Abstract
Light chain amyloidosis (AL) is a plasma cell dyscrasia associated with production of amyloidogenic immunoglobulin light chains (LC). Despite its often fatal course, the mechanism of injury remains unknown. We tested the hypothesis that AL is associated with oxidative stress by comparing serum protein carbonyl (a marker of protein oxidation and oxidative stress) in AL subjects (n=23, 60±11 years) vs. controls (n=9, 54± 2 years); we also measured superoxide production (n=11) and dilator response to sodium nitroprusside (SNP, n=6) in isolated non-AL human adipose arterioles exposed to LC (20 μg/mL) purified from AL subjects for 1 h vs. control. Protein carbonyl was higher in AL patients (0.19±0.04 vs. 0.003±0.003 nmol/mg control, p=0.002). Post-exposure to LC proteins, arteriole superoxide was higher (1.89±0.36 times control, p=0.03) with impaired dilation to SNP (10−4 M, 54±6 vs. 86±4%, p=0.01, logEC50 −3.7±0.2 vs. −6.7±0.6, p=0.002). AL is associated with systemic oxidative stress and brief acute exposure to AL light chain proteins induces oxidative stress and microvascular dysfunction in human adipose arterioles. This novel mechanism of injury may be important in AL pathophysiology.
Keywords: Amyloid, Heart failure, Microvascular dysfunction, Oxidative stress, Reactive oxygen species, Cardiomyopathy
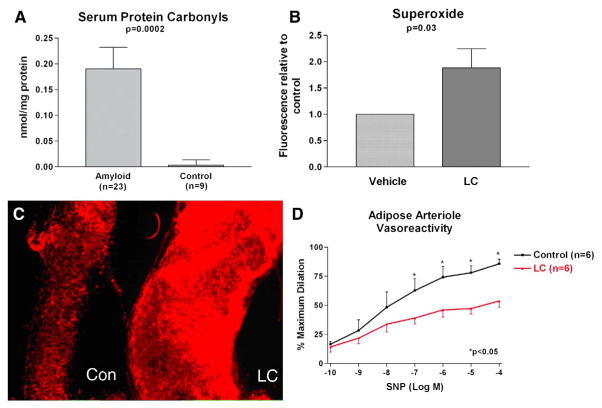
The mechanism underlying multiorgan dysfunction from light chain amyloidosis (AL) is unknown but exposure of rodent tissue to light chain proteins (LC) purified from AL patients results in oxidative stress [1,2]. We tested the hypothesis that AL is associated with systemic protein oxidation and that acute exposure to LC induces human arteriolar oxidative stress and microvascular dysfunction. First, we compared serum protein carbonyl (marker of protein oxidation/oxidative stress [3]) in 23 AL patients (all biopsy-proven AL, 60±11 years, 10 males) to those of 9 healthy subjects (54±2 years, 5 males) by dinitrophenylhydrazine ELISA (Biocell Corp., Papatoetoe, NZ) and found higher levels in AL (Fig. 1A). From 3 AL patients, we isolated and purified urine LC using dialysis, size-specific filtration (Amikon spin columns 50/30/5KD) and lyophilization. They were verified as kappa LC by Western blotting (anti-human kappa LC, Sigma, St. Louis MO). Arterioles from non-AL human adipose tissue without diabetes/known vascular disease (52±4 years) discarded during routine thoracoabdominal surgery were isolated and exposed to 20 μg/mL of LC (usual serum concentration in AL) or vehicle for 1 h (n=11) and superoxide production was measured following addition of 5 μM dihydroethidium (Molecular Probes, Eugene OR) by peak fluorescence microscopy (excitation 488/emission 620 nM). Superoxide was increased in LC treated arterioles (1.89±0.36 times control, p=0.03, Fig. 1B–C). An additional six human adipose arterioles were cannulated and pressurized to 60 mm Hg (~physiologic pressure in 100 μM arteriole) as previously described [4] and vessel diameter was measured using videomicrometer (Boeckeler VIA-100). Each arteriole was constricted ~40% with endothelin-1 (10−9–10−8 M). Baseline (vehicle) control dilation was measured following exposure to sodium nitroprusside (SNP, 10−10–10−4 M, endothelium-independent dilation). The chamber was then washed with fresh buffer and the arteriole was exposed intraluminally to 20 μg/mL LC (1 h) and after constriction with the same dose of endothelin-1, a second dilator response to SNP was measured. Dilation was impaired following exposure to LC (SNP dose logEC50 −3.7±0.2 vs. −6.7±0.6, p=0.002, Fig. 1D). Subjects who provided blood/urine samples gave informed consent while discarded/deidentified adipose tissues were obtained with waived consent with approval by the local Institutional Review Board.
Fig. 1.
A. Serum protein carbonyl was higher in AL amyloid subjects compared to healthy controls. B. Isolated adipose arterioles exposed to AL light chains (LC) show significantly higher superoxide production than vehicle controls. C. Representative arterioles exposed to LC and vehicle showing increased fluorescence and superoxide with LC treatment. D. There is reduced dilation to sodium nitroprusside (SNP) in isolated adipose arterioles exposed to LC.
We report three novel findings. One, there is evidence of increased systemic oxidative stress in AL compared to healthy subjects with increased circulating oxidized proteins. Second, acute brief exposure of human microvessels to LC increases formation of reactive oxygen species (ROS). Third, brief presence of LC impairs vascular smooth muscle relaxation to a nitric oxide donor. Our results suggest that circulating LC may play a role in disease pathogenesis.
Our human study is consistent with findings of acute LC toxicity in animal tissue. Rat cardiomyocytes exposed to LC develop impaired contractility with abnormal intracellular calcium handling and increased ROS production [1]. Mouse hearts similarly develop impaired relaxation [2]. Clinical observations support the notion that circulating LC may be tissue-toxic. Concomitant with chemotherapy-induced decline in circulating LC, clinico-biochemical markers of tissue dysfunction improved significantly [5,6] despite persistent/increased amyloid deposits on biopsy [7]. Oxidative stress as a mechanism of injury is also supported by findings in colon biopsies in AL showing that LC deposits in the perivascular wall/stroma were associated with oxidized proteins and toxic lipid peroxidation products [8]. This is the first report that AL LC causes microvascular oxidative stress and vasomotor impairment. These findings warrant further mechanistic investigations on the pathobiology of this often fatal disease and may suggest a novel therapeutic approach targeted at improving vascular function.
Acknowledgments
Funding was provided by the Amyloidosis Research Foundation, American Heart Association GIA 0855683G, Greater Milwaukee Foundation and American Cancer Society. We thank Jayashree Narayanan, Vi Nguyen-Liu, Paulette Jacobs, April Wittenburg and Rita Ellerbrook (Helena Laboratories) for their assistance. The authors of the manuscript have certified that they comply with the Principles of Ethical Publishing in the International Journal of Cardiology [9].
References
- 1.Brenner DA, Jain M, Pimentel DR, Wang B, Connors LH, Skinner M, et al. Human amyloidogenic light chains directly impair cardiomyocyte function through an increase in cellular oxidant stress. Circ Res. 2004 Apr 30;94(8):1008–10. doi: 10.1161/01.RES.0000126569.75419.74. [DOI] [PubMed] [Google Scholar]
- 2.Liao R, Jain M, Teller P, Connors LH, Ngoy S, Skinner M, et al. Infusion of light chains from patients with cardiac amyloidosis causes diastolic dysfunction in isolated mouse hearts. Circulation. 2001 Oct 2;104(14):1594–7. [PubMed] [Google Scholar]
- 3.Dalle-Donne I, Rossi R, Giustarini D, Milzani A, Colombo R. Protein carbonyl groups as biomarkers of oxidative stress. Clinica Chimica Acta; International Journal of Clinical Chemistry. 2003 Mar;329(1–2):23–38. doi: 10.1016/s0009-8981(03)00003-2. [DOI] [PubMed] [Google Scholar]
- 4.Phillips SA, Hatoum OA, Gutterman DD. The mechanism of flow-induced dilation in human adipose arterioles involves hydrogen peroxide during cad. Am J Physiol Heart Circ Physiol. 2006 Oct 13; doi: 10.1152/ajpheart.00819.2006. [DOI] [PubMed] [Google Scholar]
- 5.Dubrey S, Mendes L, Skinner M, Falk RH. Resolution of heart failure in patients with AL amyloidosis. Ann Intern Med. 1996 Sep 15;125(6):481–4. doi: 10.7326/0003-4819-125-6-199609150-00009. [DOI] [PubMed] [Google Scholar]
- 6.Palladini G, Lavatelli F, Russo P, Perlini S, Perfetti V, Bosoni T, et al. Circulating amyloidogenic free light chains and serum N-terminal natriuretic peptide type B decrease simultaneously in association with improvement of survival in AL. Blood. 2006 May 15;107(10):3854–8. doi: 10.1182/blood-2005-11-4385. [DOI] [PubMed] [Google Scholar]
- 7.Kyle RA, Wagoner RD, Holley KE. Primary systemic amyloidosis: resolution of the nephrotic syndrome with melphalan and prednisone. Arch Intern Med. 1982 Aug;142(8):1445–7. doi: 10.1001/archinte.142.8.1445. [DOI] [PubMed] [Google Scholar]
- 8.Ando Y, Nyhlin N, Suhr O, Holmgren G, Uchida K, el Sahly M, et al. Oxidative stress is found in amyloid deposits in systemic amyloidosis. Biochem Biophys Res Commun. 1997 Mar 17;232(2):497–502. doi: 10.1006/bbrc.1996.5997. [DOI] [PubMed] [Google Scholar]
- 9.Coats AJ. Ethical authorship and publishing. Int J Cardiol. 2009 Jan 9;131(2):149–50. doi: 10.1016/j.ijcard.2008.11.048. [DOI] [PubMed] [Google Scholar]