Abstract
Eccentric muscle exercise is a common cause of acute and chronic (lasting days to weeks) musculoskeletal pain. To evaluate mechanisms involved, we have employed a model in the rat, in which eccentric hind limb exercise produces both acute mechanical hyperalgesia as well as long-term changes characterized by enhanced hyperalgesia to subsequent exposure to an inflammatory mediator. Eccentric exercise of the hind limb produced mechanical hyperalgesia, measured in the gastrocnemius muscle, that returned to baseline 120 h post-exercise. When nociceptive thresholds had returned to baseline, intramuscular injection of prostaglandin E2 (PGE2) induced hyperalgesia that was unattenuated 240 h later, much longer than PGE2–induced hyperalgesia in unexercised rats (4 h). This marked prolongation of PGE2 hyperalgesia induced by eccentric exercise was prevented by spinal intrathecal injection of oligodeoxynucleotide antisense to protein kinase Cε, a second messenger in nociceptors implicated in the induction of chronic pain. Exercise-induced hyperalgesia and prolongation of PGE2 hyperalgesia was inhibited by spinal intrathecal administration of antisense for the interleukin-6, but not the tumor necrosis factor-α type-1 receptor. These findings provide further insight into the mechanism underlying exercise-induced chronic muscle pain, which suggest novel approaches for the prevention and treatment of exercise or work-related chronic musculoskeletal pain syndromes.
Keywords: Muscle, hyperalgesia, tumor necrosis factor alpha, interleukin-6, protein kinase c epsilon, prostaglandin E2
Introduction
Muscle pain can result from repetitive or eccentric activity, and may persist for days (e.g. delayed onset muscle soreness) or weeks (e.g. chronic work-related myalgia) after exposure to these activities (Alund et al., 1992). While the underlying cellular mechanisms in these pain syndromes are not well understood, inflammatory cytokines, which sensitize high threshold mechano-sensitive muscle afferents (Diehl et al., 1993) are likely to be involved (Barr & Barbe, 2002; Stauber, 2004; Sluka et al., 2007). For example, following eccentric exercise in humans (Hamada et al., 2005) and in a repetitive motion-induced muscle pain model in the rat (Barbe et al., 2003), levels of inflammatory cytokines are increased in the exercised limb (Al-Shatti et al., 2005). Furthermore, we have observed that intramuscular administration of an inflammogen, carrageenan, induces mechanical hyperalgesia in muscle (Dina et al., 2008); following recovery to baseline nociceptive threshold, hyperalgesia produced by subsequent administration of an inflammatory mediator, prostaglandin E2 (PGE2), is enhanced and markedly prolonged (Dina et al., 2008; Dina et al., 2008). This enhancement of hyperalgesia (hyperalgesic priming) is qualitatively similar to hyperalgesic priming observed in the skin (Aley et al., 2000; Parada et al., 2003a; Parada et al., 2005; Joseph et al., 2007), in that it may be dependent on protein kinase Cε (PKCε) in nociceptors (Aley et al., 2000; Parada et al., 2005; Joseph et al., 2007).
To better understand the mechanisms underlying the pain associated with strenuous activity, we employed a rat model of eccentric exercise-induced muscle pain (Taguchi et al., 2005). We also evaluated the contribution of PKCε and two inflammatory cytokines that have been implicated in muscle hyperalgesia: tumor necrosis factor α (TNFα) (Schafers et al., 2003; Beyreuther et al., 2007; Dina et al., 2009) and interleukin 6 (IL-6) (Gerdle et al., 2007; Dina et al., 2008; Shah et al., 2008).
Materials and Methods
Animals
Adult male Sprague Dawley rats (250–400 g; Charles River, Hollister, CA) were used in these experiments. They were housed in the Animal Care Facility at UCSF, under environmentally controlled conditions (lights on 7 am to 7 pm; room temperature 21–23°C) with food and water available ad libitum. Animal care and use conformed to NIH guidelines; the UCSF Committee on Animal Research approved all experimental protocols.
Eccentric exercise
The method used to eccentrically exercise the rat hind limb was similar to that described by Kano and colleagues (Kano et al., 2004), and comparable to that used by Mizumura and colleagues (Taguchi et al., 2005). Briefly, an isoflurane-anesthetized rat was placed in the supine position, on a heating pad (to maintain body temperature at 37 °C), and the right hind paw affixed to the foot bracket of the exercise apparatus (Model RU-72, NEC Medical Systems, Tokyo, Japan) with 3M Micropore® surgical paper tape (Fisher Scientific), such that the angle of the knee and ankle joints were ~90° (the paw 30° from vertical). The gastrocnemius muscle was stimulated, via subcutaneous needle-type electrodes, attached to a Model DPS-07 stimulator (Dia Medical System Inc, Tokyo, Japan) that delivered trains of rectangular pulses (100 Hz, 700 ms, 3 V) every 3 seconds, to give a total of 300 contractions. During these stimulus-induced contractions of the gastrocnemius muscle, the electromotor system rotated the foot to produce extension of the gastrocnemius muscle. We measured force output during contractions (using an NEC Dynamic Strain Amplifier, model AS1603, attached to a chart recorder, ABB model SE111) to be 98.7±12.9 mNm for the first 10 contractions and 38.3±3.1 mNm for the last 10 contractions. This initial force output is similar to that seen in other studies in the rat (Ochi et al., 2007), and a similar 30–40% decrease in force output has previously been shown with repeated electrical stimulation in the rat (Kano et al., 2008).
Measurement of hyperalgesia
Mechanical nociceptive threshold was quantified using a Chatillon digital force transducer (model DFI2, Amtek Inc., Largo, FL). Rats were lightly restrained in a cylindrical acrylic holder that allows for easy access to the hind limb, and a 6 mm diameter probe attached to the force transducer applied to the gastrocnemius muscle to deliver an increasing compression force. The nociceptive threshold was defined as the force, in Newtons, at which the rat withdrew its hind leg. The experimenter was not blind to the treatment group. Baseline withdrawal threshold was defined as the mean of 2 readings taken at 5-min intervals. Each hind limb is treated as an independent measure and each experiment performed on a separate group of rats. All behavioral testing was done between 10 am and 4 pm.
Intramuscular injection
Rats were briefly anesthetized with 3% isoflurane to facilitate the administration of PGE2 (1 μg, Sigma Chemical Co., St. Louis, MO) or vehicle (0.9% sodium chloride), in a volume of 20 μl, into the belly of the gastrocnemius muscle; skin over the injection site was marked with a fine-tip indelible pen so that the underlying injection site in the muscle could be repeatedly tested for mechanical nociceptive threshold.
Antisense oligodeoxynucleotide design and administration
To attenuate the expression of PKCε we used a 20-mer antisense ODN sequence, 5′-GCC AGC TCG ATC TTG CGC CC-3′, directed against a unique sequence of rat PKCε. The corresponding GenBank accession number and ODN position within the cDNA sequence are XM345631 and 226–245, respectively. The mismatch ODN sequence, 5′-GCC AGC GCG ATC TTT CGC CC-3′, corresponds to the PKCε subunit antisense sequence with 2 bases mismatched (indicated in bold typeface). We have previously shown that antisense ODN with this sequence (at a dose of 80 μg) protocol decreases PKCε protein in dorsal root ganglia (Dina et al., 2005). A search of EMBL and NCBI GenBank Rattus norvegicus databases to PKCε identified no homologous sequences.
To attenuate the expression of TNFα receptor type-1, the antisense oligodeoxynucleotide (ODN) sequence 5'-ACACGGTGTTCTGTTTCTCC-3' directed against a unique sequence of rat TNFα receptor type-1 was used. The mismatch ODN sequence, 5'-ACCCGTTGTTCGGTTGCTCC-3' is the antisense sequence, with four bases mismatched (denoted by bold face). We have previously shown that this ODN antisense against TNFα receptor type-1, at a dose of 80 μg, decreases TNFα receptor type-1 protein in dorsal root ganglia (Parada et al., 2003a).
To determine the contribution of IL-6, its effect on sensory neurons was disrupted by intrathecal administration of ODN antisense to the signal transducing molecule, glycoprotein 130 (gp130), a subunit of the IL-6 receptor signaling complex necessary for IL-6 receptor function (Muller-Newen, 2003). The 19-mer AS- and mismatch ODN for gp130 were purchased from Invitrogen (San Francisco, CA). The dose of ODN (80 μg) was based on prior dose–response studies (Summer et al., 2006). The antisense ODN sequence, 5′-TCC TTC CCA CCT TCT TCT G-3′, was directed against a unique sequence of rat gp130. The corresponding GenBank accession number and ODN position within the cDNA sequence are M92340 and 1834–1852, respectively (Wang et al., 1992). The mismatch ODN sequence, 5′-TAC TAC TCA CAT TCA TCA G-3′, corresponds to the gp130 subunit antisense sequence with 6 bases mismatched (denoted by bold).
The method for intrathecal ODN injection has been described previously (Khasar et al., 1996; Aley & Levine, 1997; Khasar et al., 1998; Alessandri-Haber et al., 2003; Parada et al., 2003a; Parada et al., 2003b; Alessandri-Haber et al., 2004; Dina et al., 2004). Before ODN injections, rats were briefly anesthetized with 3% isoflurane and a 30-gauge hypodermic needle inserted into the subarachnoid space, at the midline, between the L4 and L5 vertebrae. ODN (80 μg/10 μl) was slowly injected over ~15 s. This procedure was repeated daily so that ODN was administered on 3 or 6 consecutive days. Control animals received injections of mismatch ODN.
Statistics
Group data are expressed as percentage change from baseline nociceptive threshold (mean ± SEM of n distinct observations). Statistical comparisons were made by a two-tailed Student's t-test (for one or two independent populations) and by one-way ANOVA for comparing multiple treatments, using StatView statistical software. P<0.05 was considered statistically significant. To compare change from baseline, one-way repeated measures ANOVA with a Greenhouse-Geisser adjusted P-value was used (SPSS statistical software).
Results
Eccentric exercise-induces hyperalgesia
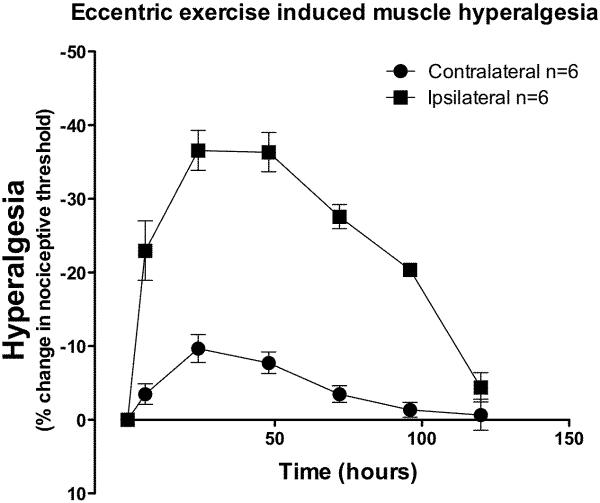
Eccentric exercise produced a decrease in mechanical nociceptive threshold in the ipsilateral gastrocnemius muscle that was statistically significant by the first measurement, 6 h after exercise (Figure 1; two-way ANOVA F1,120 = 47.3; P < 0.0001), and reached its maximum (~36% decrease in nociceptive threshold) 24 – 48 h post exercise. Hyperalgesia was still present 96 h post-exercise (~20% decrease in nociceptive threshold), returning to baseline by 120 h. Applying a Bonferroni-type correction to the alpha level (0.05/6 = 0.0083), the nociceptive threshold in the gastrocnemius muscle contralateral to the exercised leg was significantly different from pre-exercise baseline at two time points; a 9.7% lower threshold at 24 h (P = 0.005) and 7.7% lower threshold at 48 h (P = 0.004) post exercise was observed (Figure 1).
Figure 1. Eccentric exercise induces muscle hyperalgesia.
The ipsilateral hind limb was exercised eccentrically for 15 min (filled squares) and after exercise mechanical nociceptive threshold of the gastrocnemius muscle in both hind limbs measured over time. Compared to contralateral limbs (filled circle) there was a significant decrease in nociceptive threshold in the vibrated limbs, which lasted at least 120 h post-exercise.
Eccentric exercise-induces hyperalgesic priming
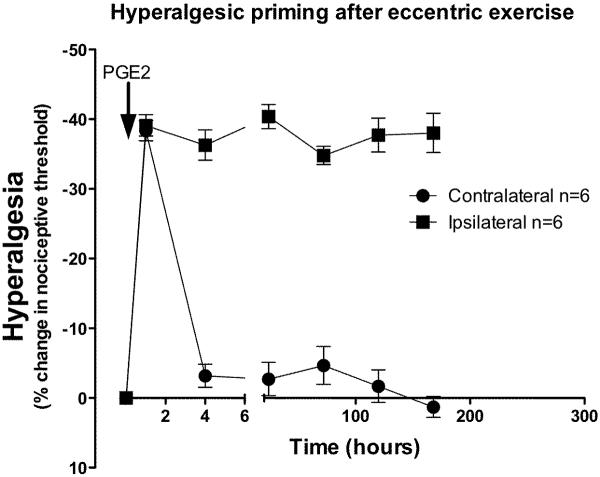
To determine if eccentric exercise induces hyperalgesic priming, one hind limb of each rat was exercised; following recovery of nociceptive threshold to baseline (5 days later), PGE2 (1 μg), the proinflammatory cytokine used to characterize hyperalgesic priming in skin (Joseph et al., 2003; Parada et al., 2003b), was injected into the gastrocnemius muscle. In the gastrocnemius muscle of the eccentric-exercised leg, PGE2 induced hyperalgesia with a rapid onset, that was still present, unattenuated, 240 h after administration. In the non-exercised hind limb, PGE2 produced a decrease in nociceptive threshold that returned to baseline by 3 h, significantly shorter duration hyperalgesia than in the exercised leg (Figure 2; F1,120 = 258.1; P < 0.0001).
Figure 2. Eccentric exercise induces hyperalgesic priming.
Seven days after eccentric exercise, following recovery of nociceptive threshold to pre-exercise baseline, PGE2 (1 μg) was injected into both the ipsilateral and contralateral gastrocnemius muscle. In non-exercised contralateral limbs (filled circles) PGE2-induced hyperalgesia had completely resolved within 4 h, while in the exercised limbs (filled squares), hyperalgesia was greatly prolonged, being undiminished 240 h after PGE2 administration.
Effect of PKCε antisense on eccentric exercise-induced hyperalgesia and priming
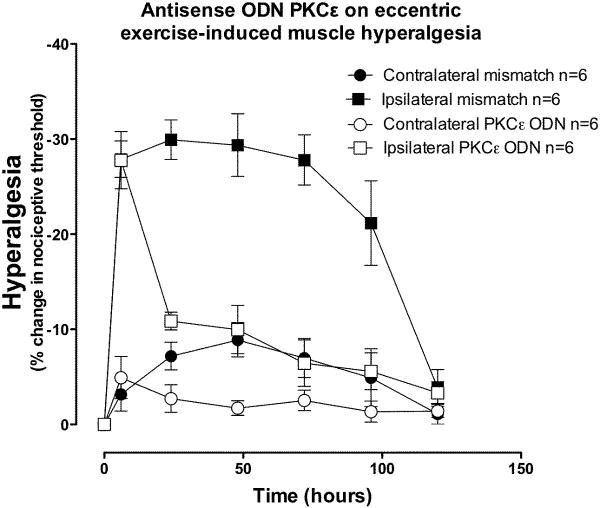
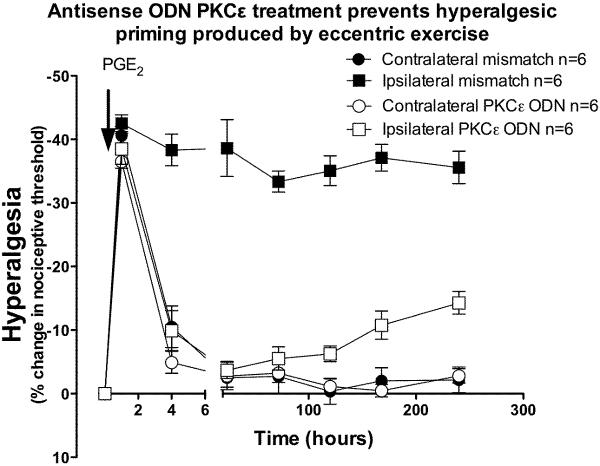
ODN antisense or mismatch to PKCε was administered once daily for 3 days prior and 3 days after the eccentric exercise protocol. In eccentrically exercised limbs, hyperalgesia was significantly greater in the mismatch-treated, compared to antisense-treated rats (Figure 3, two-way ANOVA F1,60 = 41.3; P < 0.0001), but at the 6 h, and 120 h time points, there was no significant difference (Bonferroni post-hoc test). In the contralateral non-exercised hind limb, intramuscular PGE2 produced a short-lived hyperalgesia lasting <4 h, similar to what we have observed in naïve rats (Dina et al., 2008). In the eccentrically exercised leg, however, PGE2-induced hyperalgesia was still present after 240 h, two-way ANOVA indicates that exercise produced significant hyperalgesia (Figure 4, two-way ANOVA F1,70 = 189.3; P < 0.0001). This exercise-induced `hyperalgesic priming' (Parada et al., 2003a) was prevented by administration of antisense ODN against PKCε, up to 120 h post PGE2, however 168 and 240 h post PGE2 there was significantly greater hyperalgesia in the exercised leg (Bonferroni post hoc test, P<0.001), presumably due to de novo PKCε synthesis at these later time points, as antisense administration only transiently suppresses PKCε function, which recovers within days of stopping antisense administration (Parada et al., 2003a).
Figure 3. PKCε antisense inhibits exercise-induced hyperalgesia.
Intrathecal administration of ODN antisense against PKCε for 3 days before and 3 days after eccentric exercise suppressed the acute hyperalgesia in the exercised leg (open squares), while hyperalgesia was still present in exercised leg of mismatch ODN treated rats (filled squares). There was no significant effect of treatment in the contralateral limbs (two-way ANOVA F1,60 = 3.4; P = 0.095).
Figure 4. PKCε antisense inhibits exercise-induced hyperalgesic priming.
In rats that had received ODN antisense against PKCε, for 3 days before and 3 days after vibration (open squares, n=6), PGE2–induced hyperalgesia returned to baseline by 4 h post PGE2, while in mismatch-treated rats (filled squares), PGE2 hyperalgesia remained elevated 240 h after exercise. There was no significant effect of treatment in the contralateral limbs (two-way ANOVA F1,70 = 0.4; P = 0.53).
Effect of antisense ODN against IL-6 receptor subunit gp 130 on exercise-induced hyperalgesia and priming
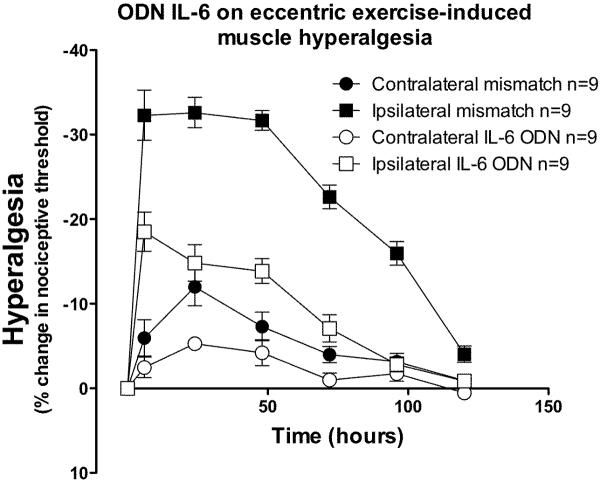
Injection of ODN antisense (80 μg/20 μl, intrathecal) to the IL-6 signal transducing molecule, gp 130, for 3 days prior to eccentric exercise, significantly reduced the magnitude of exercise-induced acute hyperalgesia compared to mismatch ODN-treated rats (Figure 5; F1,96 = 74.7; P < 0.0001), with significant differences from the 6 to the 96 h time points (Bonferroni post-hoc test, P<0.001).
Figure 5. IL-6 receptor antisense prevents exercise-induced hyperalgesia.
In rats that received antisense ODN to gp130 (open squares) for 3 days prior and 3 days after exercise, hyperalgesia in the exercised leg was significantly less than in exercised leg of rats receiving mismatch ODN to gp130 (filled squares). There was a significant effect of treatment in the contralateral limbs (two-way ANOVA F1,60 = 6.5; P = 0.021), with a significant difference only at the 24 h time point (Bonferroni post-hoc test, P<0.001).
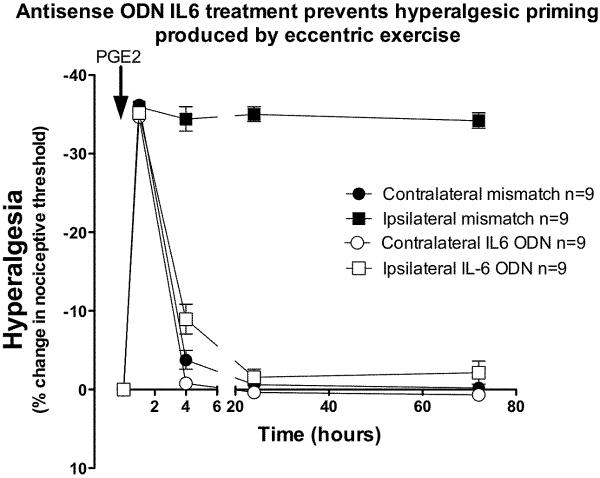
To determine the contribution of IL-6 to hyperalgesic priming produced by eccentric exercise, ODN antisense or mismatch (80 μg/20 μl, intrathecal) to gp 130 was injected 3 days prior and 3 days following the exercise protocol. After recovery of nociceptive threshold to baseline (5 days after exercise), PGE2 (1 μg) was injected into the gastrocnemius muscle. In the exercised leg of the mismatch-treated animals PGE2-induced a rapid onset hyperalgesia that remained undiminished even 72 h after administration. In exercised rats receiving antisense ODN to gp 130, PGE2 hyperalgesia was transient, returning to baseline by 4 h after administration, and was significantly different compared to exercised rats receiving mismatch ODN (Figure 6; F1,64 = 490.8 P < 0.0001), at the 4, 24 and 72 h time points (Bonferroni post-hoc test, P<0.001). In the gp 130 antisense ODN-treated rats, there was no significant difference between the exercised leg and contralateral leg.
Figure 6. IL-6 receptor antisense prevents exercise-induced hyperalgesic priming.
In rats that received ODN antisense to gp130 (open squares, n=6) for 3 days prior and 3 days after exercise, PGE2–induced hyperalgesia returned to baseline by 4 h post PGE2, in contrast to rats treated with mismatch ODN in which PGE2 produced hyperalgesia that remained enhanced 72 h after exercise. There was a significant effect of antisense treatment in the contralateral limbs (two-way ANOVA, F1,64 = 9.75; P = 0.007), with a significant difference only at the 4 h time point (Bonferroni post-hoc test, P<0.001).
Effect of TNFα receptor type-1 antisense treatment on exercise-induced hyperalgesia and priming
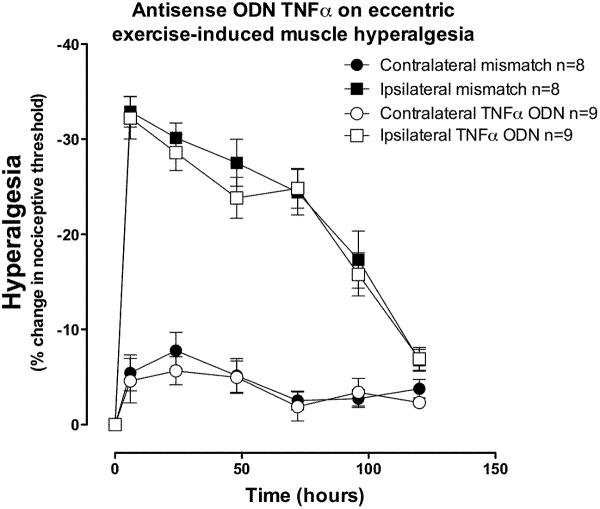
Injection of ODN antisense against TNFα receptor type-1, but not mismatch ODN (both 80 μg/20 μl, intrathecal), for 3 days prior to eccentric exercise had no significant effect on the magnitude of exercise-induced hyperalgesia (Figure 7).
Figure 7. TNFα type-1 receptor antisense does not prevent exercise-induced hyperalgesia.
In rats that received ODN antisense to TNFα receptor type 1 (open squares) for 3 days prior and 3 days after exercise, hyperalgesia in the exercised leg was not significantly different compared to that in exercised leg of rats receiving mismatch ODN (filled squares; two-way ANOVA F1,90 = 0.4; P = 0.54). There was no significant effect of treatment in the contralateral limbs (two-way ANOVA F1,90 = 0.29; P = 0.600).
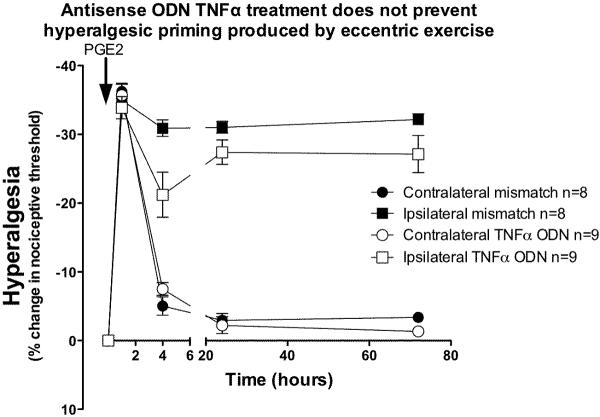
To test whether antisense to TNFα receptor prevented hyperalgesic priming produced by eccentric exercise, antisense or mismatch ODN (80 μg/20 μl, intrathecal) against TNFα receptor was injected 3 days prior and 3 days following exercise. After recovery of nociceptive threshold to baseline (5 days after exercise), PGE2 (1 μg) was injected into the gastrocnemius muscle. In exercised rats receiving ODN antisense to TNFα receptor, PGE2 hyperalgesia remained enhanced (indicating presence of hyperalgesic priming), but was significantly less than in mismatch ODN-treated rats (Figure 8; F1,60 = 4.64; P = 0.048), with significant difference only at the 4 h time point (Bonferroni post hoc test, P<0.001).
Figure 8. TNFα type-1 receptor antisense does not completely prevent exercise-induced hyperalgesic priming.
In rats that received ODN antisense to TNFα receptor type-1 (open squares) for 3 days prior and 3 days after exercise, PGE2–induced hyperalgesia remained elevated 72 h post PGE2, similar to rats treated with mismatch ODN. However, hyperalgesia was significantly less at the 4 h time point in antisense- compared to mismatch-treated rats. There was no significant effect of treatment in the contralateral limbs (two-way ANOVA F1,60 = 0.04; P = 0.84).
Discussion
In this study we show that eccentric exercise in the hind limb of the rat produces both acute mechanical hyperalgesia and enhancement of a subsequent inflammatory mediator-induced hyperalgesia (hyperalgesic priming) (Joseph et al., 2003). The time-course of the acute mechanical hyperalgesia observed in this study is very similar to that in studies of eccentric exercise in humans, which report a maximum in muscle pain at 24–48 h and duration of 3–4 d (Jones et al., 1986). Our findings of an acute change in mechanical nociceptive threshold in muscle are similar to those of Mizumura and colleagues (Taguchi et al., 2005) who observed, using a similar eccentric exercise model in the rat, a robust and long-lasting decrease in nociceptive threshold in muscle, but not skin.
Pretreatment with PKCε antisense attenuated eccentric exercise-induced acute mechanical hyperalgesia as well as the prolongation of PGE2–induced hyperalgesia, implicating this second messenger in the development of eccentric exercise-induced primary muscle hyperalgesia and hyperalgesic priming. PKCε has previously also been implicated in the development of hyperalgesic priming in the skin induced by carrageenan or TNFα (Parada et al., 2003a; Parada et al., 2003b), and in muscle following exposure to vibration (Dina et al., 2009). Of note, hyperalgesia 6 h post-eccentric exercise was not attenuated in PKCε antisense-treated rats, suggesting that PKCε-independent mechanisms mediate early (≤6 h) post-exercise hyperalgesia. Our observation that there was mild hyperalgesia (<10% change in nociceptive threshold) in the limb contralateral to the exercised limb (only in mismatch-treated animals), may have been due to a contralateral reflex response, similar to what we and others have previously observed following mild injury in the contralateral limb (Levine et al., 1985; Koltzenburg et al., 1999).
Intrathecal administration of antisense to gp 130, which decreases the level of a functional subunit of the IL-6 receptor level in sensory neurons (Summer et al., 2007), significantly attenuated the magnitude of eccentric exercise-induced hyperalgesia and eliminated the development of hyperalgesic priming. These findings support the suggestion that IL-6 is involved in exercise-induced acute and chronic muscle hyperalgesia, consistent with previous studies demonstrating increased concentration of IL-6 protein and gene expression in skeletal muscle after eccentric exercise (Tomiya et al., 2004; Pedersen & Febbraio, 2008; Betts et al., 2009; Buford et al., 2009; Pedersen, 2009) and increased muscle IL-6 with increased exercise pain intensity in patients with chronic trapezius myalgia (Rosendal et al., 2005a). Furthermore, we have previously shown that administration of IL-6 into the gastrocnemius muscle of the rat produces acute mechanical hyperalgesia, which resolves within 5 days, and a subsequent administration of PGE2 into the muscle produces long-lasting (>14 days) mechanical hyperalgesia (i.e., hyperalgesic priming) (Dina et al., 2008).
In contrast to the role of IL-6, antisense ODN treatment to decrease TNFα receptor type-1 in the primary afferent nociceptor innervating the gastrocnemius muscle had no effect on the magnitude of eccentric exercise-induced hyperalgesia, and did not affect the development of hyperalgesic priming. This also contrasts with our earlier observation that intramuscular administration of TNFα (100 ng) induces hyperalgesia in the gastrocnemius muscle and ODN antisense to the TNFα receptor type-1 significantly inhibits mechanical hyperalgesia in muscle. It also contrasts with the finding that induction of hyperalgesic priming in another ergonomic muscle pain model, exposure to hind limb vibration, was prevented by TNFα type-1 receptor antisense (Dina et al., 2009). These differences from our current study may be related to vibration producing a greater release of TNFα compared to eccentric exercise. While TNFα has been implicated in several chronic muscle pain syndromes (Schafers et al., 2003; Hoheisel et al., 2005; Rosendal et al., 2005b; Shah et al., 2005), the literature describing the effect of eccentric exercise on TNFα levels in muscle is contradictory, with reports showing either an increase (Hamada et al., 2005; Rosa Neto et al., 2009; Liao et al., 2010) or no change (Buford et al., 2009; Croft et al., 2009) in TNFα concentration in muscle and plasma after exercise, and neutralizing TNFα antibodies not attenuating exercise-induced muscle pain (Rice et al., 2008).
While intrathecal antisense administration decreases expression of target molecules in the sensory neurons (Khasar et al., 1996; Aley & Levine, 1997; Khasar et al., 1998; Alessandri-Haber et al., 2003; Parada et al., 2003a; Parada et al., 2003b; Alessandri-Haber et al., 2004; Dina et al., 2004), our data do not exclude the possibility that this protocol also decreases expression of these target molecules in cells within the spinal cord (i.e. neurons and glia), which may be important given the likely presence of cytokines in the cord. However, since IL-6 levels are increased in muscle following a similar exercise protocol to ours (Jonsdottir et al., 2000), whereas IL-6 levels in cerebrospinal fluid are unchanged following strenuous exercise (Dalsgaard et al., 2004; Steensberg et al., 2006), we suggest that the sensory neuron is more likely to be the functionally important site in the present experiment.
Recent work by Sluka and colleagues has provided key information on the role of gender and sex steroids in muscle nociceptive threshold (Sluka & Rasmussen, 2010). However, their study differs from ours in several ways, including the use of an exercise protocol of voluntary running (a concentric exercise), in mice, which may not be as intense as the eccentric muscle contraction protocol employed in our studies. Of note, in this regard, in contrast to our observations, Sluka and colleagues did not observe any change in nociceptive threshold immediately or 24 h after exercise.
Recently, Mizumura and colleagues (Murase et al., 2010) observed that lengthening contraction (i.e., eccentric exercise) in rats produced muscle hyperalgesia that could be prevented by administration of a bradykinin B2 receptor antagonist, HOE 140. They also observed an increase in IL-6 mRNA in the muscle immediately and 12 h after both lengthening contraction. However, after shortening contraction (i.e., concentric exercise) it was increased immediately, but not 12 h after exercise. Administration of HOE 140 prevented the increase in IL-6 12 h after lengthening contraction. They suggest that IL-6 is unlikely to be responsible for the muscle hyperalgesia since intramuscular administration of an IL-6 antibody two days after lengthening contraction did not reverse hyperalgesia. This may, however, be due to IL-6 having already activated a downstream signaling pathway in the nociceptor (i.e., IL-6 had already exerted its effect before antibody administration), or due to inadequate penetration of the antibody into the muscle. In contrast to IL-6, HOE 140 had no effect on TNFα mRNA in muscle, which was increased immediately and 12 h after lengthening contraction.
Evidence from the clinical literature provides additional indications for a role for IL-6 in exercise-induced pain. Thus, eccentric exercise in humans results in increased levels of IL-6 (Steensberg et al., 2000; Tomiya et al., 2004; Rosendal et al., 2005b; Buford et al., 2009; Meckel et al., 2009), as well as increased expression of IL-6 receptor (Keller et al., 2005a; Keller et al., 2005b) in muscle. Furthermore, post eccentric exercise pain and serum IL-6 were significantly greater in untrained subjects compared to after a second test session (Smith et al., 2007). IL-6 is believed to play a key role in muscle repair after intense exercise (Toft et al., 2002; Meckel et al., 2009), and it is likely that it contributes to post-exercise muscle pain (Mortensen et al., 2008).
In conclusion, we have observed that eccentric exercise can produce IL-6–mediated acute muscle hyperalgesia and hyperalgesic priming. This IL-6-dependent effect is mediated by its receptors on the primary afferent nociceptor and downstream of this receptor by a PKCε-dependent signaling pathway. Our studies do not exclude the possibility that spinal neurons or glia are also involved. Unlike the ergonomic-related muscle pain associated with exposure to vibration, TNFα does not appear to play a role in hyperalgesia or hyperalgesic priming in strenuous eccentric exercise. These findings provide further insight into the mechanisms underlying exercise-induced chronic muscle pain, which may lead to novel approaches for the prevention and treatment of specific exercise or work-related chronic musculoskeletal pain syndromes.
Acknowledgments
This research was supported by a grant from NIAMS AR054635.
Abbreviations
- PGE2
Prostaglandin E2
- ODN
oligodeoxynucleotide
- IL-6
interleukin-6
- TNFα
tumor necrosis factor-α
- PKCε
protein kinase Cε
- gp130
glycoprotein 130
- ANOVA
analysis of variance
References
- Al-Shatti T, Barr AE, Safadi FF, Amin M, Barbe MF. Increase in inflammatory cytokines in median nerves in a rat model of repetitive motion injury. J. Neuroimmunol. 2005;167:13–22. doi: 10.1016/j.jneuroim.2005.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alessandri-Haber N, Dina OA, Yeh JJ, Parada CA, Reichling DB, Levine JD. Transient receptor potential vanilloid 4 is essential in chemotherapy-induced neuropathic pain in the rat. J. Neurosci. 2004;24:4444–4452. doi: 10.1523/JNEUROSCI.0242-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alessandri-Haber N, Yeh JJ, Boyd AE, Parada CA, Chen X, Reichling DB, Levine JD. Hypotonicity induces TRPV4-mediated nociception in rat. Neuron. 2003;39:497–511. doi: 10.1016/s0896-6273(03)00462-8. [DOI] [PubMed] [Google Scholar]
- Aley KO, Levine JD. Multiple receptors involved in peripheral alpha 2, mu, and A1 antinociception, tolerance, and withdrawal. J. Neurosci. 1997;17:735–44. doi: 10.1523/JNEUROSCI.17-02-00735.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aley KO, Messing RO, Mochly-Rosen D, Levine JD. Chronic hypersensitivity for inflammatory nociceptor sensitization mediated by the epsilon isozyme of protein kinase C. J. Neurosci. 2000;20:4680–4685. doi: 10.1523/JNEUROSCI.20-12-04680.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alund M, Larsson SE, Lewin T. Work-related chronic neck impairment. Neck motion analysis in female traverse crane operators. Scand J Rehabil Med. 1992;24:133–139. [PubMed] [Google Scholar]
- Barbe MF, Barr AE, Gorzelany I, Amin M, Gaughan JP, Safadi FF. Chronic repetitive reaching and grasping results in decreased motor performance and widespread tissue responses in a rat model of MSD. J Orthop Res. 2003;21:167–176. doi: 10.1016/S0736-0266(02)00086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr AE, Barbe MF. Pathophysiological tissue changes associated with repetitive movement: a review of the evidence. Phys Ther. 2002;82:173–187. doi: 10.1093/ptj/82.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betts JA, Toone RJ, Stokes KA, Thompson D. Systemic indices of skeletal muscle damage and recovery of muscle function after exercise: effect of combined carbohydrate-protein ingestion. Appl Physiol Nutr Metab. 2009;34:773–784. doi: 10.1139/H09-070. [DOI] [PubMed] [Google Scholar]
- Beyreuther BK, Geis C, Stohr T, Sommer C. Antihyperalgesic efficacy of lacosamide in a rat model for muscle pain induced by TNF. Neuropharmacology. 2007;52:1312–1317. doi: 10.1016/j.neuropharm.2007.01.012. [DOI] [PubMed] [Google Scholar]
- Buford TW, Cooke MB, Shelmadine BD, Hudson GM, Redd L, Willoughby DS. Effects of eccentric treadmill exercise on inflammatory gene expression in human skeletal muscle. Appl Physiol Nutr Metab. 2009;34:745–753. doi: 10.1139/H09-067. [DOI] [PubMed] [Google Scholar]
- Croft L, Bartlett JD, Maclaren DP, Reilly T, Evans L, Mattey DL, Nixon NB, Drust B, Morton JP. High-intensity interval training attenuates the exercise-induced increase in plasma IL-6 in response to acute exercise. Appl Physiol Nutr Metab. 2009;34:1098–1107. doi: 10.1139/H09-117. [DOI] [PubMed] [Google Scholar]
- Dalsgaard MK, Ott P, Dela F, Juul A, Pedersen BK, Warberg J, Fahrenkrug J, Secher NH. The CSF and arterial to internal jugular venous hormonal differences during exercise in humans. Exp Physiol. 2004;89:271–277. doi: 10.1113/expphysiol.2003.026922. [DOI] [PubMed] [Google Scholar]
- Diehl B, Hoheisel U, Mense S. The influence of mechanical stimuli and of acetylsalicylic acid on the discharges of slowly conducting afferent units from normal and inflamed muscle in the rat. Exp Brain Res. 1993;92:431–440. doi: 10.1007/BF00229031. [DOI] [PubMed] [Google Scholar]
- Dina OA, Hucho T, Yeh J, Malik-Hall M, Reichling DB, Levine JD. Primary afferent second messenger cascades interact with specific integrin subunits in producing inflammatory hyperalgesia. Pain. 2005;115:191–203. doi: 10.1016/j.pain.2005.02.028. [DOI] [PubMed] [Google Scholar]
- Dina OA, Parada CA, Yeh J, Chen X, McCarter GC, Levine JD. Integrin signaling in inflammatory and neuropathic pain in the rat. Eur J Neurosci. 2004;19:634–642. doi: 10.1111/j.1460-9568.2004.03169.x. [DOI] [PubMed] [Google Scholar]
- Dina OA, Green PG, Levine JD. Role of interleukin-6 in chronic muscle hyperalgesic priming. Neuroscience. 2008;152:521–525. doi: 10.1016/j.neuroscience.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dina OA, Joseph EK, Levine JD, Green PG. Mechanisms Mediating Vibration-induced Chronic Musculoskeletal Pain Analyzed in the Rat. J Pain. 2009 doi: 10.1016/j.jpain.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dina OA, Levine JD, Green PG. Muscle inflammation induces a protein kinase cepsilon-dependent chronic-latent muscle pain. J Pain. 2008;9:457–462. doi: 10.1016/j.jpain.2008.01.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdle B, Lemming D, Kristiansen J, Larsson B, Peolsson M, Rosendal L. Biochemical alterations in the trapezius muscle of patients with chronic whiplash associated disorders (WAD) - A microdialysis study. Eur J Pain. 2007 doi: 10.1016/j.ejpain.2007.03.009. [DOI] [PubMed] [Google Scholar]
- Hamada K, Vannier E, Sacheck JM, Witsell AL, Roubenoff R. Senescence of human skeletal muscle impairs the local inflammatory cytokine response to acute eccentric exercise. FASEB J. 2005;19:264–266. doi: 10.1096/fj.03-1286fje. [DOI] [PubMed] [Google Scholar]
- Hoheisel U, Unger T, Mense S. Excitatory and modulatory effects of inflammatory cytokines and neurotrophins on mechanosensitive group IV muscle afferents in the rat. Pain. 2005;114:168–176. doi: 10.1016/j.pain.2004.12.020. [DOI] [PubMed] [Google Scholar]
- Jones DA, Newham DJ, Round JM, Tolfree SE. Experimental human muscle damage: morphological changes in relation to other indices of damage. J. Physiol. 1986;375:435–448. doi: 10.1113/jphysiol.1986.sp016126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsdottir IH, Schjerling P, Ostrowski K, Asp S, Richter EA, Pedersen BK. Muscle contractions induce interleukin-6 mRNA production in rat skeletal muscles. J. Physiol. 2000;528(Pt 1):157–163. doi: 10.1111/j.1469-7793.2000.00157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph EK, Parada CA, Levine JD. Hyperalgesic priming in the rat demonstrates marked sexual dimorphism. Pain. 2003;105:143–150. doi: 10.1016/s0304-3959(03)00175-1. [DOI] [PubMed] [Google Scholar]
- Joseph EK, Bogen O, Alessandri-Haber N, Levine JD. PLC-beta 3 signals upstream of PKC epsilon in acute and chronic inflammatory hyperalgesia. Pain. 2007;132:67–73. doi: 10.1016/j.pain.2007.01.027. [DOI] [PubMed] [Google Scholar]
- Kano Y, Masuda K, Furukawa H, Sudo M, Mito K, Sakamoto K. Histological skeletal muscle damage and surface EMG relationships following eccentric contractions. J Physiol Sci. 2008;58:349–355. doi: 10.2170/physiolsci.RP004908. [DOI] [PubMed] [Google Scholar]
- Kano Y, Sampei K, Matsudo H. Time course of capillary structure changes in rat skeletal muscle following strenuous eccentric exercise. Acta Physiol Scand. 2004;180:291–299. doi: 10.1111/j.0001-6772.2003.01250.x. [DOI] [PubMed] [Google Scholar]
- Keller C, Steensberg A, Hansen AK, Fischer CP, Plomgaard P, Pedersen BK. Effect of exercise, training, and glycogen availability on IL-6 receptor expression in human skeletal muscle. J Appl Physiol. 2005a;99:2075–2079. doi: 10.1152/japplphysiol.00590.2005. [DOI] [PubMed] [Google Scholar]
- Keller P, Penkowa M, Keller C, Steensberg A, Fischer CP, Giralt M, Hidalgo J, Pedersen BK. Interleukin-6 receptor expression in contracting human skeletal muscle: regulating role of IL-6. FASEB J. 2005b;19:1181–1183. doi: 10.1096/fj.04-3278fje. [DOI] [PubMed] [Google Scholar]
- Khasar SG, Gold MS, Dastmalchi S, Levine JD. Selective attenuation of muopioid receptor-mediated effects in rat sensory neurons by intrathecal administration of antisense oligodeoxynucleotides. Neurosci. Lett. 1996;218:17–20. doi: 10.1016/0304-3940(96)13111-6. [DOI] [PubMed] [Google Scholar]
- Khasar SG, Gold MS, Levine JD. A tetrodotoxin-resistant sodium current mediates inflammatory pain in the rat. Neurosci. Lett. 1998;256:17–20. doi: 10.1016/s0304-3940(98)00738-1. [DOI] [PubMed] [Google Scholar]
- Koltzenburg M, Wall PD, McMahon SB. Does the right side know what the left is doing? Trends Neurosci. 1999;22:122–127. doi: 10.1016/s0166-2236(98)01302-2. [DOI] [PubMed] [Google Scholar]
- Levine JD, Dardick SJ, Basbaum AI, Scipio E. Reflex neurogenic inflammation. I. Contribution of the peripheral nervous system to spatially remote inflammatory responses that follow injury. J. Neurosci. 1985;5:1380–1386. doi: 10.1523/JNEUROSCI.05-05-01380.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao P, Zhou J, Ji LL, Zhang Y. Eccentric Contraction Induces Inflammatory Responses in Rat Skeletal Muscle: Role of Tumor Necrosis Factor {alpha} Am J Physiol Regul Integr Comp Physiol. 2010;298:R599–607. doi: 10.1152/ajpregu.00480.2009. [DOI] [PubMed] [Google Scholar]
- Meckel Y, Eliakim A, Seraev M, Zaldivar F, Cooper DM, Sagiv M, Nemet D. The effect of a brief sprint interval exercise on growth factors and inflammatory mediators. J Strength Cond Res. 2009;23:225–230. doi: 10.1519/JSC.0b013e3181876a9a. [DOI] [PubMed] [Google Scholar]
- Mortensen OH, Andersen K, Fischer C, Nielsen AR, Nielsen S, Akerstrom T, Aastrom MB, Borup R, Pedersen BK. Calprotectin is released from human skeletal muscle tissue during exercise. J. Physiol. 2008;586:3551–3562. doi: 10.1113/jphysiol.2008.153551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller-Newen G. The cytokine receptor gp130: faithfully promiscuous. Sci STKE. 2003;2003:PE40. doi: 10.1126/stke.2003.201.pe40. [DOI] [PubMed] [Google Scholar]
- Murase S, Terazawa E, Queme F, Ota H, Matsuda T, Hirate K, Kozaki Y, Katanosaka K, Taguchi T, Urai H, Mizumura K. Bradykinin and nerve growth factor play pivotal roles in muscular mechanical hyperalgesia after exercise (delayed-onset muscle soreness) J. Neurosci. 2010;30:3752–3761. doi: 10.1523/JNEUROSCI.3803-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochi E, Nakazato K, Ishii N. Effects of eccentric exercise on joint stiffness and muscle connectin (titin) isoform in the rat hindlimb. J Physiol Sci. 2007;57:1–6. doi: 10.2170/physiolsci.RP008806. [DOI] [PubMed] [Google Scholar]
- Parada CA, Yeh JJ, Joseph EK, Levine JD. Tumor necrosis factor receptor type-1 in sensory neurons contributes to induction of chronic enhancement of inflammatory hyperalgesia in rat. Eur J Neurosci. 2003a;17:1847–1852. doi: 10.1046/j.1460-9568.2003.02626.x. [DOI] [PubMed] [Google Scholar]
- Parada CA, Yeh JJ, Reichling DB, Levine JD. Transient attenuation of protein kinase Cepsilon can terminate a chronic hyperalgesic state in the rat. Neuroscience. 2003b;120:219–226. doi: 10.1016/s0306-4522(03)00267-7. [DOI] [PubMed] [Google Scholar]
- Parada CA, Reichling DB, Levine JD. Chronic hyperalgesic priming in the rat involves a novel interaction between cAMP and PKCepsilon second messenger pathways. Pain. 2005;113:185–190. doi: 10.1016/j.pain.2004.10.021. [DOI] [PubMed] [Google Scholar]
- Pedersen BK. Edward F. Adolph distinguished lecture: muscle as an endocrine organ: IL-6 and other myokines. J Appl Physiol. 2009;107:1006–1014. doi: 10.1152/japplphysiol.00734.2009. [DOI] [PubMed] [Google Scholar]
- Pedersen BK, Febbraio MA. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol. Rev. 2008;88:1379–1406. doi: 10.1152/physrev.90100.2007. [DOI] [PubMed] [Google Scholar]
- Rice TL, Chantler I, Loram LC. Neutralisation of muscle tumour necrosis factor alpha does not attenuate exercise-induced muscle pain but does improve muscle strength in healthy male volunteers. Br J Sports Med. 2008;42:758–762. doi: 10.1136/bjsm.2007.038067. [DOI] [PubMed] [Google Scholar]
- Rosa Neto JC, Lira FS, Oyama LM, Zanchi NE, Yamashita AS, Batista MLJ, Oller do Nascimento CM, Seelaender M. Exhaustive exercise causes an anti-inflammatory effect in skeletal muscle and a pro-inflammatory effect in adipose tissue in rats. Eur J Appl Physiol. 2009;106:697–704. doi: 10.1007/s00421-009-1070-1. [DOI] [PubMed] [Google Scholar]
- Rosendal L, Kristiansen J, Gerdle B, Sogaard K, Peolsson M, Kjaer M, Sorensen J, Larsson B. Increased levels of interstitial potassium but normal levels of muscle IL-6 and LDH in patients with trapezius myalgia. Pain. 2005a;119:201–209. doi: 10.1016/j.pain.2005.09.026. [DOI] [PubMed] [Google Scholar]
- Rosendal L, Sogaard K, Kjaer M, Sjogaard G, Langberg H, Kristiansen J. Increase in interstitial interleukin-6 of human skeletal muscle with repetitive low-force exercise. J Appl Physiol. 2005b;98:477–481. doi: 10.1152/japplphysiol.00130.2004. [DOI] [PubMed] [Google Scholar]
- Schafers M, Sorkin LS, Sommer C. Intramuscular injection of tumor necrosis factor-alpha induces muscle hyperalgesia in rats. Pain. 2003;104:579–588. doi: 10.1016/S0304-3959(03)00115-5. [DOI] [PubMed] [Google Scholar]
- Shah JP, Phillips TM, Danoff JV, Gerber LH. An in vivo microanalytical technique for measuring the local biochemical milieu of human skeletal muscle. J Appl Physiol. 2005;99:1977–1984. doi: 10.1152/japplphysiol.00419.2005. [DOI] [PubMed] [Google Scholar]
- Shah JP, Danoff JV, Desai MJ, Parikh S, Nakamura LY, Phillips TM, Gerber LH. Biochemicals associated with pain and inflammation are elevated in sites near to and remote from active myofascial trigger points. Arch Phys Med Rehabil. 2008;89:16–23. doi: 10.1016/j.apmr.2007.10.018. [DOI] [PubMed] [Google Scholar]
- Sluka KA, Radhakrishnan R, Benson CJ, Eshcol JO, Price MP, Babinski K, Audette KM, Yeomans DC, Wilson SP. ASIC3 in muscle mediates mechanical, but not heat, hyperalgesia associated with muscle inflammation. Pain. 2007;129:102–112. doi: 10.1016/j.pain.2006.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluka KA, Rasmussen LA. Fatiguing exercise enhances hyperalgesia to muscle inflammation. Pain. 2010;148:188–197. doi: 10.1016/j.pain.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LL, McKune AJ, Semple SJ, Sibanda E, Steel H, Anderson R. Changes in serum cytokines after repeated bouts of downhill running. Appl Physiol Nutr Metab. 2007;32:233–240. doi: 10.1139/h06-106. [DOI] [PubMed] [Google Scholar]
- Stauber WT. Factors involved in strain-induced injury in skeletal muscles and outcomes of prolonged exposures. J Electromyogr Kinesiol. 2004;14:61–70. doi: 10.1016/j.jelekin.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Steensberg A, Dalsgaard MK, Secher NH, Pedersen BK. Cerebrospinal fluid IL-6, HSP72, and TNF-alpha in exercising humans. Brain Behav. Immun. 2006;20:585–589. doi: 10.1016/j.bbi.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Steensberg A, van Hall G, Osada T, Sacchetti M, Saltin B, Klarlund Pedersen B. Production of interleukin-6 in contracting human skeletal muscles can account for the exercise-induced increase in plasma interleukin-6. J. Physiol. 2000;529(Pt 1):237–242. doi: 10.1111/j.1469-7793.2000.00237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summer GJ, Romero-Sandoval EA, Bogen O, Dina OA, Khasar SG, Levine JD. Proinflammatory cytokines mediating burn-injury pain. Pain. 2007 doi: 10.1016/j.pain.2007.05.012. [DOI] [PubMed] [Google Scholar]
- Taguchi T, Matsuda T, Tamura R, Sato J, Mizumura K. Muscular mechanical hyperalgesia revealed by behavioural pain test and c-Fos expression in the spinal dorsal horn after eccentric contraction in rats. J. Physiol. 2005;564:259–268. doi: 10.1113/jphysiol.2004.079483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toft AD, Jensen LB, Bruunsgaard H, Ibfelt T, Halkjaer-Kristensen J, Febbraio M, Pedersen BK. Cytokine response to eccentric exercise in young and elderly humans. Am J Physiol Cell Physiol. 2002;283:C289–95. doi: 10.1152/ajpcell.00583.2001. [DOI] [PubMed] [Google Scholar]
- Tomiya A, Aizawa T, Nagatomi R, Sensui H, Kokubun S. Myofibers express IL-6 after eccentric exercise. Am J Sports Med. 2004;32:503–508. doi: 10.1177/0095399703258788. [DOI] [PubMed] [Google Scholar]