Abstract
Purpose
In this study we investigated the effect of platelet-rich plasma (PRP) and bone-marrow derived stromal cell (BMSC)-seeded interposition in an in vitro canine tendon repair model.
Methods
Bone marrow, peripheral blood, and tendons were harvested from mixed breed dogs. BMSC were cultured and passaged from adherent cells of bone marrow suspension. PRP was purified from peripheral blood using a commercial kit. 192 flexor digitorum profundus tendons were used for the study. Tendons repaired with a simple suture were used as a control group. In treatment groups, a collagen gel patch was interposed at the tendon repair site prior to suture. There were three treatment groups according to the type of collagen patch; a patch with PRP, a patch with BMSC, and a patch with PRP and BMSC. The repaired tendons were evaluated by biomechanical testing and by histological survey after 2 and 4 weeks in tissue culture. To evaluate viability, cells were labeled with PKH26 and surveyed under confocal microscopy after culture.
Results
The maximum breaking strength and stiffness of the healing tendons with the BMSC-seeded PRP patch was significantly higher than the healing tendons without a patch or with a cell-seeded patch (p<0.02). Viable BMSC were present at both 2 and 4 weeks.
Conclusions
PRP enhanced the effect of BMSC-seeded collagen gel interposition in this in vitro model. Based on these results we now plan to investigate this effect in vivo.
Keywords: Bone Marrow Derived Stromal Cells, Canine, In Vitro, Platelet-Rich Plasma, Tendon Healing
INTRODUCTION
Flexor tendon injuries in the hand are common and associated with substantial socioeconomic costs and disability (1). Functional outcomes of these injuries have improved with new surgical repair methods and rehabilitation regimens, but complications such as adhesions and repair ruptures remain problematic in some cases (2, 3). These complications are believed to be related to an inherent weakness in the healing capacity of tendons, which are hypocellular, and for which the usual mode of healing involves recruitment of extratendinous fibroblastic cells. As these cells attach to the healing tendon, restrictive adhesions develop. Rehabilitation methods to disrupt the adhesions with mobilization may result in failure of the repair (4).
To overcome the problems associated with tendon hypocellularity and improve tendon healing, bone marrow derived stromal cells (BMSCs) have been used as a source of additional cells at the tendon repair site (5, 6). BMSCs are nonhematopoietic stromal cells obtained from a subset of clonogenic adherent marrow-derived cells that undergo replication in culture. BMSCs are able to differentiate into a spectrum of cell types including osteoblasts, chondrocytes, adipocytes, fibroblasts and tenocytes (7, 8). Indeed, the extratendinous fibroblasts that appear at the repair site during normal tendon healing may be of BMSC origin (9). Growth factors are also important in tendon healing (10, 11). Growth factors can enhance tendon repair when applied to the site of injury(12), but it is difficult to supply an effective sustained concentration of necessary growth factors in the normal sequence of their appearance. To overcome this problem, investigators have started to focus on a natural packet of multiple growth factors, the platelet, delivered in the form of platelet-rich plasma (PRP) (13, 14).
The growth factors in platelets include not only platelet-derived growth factor (PDGF), but also transforming growth factor β (TGF- β), vascular endothelial growth factor (VEGF), insulin-like growth factor-1 (IGF-1), and epidermal growth factor (EGF) (15). The use of PRP to improve healing has been explored considerably during the last decade. PRP is used for both bone and soft tissue healing enhancement, including tendon (16, 17). PRP also enhances proliferation of BMSC (18), and some growth factors known to be present in PRP, such as IGF-1 and TGF- β, augment the effect of BMSC on tendon (19, 20). Therefore, the purpose of the current study was to test the hypothesis that the use of BMSCs and PRP at the tendon repair site would accelerate tendon healing, using an in vitro canine tendon tissue culture model. We further hypothesized that PRP would enhance the effect of BMSC on tendon healing site and increase tendon healing strength, beyond the effect of BMSCs alone.
MATERIALS AND METHODS
Study Design
A total of 192 flexor digitorum profundus (FDP) tendons from the 2nd to 5th digits of both forepaws and hind paws were immediately harvested from 12 dogs after sacrifice for other, IACUC approved, studies. The FDP tendons were then immediately immersed into cell culture medium to maintain tissue viability. The tendons were randomly assigned to one of four treatment groups and two time points, for a total of eight study groups with 24 tendons in each group (Table 1). The FDP tendons were fully lacerated and surgically repaired with one of four different interposition patches placed within the laceration site prior to suture. All procedures were carried out under aseptic conditions. After 2 or 4 weeks in tissue culture, the tendons were evaluated for mechanical strength, cell viability, and histology.
Table 1.
Experimental Design
| Groups | Culture 2 weeks | Culture 4 weeks | ||
|---|---|---|---|---|
| MT | HIS/CV | MT | HIS/CV | |
| No patch | 20 | 4 | 20 | 4 |
| PRP alone patch | 20 | 4 | 20 | 4 |
| BMSC in collagen patch | 20 | 4 | 20 | 4 |
| BMSC in PRP patch | 20 | 4 | 20 | 4 |
ME-mechanical testing; HIS-histological analysis; CV-cell viability analysis.
Preparation of PRP
Whole blood (55 ml) was withdrawn into a sterile syringe containing citric acid-citrate-dextrose anticoagulant (ACD-A) at ratio of 10:1 (16). The blood was then processed within 1 hour after harvest. PRP preparation from blood was carried out using the GPS III System (Biomet Biologic, Warsaw, IN), according to the manufacturer’s directions. A solution of 1000 units of bovine thrombin (BioPharm, Alpine, UT) per milliliter of 10% calcium chloride (Sigma, St. Louis, MO) was used to activate the PRP (16), at a ratio of 6 ml of PRP to 1 ml of the thrombin/calcium chloride mix. This mixture was then left at room temperature for one hour to lyse the platelets and release the growth factors. The solutions were centrifuged for 5 minutes at 1500 rpm and the supernatant was used in the next step. Platelets within both whole blood and the PRP were counted for comparison according to the method of Brecher and Cronkite (21).
BMSC Harvest and Suspension
The BMSC were isolated from bone marrow aspirates obtained from canine tibia. 8.0 ml of bone marrow was aspirated aseptically using a 20 ml syringe containing 2.0ml of heparin solution immediately prior to euthanasia. The heparin was removed by centrifugation at 1500 rpm for 5 minutes at room temperature, and the bone marrow pellet was then resuspended in cell culture medium and divided into three equal aliquots and placed in 100-mm cell culture dishes with 10mL of standard medium, which consists of minimal essential medium (MEM) with Earle’s salts (GIBCO, Grand Island, NY), 10% fetal calf serum, and 1% antibiotics (Antibiotic-Antimycotic, GIBCO, Grand Island, NY).
The bone marrow cells were then incubated at 37°C with 5% CO2 and 95% air at 100% humidity. After 3 days, the medium containing floating cells was removed and new medium was added to the remaining adherent cells. These adherent cells were considered to be bone marrow stromal cells (BMSCs) (22). When reaching 70–80% of confluence, the BMSCs were released with trypsin-EDTA and subcultured. A homogenous BMSCs population was obtained after 3weeks of culture and BMSCs (passage 3) were harvested for further use.
Preparation of Cell-Seeded patch
PureCol bovine dermal collagen (2.9mg/ml, Inamed Corporation, Milmont Drive Fremont, CA) was prepared following the company’s instructions. Briefly, 5.50ml of sterile, chilled PureCol collagen was mixed with 1.6 ml of sterile 10 × MEM, 7 µl of sterile 5M NaOH and 893 µl distilled H2O to adjust the pH to 7.4 ± 0.2, making 8 ml temporary collagen/MEM solution on ice. The solution was then stored on ice for no longer than 1 hour until use.
BMSCs in passage 3 were washed twice with sterile PBS and trypsinized. The cells were counted with a hemocytometer and centrifuged to remove the media and leave behind a cell pellet with a known number of cells. The amounts of collagen and cell density were adjusted to a final collagen concentration of 0.5 mg/ml and initial cell density 1.0 × 106 cells/ml. A 2ml aliquot of the cell-seeded collagen solution was added to a sterile 35 mm Petri dish.
After incubating at 37°C in a 5% CO2 humidified incubator for one day for gelation, the BMSC-seeded patch was cut to a similar cross-sectional shape as the tendon ends (roughly 2×4 mm), and used immediately.
Preparation BMSC-Seeded PRP Patch
Cells and collagen solution were prepared in the same fashion described above. The amount of collagen and cell density were adjusted to a final collagen concentration of 0.5 mg/ml and initial cell density 1.0 × 106 cells/ml using 1 ml of the PRP supernatant and 1ml of the collagen solution. A 2 ml aliquot of the BMSC-seeded PRP collagen solution was added to a sterile 35 mm Petri dish.
After incubating at 37°C in a 5% CO2 humidified incubator for one day for gelation, the gel was cut and used immediately. For the PRP patch group, the PRP patch was prepared similarly, but without the addition of BMSC.
Tendon Repair and Tissue Culture
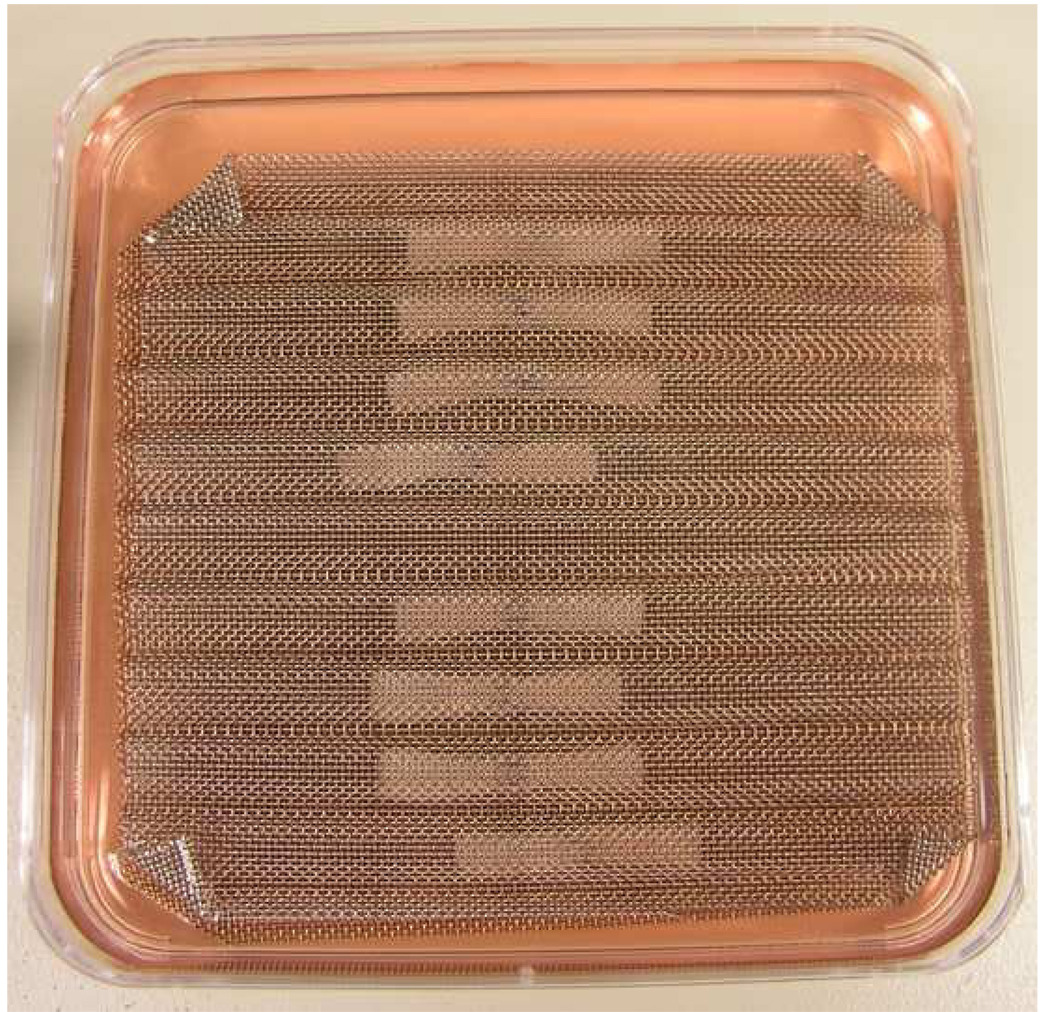
Each tendon was transected 6 mm distal to the distal edge of A2 pulley and shortened by cutting to a standardized length of 30 mm, with the repair site located centrally at the zone II D level (23). The gel was placed between the lacerated tendon ends. Then the tendon ends were apposed with two simple loop sutures of 6-0 Prolene (Ethicon, Somerville NJ). The sutures were set carefully not to directly cross tendon apposition site (Figure 3B). The repaired tendons were mounted on a wire mesh designed to maintain the tendons in a straight position (Figure 1). The mesh was then placed into a 100 mm Petri dish with 50ml of minimal essential medium (MEM), Earle’s salts (GIBCO, Grand Island, NY), 10% fetal calf serum, and 1% antibiotics (Antibiotic-Antimycotic, GIBCO, Grand Island, NY), and incubated at 37°C in a 5% CO2 humidified atmosphere. Tendons were cultured for 2 or 4 weeks. Culture medium was changed every 3 days.
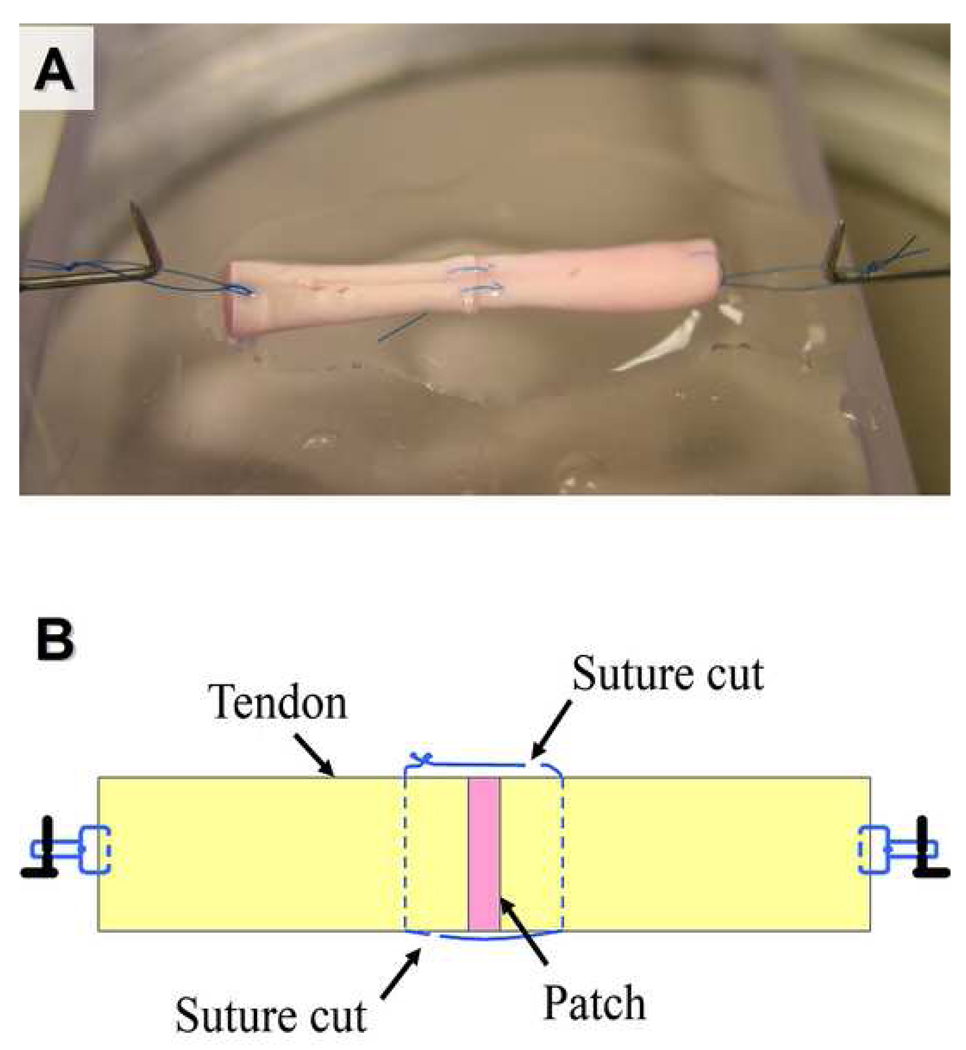
Figure 3.
(A) Appearance of the repaired tendon at mechanical testing. Both sutures were cut prior to testing. (B) Schema of the sutured tendon after suture cut. See the relation between suture, apposition site, and the site of suture cut.
Figure 1.
Tissue culture of the repaired tendon on custom made wire mesh.
Biomechanical Testing
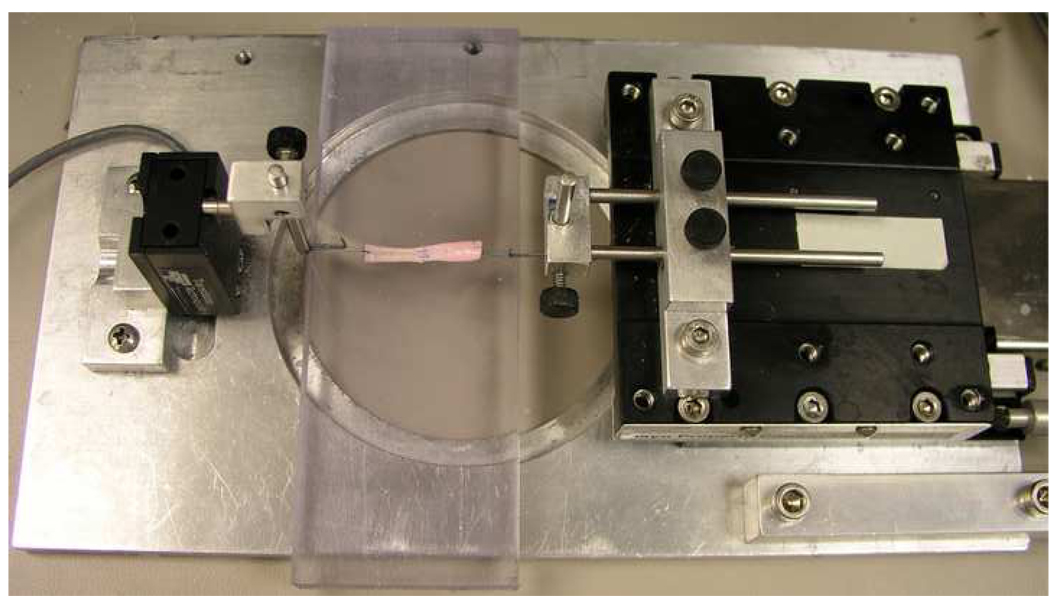
After culture, tendons were removed from the culture dish. A single loop suture was placed at each end of the test specimen to connect the tendon to a custom-designed micro-tester for mechanical evaluation (Figure 2). The testing apparatus included a load transducer (Techniques Inc., Temecula, CA) which connected to the one of the tendon loops, and a motor and potentiometer (Parker Hannifin Corp., Rohnert Park, CA) which were connected to the other loop. Before testing, the tendon apposition sutures were cut on both sides of the tendon, without disrupting the repair site, in order to assess the strength of the healing tissue rather than the suture strength (Figure 3).
Figure 2.
Healing tendon mounted on the micro-tester for mechanical testing.
To minimize influence on the repair site, sutures were carefully cut at a point away from the apposition site. For the same reason, the sutures were not removed during testing.
The tendon was placed on a flat plastic platform moistened with saline. The specimen was then distracted at a rate of 0.1 mm/second. The displacement and maximum strength to failure were measured by the transducer and recorded for data analysis. The failure was confirmed by two separate observations, the complete loss of force detected by the load transducer and gross observation of disruption of the connecting tissues.
Cell Viability Assessment
In order to assess the cell viability and distinguish the BMSCs from the cells existing in the native tendon, the BMSCs were labeled with PKH26 red fluorescent cell linker (Sigma, St. Louis, MO) before seeding in the gel patch. The patches with the labeled BMSC were implanted between the cut tendon ends. After tissue culture for 2 or 4 weeks, the tendon samples were frozen immediately on dry ice and mounted with O.C.T. compound (Tissue-Tek; Sakura Finetek, Torance, CA). Sections of 6 µm were cut in the sagittal plane using a cryostat (Leica, Bannockburn, IL) and were mounted on glass slides with cyanoacrylate ester glue (Elmer’s product, Columbia, OH). Acquired specimens were observed using laser scanning confocal microscopy (LSM510; Zeiss, Thornwood, NY).
Histology
From each test group, four tendon segments, including the repair site, were collected and fixed in 10% neutral buffered formalin for 24hours. The tendon samples were soaked in 10% to 20% of sucrose/0.1M PBS solution gradually. Sections of 6 µm were cut in the sagittal plane using a cryostat (Leica, Bannockburn, IL). The sections were mounted on glass slides and stained with hematoxylin and eosin (H&E). The morphology was evaluated with light microscopy.
Statistical Considerations
Design
The experimental design was based on a paired or repeated design; 16 tendons from each dog were allocated in a balanced manner to each of the 4 repair methods and each of the 2 time conditions, such that each animal contributed 2 tendons to each of the 8 combinations of repair method and time condition.
Analysis
The outcome of primary interest was the biomechanically measured ultimate strength of the healing tendons with the repair sutures removed. The effect of 2 factors on this outcome was studied: 1) patch type at 4 levels (no patch, PRP patch, BMSC in collagen patch, and BMSC in PRP collagen patch) and 2) time at 2 levels (2 weeks and 4 weeks). As each animal contributed 2 tendons to each of the 8 combinations of repair method and time condition, the data was analyzed using a 2-factor analysis of variance (ANOVA) with repeated measures on both factors. All statistical tests were two-sided and p-values less than 0.05 were considered significant.
Sample Size and Power
The overall experiment was designed with two factors having 4 and 2 levels, respectively. However, since each animal contributed tendons to each experimental condition, a conservative approach to the power analysis considered the number of unique animals contributing to the comparison of any two experimental conditions based on a paired t-test. Based on preliminary data from a similar experiment, the standard deviation of the ultimate strength was calculated to be 25mN. Assuming similar variability in the proposed study, a sample of 10 animals (160 tendons) provides 80% power to detect a difference of 25mN in ultimate strength between any two experimental conditions (α= 0.05). These estimates were based on a conservative approach, so actual detectable differences might be smaller, given the more efficient and powerful nature of the two-factor model.
RESULTS
Platelets Count
The mean platelet count in the whole blood and PRP was 243 × 103/µl (range 198–324 × 103/µl; SD 49 × 103/µl) and 1316 × 103/µl (range 919–1594 × 103/µl; SD 263 × 103/µl) (p=0.0006) respectively. Platelet counts were 5.41-fold greater in the PRP compared to whole blood (range 4.15–6.75-fold increase; SD 1.07).
Maximum Strength and Stiffness of the Healing Tendons
Analysis using a 2-factor ANOVA with repeated measures showed that the repaired method had a significant effect on both maximum strength and stiffness of the healing tendons with the repair sutures removed. The effect of time was not significant in either maximum strength or stiffness. Since the interaction between repair method and time was not significant, the comparison between each patch method was tested using the Tukey-Kramer post-hoc test.
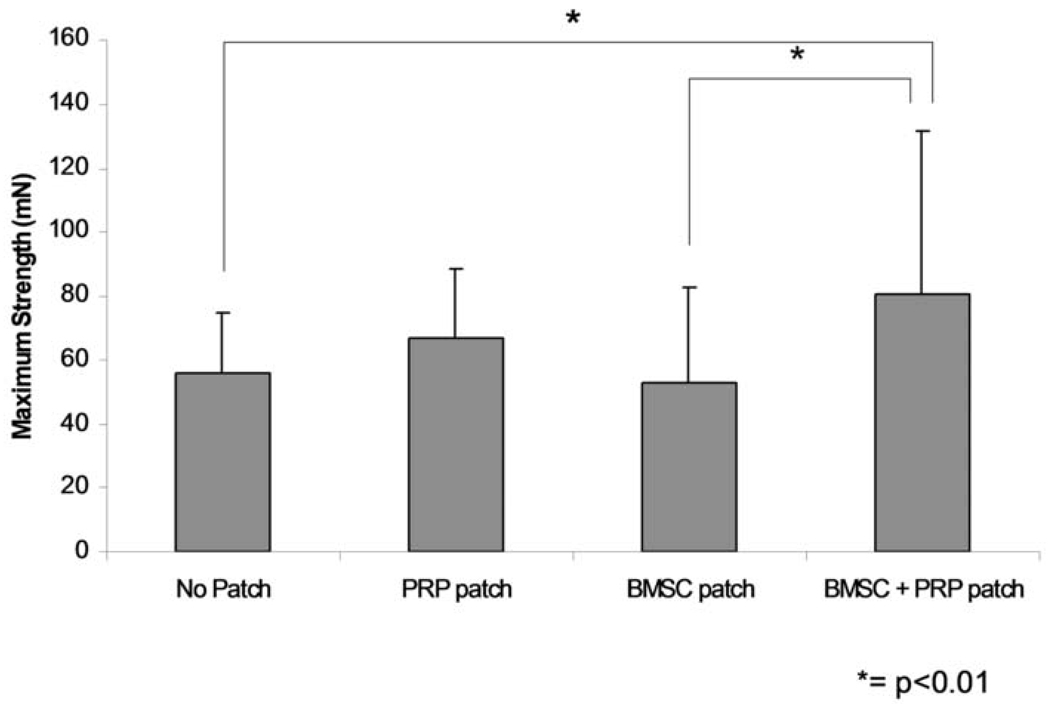
The maximum breaking strength of the healing tendons was 55.6 mN (SD 19.1), 67.0 mN (SD 21.3), 52.4 mN (SD 30.3), and 80.9 mN (SD 50.3) for the tendons without a patch, with a PRP patch, with a BMSC-seeded patch, and with a BMSC-seeded PRP patch, respectively (Figure 4). The maximum strength of the healing tendons with the BMSC-seeded PRP patch was significantly higher than the healing tendons without a patch (p=0.0077) or with a cell-seeded patch (p=0.0025).
Figure 4.
Maximum strength of the repaired tendon. (mean ± SD. * =p< 0.01, ** = p < 0.02)
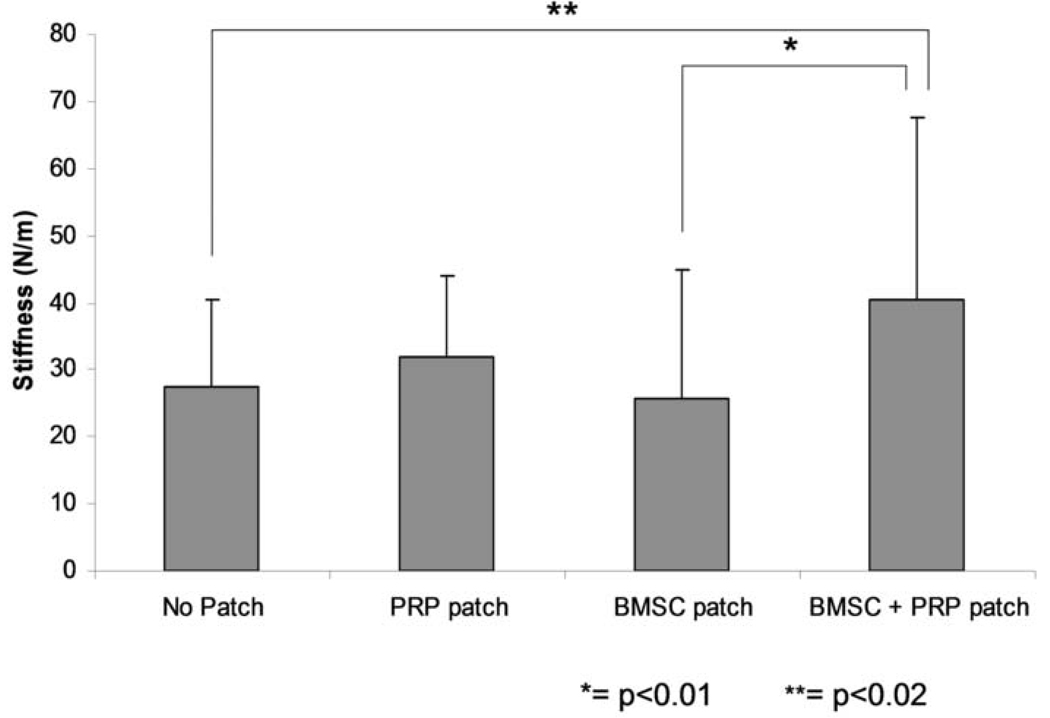
The stiffness of the healing tendons followed a similar trend. The stiffness of the healing tendons was 27.5 N/m (SD 12.8), 31.7 N/m (SD 12.1), 25.7 N/m (SD 19.2), and 40.6 N/m (SD 27.1) for healing tendon without a patch, with a PRP patch, with a BMSC-seeded patch, and with a BMSC-seeded PRP patch, respectively (Figure 5).The stiffness of the healing tendons with a BMSC-seeded PRP patch was significantly higher than the healing tendons without a patch (p=0.018) or with a cell-seeded patch (p=0.0062).
Figure 5.
Stiffness of the repaired tendon. (mean ± SD. * =p< 0.01, ** = p < 0.02)
Cell Viability and Histology
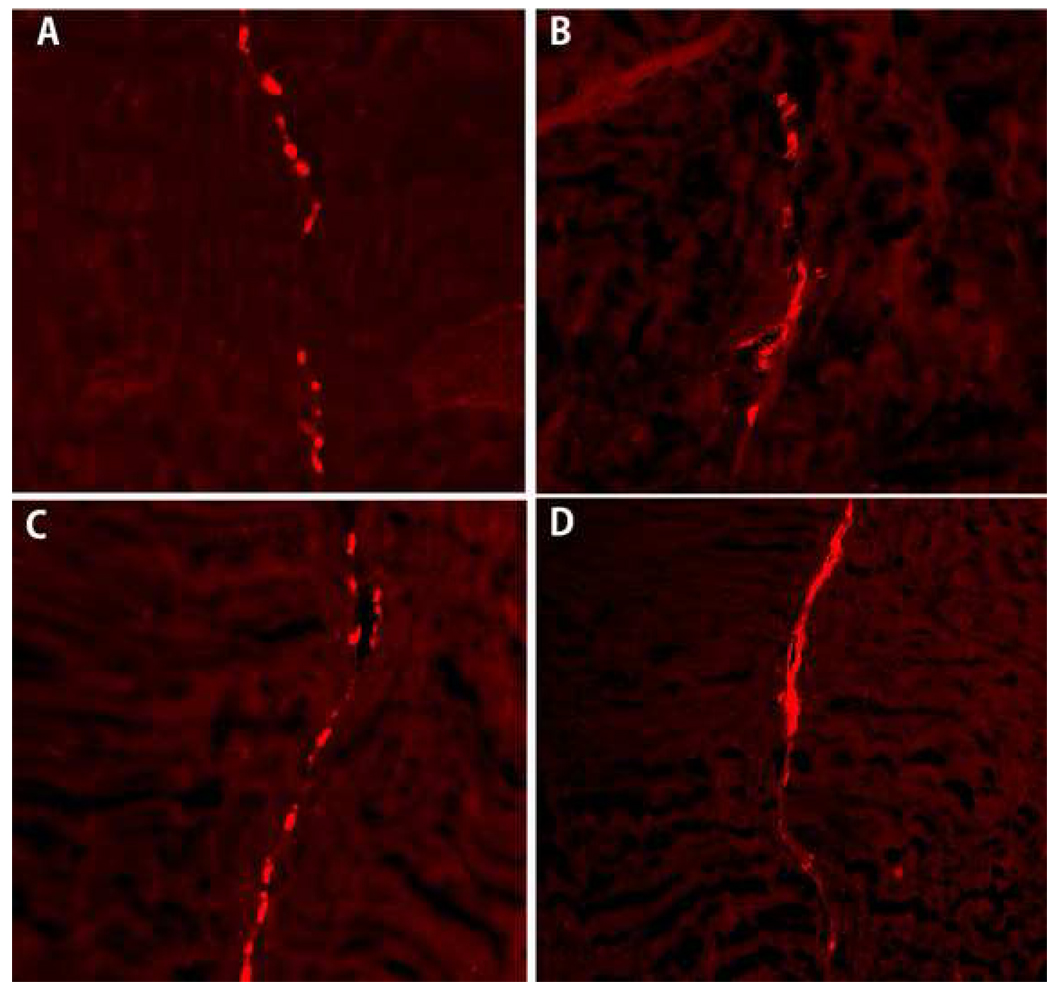
Qualitative observation by confocal microscopy revealed that labeled viable BMSCs were present among the repair site in both the BMSC-seeded patch and BMSC-seeded PRP patch group after 2 and 4 weeks of tissue culture (Figure 6). We did not quantify the number of cells in each section, but do note a suggestion of more cells in the PRP treated patch at 4 weeks (Figure 5D), as compared to the untreated patch (Figure 6C).
Figure 6.
The labeled BMSC with PKH26 cell linker was observed under confocal microscopy with red fluorescence. (A) BMSC-seeded patch at 2 weeks, (B) BMSC-seeded PRP patch at 2 weeks, (C) BMSC-seeded patch at 4 weeks, (D) BMSC-seeded PRP patch at 4 weeks.
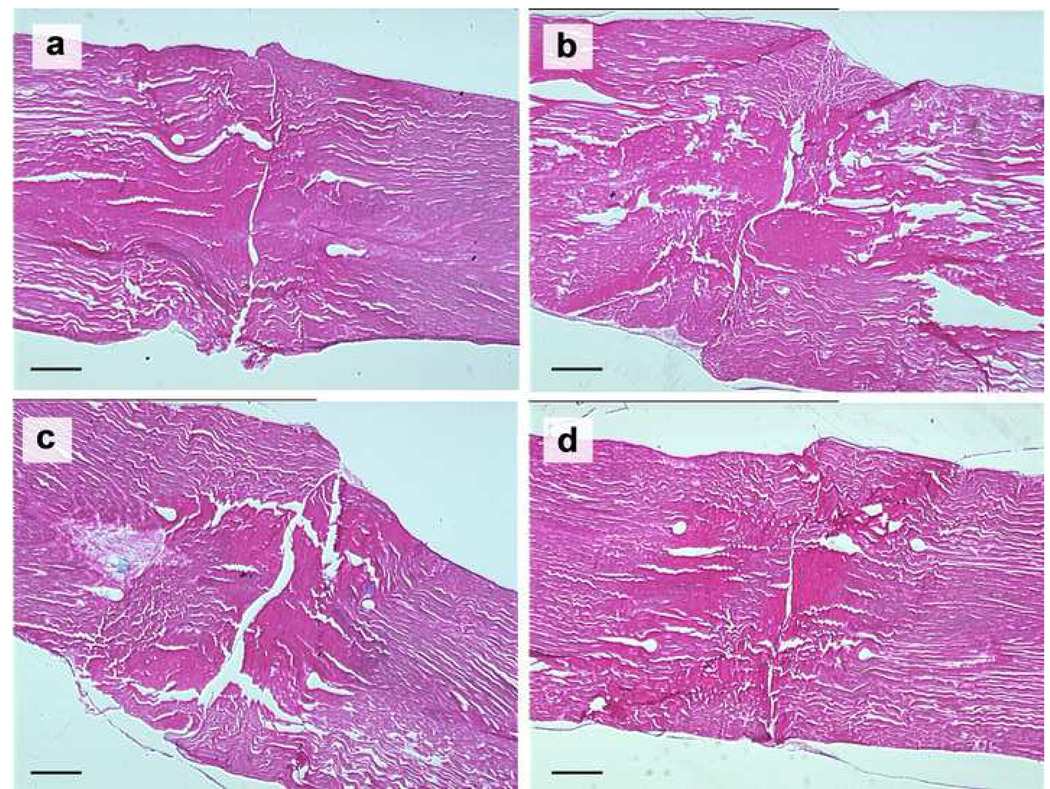
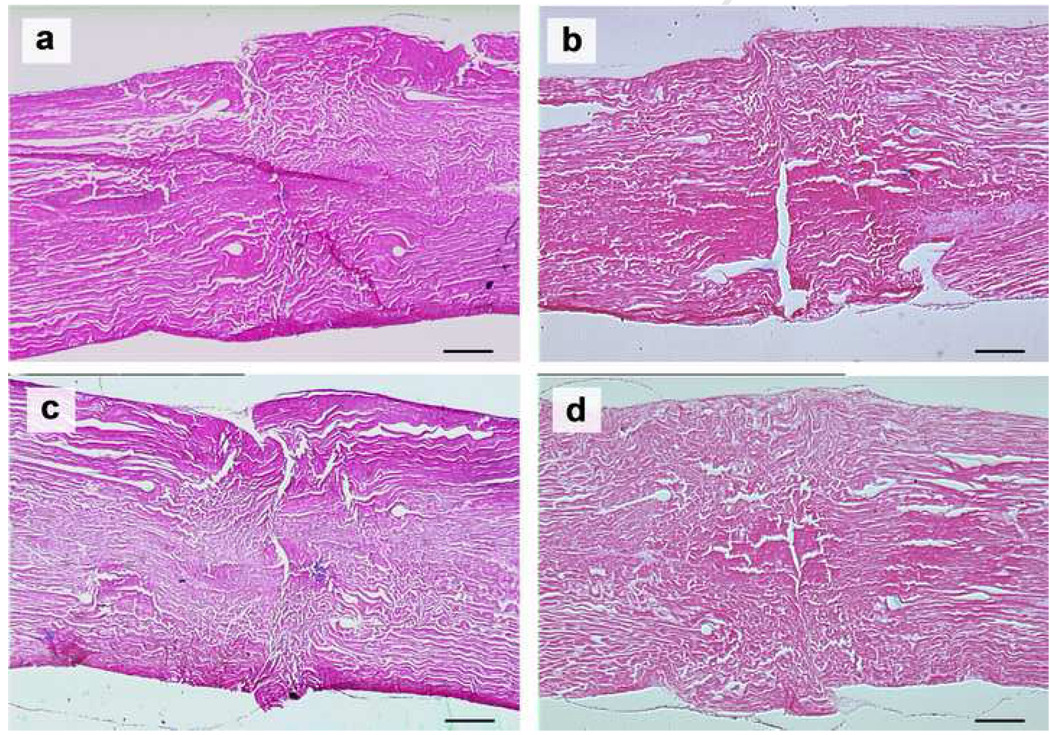
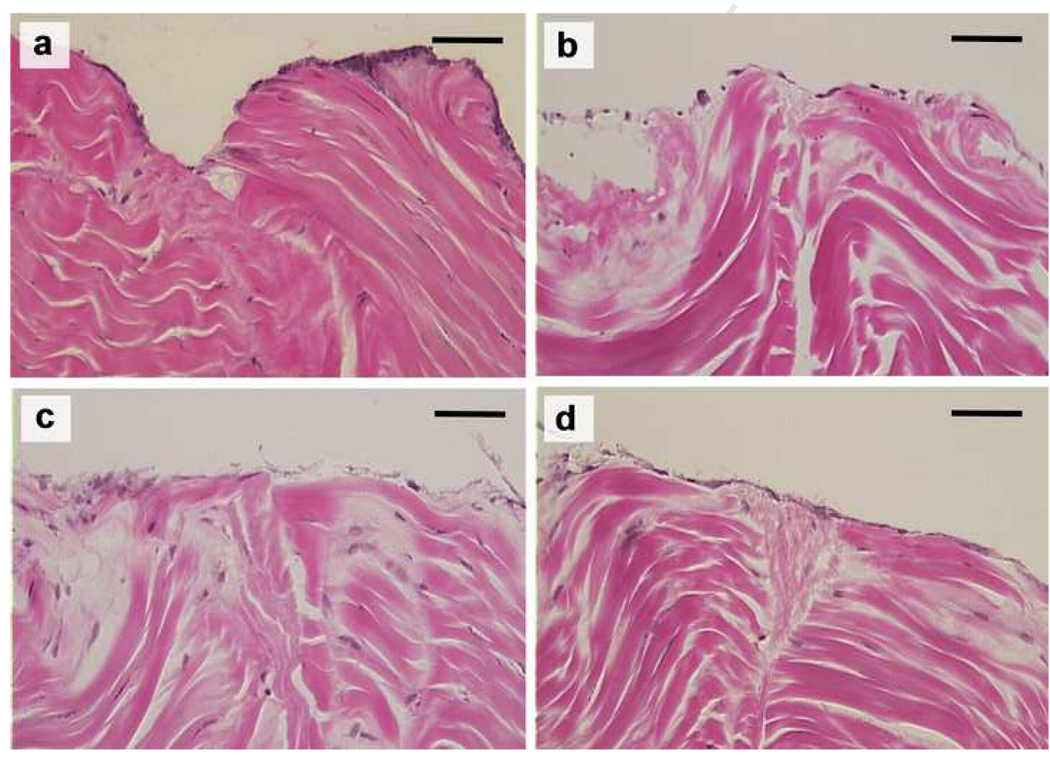
Under light microscopy, the tendon apposition site was bridged with an epitenon cell layer in all groups cultured for 2 weeks, but there was no connection of collagen bundles within the opposed tendon ends (Figure 7). In the 4 week samples, epitenon cells bridged the healing site in all groups, as in the 2 weeks samples. In addition, every group showed partial connection of collagen bundles between the opposed tendon ends (Figure 8 and 9).
Figure 7.
The repaired tendons stained with hematoxylin and eosin at 2 weeks in lower magnification (original photo taken at 90× magnification). (a) No patch group, (b) PRP patch group, (c) BMSC-seeded patch group, and (d) BMSC-seeded PRP patch group. Scale = 0.5 mm.
Figure 8.
The repaired tendons stained with hematoxylin and eosin at 4 weeks in lower magnification (original photo taken at 90× magnification). (a) No patch group, (b) PRP patch group, (c) BMSC-seeded patch group, and (d) BMSC-seeded PRP patch group. Scale = 0.5 mm.
Figure 9.
The repaired tendons stained with hematoxylin and eosin at 4 weeks in higher magnification (original photo taken at 450× magnification). Border of the collagen fibers within apposition site is partially indistinguishable in every group.(a) No patch group, (b) PRP patch group, (c) BMSC-seeded patch group, and (d) BMSC-seeded PRP patch group. Scale = 0.1 mm.
DISCUSSION
The growth factors found in platelets are stored in an inactive state within a cytoplasmic organelle called the α–granule (24). When the clotting process activates the platelets, the α–granules fuse to the cell membrane and the growth factors are secreted and converted to a bioactive state by the addition of histones and carbohydrate side chains (24). PRP can similarly be activated by a variety of methods, including repeated freeze-thaw cycles (25), CaCl2 activation, (14), and thrombin/CaCl2 activation (26). There is controversy regarding which method is most effective. In our study, thrombin/CaCl2 was used to activate the platelets (14, 24, 27).
In this study, maximal strength was significantly higher in tendons with a BMSC patch with PRP than with no patch, a PRP only patch, or a BMSC only patch. Although this was a tissue culture study, this result suggests the potential of a combination of PRP and BMSC to augment tendon healing.
The attaching force measured between the tendon ends was in the order of mN. This is much lower, of course, than the force measured in a sutured tendon, usually a value several orders of magnitude larger, as is typically done in vivo studies of tendon healing, or in cadaver studies of different suture designs. The difference from such studies is that an aim of this study was to gain insights regarding early tendon-tendon healing, in an attempt to hasten the time when the strength of the healing tendon exceeds the strength of the suture material used to support the healing tendon. In order to do this, the suture effect must be eliminated. The data showed that these initial strengths are very low, at least in our tissue culture model. Of course it is known that over 6–12 weeks in vivo the strength of the healing tissue between the tendon ends, even though it starts out at the mN level, grows by four orders of magnitude, eventually exceeding that of the tendon suture. It is possible that that these low initial strengths can be increased, both with new stimuli and over time, especially in vivo, to hasten the moment when the tendon strength exceeds the suture strength, and the patient with a tendon injury can safely resume full activity. As a start, this study showed the strength can indeed be improved with use of a BMSC patch with PRP.
One of the limitations of the current study was that number of transplanted BMSCs was not exactly known. Although the patches were similar in size, there might be different numbers of cells within each patch. In future studies it would be helpful to quantify cell number more precisely. A second limitation of the current study was that no quantitative comparison was done for cell viability or our histological measures. A third limitation was that specific markers of tenocyte or fibroblast differentiation, such as tenomodulin, tenascin-C, type I collagen and type III collagen expression were not examined. If quantitative assays had been done, those data might have given a clue to understand the basis for the differences between groups. Instead, the focus of this initial study was on the mechanical properties of the healing tissue, thinking that if no differences were observed there would be little value in such additional studies. But it is obvious that future studies should assess these factors as well. A fourth limitation was that this was an ex vivo model, which might be quite different from the in vivo condition. This was done for several reasons, not least to minimize animal use until we were confident that a positive effect would be observed. In addition, tissue culture is a more controlled environment, important in setting initial conditions.
In this study, BMSCs were implanted directly between cut flexor tendon ends using a collagen patch containing PRP. The BMSC patch with PRP improved maximal strength and stiffness of the tissue between the tendon ends in vitro. Though further studies are needed, this data suggests that a BMSC-seeded patch with PRP has the potential to enhance flexor tendon healing. Future in vivo studies are planned.
Acknowledgments
This project was funded by Mayo Foundation, and by a grant from NIH (NIAMS AR 44391).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Kelsey J. Upper extremity disorders: frequency, impact, and cost. New York: Churchill Livingstone; 1997. pp. 41–70. [Google Scholar]
- 2.Harris SB, Harris D, Foster AJ, Elliot D. The aetiology of acute rupture of flexor tendon repairs in zones 1 and 2 of the fingers during early mobilization. J Hand Surg Br. 1999;24(3):275–280. doi: 10.1054/jhsb.1998.0212. [DOI] [PubMed] [Google Scholar]
- 3.Elliot D. Primary flexor tendon repair--operative repair, pulley management and rehabilitation. J Hand Surg Br. 2002;27(6):507–513. doi: 10.1054/jhsb.2002.0800. [DOI] [PubMed] [Google Scholar]
- 4.Tang JB. Clinical outcomes associated with flexor tendon repair. Hand Clin. 2005;21(2):199–210. doi: 10.1016/j.hcl.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 5.Omae H, Zhao C, Sun YL, An KN, Amadio PC. Multilayer tendon slices seeded with bone marrow stromal cells: a novel composite for tendon engineering. J Orthop Res. 2009;27(7):937–942. doi: 10.1002/jor.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao C, Chieh HF, Bakri K, Ikeda J, Sun YL, Moran SL, et al. The effects of bone marrow stromal cell transplants on tendon healing in vitro. Med Eng Phys. 2009;31(10):1271–1275. doi: 10.1016/j.medengphy.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25(11):2739–2749. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- 8.Wang QW, Chen ZL, Piao YJ. Mesenchymal stem cells differentiate into tenocytes by bone morphogenetic protein (BMP) 12 gene transfer. J Biosci Bioeng. 2005;100(4):418–422. doi: 10.1263/jbb.100.418. [DOI] [PubMed] [Google Scholar]
- 9.Caplan AI. The mesengenic process. Clin Plast Surg. 1994;21(3):429–435. [PubMed] [Google Scholar]
- 10.Hsu C, Chang J. Clinical implications of growth factors in flexor tendon wound healing. J Hand Surg Am. 2004;29(4):551–563. doi: 10.1016/j.jhsa.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 11.James R, Kesturu G, Balian G, Chhabra AB. Tendon: biology, biomechanics, repair, growth factors, and evolving treatment options. J Hand Surg Am. 2008;33(1):102–112. doi: 10.1016/j.jhsa.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 12.Woo SL, Hildebrand K, Watanabe N, Fenwick JA, Papageorgiou CD, Wang JH. Tissue engineering of ligament and tendon healing. Clin Orthop Relat Res. 1999 367; Suppl:S312–S323. doi: 10.1097/00003086-199910001-00030. [DOI] [PubMed] [Google Scholar]
- 13.Marx RE. Platelet-rich plasma (PRP): what is PRP and what is not PRP? Implant Dent. 2001;10(4):225–228. doi: 10.1097/00008505-200110000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Anitua E. Plasma rich in growth factors: preliminary results of use in the preparation of future sites for implants. Int J Oral Maxillofac Implants. 1999;14(4):529–535. [PubMed] [Google Scholar]
- 15.Eppley BL, Woodell JE, Higgins J. Platelet quantification and growth factor analysis from platelet-rich plasma: implications for wound healing. Plast Reconstr Surg. 2004;114(6):1502–1508. doi: 10.1097/01.prs.0000138251.07040.51. [DOI] [PubMed] [Google Scholar]
- 16.Pietrzak WS, Eppley BL. Platelet rich plasma: biology and new technology. J Craniofac Surg. 2005;16(6):1043–1054. doi: 10.1097/01.scs.0000186454.07097.bf. [DOI] [PubMed] [Google Scholar]
- 17.Sanchez M, Anitua E, Azofra J, Andia I, Padilla S, Mujika I. Comparison of surgically repaired Achilles tendon tears using platelet-rich fibrin matrices. Am J Sports Med. 2007;35(2):245–251. doi: 10.1177/0363546506294078. [DOI] [PubMed] [Google Scholar]
- 18.Mishra A, Tummala P, King A, Lee B, Kraus M, Tse V, et al. Buffered Platelet-Rich Plasma Enhances Mesenchymal Stem Cell Proliferation and Chondrogenic Differentiation. Tissue Eng Part C Methods. 2009;15(3):431–435. doi: 10.1089/ten.tec.2008.0534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schnabel LV, Lynch ME, van der Meulen MC, Yeager AE, Kornatowski MA, Nixon AJ. Mesenchymal stem cells and insulin-like growth factor-I gene-enhanced mesenchymal stem cells improve structural aspects of healing in equine flexor digitorum superficialis tendons. J Orthop Res. 2009;27(10):1392–1398. doi: 10.1002/jor.20887. [DOI] [PubMed] [Google Scholar]
- 20.Hou Y, Mao Z, Wei X, Lin L, Chen L, Wang H, et al. Effects of transforming growth factor-beta1 and vascular endothelial growth factor 165 gene transfer on Achilles tendon healing. Matrix Biol. 2009;28(6):324–335. doi: 10.1016/j.matbio.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 21.Brecher G, Cronkite EP. Morphology and enumeration of human blood platelets. J Appl Physiol. 1950;3(6):365–377. doi: 10.1152/jappl.1950.3.6.365. [DOI] [PubMed] [Google Scholar]
- 22.Pittenger MF. Mesenchymal stem cells from adult bone marrow. Methods Mol Biol. 2008;449:27–44. doi: 10.1007/978-1-60327-169-1_2. [DOI] [PubMed] [Google Scholar]
- 23.Amadio PC, Berglund LJ, An KN. Biochemically discrete zones of canine flexor tendon: evaluation of properties with a new photographic method. J Orthop Res. 1992;10(2):198–204. doi: 10.1002/jor.1100100206. [DOI] [PubMed] [Google Scholar]
- 24.Marx RE. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg. 2004;62(4):489–496. doi: 10.1016/j.joms.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 25.Weibrich G, Kleis WK, Hafner G, Hitzler WE. Growth factor levels in platelet-rich plasma and correlations with donor age, sex, and platelet count. J Craniomaxillofac Surg. 2002;30(2):97–102. doi: 10.1054/jcms.2002.0285. [DOI] [PubMed] [Google Scholar]
- 26.Marx RE, Carlson ER, Eichstaedt RM, Schimmele SR, Strauss JE, Georgeff KR. Plateletrich plasma: Growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85(6):638–646. doi: 10.1016/s1079-2104(98)90029-4. [DOI] [PubMed] [Google Scholar]
- 27.Han B, Woodell-May J, Ponticiello M, Yang Z, Nimni M. The effect of thrombin activation of platelet-rich plasma on demineralized bone matrix osteoinductivity. J Bone Joint Surg Am. 2009;91(6):1459–1470. doi: 10.2106/JBJS.H.00246. [DOI] [PubMed] [Google Scholar]