1.17 (0.30) | ||
| Median daily use (grams/day) | 0.50 | 0.76 | ||
| Duration of use (months) | 32.38 (9.85) | 25.76 (3.79) | ||
| Duration of abstinence (days) | 139.63 (81.90) | 337.72 (97.84) | ||
| Lifetime exposure (grams) | 681.65 (301.89) | 916.39 (415.19) | ||
The top half of presents the demographic characteristics of the subject sample divided by group and sex. Also presented in the top half is the number of subjects in the younger vs. older subject grouping, as determined by a median split. For female subjects, younger is defined as age ≤17 years while older is >17 years. For male subjects, younger is defined as age ≤18 years, while older is >18 years. The bottom half presents the METH use patterns for the METH users, separately by sex
SCL 90 data
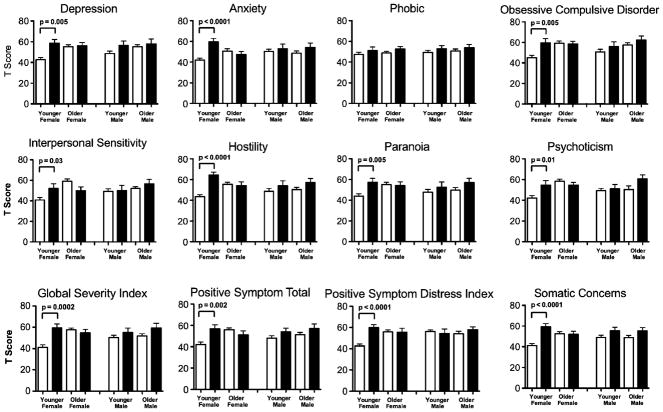
METH users had greater severity of symptoms on every dimension and on the global scales as compared to the control subjects, except for the interpersonal sensitivity and phobic dimensions (Table 2, tFig. 1). Bonferroni’s post hoc tests showed that for most dimensions or scales that showed significance on the three-way interactions, the younger female METH users had significantly higher scores than younger female control subjects (see Fig. 1). No measure of METH usage (e.g., months of METH use and cumulative lifetime grams of METH exposed) correlated with the psychiatric symptomology (Table 2 and Fig. 1).
Table 2.
F values from the three-way ANCOVA on the SCL-90 scores
| Dimension | METH status | Age | Sex | METH×age | METH×sex | METH×sex×age |
|---|---|---|---|---|---|---|
| Somatic | 17.18*** | 0.46 | 0.28 | 7.03** | 0.56 | 4.95* |
| OCD | 8.24** | 10.41** | 0.40 | 3.83 | 0.29 | 3.70 |
| Interpersonal Sensitivity | 1.01 | 10.61** | 0.71 | 5.21* | 0.06 | 7.12** |
| Depression | 11.74*** | 5.69* | 0.56 | 6.73* | 0.75* | 2.05 |
| Anxiety | 7.85** | 0.25 | 0.77 | 4.74* | 0.80 | 8.59** |
| Hostility | 14.93*** | 0.82 | 0.56 | 6.93** | 1.04 | 8.68** |
| Phobia | 3.98* | 0.85 | 1.05 | 0.07 | 0.03 | 0.04 |
| Paranoia | 7.23** | 2.83 | 0.08 | 2.06 | 0.01 | 3.81* |
| Psychoticism | 5.82* | 10.08** | 0.14 | 1.14 | 0.06 | 8.82** |
| Global severity index | 11.76*** | 5.30* | 0.33 | 5.86* | 0.26 | 9.50** |
| Positive symptom distress index | 6.99** | 2.25 | 1.59 | 3.04 | 4.52* | 10.05** |
| Positive symptom total | 7.70** | 4.08* | 0.44 | 6.97** | 0.002 | 6.81** |
F values from three-way ANCOVAs performed on each dimension of the SCL-90. The main factors for the three-way ANOVAs were Meth status (user vs. control), age (younger vs. older), and sex (male vs. female). Education was the covariate for each ANCOVA. The significant F values are boldfaced
p<0.05,
p<0.01,
p<0.001
Fig. 1.
T scores (mean ± SE) for each of the psychiatric symptom domains from the Symptom Checklist-90, shown separately for each group, sex, and age grouping. The white bars represent the control subjects and the black bars represent the METH users. See Table 2 for three-way ANOVA results. The post hoc group comparisons were evaluated by t tests
BPRS data
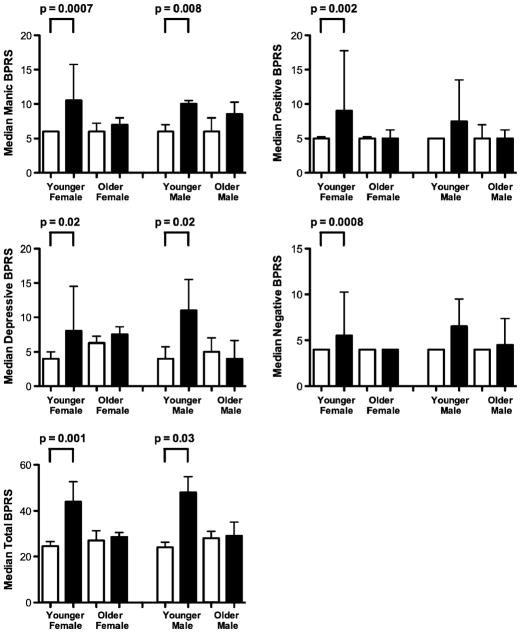
Similar to the results for the SCL-90, METH users as a group had significantly higher composite scores as compared to control subjects. In addition, younger female METH users had significantly higher composite scores than younger female control subjects for all composite scores, while younger male METH users had significantly higher negative and total BPRS composite scores than younger male control subjects (Fig. 2).
Fig. 2.
The median scores and interquartile ranges for the composite scales of the Brief Psychiatric Rating Scale, shown separately for each group, sex, and age grouping (white bars= control subjects; black bars= METH users). Group comparisons were evaluated by nonpara-metric Mann-Whitney U tests
Cortisol data
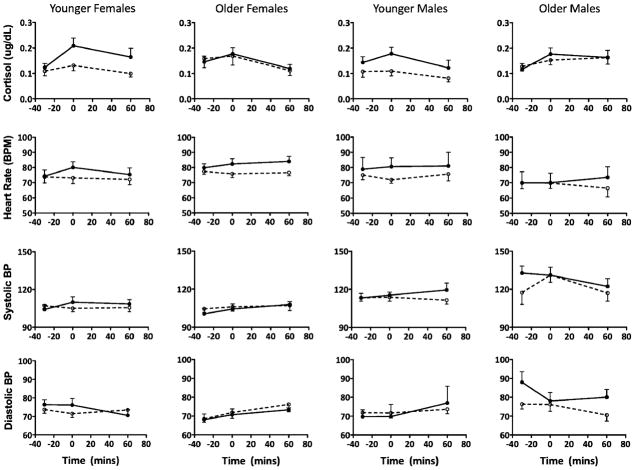
The cortisol level, pulse rate, systolic, and diastolic blood pressures are shown separately for each group and sex across the three collection time points. The result of a mixed-model ANOVA on the cortisol data, with METH status, age, and sex as the between-subjects factor and time as the repeated measures show that although the METH users and controls had similar baseline cortisol levels [METH status: F(1, 99)=1.59, p=0.22], and there were no sex differences across all subjects [sex: F(1, 98)= 0.00, p=0.96], the METH users exhibited a significantly enhanced cortisol response to the TSST as compared to the control subjects [time: F(2, 198)=14.46, p<0.0001]. However, this enhanced cortisol response to the TSST in the METH subjects was not different between the male and female METH users (group×sex: F(1, 98)=0.01, p=0.94; time×sex: F(2, 194)=1.58, p=0.21; group×time×sex: F(2, 194)=0.04, p=0.96). A paired t test, comparing baseline cortisol and cortisol immediately after the TSST, was significant for both the younger female (t(12)=3.86, p= 0.002) and male (t(7)=2.61, p=0.04) METH users. A t test comparing the percent change in cortisol release from baseline to post-TSST between the younger female and male METH users was marginally significant (t(19)=1.90, p=0.07; Fig. 3).
Fig. 3.
Means and standard errors for the cortisol levels, heart rate, systolic, and diastolic blood pressure separately for each group, sex, and age grouping 30 min before, immediately after, and 60 min after the Trier Social Stress Test. The white circles represent the control subjects and the black-filled circles represent the METH users
Heart rate and systolic and diastolic blood pressure
Heart rate was elevated significantly after the TSST in all subjects indicating a significant main effect of time [time: F(1, 77)=19.65, mixed-model ANOVA, p<0.0001], but the blood pressure did not show a significant change after the TSST (Fig. 3).
Correlations between cortisol, METH use characteristics, and psychiatric symptoms
Basal cortisol levels did not correlate with the SCL-90 in either the control or METH-using subjects. Cortisol levels (either basal or after the TSST) also did not correlate with METH use characteristics. Heart rate correlated with cortisol levels immediately following the TSST in the female METH users (r=0.65, p= 0.003) but not in the male METH users (r=0.21, p=0.46).
Discussion
Psychiatric symptoms were more common in a group of adolescent METH users than in comparison nondrug user subjects on the Symptom Checklist-90 and Brief Psychiatric Rating Scale. On both assessment tools, METH users had significantly higher scores than controls. Further analyses found that the higher scores were most pronounced in the younger (<17 years) female METH users compared to the corresponding younger female controls. In addition, this group of METH users exhibited a significantly enhanced cortisol response to the TSST as compared to controls, although they show no differences in their basal cortisol levels.
Psychiatric symptoms
The greater psychiatric symptoms in these adolescent METH users are consistent with those found in adult METH users, who have higher incidences of psychotic symptoms and paranoia (Chen et al. 2005). In adult METH users, they were also significantly more prone to psychotic episodes even after years of abstinence (Ujike and Sato 2004). Since this is a cross-sectional study, we will need to perform longitudinal follow-up studies to determine whether these adolescents might be more likely to experience psychotic episodes later in life and whether such episodes would be related to the amount of duration of METH use in these individuals.
In our study, the younger adolescent female METH users experienced significantly more depressive symptoms than younger adolescent female comparison subjects, which is also consistent with the results reported in adult female METH users (Semple et al. 2007). Several factors may contribute to depression in adolescent females, regardless of METH use status, including poor body image, low self-esteem, lack of social support, and eating disorders (Santos et al. 2007). Our adolescent subjects were screened for major psychiatric conditions, such as psychosis or depression, but were not explicitly screened for eating disorders or body-image issues. However, none of the subjects had obvious signs for eating disorders on their physical examination or verbally reported these conditions during their psychiatric interviews. Nevertheless, our female METH subjects might have had some of these co-morbid conditions that could have contributed to their relatively greater depressive symptoms.
Cortisol results
The adolescent METH users did not have altered basal cortisol levels, which was not consistent with our initial hypothesis. The current results are also different from prior studies of abstinent adult MDMA users, who exhibited enhanced basal cortisol levels and a blunted cortisol response to the TSST (Gerra et al. 2003). The different findings in the different drug user populations indicate the differential effects between METH and MDMA on the HPA axis. MDMA has greater serotonergic effects than METH, such that MDMA consumption can cause long-term 5-HT depletions with subsequent effects on behavior and hormone secretion (Baumann et al. 2007). Thus, the difference in cortisol response between MDMA and METH users may reflect differences in dopaminergic function between these drug users.
The METH users did exhibit an enhanced cortisol response to the social stressor, which was also contrary to our initial prediction for the current study. However, this enhanced response was only present in the younger METH users and was larger in the younger female METH users than the younger male METH users. The overall pattern of results suggests that younger female METH users may be more susceptible to, and stressed by, social situations. These subjects exhibited an enhanced cortisol response, a significant correlation between cortisol levels and heart rate following the TSST, and had significantly higher interpersonal sensitivity and anxiety scores on the SCL-90 compared to younger female controls.
The enhanced cortisol response to a social stressor and the possible relationship to the dopaminergic system have implications for the treatment adolescent METH abusers. For example, recent [11C]raclopride PET studies indicate that cortisol and ventral tegmental area dopamine secretion in response to AMPH administration are correlated (Oswald et al. 2005) and that following the TSST, cortisol and dopamine secretion and positive subjective effects of AMPH were all correlated (Wand et al. 2007). As discussed above, stress can induce drug consumption and reinstate the extinguished drug consumption behavior (Marinelli and Piazza 2002). Furthermore, stress can increase drug craving (Sinha et al. 1999). Thus, adolescent METH users, particularly younger females, are susceptible to stress-induced craving and relapse to METH use. Therefore, treatment strategies should focus on stress-coping skills, prevention of life stressors, or pharmacological approaches that target the HPA axis or the dopaminergic system, which might minimize relapse in these individuals.
Cortisol and psychiatric symptoms
In the current experiment, there were no correlations between cortisol levels and psychiatric symptoms. This result is somewhat surprising, considering the relationship between HPA axis hyperactivity and depression (Piwowarska et al. 2009). However, the absence of a correlation may reflect the fact that the adolescents in the current study did not have any clinically diagnosable disorder: had a subject been clinically depressed, they would have been excluded from the experiment. Thus, it may be the case that the correlation between cortisol and symptomology exists only when a clinical syndrome is present.
Although there were no correlations between cortisol and self-reported symptoms, the psychiatric symptoms in the METH subjects, particularly the younger female METH users, may be related to the effects of stress/glucocorticoid exposure on the prefrontal cortex. Prolonged stress/glucocorticoid exposure impairs hippocampal and prefrontal cortex function, resulting in disturbed inhibitory regulation of the HPA axis and regulation of behavior (McEwen 2004). For example, glucocorticoids have been shown to enhance pro-inflammatory cytokines in the hippocampal cell cultures (MacPherson et al. 2005), while subacute glucocorticoid administration enhances ischemia-induced hippocampal cell loss (Sapolsky and Pulsinelli 1985). Indeed, the literature clearly indicates that under some circumstances, glucocorticoid release can enhance neuronal cell loss in response to injury (MacPherson et al. 2005).
Since stress and glucocorticoids can impair prefrontal cortical function, as discussed above, the subsequent effects on the prefrontal cortex may be responsible for the depression, negative affective states such as anger and hatred and anxiety seen in the current subjects. Depressed individuals were shown to have reduced metabolic activities of their prefrontal cortex (Baxter et al. 1989), while hypermetabolic activities in the anterior cingulate also have been linked to depressive symptoms in METH users (London et al. 2004; Dluzen and Liu 2008). The prefrontal cortex is also involved in regulation of negative affective states (Zald et al. 2002; Dougherty et al. 2004). For example, individuals with major depression plus anger attacks exhibited reduced left ventromedial prefrontal cortical blood flow compared to controls. Lastly, the prefrontal cortex has been involved in anxiety, which correlated inversely with medial prefrontal cortical blood flow (Davidson 2002; Simpson et al. 2001). Thus, the cluster of symptoms exhibited by the current METH subjects may be the result of altered prefrontal function or neurochemical alterations that are induced by METH consumption and METH-induced glucocorticoid release.
Limitations
Two limitations in the current study may be improved in future studies: first, the sample size is moderate, particularly for the male subjects and for the younger age groups. A larger sample size would provide more stable subgroup comparisons. Second, the accuracy of the self-reported data, particularly regarding METH usage, may be limited. We did perform urine toxicology on these subjects, but more detail METH usage history with timeline-follow back interviews (Brecht et al. 2007) and hair analyses also may be useful.
In conclusion, adolescent METH users have greater psychiatric symptoms, particularly in the younger female METH users. In addition, METH use was associated with an increased cortisol response to a social stressor. These results suggest that METH-mediated neurotoxicity may lead to greater psychiatric symptoms and may affect the HPA axis. In addition, METH-mediated dopaminergic neurotoxicity also may lead to greater psychiatric symptoms (Dailly et al. 2004). Therefore, treatments should focus on pharmacological approach on the HPA axis and the dopaminergic system, as well as stress prevention and coping strategies for stress management to decrease the probability of relapse and psychiatric symptoms in adolescent METH users.
Acknowledgments
Grant Support This study is jointly supported by National Institute of Neurological Disorders and Stroke (2U54 NS039406; U54 NS56883), National Institute of Drug Abuse (U54 NS56883-01; 1K24-DA016170, K01-DA021203), National Center for Research Resources (G12RR003061 and P20RR11091), the Office of National Drug Control Policy, and the Queen’s Medical Center.
Footnotes
Previous presentation Parts of these data were presented at the Annual Conference of the Specialized Neuroscience Research Programs, New York, NY, USA on August 19-22.
Disclosures The authors report no competing interests.
Contributor Information
George King, Email: grking@hawaii.edu, Department of Medicine, John A. Burns School of Medicine, Honolulu, HI 96812, USA. 1356 Lusitana St. 7th Floor, Honolulu, HI 96813, USA.
Daniel Alicata, Department of Medicine, John A. Burns School of Medicine, Honolulu, HI 96812, USA.
Christine Cloak, Department of Medicine, John A. Burns School of Medicine, Honolulu, HI 96812, USA.
Linda Chang, Department of Medicine, John A. Burns School of Medicine, Honolulu, HI 96812, USA.
References
- Baumann MH, Wang X, Rothman RB. 3, 4-Methylenedioxyme-thamphetamine (MDMA) neurotoxicity in rats: a reappraisal of past and present findings. Psychopharmacology (Berl) 2007;189(4):407–424. doi: 10.1007/s00213-006-0322-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter LR, Jr, et al. Reduction of prefrontal cortex glucose metabolism common to three types of depression. Arch Gen Psychiatry. 1989;46(3):243–250. doi: 10.1001/archpsyc.1989.01810030049007. [DOI] [PubMed] [Google Scholar]
- Brecht ML, Greenwell L, Anglin MD. Substance use pathways to methamphetamine use among treated users. Addict Behav. 2007;32 (1):24–38. doi: 10.1016/j.addbeh.2006.03.017. [DOI] [PubMed] [Google Scholar]
- Cadet JL, Jayanthi S, Deng X. Speed kills: cellular and molecular bases of methamphetamine-induced nerve terminal degeneration and neuronal apoptosis. FASEB J. 2003;17(13):1775–1788. doi: 10.1096/fj.03-0073rev. [DOI] [PubMed] [Google Scholar]
- Chen EY, et al. Self-administered instrument to measure the patient’s experience of recovery after first-episode psychosis: development and validation of the Psychosis Recovery Inventory. Aust NZ J Psychiatry. 2005;39(6):493–499. doi: 10.1080/j.1440-1614.2005.01609.x. [DOI] [PubMed] [Google Scholar]
- Dailly E, et al. Dopamine, depression and antidepressants. Fundam Clin Pharmacol. 2004;18(6):601–607. doi: 10.1111/j.1472-8206.2004.00287.x. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Anxiety and affective style: role of prefrontal cortex and amygdala. Biol Psychiatry. 2002;51(1):68–80. doi: 10.1016/s0006-3223(01)01328-2. [DOI] [PubMed] [Google Scholar]
- Davidson C, et al. Methamphetamine neurotoxicity: necrotic and apoptotic mechanisms and relevance to human abuse and treatment. Brain Res Brain Res Rev. 2001;36(1):1–22. doi: 10.1016/s0165-0173(01)00054-6. [DOI] [PubMed] [Google Scholar]
- Diorio D, Viau V, Meaney MJ. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. J Neurosci. 1993;13(9):3839–3847. doi: 10.1523/JNEUROSCI.13-09-03839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dluzen DE, Liu B. Gender differences in methamphetamine use and responses: a review. Gen Med. 2008;5(1):24–35. doi: 10.1016/s1550-8579(08)80005-8. [DOI] [PubMed] [Google Scholar]
- Dougherty DD, et al. Ventromedial prefrontal cortex and amygdala dysfunction during an anger induction positron emission tomography study in patients with major depressive disorder with anger attacks. Arch Gen Psychiatry. 2004;61(8):795–804. doi: 10.1001/archpsyc.61.8.795. [DOI] [PubMed] [Google Scholar]
- Ernst T, et al. Evidence for long-term neurotoxicity associated with methamphetamine abuse: A 1H MRS study. Neurology. 2000;54 (6):1344–1349. doi: 10.1212/wnl.54.6.1344. [DOI] [PubMed] [Google Scholar]
- Freese TE, Miotto K, Reback CJ. The effects and consequences of selected club drugs. J Subst Abuse Treat. 2002;23(2):151–156. doi: 10.1016/s0740-5472(02)00267-2. [DOI] [PubMed] [Google Scholar]
- Gerra G, et al. Hypothalamic-pituitary-adrenal axis responses to stress in subjects with 3, 4-methylenedioxy-methamphetamine (“ecstasy”) use history: correlation with dopamine receptor sensitivity. Psychiatry Res. 2003;120(2):115–124. doi: 10.1016/s0165-1781(03)00175-6. [DOI] [PubMed] [Google Scholar]
- Gouzoulis-Mayfrank E, et al. Psychopathological, neuroendocrine and autonomic effects of 3, 4-methylenedioxyethylamphetamine (MDE), psilocybin and d-methamphetamine in healthy volunteers. Results of an experimental double-blind placebo-controlled study. Psychopharmacology (Berl) 1999;142(1):41–50. doi: 10.1007/s002130050860. [DOI] [PubMed] [Google Scholar]
- Grace CE, et al. (+)–Methamphetamine increases corticosterone in plasma and BDNF in brain more than forced swim or isolation in neonatal rats. Synapse. 2008;62(2):110–121. doi: 10.1002/syn.20470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris DS, et al. Repeated psychological stress testing in stimulant-dependent patients. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29(5):669–677. doi: 10.1016/j.pnpbp.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Kieburtz K, et al. Cognitive performance and regional brain volume in human immunodeficiency virus type 1 infection. Arch Neurol. 1996;53(2):155–158. doi: 10.1001/archneur.1996.00550020059016. [DOI] [PubMed] [Google Scholar]
- King G, Ellinwood E. Amphetamines and other stimulants. In: Ruiz P, Millman R, editors. Substance abuse: a comprehensive textbook. Williams and Wilkins; Baltimore: 2004. pp. 277–301. [Google Scholar]
- Lenroot RK, et al. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage. 2007;36 (4):1065–1073. doi: 10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London ED, et al. Mood disturbances and regional cerebral metabolic abnormalities in recently abstinent methamphetamine abusers. Arch Gen Psychiatry. 2004;61(1):73–84. doi: 10.1001/archpsyc.61.1.73. [DOI] [PubMed] [Google Scholar]
- MacPherson A, Dinkel K, Sapolsky R. Glucocorticoids worsen excitotoxin-induced expression of pro-inflammatory cytokines in hippocampal cultures. Exp Neurol. 2005;194(2):376–383. doi: 10.1016/j.expneurol.2005.02.021. [DOI] [PubMed] [Google Scholar]
- Madden LJ, et al. Modeling human methamphetamine exposure in nonhuman primates: chronic dosing in the rhesus macaque leads to behavioral and physiological abnormalities. Neuropsychopharmacology. 2005;30(2):350–359. doi: 10.1038/sj.npp.1300575. [DOI] [PubMed] [Google Scholar]
- Marinelli M, Piazza PV. Interaction between glucocorticoid hormones, stress and psychostimulant drugs. Eur J NeuroSci. 2002;16 (3):387–394. doi: 10.1046/j.1460-9568.2002.02089.x. [DOI] [PubMed] [Google Scholar]
- McCann UD, et al. Positron emission tomographic studies of brain dopamine and serotonin transporters in abstinent (±)3, 4-methylenedioxymethamphetamine (“ecstasy”) users: relationship to cognitive performance. Psychopharmacology (Berl) 2008;200 (3):439–450. doi: 10.1007/s00213-008-1218-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Protection and damage from acute and chronic stress: allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders. Ann N Y Acad Sci. 2004;1032:1–7. doi: 10.1196/annals.1314.001. [DOI] [PubMed] [Google Scholar]
- Meredith CW, et al. Implications of chronic methamphetamine use: a literature review. Harv Rev Psychiatry. 2005;13(3):141–154. doi: 10.1080/10673220591003605. [DOI] [PubMed] [Google Scholar]
- Millan MJ. The neurobiology and control of anxious states. Prog Neurobiol. 2003;70(2):83–244. doi: 10.1016/s0301-0082(03)00087-x. [DOI] [PubMed] [Google Scholar]
- Mirecki A, et al. Brain antioxidant systems in human methamphetamine users. J Neurochem. 2004;89(6):1396–1408. doi: 10.1111/j.1471-4159.2004.02434.x. [DOI] [PubMed] [Google Scholar]
- Moon M, et al. Memory impairment in methamphetamine dependent patients. Int J Neurosci. 2007;117(1):1–9. doi: 10.1080/00207450500535503. [DOI] [PubMed] [Google Scholar]
- Nakama H, et al. Association between psychiatric symptoms and craving in methamphetamine users. Am J Addict. 2008;17(5):441–446. doi: 10.1080/10550490802268462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswald LM, et al. Relationships among ventral striatal dopamine release, cortisol secretion, and subjective responses to amphetamine. Neuropsychopharmacology. 2005;30(4):821–832. doi: 10.1038/sj.npp.1300667. [DOI] [PubMed] [Google Scholar]
- Overall JE, Gorham DR. The brief psychiatric rating scale. Psychol Rep. 1962;10:799–812. [Google Scholar]
- Piwowarska J, et al. Serum cortisol concentration in patients with major depression after treatment with clomipramine. Pharmacol Rep. 2009;61(4):604–611. doi: 10.1016/s1734-1140(09)70112-4. [DOI] [PubMed] [Google Scholar]
- Santos M, Richards CS, Bleckley MK. Comorbidity between depression and disordered eating in adolescents. Eat Behav. 2007;8 (4):440–449. doi: 10.1016/j.eatbeh.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Pulsinelli WA. Glucocorticoids potentiate ischemic injury to neurons: therapeutic implications. Science. 1985;229(4720):1397–1400. doi: 10.1126/science.4035356. [DOI] [PubMed] [Google Scholar]
- Scott JC, et al. Neurocognitive effects of methamphetamine: a critical review and meta-analysis. Neuropsychol Rev. 2007;17(3):275–297. doi: 10.1007/s11065-007-9031-0. [DOI] [PubMed] [Google Scholar]
- Sekine Y, et al. Methamphetamine causes microglial activation in the brains of human abusers. J Neurosci. 2008;28(22):5756–5761. doi: 10.1523/JNEUROSCI.1179-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple SJ, Grant I, Patterson TL. Female methamphetamine users: social characteristics and sexual risk behavior. Women Health. 2004;40(3):35–50. doi: 10.1300/j013v40n03_03. [DOI] [PubMed] [Google Scholar]
- Semple SJ, et al. Psychosocial and behavioral correlates of depressed mood among female methamphetamine users. J Psychoact Drugs. 2007;4:353–366. doi: 10.1080/02791072.2007.10399897. [DOI] [PubMed] [Google Scholar]
- Shaw P, et al. Neurodevelopmental trajectories of the human cerebral cortex. J Neurosci. 2008;28(14):3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson JR, Jr, et al. Emotion-induced changes in human medial prefrontal cortex: II. During anticipatory anxiety. Proc Natl Acad Sci USA. 2001;98(2):688–693. doi: 10.1073/pnas.98.2.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Catapano D, O’Malley S. Stress-induced craving and stress response in cocaine dependent individuals. Psychopharmacology (Berl) 1999;142(4):343–351. doi: 10.1007/s002130050898. [DOI] [PubMed] [Google Scholar]
- Sinha R, et al. Psychological stress, drug-related cues and cocaine craving. Psychopharmacology (Berl) 2000;152(2):140–148. doi: 10.1007/s002130000499. [DOI] [PubMed] [Google Scholar]
- Sinha R, et al. Hypothalamic-pituitary-adrenal axis and sympatho-adreno-medullary responses during stress-induced and drug cue-induced cocaine craving states. Psychopharmacology (Berl) 2003;170(1):62–72. doi: 10.1007/s00213-003-1525-8. [DOI] [PubMed] [Google Scholar]
- Soderpalm A, Nikolayev L, de Wit H. Effects of stress on responses to methamphetamine in humans. Psychopharmacology (Berl) 2003;170(2):188–199. doi: 10.1007/s00213-003-1536-5. [DOI] [PubMed] [Google Scholar]
- Ujike H, Sato M. Clinical features of sensitization to methamphetamine observed in patients with methamphetamine dependence and psychosis. Ann N Y Acad Sci. 2004;1025:279–287. doi: 10.1196/annals.1316.035. [DOI] [PubMed] [Google Scholar]
- Ventura J, et al. Symptom dimensions in recent-onset schizophrenia and mania: a principal components analysis of the 24-item Brief Psychiatric Rating Scale. Psychiatry Res. 2000;97 (2–3):129–135. doi: 10.1016/s0165-1781(00)00228-6. [DOI] [PubMed] [Google Scholar]
- Wand GS, et al. Association of amphetamine-induced striatal dopamine release and cortisol responses to psychological stress. Neuropsychopharmacology. 2007;32(11):2310–2320. doi: 10.1038/sj.npp.1301373. [DOI] [PubMed] [Google Scholar]
- Zald DH, Mattson DL, Pardo JV. Brain activity in ventromedial prefrontal cortex correlates with individual differences in negative affect. Proc Natl Acad Sci USA. 2002;99(4):2450–2454. doi: 10.1073/pnas.042457199. [DOI] [PMC free article] [PubMed] [Google Scholar]