Abstract
TCDD (2,3,7,8-tetrachlorodibenzo-p-dioxin), the prototypic ligand for the aryl hydrocarbon receptor (AhR), promotes tumor formation in some model systems. However with regard to breast cancer, epidemiological and animal studies are inconclusive as to whether exposure increases tumor incidence or may instead be protective. We have previously reported that mice exposed to TCDD during pregnancy have impaired differentiation of mammary tissue, including decreased branching and poor development of lobuloalveolar structures. Because normal pregnancy-induced mammary differentiation may protect against subsequent neoplastic transformation, we hypothesized that TCDD-treated mice would be more susceptible to chemical carcinogenesis after parturition. To test this, mice were treated with TCDD or vehicle during pregnancy. Four weeks later, DMBA (7,12-dimethylbenz[a]anthracene) was administered to induce mammary tumor formation. Contrary to our hypothesis, TCDD-exposed parous mice showed a four-week delay in tumor formation relative to controls, and had a lower tumor incidence throughout the 27-week time course. The same results were obtained in nulliparous mice given TCDD and DMBA on the same schedule. We next addressed whether the delayed tumor incidence was a reflection of decreased tumor initiation, by testing the formation of DMBA-DNA adducts and preneoplastic lesions, induction of cytochrome P450s, and cell proliferation. None of these markers of tumor initiation differed between vehicle- and TCDD-treated animals. The expression of CXCL12 and CXCR4 was also measured to address their possible role in tumorigenesis. Taken together, our results suggest that AhR activation by TCDD slows the promotion of preneoplastic lesions to overt mammary tumors.
Keywords: Aryl hydrocarbon receptor, breast cancer, pregnancy, TCDD
Introduction
The aryl hydrocarbon receptor (AhR) is an orphan nuclear receptor that belongs to the PAS (per-arnt-sim) family of transcriptional regulators.1 For many years this receptor has been studied primarily because of its role in the toxic effects of dioxin-like compounds, which are ubiquitous and long-lived contaminants in the environment.2 Recently however, interest in further understanding this receptor has been bolstered by numerous reports indicating that the AhR plays a role in normal development, carcinogenesis and cell cycle regulation, and likely has endogenous ligands with important biological functions.3–6 Because of such discoveries, the development of non-toxic analogs of known AhR ligands for therapeutic use has begun to be explored.7–9
TCDD (2,3,7,8-tetrachlordibenzo-p-dioxin) is the best-characterized AhR ligand, and it affects many organ systems and deregulates numerous cellular pathways.2,10 TCDD is also classified as a class 1 carcinogen by the International Agency for Research on Cancer,11 and is established as a promoter for certain tumors including liver and skin.12,13 For breast cancer however, the relationship between exposure to AhR ligands and cancer risk is unclear. There are conflicting reports as to whether exposure to AhR ligands is associated with increased incidence of mammary neoplasias, or if it may in fact protect against breast cancer. Investigations of human populations exposed to dioxin-like chemicals report mixed findings of increased and decreased association with breast cancer risk, while others fail to show any correlation at all. 14–17
Collectively, studies conducted in rat models indicate that susceptibility to mammary tumors likely correlates with the age of the animal, and by extension the differentiation state of the mammary gland, at the time of TCDD exposure. For example, studies conducted by Safe and colleagues using mature adult rats demonstrated that TCDD, and other AhR agonists, can actually cause regression of existing chemical-induced tumors.18–20 Similarly, Kociba et al21 found reduced incidence of spontaneous mammary tumors in a long-term feeding with TCDD, despite increases in incidence of tumors at other locations. In contrast to the experiments wherein TCDD is administered to adult rats, other studies addressing developmental exposure to TCDD have demonstrated increased sensitivity to mammary tumorigenesis. Specifically, Lamartiniere and colleagues have shown that prenatal exposure to TCDD, which alters normal development of the mammary gland, increased the incidence of 7,12-dimethylbenz[a]anthracene (DMBA)-induced mammary tumors later in life.22,23 Finally, during the neonatal and weaning period, administration of AhR agonists may also influence subsequent development of mammary tumors, with somewhat conflicting results depending on the timing of exposure and the mixture administered.24, 25
One emerging paradigm to reconcile these seemingly conflicting results is that the ultimate susceptibility of mammary tissue to carcinogenic insult is dependent upon the status of the tissue at the time of AhR activation. More specifically, one way by which AhR ligands such as TCDD influence mammary tumorigenesis is by interfering with normal timing of development and differentiation of the gland, such as during fetal development.26,27 In support of this idea, Lamartiniere and colleagues have shown that developmental exposure to TCDD significantly increases the relative number of terminal end buds (TEB) compared to developed type II lobules at postnatal day 50.22 Because TEB are very susceptible to carcinogenic transformation, this hypothesis fits well with the observed increase in sensitivity to DMBA-induced tumorigenesis in rats exposed developmentally to TCDD.22,23
The differentiation of mammary tissue that occurs during pregnancy represents another critical window during which TCDD exposure causes profound suppression of normal development. Specifically, we have shown that mice treated with TCDD starting at the very beginning of pregnancy have observable defects in branching as early as day 6, and severely stunted development of lobuloalveolar structures with concomitant suppression of milk production by the end of pregnancy.28,29 Given that AhR activation impairs pregnancy-associated mammary gland development, we hypothesized that these defects would influence susceptibility to tumorigenesis postpartum. The rationale for this idea is that pregnancy-induced changes in the mammary gland may provide protection against breast cancer later in life.30–32 Although the specific alterations responsible for this protection are not fully understood, this protective effect is thought to result from permanent changes in the differentiation status or fate of the mammary cells that are induced by pregnancy.30–32
The goal of the studies presented here was to determine whether mice treated with TCDD during pregnancy demonstrate increased susceptibility to a mammary carcinogen administered after parturition. In other words, we considered that our discovery that TCDD suppresses pregnancy-induced mammary differentiation provides at least one mechanistic explanation for the correlation between AhR-mediated perturbation of normal mammary differentiation and altered incidence of breast cancer. Interestingly, our results showed that AhR activation delayed tumorigenesis regardless of pregnancy status and despite the suppression of pregnancy-induced glandular development. Further exploration into the mechanism of the delay suggested that prior AhR activation does not reduce carcinogen-induced DNA damage and the initiation phase of carcinogenesis. Instead, we postulate that the fate of the mammary epithelial cells is persistently altered by AhR activation to slow the proliferation and promotion of initiated cells.
Materials and Methods
Animals
Female CB6F1 mice were obtained from NCI Charles River. Both parental strains (BALB/c and C57Bl/6) express the high affinity AhRb allele that confers sensitivity to TCDD.33 Animals were given food and water ad libitum, and were maintained on a 12:12 hour light cycle. For experiments conducted in pregnant animals, female mice (age 6–7 weeks) were housed with males and checked daily for presence of vaginal plugs. Day 0 of pregnancy was designated as the day the vaginal plug was found. All treatment was in accordance with protocols approved by the Washington State University Institutional Care and Use Committee.
Chemicals and animal exposures
TCDD was dissolved in anisole and diluted in peanut oil to a concentration for dosing at 10 µl per gram body weight. Mice were administered TCDD in three weekly doses of 10 µg/kg (on day 0 and 7) and 5 µg/kg (on day 14). Preliminary dose-response studies indicated that 10 µg/kg TCDD on days 0 and 7 was necessary for substantial suppression of pregnancy-induced gland differentiation in this strain. The dose given on day 14 was decreased in order to speed clearance of TCDD from the body after parturition. Mice were treated every 7 days in order to maintain a relatively constant body burden of TCDD throughout pregnancy; the half-life of TCDD in mice is 11 days.34 Nulliparous mice were treated under the same TCDD paradigm as pregnant mice to serve as controls for the effect of pregnancy. Nulliparous mice were also used for some of the mechanistic studies, due to the fact that pretreatment with TCDD was determined to affect tumor formation equivalently in parous and nulliparous mice, and because it is logistically easier to maintain and treat nulliparous mice for tissue collection. Vehicle control for the TCDD treatments consisted of peanut oil containing an equivalent concentration of anisole. DMBA (7,12-dimethylbenz[a]anthracene, Sigma, St. Louis, MO) was dissolved in sesame oil under gentle heat to a final concentration of 5 mg/ml (for administering 1 mg in 200 µl).35 The solution was stored at 4°C and kept protected from light at all times. Sesame oil was given as the vehicle control for the DMBA treatments. Both TCDD and DMBA, and their vehicle controls, were administered by gavage.
Tumor study
Experimental design overview
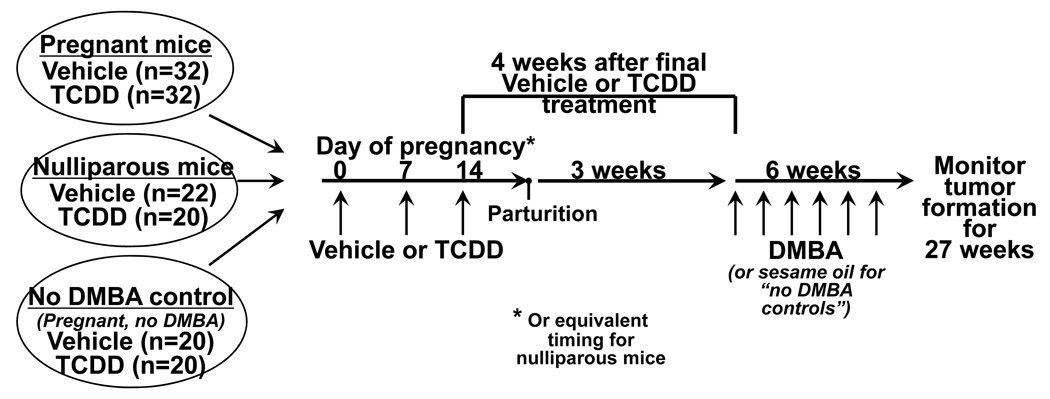
A graphic of the experimental design is shown in Figure 1. Female mice CB6F1 mice (age 6–7 weeks) were housed with males and checked daily for presence of vaginal plugs. Pregnant mice (n=32 per treatment group) were treated with 10 µg/kg of TCDD, or peanut oil vehicle, on days 0 and 7 of pregnancy, and with 5 µg/kg on day 14. Pups were removed at birth in order to eliminate lactation as a potential confounding factor (dams treated with these doses of TCDD do not lactate and their pups would not survive 28). The dams received no further treatment for 4 weeks after the final TCDD treatment, which is equivalent to 3 weeks after parturition, to decrease the residual body burden of TCDD and to permit the glands to regress. Following the no exposure period, mice were treated with 1 mg DMBA per week for six weeks.36 Nulliparous age-matched animals were treated with the same doses of TCDD (n=20) or its vehicle (n=22) and with DMBA on the same schedule as the parous mice. Control groups of vehicle- and TCDD-treated parous mice (n=20 per treatment group) were not given DMBA, but instead were administered sesame oil vehicle. These non-DMBA control animals never developed palpable tumors.
Figure 1. Tumor study design and animal treatment.
Six groups of CB6F1 mice (age 6–7 weeks at the start of the experiment) were included in this study. Pregnant mice were treated with vehicle (n=32) or TCDD (n=32) at 3 time points during pregnancy, followed by 6 weekly doses of DMBA beginning 4 weeks after the final TCDD treatment (equal to 3 weeks after parturition). Nulliparous age-matched mice were treated with vehicle (n=22) or TCDD (n=20) and DMBA on the same schedule as the pregnant mice. Finally, vehicle- and TCDD-treated control groups that did not receive DMBA were also included. For these “no DMBA” controls, pregnant mice were treated with vehicle (n=20) or TCDD (n=20) during pregnancy, then given sesame oil (the vehicle control for DMBA) beginning three weeks after parturition. No tumors developed in the mice that did not receive DMBA. See Methods for additional details about the TCDD and DMBA treatments.
Tumor assessment
Mammary tumor formation was monitored by physical palpation twice weekly, beginning five weeks after the final administration of DMBA. The location of the tumors was recorded. Once a tumor had enough 3-dimensional structure to measure, the size was monitored weekly using a vernier caliper. Two perpendicular diameters, termed length (L) and width (W), were determined, with length defined as the larger of the two measurements. Volume was calculated using the formula 4/3 × π x (L/2) x (W/2)2.37 The multiplicity of tumors was determined as follows: (the total number tumors per group) / (number of mice with tumors in that group).
Termination
Mice were euthanized by carbon dioxide overdose when a tumor length reached 25 mm, or if at any time the animal appeared uncomfortable or moribund. The tumor study was terminated at 27 weeks after the final DMBA administration. At that time, greater than 90% of the mice in each DMBA group had either developed palpable tumors or had died of other causes. DMBA is a multisite carcinogen, and some of the mice developed other malignancies (often thymoma, also ovarian, lung, etc) that contributed to morbidity and mortality.
Evaluation of preneoplastic lesions
Preneoplastic lesions, including hyperplastic alveolar nodules (HAN), ductal carcinoma in situ (DCIS), and ductal hyperplasia (DH) in the mammary whole mounts were quantified.38,39 Additionally, non-preneoplastic lesions in the gland were quantified, including cystic nodules (CN), fine duct hyperplasias (FDH), dense nodules (DN), and mammary tumors (MT). Cystic nodules were defined as focal areas of dilated alveoli. Dense nodules were small highly stained areas comprised of epithelial and stromal cells surrounded by dense connective tissue and with a very low mitotic index. Mammary tumors were microscopic foci of epithelial cells that proliferated into the surrounding stroma. Samples of all types of lesions were verified by histological sections. Lesions were identified in a subset of mice (n=11–19 per group) that were sacrificed within a consistent window of time relative to DMBA administration (≥22 weeks after final DMBA treatment).
Whole mounts
Mammary whole mounts were prepared as described previously.28 Briefly, the 4th and 5th mammary glands were mounted onto a glass slide under weight, and fixed in Carnoy's fixative. Fixed glands were transferred to 70% ethanol, rehydrated, and stained with carmine alum. Glands were then dehydrated, cleared with xylenes, and mounted using Permount (Fisher Scientific, Pittsburgh, PA). Whole mounts were examined and given a development score based on a four-point scale. The development scores considered epithelial branching, development of lobuloalveolar units, and the size of the structures (1=poor development, 4=excellent development). Photographs were taken at 3.1x using a Jenoptik ProgRes C12plus digital camera attached to a Wild Dissecting Microscope.
Western Blotting
The levels of cytochrome P450s (Cyps) 1a1 and 1b1 and of AhR were assessed in protein extracts from mammary gland and/or liver. Tissues were homogenized in RIPA buffer and protease inhibitors using a Tissue Tearor (Biospec Products, Inc). The proteins in the supernatant were quantified and boiled in SDS-PAGE sample buffer. Thirty µg of protein was separated on 8% acrylamide gels, and transferred to PVDF membranes. Primary antibodies for Cyp1a1, Cyp1b1, and Actin were purchased from Santa Cruz Biotech (Santa Cruz, CA), and the primary antibody for AhR was purchased from BIOMOL International (now Enzo Life Sciences, Plymouth Meeting, PA). Secondary antibodies included IRDye™ 700DX Conjugated anti-goat IgG (Li-Cor, Lincoln, Nebraska) and HRP-conjugated anti-rabbit antibody (DakoCytomation, Glostrup, Denmark). Bands were visualized using the Li-Cor Odyssey ™ Infrared Imaging System (for IRDye reagents), or by exposure to x-ray film following incubation with chemiluminescent ECL reagents (for HRP-conjugated reagents). The intensity of the bands was evaluated using the Li-Cor software or from scans of x-ray films using Quantity One (BioRad, Hercules, CA), as appropriate for each secondary antibody conjugate.
DMBA-DNA adduct assay
Genomic DNA from homogenized mammary glands was isolated by a standard phenol-chloroform extraction method.40 DNA adducts were determined for each DNA sample using the nuclease P1 enrichment version of the 32P-postlabeling method as described previously.41, 42 Briefly DNA samples (4 µg) were digested with micrococcal nuclease (120 mU) and calf spleen phosphodiesterase (40 mU), enriched and labeled as reported. Chromatographic conditions for thin-layer chromatography (TLC) on polyethyleneimine-cellulose (PEI-cellulose) (10 cm × 20 cm; Macherey-Nagel, Düren, Germany) were: D1, 1.0 M sodium phosphate, pH 6; D3, 3.5 M lithium-formate, 8.5 M urea, pH 3.5; D4, 0.8 M lithium chloride, 0.5 M Tris-HCl, 8.5 M urea, pH 8. After chromatography, TLC sheets were scanned using a Packard Instant Imager (Dowers Grove, IL, USA) and DNA adduct levels (RAL, relative adduct labeling) were calculated from adduct cpm, the specific activity of [γ-32P]ATP and the amount of DNA (pmol of DNA-P) used. Results were expressed as DNA adducts/108 nucleotides.
Cell Proliferation Analysis
The incorporation of BrdU (bromodeoxyuridine) was used to measure the proliferation status of mammary epithelial cells. An intraperitoneal injection of BrdU (50 mg/kg, Sigma-Aldrich, St. Louis, MO) was given one day prior to sacrifice. Abdominal-inguinal mammary glands were formalin-fixed, paraffin-embedded, and sliced for immunohistochemical (IHC) analysis of BrdU incorporation. A BrdU IHC System Kit (Calbiochem, San Diego, CA) was used to stain the BrdU positive cells according to the manufacturer’s instructions. To count the BrdU positive epithelial cells in each tissue slice, 10 images were generated for one slice of each gland using QCapture Pro51 software (Qimaging, Surrey, BC, Canada) and a Nikon microscope at magnification 400x.
Real-time PCR
Quantitative real-time PCR (qRT-PCR) analyses were performed to measure mRNA levels of the chemokine CXCL12 and its receptor CXCR4 in mammary tissue and tumors. Actin was used as an endogenous control. Total RNA was extracted from mammary glands homogenized in TRIzol reagent (Invitrogen, Carlsbad, CA). One µg of total RNA was reverse-transcribed to single strand cDNA using High-Capacity cDNA Reverse Transcription kit (Applied Biosystems Inc. [ABI], Foster City, CA). qRT-PCR reactions were performed using the ABI Prism 7000 Sequence Detection System. TaqMan reagents and gene expression assays were from ABI. A 25 µl reaction mixture containing 5 µl of cDNA template, 12.5 µl TaqMan Universal PCR master mix and 1.25 µl primer probe mixture was amplified using the following thermal cycler parameters: denaturation at 95°C for 10 min, then 40 cycles of the amplification step (denaturation at 95°C for 15 sec and annealing/extension at 60°C for 1 min). Raw CT (cycle threshold) values were obtained from the ABI7000 software, and used to compare the mRNA levels between the vehicle- and TCDD-treated groups. The fold-change between the treatment groups was calculated by first determining the ΔCT value (ΔCT = CT(target RNA) -CT(endogenous control)) for each sample. The unlogged ΔCT (= 2−ΔCT) was then determined for each vehicle-treated animal, then normalized to 1 using the average unlogged ΔCT of all samples in that group. The relative fold-change for each TCDD-treated animal was calculated by comparison to the average of the vehicle-treated group. The unlogged ΔCT (= 2−ΔCT) for each animal was used for statistical analysis.
Statistics
Tumor incidence was analyzed by Fisher’s exact test and by ANOVA, using Tukey’s test for pairwise comparisons. Other comparisons were made using Students t test. A p-value of ≤ 0.05 was considered significant. Statistical analyses were performed using Prism (GraphPad Software, La Jolla, CA) or Minitab (Minitab Inc., State College, PA).
Results
Part 1: Effect of AhR activation on mammary gland differentiation and tumor incidence
AhR activation suppresses pregnancy-induced differentiation of mammary glands in CB6F1 mice
Previous studies conducted in C57Bl/6 mice have demonstrated that AhR activation during pregnancy suppresses normal branching and lobuloalveolar development in the mammary gland. The central hypothesis for the current study was that this suppressed differentiation would result in increased susceptibility to mammary tumorigenesis postpartum. Because C57Bl/6 mice are less susceptible to chemical-induced carcinogenesis than the Balb/c strain,43 CB6F1 mice (Balb/c X C57Bl/6) were used for these tumorigenesis studies. Therefore, the first objective was to demonstrate the suppressive effect of TCDD on pregnancy-induced gland differentiation in this particular mouse strain.
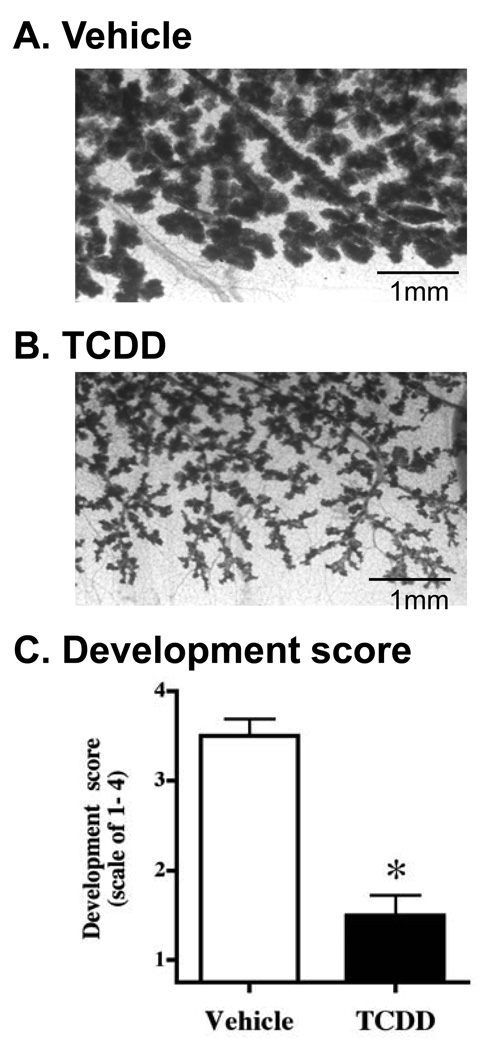
Impregnated mice were treated with 10 µg/kg TCDD on days 0 and 7 of pregnancy, and with 5 µg/kg on day 14. Mammary whole mounts were made from glands taken on day 17 of pregnancy. As shown in Figure 2, AhR activation decreased the normal branching that occurs during pregnancy and resulted in poor development of lobuloalveolar structures. Development scores quantifying the amount of differentiation in glands showed that the suppression caused by TCDD treatment was statistically significant (Figure 2C). The effects presented in Figure 2 are similar to those reported previously in TCDD-treated C57Bl/6 mice.28, 29
Figure 2. Mammary gland differentiation that occurs during pregnancy is disrupted by AhR activation.
Impregnated CB6F1 mice were treated with vehicle (A; n=7) or TCDD (B; n=5) at three times during pregnancy (10 µg/kg on day 0 and day 7, and 5 µg/kg on day 14). Whole mounts of the abdominal mammary glands were made on day 17 of pregnancy, fixed, and stained with carmine alum. (C) Glands were given scores that considered epithelial branching, development of lobuloalveolar units, and the size of the structures. Bars indicate the mean score for each treatment group (± SEM). * p ≤ 0.05. Representative photos of glands from vehicle-treated (A, developmental score = 4) and TCDD-treated (B, developmental score 1.5) mice were taken at 3.1x
Prior AhR activation decreases susceptibility to DMBA-induced mammary tumorigenesis
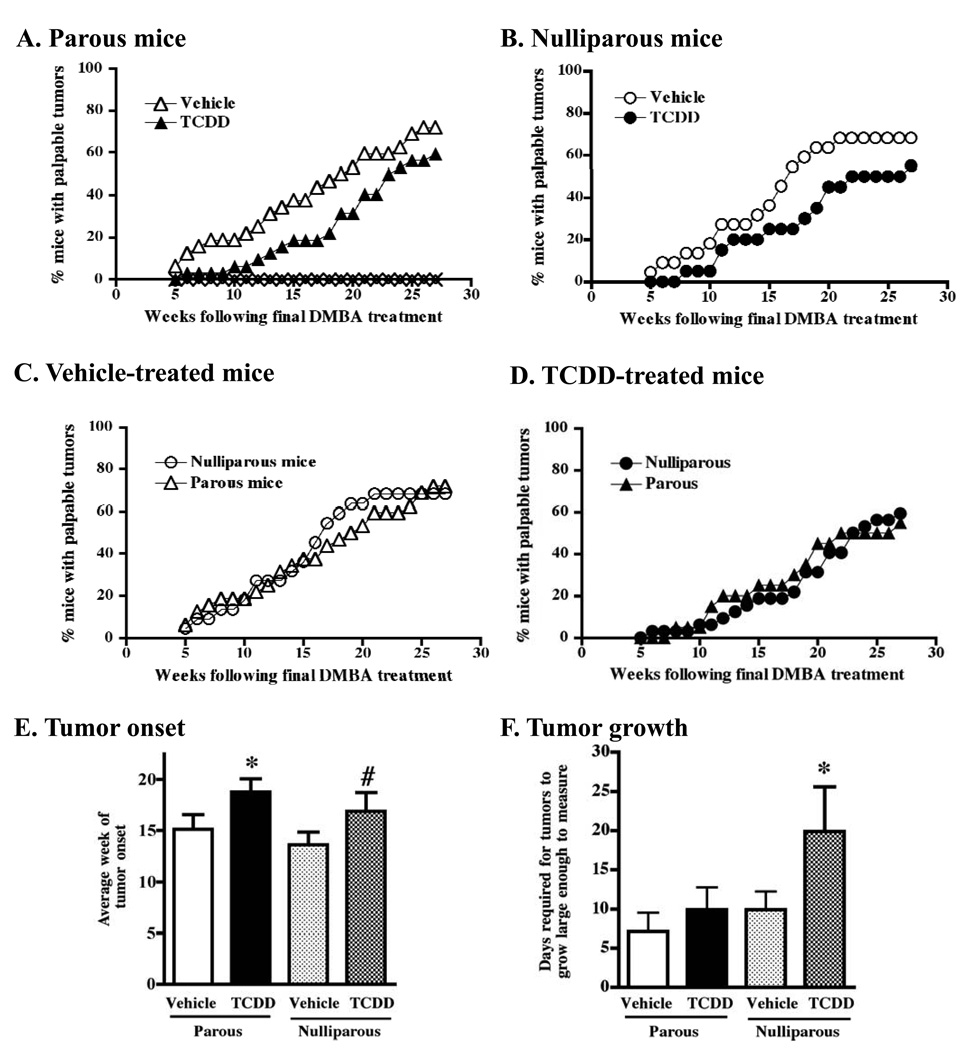
Evidence in both humans and rodents suggests that pregnancy may cause changes in mammary cells that reduce the probability of developing mammary tumors later in life.32,44–46 Because TCDD suppresses differentiation of the mammary gland when given to mice during pregnancy, we tested whether these mice would be more susceptible to DMBA-induced tumor formation later on. To accomplish this, mice were treated with TCDD or vehicle control during pregnancy, pups were removed at birth and the dams remained untreated for 3 weeks post-partum. DMBA was administered once a week for 6 weeks, and palpable tumors were evaluated over time. Interestingly, we found that mice treated with TCDD during pregnancy had a decreased tumor incidence relative to the vehicle-treated parous animals at all times examined (Figure 3A). Specifically, the number of tumor-positive mice at a given time point was decreased by an average of 17% in the TCDD-treated group compared to the vehicle-treated group (range 9–22% lower, depending on the week). The difference between the two groups was highly significant (p < 0.0001). Furthermore, the average week of tumor onset (relative to the final DMBA treatment) was delayed by nearly four weeks in the TCDD-treated animals (Figure 3E, parous mice, p < 0.05).
Figure 3. Prior treatment with TCDD delays tumor formation in DMBA-treated parous and nulliparous mice.
The development of mammary tumors was monitored by physical palpation beginning 5 weeks after the final treatment with DMBA and continued for 27 weeks. (A) Incidence of mammary tumors in DMBA-treated parous mice (triangles) that had been treated with vehicle (open triangles, n=32) or TCDD (filled triangles, n=32) during pregnancy. The difference between the two groups was highly significant (p < 0.0001 as analyzed by ANOVA using a Tukey’s test for pairwise comparisons). No tumors formed in parous control mice that were not given DMBA (vehicle group = open diamonds, n=20; TCDD group = X symbol, n=20). (B) Tumor incidence in DMBA-treated nulliparous mice (circles) that had been treated with vehicle (open circles, n=22) or TCDD (filled circles, n=20) and DMBA on the same schedule as the parous mice. The difference between the two groups was highly significant (p < 0.0001). (C and D) The same experimental groups shown in panels A and B were re-graphed to demonstrate the observed lack of effect of pregnancy on subsequent tumor incidence. The incidence of mammary tumors in DMBA-treated parous (triangles) and nulliparous mice (circles) is shown both for mice pretreated with vehicle control (C) or with TCDD (D). Statistical comparisons showed no difference between the parous and nulliparous groups (p = 0.30 for C; and p = 0.18 for D). (E) Average time of tumor onset (± SEM) relative to the final DMBA treatment. (F) Growth rate was assessed by determining the number of days between the time each tumor was first detected by palpation until it grew large enough to physically measure with vernier calipers. * p ≤ 0.05; # p = 0.065.
The same beneficial effect of prior AhR activation was observed in nulliparous mice treated with DMBA (Figure 3B, p < 0.0001). Specifically, the number of tumor-positive mice at a given time point was decreased by an average of 18% in the TCDD-treated group compared to the vehicle-treated group (range 7–30% lower, depending on the week). The average time of tumor onset was also delayed by over three weeks in mice with prior TCDD exposure (Figure 3E, nulliparous mice, p = 0.065).
Parity status alone does not alter tumor incidence
A separate but important consideration in these studies was the effect of parity status on tumor incidence. Pregnancy is hypothesized to protect against tumor development in rats and humans, and therefore nulliparous mice were included in the study to serve as controls for the parous animals. However, our results showed that a single pregnancy did not protect against tumor development, because tumor formation in nulliparous mice was essentially the same as for the parous animals. In other words, there were no statistically significant differences in the tumor incidence curves between the vehicle-treated parous mice and the vehicle-treated nulliparous animals (Figure 3C; p=0.30). Neither did we observe differences between the TCDD-treated parous animals and the TCDD-treated nulliparous mice (Figure 3D; p=0.18).
Tumor size and multiplicity are not significantly altered by prior AhR activation
We next determined whether growth of the mammary tumors was affected by prior exposure to TCDD. It is important to emphasize that we intentionally addressed the growth rate of tumors once they had formed. That is, given that there was an overall delay in tumor formation in the TCDD-treated mice, the number and size of tumors in TCDD groups were smaller at a given week following DMBA exposure. Therefore, the growth of each tumor was monitored relative to the time of initial onset in that animal.
Tumor growth was assessed in three ways. First, we calculated the number of days between the time each tumor was first detectable by palpation and when it grew large enough to measure with calipers. As shown in Figure 3F, prior TCDD exposure caused a statistically significant retardation of tumor growth in the nulliparous mice, but not in parous animals. This result is suggestive that prior AhR activation slows tumor growth in addition to the timing of tumor onset, and that the protective effect is more pronounced in nulliparous animals. However, for the other two methods used to evaluate tumor growth we did not observe a difference based on parity or TCDD pretreatment. Specifically, when we determined the volume of each tumor on days 7, 14, and 21 after the tumor was first large enough to measure, neither prior exposure to TCDD nor parity status affected tumor volume at any time point (data not shown). Likewise, the number of tumors that formed in each affected animal was not significantly affected by either TCDD treatment or parity (tumor multiplicity: Vehicle-Parous = 1.13, TCDD-Parous = 1.37; Vehicle-Nulliparous = 1.53, TCDD-Nulliparous = 1.45).
Part 2: Effect of AhR activation on tumor initiation
Chemical carcinogenesis is considered a multi-step process, consisting of initiation, promotion, and progression. In the next series of experiments we addressed the hypothesis that prior activation of the AhR specifically alters tumor initiation. Initiation parameters examined included P450 induction and DNA damage, carcinogen-susceptible proliferating epithelial cells, and formation of preneoplastic lesions.
Persistent AhR activation and Cytochrome P450 induction is unlikely at the time of carcinogen administration
In the experimental design for the tumor study, administration of the carcinogen (DMBA) began four weeks after the final treatment with TCDD. The intent of this delay was in part to allow time for the TCDD to be cleared and reduce/eliminate the influence of persistent AhR activation at the time the tumors were forming. In other words, our intent was to test whether the fate of the mammary epithelial cells was changed by prior AhR activation, not to examine the effect of concurrent AhR activation on a carcinogenic insult. However, it remained possible that the AhR was still activated four weeks after TCDD treatment, such that persistent induction of P450 enzymes could alter the biotransformation of the carcinogen DMBA.
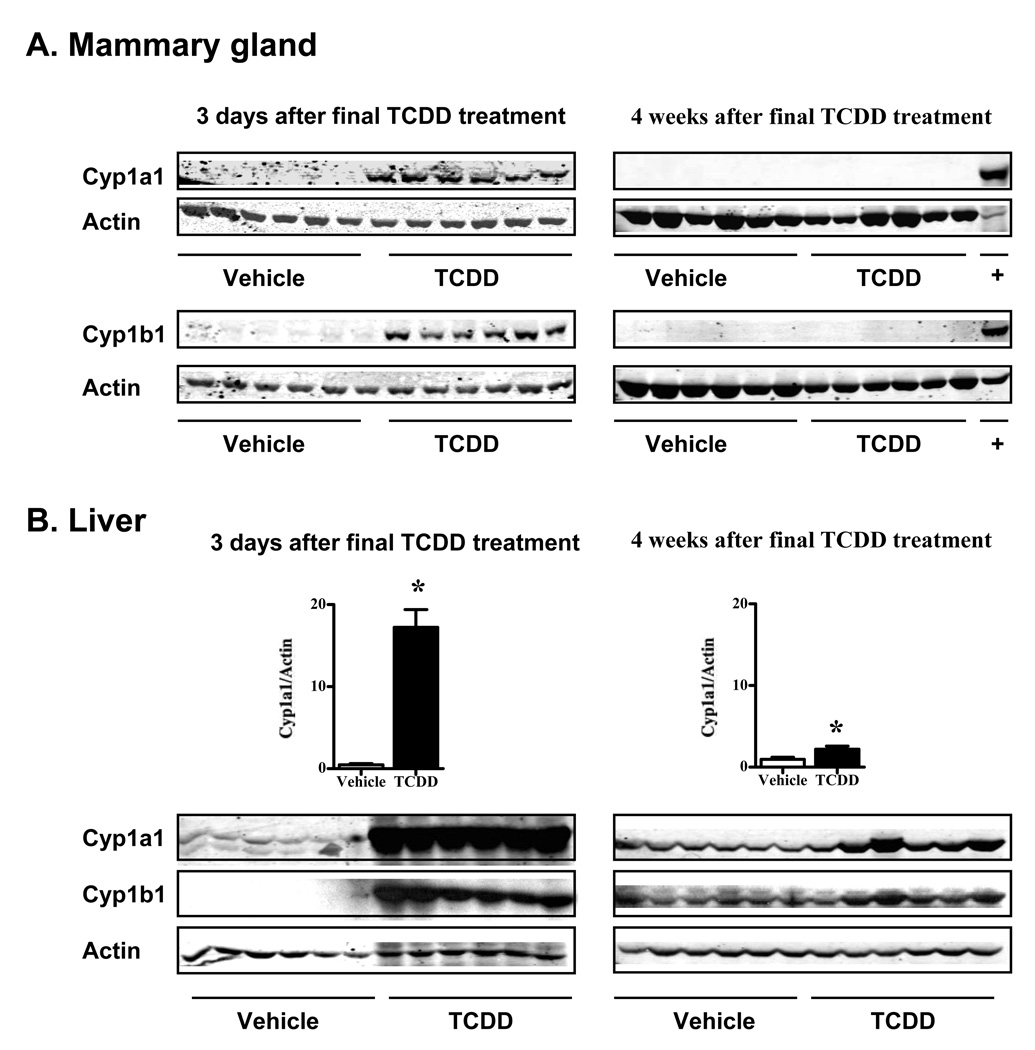
To address AhR activation status at the time of DMBA administration, the levels of Cyp1a1 and Cyp1b1 proteins were measured four weeks after the final treatment with TCDD, using a cohort of mice separate from the tumor study. For comparison, the induction of these enzymes was also determined three days after the final treatment with TCDD. As expected, Cyp1a1 and Cyp1b1 were highly induced in both the mammary gland and liver three days following TCDD treatment (Figure 4, left panels). However, in the mammary gland these enzymes were completely absent by four weeks after treatment (Figure 4A, right panels). Similar results were observed in the liver samples, where Cyp induction was markedly reduced, although still detectable and statistically different (p < 0.05), by four weeks post-exposure (Figure 4B, right panels). These results strongly support the conclusion that persistent AhR activation, particularly in the mammary gland itself, is not responsible for the delay in tumor formation. Furthermore, the absence of persistent induction of Cyp enzymes suggests that differences in DMBA metabolism are unlikely to account for the delay in tumor formation in the mice pretreated with TCDD.
Figure 4. Cytochrome P450 induction is transient and returns to baseline by four weeks following TCDD treatment.
Cyp1a1 (56kDa) and Cyp1b1 (57kDa) were assessed in protein extracts from (A) mammary gland and (B) liver using Western blotting. Mice were treated with three weekly doses of vehicle or TCDD, as described in the Methods. Tissues were collected at two time points, including three days after, and four weeks after the final vehicle or TCDD treatment. The positive (+) control for Cyp1a1 is liver extract from a TCDD-treated mouse, and for Cyp1b1 is protein from cultured mammary epithelial cells (SCp2 cells) treated with 10−9M TCDD for 5 days. Actin (43kDa) was used as a loading control. Bar graphs in (B) illustrate the magnitude of the difference in Cyp1a1 induction present at 3 days vs 4 weeks following TCDD exposure. Similar results were obtained for Cyp1b1. Tissues from pregnant mice were used in the three day time point, and nulliparous mice were the source of tissues taken at 4 weeks. Parity status did not substantially affect baseline Cyp expression in the liver of vehicle-treated animals when proteins were compared on the same blots (≤14% difference, not shown). * p ≤ 0.05.
Given that TCDD treatment is known to cause rapid degradation of the AhR, which could likewise affect the response to DMBA, we also examined the level of AhR protein in the mammary glands. Consistent with other reports showing diminished AhR expression in cultured cell lines, mammary gland, and other tissues,47–50 we found that AhR levels in the mammary gland were significantly lower in TCDD-treated mice when assessed 3 days following treatment (Supplementary Figure 1). However AhR levels had recovered within the 4 weeks following the final TCDD exposure, and there was no persistent difference in receptor levels between the treatment groups at the time DMBA was administered.
Influence of TCDD on DMBA-DNA adduct levels
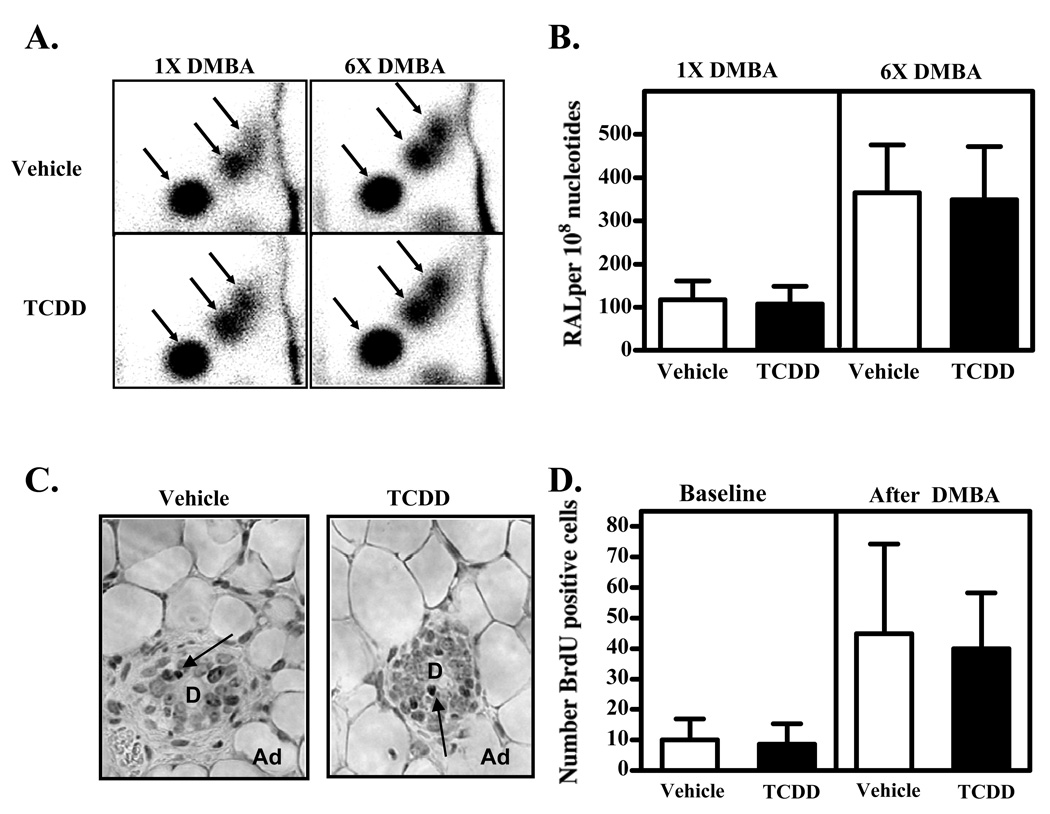
The initiation phase of chemical carcinogenesis begins with damage to the DNA bases. For DMBA, metabolites of the chemical form covalent adducts with DNA that can result in DNA mismatch and mutation. To directly test whether prior exposure to TCDD alters the formation of these lesions, the amount of DMBA-DNA adducts in the mammary glands was investigated by 32P-postlabeling analysis. Representative TLC autoradiographs are shown in Figure 5A. The adduct pattern observed after oral administration of DMBA (in the presence or absence of TCDD) was similar to that found in mouse skin epidermis after topical application.41 It consisted of three major adduct spots, which have been shown to represent DMBA-DNA adducts formed by bay-region diol epoxides. No DNA adducts were detected in non-DMBA control tissues (data not shown). Total DMBA-DNA adduct levels are illustrated in Figure 5B. The level of DMBA-DNA adducts increased in response to the number of DMBA treatments the animals received, at around 120 and 350 adducts per 108 nucleotides after one and six DMBA doses, respectively. However, prior treatment with TCDD did not change the damage to the DNA bases (adducts) caused by DMBA.
Figure 5. Prior activation of the AhR does not change the level of DMBA-DNA adducts in the gland or the proliferative status of mammary epithelial cells.
Nulliparous mice were treated with three weekly doses of vehicle or TCDD, as described in the Methods. (A, B) Four weeks after the final vehicle or TCDD treatment, the carcinogen DMBA was administered to initiate tumor formation. Mice were sacrificed 24 hours after receiving either one dose of DMBA, or after receiving six weekly doses of DMBA (n=6 mice per treatment group at each time point). The number of DNA adducts in DNA extracted from the mammary glands was determined by 32P-postlabeling. (A) The patterns of DMBA-DNA adducts are shown in representative TLC autoradiographs. Arrows indicate the three major adduct spots, which represent adducts formed by DMBA bay-region diol epoxides. (B) Levels of adducts (RAL = relative adduct labeling) in the vehicle- and TCDD-pretreated mice that were subsequently given one or six doses of DMBA. (C, D) Four weeks after the final vehicle or TCDD treatment, the proliferating mammary epithelial cells were labeled by injecting the animals with BrdU (“Baseline”). Separate groups of vehicle- and TCDD-exposed animals were also administered a single dose of DMBA and sacrificed six days afterward (“DMBA”) (n=7 mice for each treatment group). BrdU positive cells in mammary gland slices were detected by immunohistochemistry. (C) Examples of tissue sections showing the BrdU stained cells (arrows) from vehicle- and TCDD-pretreated mice following DMBA exposure. Photos were taken at 400x. “D” indicates a duct, “Ad” indicates adipocytes. (D) Quantification of the proliferating cells was conducted by counting the total number of BrdU stained cells in 10 separate images from each gland.
Proliferation status of mammary epithelial cells at the time of carcinogen exposure
Mammary epithelial cells that are rapidly proliferating may be more susceptible to damage by carcinogens, as evidenced by the correlation between mammary tumor incidence and number of proliferating cells in the ducts and end buds.22,31,45 To test whether prior exposure to TCDD reduced the number of proliferating mammary epithelial cells (i.e., those cells possibly most susceptible to DNA damage and mutation), BrdU incorporation was examined by immunohistochemistry. Mice that had been treated with three weekly doses of vehicle or TCDD were injected with a single dose of BrdU 4 weeks after their final treatment. Proliferating cells were quantified by counting the number of cells that stained positively for BrdU (Figure 5C). The analysis revealed that prior AhR activation did not diminish the number of proliferating mammary epithelial cells 4 weeks later (Figure 5D, left columns, “Baseline”), which was the time at which the DMBA was administered.
In separate groups of pretreated animals, a single dose of DMBA was given (Figure 5D, right columns, “DMBA”). The goal was to determine if prior AhR activation dampens the proliferative response of the mammary epithelial cells resulting from DNA damage. As expected, the DMBA treatment itself increased the number of proliferating cells relative to cells from mice not given DMBA (Baseline); however there was no effect of prior treatment with TCDD. Taken together, these results show that TCDD does not cause persistent suppression in the baseline proliferative status of mammary epithelial cells, nor in their initial proliferative response to DNA damage.
Evaluation of preneoplastic and other microscopic lesions
In addition to macroscopic measurable tumors, DMBA and other carcinogens induce the formation of other lesions in the mammary gland, some of which are preneoplasias. To ascertain whether prior activation of the AhR influences formation of these microscopic lesions, six types of lesions were evaluated in mammary glands from mice in the tumor study. Specifically, these lesions included two preneoplastic lesions (hyperplastic alveolar nodules and ductal carcinoma in situ/ductal hyperplasia) and four others (cystic nodules, fine duct hyperplasias, dense nodules, and mammary tumors). Table I shows the average number of each type of lesion found in each animal, the percentage of animals in each group with that lesion, and the multiplicity of each lesion type. Overall, there were no consistent differences in the incidence of these lesions between the different treatment groups. These results further support the conclusion that tumor initiation is not diminished by prior activation of the AhR, because the number of preneoplastic and other lesions in the TCDD-treated mice is similar, and in some cases even slightly higher, than in vehicle control mice.
Table 1.
Incidence of preneoplastic and other mammary lesions
| Average number of each lesion per animal1 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Preneoplastic lesions (PNL) |
Non-preneoplastic lesions |
|||||||
| Group | DCIS/DH2 | HAN | All PNL3 | MT | FDH | DN | CN | All lesions (ave # per |
| Vehicle - Parous | 1.0 | 0.4 | 1.4 | 1.0 | 0.2 | 2.2 | 4.0 | 8.8 |
| TCDD - Parous | 1.8 | 1.0 | 2.9 | 0.9 | 0.1 | 1.5 | 11.4 | 16.7 |
| Vehicle - Nulliparous | 2.4 | 0.7 | 3.1 | 1.0 | 0.6 | 0.8 | 4.7 | 11.9 |
| TCDD - Nulliparous | 1.2 | 1.9 | 3.1 | 1.1 | 0.2 | 1.1 | 4.5 | 9.9 |
| Percentage of animals per group with each lesion type | ||||||||
| Preneoplastic lesions (PNL) |
Non-preneoplastic lesions |
|||||||
| Group | DCIS/DH2 | HAN | All PNL3 | MT | FDH | DN | CN | |
| Vehicle - Parous | 45 | 36 | 54.5 | 55 | 18 | 64 | 73 | |
| TCDD - Parous | 63 | 37 | 73.7 | 47 | 11 | 58 | 100 | |
| Vehicle - Nulliparous | 64 | 36 | 54.5 | 55 | 36 | 55 | 82 | |
| TCDD - Nulliparous | 36 | 45 | 54.5 | 18 | 9 | 55 | 91 | |
| Multiplicity4 of each lesion type | ||||||||
| Preneoplastic lesions (PNL) |
Non-preneoplastic lesions |
|||||||
| Group | DCIS/DH2 | HAN | All PNL3 | MT | FDH | DN | CN | |
| Vehicle - Parous | 2.2 | 1.0 | 2.5 | 1.9 | 1.0 | 3.5 | 5.5 | |
| TCDD - Parous | 2.9 | 2.8 | 3.9 | 1.8 | 1.2 | 2.6 | 11.4 | |
| Vehicle - Nulliparous | 3.8 | 1.8 | 5.6 | 1.9 | 1.6 | 1.6 | 5.8 | |
| TCDD - Nulliparous | 3.3 | 4.1 | 5.6 | 6.0 | 2.0 | 1.9 | 5.0 | |
Sample size: Vehicle-Parous (n=ll), TCDD-Parous (n=19), Vehicle-non-Parous (n=ll), TCDD-non-Parous (n=ll)
Abbreviations: ductal carcinoma in situ (DCIS), ductal hyperplasia (DH), hyperplastic alveolar nodule (HAN), preneoplastic lesion (PNL), mammary tumor (MT), fine duct hyperplasias (FDH), dense nodules (DN), and cystic nodules (CN).
DCIS, DH, and HAN are considered preneoplastic lesions.
Multiplicity = (# lesions per group) / (# animals with that lesion per group)
Part 3: Effect of TCDD treatment on CXCL12 and CXCR4 in vivo
In a recent publication by Hsu et al,51 the authors reported that TCDD treatment of MCF-7 cells reduced the expression of the chemokine CXCL12 and its receptor CXCR4. This discovery may be significant because these two factors have been implicated in breast cancer metastasis, and a reduction in their expression was postulated to play a role in the protective role of AhR activation in breast cancer.
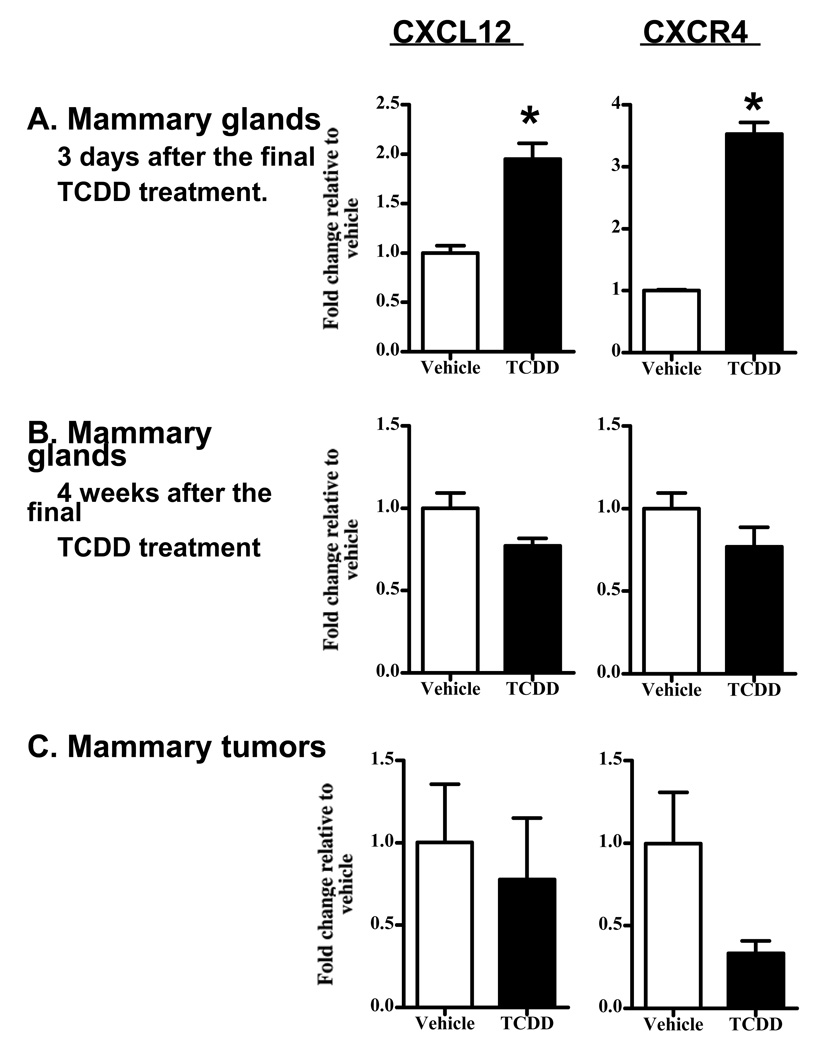
To address the potential for changes in CXCL12 and CXCR4 to contribute to the delay in tumor growth in the DMBA model, as well as to further characterize the expression of these factors in vivo, we determined their mRNA levels using qRT-PCR. Mammary glands were collected from mice treated with three weekly doses of vehicle or TCDD. In one group of mice, CXCL12 and CXCR4 levels were examined three days after the final treatment with vehicle or TCDD. At this time point, both CXCL12 and CXCR4 levels were significantly increased in the TCDD-treated animals, by 2-fold and 3.5-fold respectively (Figure 6A). However, when we examined message levels of CXCL12 and CXCR4 four weeks later, the TCDD-mediated increase did not persist (Figure 6B). Moreover, the levels of the mRNAs in the DMBA-induced mammary tumors themselves were not found to differ between the vehicle- and TCDD-treated groups (Figure 6C). There was suggestive evidence for a decrease in CXCR4 expression in tumors from TCDD-treated mice, however the difference was not technically statistically significant.
Figure 6. Effect of TCDD on CXCL12 and CXCR4 expression.
Parous mice were treated with three weekly doses of vehicle or TCDD, as described in the Methods. CXCL12 and CXCR4 expression were analyzed by qRT-PCR using RNA isolated from mammary glands of mice (n=5–6) sacrificed either 3 days (A), or 4 weeks (B), after the final TCDD treatment. (C) mRNA isolated from mammary tumors collected during the tumor study shown in Figure 3 (n=4) were also analyzed. * p ≤ 0.05 and a fold-change of ≥ 2 relative to vehicle. [The decrease in CXCL12 expression in (B) is technically statistically significant, but is substantially less than 2-fold. The p value for the decrease in CXCR4 expression in (C) is 0.07].
Discussion
Breast cancer is among the leading killers of women world-wide52 and there is a clear need for developing novel therapeutic options and preventative measures. One strategy that has been proposed for treating breast cancer is the use of selective AhR modulators (SAhRMs). The use of such compounds is based on the fact that AhR is expressed in mammary cells and that AhR activation antagonizes estrogen receptor (ER) signaling and inhibits tumor growth.8,18–21 Additionally, new evidence suggests that SAhRMs may also be effective in treating estrogen receptor-negative tumors.9 The goal of the current studies was to test whether AhR activation during pregnancy affects susceptibility to a genotoxic mammary carcinogen administered weeks later. Interestingly, we found that prior exposure to TCDD delayed tumor onset and reduced tumor incidence in adult mice. Furthermore, this beneficial effect of AhR activation occurred regardless of whether TCDD was given during pregnancy or to nulliparous animals. These interesting results provide additional support and insight for a protective role of AhR activation in mammary tumorigenesis.
In our studies, TCDD was administered 4 weeks before the animals were given DMBA and ≥ 14 weeks had elapsed by the time the first tumors formed. Therefore it was important to consider whether the beneficial effects resulted from persistent levels of TCDD in the body or from persistent changes in the fate of the mammary cells caused by previous AhR activation. To address this, we examined the level of Cyp1a1 and Cyp1b1, which are sensitive and commonly-used biomarkers of AhR activation. Our results showed that Cyp expression in the mammary gland itself had returned to background levels within the 4 weeks following TCDD treatment. Given that only minute amounts of TCDD are needed to induce Cyp1a1 and Cyp1b1,53 our results do not support the idea that residual TCDD is directly affecting tumor growth in our studies. Further support for this interpretation is provided by knowledge that the half-life of TCDD in AhRb mice is 7–10 days,54,55 so the majority of the TCDD was cleared prior to the growth phase of the tumors. Therefore we speculate that the protection afforded by prior AhR activation results from a change in the fate of the mammary epithelial cells that causes resistance to neoplastic transformation.
While numerous studies have shown that AhR activation is protective against mammary carcinogenesis, the precise mechanism of the protection remains unclear. We began our investigation into the mechanism of delayed tumor formation by examining the impact of prior AhR activation on the initiation stage of carcinogenesis. Specifically we examined whether mice treated with TCDD four weeks prior had (i) persistent induction of metabolic enzymes that biotransform DMBA, (ii) decreased numbers of DMBA-DNA adducts, or (iii) diminished proliferation status of the mammary epithelial cells at the time of carcinogen administration. We also compared (iv) the levels of preneoplastic lesions in the mammary glands of the TCDD- and vehicle-exposed animals. Each of these four markers relevant to tumor initiation was equivalent in mice from both treatment groups, suggesting that DNA damage and tumor initiation were not diminished by prior exposure to the AhR agonist. Taken together, these data strongly suggest that prior AhR activation is not altering tumor initiation in this model.
Instead, we speculate that prior AhR activation is interfering with the second stage of chemical carcinogenesis, tumor promotion. This is supported by the findings that the level of DMBA-DNA adducts and the incidence of preneoplastic lesions was the same in the TCDD- and vehicle-treated mice, yet the ultimate development of palpable tumors was suppressed in TCDD-exposed animals. TCDD is known to influence tumor promotion in other models, and is in fact established as a tumor promoter in skin and liver.4,56,57 While these opposite effects in different tissues may at first appear to be irreconcilable, it is plausible that they reflect cell- and context-dependent differences in AhR-mediated effects on cell cycle regulation and apoptosis. For example, AhR activation is known to influence cell proliferation and apoptosis in many cell lines and tissues; however, the precise nature of the effects (increased versus decreased) vary depending on the cell as well as by the treatment conditions.12,13,58–61 Thus in summary, because the TCDD-exposed mice in our studies had a longer latency period and decreased mammary tumor incidence at any given time point, it is more logical that TCDD decreases promotion in this model. Furthermore AhR-mediated suppression, or even reversal, of tumor promotion is a reasonable explanation for the regression of pre-existing mammary tumors reported previously. 18–20
While many studies in rodents show that AhR activation reduces mammary tumor growth, the complex relationship between mammary tumorigenesis, constitutive expression and function of AhR in tumors, and activation of the receptor by exogenous ligands is not fully elucidated. Studies conducted by Dave Sherr and colleagues demonstrate that AhR is overexpressed in mammary glands and tumors from DMBA-treated animals,62,63 and the receptor is also detected in many breast cancer cell lines and human tumors.64–67 Endogenous expression and activation of AhR in mammary tumors likely influences cell cycle regulation, and may promote tumor growth by stimulating cell proliferation and inhibiting apoptosis.68,69 On the other hand, AhR activation with TCDD and SAhRMs typically inhibits breast cancer cell growth in rodent models. This may be explained, at least for ER+ cancer cells, by cross-talk between AhR and ER. For example, exogenous AhR activation perturbs the expression or function of numerous genes that influence proliferation of breast cancer cells, including c-fos, TGF-β, TGF-α, and receptors for progesterone, prolactin and estrogen (reviewed in 70). Mechanisms for this antiestrogenic activity may include competition between activated AhR and ER for shared cofactors, binding of AhR to inhibitory response elements in promoter regions of ER-inducible genes, or AhR-enhanced proteosomal degradation of the ER.70–72 In future studies we will examine the status of AhR, hormone receptors, oncogenes, and other modulators that influence growth of cancer cells. This will require collecting lesions and tumors from vehicle and TCDD pretreated mice systematically throughout the course of tumor growth, and is part of our ongoing investigation into the underlying molecular pathways that are disrupted by prior TCDD treatment to delay tumor onset.
A separate issue raised by the current studies is unrelated to the effect of AhR activation; specifically the observation that pregnancy alone did not protect against tumor formation. In other words, the tumor incidence curve for the vehicle-treated parous mice was not different from that of the vehicle-treated nulliparous animals. Nor was there a difference in tumor formation between the TCDD-parous and TCDD-nulliparous mice. This finding was somewhat surprising, since pregnancy is hypothesized to protect against breast cancer in humans and has been demonstrated in rat models.30–32 However, we are aware of only one report that has directly addressed the protective effect of pregnancy in an analogous chemical carcinogen-treated mouse model. Specifically, Medina and Smith46 found that pregnancy plus lactation reduced the incidence of DMBA-induced tumors by approximately 3-fold and lengthened the latency period by 10 weeks. Although our current study did not show evidence of protection provided by pregnancy, differences in the experimental designs may explain the different outcomes. Specifically, in the Medina and Smith study the dams nursed their pups for one week, whereas in the current study we removed the pups at birth. Given that lactation is thought to further reduce the incidence of mammary tumors above pregnancy alone,73 it is highly probable that this explains the differing outcomes in the two studies.
Our results also provide insight into understanding the mechanism of protection against breast cancer afforded by pregnancy. Specifically, we found that mammary cell differentiation alone, at least differentiation that contributes to branching and lobuloalveolar development, is not likely a causal factor in parity-induced protection against tumor formation. In this model, AhR activation during pregnancy dramatically suppressed branching, lobuloalveolar development, and milk production (Figure 2 and 28,29). However, suppressed glandular development did not correlate with increased risk of developing mammary tumors, which suggests that additional factors other than differentiation must play a role.
The chemokine CXCL12 and its receptor CXCR4 are known to function in leukocyte migration to inflammatory sites.74 With regard to tumorigenesis, CXCR4 is often over-expressed in human cancers, and CXCL12 may also play a role in survival, proliferation or angiogenesis in the primary tumor.75,76 Additionally they are believed to play a role in directing the migration of cancer cells to secondary metastatic sites.77 Hsu et al51 recently reported that TCDD treatment reduced the expression of these factors in MCF-7 cells, and postulated that this may contribute to the protective effect of AhR activation in breast cancer. A subsequent report by Hall et al described similar suppression of CXCR4 by TCDD in additional ER+ and ER- breast cancer cell lines.67 In our studies, TCDD treatment caused a transient increase in the expression of both molecules in the mammary gland. The cause of this increase in the absence of antigen challenge is unclear, although it could reflect an enhanced inflammatory response that is commonly observed in TCDD-treated animals.78–80 Regardless, this increase was transient and did not persist at the time of carcinogen administration. There was suggestive evidence that CXCR4 expression was diminished in tumors from the TCDD-treated mice, which would be consistent with observations by Hsu et al and Hall et al in the cultured tumor cells. However the decrease was not technically statistically significant. Taken together our results do not provide strong support for a link between delayed tumor formation and changes in CXCL12 or CXCR4, although the potential for these molecules to influence continued tumor growth or metastasis warrants further exploration.
As discussed previously, Lamartiniere and colleagues have demonstrated that developmental exposure to TCDD alters gland development and also increases susceptibility to mammary tumorigenesis.22,23 Thus it is reasonable that the differentiation state of cells in the mammary gland at the time of AhR activation will influence susceptibility to neoplastic transformation. Our original intention was to test whether the AhR-mediated suppression of normal pregnancy-induced mammary differentiation would likewise increase susceptibility to tumor development. However, our results did not support this hypothesis for glandular differentiation caused by pregnancy. We did not find that altered glandular development caused by TCDD influenced the development of DMBA-induced tumors; tumor development was delayed by TCDD treatment regardless of whether the animal was pregnant or not at the time of exposure.
In summary, our results show that AhR activation causes a persistent change in mammary cells that delays tumor formation, and add to the growing number of studies that have demonstrated that AhR activation reduces mammary tumor incidence or causes regression of existing tumors. In combination with other findings such as overexpression of AhR in mammary tumors,62,63,68 effects of AhR activation on cell cycle regulation and invasiveness of breast cancer cells,67,81 and the discovery that a metabolite of tamoxifen activates AhR,82 this report underscores the importance of understanding the role of the receptor in breast cancer. We hypothesize that the beneficial effect observed in our studies occurs via inhibiting tumor promotion, specifically by slowing the growth of preneoplastic lesions and initial tumors. This is promising because it provides an opportunity for biologic intervention in situations where initiation has already occurred, and provides tantalizing evidence to support continued exploration into the use of SAhRMs in breast cancer treatment and prevention.
Supplementary Material
Acknowledgements
The authors would like to thank Dr. Nairangana Dasgupta (Department of Statistics, WSU) for assistance with statistical analyses; Dr. Betina Lew (University of Rochester, NY) for technical advice; Dr. Matt Settles and Derek Pouchnik (Molecular Biosciences, WSU) for technical support and statistical evaluation of qRT-PCR data; and core facilities supported by the WSU Center for Reproductive Biology and Center for Integrated Biotechnology. Work at the Institute of Cancer Research was supported by Cancer Research UK. VMA is a member of ECNIS (Environmental Cancer Risk, Nutrition and Individual Susceptibility), a Network of Excellence operating with the European Union 6th Framework Program, Priority 5: “Food Quality and Safety” (Contract No. 513943). This research was supported by a grant from the National Institutes for Environmental Health Sciences (ES14422 to BV).
Abbreviations
- AhR
Aryl hydrocarbon receptor
- TCDD
2,3,7,8-tetrachlorodibenzo-p-dioxin
- DMBA
(7,12-dimethylbenz[a]anthracene
- Cyp
Cytochrome P450
- HAN
hyperplastic alveolar nodules
- DCIS
ductal carcinoma in situ
- DH
ductal hyperplasia
- CN
cystic nodules
- FDH
fine duct hyperplasias
- DN
dense nodules
- MT
mammary tumors
- TEB
terminal end buds
- BrdU
bromodeoxyuridine
- qRT-PCR
Quantitative real-time PCR
- IHC
immunohistochemistry
- RAL
relative adduct labeling
References
- 1.Gu YZ, Hogenesch JB, Bradfield CA. The PAS superfamily: sensors of environmental and developmental signals. Annu Rev Pharmacol Toxicol. 2000;40:519–561. doi: 10.1146/annurev.pharmtox.40.1.519. [DOI] [PubMed] [Google Scholar]
- 2.Mandal PK. Dioxin: a review of its environmental effects and its aryl hydrocarbon receptor biology. J Comp Physiol [B] 2005;175:221–230. doi: 10.1007/s00360-005-0483-3. [DOI] [PubMed] [Google Scholar]
- 3.Puga A, Tomlinson CR, Xia Y. Ah receptor signals cross-talk with multiple developmental pathways. Biochem Pharmacol. 2005;69:199–207. doi: 10.1016/j.bcp.2004.06.043. [DOI] [PubMed] [Google Scholar]
- 4.Marlowe JL, Puga A. Aryl hydrocarbon receptor, cell cycle regulation, toxicity, and tumorigenesis. J Cell Biochem. 2005;96:1174–1184. doi: 10.1002/jcb.20656. [DOI] [PubMed] [Google Scholar]
- 5.Barouki R, Coumoul X, Fernandez-Salguero PM. The aryl hydrocarbon receptor, more than a xenobiotic-interacting protein. FEBS Lett. 2007;581:3608–3615. doi: 10.1016/j.febslet.2007.03.046. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen LP, Bradfield CA. The search for endogenous activators of the aryl hydrocarbon receptor. Chem Res Toxicol. 2008;21:102–116. doi: 10.1021/tx7001965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lawrence BP, Denison MS, Novak H, Vorderstrasse BA, Harrer N, Neruda W, Reichel C, Woisetschlager M. Activation of the aryl hydrocarbon receptor is essential for mediating the anti-inflammatory effects of a novel low molecular weight compound. Blood. 2008;112:1158–1165. doi: 10.1182/blood-2007-08-109645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Safe S, McDougal A. Mechanism of action and development of selective aryl hydrocarbon receptor modulators for treatment of hormone-dependent cancers (Review) Int J Oncol. 2002;20:1123–1128. [PubMed] [Google Scholar]
- 9.Zhang S, Lei P, Liu X, Li X, Walker K, Kotha L, Rowlands C, Safe S. The aryl hydrocarbon receptor as a target for estrogen receptor-negative breast cancer chemotherapy. Endocr Relat Cancer. 2009;16:835–844. doi: 10.1677/ERC-09-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schecter A, Birnbaum L, Ryan JJ, Constable JD. Dioxins: an overview. Environ Res. 2006;101:419–428. doi: 10.1016/j.envres.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 11.McGregor DB, Partensky C, Wilbourn J, Rice JM. An IARC evaluation of polychlorinated dibenzo-p-dioxins and polychlorinated dibenzofurans as risk factors in human carcinogenesis. Environ Health Perspect. 1998;106 Suppl 2:755–760. doi: 10.1289/ehp.98106755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hebert CD, Harris MW, Elwell MR, Birnbaum LS. Relative toxicity and tumor-promoting ability of 2,3,7,8- tetrachlorodibenzo-p-dioxin (TCDD), 2,3,4,7,8-pentachlorodibenzofuran (PCDF), and 1,2,3,4,7,8-hexachlorodibenzofuran (HCDF) in hairless mice. Toxicol Appl Pharmacol. 1990;102:362–377. doi: 10.1016/0041-008x(90)90033-q. [DOI] [PubMed] [Google Scholar]
- 13.Watson MA, Devereux TR, Malarkey DE, Anderson MW, Maronpot RR. H-ras oncogene mutation spectra in B6C3F1 and C57BL/6 mouse liver tumors provide evidence for TCDD promotion of spontaneous and vinyl carbamate-initiated liver cells. Carcinogenesis. 1995;16:1705–1710. doi: 10.1093/carcin/16.8.1705. [DOI] [PubMed] [Google Scholar]
- 14.Pesatori AC, Consonni D, Rubagotti M, Grillo P, Bertazzi PA. Cancer incidence in the population exposed to dioxin after the "Seveso accident": twenty years of follow-up. Environ Health. 2009;8:39. doi: 10.1186/1476-069X-8-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Viel JF, Clement MC, Hagi M, Grandjean S, Challier B, Danzon A. Dioxin emissions from a municipal solid waste incinerator and risk of invasive breast cancer: a population-based case-control study with GIS-derived exposure. Int J Health Geogr. 2008;7:4. doi: 10.1186/1476-072X-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kogevinas M, Saracci R, Winkelmann R, Johnson ES, Bertazzi PA, Bueno de Mesquita BH, Kauppinen T, Littorin M, Lynge E, Neuberger M, et al. Cancer incidence and mortality in women occupationally exposed to chlorophenoxy herbicides, chlorophenols, and dioxins. Cancer Causes Control. 1993;4:547–553. doi: 10.1007/BF00052430. [DOI] [PubMed] [Google Scholar]
- 17.Negri E, Bosetti C, Fattore E, La Vecchia C. Environmental exposure to polychlorinated biphenyls (PCBs) and breast cancer: a systematic review of the epidemiological evidence. Eur J Cancer Prev. 2003;12:509–516. doi: 10.1097/00008469-200312000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Holcomb M, Safe S. Inhibition of 7,12-dimethylbenzanthracene-induced rat mammary tumor growth by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Cancer Lett. 1994;82:43–47. doi: 10.1016/0304-3835(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 19.McDougal A, Wilson C, Safe S. Inhibition of 7,12-dimethylbenz[a]anthracene-induced rat mammary tumor growth by aryl hydrocarbon receptor agonists. Cancer Lett. 1997;120:53–63. doi: 10.1016/s0304-3835(97)00299-1. [DOI] [PubMed] [Google Scholar]
- 20.Chen I, McDougal A, Wang F, Safe S. Aryl hydrocarbon receptor-mediated antiestrogenic and antitumorigenic activity of diindolylmethane. Carcinogenesis. 1998;19:1631–1639. doi: 10.1093/carcin/19.9.1631. [DOI] [PubMed] [Google Scholar]
- 21.Kociba RJ, Keyes DG, Beyer JE, Carreon RM, Wade CE, Dittenber DA, Kalnins RP, Frauson LE, Park CN, Barnard SD, Hummel RA, Humiston CG. Results of a two-year chronic toxicity and oncogenicity study of 2,3,7,8-tetrachlorodibenzo-p-dioxin in rats. Toxicol Appl Pharmacol. 1978;46:279–303. doi: 10.1016/0041-008x(78)90075-3. [DOI] [PubMed] [Google Scholar]
- 22.Brown NM, Manzolillo PA, Zhang JX, Wang J, Lamartiniere CA. Prenatal TCDD and predisposition to mammary cancer in the rat. Carcinogenesis. 1998;19:1623–1629. doi: 10.1093/carcin/19.9.1623. [DOI] [PubMed] [Google Scholar]
- 23.Jenkins S, Rowell C, Wang J, Lamartiniere CA. Prenatal TCDD exposure predisposes for mammary cancer in rats. Reprod Toxicol. 2007;23:391–396. doi: 10.1016/j.reprotox.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lesca P, Perrot N, Peryt B. Modulating effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on skin carcinogenesis initiated by the weak inducer 7,12-dimethylbenz(a)anthracene. Drug Metabol Drug Interact. 1994;11:37–57. doi: 10.1515/dmdi.1994.11.1.37. [DOI] [PubMed] [Google Scholar]
- 25.Desaulniers D, Leingartner K, Russo J, Perkins G, Chittim BG, Archer MC, Wade M, Yang J. Modulatory effects of neonatal exposure to TCDD, or a mixture of PCBs, p,p'-DDT, and p-p'-DDE, on methylnitrosourea-induced mammary tumor development in the rat. Environ Health Perspect. 2001;109:739–747. doi: 10.1289/ehp.01109739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lamartiniere CA. Timing of exposure and mammary cancer risk. J Mammary Gland Biol Neoplasia. 2002;7:67–76. doi: 10.1023/a:1015722507237. [DOI] [PubMed] [Google Scholar]
- 27.Birnbaum LS, Fenton SE. Cancer and developmental exposure to endocrine disruptors. Environ Health Perspect. 2003;111:389–394. doi: 10.1289/ehp.5686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vorderstrasse BA, Fenton SE, Bohn AA, Cundiff JA, Lawrence BP. A novel effect of dioxin: exposure during pregnancy severely impairs mammary gland differentiation. Toxicol Sci. 2004;78:248–257. doi: 10.1093/toxsci/kfh062. [DOI] [PubMed] [Google Scholar]
- 29.Lew BJ, Collins LL, O'Reilly MA, Lawrence BP. Activation of the aryl hydrocarbon receptor (AhR) during different critical windows in pregnancy alters mammary epithelial cell proliferation and differentiation. Toxicol Sci. 2009;111:151–162. doi: 10.1093/toxsci/kfp125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Russo J, Balogh GA, Heulings R, Mailo DA, Moral R, Russo PA, Sheriff F, Vanegas J, Russo IH. Molecular basis of pregnancy-induced breast cancer protection. Eur J Cancer Prev. 2006;15:306–342. doi: 10.1097/00008469-200608000-00006. [DOI] [PubMed] [Google Scholar]
- 31.Medina D. Mammary developmental fate and breast cancer risk. Endocr Relat Cancer. 2005;12:483–495. doi: 10.1677/erc.1.00804. [DOI] [PubMed] [Google Scholar]
- 32.Medina D. Breast cancer: the protective effect of pregnancy. Clin Cancer Res. 2004;10:380S–384S. doi: 10.1158/1078-0432.ccr-031211. [DOI] [PubMed] [Google Scholar]
- 33.Poland A, Glover E. Characterization and strain distribution pattern of the murine Ah receptor specified by the Ahd and Ahb-3 alleles. Mol Pharmacol. 1990;38:306–312. [PubMed] [Google Scholar]
- 34.Gasiewicz TA, Geiger LE, Rucci G, Neal RA. Distribution, excretion, and metabolism of 2,3,7,8-tetrachlorodibenzo-p-dioxin in C57BL/6J, DBA/2J, and B6D2F1/J mice. Drug Metab Dispos. 1983;11:397–403. [PubMed] [Google Scholar]
- 35.Thompson HJ. Methods for the induction of mammary carcinogenesis in the rat using eithher 7,12-dimethylbenzanthracene or 1-methyl-1-nitrosourea. In: Ip MM, Asch BB, editors. Methods in Mammary Gland Biology and Breast Cancer Research. New York: Kluwer Academic/Plenum Publishers; 2000. pp. 19–29. [Google Scholar]
- 36.Medina D, Kittrell FS. p53 function is required for hormone-mediated protection of mouse mammary tumorigenesis. Cancer Res. 2003;63:6140–6143. [PubMed] [Google Scholar]
- 37.Yin JJ, Selander K, Chirgwin JM, Dallas M, Grubbs BG, Wieser R, Massague J, Mundy GR, Guise TA. TGF-beta signaling blockade inhibits PTHrP secretion by breast cancer cells and bone metastases development. J Clin Invest. 1999;103:197–206. doi: 10.1172/JCI3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Medina D. Mammary Tumors. In: Foster HL, Small JD, Fox JG, editors. The Mouse in Biomedical Research. vol. IV. New York: Academic Press, Inc; 1982. pp. 373–396. [Google Scholar]
- 39.Medina D. The preneoplastic phenotype in murine mammary tumorigenesis. J Mammary Gland Biol Neoplasia. 2000;5:393–407. doi: 10.1023/a:1009529928422. [DOI] [PubMed] [Google Scholar]
- 40.Arlt VM, Schmeiser HH, Pfeifer GP. Sequence-specific detection of aristolochic acid-DNA adducts in the human p53 gene by terminal transferase-dependent PCR. Carcinogenesis. 2001;22:133–140. doi: 10.1093/carcin/22.1.133. [DOI] [PubMed] [Google Scholar]
- 41.Schmeiser HH, Furstenberger G, Takamura-Enya T, Phillips DH, Arlt VM. The genotoxic air pollutant 3-nitrobenzanthrone and its reactive metabolite N-hydroxy-3-aminobenzanthrone lack initiating and complete carcinogenic activity in NMRI mouse skin. Cancer Lett. 2009;284:21–29. doi: 10.1016/j.canlet.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 42.Phillips DH, Arlt VM. The 32P–postlabeling assay for DNA adducts. Nat Protoc. 2007;2:2772–2781. doi: 10.1038/nprot.2007.394. [DOI] [PubMed] [Google Scholar]
- 43.Medina D. Mouse Models for Mammary Cancer. In: Ip MM, Asch BB, editors. Methods in Mammary Gland Biology and Breast Cancer Research. New York: Kluwer Academic/Plenum Publishers; 2000. pp. 3–17. [Google Scholar]
- 44.Brisken C. Hormonal control of alveolar development and its implications for breast carcinogenesis. J. Mammary Gland Biol. Neoplasia. 2002;7:39–48. doi: 10.1023/a:1015718406329. [DOI] [PubMed] [Google Scholar]
- 45.Russo J, Mailo D, Hu YF, Balogh G, Sheriff F, Russo IH. Breast differentiation and its implication in cancer prevention. Clin Cancer Res. 2005;11:931s–936s. [PubMed] [Google Scholar]
- 46.Medina D, Smith GH. Chemical carcinogen-induced tumorigenesis in parous, involuted mouse mammary glands. J Natl Cancer Inst. 1999;91:967–969. doi: 10.1093/jnci/91.11.967. [DOI] [PubMed] [Google Scholar]
- 47.Giannone JV, Li W, Probst M, Okey AB. Prolonged depletion of AH receptor without alteration of receptor mRNA levels after treatment of cells in culture with 2,3, 7,8- tetrachlorodibenzo-p-dioxin. Biochem Pharmacol. 1998;55:489–497. doi: 10.1016/s0006-2952(97)00493-0. [DOI] [PubMed] [Google Scholar]
- 48.Pollenz RS. The mechanism of AH receptor protein down-regulation (degradation) and its impact on AH receptor-mediated gene regulation. Chem Biol Interact. 2002;141:41–61. doi: 10.1016/s0009-2797(02)00065-0. [DOI] [PubMed] [Google Scholar]
- 49.Collins LL, Lew BJ, Lawrence BP. TCDD exposure disrupts mammary epithelial cell differentiation and function. Reprod Toxicol. 2009;28:11–17. doi: 10.1016/j.reprotox.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ma Q, Baldwin KT. 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced degradation of aryl hydrocarbon receptor (AhR) by the ubiquitin-proteasome pathway. Role of the transcription activaton and DNA binding of AhR. J Biol Chem. 2000;275:8432–8438. doi: 10.1074/jbc.275.12.8432. [DOI] [PubMed] [Google Scholar]
- 51.Hsu EL, Yoon D, Choi HH, Wang F, Taylor RT, Chen N, Zhang R, Hankinson O. A proposed mechanism for the protective effect of dioxin against breast cancer. Toxicol Sci. 2007;98:436–444. doi: 10.1093/toxsci/kfm125. [DOI] [PubMed] [Google Scholar]
- 52.McPherson K, Steel CM, Dixon JM. ABC of breast diseases. Breast cancer-epidemiology, risk factors, and genetics. BMJ. 2000;321:624–628. doi: 10.1136/bmj.321.7261.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Santostefano MJ, Richardson VM, Walker NJ, Blanton J, Lindros KO, Lucier GW, Alcasey SK, Birnbaum LS. Dose-dependent localization of TCDD in isolated centrilobular and periportal hepatocytes. Toxicol Sci. 1999;52:9–19. doi: 10.1093/toxsci/52.1.9. [DOI] [PubMed] [Google Scholar]
- 54.Birnbaum LS. Distribution and excretion of 2,3,7,8-tetrachlorodibenzo-p-dioxin in congenic strains of mice which differ at the Ah locus. Drug Metab Dispos. 1986;14:34–40. [PubMed] [Google Scholar]
- 55.Weber H, Birnbaum LS. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) and 2,3,7,8-tetrachlorodibenzofuran (TCDF) in pregnant C57BL/6N mice: distribution to the embryo and excretion. Arch Toxicol. 1985;57:159–162. doi: 10.1007/BF00290880. [DOI] [PubMed] [Google Scholar]
- 56.Bock KW, Kohle C. Ah receptor: dioxin-mediated toxic responses as hints to deregulated physiologic functions. Biochem Pharmacol. 2006;72:393–404. doi: 10.1016/j.bcp.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 57.Mitchell KA, Lockhart CA, Huang G, Elferink CJ. Sustained aryl hydrocarbon receptor activity attenuates liver regeneration. Mol Pharmacol. 2006;70:163–170. doi: 10.1124/mol.106.023465. [DOI] [PubMed] [Google Scholar]
- 58.Oenga GN, Spink DC, Carpenter DO. TCDD and PCBs inhibit breast cancer cell proliferation in vitro. Toxicol In Vitro. 2004;18:811–819. doi: 10.1016/j.tiv.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 59.Park S, Mazina O, Kitagawa A, Wong P, Matsumura F. TCDD causes suppression of growth and differentiation of MCF10A, human mammary epithelial cells by interfering with their insulin receptor signaling through c-Src kinase and ERK activation. J Biochem Mol Toxicol. 2004;18:322–331. doi: 10.1002/jbt.20040. [DOI] [PubMed] [Google Scholar]
- 60.Ahn NS, Hu H, Park JS, Park JS, Kim JS, An S, Kong G, Aruoma OI, Lee YS, Kang KS. Molecular mechanisms of the 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced inverted U-shaped dose responsiveness in anchorage independent growth and cell proliferation of human breast epithelial cells with stem cell characteristics. Mutat Res. 2005;579:189–199. doi: 10.1016/j.mrfmmm.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 61.Ray S, Swanson HI. Activation of the aryl hydrocarbon receptor by TCDD inhibits senescence: a tumor promoting event? Biochem Pharmacol. 2009;77:681–688. doi: 10.1016/j.bcp.2008.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang X, Solomon S, Fraser LR, Trombino AF, Liu D, Sonenshein GE, Hestermann EV, Sherr DH. Constitutive regulation of CYP1B1 by the aryl hydrocarbon receptor (AhR) in pre-malignant and malignant mammary tissue. J Cell Biochem. 2008;104:402–417. doi: 10.1002/jcb.21630. [DOI] [PubMed] [Google Scholar]
- 63.Trombino AF, Near RI, Matulka RA, Yang S, Hafer LJ, Toselli PA, Kim DW, Rogers AE, Sonenshein GE, Sherr DH. Expression of the aryl hydrocarbon receptor/transcription factor (AhR) and AhR-regulated CYP1 gene transcripts in a rat model of mammary tumorigenesis. Breast Cancer Res Treat. 2000;63:117–131. doi: 10.1023/a:1006443104670. [DOI] [PubMed] [Google Scholar]
- 64.Spink DC, Spink BC, Cao JQ, DePasquale JA, Pentecost BT, Fasco MJ, Li Y, Sutter TR. Differential expression of CYP1A1 and CYP1B1 in human breast epithelial cells and breast tumor cells. Carcinogenesis. 1998;19:291–298. doi: 10.1093/carcin/19.2.291. [DOI] [PubMed] [Google Scholar]
- 65.Wang X, Thomsen JS, Santostefano M, Rosengren R, Safe S, Perdew GH. Comparative properties of the nuclear aryl hydrocarbon (Ah) receptor complex from several human cell lines. Eur J Pharmacol. 1995;293:191–205. doi: 10.1016/s0922-4106(05)80044-6. [DOI] [PubMed] [Google Scholar]
- 66.Yang X, Liu D, Murray TJ, Mitchell GC, Hesterman EV, Karchner SI, Merson RR, Hahn ME, Sherr DH. The aryl hydrocarbon receptor constitutively represses c-myc transcription in human mammary tumor cells. Oncogene. 2005;24:7869–7881. doi: 10.1038/sj.onc.1208938. [DOI] [PubMed] [Google Scholar]
- 67.Hall JM, Barhoover MA, Kazmin D, McDonnell DP, Greenlee WF, Thomas RS. Activation of the aryl-hydrocarbon receptor inhibits invasive and metastatic features of human breast cancer cells and promotes breast cancer cell differentiation. Mol Endocrinol. 2010;24:359–369. doi: 10.1210/me.2009-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schlezinger JJ, Liu D, Farago M, Seldin DC, Belguise K, Sonenshein GE, Sherr DH. A role for the aryl hydrocarbon receptor in mammary gland tumorigenesis. Biol Chem. 2006;387:1175–1187. doi: 10.1515/BC.2006.145. [DOI] [PubMed] [Google Scholar]
- 69.Currier N, Solomon SE, Demicco EG, Chang DL, Farago M, Ying H, Dominguez I, Sonenshein GE, Cardiff RD, Xiao ZX, Sherr DH, Seldin DC. Oncogenic signaling pathways activated in DMBA-induced mouse mammary tumors. Toxicol Pathol. 2005;33:726–737. doi: 10.1080/01926230500352226. [DOI] [PubMed] [Google Scholar]
- 70.Safe S, Wormke M. Inhibitory aryl hydrocarbon receptor-estrogen receptor alpha cross-talk and mechanisms of action. Chem Res Toxicol. 2003;16:807–816. doi: 10.1021/tx034036r. [DOI] [PubMed] [Google Scholar]
- 71.Beischlag TV, Luis Morales J, Hollingshead BD, Perdew GH. The aryl hydrocarbon receptor complex and the control of gene expression. Crit Rev Eukaryot Gene Expr. 2008;18:207–250. doi: 10.1615/critreveukargeneexpr.v18.i3.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Swedenborg E, Pongratz I. AhR and ARNT modulate ER signaling. Toxicology. 2010;268:132–138. doi: 10.1016/j.tox.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 73.Yang J, Yoshizawa K, Nandi S, Tsubura A. Protective effects of pregnancy and lactation against N-methyl-N-nitrosourea-induced mammary carcinomas in female Lewis rats. Carcinogenesis. 1999;20:623–628. doi: 10.1093/carcin/20.4.623. [DOI] [PubMed] [Google Scholar]
- 74.Vandercappellen J, Van Damme J, Struyf S. The role of CXC chemokines and their receptors in cancer. Cancer Lett. 2008;267:226–244. doi: 10.1016/j.canlet.2008.04.050. [DOI] [PubMed] [Google Scholar]
- 75.Koizumi K, Hojo S, Akashi T, Yasumoto K, Saiki I. Chemokine receptors in cancer metastasis and cancer cell-derived chemokines in host immune response. Cancer Sci. 2007;98:1652–1658. doi: 10.1111/j.1349-7006.2007.00606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wendt MK, Cooper AN, Dwinell MB. Epigenetic silencing of CXCL12 increases the metastatic potential of mammary carcinoma cells. Oncogene. 2008;27:1461–1471. doi: 10.1038/sj.onc.1210751. [DOI] [PubMed] [Google Scholar]
- 77.Arya M, Ahmed H, Silhi N, Williamson M, Patel HR. Clinical importance and therapeutic implications of the pivotal CXCL12-CXCR4 (chemokine ligand-receptor) interaction in cancer cell migration. Tumour Biol. 2007;28:123–131. doi: 10.1159/000102979. [DOI] [PubMed] [Google Scholar]
- 78.Moos AB, Baecher-Steppan L, Kerkvliet NI. Acute inflammatory response to sheep red blood cells in mice treated with 2,3,7,8-tetrachlorodibenzo-p-dioxin: the role of proinflammatory cytokines, IL-1 and TNF. Toxicol. Appl. Pharmacol. 1994;127:331–335. doi: 10.1006/taap.1994.1169. [DOI] [PubMed] [Google Scholar]
- 79.Clark GC, Taylor MJ. Tumor necrosis factor involvement in the toxicity of TCDD: the role of endotoxin in the response. Immunogenet. 1994;11:136–141. doi: 10.1159/000424204. [DOI] [PubMed] [Google Scholar]
- 80.Teske S, Bohn AA, Regal JF, Neumiller JJ, Lawrence BP. Activation of the aryl hydrocarbon receptor increases pulmonary neutrophilia and diminishes host resistance to influenza A virus. Am J Physiol Lung Cell Mol Physiol. 2005;289:L111–L124. doi: 10.1152/ajplung.00318.2004. [DOI] [PubMed] [Google Scholar]
- 81.Barhoover MA, Hall JM, Greenlee WF, Thomas RS. The AHR regulates cell cycle progression in human breast cancer cells via a functional interaction with CDK4. Mol Pharmacol. 2010;77:195–201. doi: 10.1124/mol.109.059675. [DOI] [PubMed] [Google Scholar]
- 82.Dusell CD, Nelson ER, Wittmann BM, Fretz JA, Kazmin D, Thomas RS, Pike JW, McDonnell DP. Regulation of Aryl Hydrocarbon Receptor Function by Selective Estrogen Receptor Modulators. Mol Endocrinol. 2010;24:33–46. doi: 10.1210/me.2009-0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.