Abstract
Sphingosine-1-phosphate (S1P) constricts cerebral arteries through S1P3 receptor stimulation. Because the activity of the key S1P-synthesizing enzyme, sphingosine kinase (SPK), can be stimulated by agonists of various G protein-coupled receptors, it is likely that S1P also acts as a second messenger for other vasoconstrictors. We investigated the effect of SPK inhibitors and SPK gene deletion on the contractile responses of isolated vessels to vasoactive agonists and KCl-induced depolarization. Basilar and femoral arteries of rat, mounted in a wire myograph, were incubated with dimethylsphingosine (DMS), 2-(p-hydroxyanilino)-4-(p-chlorophenyl) thiazole (Compound 2) or FTY720, and exposed to KCl, 5-hydroxytryptamine (5-HT), S1P or phenylephrine (PE). Vasomotor responses in basilar artery were decreased by DMS, Compound 2 and FTY720, while they were not affected in femoral artery. Basilar artery from SPK1−/− mice exhibited weaker vasoconstriction to both KCl and agonists (S1P and the prostanoid U46619) when compared to either wild type (WT) or SPK2−/−. In contrast, in mesenteric resistance arteries, neither the contraction to KCl nor the maximum contraction to PE and S1P significantly differed among WT, SPK1−/− and SPK2−/−. Quantitative analysis of SPK mRNA (reverse transcription and real time polymerase chain reaction) in mouse arteries showed 40–80-fold higher SPK1 expression in cerebral arteries than in aorta or mesenteric arteries. SPK1 critically modulates the reactivity of cerebral vasculature to vasoconstrictors. S1P plays a specific role as modulator of cerebral blood flow, potentially acting either directly outside vascular smooth muscle cells on S1P3 receptors, or indirectly after being generated inside the cell in response to vasoconstrictors.
Keywords: sphingosine kinase, vasoconstriction, basilar artery
1 Introduction
Sphingosine-1-phosphate (S1P) binds to G protein-coupled receptors and induces diverse cell responses [1]. In particular, S1P is an important signaling molecule in the cardiovascular system [2,3], where it may affect both cardiac [4] and vascular [5] physiology. We have shown that S1P induces robust vasoconstriction in cerebral arteries, in vitro and in vivo, by stimulating the S1P3 receptor subtype [6,7], but poorly constricts peripheral arteries [6]. S1P, generated within the cell by sphingosine kinases (SPK), may also signal as a second messenger [8,9] and may be involved in excitation-contraction coupling. Indeed, SPK1 activity increases following membrane depolarization and activation of voltage-gated Ca2+-channels [10], whereas SPK inhibitors block endothelin-1-induced myometrium contraction [11]. In isolated resistance arteries, resting tone and myogenic response are increased by overexpression and activity of SPK1, suggesting that SPK1 plays a role in translating mechanical forces into intracellular signals [12]. Interestingly, in endothelial cells, SPK inhibitors reduce NO production and thereby angiotensin II-induced endothelium-dependent vasodilatation [13].
The aim of the present study was to establish the role of SPK as effector of vasoconstrictor stimuli in different vascular beds. To do so, we first inhibited SPK activity pharmacologically in different arteries isolated from rat; we then used arteries from SPK null mice to discriminate the relative importance of the two SPK isoforms. On the basis of the effect of SPK inhibitors and of the vasomotor responses in arteries from SPK null mice, we conclude that SPK1 is the isoform that critically modulates the reactivity of cerebral vasculature to vasoconstrictors.
2 Materials and Methods
2.1 Animals
The investigation conforms with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996) and approved by the Institutional Animal Care and Use Committee. Rats were male Sprague-Dawley, weighing 250–350 g. SPK1−/− and SPK2−/− mice were generously provided by Dr. Richard Proia (National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD) [14,15]. These mice were bred and housed in our animal facility. Animals had free access to water and food. SPK1−/− and SPK2−/− have been back-crossed more than 10 times on a C57 black 6/j background; both wild-type (WT) littermates and commercial C57 black 6/j were used as controls. The genotype of each mouse was confirmed by polymerase chain reaction (PCR).
2.2 Myograph experiments
Rats or mice were euthanized by chloroform anesthesia followed by decapitation. The brain was removed and immersed in physiological solution (composition, mmol/L: NaCl, 118; KCl, 4.6; NaHCO3, 25; MgSO4, 1.2; KH2PO4, 1.2; CaCl2, 1.2; glucose, 10; EDTA, 0.025; pH 7.4 at 37 °C). Basilar and femoral arteries in rat, basilar and mesenteric resistance (third branch) arteries in mouse were dissected, cut into 1.5 – 2 mm long segments and threaded onto 40 µm stainless steel wires (rat) or 15 µm tungsten (mouse basilar) and 25 µm stainless steel wires (mouse mesenteric artery). Each segment was mounted in one of the four organ chambers of an isometric myograph (610M, Danish Myo Technology, Aarhus, Denmark). For mice, an entire basilar artery was mounted in each organ chamber. After mounting, each preparation was equilibrated, unstretched, for 30 min, in physiological solution, maintained at 37°C and aerated with a gas mixture of 95% O2 - 5% CO2. The normalized passive resting force and the corresponding diameter were then determined for each preparation from its own length-pressure curve, according to Mulvany and Halpern [16]. Responses were recorded by using a computerized data acquisition and recording software (Myodaq and Myodata, Danish Myo Technology). After normalization and 30-min equilibration in physiological solution, the preparations were stimulated with 100 mmol/L KCl isotonic depolarizing solution (composition, mmol/L: NaCl, 22.6; KCl, 100; NaHCO3, 25; MgSO4, 1.2; KH2PO4, 1.2; CaCl2, 1.2; glucose, 10; EDTA, 0.025, pH 7.4 at 37°C). After washout, rat vessels were incubated for 1 h with vehicle or putative sphingosine kinase (SPK) inhibitors (dimethylsphingosine, DMS; 2-(p-hydroxyanilino)-4-(p-chlorophenyl) thiazole, Compound 2; FTY720) and exposed again to 100 mmol/L KCl or cumulative concentrations of vasoconstrictor agonists: 5-hydroxytryptamine (5-HT), sphingosine-1-phosphate (S1P), phenylephrine (PE), or U46619 (a synthetic prostanoid). The first contraction induced by KCl in the absence of SPK inhibitor was taken as internal control (i.e. subsequent contractions in the presence of SPK inhibitor were expressed in % of the first one).
2.3 Pressurized posterior cerebral arteries
Adult male C57BL/6 mice were anesthetized using chloroform and killed by decapitation. The brain was removed and placed in a dissection dish filled with cold physiologic salt solution (composition, mM): NaCl, 141; KCl, 4.6; MgSO4, 1.7; EDTA, 0.51; CaCl2, 2.7; HEPES, 1.0; KH2PO4, 1.1; Glucose, 4.9; pH 7.4). Posterior cerebral arteries (PCA) were dissected free from connective tissue. A segment of the PCA was cannulated, pressurized and mounted in an arteriograph (Living Systems Instrumentation, Burlington, VT) that contained physiological solution (37°C, pH 7.4) for 30 min equilibration. The arterial diameter was recorded using the Video Dimension analysis system and transmural pressure was measured and controlled using a pressure servomechanism. Pressure was produced by a peristaltic pump linked to the cannula via silicone tubing and measured using an inline transducer (Living Systems Instrumentation), pressure was set at 60 mmHg for all experiments.
For experiments with caged S1P, posterior cerebral arteries from mice were mounted in the arteriograph as described above and pressurized to 10 mmHg. Caged S1P (caged D-erythro-sphingosine-1-phosphate, Alexis Biochemicals, Plymouth Meeting, PA) was loaded into vessels for 30 minutes, pressure was then raised to 60 mmHg. Vessels were washed 3 times immediately before the start of the experiment. After a 30 second equilibration, photolysis was performed by a 12 second UV pulse (using a 400DCLP dichroic mirror from Chroma, Brattleboro, VT). Vessel diameter was measured on a Nikon Eclipse TEi inverted epifluorescence microscope (Nikon, Melville, NY) equipped with a 10× objective. Images were acquired and analyzed using NIS elements (Nikon).
2.4 Drugs
DMS and S1P were from Avanti Polar Lipids Inc. (Alabaster, AL); DMS was solubilized in ethanol as 10 mmol/L stock solution; S1P was solubilized as 1 mmol/L stock solution in a buffer containing 100 mmol/L Tris pH 9.0, 145 mmol/L NaCl, 4 mg/ml fatty acid free bovine serum albumin, as previously described [7]. Compound 2 (Calbiochem, San Diego, CA) was dissolved as a 10 mmol/L stock solution in dimethylsulphoxide (DMSO). U46619 (Sigma-Aldrich, St. Louis, MO) was dissolved in ethanol as a 30 mmol/L stock solution. FTY720 was from Cayman Chemical (Ann Arbor, MI); it was dissolved in H2O as a 10 mmol/L stock solution; PE and 5-HT were from Sigma-Aldrich; they were dissolved as 10 mmol/L stock solution in H2O.
2.5 Analysis of Gene Expression by Real-Time PCR
Total RNA extraction of mouse tissues were performed using the RNAspin Mini kit (GE Healthcare, Piscataway, NJ) according to the manufacturer’s protocol. DNase treatment was performed to avoid DNA contamination of RNA samples. cDNA was synthesized from total RNA using Super-ScriptIII reverse transcriptase. Real-time PCR reactions were completed in triplicates using an ABI Prism 7000 from Applied Biosystems (Foster City, CA, USA) with 100 ng/mL cDNA aliquots as template, together with primers and probes (Applied Biosystems, Taqman) for SPK1 (Mm01252544_m1), SPK2 (Mm000445021_m1) and 18S ribosomal RNA (used to normalize mRNA expression). The gene expression data were analyzed using Qgene, a publicly available Excel script package [17].
2.6 Statistical analysis
In rat arteries, concentration-response curves to agonists carried out in the presence of vehicle (control) or SPK inhibitor were plotted as a percentage of the previous vasoconstriction induced by KCl in the same arteries in the absence of inhibitor against log mol/L concentration of drug. In mouse arteries, concentration-response curves to agonists were carried out in the absence of SPK inhibitors (or other treatments) and were therefore plotted as mN/mm against log molar concentration of drug. The use of KCl-induced vasoconstriction as internal control was applied to rat arteries, because the starting condition (absence of treatment, naïve preparations), when KCl challenge was carried out, was identical for any preparation; the same paradigm, however, could not be applied to mouse arteries, because in the starting condition, when KCl challenge was carried out, the “gene-effect” (i.e. the genotype that supposedly produced a vascular phenotype) was already present and was different in different groups; for this reason, contractile responses in mouse arteries were expressed as raw data, in mN/mm (rather than in % of KCl as in rat arteries). Each set of data points was curve-fitted by a non-linear regression, best-fit, sigmoidal dose-response curve with no constraints, with the use of GraphPad Prism (GraphPad Software, San Diego, CA). Pharmacological parameters (concentration producing 50% of maximum effect or EC50 and maximum effect or Emax) were calculated from these non-linear fits. Each curve represents preparations from at least 5 different animals. Whenever it was possible, arterial segments from the same animal were represented in the different experimental conditions. For example, we typically cut one basilar artery of rat in four segments, one was incubated with vehicle (control), the other three with DMS 5 µmol/L, 10 µmol/L and 20 µmol/L). Whole curves were compared by two-way analysis of variance (ANOVA). Contractions induced by KCl and PCR data were compared by one-way ANOVA followed by Tukey’s test, with significance set at p<0.05.
3 Results
3.1 Effect of structurally unrelated sphingosine kinase inhibitors on constriction of isolated arteries
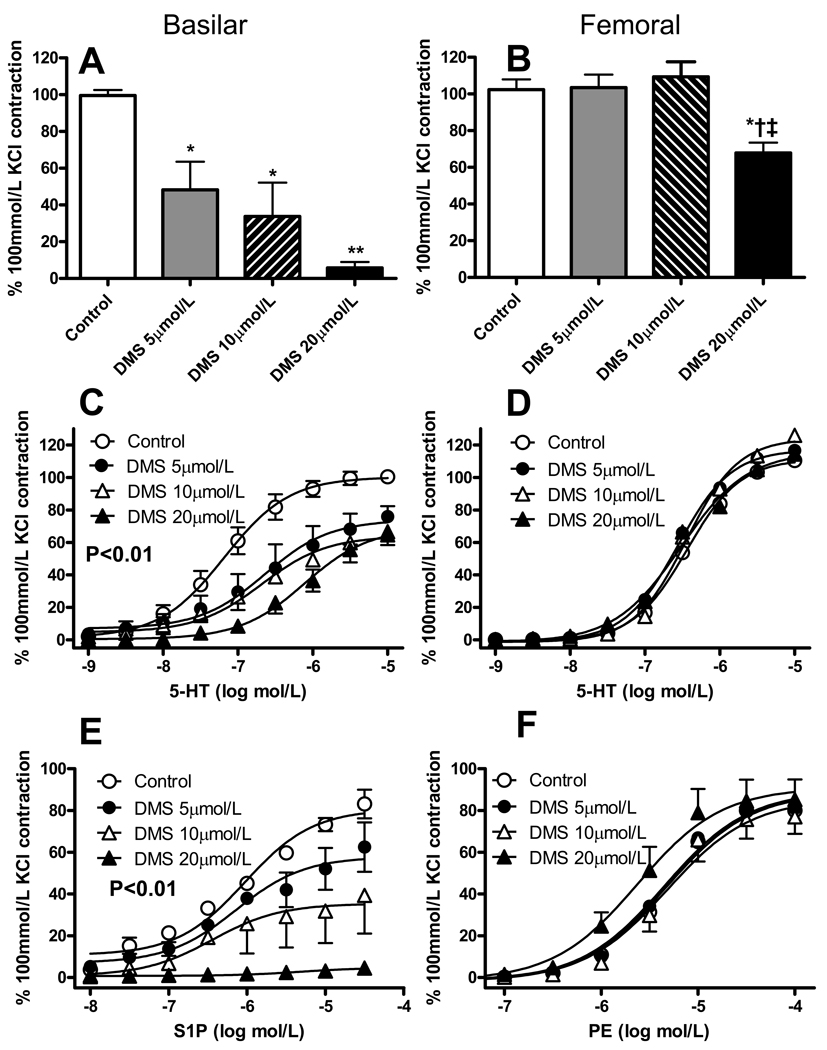
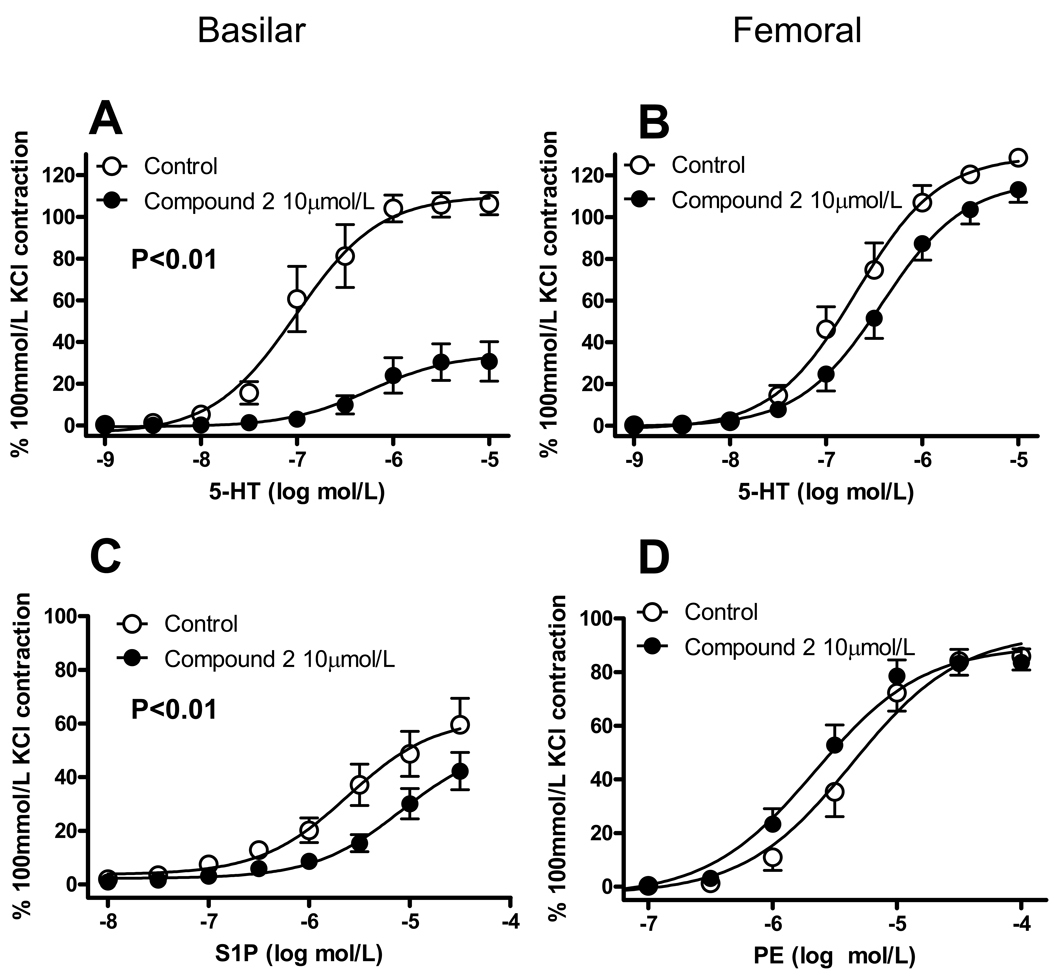
To investigate the role of SPK in vasomotor responses, we first exposed isolated segments of basilar or femoral artery of rat to a depolarizing 100 mmol/L KCl solution; this first contraction obtained in the absence of SPK inhibition was taken as internal control (i.e. subsequent contractions induced after SPK inhibition were expressed in % of this first one). The preparations were then incubated for 60 min in the presence of the SPK inhibitors DMS and Compound 2 (Fig. 1), before being challenged again with KCl or with vasoconstrictor agonists (5-HT, S1P, PE). DMS does not discriminate between SPK1 and SPK2 [18]; Compound 2 is a selective SPK inhibitor (IC50 0.5 µmol/L, while it does affect ERK2, PKC-α and PI3K when tested up to 50 µmol/L), but no information is available about its relative selectivity for the two SPK isoforms [19]. DMS and Compound 2 did not the basal tone of the artery (not shown). We tested KCl and 5-HT in both arteries, S1P only in basilar and PE only in femoral artery, because, as previously reported, basilar artery poorly constricts to α-adrenergic agonists, while femoral artery poorly constricts to S1P [6]. As shown in Fig. 2, DMS concentration-dependently inhibited vasoconstriction induced by KCl, 5-HT or S1P in basilar artery, but barely altered vasoconstriction to KCl, 5-HT or PE in femoral artery (at the highest concentration tested, 20 µmol/L DMS inhibited KCl-induced contraction by about 30%). Similarly, Compound 2 (10 µmol/L) significantly inhibited vasoconstriction to 5-HT or S1P in basilar artery, but affected only slightly vasoconstriction to 5-HT or PE in femoral artery (Fig. 3). The inhibition of basilar artery contraction to agonists by either DMS or Compound 2 generally produced a significant reduction of the maximum effect (Emax), barely affecting the concentration of agonist producing 50% of maximum effect (EC50; Tab. 1 and 2).
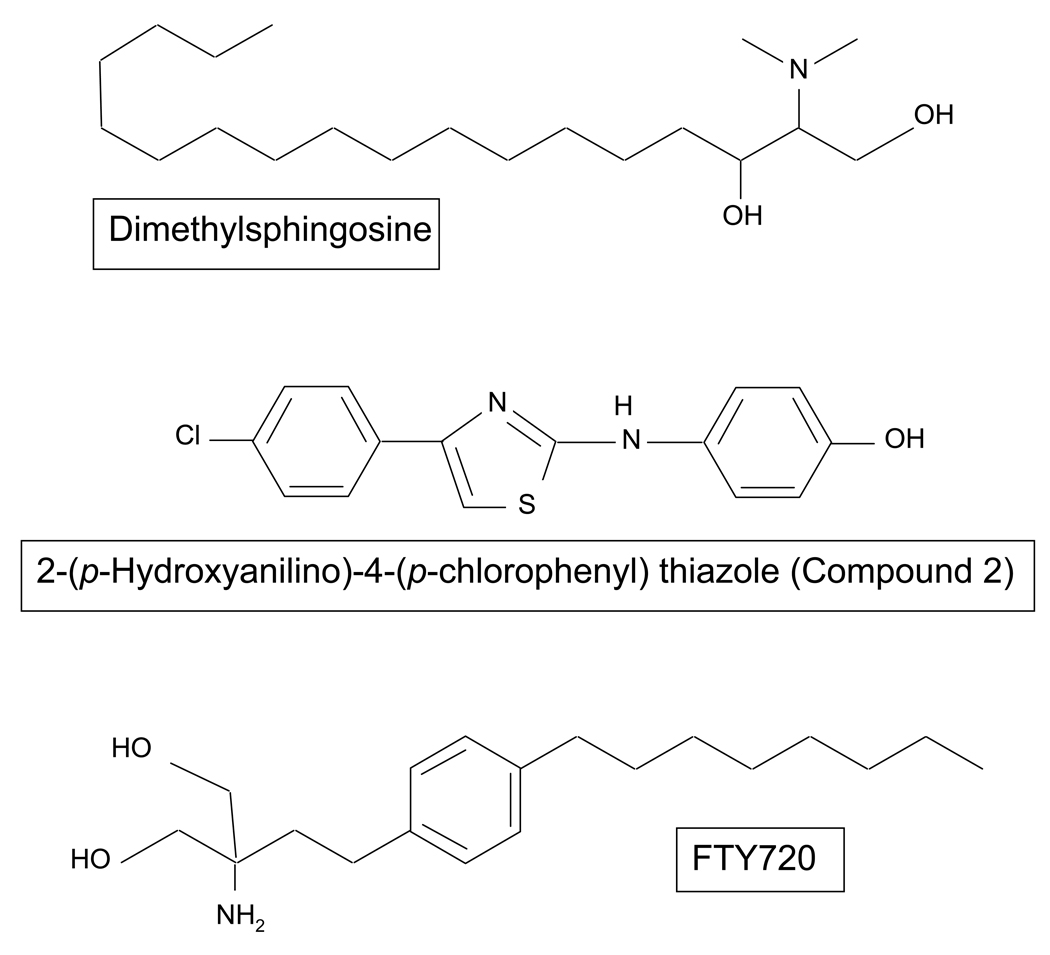
Figure 1.
Chemical structure of the three putative sphingosine kinase inhibitors used in the present study.
Figure 2.
Effect of dimethylsphingosine (DMS) on vasomotor responses of isolated vessels. Contractile responses of basilar and femoral arteries of rat to either 100 mmol/L KCl (A, B) or cumulative concentrations of 5-hydroxytryptamine (5-HT; C, D), sphingosine-1-phosphate (S1P, E) or phenylephrine (PE, F) following 1h incubation with vehicle (ethanol, 0.1%), 5, 10 or 20 µmol/L DMS. Contractile responses are normalized to vasoconstriction induced by exposure to 100 mmol/L KCl in the same preparation before DMS-treatment. Data are mean ± SEM of 5 independent measurements.
In A and B *P<0.05, **P<0.01 versus Control, † P<0.05 vs. DMS 5 µmol/L, ‡ vs. DMS 10 µmol/L; one-way ANOVA and Tukey test. In B and E, P<0.01 DMS vs. control and DMS 20 µmol/L vs. DMS 5 µmol/L; two-way ANOVA.
Figure 3.
Effect of 2-(p-hydroxyanilino)-4-(p-chlorophenyl) thiazole (Compound 2) on vasomotor responses of isolated vessels. Basilar and femoral arteries of rat were challenged with cumulative concentrations of 5-hydroxytryptamine (5-HT; A, B), sphingosine-1-phosphate (S1P, C) or phenylephrine (PE, D) following 1h incubation with vehicle (dimethylsulfoxide, 0.1%) or 10 µmol/L Compound 2. Contractile responses are normalized to vasoconstriction induced by exposure to 100 mmol/L KCl in the same preparation before treatment with Compound 2. Data are mean ± SEM of 7–12 independent measurements. In A and C P<0.01; two-way ANOVA.
Table 1.
Effect of DMS on vasomotor responses to 5-HT, S1P and PE in basilar and femoral arteries of rat: pharmacological parameters.
| pD2 | EC50 (µmol/L) | Emax (% K+) | |
|---|---|---|---|
| 5-HT | |||
| Basilar | |||
| Control | 7.18 ± 0.09 | 0.07 (0.04–0.10) | 100.3 ± 3.2 |
| DMS 5 µmol/L | 6.64 ± 0.22 | 0.22 (0.08–0.64) | 73.9 ± 6.6** |
| DMS 10 µmol/L | 6.68 ± 0.39 | 0.21 (0.03–1.26) | 64.0 ± 10.0** |
| DMS 20 µmol/L | 6.11 ± 0.13** | 0.77 (0.43–1.40) | 69.0 ± 4.9** |
| Femoral | |||
| Control | 6.44 ± 0.11 | 0.36 (0.22–0.60) | 111.4 ± 8.3 |
| DMS 5 µmol/L | 6.56 ± 0.11 | 0.27 (0.17–0.46) | 117.2 ± 8.2 |
| DMS 10 µmol/L | 6.45 ± 0.08 | 0.36 (0.24–0.52) | 124.1 ± 7.1 |
| DMS 20 µmol/L | 6.52 ± 0.10 | 0.30 (0.19–0.48) | 115.9 ± 7.3 |
| S1P | |||
| Basilar | |||
| Control | 6.03 ± 0.11 | 0.94 (0.57–1.55) | 80.7 ± 3.3 |
| DMS 5 µmol/L | 6.14 ± 0.26 | 0.73 (0.21–2.47) | 57.8 ± 5.6** |
| DMS 10 µmol/L | 6.44 ± 0.60 | 0.36 (0.02–5.97) | 35.4 ± 7.5** |
| DMS 20 µmol/L | 5.30 ± 0.72 | 5.04 (0.18–1000) | 4.8 ± 2.0** |
| PE | |||
| Femoral | |||
| Control | 5.32 ± 0.13 | 4.77 (2.59–8.77) | 88.8 ± 6.3 |
| DMS 5 µmol/L | 5.35 ± 0.11 | 4.50 (2.70–7.53) | 89.5 ± 5.3 |
| DMS 10 µmol/L | 5.32 ± 0.11 | 4.78 (2.90–7.89) | 85.7 ± 5.0 |
| DMS 20 µmol/L | 5.63 ± 0.11 | 2.37 (1.40–4.02) | 91.0 ± 5.0 |
Pharmacological parameters are from non linear regression; pD2 is the negative logarithm of the concentration producing 50% of the maximum effect, EC50 is the concentration producing 50% of the maximum effect (95% confidence limits are given in parenthesis), Emax is the maximum effect (in % of vasoconstriction evoked in the same preparations by 100 mmol/L K+).
Data are mean ± SEM of 5–12 independent measurements. Statistical comparisons were made on the whole curves by two-way ANOVA;
P<0.01 versus Control.
Table 2.
Effect of 10 µmol/L Compound 2 on vasomotor responses to 5-HT, S1P and PE in basilar and femoral arteries of rat: pharmacological parameters.
| pD2 | EC50 (µmol/L) | Emax (% K+) | |
|---|---|---|---|
| 5-HT | |||
| Basilar | |||
| Control | 7.01 ± 0.12 | 0.10 (0.06–0.17) | 110.1 ± 5.2 |
| Compound 2 | 6.24 ± 0.29 | 0.57 (0.16–2.10) | 34.4 ± 5.3** |
| Femoral | |||
| Control | 6.69 ± 0.09 | 0.20 (0.14–0.30) | 129.4 ± 4.9 |
| Compound 2 | 6.42 ± 0.09 | 0.38 (0.25–0.57) | 117.4 ± 5.2 |
| S1P | |||
| Basilar | |||
| Control | 5.62 ± 0.19 | 2.41 (0.99–5.89) | 62.2 ± 6.2 |
| Compound 2 | 5.09 ± 0.19 | 8.14 (3.42–19.4) | 52.5 ± 7.4 |
| PE | |||
| Femoral | |||
| Control | 5.36 ± 0.09 | 4.36 (2.86–6.64) | 94.6 ± 4.7 |
| Compound 2 | 5.66 ± 0.08 | 2.21 (1.50–3.24) | 89.7 ± 3.5 |
Pharmacological parameters are from non linear regression; pD2 is the negative logarithm of the concentration producing 50% of the maximum effect, EC50 is the concentration producing 50% of the maximum effect (95% confidence limits are given in parenthesis), Emax is the maximum effect (in % of vasoconstriction evoked in the same preparations by 100 mmol/L K+).
Data are mean ± SEM of 5–12 independent measurements. Statistical comparisons were made on the whole curves by two-way ANOVA;
P<0.01 versus Control.
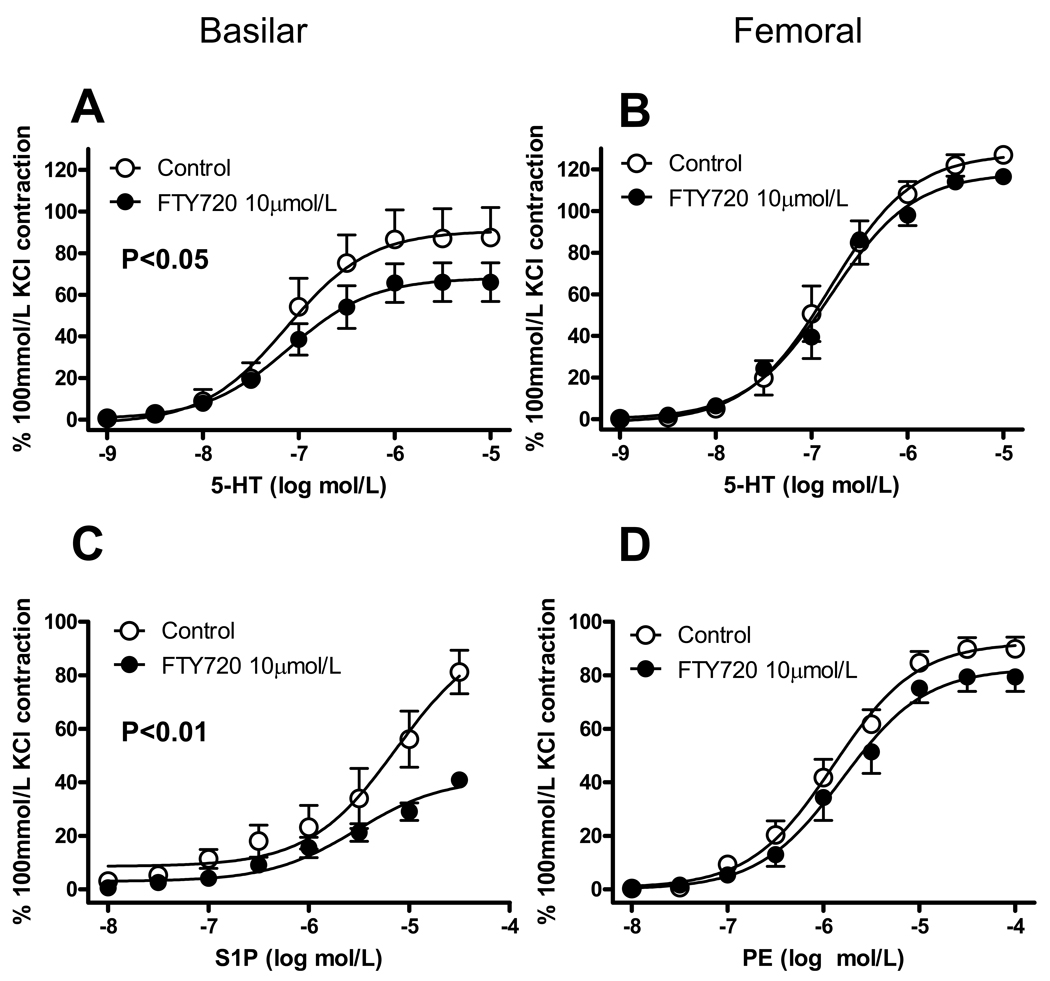
FTY720 has been reported to inhibit SPK1 but not SPK2 [20,21]. In our previous experiments with rat basilar artery, however, the effect of 30-min incubation with 10 µmol/L FTY720 on the tone induced by either 5-HT or high KCl did not reach statistical significance [7]. Therefore, in the present study, we decided to test a longer-lasting FTY incubation, i.e. 60 min (as for DMS and Compound 2). As observed with DMS and Compound 2, 10 µmol/L FTY720 significantly decreased 5-HT- and S1P-induced vasoconstriction in basilar artery, but did not affect vasoconstriction to 5-HT and PE in femoral artery (Fig. 4, Tab. 3).
Figure 4.
Effect of FTY720 on vasomotor responses of isolated vessels. Basilar and femoral arteries of rat were challenged with cumulative concentrations of 5-hydroxytryptamine (5-HT; A, B), sphingosine-1-phosphate (S1P, C) or phenylephrine (PE, D) following 1h incubation with vehicle (H2O) or 10 µmol/L FTY720. Contractile responses are normalized to vasoconstriction induced by exposure to 100 mmol/L KCl in the same preparation before FTY720-treatment. Data are mean ± SEM of 5 independent measurements. In A and C P<0.05 and P<0.01, respectively; two-way ANOVA.
Table 3.
Effect of 10 µmol/L FTY720 on vasomotor responses to 5-HT, S1P and PE in basilar and femoral arteries of rat: pharmacological parameters.
| pD2 | EC50 (µmol/L) | Emax (% K+) | |
|---|---|---|---|
| 5-HT | |||
| Basilar | |||
| Control | 7.13 ± 0.19 | 0.07 (0.03–0.18) | 90.8 ± 6.4 |
| FTY720 | 7.10 ± 0.17 | 0.08 (0.08–0.64) | 68.1 ± 4.1* |
| Femoral | |||
| Control | 6.81 ± 0.09 | 0.16 (0.14–0.23) | 127.4 ± 4.7 |
| FTY720 | 6.81 ± 0.09 | 0.16 (0.10–0.24) | 118.4 ± 4.6 |
| S1P | |||
| Basilar | |||
| Control | 5.13 ± 0.21 | 7.41 (2.72–20.2) | 96.4 ± 14.1 |
| FTY720 | 5.52 ± 0.14 | 3.01 (1.57–5.77) | 41.6 ± 3.1** |
| PE | |||
| Femoral | |||
| Control | 5.90 ± 0.08 | 1.27 (0.89–1.82) | 92.3 ± 2.8 |
| FTY720 | 5.80 ± 0.10 | 1.57 (0.96–2.56) | 82.7 ± 3.6 |
Pharmacological parameters are from non linear regression; pD2 is the negative logarithm of the concentration producing 50% of the maximum effect, EC50 is the concentration producing 50% of maximum the effect (95% confidence limits are given in parenthesis), Emax is the maximum effect (in % of vasoconstriction evoked in the same preparations by 100 mmol/L K+).
Data are mean ± SEM of 5–12 independent measurements. Statistical comparisons were made on the whole curves by two-way ANOVA;
P<0.05
P<0.01 versus Control.
Because S1P generated by SPK has been proposed to act extracellularly on membrane S1P receptors [22], and we have shown that S1P3 receptors mediate vasoconstriction of cerebral arteries to exogenous S1P [7], we tested the vasoconstriction to the prostanoid U46619 in cerebral arteries from S1P3 receptor knockout mice subjected to SPK inhibition. In perfused posterior communicating arteries from S1P3 null mice, vasoconstriction to 10 µmol/L U46619 was 102±13% of that induced by 100 mM KCl, while after 30 min incubation with 10 µmol/L DMS it was reduced to 6±3% of KCl-induced constriction (n=4, P<0.01).
3.2 Analysis of vasoconstriction of arteries isolated from SPK1−/− and SPK2−/− mice
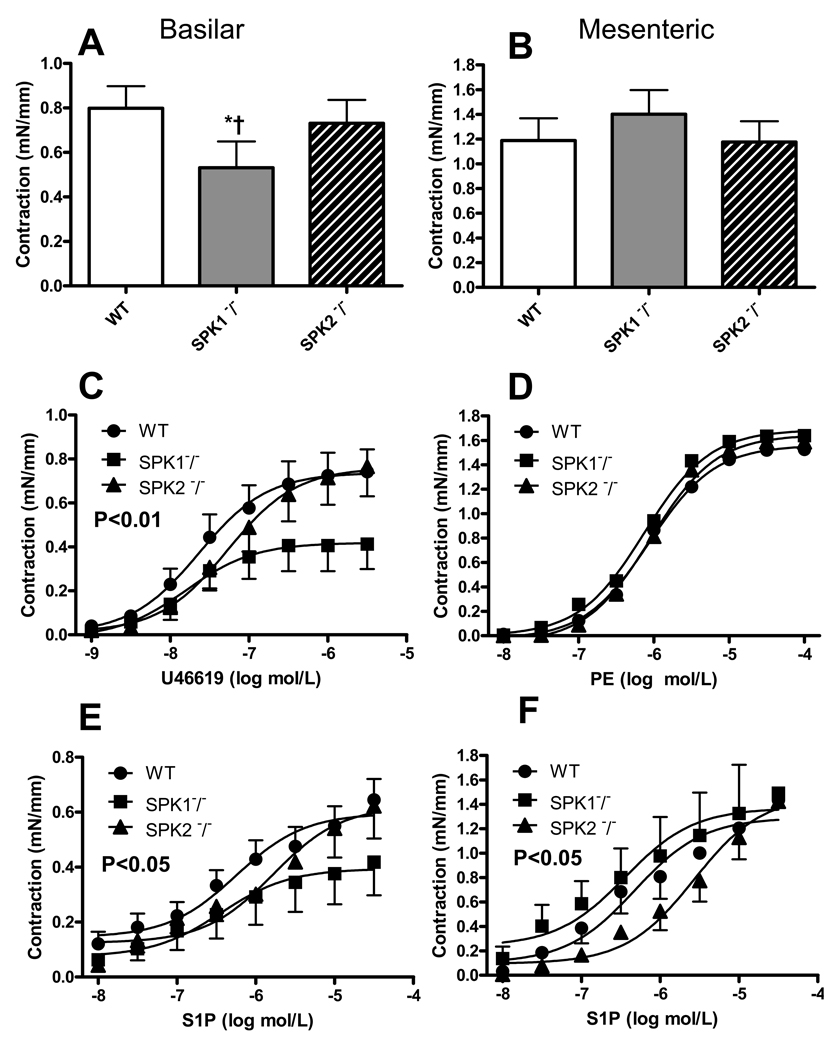
We have investigated the effect of different constrictor stimuli in basilar and third branch mesenteric arteries of SPK1−/− and SPK2−/− mice. The normalized diameter of these vessels did not show significant genotype-related differences (not shown). Contractile force was analyzed and compared, among groups, as raw data (mN/mm, see Material and Methods). Basilar arteries isolated from SPK1−/− showed weaker contractions to either KCl or agonists (U46619 and S1P) compared to WT and SPK2−/− (Fig. 5 and Tab. 4). In particular, in basilar arteries of SPK1−/−, the contractile response to KCl as well as Emax to U46619 and S1P were reduced by 34%–44% (P<0.01), without significant changes in the EC50 values. In contrast, in mesenteric arteries, neither the contractile response to KCl nor Emax to PE and S1P significantly differ among WT, SPK1−/− and SPK2−/−. In SPK2−/−, there was a general tendency toward a rightward shift of the concentration-contraction curves to agonists; however, when compared to WT and SPK1−/−, the increase in EC50 values in SPK2−/− did not reach statistical significance, except in mesenteric resistance arteries (P<0.05; Fig. 5 and Tab. 4).
Figure 5.
Effect of different constrictor stimuli in basilar and third branch mesenteric arteries of wild type (WT), SPK1−/− and SPK2−/− mice. Contractile responses were induced with 100 mmol/L KCl (A, B) or cumulative concentrations of the tromboxane analog U46619 (C), phenylephrine (PE, D) or sphingosine-1-phosphate (S1P, E, F). Notice that contractile force is expressed in mN/mm (instead of as % of KCl, see also Material and Methods). Data are mean ± SEM of 5–12 independent measurements. In A *P<0.05 versus WT, † P<0.05 versus SPK2−/− one-way ANOVA and Tukey test.
In C P<0.01 SPK1−/− versus control; in E P<0.05 SPK1−/− versus control; in F P<0.05 SPK2−/− versus control and SPK1−/− two-way ANOVA.
Table 4.
Pharmacological parameters of vasomotor responses to U46619, PE and S1P in arteries of WT, SPK1−/− and SPK2−/− mice.
| pD2 | EC50 (µmol/L) | Emax (mN/mm) | |
|---|---|---|---|
| U46619 | |||
| Basilar | |||
| WT | 7.64 ± 0.25 | 0.02 (0.01–0.07) | 0.74 ± 0.06 |
| SPK1−/− | 7.80 ± 0.38 | 0.02 (0.01–0.09) | 0.42 ± 0.05** |
| SPK2−/− | 7.27 ± 0.30 | 0.05 (0.01–0.21) | 0.76 ± 0.08 |
| PE | |||
| Mesenteric | |||
| WT | 6.06 ± 0.16 | 0.87 (0.41–1.84) | 1.56 ± 0.10 |
| SPK1−/− | 6.13 ± 0.26 | 0.75 (0.22–2.53) | 1.69 ± 0.16 |
| SPK2−/− | 6.05 ± 0.17 | 0.90 (0.41–1.96) | 1.65 ± 0.11 |
| S1P | |||
| Basilar | |||
| WT | 6.25 ± 0.25 | 0.56 (0.18–1.78) | 0.60 ± 0.05 |
| SPK1−/− | 6.44 ± 0.50 | 0.36 (0.04–3.63) | 0.39 ± 0.06* |
| SPK2−/− | 5.78 ± 0.29 | 1.65 (0.43–6.36) | 0.62 ± 0.07 |
| Mesenteric | |||
| WT | 6.32 ± 0.25 | 0.47 (0.15–1.47) | 1.29 ± 0.11 |
| SPK1−/− | 6.45 ± 0.46 | 0.36 (0.04–2.99) | 1.37 ± 0.19 |
| SPK2−/− | 5.58 ± 0.18* | 2.69 (1.18–6.14) | 1.48 ± 0.14 |
Pharmacological parameters are from non linear regression; pD2 is the negative logarithm of the concentration producing 50% of the maximum effect, EC50 is the concentration producing 50% of the maximum effect (95% confidence limits are given in parenthesis), Emax is the maximum effect.
Data are mean ± SEM of 5–12 independent measurements. Statistical comparisons were made on the whole curves by two-way ANOVA;
P<0.05
P<0.01 versus Control.
3.3 Effect of intracellular application of S1P (caged S1P) on the constriction of isolated mouse posterior cerebral arteries
Because measuring S1P concentrations and/or SPK activity in rodent cerebral arteries would require pooling a very large number of vessels, as an alternative strategy we used caged S1P to determine if SPK activation and the resulting intracellular S1P production could in fact induce vessel constriction. S1P was released inside the cell using a 12 second UV flash after loading caged S1P (10µM) for 30 minutes and washing immediately before the experiment. Constrictions are represented as a percentage of the constriction to KCl (100mM). Vessels loaded with caged S1P constricted to 51% of the maximum constriction obtained with KCl, while controls (exposed to BSA instead of caged S1P) only constricted to 15% of the KCl value (Fig. 6). Arteries from S1P2−/− mice also constricted to caged S1P, but arteries from S1P3−/− mice showed a constriction similar to that of the control (BSA, 1mg/ml).
Figure 6.
Caged sphingosine-1-phosphate was used to determine whether intracellular S1P generation would induce vessel constriction. S1P was released inside the cell using a 12 second UV flash after loading caged S1P (10µM) for 30 minutes and washing immediately before the experiment. Constrictions are represented as a percentage of the constriction to KCl (100mM). Vessels were loaded with caged S1P constricted to 51% of the maximum constriction obtained with KCl, while controls loaded with vehicle only (BSA, 1 mg/ml) only constricted to 15% of the KCl value. Vessels from S1P2−/− mice also constricted to caged S1P, but the vessels from S1P3−/− mice showed a similar constriction to that of the BSA control.
3.4 Quantitative analysis of relative mRNA expression of SPK1 and SPK2 in different vascular beds
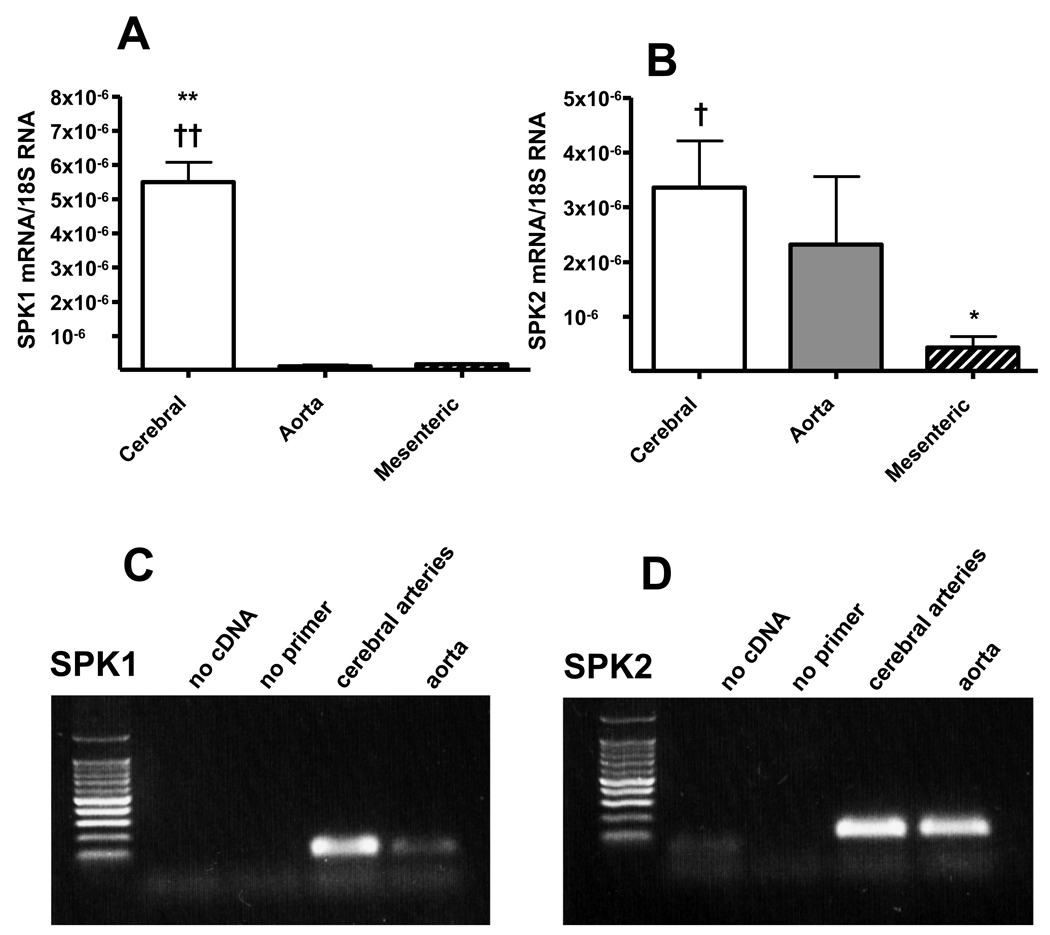
mRNA isolated from mouse arteries was submitted to reverse transcription and quantitative real-time PCR to assess the expression levels of the two SPK isoforms. As shown in Fig. 7, SPK1 mRNA expression was much higher (about 40–80-fold) in cerebral arteries than in aorta or mesenteric arteries; in contrast, SPK2 expression was similar in cerebral arteries and aorta, but lower (4–8-fold) in mesenteric artery.
Figure 7.
SPK1 (A) and SPK2 (B) mRNA expression in rat arteries detected by real-time PCR. “Cerebral” refers to Circle of Willis and proximal middle cerebral artery, “aorta” to thoracic aorta and “mesenteric” to superior mesenteric artery and secondary branches. Aortic and cerebral vessels were taken from 6 animals. Cycle thresholds for 18S rRNA from cerebral and aorta samples did not statistically differ. Data are expressed as average of ratios over 18S rRNA amplified from the same samples. *P<0.05, **P<0.01 versus aorta, † P<0.05, †† P<0.01 versus mesenteric; one-way ANOVA and Tukey test.
C, D, control agarose gel electrophoresis of PCR products, shows single bands for either SPK1 or SPK2.
4 Discussion
We have investigated the effects of SPK inhibitors and of SPK gene deletion on the contractile responses of isolated vessels to vasoactive agonists and KCl-induced depolarization. Our data show that three structurally unrelated SPK inhibitors decreased vasomotor responses in basilar artery, but not (or minimally) in femoral artery. The limited structural homologies between these three inhibitors, the fact that they do not compete for the highly conserved nucleotide binding site of the kinase [19] (which would cause non specific inhibition of other ATP-dependent enzymes), taken together with the consistent results obtained in vessels from SPK1−/− mice strongly increase our confidence that our observations are indeed accounted for by specific SPK inhibition. SPK activity is known to be stimulated by agonists of various G protein-coupled receptors (muscarinic, formyl peptide, nucleotides, lysophosphatidic acid and bradykinin [23]) as well as by depolarization-induced Ca2+ entry [10]. SPK activation may in turn activate Ca2+-sensitizing mechanisms, such as RhoA/Rho kinase pathway [12], which lead to increased myosin light chain phosphorylation in vascular smooth muscle cells and contraction [24]. Consistent with this view, we observed that SPK inhibition reduces both KCl-induced vasoconstriction (which depends on depolarization-induced Ca2+ entry) and vasoconstriction induced by G protein-coupled receptor stimulation (by agonists such as 5-HT and S1P). However, based on the inhibition of vasoconstriction by SPK inhibitors in different arteries, we hypothesize that, in cerebral arteries, SPK activation is required for vasoconstriction to occur, whereas in peripheral arteries, SPK activation is less critical. It is interesting to note that SPK inhibitors blocked vasoconstriction evoked by exogenous S1P. This effect, similar to the previous observation that SPK inhibitors block S1P receptor-mediated intracellular Ca2+ mobilization in HEK-293 cells [25], suggests that exogenously added S1P does not access the intracellular compartment in concentrations sufficient to act as a second messenger.
Two different SPK isoforms (termed SPK1 and SPK2) with distinct enzymatic properties, subcellular localization and patterns of tissue distribution, have been found thus far in mammals [26]. SPK1 over-expression in vascular smooth muscle cells has been reported to increase resting tone and myogenic responses of isolated resistance arteries through RhoA/Rho kinase [12], but SPK2 has not been investigated. At least in theory, these SPK isoforms might have some functional redundancy in isolated arteries, since they show reciprocal compensatory roles during vascular development, as indicated by the observation that mice deficient in either SPK1 or SPK2 do not show abnormalities, whereas double-knockout animals are lethal prior to E13.5 with severe vascular defects [14,15].
Using currently available agents, pharmacological SPK inhibition does not enable to discriminate the role of the two isoforms, because SPK inhibitors lack sufficient selectivity and conflicting data on DMS selectivity exist in literature, possibly accounted for by the different biological material used for SPK characterization (recombinant vs. native or crude versus purified enzymatic preparations [18–20]). The data we obtained with FTY720 may be helpful in this respect, since the information available so far on FTY720 reports inhibition of SPK1 and not of SPK2 [20,21]. Inhibition of vasoconstriction by FTY720 in isolated basilar artery therefore suggests a role for SPK1 rather than for SPK2. A potential confounding factor in analyzing the SPK-dependent effects of FTY720 could be related to its conversion to phospho-FTY720 by the SPK2 isoform [27]; phospho-FTY720 is a potent agonist for all S1P receptors but S1P2 [28] and induces S1P3-mediated vasoconstriction in isolated cerebral arteries [7]. Potential production of phospho-FTY720 over time is expected to induce vasoconstriction rather than inhibit contraction, and we did not observe any development of vascular tone during incubation with FTY720 (lasting over 1 h), which suggests that SPK2 activity does not produce functionally relevant levels of phospho-FTY720 in isolated vessels over this time.
We then studied vasoconstriction in cerebral (basilar) and peripheral (third branch mesenteric resistance) vessels isolated from mice with selective SPK1 and SPK2 gene deletion. Basilar artery, but not mesenteric resistance arteries from SPK1−/− mice exhibited weaker contractions to either KCl or agonists (U46619 and S1P) compared to either WT or SPK2−/−. This observation strengthens the idea that SPK1 is involved in cerebrovascular constriction and that the effect of SPK inhibitors on cerebral vessels is mostly related to SPK1 inhibition. Of note, there was a general tendency toward a rightward shift of concentration-contraction curves to agonists in SPK2−/− that reached statistical significance in mesenteric resistance arteries. This finding suggests that the SPK2 isoform might also play in the constriction of some vessels and deserves further analysis. However, it must be noted that the effect of SPK2 gene deletion differed from that of SPK1 deletion in various aspects: 1) its effect was of much weaker magnitude than that of SPK1 gene deletion, 2) it did not affect KCl-induced contraction (at variance with what was observed in basilar artery of SPK1−/− and with the effect of inhibitors on rat basilar artery), 3) it did not alter Emax to agonists (Emax to agonists was depressed in basilar artery of SPK1−/− 4) it affected mesenteric resistance arteries rather than basilar (at odds with the effect of pharmacological SPK inhibition, which was much stronger in basilar artery).
The fact that intracellularly applied S1P (caged S1P experiments) constricted vessels supports the hypothesis that stimulation of the receptors for various constrictors leads to the generation of intracellular S1P, which could then either act as a second messenger, or as a paracrine/autocrine mediator, stimulating membrane S1P receptors. The fact that caged S1P did not constrict vessels from S1P3 receptor knockout mice seems to validate the later hypothesis. It is interesting to note that contraction to U46619 was decreased in basilar from SPK1 knockouts and U46619-induced constriction was inhibited by DMS in S1P3 knockout mice, in apparent discrepancy with our previous observation that U46619 and endothelin-1 constrict S1P3−/− vessels just as much as wild-type vessels [7]. S1P generated by SPK1 might be more concentrated in the vicinity of the enzyme, while photolysis of the caged compound is likely to generate S1P with a different intracellular localization pattern. In addition, S1P degrading enzymes might further restrict the intracellular localization of S1P; S1P phosphohydrolase 1 in particular has been shown to counteract the contractile effect of overexpressed SPK1 [22]. We therefore hypothesize that S1P generated by photolysis can activate membrane S1P3 receptors, while endogenously-produced S1P is more likely to act on intracellular targets, because the combined specific intracellular localizations of SPK1 and S1P-degrading enzymes limits endogenous S1P concentration in the vicinity of membrane S1P receptors.
When analyzing SPK mRNA expression in mouse arteries we found SPK1 mRNA to be 40–80-fold higher in cerebral arteries than in aorta or mesenteric artery, while much smaller vessel-related differences were observed for SPK2 expression. Such a high expression of SPK1 in cerebral arteries is consistent with the explanation that all SPK inhibitors tested in this study acted through inhibition of SPK1, and may account for the apparent cerebrovascular selectivity of these agents, that appeared almost devoid of activity in isolated femoral artery. Interestingly, brain vessels behave as “resistance vessels” at a size range where other vascular beds see relatively minor drops in perfusion pressure [29]. This peculiarity, as deduced from in vivo measurements [30], might be part of the autoregulatory mechanism which maintains cerebral blood flow relatively constant over a broad range of systemic blood pressure and is likely to involve specific signal-transduction mechanisms, possibly involving vessel-specific S1P signaling pathways. In this respect we have previously shown that extracellularly applied S1P is more potent in constricting cerebral rather than peripheral vessels and demonstrated that this occurs through S1P3 receptor stimulation, by using S1P3 receptor knockout mice [6,7]. The role of S1P3 receptors in dog coronary and cerebral arteries was recently confirmed using a new S1P3 receptor antagonist [31]. It is worth noting that the S1P receptor subtype mediating vasoconstriction may be species- and/or vessel-specific, because antisense oligonucleotides against S1P2 mRNA decrease the constriction of hamster gracilis muscle resistance arteries to S1P [22]. Here we show that SPK1, one of the two isoenzymes that generate S1P inside the cell, has a major involvement in modulating vasoconstriction of cerebral vessels and a minor role in peripheral vessels.
The molecular basis for the specific role of SPK1 in the constriction of cerebral vessels remains to be elucidated, but might involve activation of the RhoA/Rho kinase pathway [12]. The importance of Ca2+-sensitizing mechanisms, such as RhoA/Rho kinase pathway, in cerebral vascular smooth muscle cells is underscored by the observation that systemic administration of Rho kinase inhibitors increases cerebral blood flow in experimental brain ischemia, without reducing blood pressure, i.e. these inhibitors reduce cerebrovascular resistance at doses that do not substantially alter peripheral vascular resistances [32,33]. If SPK1 is functionally coupled to RhoA/Rho kinase [12], a high SPK activity in brain arteries, either basal or following vasoconstrictor stimuli, would be consistent with the relative selectivity of Rho kinase inhibitors in this vascular bed.
Understanding novel factors and mediators that regulate the caliber of cerebral vessels may offer new therapeutic targets for treating conditions in which cerebro-vascular regulation is altered. S1P is a particularly interesting constrictor because it exerts a more potent action on cerebral than on peripheral vessels when stimulating membrane G Protein Coupled Receptors, and because one of the two S1P-synthesizing isoenzymes (SPK1) is more abundantly expressed in cerebral arteries and critically control their responsiveness to vasoconstrictor stimuli.
Acknowledgements
This work was supported grants from by the National Institutes of Health [grant numbers R01NS049263, P01NS055104] to C.W. and [5T32AG00277] to K.P.
The Authors are grateful to Dr. Richard Proia (National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD) who generously provided SPK1 and SPK2 null mice, and to Dr. Cenk Ayata (Department of Neurology, Massachusetts General Hospital, Charlestown, MA) for critically reading the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kim RH, Takabe K, Milstien S, Spiegel S. Export and functions of sphingosine-1-phosphate. Biochim Biophys Acta. 2009;1791:692–696. doi: 10.1016/j.bbalip.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Waeber C, Blondeau N, Salomone S. Vascular sphingosine-1-phosphate S1P1 and S1P3 receptors. Drug News Perspect. 2004;17:365–382. doi: 10.1358/dnp.2004.17.6.829028. [DOI] [PubMed] [Google Scholar]
- 3.Igarashi J, Michel T. Sphingosine-1-phosphate and modulation of vascular tone. Cardiovasc Res. 2009;82:212–220. doi: 10.1093/cvr/cvp064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sugiyama A, Yatomi Y, Ozaki Y, Hashimoto K. Sphingosine 1-phosphate induces sinus tachycardia and coronary vasoconstriction in the canine heart. Cardiovasc Res. 2000;46:119–125. doi: 10.1016/s0008-6363(00)00013-4. [DOI] [PubMed] [Google Scholar]
- 5.Igarashi J, Erwin PA, Dantas AP, Chen H, Michel T. VEGF induces S1P1 receptors in endothelial cells: Implications for cross-talk between sphingolipid and growth factor receptors. Proc Natl Acad Sci U S A. 2003;100:10664–10669. doi: 10.1073/pnas.1934494100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salomone S, Yoshimura S, Reuter U, Foley M, Thomas SS, Moskowitz MA, Waeber C. S1P3 receptors mediate the potent constriction of cerebral arteries by sphingosine-1-phosphate. Eur J Pharmacol. 2003;469:125–134. doi: 10.1016/s0014-2999(03)01731-x. [DOI] [PubMed] [Google Scholar]
- 7.Salomone S, Potts EM, Tyndall S, Ip PC, Chun J, Brinkmann V, Waeber C. Analysis of sphingosine 1-phosphate receptors involved in constriction of isolated cerebral arteries with receptor null mice and pharmacological tools. Br J Pharmacol. 2008;153:140–147. doi: 10.1038/sj.bjp.0707581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pyne S, Pyne NJ. Sphingosine 1-phosphate signalling in mammalian cells. Biochem J. 2000;349:385–402. doi: 10.1042/0264-6021:3490385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Brocklyn JR, Lee MJ, Menzeleev R, Olivera A, Edsall L, Cuvillier O, Thomas DM, Coopman PJ, Thangada S, Liu CH, Hla T, Spiegel S. Dual actions of sphingosine-1-phosphate: Extracellular through the Gi-coupled receptor Edg-1 and intracellular to regulate proliferation and survival. J Cell Biol. 1998;142:229–240. doi: 10.1083/jcb.142.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alemany R, Kleuser B, Ruwisch L, Danneberg K, Lass H, Hashemi R, Spiegel S, Jakobs KH, Meyer zu Heringdorf D. Depolarisation induces rapid and transient formation of intracellular sphingosine-1-phosphate. FEBS Lett. 2001;509:239–244. doi: 10.1016/s0014-5793(01)03168-4. [DOI] [PubMed] [Google Scholar]
- 11.Leiber D, Banno Y, Tanfin Z. Exogenous sphingosine 1-phosphate and sphingosine kinase activated by endothelin-1 induced myometrial contraction through differential mechanisms. Am J Physiol Cell Physiol. 2007;292:C240–C250. doi: 10.1152/ajpcell.00023.2006. [DOI] [PubMed] [Google Scholar]
- 12.Bolz SS, Vogel L, Sollinger D, Derwand R, Boer C, Pitson SM, Spiegel S, Pohl U. Sphingosine kinase modulates microvascular tone and myogenic responses through activation of Rhoa/Rho kinase. Circulation. 2003;108:342–347. doi: 10.1161/01.CIR.0000080324.12530.0D. [DOI] [PubMed] [Google Scholar]
- 13.Mulders AC, Hendriks-Balk MC, Mathy MJ, Michel MC, Alewijnse AE, Peters SL. Sphingosine kinase-dependent activation of endothelial nitric oxide synthase by angiotensin II. Arterioscler Thromb Vasc Biol. 2006;26:2043–2048. doi: 10.1161/01.ATV.0000237569.95046.b9. [DOI] [PubMed] [Google Scholar]
- 14.Allende ML, Sasaki T, Kawai H, Olivera A, Mi Y, van Echten-Deckert G, Hajdu R, Rosenbach M, Keohane CA, Mandala S, Spiegel S, Proia RL. Mice deficient in sphingosine kinase 1 are rendered lymphopenic by FTY720. J Biol Chem. 2004;279:52487–52492. doi: 10.1074/jbc.M406512200. [DOI] [PubMed] [Google Scholar]
- 15.Mizugishi K, Yamashita T, Olivera A, Miller GF, Spiegel S, Proia RL. Essential role for sphingosine kinases in neural and vascular development. Mol Cell Biol. 2005;25:11113–11121. doi: 10.1128/MCB.25.24.11113-11121.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mulvany MJ, Halpern W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ Res. 1977;41:19–26. doi: 10.1161/01.res.41.1.19. [DOI] [PubMed] [Google Scholar]
- 17.Muller PY, Janovjak H, Miserez AR, Dobbie Z. Processing of gene expression data generated by quantitative real-time RT-PCR. Biotechniques. 2002;32:1372–1379. [PubMed] [Google Scholar]
- 18.Kim JW, Kim YW, Inagaki Y, Hwang YA, Mitsutake S, Ryu YW, Lee WK, Ha HJ, Park CS, Igarashi Y. Synthesis and evaluation of sphingoid analogs as inhibitors of sphingosine kinases. Bioorg Med Chem. 2005;13:3475–3485. doi: 10.1016/j.bmc.2005.02.053. [DOI] [PubMed] [Google Scholar]
- 19.French KJ, Schrecengost RS, Lee BD, Zhuang Y, Smith SN, Eberly JL, Yun JK, Smith CD. Discovery and evaluation of inhibitors of human sphingosine kinase. Cancer Res. 2003;63:5962–5969. [PubMed] [Google Scholar]
- 20.Vessey DA, Kelley M, Zhang J, Li L, Tao R, Karliner JS. Dimethylsphingosine and FTY720 inhibit the SK1 form but activate the SK2 form of sphingosine kinase from rat heart. J Biochem Mol Toxicol. 2007;21:273–279. doi: 10.1002/jbt.20193. [DOI] [PubMed] [Google Scholar]
- 21.Tonelli F, Lim KG, Loveridge C, Long J, Pitson SM, Tigyi G, Bittman R, Pyne S, Pyne NJ. FTY720 and (S)-FTY720 vinylphosphonate inhibit sphingosine kinase 1 and promote its proteasomal degradation in human pulmonary artery smooth muscle, breast cancer and androgen-independent prostate cancer cells. Cell Signal. doi: 10.1016/j.cellsig.2010.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peter BF, Lidington D, Harada A, Bolz HJ, Vogel L, Heximer S, Spiegel S, Pohl U, Bolz SS. Role of sphingosine-1-phosphate phosphohydrolase 1 in the regulation of resistance artery tone. Circ Res. 2008;103:315–324. doi: 10.1161/CIRCRESAHA.108.173575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alemany R, van Koppen CJ, Danneberg K, Ter Braak M, Meyer Zu Heringdorf D. Regulation and functional roles of sphingosine kinases. Naunyn Schmiedeberg's Arch Pharmacol. 2007;374:413–428. doi: 10.1007/s00210-007-0132-3. [DOI] [PubMed] [Google Scholar]
- 24.Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: Modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev. 2003;83:1325–1358. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
- 25.Meyer zu Heringdorf D, Lass H, Kuchar I, Lipinski M, Alemany R, Rumenapp U, Jakobs KH. Stimulation of intracellular sphingosine-1-phosphate production by G-protein-coupled sphingosine-1-phosphate receptors. Eur J Pharmacol. 2001;414:145–154. doi: 10.1016/s0014-2999(01)00789-0. [DOI] [PubMed] [Google Scholar]
- 26.Spiegel S, Milstien S. Functions of the multifaceted family of sphingosine kinases and some close relatives. J Biol Chem. 2007;282:2125–2129. doi: 10.1074/jbc.R600028200. [DOI] [PubMed] [Google Scholar]
- 27.Paugh SW, Payne SG, Barbour SE, Milstien S, Spiegel S. The immunosuppressant FTY720 is phosphorylated by sphingosine kinase type 2. FEBS Lett. 2003;554:189–193. doi: 10.1016/s0014-5793(03)01168-2. [DOI] [PubMed] [Google Scholar]
- 28.Butler J, Lana D, Round O, LaMontagne K. Functional characterization of sphingosine 1-phosphate receptor agonist in human endothelial cells. Prostaglandins Other Lipid Mediat. 2004;73:29–45. doi: 10.1016/j.prostaglandins.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 29.Christensen KL, Mulvany MJ. Location of resistance arteries. J Vasc Res. 2001;38:1–12. doi: 10.1159/000051024. [DOI] [PubMed] [Google Scholar]
- 30.Faraci FM, Mayhan WG, Schmid PG, Heistad DD. Effects of arginine vasopressin on cerebral microvascular pressure. Am J Physiol. 1988;255:H70–H76. doi: 10.1152/ajpheart.1988.255.1.H70. [DOI] [PubMed] [Google Scholar]
- 31.Murakami A, Takasugi H, Ohnuma S, Koide Y, Sakurai A, Takeda S, Hasegawa T, Sasamori J, Konno T, Hayashi K, Watanabe Y, Mori K, Sato Y, Takahashi A, Mochizuki N, Takakura N. Sphingosine 1-phosphate (S1P) regulates vascular contraction via S1P3 receptor: Investigation based on a new S1P3 receptor antagonist. Mol Pharmacol. 2010;77:704–713. doi: 10.1124/mol.109.061481. [DOI] [PubMed] [Google Scholar]
- 32.Shin HK, Salomone S, Potts EM, Lee SW, Millican E, Noma K, Huang PL, Boas DA, Liao JK, Moskowitz MA, Ayata C. Rho-kinase inhibition acutely augments blood flow in focal cerebral ischemia via endothelial mechanisms. J Cereb Blood Flow Metab. 2007;27:998–1009. doi: 10.1038/sj.jcbfm.9600406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shin HK, Salomone S, Ayata C. Targeting cerebrovascular Rho-kinase in stroke. Expert Opin Ther Targets. 2008;12:1547–1564. doi: 10.1517/14728220802539244. [DOI] [PubMed] [Google Scholar]