Abstract
Extracellular ADP is known to play many important physiological roles. In this study, we identified the P2Y13 receptor in a rat mast cell line (RBL-2H3) and explored the functional role of ADP, its endogenous agonist. ADP induced both intracellular calcium mobilization and release of hexosaminidase (Hex). In an assay of intracellular calcium, ADP was 100-fold less potent than and equally efficacious as the P2Y1 receptor-selective agonist MRS2365. However, ADP was more potent and efficacious than MRS2365 in inducing Hex release and in enhancing antigen-induced Hex release. ADP-induced intracellular calcium mobilization was blocked by phospholipase C inhibitor U73122 and by P2Y1 receptor selective antagonist MRS2500, but not by pertussis toxin (PTX), suggesting a mechanism mediated by the Gq-coupled P2Y1 receptor, but not P2Y13 (Gi-coupled) or P2X receptors. ADP-induced Hex release was blocked by PTX and a selective P2Y13 receptor antagonist MRS2211, but not by MRS2500 or P2Y1 receptor-specific siRNA, suggesting a Gi-coupled P2Y13 receptor-related mechanism. Measurement of gene expression confirmed high expression of both P2Y1 and P2Y13 receptors (in comparison to a previously reported P2Y14 receptor) in RBL-2H3 cells. Thus, we demonstrated that ADP-mediated intracellular calcium mobilization and Hex release in RBL-2H3 cells are via P2Y1 and P2Y13 receptors, respectively. Selective antagonists of the P2Y13 receptor might be novel therapeutic agents for various allergic conditions.
Keywords: P2Y13 receptor, nucleotide, mast cell, degranulation, allergy, G protein-coupled receptor
1. Introduction
Mast cell degranulation plays a crucial role in the development of allergic diseases, which has traditionally been thought to be mediated via the FcεRI receptor [1-3]. However, recent evidence suggests that a growing number of G protein-coupled receptors (GPCRs), such as sphingosine-1-phosphate, C3a, C5a, adenosine, and UDP-glucose (P2Y14) receptors, are also involved in this process [4-6]. Understanding the regulatory mechanisms of mast cell degranulation would help reduce unwanted allergic reactions in the development of novel therapeutic agents.
The role of extracellular nucleotides acting at P2X receptors (ion channels) and at P2Y receptors (GPCRs) in mast cells has been explored [7]. The P2Y receptors, which are activated by a range of extracellular mono- and dinucleotides, are widespread in the immune system [8] and are an increasingly important focus of preclinical research [9]. In a recent study, we have shown that an extracellular nucleotide-sugar, UDP-glucose, can enhance antigen-induced β-hexosaminidase (Hex) release in rat basophilic leukemia (RBL)-2H3 cells via the P2Y14 receptor [6]. Previously, some other nucleotides, including ATP and ADP, have also been indicated to be involved in mast cell function, although the P2Y receptor subtypes responsible for degranulation in various cases have not been fully identified partly due to the lack of proper pharmacological and biochemical tools [10,11,12]. For example, ADP has been shown to enhance IgE-mediated secretion of [3H]5-hydroxytryptamine by IL-3-dependent bone marrow-derived mast cells (BMMC) in a pertussis toxin-sensitive manner, but the receptor subtype involved could not be demonstrated [12]. ATP-induced histamine release in rat peritoneal mast cells has been ascribed to a mechanism mediated by phospholipase A2 or a P2X receptor [10].
ADP has been identified as the endogenous agonist of both human [13] and rat [14] P2Y13 receptors and also activates P2Y1 and P2Y12 receptors. Cord blood-derived human mast cells were found to express mRNA encoding all three of these ADP-responsive receptors [11]. High expression levels of the P2Y13 receptor in hematopoietic cells and the immune system, such as spleen, lymph nodes, and bone marrow, suggest a role for this subtype in inflammation-related disorders [13] and potentially in mast cell degranulation.
In the present study, we compared the expression levels of P2Y receptor subtypes using quantitative real-time PCR and found that P2Y13 and P2Y1 receptors are equally highly expressed in RBL-2H3 cells. We found that the P2Y13 receptor is responsible for ADP-mediated Hex release, whereas the P2Y1 receptor mediates ADP-induced intracellular calcium mobilization.
2. Materials and Methods
2.1. Materials
MRS2211 (2-[(2-Chloro-5-nitrophenyl)azo]-5-hydroxy-6-methyl-3-[(phosphonooxy)methyl]-4-pyridinecarboxaldehyde disodium salt) [15]; MRS2365 ([[(1R,2R,3S,4R,5S)-4-[6-amino-2-(methylthio)-9H-purin-9 -yl]-2,3-dihydroxybicyclo[3.1.0]hex-1-yl]methyl]diphosphoric acid mono ester trisodium salt) and MRS2500 ((1R,2S,4S,5S)-4-[2-iodo-6-(methylamino)-9H-purin-9-yl]- 2-(phosphonooxy)bicyclo[3.1.0]hexane-1-methanol dihydrogen phosphate ester tetraammonium salt) were from Tocris (St. Louis, MO). ADP, ATP, 2-MeSADP, U73122, pertussis toxin, p-nitrophenyl-N-acetyl-β-D-glucosaminide, and 2,4-dinitrophenyl-bovine serum albumin (DNP-BSA), Triton X-100 and anti-DNP antibody were obtained from Sigma (St. Louis, MO). Taqman Universal PCR master mix, Taqman Gene Expression Assays, High Capacity cDNA Reverse Transcription Kit and Taqman Rodent glyceraldehyde 3-phosphate dehydrogenase (GAPDH) control were purchased from Applied Biosystems (Foster City, CA). Calcium assay kit was from Molecular Devices (Sunnyvale, CA). All other reagents were from standard sources and are of analytical grade.
2.2. Cell culture and detection of gene expression of P2Y receptors
Cells were cultured in DMEM supplemented with 10% FBS, 100 units/ml penicillin, 100 μg/ml streptomycin, 3 μmol/ml glutamine in a humidified atmosphere of 5% CO2 at 37°C. Total cellular RNA was isolated from RBL-2H3 cells using an RNA isolation kit (RNeasy, Qiagen, Valencia, CA) and was reversed-transcribed with the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA) according to the manufacturer's protocol. Real-time PCR detection of the expression of the rat P2Y receptor gene and the endogenous reference GAPDH mRNA was performed using the 7900HT Fast System (Applied Biosystems, Foster City, CA). Quantitative analysis of data was performed by using the 2-ΔΔCt method [16]. Values were normalized to GAPDH and were expressed as relative expression levels.
2.3. Measurement of release of Hex
We measured the release of Hex, a granule-associated protein that parallels histamine release and is an indicator of degranulation of RBL-2H3 cells, as previously described [6]. In brief, RBL-2H3 cells were split to 24-well plates (105 cells/ml) and incubated overnight with 0.1 μg/ml DNP-specific IgE antibody. Cells were washed twice and then stimulated with DNP-BSA, ADP, or both for 30 min. Hex was measured in the medium and in cell lysates (in 0.1% Triton X-100) by a colorimetric assay. Aliquots (20 μl) of samples were incubated with 20 μl of 1 mM p-nitrophenyl-N-acetyl-β-d-glucosaminide at 37°C in 0.1 M sodium citrate buffer (pH 4.5) for 1 h. The product, p-nitrophenol, was converted to the chromophore, 4-nitrophenoxide, by addition of 200 μl of a 0.1 M Na2CO3/0.1 M NaHCO3 buffer. Absorbance was read at 405 nm using a spectrophotometer. The P2Y1 receptor-specific siRNA assay was performed as previously described [6]. Results are reported as the percentage of intracellular Hex that was released into the medium.
2.5. Intracellular intracellular calcium mobilization
RBL-2H3 cells were grown overnight in 100 μl media in 96-well flat bottom plates at 37°C at 5% CO2 or until 80-90% confluency. The calcium assay kit (Molecular Devices, Sunnyvale, CA) was used as directed without washing cells, and with probenecid added to the loading dye at a final concentration of 2.5 mM to increase dye retention. Cells were loaded with 50 μl dye with probenecid to each well and incubated for 60 min at room temperature. The compound plate was prepared using dilutions of various compounds in Hanks Buffer (pH 7.4). Samples were run in duplicate at room temperature. Cell fluorescence was measured (excitation at 485 nm; emission at 525 nm) following exposure to agonists. Increases in intracellular calcium are reported as the maximum fluorescence value after exposure minus the basal fluorescence value before exposure.
2.8. Statistical analysis
EC50 values were calculated with Prism 5 (GraphPad, San Diego, CA). Data were analyzed by analysis of variance (ANOVA) (followed by post-hoc analysis) to check the statistical difference among groups with P value less than 0.05 being considered significant. Results were expressed as mean ± S.E.
Results
3.1. ADP induced intracellular calcium mobilization in RBL-2H3 cells
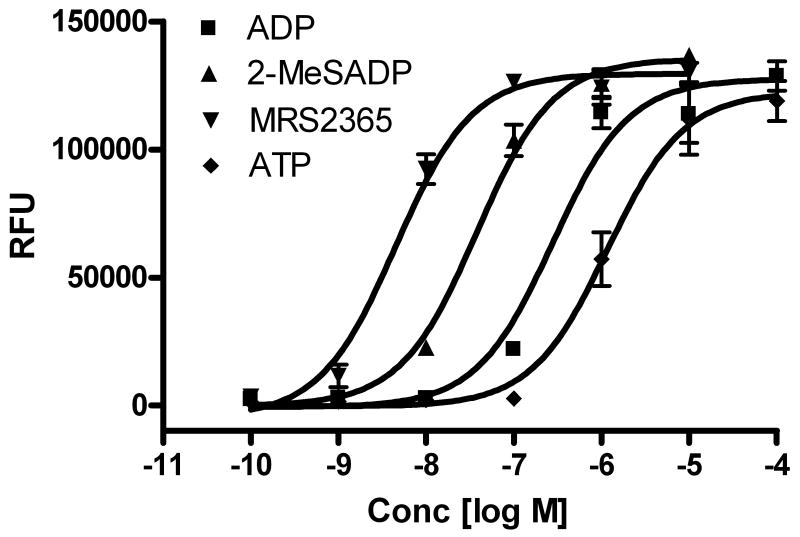
In an initial experiment, it was found that ADP induced intracellular calcium mobilization in a concentration-dependent manner with an EC50 value of 278 ± 42 nM (Figure 1). ATP was also shown to induce intracellular calcium increase, albeit less potently (EC50=1170 ± 220 nM). As ADP is the endogenous agonist for three subtypes of P2Y receptors (P2Y1, P2Y12 and P2Y13), it was not clear which subtype mediated this effect. We subsequently examined the effect of MRS2365, a potent and selective agonist of the P2Y1 receptor [17]. The EC50 of MRS2365 was calculated to be 4.5 ± 0.8 nM (Figure 1), which was 60-fold more potent than ADP, and it was equally efficacious, indicating full agonism. The EC50 value of the potent, non-selective agonist 2-MeSADP was 37.8 ± 11.3 nM. The rank order of agonist potency was MRS2365 > 2-MeSADP > ADP > ATP, consistent with a P2Y1 receptor-mediated effect [17].
Figure 1.
P2Y1 receptor agonist-induced intracellular calcium mobilization in RBL-2H3 cells. Results are expressed as mean ± S.E. from a triplicate determination representative of three independent experiments performed in duplicate or triplicate. The calcium mobilization was measured 20 s after addition of agonists and lasted for 120 s. The protocol used was similar to previously described [29]. The mean EC50 values of agonists are listed in the text.
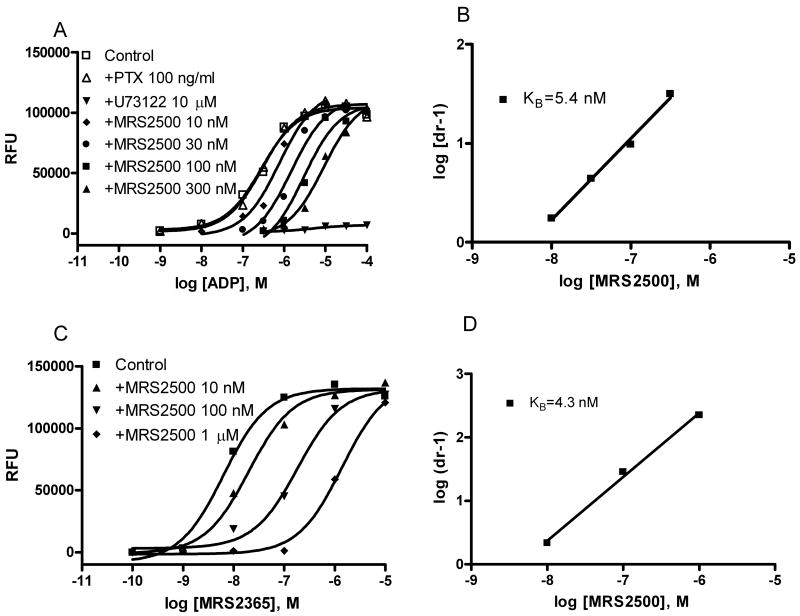
The calcium transients induced by ADP were blocked by an inhibitor of phospholipase C (PLC), U73122, further supporting a Gq-coupled P2Y1, not the P2X receptor-mediated mechanism. The calcium transients were not prevented by pretreatment with pertussis toxin (PTX) (Figure 2), which inhibits the function of the α-subunit of Gi protein, including the release of Gβγ subunits, but not Gq protein. ADP-induced calcium transients were antagonized by a selective antagonist of the P2Y1 receptor, MRS2500. The inhibition corresponded to a KB value of 5.4 nM by Schild analysis (Figure 2), consistent with the previously reported affinity of this antagonist at the P2Y1 receptor [18]. MRS2500 also concentration-dependently shifted the concentration-response curve of MRS2365-induced calcium release corresponding to a KB value of 4.3 nM.
Figure 2.
Effects of various inhibitors on agonist-induced intracellular calcium mobilization in RBL-2H3 cells. A. Effects of pretreatment with PTX, a phospholipase C inhibitor U73122 and various concentrations of the P2Y1 receptor antagonist MRS2500. Cells were pretreated with PTX (100 ng/ml) for 24 h or with U73122 (10 μM) and MRS2500 for 20 min before the measurements. B. Schild analysis of antagonism by MRS2500 of the agonist effect of ADP. C. Effect of MRS2500 on the selective P2Y1 receptor agonist MRS2365-induced calcium release. D. Schild analysis of antagonism by MRS2500 of the agonist effect of MRS2365. The calcium mobilization was measured 20 s after addition of agonists and lasted for 120 s. The protocol used for measurement of calcium mobilization was similar to previously described [29]. The EC50 values (nM) of ADP and MRS2365 were 278 ± 42 and 4.5 ± 0.8 nM, respectively.
3.2. ADP enhanced antigen-induced Hex release
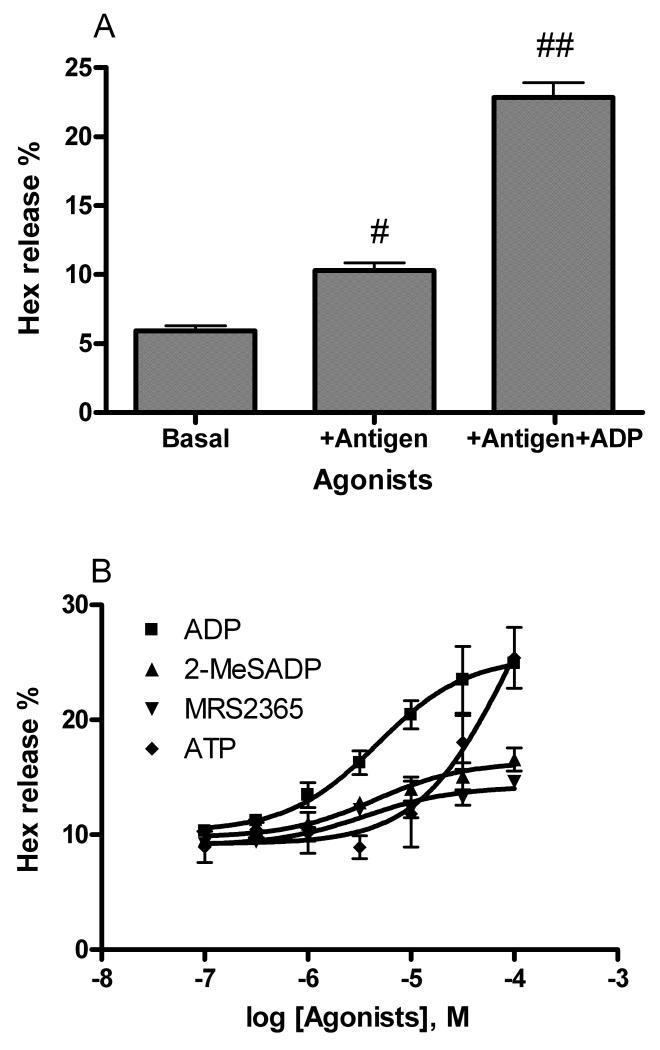
In an initial study, a sub-maximal concentration of antigen (DNP-BSA, 10 ng/ml) induced about 10% release of Hex from RBL-2H3 cells (Figure 3A, P < 0.05, compared with control). ADP (100 μM) caused a significant enhancement of the effect of antigen (Figure 3A, P < 0.01, compared with the antigen group).
Figure 3.
Hex release from RBL-2H3 cell induced by antigen (10 ng/ml) in the presence and absence of P2Y receptor agonists. A. Initial testing of the effect of antigen (10 ng/ml) in the absence and presence of ADP (100 μM). B. Enhancement by various agonists of antigen-mediated Hex release. Cells were primed with anti-DNP-BSA antibody for 24 h before testing for the release of Hex. Results are expressed as mean ± S.E. and were from three separate experiments performed in duplicate. #P < 0.05; ##P < 0.01, compared with control.
We next compared the effect of several P2Y1 receptor agonists on antigen-induced Hex release in RBL-2H3 cells. Unlike the rank order of potencies in calcium release, ADP was more potent (EC50 = 4.7 ± 0.8 μM,) and more efficacious than either 2-MeSADP or MRS2365 in enhancing Hex release (Figure 3B). ATP was much weaker than ADP in enhancing antigen-induced Hex release (EC50= 85 ± 23 μM).
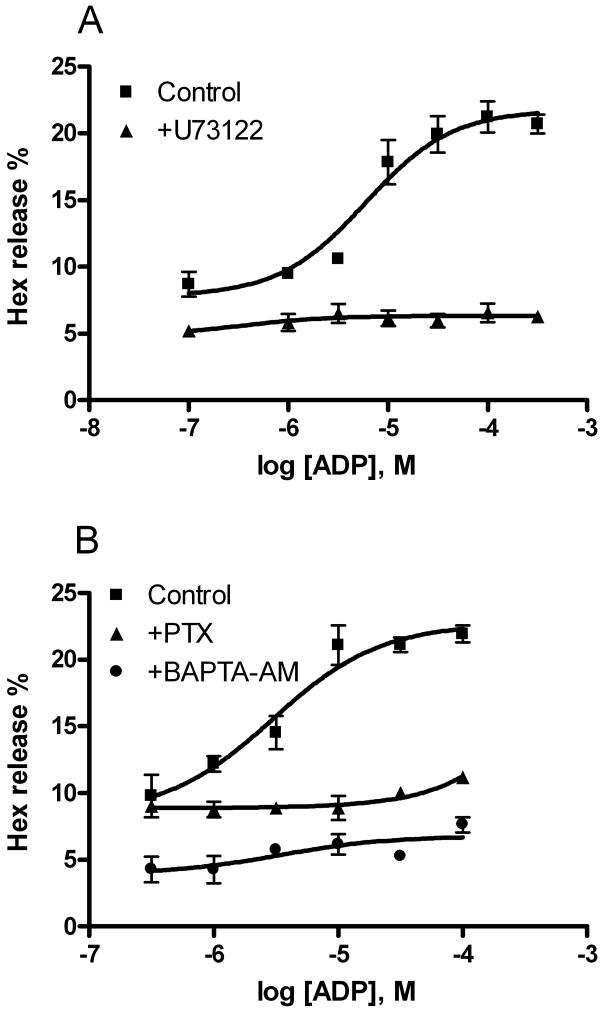
Pretreatment of RBL-2H3 cells with a PLC inhibitor, U73122 (10 μM), blocked both antigen-induced and ADP-enhanced Hex release (Figure 4A). Pretreatment with a calcium chelator, BAPTA-AM, produced an inhibitory effect similar to U73122. However, PTX diminished only ADP-elicited enhancement but not the effect of antigen, suggesting that the ADP-induced enhancement is via a Gi protein-mediated mechanism, most likely through the activation of either P2Y12 or P2Y13 receptors or both. ATP-induced enhancement of Hex release was also diminished by PTX treatment (data not shown).
Figure 4.
Effects of the phospholipase C inhibitor, U73122 (A, 10 μM) and pertussis toxin (PTX, 100 ng/ml) (B) and a calcium chelator, BAPTA-AM (10 μM) on antigen-mediated Hex release. Results are expressed as mean ± SE and are from three independent experiments performed in duplicate. The basal value of percentage Hex release was about 5%. U73122 and BAPTA-AM inhibited both antigen-induced and ADP-enhanced Hex release, whereas PTX only blocked ADP-enhanced Hex release.
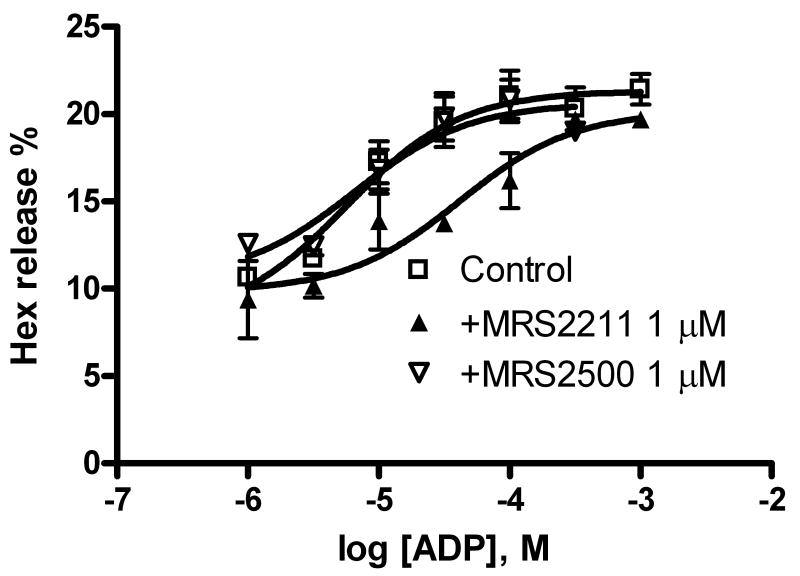
In order to identify the receptor subtype responsible for the enhancement of Hex release, we used several selective antagonists of various P2Y receptor subtypes. Figure 5A shows that the concentration-response curve of ADP-mediated Hex release was significantly right-shifted by the P2Y13 receptor antagonist MRS2211 (1 μM) but not by a selective antagonist of the P2Y1 receptor (MRS2500, 1 μM), suggesting a mechanism mediated by the P2Y13 receptor. MRS2211 increased the EC50 of ADP to 41 ± 12 μM, which represents an order of magnitude right-shift.
Figure 5.
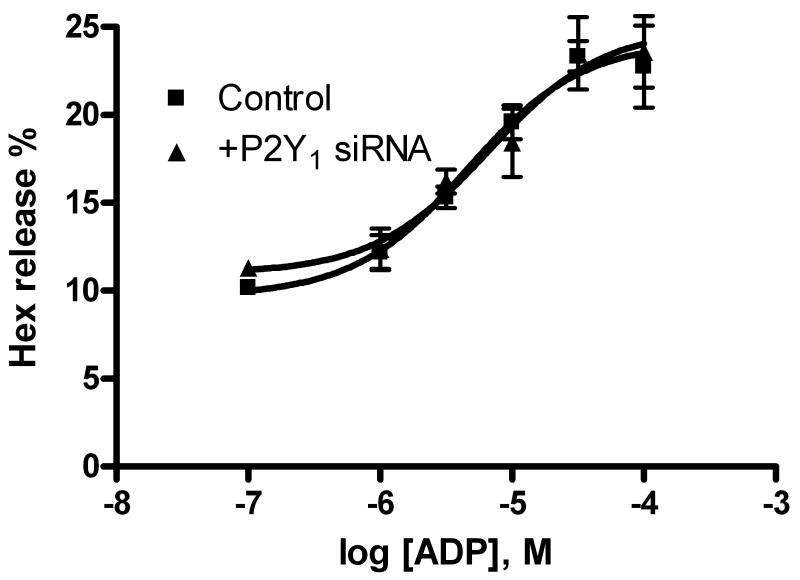
A. Effects of P2Y receptor antagonists on ADP-induced enhancement of antigen-mediated Hex release in RBL-2H3 cells. MRS2500 and MRS2211 are antagonists for the P2Y1 and P2Y13 receptors, respectively. Cells were primed with anti-DNP-BSA antibody for 24 h before testing for the release of Hex. Results are expressed as mean ± S.E. and were from 3-4 separate experiments performed in duplicate. B. Effect of P2Y1 receptor-specific siRNA on ADP-induced enhancement of antigen-induced Hex release. The experimental procedures were as described previously [6].
To confirm that the P2Y1 receptor is not involved in degranulation, a P2Y1 receptor specific siRNA was used in the Hex release assay. Figure 5B shows that the concentration-response curve of ADP-induced enhancement of antigen-induced Hex release was not affected by the inhibition with the P2Y1 receptor-specific siRNA.
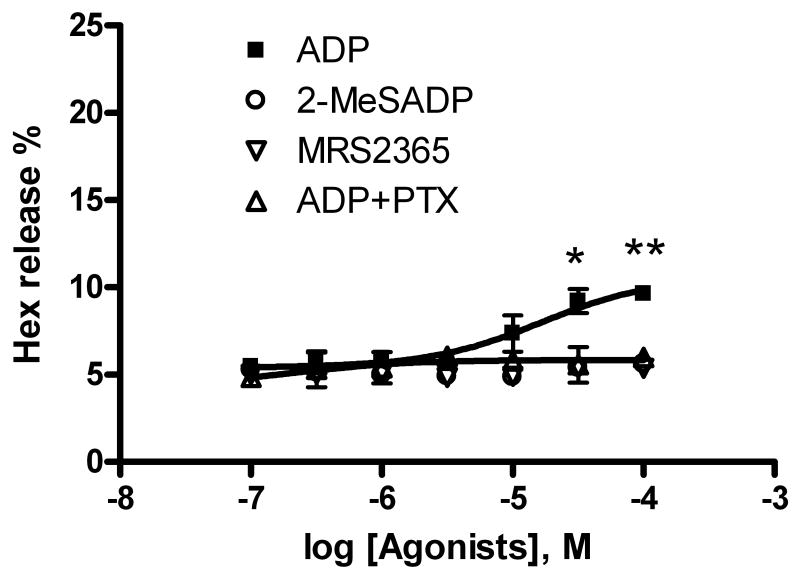
In a previous study [6], both the native agonist of the P2Y14 receptor UDP-glucose and a potent, synthetic P2Y14 receptor-selective agonist MRS2690 were able to enhance antigen-induced Hex release, although they were inactive in the absence of antigen. In the present study, we also examined whether ADP alone was able to induce Hex release in addition to its enhancing effect on antigen-induced Hex release. Figure 6 shows that ADP concentration-dependently induced a modest but significant amount of Hex release in the absence of antigen (P < 0.05 compared with basal value) corresponding to an EC50 of 16.7 ± 4.8 μM (n=3). Furthermore, the modest amount of Hex release was completely blocked by pretreatment with PTX (Figure 6). By contrast, the release of Hex induced by MRS2365 or 2-MeSADP alone was shown to be insignificant (P>0.05, comparing the percentage of Hex release at 10 μM with basal values).
Figure 6.
ADP-induced Hex release in the absence of antigen DNP-BSA. Results were from 3-4 experiments performed in duplicate. *Significantly different from basal Hex release, P < 0.05.
3.3. Measurement of the mRNA expression level of ADP-activated P2Y receptors
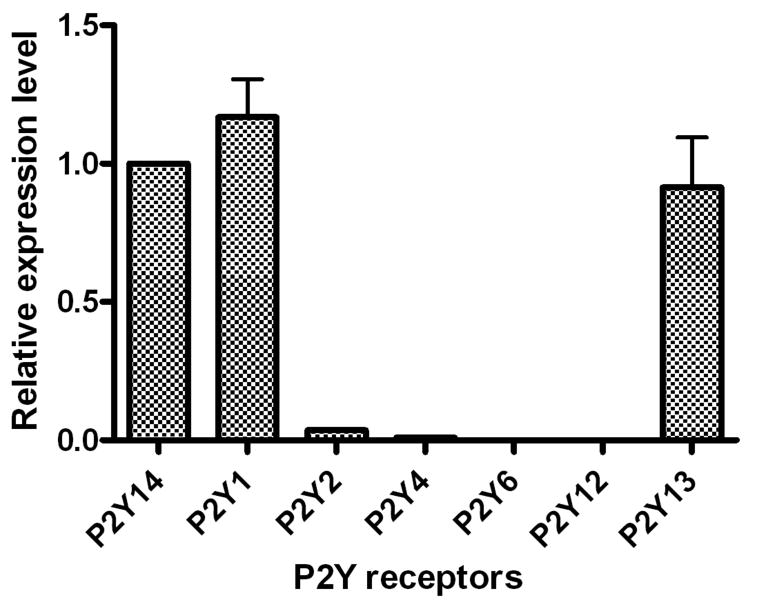
We compared the gene expression levels of all three P2Y receptor subtypes that respond to ADP using quantitative real-time polymerase chain reaction (qRT-PCR) analysis. It was found that the gene expression levels of P2Y1 and P2Y13 receptors were comparable to that of the previously reported P2Y14 receptor [6], while the P2Y12 receptor was undetectable (Figure 7). Of other P2Y receptor subtypes, as previously reported [6], the gene expression of the P2Y2 receptor was 26-fold lower, and the P2Y4 and P2Y6 receptors were over 100-fold lower, compared with the P2Y13 receptor. The gene expression of the P2Y11 receptor could not be measured, due to the lack of this subtype in rodents [9].
Figure 7.
Expression of P2Y1, P2Y12 and P2Y13 receptor genes determined using qRT-PCR in RBL-2H3 cells. Results are expressed as mean ± SE from three separate experiments performed in triplicate. The GAPDH was used as an endogenous positive control. The expression level of the known P2Y14 receptor [ref. 6] was expressed as 1. The gene expression levels of P2Y1, P2Y12 and P2Y13 receptors were expressed as their relative values to that of the P2Y14. The gene expression of the P2Y12 receptor was essentially undetectable in RBL-2H3 cells. The P2Y11 gene is absent in rodents.
4. Discussion
Results from the present study suggested that the P2Y13 receptor is the receptor subtype responsible for ADP-mediated Hex release. The P2Y1 receptor, which was highly expressed in RBL-2H3 mast cells and to the same degree as the P2Y13 receptor, did not mediate Hex release. However, ADP induced a robust elevation of intracellular calcium ions in a P2Y1 receptor-dependent manner. The P2Y1 receptor is known to couple through Gq protein to activate PLCβ, and consistent with this proposed mechanism the elevation of intracellular calcium ions was blocked by the PLC inhibitor U73122 but not by PTX.
The agonist profile demonstrated in the present study indicating that ADP is more effective than 2-MeSADP in mediating Hex release is consistent with the result from an earlier study of the cloned rat P2Y13 receptor [14]. Also, the use of more selective ligand tools, such as the P2Y13 receptor antagonist MRS2211, has enabled the delineation of action at specific receptor subtypes. MRS2365 is an ADP analogue that has been shown to be a potent and full agonist of the human P2Y1 receptor and a weak partial agonist of the human P2Y13 receptor, without any obvious effects at the human P2Y12 receptor, such as stimulation of the production of inositol phosphates in transiently transfected COS-7 cells [17]. The antagonist profile from this study is also in line with that reported by Fumagalli et al. [14] who showed that a P2Y1 receptor antagonist MRS2179 did not alter the effect of ADP at the rat P2Y13 receptor.
Unlike UDPG acting at the P2Y14 receptor in RBL-2H3 cells, which enhanced only antigen-enhanced Hex release [6], ADP, in addition to enhancing antigen-induced Hex release, seemed to induce Hex release via the P2Y13 receptor on its own, albeit to a modest extent, suggesting the P2Y13 receptor may mediate the Hex release via a mechanism different from that used by the P2Y14 receptor and could play a more important role in allergic or inflammatory conditions.
It has been suggested that nucleotides may be involved in mast cell degranulation in different assay systems and cells [19,20]. ATP and UTP have been reported to enhance histamine release induced by antigen but not by the calcium ionophore A23187 in human lung mast cells [19]. In that study the P2Y1 receptor was clearly expressed, and the P2Y2 receptor was detectable but at a lower expression level. However, the expression levels of other P2Y subtypes were not established. In mouse mc9 mast cells, ATP was shown to induce Hex release [20], although this effect required very high concentrations (1 mM) of ATP, which could act through multiple mechanisms. This ATP-induced [Ca2+]i elevation is through P2X receptor-induced influx and is independent of inositol phosphates [19]. Adenine nucleotides have been suggested to inhibit cytokine release in human mast cells and to be involved in pulmonary inflammation [11,21], although the role of P2Y13 receptors was not explored. ADP has been shown to enhance IgE-mediated secretion of [3H]5-hydroxytryptamine by IL-3-dependent BMMC in a pertussis toxin-sensitive manner, although the receptor subtype involved was not demonstrated [12], which is consistent the present finding that the P2Y13 subtype is the responsible for Hex release.
The results that the Gq-coupled P2Y1 receptor mediated the intracellular calcium mobilization but did not contribute directly to the Hex release suggest that, in RBL-2H3 cells, the P2Y1 receptor-induced calcium signal alone is not sufficient to induce Hex release. This finding is consistent with a previous study by Alfonso et al. [22] suggesting that intracellular calcium release is not a sufficient signal for degranulation of rat mast cells. Earlier studies also indicated that IgE-dependent mast cell degranulation is strictly dependent on extracellular calcium, whereas extracellular calcium may not be required in degranulation induced via non-IgE mechanisms [22,23]. However, in the present study, BAPTA-AM completely removed both antigen-induced Hex release and ADP-induced enhancement. It is not clear whether the elimination of the ADP-induced enhancement is a consequence of the inhibition of the antigen-induced release or not. Antigen-induced calcium signals could be triggered by different phospholipid pathways in antigen stimulation. IgE-antigen stimulation of mast cells induces a fast and transient calcium release from intracellular stores depending on the activation of sphingosine kinase 1 (which is dependent on the activity of phospholipase D1). PLCγ1 triggers a second (slower) wave of calcium release from intracellular stores. FcξRI–mediated degranulation depends on the first wave [24]. In RBL-2H3 cells, both IP3 and sphingosine kinase are required for the FcξRI-mediated calcium release, but only IP3 is required for the NECA-induced release [25]. PI3K has been shown to be a crucial participant in the augmentation of FcξRI–mediated degranulation by adenosine [26,27]. It remains to be explored which signal pathways are involved in ADP-mediated Hex release. The finding from the present study is also reminiscent of a recent report on the opposing effects of ADP in modulating nociceptive signaling and inflammatory pain behavior via acting Gi and Gq-coupled P2Y receptors [28].
Taken together, the detection of P2Y13, but not P2Y12, receptor gene expression, the antagonism by the P2Y13 receptor antagonist MRS2211, and the inhibitory effect of PTX, all suggest that the P2Y13 receptor is the subtype responsible for ADP-mediated degranulation in RBL-2H3 cells. However, for ADP-induced intracellular calcium mobilization, the expression of the P2Y1 receptor gene, activation and antagonism by P2Y1 selective ligands, the lack of effect by PTX treatment, and the blockade by U73122 all indicate that the P2Y1 receptor is the subtype responsible. The expression of the P2Y13 receptor in mast cells and its role in mast cell degranulation suggest that selective antagonists of the P2Y13 receptor might be novel therapeutic agents for various allergic and inflammatory conditions. The role of the P2Y13 receptor in primary human mast cells remains to be determined.
Acknowledgments
Supported by the NIDDK Intramural Research Program, National Institutes of Health.
Abbreviations
- BAPTA-AM
1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid acetoxymethyl ester
- BSA
bovine serum albumin
- CHAPS
3-[(3-cholamidopropyl) dimethylammonio]propanesulfonate
- DMEM
Dulbecco's modified Eagle's medium
- DNP
2,4-dinitrophenyl
- FBS
fetal bovine serum
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- GPCR
G protein-coupled receptor
- Hex
β-hexosaminidase
- MRS2211
2-[(2-chloro-5-nitrophenyl)azo]-5-hydroxy-6-methyl-3-[(phosphonooxy)methyl]-4-pyridinecarboxaldehyde
- PLC
phospholipase C
- PTX
pertussis toxin
- qRT-PCR
quantitative real time polymerase chain reaction
- RBL
rat basophilic leukemia
- U73122
1-[6-[((17β)-3-methoxyestra-1,3,5[10]-trien-17-yl)amino]hexyl]-1H-pyrrole-2,5-dione
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gilfillan AM, Tkaczyk C. Integrated signalling pathways for mast-cell activation. Nat Rev Immunol. 2006;6:218–30. doi: 10.1038/nri1782. [DOI] [PubMed] [Google Scholar]
- 2.Metcalfe DD. Mast cells and mastocytosis. Blood. 2008;112:946–56. doi: 10.1182/blood-2007-11-078097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galli SJ, Tsai M, Piliponsky AM. The development of allergic inflammation. Nature. 2008;454:445–54. doi: 10.1038/nature07204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okayama Y, Saito H, Ra C. Targeting human mast cells expressing g-protein-coupled receptors in allergic diseases. Allergol Int. 2008;57:197–203. doi: 10.2332/allergolint.R-08-163. [DOI] [PubMed] [Google Scholar]
- 5.Gilfillan AM, Peavy RD, Metcalfe DD. Amplification mechanisms for the enhancement of antigen-mediated mast cell activation. Immunol Res. 2009;43:15–24. doi: 10.1007/s12026-008-8046-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gao ZG, Ding Y, Jacobson KA. UDP-glucose acting at P2Y14 receptors is a mediator of mast cell degranulation. Biochem Pharmacol. 2010;79:873–9. doi: 10.1016/j.bcp.2009.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bulanova E, Bulfone-Paus S. P2 receptor-mediated signaling in mast cell biology. Purinergic Signal. 2010;6:3–17. doi: 10.1007/s11302-009-9173-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Virgilio F, Chiozzi P, Ferrari D, Falzoni S, Sanz JM, Morelli A, Torboli M, Bolognesi G, Baricordi OR. Nucleotide receptors: an emerging family of regulatory molecules in blood cells. Blood. 2001;97:587–600. doi: 10.1182/blood.v97.3.587. [DOI] [PubMed] [Google Scholar]
- 9.Jacobson KA, Boeynaems JM. P2Y nucleotide receptors: Promise of therapeutic applications. Drug Disc Today. 2010;15:570–578. doi: 10.1016/j.drudis.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee YH, Lee SJ, Seo MH, Kim CJ, Sim SS. ATP-induced histamine release is in part related to phospholipase A2-mediated arachidonic acid metabolism in rat peritoneal mast cells. Arch Pharm Res. 2001;24:552–6. doi: 10.1007/BF02975164. [DOI] [PubMed] [Google Scholar]
- 11.Feng C, Mery AG, Beller EM, Favot C, Boyce JA. Adenine nucleotides inhibit cytokine generation by human mast cells through a Gs-coupled receptor. J Immunol. 2004;173:7539–47. doi: 10.4049/jimmunol.173.12.7539. [DOI] [PubMed] [Google Scholar]
- 12.McCloskey MA, Qian YX. Selective expression of potassium channels during mast cell differentiation. J Biol Chem. 1994;269:14813–9. [PubMed] [Google Scholar]
- 13.Communi D, Gonzalez NS, Detheux M, Brézillon S, Lannoy V, Parmentier M, Boeynaems JM. Identification of a novel human ADP receptor coupled to Gi. J Biol Chem. 2001;276:41479–85. doi: 10.1074/jbc.M105912200. [DOI] [PubMed] [Google Scholar]
- 14.Fumagalli M, Trincavelli L, Lecca D, Martini C, Ciana P, Abbracchio MP. Cloning, pharmacological characterisation and distribution of the rat G-protein-coupled P2Y13 receptor. Biochem Pharmacol. 2004;68:113–24. doi: 10.1016/j.bcp.2004.02.038. [DOI] [PubMed] [Google Scholar]
- 15.Kim YC, Lee JS, Sak K, Marteau F, Mamedova L, Boeynaems JM, Jacobson KA. Synthesis of pyridoxal phosphate derivatives with antagonist activity at the P2Y13 receptor. Biochem Pharmacol. 2005;70:266–74. doi: 10.1016/j.bcp.2005.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 17.Chhatriwala M, Ravi RG, Patel RI, Boyer JL, Jacobson KA, Harden TK. Induction of novel agonist selectivity for the ADP-activated P2Y1 receptor versus the ADP-activated P2Y12 and P2Y13 receptors by conformational constraint of an ADP analog. J Pharmacol Exp Ther. 2004;311:1038–43. doi: 10.1124/jpet.104.068650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boyer J, Adams M, Ravi RG, Jacobson KA, Harden TK. 2-Chloro-N6-methyl-(N)-methanocarba-2′-deoxyadenosine-3′,5′-bisphosphate is a selective high affinity P2Y1 receptor antagonist. Br J Pharmacol. 2002;135:2004–10. doi: 10.1038/sj.bjp.0704673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schulman ES, Glaum MC, Post T, Wang Y, Raible DG, Mohanty J, Butterfield JH, Pelleg A. ATP modulates anti-IgE-induced release of histamine from human lung mast cells. Am J Respir Cell Mol Biol. 1999;20:530–7. doi: 10.1165/ajrcmb.20.3.3387. [DOI] [PubMed] [Google Scholar]
- 20.Sudo N, Tanaka K, Koga Y, Okumura Y, Kubo C, Nomoto K. Extracellular ATP activates mast cells via a mechanism that is different from the activation induced by the cross-linking of Fc receptors. J Immunol. 1996;156:3970–9. [PubMed] [Google Scholar]
- 21.Paruchuri S, Tashimo H, Feng C, Maekawa A, Xing W, Jiang Y, Kanaoka Y, Conley P, Boyce JA. Leukotriene E4-induced pulmonary inflammation is mediated by the P2Y12 receptor. J Exp Med. 2009;206:2543–55. doi: 10.1084/jem.20091240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alfonso A, Cabado AG, Vieytes MR, Botana LM. Calcium-pH crosstalks in rat mast cells: cytosolic alkalinization, but not intracellular calcium release, is a sufficient signal for degranulation. Br J Pharmacol. 2000;130:1809–16. doi: 10.1038/sj.bjp.0703490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suzuki Y, Yoshimaru T, Inoue T, Ra C. Mitochondrial Ca2+ flux is a critical determinant of the Ca2+ dependence of mast cell degranulation. J Leukoc Biol. 2006;79:508–18. doi: 10.1189/jlb.0705412. [DOI] [PubMed] [Google Scholar]
- 24.Melendez AJ, Khaw AK. Dichotomy of Ca2+ signals triggered by different phospholipid pathways in antigen stimulation of human mast cells. J Biol Chem. 2002;277:17255–62. doi: 10.1074/jbc.M110944200. [DOI] [PubMed] [Google Scholar]
- 25.Lee HS, Park CS, Lee YM, Suk HY, Clemons TC, Choi OH. Antigen-induced Ca2+ mobilization in RBL-2H3 cells: role of I(1,4,5)P3 and S1P and necessity of I(1,4,5)P3 production. Cell Calcium. 2005;38:581–92. doi: 10.1016/j.ceca.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 26.Bohnacker T, Marone R, Collmann E, Calvez R, Hirsch E, Wymann MP. PI3Kgamma adaptor subunits define coupling to degranulation and cell motility by distinct PtdIns(3,4,5)P3 pools in mast cells. Sci Signal. 2009;2:ra27. doi: 10.1126/scisignal.2000259. [DOI] [PubMed] [Google Scholar]
- 27.Nunomura S, Gon Y, Yoshimaru T, Kashiwakura J, Kawakami T, Ra C. FcepsilonRI beta-chain ITAM amplifies PI3K-signaling to ensure synergistic degranulation response via FcepsilonRI and adenosine receptors. Eur J Immunol. 2010;40:1205–17. doi: 10.1002/eji.200939651. [DOI] [PubMed] [Google Scholar]
- 28.Malin SA, Molliver DC. Gi- and Gq-coupled ADP (P2Y) receptors act in opposition to modulate nociceptive signaling and inflammatory pain behavior. Mol Pain. 2010;6:21. doi: 10.1186/1744-8069-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gao ZG, Hechler B, Besada P, Gachet C, Jacobson KA. Caged agonist of P2Y1 and P2Y12 receptors for light-directed facilitation of platelet aggregation. Biochem Pharmacol. 2008;75:1341–7. doi: 10.1016/j.bcp.2007.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]