Abstract
The present study was designed to examine the question of whether or not there is a natriuretic hormonal substance involved in the renal regulation of sodium balance.
For this purpose, procedures for concentration and fractionation of plasma and urine samples and a sensitive bioassay for demonstrating changes in renal sodium excretion were developed. The natriuretic assay utilized rats with mild diabetes insipidus which were maintained in salt and water balance.
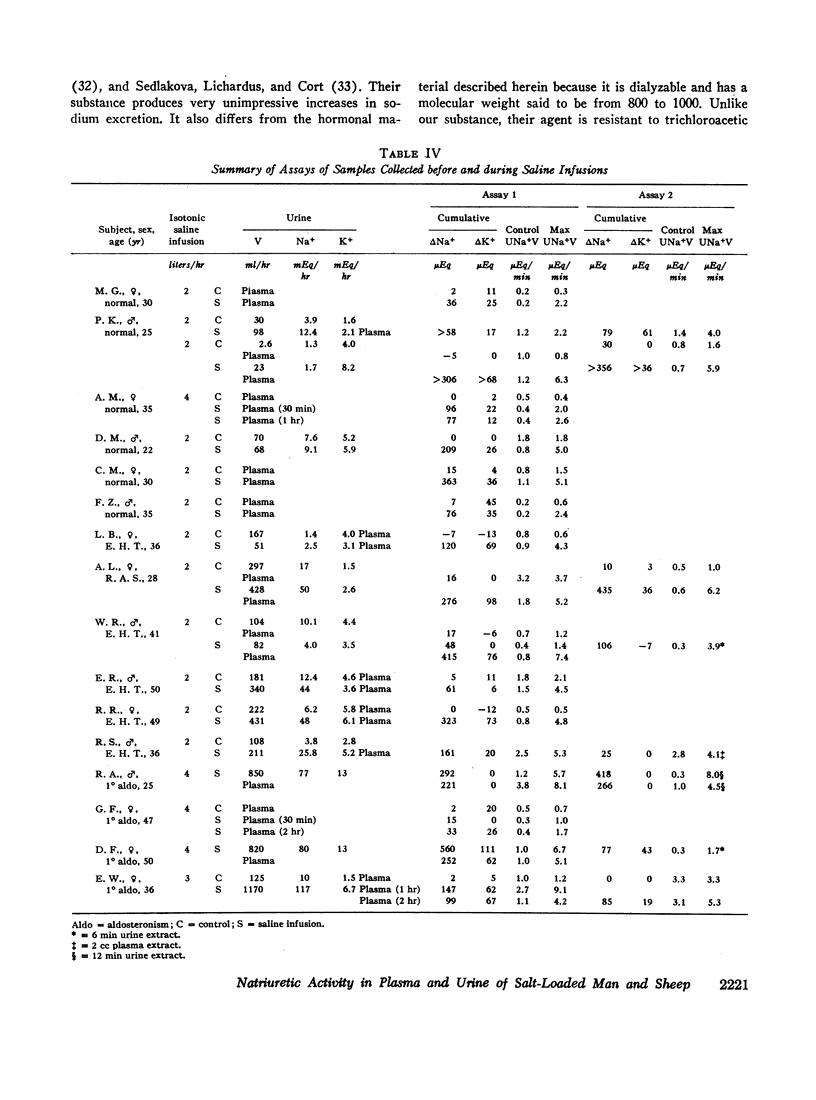
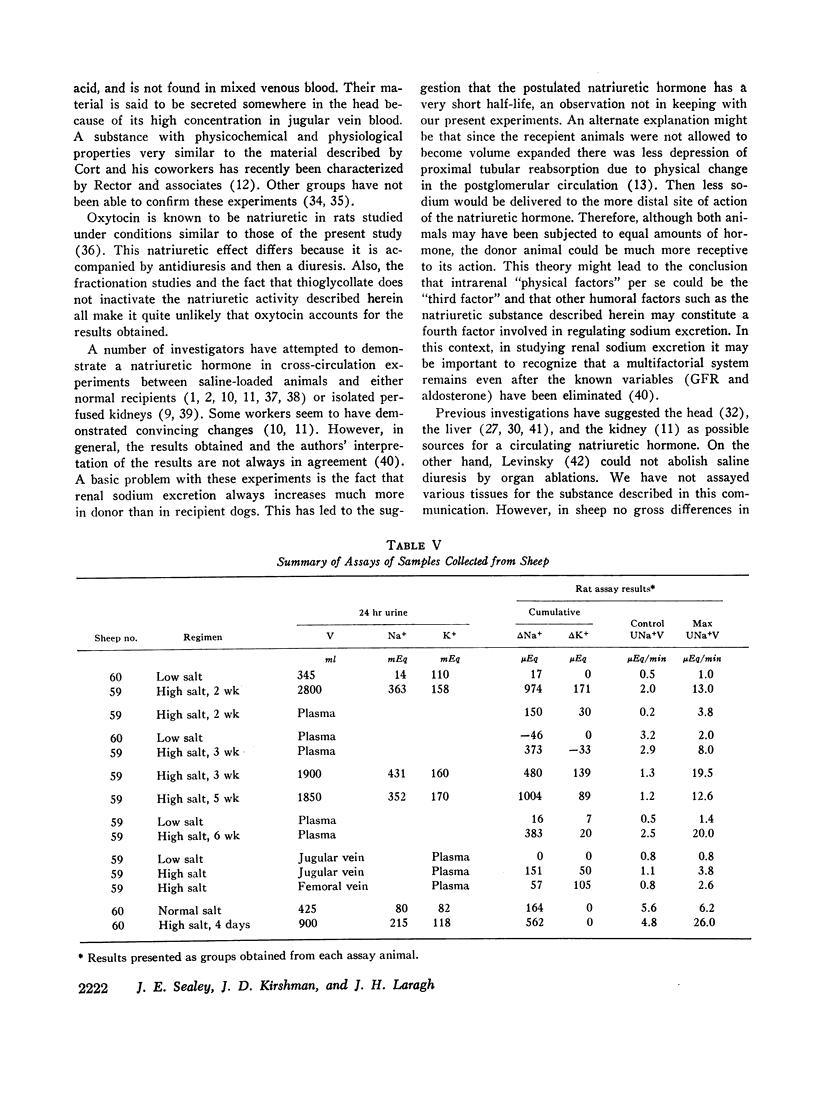
Using these approaches a natriuretic humoral substance was demonstrated in plasma and urine from normal man and sheep, and in patients with primary aldosteronism or essential hypertension.
It seems likely that this substance participates in day to day regulation of sodium balance because it was not detectable in sodium-depleted subjects and it consistently appeared in the sodium-loaded subjects.
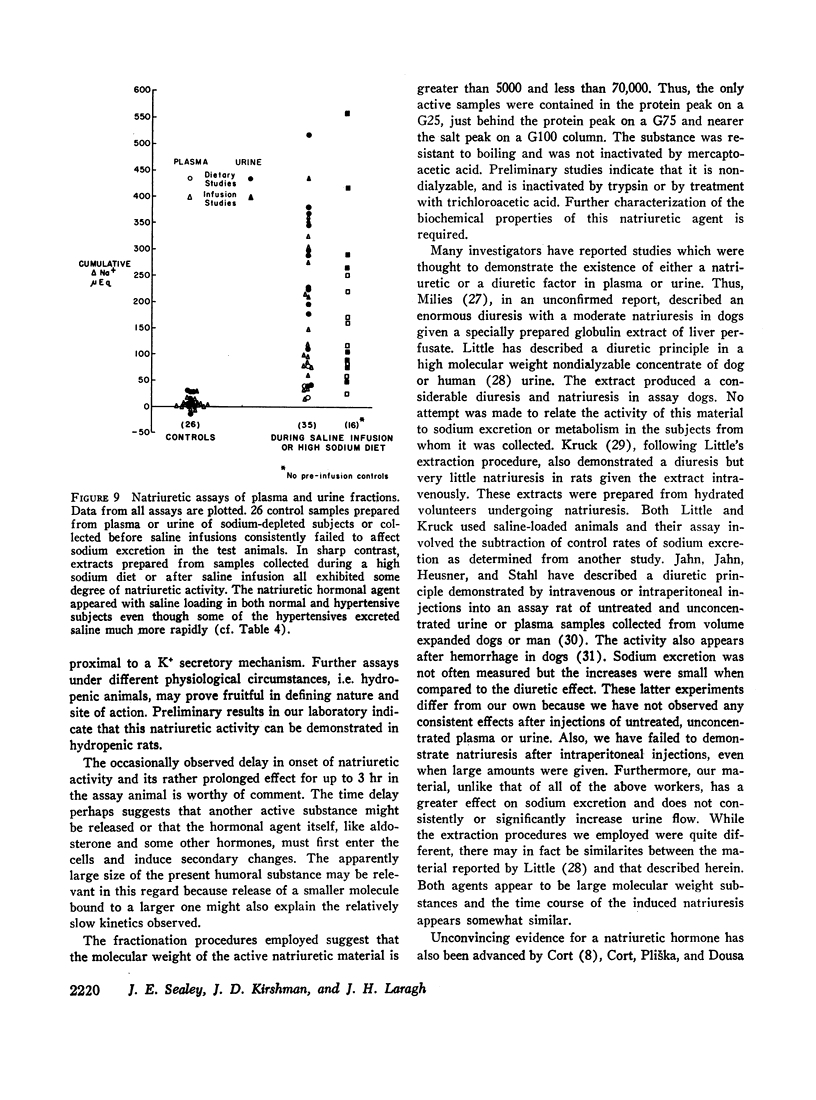
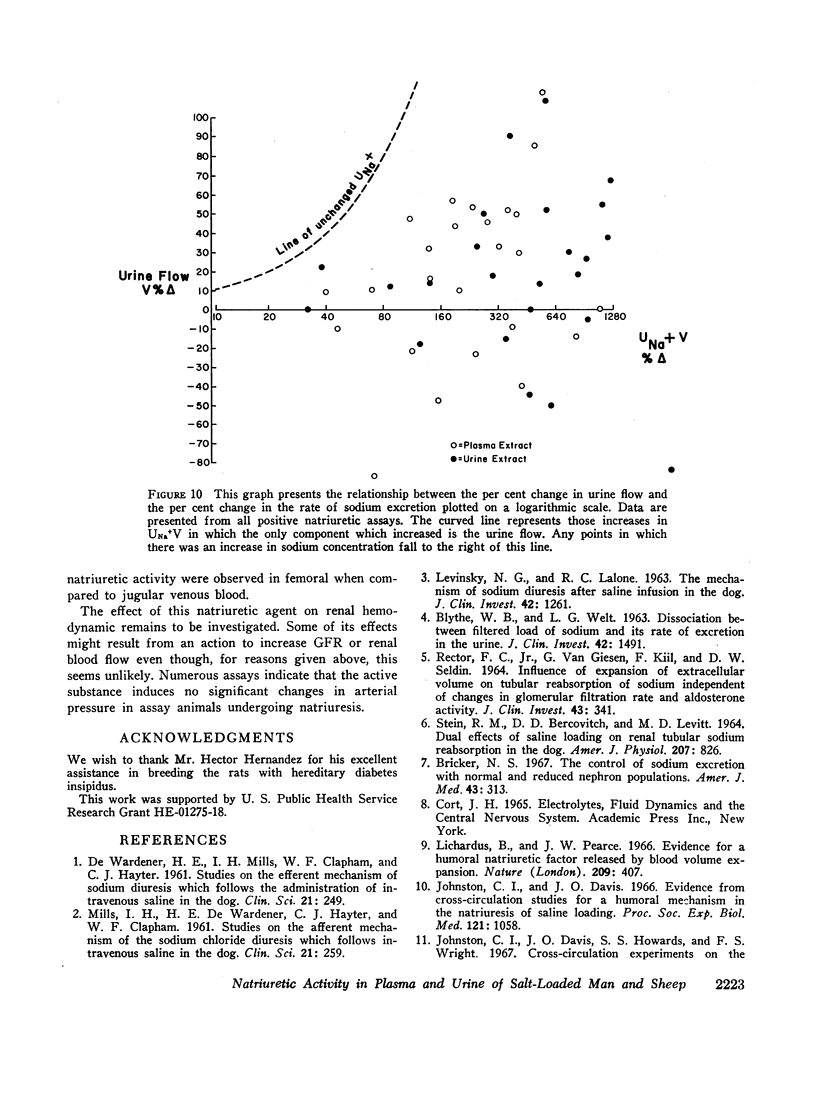
The hormonal agent may not act immediately and its activity can be apparent for up to 3 hr. Full expression of its activity requires that the assay animals be appropriately volume expanded. This suggests that the increases in sodium excretion mediated by this hormonal substance depend in part on the coparticipation of other physical and perhaps humoral factors.
This natriuretic substance appears to be of large molecular weight or carried by a large molecule. The data suggest that it acts, at least in part, to block sodium reabsorption in a more distal portion of the tubule.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLYTHE W. B., WELT L. G. DISSOCIATION BETWEEN FILTERED LOAD OF SODIUM AND ITS RATE OF EXCRETION IN THE URINE. J Clin Invest. 1963 Sep;42:1491–1496. doi: 10.1172/JCI104833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bank N., Koch K. M., Aynedjian H. S., Aras M. Effect of changes in renal perfusion pressure on the suppression of proximal tubular sodium reabsorption due to saline loading. J Clin Invest. 1969 Feb;48(2):271–283. doi: 10.1172/JCI105983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barger A. C. Renal hemodynamic factors in congestive heart failure. Ann N Y Acad Sci. 1966 Nov 22;139(2):276–284. doi: 10.1111/j.1749-6632.1966.tb41202.x. [DOI] [PubMed] [Google Scholar]
- Berliner R. W. Intrarenal mechanisms in the control of sodium excretion. Fed Proc. 1968 Sep-Oct;27(5):1127–1131. [PubMed] [Google Scholar]
- Bricker N. S. The control of sodium excretion with normal and reduced nephron populations. The pre-eminence of third factor. Am J Med. 1967 Sep;43(3):313–321. doi: 10.1016/0002-9343(67)90188-x. [DOI] [PubMed] [Google Scholar]
- Chan W. Y. Effects of neurohypophysial hormones and their deamino analogues on renal excretion of Na, K and water in rats. Endocrinology. 1965 Dec;77(6):1097–1104. doi: 10.1210/endo-77-6-1097. [DOI] [PubMed] [Google Scholar]
- DE WARDENER H. E., MILLS I. H., CLAPHAM W. F., HAYTER C. J. Studies on the efferent mechanism of the sodium diuresis which follows the administration of intravenous saline in the dog. Clin Sci. 1961 Oct;21:249–258. [PubMed] [Google Scholar]
- DIRKS J. H., CIRKSENA W. J., BERLINER R. W. THE EFFECTS OF SALINE INFUSION ON SODIUM REABSORPTION BY THE PROXIMAL TUBULE OF THE DOG. J Clin Invest. 1965 Jul;44:1160–1170. doi: 10.1172/JCI105223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugharty T. M., Belleau L. J., Martino J. A., Earley L. E. Interrelationship of physical factors affecting sodium reabsorption in the dog. Am J Physiol. 1968 Dec;215(6):1442–1447. doi: 10.1152/ajplegacy.1968.215.6.1442. [DOI] [PubMed] [Google Scholar]
- DeHaven J. C., Shapiro N. Z. On the control of urine formation. Nephron. 1967;4(Suppl):1–63. [PubMed] [Google Scholar]
- Gocke D. J., Gerten J., Sherwood L. M., Laragh J. H. Physiological and pathological variations of plasma angiotensin II in man. Correlation with renin activity and sodium balance. Circ Res. 1969 May;24(5 Suppl):131–148. [PubMed] [Google Scholar]
- Horster M., Thurau K. Micropuncture studies on the filtration rate of single superficial and juxtamedullary glomeruli in the rat kidney. Pflugers Arch Gesamte Physiol Menschen Tiere. 1968;301(2):162–181. doi: 10.1007/BF00362733. [DOI] [PubMed] [Google Scholar]
- Howards S. S., Davis B. B., Knox F. G., Wright F. S., Berliner R. W. Depression of fractional sodium reabsorption by the proximal tubule of the dog without sodium diuresis. J Clin Invest. 1968 Jul;47(7):1561–1572. doi: 10.1172/JCI105848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn H., Jahn M., Heusner A. A., Stahl J. Mise en évidence chez l'homme et chez le chien d'un facteur diurétique et natriurétique dans le sang et les urines après expansion du volume extracellulaire. C R Acad Sci Hebd Seances Acad Sci D. 1967 Oct 16;265(16):1145–1148. [PubMed] [Google Scholar]
- Johnston C. I., Davis J. O. Evidence from cross circulation studies for a humoral mechanism in the natriuresis of saline loading. Proc Soc Exp Biol Med. 1966 Apr;121(4):1058–1063. doi: 10.3181/00379727-121-30965. [DOI] [PubMed] [Google Scholar]
- Krück F. Biologischer Nachweis eines humoralen natriuretischen Prinzips im Urin gesunder Menschen. Klin Wochenschr. 1967 Jan 1;45(1):30–34. doi: 10.1007/BF01745735. [DOI] [PubMed] [Google Scholar]
- LEVINSKY N. G., LALONE R. C. THE MECHANISM OF SODIUM DURESIS AFTER SALINE INFUSION IN THE DOG. J Clin Invest. 1963 Aug;42:1261–1276. doi: 10.1172/JCI104811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LITTLE J. M. RENAL HEMODYNAMIC AND ELECTROLYTE EXCRETION EFFECTS OF THE URINARY DIURETIC FACTOR (UDF). J Pharmacol Exp Ther. 1965 Jun;148:363–366. [PubMed] [Google Scholar]
- Levinsky N. G. Nonaldosterone influences on renal sodium transport. Ann N Y Acad Sci. 1966 Nov 22;139(2):295–303. doi: 10.1111/j.1749-6632.1966.tb41204.x. [DOI] [PubMed] [Google Scholar]
- Lewy J. E., Windhager E. E. Peritubular control of proximal tubular fluid reabsorption in the rat kidney. Am J Physiol. 1968 May;214(5):943–954. doi: 10.1152/ajplegacy.1968.214.5.943. [DOI] [PubMed] [Google Scholar]
- Lichardus B., Pearce J. W. Evidence for a humoral natriuretic factor released by blood volume expansion. Nature. 1966 Jan 22;209(5021):407–409. doi: 10.1038/209407a0. [DOI] [PubMed] [Google Scholar]
- MILIES E. A new diuretic factor of hepatic origin. Acta Physiol Lat Am. 1960;10:178–193. [PubMed] [Google Scholar]
- MILLS I. H., DE WARDENER H. E., HAYTER C. J., CLAPHAM W. F. Studies on the afferent mechanism of the sodium chloride diuresis which follows intravenous saline in the dog. Clin Sci. 1961 Oct;21:259–264. [PubMed] [Google Scholar]
- Newton M. A., Laragh J. H. Effect of corticotropin on aldosterone excretion and plasma renin in normal subjects, in essential hypertension and in primary aldosteronism. J Clin Endocrinol Metab. 1968 Jul;28(7):1006–1013. doi: 10.1210/jcem-28-7-1006. [DOI] [PubMed] [Google Scholar]
- Pearce J. W., Sonnenberg H., Veress A. T., Ackermann U. Evidence for a humoral factor modifying the renal response to blood volume expansion in the rat. Can J Physiol Pharmacol. 1969 Apr;47(4):377–386. doi: 10.1139/y69-066. [DOI] [PubMed] [Google Scholar]
- RECTOR F. C., Jr, VANGIESEN G., KIIL F., SELDIN D. W. INFLUENCE OF EXPANSION OF EXTRACELLULAR VOLUME ON TUBULAR REABSORPTION OF SODIUM INDEPENDENT OF CHANGES IN GLOMERULAR FILTRATION RATE AND ALDOSTERONE ACTIVITY. J Clin Invest. 1964 Mar;43:341–348. doi: 10.1172/JCI104919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rector F. C., Jr, Martinez-Maldonado M., Kurtzman N. A., Sellman J. C., Oerther F., Seldin D. W. Demonstration of a hormonal inhibitor of proximal tubular reabsorption during expansion of extracellular volume with isotonic saline. J Clin Invest. 1968 Apr;47(4):761–773. doi: 10.1172/JCI105771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAWYER W. H. Biologic assays for oxytocin and vasopressin. Methods Med Res. 1961;9:210–219. [PubMed] [Google Scholar]
- STEIN R. M., BERCOVITCH D. D., LEVITT M. F. DUAL EFFECTS OF SALINE LOADING ON RENAL TUBULAR SODIUM REABSORPTION IN THE DOG. Am J Physiol. 1964 Oct;207:826–834. doi: 10.1152/ajplegacy.1964.207.4.826. [DOI] [PubMed] [Google Scholar]
- Sedláková E., Lichardus B., Cort J. H. Plasma saluretic activity: its nature and relation to oxytocin analogs. Science. 1969 May 2;164(3879):580–582. doi: 10.1126/science.164.3879.580. [DOI] [PubMed] [Google Scholar]
- Stahl J., Jahn H., Jahn M., Kieny R. Origine hépatique du facteur diurétique et natriurétique produit par stimulation des volorécepteurs intrathoraciques (respiration en pression négative) C R Seances Soc Biol Fil. 1968 Jul;162(1):264–266. [PubMed] [Google Scholar]
- Tobian L., Coffee K., McCrea P. Evidence for a humoral factor of non-renal and non-adrenal origin which influences renal sodium excretion. Trans Assoc Am Physicians. 1967;80:200–206. [PubMed] [Google Scholar]
- Valtin H. Hereditary hypothalamic diabetes insipidus in rats (Brattleboro strain). A useful experimental model. Am J Med. 1967 May;42(5):814–827. doi: 10.1016/0002-9343(67)90098-8. [DOI] [PubMed] [Google Scholar]
- Wilson D. R., Thiel G., Arce M. L., Oken D. E. The role of the concentration mechanism in the development of acute renal failure: micropuncture studies using diabetes insipidus rats. Nephron. 1969;6(2):128–139. doi: 10.1159/000179721. [DOI] [PubMed] [Google Scholar]
- Wright F. S., Brenner B. M., Bennett C. M., Keimowitz R. I., Berliner R. W., Schrier R. W., Verroust P. J., De Wardener H. E., Holzgreve H. Failure to demonstrate a hormonal inhibitor of proximal sodium reabsorption. J Clin Invest. 1969 Jun;48(6):1107–1113. doi: 10.1172/JCI106067. [DOI] [PMC free article] [PubMed] [Google Scholar]


