Abstract
Objectives
To examine the impact of HIV on lung cancer incidence and survival.
Design
Prospective study of 2,495 HIV-infected and HIV-uninfected injection drug users in Baltimore, MD.
Methods
Cancer data were obtained from the Maryland Cancer Registry. We estimated hazard ratios (HRs) and 95% confidence intervals (CIs) for lung cancer in two strata of packs smoked per day by HIV serostatus, and for mortality by HIV serostatus.
Results
HIV-infected participants had twice the risk (HR=2.3; 95% CI: 1.1-5.1) of lung cancer. There was no evidence of an interaction between HIV and packs of cigarettes smoked per day (p-interaction=0.18). Compared to participants who smoked <1.43 packs per day, among HIV-uninfected individuals lung cancer risk was six times greater (HR=5.9; 95% CI 2.1-17) and among HIV-infected individuals lung cancer risk was doubled (HR=2.1; 95% CI 0.63-6.8) in persons who smoked ≥1.43 per day. Additionally, HIV was associated with four times the risk of death (HR=3.8; 95% CI 0.92-15) in lung cancer cases.
Conclusions
HIV was associated with increased risk of lung cancer, after adjusting for smoking. However, no evidence was observed for synergistic effects of HIV and smoking. Further, HIV was associated with poorer lung cancer survival, after accounting for cancer stage.
Keywords: Cancer, Lung, Smoking, Survival, Drug Users
INTRODUCTION
After Kaposi sarcoma and non-Hodgkin lymphoma, lung cancer is the most common cancer among HIV-infected individuals,1;2 with an incidence rate that is two to three times higher among HIV-infected individuals than in the general population.3;4 However, it is likely that the increased risk of lung cancer is partially driven by the high prevalence of cigarette smoking in HIV-infected individuals. For example, the prevalence of cigarette smoking was 59% among persons with HIV/AIDS in New York State, three times the prevalence in the general U.S. population.5 Further, the prevalence of smoking among HIV-infected women in the Women’s Interagency HIV Study was 56%,6 and the prevalence of smoking among injection drug users (IDUs) in the Swiss HIV Cohort Study was 96%.7
Several recent studies have suggested that HIV infection is associated with lung cancer, after adjusting for cigarette smoking.8-10 As in the general population, the vast majority of lung cancers in HIV-infected individuals occur among cigarette smokers.10-12 Consequently, HIV infection might enhance the effects of cigarette smoke rather than being independently carcinogenic.8;9 One hypothesized mechanism asserts that increased HIV-associated inflammation in the lungs could lead to a greater predisposition to smoking-related lung damage.9 Previous studies have not fully examined whether HIV interacts with cigarette smoking, potentially enhancing its carcinogenic effects.
Based on previous literature, it also remains unclear whether survival following a lung cancer diagnosis is differentially impacted by HIV infection. One prior study reported an increased risk of death among HIV-infected lung cancer patients compared to HIV-indeterminate lung cancer patients. However, the difference in mortality was primarily due to differences in cancer stage at diagnosis as no difference was observed by HIV status after adjusting for stage and other covariates.11
In the present study, we examined whether the association between cigarette smoking and the risk of lung cancer differs by HIV status and whether HIV impacts survival in a large, well-characterized prospective cohort study of HIV-infected and HIV-uninfected IDUs.
METHODS
Study Population
The AIDS Link to the Intravenous Experience (ALIVE) study is a prospective cohort study that follows community-recruited, HIV-infected and HIV-uninfected IDUs in Baltimore, MD. Through original enrollment in 1988-89 and subsequent recruitments in 1994-1995, 1998, 2000 and 2005-07, ALIVE has enrolled 4,311 participants. The original cohort included individuals who were at least 18 years old with a history of injection drug use. Additionally, HIV-infected participants had to be AIDS-free at enrollment. Participants are interviewed and data are collected on behavioral practices and medical care. Additionally, blood samples are collected for HIV antibody testing or measurement of CD4 cell counts and HIV-1 RNA levels, depending on HIV status. The current analysis included 2,495 participants with at least two years of follow-up. The ALIVE study has maintained institutional review board approval through the Johns Hopkins School of Public Health and all participants provided written informed consent.
Lung Cancer Ascertainment
Lung cancer cases were ascertained through linkage of ALIVE cohort participants with the Maryland State Cancer Registry. Three additional lung cancer cases were identified through death certificate review. The Maryland State Cancer Registry has collected information on incident cancers since 1982, with reporting mandated since July 1, 1991.13 Information on primary cancer sites, dates of cancer diagnoses, and tumor stage were available.
Exposure Assessment
ALIVE participants were tested for HIV at baseline, and HIV-uninfected participants were tested for HIV antibodies with commercial tests at subsequent visits. For seroconverters, date of HIV seroconversion was estimated to be the midpoint between the last HIV-negative visit and first HIV-positive visit. The median seroconversion window was 0.51 years (interquartile range [IQR]: 0.49, 0.61 years). Information about cigarette smoking was collected through semi-annual questionnaires. Participants were asked if they smoked cigarettes and how many cigarettes they smoked in the past six months. Average packs of cigarettes per day were calculated with data from baseline through two years prior to the end of follow-up, to allow for a lag prior to cancer diagnosis. Additionally, information on birth date, gender, race, insurance status, and use of injected and inhaled illicit drugs were collected by questionnaire. Information on cause of death was obtained from review of death certificates and linkage with the National Death Index.
Statistical Analysis
We examined whether the association between the number of packs of cigarettes smoked per day and lung cancer risk was modified by HIV infection. Participants were censored at the earliest of the following events: death, two years after loss to follow-up (to allow for additional cancer ascertainment), or December 31, 2006. Cox proportional hazards regression was used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for lung cancer across categories of the average number of packs of cigarettes smoked per day, dichotomized based on the median value among lung cancer cases: 0-1.42 and 1.43-2.50 packs per day. An interaction term between category of packs smoked per day and HIV was added to the model to test for the presence of effect modification. Joint categories of HIV and packs smoked per day were assessed in a separate model. Models were adjusted for age at baseline and gender. HIV status was treated as a time-varying covariate. Age at baseline was modeled as a restricted cubic spline with knots at the 5th, 50th and 95th percentiles (25, 35 and 48 years) to allow non-linear associations with packs smoked per day,14 and gender (male vs. female) was treated as an indicator variable. Inhaled drug use was examined, but not included in the final models, as its inclusion did not alter the associations of interest. Further, we tested for the presence of an interaction between HIV and cigarette smoking on the risk of all smoking-associated cancers combined (i.e., lung, pancreas, larynx, cervix, stomach, and kidney cancers; n=44).
Weighted Kaplan-Meier curves were generated to compare adjusted survival from lung cancer to death by HIV status.15 Cox proportional hazards regression was used to compare mortality among HIV-infected and HIV-uninfected lung cancer cases. HRs and 95% CIs were estimated with HIV as the exposure and time from lung cancer diagnosis until death as the outcome of interest. Regression models were adjusted for age at diagnosis, gender, distant cancer stage, average packs smoked per day, current injection drug use at diagnosis, and insurance status at diagnosis. Age was modeled with restricted cubic splines, with knots at the 5th, 50th and 95th percentiles (40, 53 and 60 years). Gender (male vs. female), distant stage (distant vs. regional and local), current injection drug use at diagnosis (yes vs. no) and insurance status (yes vs. no) were modeled as indicator variables and packs of cigarettes smoked per day was modeled as a linear term. An additional sub-analysis was restricted to those with distant stage lung cancers, excluding regional and local stage cancers. Data was analyzed with SAS version 9.1 (SAS Institute, Cary, NC).
RESULTS
A total of 2,495 ALIVE participants (1,423 HIV-uninfected, 740 HIV-infected at baseline and 332 who seroconverted during follow-up) were followed for 25,708 person-years between 1988 and December 31, 2006. Tweny-nine lung cancer cases occurred among participants with at least two years of follow-up. Participants that developed lung cancer were older at baseline (median age: 46 years vs. 35 years; Table 1). The proportion of men (72% vs. 74%, respectively) and African Americans (97 vs. 94%, respectively) was similar among those with and without lung cancer. The proportion of participants that had ever used inhaled drugs was lower in lung cancer cases (48 vs. 56%). Nearly all ALIVE participants (94%) were ever smokers. All participants that developed lung cancer had a self-reported history of smoking while enrolled in the study, and smoked a greater median number of packs of cigarettes per day than the remainder of the ALIVE cohort (1.4 vs. 1.0 packs per day). The average number of packs smoked per day during follow-up was the same (i.e., 1.0 pack per day) for HIV-infected and HIV-uninfected participants.
Table 1.
Characteristics of 2,495 men and women enrolled in the ALIVE study, 29 of whom develop lung cancer during follow up between 1988 and 2006
| Lung cancer cases (n=29) |
Participants without lung cancer (n=2466) |
|
|---|---|---|
| Age at baseline (years), median (IQR) | 46 (40, 49) | 35 (31, 40) |
| Person-years of follow-up, median (IQR) | 7.3 (5.4, 9.8) | 9.8 (5.7, 15) |
| Men, n (%) | 21 (72) | 1,819 (74) |
| African American, n (%) | 28 (97) | 2,315 (94) |
| Ever smoker, n (%) | 29 (100) | 2,324 (94) |
| Average packs smoked per day, median (IQR) | 1.4 (1.1, 1.6) | 1.0 (0.65, 1.4) |
| Inhaled drugs use, n (%) | 14 (48) | 1,369 (56) |
| HIV status: | ||
| Negative, n (%) | 16 (55) | 1,407 (57) |
| Positive, n (%) | 9 (31) | 731 (30) |
| Seroconverter, n (%) | 4 (14) | 328 (13) |
IQR: interquartile range
Of the 29 participants that developed lung cancer, 13 were HIV-infected and 16 were HIV-uninfected. At cancer diagnosis, the median CD4 cell count among HIV-infected lung cancer cases was 267 cells/mm3 (IQR: 199, 437) and 46% had a history of highly active antiretroviral therapy (HAART) use (n=6). HIV-infected participants had an increased risk (HR=2.3; 95% CI: 1.1-5.1) of developing lung cancer compared to HIV-uninfected participants, after adjusting for age, gender, and average packs smoked per day. Without adjustment for cigarette smoking, the association was similar (HR=2.0; 95% CI: 0.92-4.2). The results were comparable for all smoking-associated cancers combined (HR=2.0; 95% CI: 1.1-3.7).
We compared the risk of lung cancer in binary categories of average packs of cigarettes smoked per day among HIV-infected and HIV-uninfected individuals, after adjusting for age and gender (Table 2). For HIV-uninfected individuals the risk of lung cancer was six times greater (HR=5.9; 95% CI 2.1-17) and in HIV-infected individuals the risk of lung cancer was doubled (HR=2.1; 95% CI 0.63-6.8) in persons who smoked ≥1.43 packs of cigarettes per day, compared to those who smoked <1.43 packs of cigarettes per day. Though the association between packs smoked per day and lung cancer was stronger among HIV-uninfected individuals, there was no evidence for an interaction between HIV status and packs smoked per day (p-interaction=0.18). When we treated average packs of cigarettes per day as a continuous variable, we also observed no evidence of interaction (p-interaction=0.85). Compared to HIV-uninfected individuals who smoked <1.43 packs per day, HIV-infected individuals who smoked <1.43 packs per day had greater than triple the risk (HR=3.5; 95% CI 1.2-10) of lung cancer and HIV-infected individuals who smoked ≥1.43 packs per day had greater than seven times the risk (HR=7.2; 95% CI 2.0-26) of developing lung cancer. When the analysis was expanded to include 44 smoking-associated cancer cases with at least two years of follow-up, there was also no significant interaction between HIV status and average packs of cigarettes per day in categorical (p-interaction=0.90) or continuous analyses (p-interaction=0.94).
Table 2.
Hazard ratios and 95% confidence limits for lung cancer by average number of packs smoked per day, 2,495 men and women enrolled in the ALIVE study and followed between 1988 and 2006
| Packs per day | Cases | Person-years | Hazard Ratio1,2, 3 (95% CI1) |
Hazard Ratio1,2,4 (95% CI1) |
|
|---|---|---|---|---|---|
| HIV-uninfected | |||||
| <1.43 | 6 | 12,392 | 1.0 | 1.0 | |
| ≥1.43 | 10 | 3,622 | 5.9 (2.1, 17) | 5.9 (2.1, 17) | |
| HIV-infected | |||||
| <1.43 | 9 | 7,706 | 1.0 | 3.5 (1.2, 16) | |
| ≥1.43 | 4 | 1,989 | 2.1 (0.63, 6.8) | 7.2 (2.0, 26) |
p-interaction=0.18
Confidence intervals, CI
Hazard ratio adjusted for age and gender.
Hazard ratios compares lung cancer risk for those who smoke ≥1.43 packs per day compared to those who smoke <1.43 packs per day, stratified by HIV status.
Hazard ratios compare people who are HIV-uninfected and smoke ≥1.43 packs per day, people who are HIV-infected and smoke <1.43 packs per day and people who are HIV-infected and smoke ≥1.43 packs per day to those who are HIV-uninfected and smoke <1.43 packs per day.
Of the 29 incident lung cancer cases that occurred during follow-up, 26 were identified by the Maryland Cancer Registry and 3 were identified by death certificate (1 HIV-infected and 2 HIV-uninfected). Of the 12 HIV-infected incident cases, the most common histological type of lung cancer was squamous cell carcinoma (n=5, 42%). Of the 14 HIV-uninfected incident lung cancer cases, adenocarcinoma was the most common histological type (n=7, 50%). One adenocarcinoma occurred among HIV-infected cases and three squamous cell carcinomas occurred among HIV-uninfected cases. Among HIV-infected cases, 4 (33%) were regional stage and 8 (64%) were distant stage compared to 2 (14%) local stage, 5 (36%) regional stage, 6 (43%) distant stage and 1 (7%) unstaged among HIV-uninfected cases.
Three additional lung cancers not meeting the inclusion criteria for the interaction analysis were included in the lung cancer survival analysis (two cases that occurred more than two years after their last ALIVE visit, and one case with less than 2 years of follow-up). Among the 29 incident lung cancer cases, 26 deaths occurred in 33.3 person-years of follow-up (Table 3). Of the three survivors, two were HIV-uninfected (one regional stage cancer diagnosed in 2000 and one distant stage cancer in 2006) and one was HIV-infected (regional stage cancer diagnosed in 2006). According to death certificate review, 24 of the deaths were due to cancer. One death related to a fall occurred in an HIV-infected case and another death related to a bleeding disorder occurred in an HIV-uninfected case; none of the deaths were due to AIDS. Five (38%) of the HIV-infected and seven (44%) of the HIV-uninfected cases had evidence of receiving chemotherapy, radiation therapy or surgery.
Table 3.
Hazard ratios (95% CI) for mortality among those who were HIV-infected compared to those who were HIV-uninfected among 29 lung cancer cases in the ALIVE study, followed between 1988 and 2006
| All Lung Cancer Cases (n=29) |
Distant Stage Lung Cancer Cases (n=17) |
|||||
|---|---|---|---|---|---|---|
| Deaths | Age-adjusted HR (95% CI) |
Adjusted HR (95% CI)* |
Deaths | Age-adjusted HR (95% CI) |
Adjusted HR (95% CI)† |
|
| HIV− | 14 | 1 | 1 | 7 | 1 | 1 |
| HIV+ | 12 | 2.9 (1.0, 8.2) | 3.8 (0.92, 15) | 9 | 3.5 (0.85, 15) | 4.3 (0.84,22) |
ALIVE: AIDS Link to Intravenous Experience; HR: hazard ratio; CI: confidence intervals; IQR: interquartile range
adjusted for age, gender, distant stage, average packs smoked per day, current injection drug use at diagnosis and insurance status at diagnosis
adjusted for age, gender, average packs smoked per day, current injection drug use at diagnosis and insurance status at diagnosis
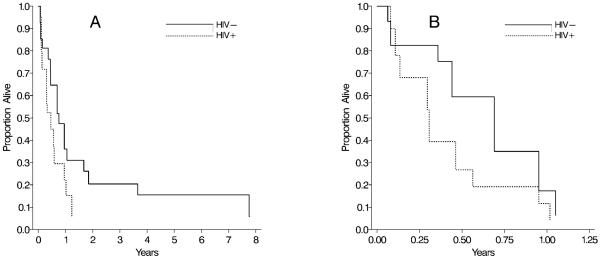
HIV-infected lung cancer cases had a shorter median survival time (0.39 years; IQR: 0.21; 0.77) than HIV-uninfected cases (0.72 years; IQR: 0.36; 1.7), and a lower one-year survival rate (18 vs. 42%) (Figure 1A). After adjusting for age, gender, distant stage, average packs smoked per day, current injection drug use and insurance status (Table 3), the HR of death was 3.8 (95% CI: 0.92-15) for HIV-infected compared to HIV-uninfected lung cancer cases. When the analysis was restricted to the 17 lung cancer cases with distant stage disease at diagnosis, the median survival times were 0.31 (IQR: 0.13, 0.56) and 0.44 (0.08, 0.95) years, and the one-year survival rates were 9% and 17% for HIV-infected and HIV-uninfected distant stage lung cancer cases (Figure 1B). The adjusted HR of death was 4.3 (95% CI: 0.84-22; Table 3).
Figure 1.
Adjusted survival curves for HIV-infected and HIV-uninfected lung cancer cases (A) and distant stage lung cancer cases (B) in the ALIVE study, 1988-2006.
DISCUSSION
HIV infection was associated with greater than twice risk of developing lung cancer, after adjustment for average number of packs of cigarettes smoked per day and other covariates. However, we observed no evidence for modification of the effect of cigarette smoking in development of lung cancer by HIV status. Lung cancer cases occurring in this urban, predominantly African American cohort of IDUs generally presented at advanced stages and survival was almost uniformly dismal with cancer reported as the underlying cause of death. We observed HIV-infected lung cancer cases to have shorter survival than HIV-uninfected lung cancer cases, though with limited cases this was not statistically significant.
The incidence rates of lung cancer have been shown to be elevated among HIV-infected individuals in a number of studies.1;3;4;16 In data from the Johns Hopkins HIV clinical cohort in Baltimore, the standardized incidence ratio (SIR) for lung cancer was 5.5.16 Additionally, a meta-analysis showed that HIV-infected individuals have 2.7 times the risk of lung cancer, compared to the general population.3 The differences in lung cancer rates are likely partially attributed to differences in smoking prevalence between HIV-infected individuals and the general population.7;17-20 However, we observed HIV infection to be associated with 2.3-times the risk of developing lung cancer, even after controlling for packs smoked per day. The association between HIV infection and incident lung cancer is attenuated compared to a previous study in ALIVE, which found HIV to increase the risk of lung cancer death by nearly four-fold, after adjustment for smoking and other covariates.10 As we observed HIV-infected individuals with lung cancer to have a shorter survival than HIV-uninfected individuals, this attenuation is likely due to the use of lung cancer mortality as the outcome in the prior study. Two studies have also found associations between HIV or AIDS and lung cancer risk in the absence of complete smoking data, by using alternate methods for adjustment of cigarette smoking.8;9 The Women’s Interagency HIV Study also found twice the risk of lung cancer when comparing HIV-infected to HIV-uninfected women, but this result was not statistically significant, and was based on only 14 lung cancer cases.21 Given the extremely high prevalence of smoking5-7 in HIV-infected individuals and the decreasing risk of AIDS-related death,22 the incidence of lung cancer and the number of deaths due attributed to lung cancer among HIV-infected individuals may increase over time.
Sparse data exist regarding mechanisms by which HIV may increase risk for lung cancer; however, several hypotheses have been proposed. For example, HIV-associated immune suppression may lead to reduced tumor surveillance, allowing tumors to continue to develop when they ordinarily would be eliminated by the immune system.23;24 Additionally, HIV-induced chronic inflammation of the lung and repeated lung infections may increase the risk of lung cancer among HIV-infected individuals.12 Another hypothesis is that HIV may enhance the carcinogenic effects of cigarette smoking.9;12 However, we observed no evidence for multiplicative interaction between packs smoked per day and HIV infection on the risk of developing a lung cancer, though interpretation of interaction should be performed cautiously with the limited numbers of cases.
We observed HIV infection to be associated with four times the hazard of death among lung cancer cases and distant stage lung cancer cases, although limited power precluded these findings from being statistically significant. Several previous studies have shown poorer survival among those with HIV or AIDS,25-28 though one study found no difference between HIV-infected and HIV-indeterminate patients after accounting for stage at presentation.11
The differences that we observed in survival were not due to AIDS or other competing causes of death. For both HIV-infected and HIV-uninfected individuals, all but two of the deaths were due to cancer. We were unable to thoroughly examine whether differential cancer treatment may have contributed to the differences in survival. Though treatment data was provided by the Maryland Cancer Registry, data is only collected on the first course of cancer treatment. Thus, we are limited in drawing any conclusions from this study regarding the role of treatment in the shorter survival of HIV-infected lung cancer cases. Unlike a prior study,11 the differences in survival by HIV status that we observed were not due to later stage at diagnosis among HIV-infected individuals. When restricted to distant stage cancer cases, we continued to observe an increased risk of death among those with HIV. It has also been suggested that the differences in survival may be due to HIV infection leading to a more aggressive form of lung cancer.11 It is important to note that lung cancer survival was poor among both HIV-infected and HIV-uninfected participants, perhaps reflecting limited access to care among IDUs, irrespective of HIV status.
Our use of cohort data that included collection of smoking exposure among both HIV-infected and HIV-uninfected individuals followed through identical methods provides an appropriate comparison group and is a main strength of this report. The lack of available data from HIV-uninfected individuals drawn from the same source population as HIV-infected individuals is a central limitation of many prior studies that have examined HIV and cancer risk. The availability of this data allowed for adjustment of covariates, including average number of packs of cigarettes smoked during follow-up, and supports our findings that HIV infection increases risk for lung cancer independent of smoking.
Our study had several limitations. Although we evaluated a large cohort with extensive follow-up, we identified a relatively small number of cancer cases, which limited the precision of our estimates and the power to detect effect modification. Additionally, cancers may not have been ascertained if study participants lived outside of Maryland. However, >98% of ALIVE participants live in Maryland, and we do not believe under-ascertainment would be differential by HIV status, thus this limitation should not have biased our results. The baseline smoking data collected in the ALIVE study did not include smoking history prior to study enrollment. Thus, we were unable to include a measure of duration of smoking as a covariate. However, In recent data from active ALIVE participants, we have demonstrated equivalent age at initiation (14 vs. 14 years) and cumulative pack-years of smoking (20 vs. 19) among HIV-uninfected and HIV-infected participants, respectively (Marshall, M.M.; unpublished work).
In conclusion, we observed that HIV infection was associated with an increased risk of lung cancer, after adjustment for individual smoking exposure and other covariates. Our data did not suggest any synergistic effect of HIV infection in enhancing the effect of cigarette smoke on cancer risk. Finally, we observed that lung cancer cases with HIV have may have shorter survival than those without HIV, independent of stage at diagnosis. These data highlight the need for continued investigation of the mechanisms involved in HIV infection influencing the development and subsequent survival of lung cancer.
Acknowledgments
This work was supported by Public Health Service Grants from the National Institute on Drug Abuse: R01 DA12568, R01 DA04334, and the National Heart, Lung and Blood Institute (R01 HL090483). Dr. Shiels was supported by the National Institutes of Health Research Service Award T32 CA009314.
Footnotes
Data presented previously at the 42nd Annual Society for Epidemiologic Research meeting, Anaheim, CA (June 2009) and published as abstract in the American Journal of Epidemiology 2009; 169 (Suppl): S54.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Engels EA, Pfeiffer RM, Goedert JJ, Virgo P, McNeel TS, Scoppa SM, Biggar RJ. Trends in cancer risk among people with AIDS in the United States 1980-2002. AIDS. 2006;20:1645–54. doi: 10.1097/01.aids.0000238411.75324.59. [DOI] [PubMed] [Google Scholar]
- 2.Engels EA, Biggar RJ, Hall HI, Cross H, Crutchfield A, Finch JL, Grigg R, Hylton T, Pawlish KS, McNeel TS, Goedert JJ. Cancer risk in people infected with human immunodeficiency virus in the United States. Int J Cancer. 2008;123:187–94. doi: 10.1002/ijc.23487. [DOI] [PubMed] [Google Scholar]
- 3.Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet. 2007;370:59–67. doi: 10.1016/S0140-6736(07)61050-2. [DOI] [PubMed] [Google Scholar]
- 4.Shiels MS, Cole SR, Kirk GD, Poole C. A meta-analysis of the incidence of non-AIDS cancers in HIV-infected individuals. J Acquir Immune Defic Syndr. 2009;52:611–22. doi: 10.1097/QAI.0b013e3181b327ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tesoriero JM, Gieryic SM, Carrascal A, Lavigne HE. Smoking Among HIV Positive New Yorkers: Prevalence, Frequency, and Opportunities for Cessation. AIDS and Behavior. 2008 doi: 10.1007/s10461-008-9449-2. [DOI] [PubMed] [Google Scholar]
- 6.Goldberg D, Weber KM, Orsi J, Hessol NA, D’Souza G, Watts DH, Schwartz R, Liu C, Glesby M, Burian P, Cohen MH. Smoking cessation among women with and at risk for HIV: are they quitting? J Gen Intern Med. 2010;25:39–44. doi: 10.1007/s11606-009-1150-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clifford GM, Polesel J, Rickenbach M, Dal Maso L, Keiser O, Kofler A, Rapiti E, Levi F, Jundt G, Fisch T, Bordoni A, De Weck D, Franceschi S. Cancer risk in the Swiss HIV Cohort Study: associations with immunodeficiency, smoking, and highly active antiretroviral therapy. J Natl Cancer Inst. 2005;97:425–32. doi: 10.1093/jnci/dji072. [DOI] [PubMed] [Google Scholar]
- 8.Chaturvedi AK, Pfeiffer RM, Chang L, Goedert JJ, Biggar RJ, Engels EA. Elevated risk of lung cancer among people with AIDS. AIDS. 2007;21:207–13. doi: 10.1097/QAD.0b013e3280118fca. [DOI] [PubMed] [Google Scholar]
- 9.Engels EA, Brock MV, Chen J, Hooker CM, Gillison M, Moore RD. Elevated incidence of lung cancer among HIV-infected individuals. J Clin Oncol. 2006;24:1383–88. doi: 10.1200/JCO.2005.03.4413. [DOI] [PubMed] [Google Scholar]
- 10.Kirk GD, Merlo C, O’ Driscoll P, Mehta SH, Galai N, Vlahov D, Samet J, Engels EA. HIV infection is associated with an increased risk for lung cancer, independent of smoking. Clin Infect Dis. 2007;45:103–10. doi: 10.1086/518606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brock MV, Hooker CM, Engels EA, Moore RD, Gillison ML, Alberg AJ, Keruly JC, Yang SC, Heitmiller RF, Baylin SB, Herman JG, Brahmer JR. Delayed diagnosis and elevated mortality in an urban population with HIV and lung cancer: implications for patient care. J Acquir Immune Defic Syndr. 2006;43:47–55. doi: 10.1097/01.qai.0000232260.95288.93. [DOI] [PubMed] [Google Scholar]
- 12.Engels EA. Inflammation in the development of lung cancer: epidemiological evidence. Expert Review of Anticancer Therapy. 2008;8:605–15. doi: 10.1586/14737140.8.4.605. [DOI] [PubMed] [Google Scholar]
- 13.Maryland Cancer Registry . Family Health Administration Department of Health and Mental Hygiene. Baltimore, MD: [Accessed on 1-26-2009]. [Google Scholar]
- 14.Harrell FE. Regression Modeling Strategies. Springer-Verlag New York, Inc.; New York City: 2001. [Google Scholar]
- 15.Cole SR, Hernan MA. Adjusted survival curves with inverse probability weights. Comput Methods Programs Biomed. 2004;75:45–49. doi: 10.1016/j.cmpb.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 16.Long JL, Engels EA, Moore RD, Gebo KA. Incidence and outcomes of malignancy in the HAART era in an urban cohort of HIV-infected individuals. AIDS. 2008;22:489–96. doi: 10.1097/QAD.0b013e3282f47082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dal Maso L, Polesel J, Serraino D, Lise M, Piselli P, Falcini F, Russo A, Intrieri T, Vercelli M, Zambon P, Tagliabue G, Zanetti R, Federico M, Limina RM, Mangone L, De Lisi V, Stracci F, Ferretti S, Piffer S, Budroni M, Donato A, Giacomin A, Bellu F, Fusco M, Madeddu A, Vitarelli S, Tessandori R, Tumino R, Suligoi B, Franceschi S. Pattern of cancer risk in persons with AIDS in Italy in the HAART era. Br J Cancer. 2009;100:840–847. doi: 10.1038/sj.bjc.6604923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galceran J, Marcos-Gragera R, Soler M, Romaguera A, Ameijide A, Izquierdo A, Borras J, de Sanjose S, Casabona J. Cancer incidence in AIDS patients in Catalonia, Spain. Eur J Cancer. 2007;43:1085–91. doi: 10.1016/j.ejca.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 19.Herida M, Mary-Krause M, Kaphan R, Cadranel J, Poizot-Martin I, Rabaud C, Plaisance N, Tissot-Dupont H, Boue F, Lang JM, Costagliola D. Incidence of non-AIDS-defining cancers before and during the highly active antiretroviral therapy era in a cohort of human immunodeficiency virus-infected patients. J Clin Oncol. 2003;21:3447–53. doi: 10.1200/JCO.2003.01.096. [DOI] [PubMed] [Google Scholar]
- 20.Serraino D, Boschini A, Carrieri P, Pradier C, Dorrucci M, Dal Maso L, Ballarini P, Pezzotti P, Smacchia C, Pesce A, Ippolito G, Franceschi S, Rezza G. Cancer risk among men with, or at risk of, HIV infection in southern Europe. AIDS. 2000;14:553–59. doi: 10.1097/00002030-200003310-00011. [DOI] [PubMed] [Google Scholar]
- 21.Levine AM, Seaberg EC, Hessol NA, Preston-Martin S, Silver S, Cohen MH, Anastos K, Minkoff H, Orenstein J, Dominguez G, Watts DH. HIV as a risk factor for lung cancer in women: data from the Women’s Interagency HIV Study. J Clin Oncol. 2010;28:1514–19. doi: 10.1200/JCO.2009.25.6149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lewden C, May T, Rosenthal E, Burty C, Bonnet F, Costagliola D, Jougla E, Semaille C, Morlat P, Salmon D, Cacoub P, Chene G. Changes in causes of death among adults infected by HIV between 2000 and 2005: The “Mortalite 2000 and 2005” surveys (ANRS EN19 and Mortavic) J Acquir Immune Defic Syndr. 2008;48:590–598. doi: 10.1097/QAI.0b013e31817efb54. [DOI] [PubMed] [Google Scholar]
- 23.Bower M, Powles T, Nelson M, Shah P, Cox S, Mandelia S, Gazzard B. HIV-related lung cancer in the era of highly active antiretroviral therapy. AIDS. 2003;17:1085–91. doi: 10.1097/00002030-200302140-00011. [DOI] [PubMed] [Google Scholar]
- 24.Engels EA. Human immunodeficiency virus infection, aging, and cancer. Journal of Clinical Epidemiology. 2001;54(Suppl 1):s29–s34. doi: 10.1016/s0895-4356(01)00444-9. [DOI] [PubMed] [Google Scholar]
- 25.Biggar RJ, Engels EA, Ly S, Kahn A, Schymura MJ, Sackoff J, Virgo P, Pfeiffer RM. Survival after cancer diagnosis in persons with AIDS. J Acquir Immune Defic Syndr. 2005;39:293–99. doi: 10.1097/01.qai.0000164033.02947.e3. [DOI] [PubMed] [Google Scholar]
- 26.Sridhar KS, Flores MR, Raub WA, Jr., Saldana M. Lung cancer in patients with human immunodeficiency virus infection compared with historic control subjects. Chest. 1992;102:1704–8. doi: 10.1378/chest.102.6.1704. [DOI] [PubMed] [Google Scholar]
- 27.Tirelli U, Spina M, Sandri S, Serraino D, Gobitti C, Fasan M, Sinicco A, Garavelli P, Ridolfo AL, Vaccher E. Lung carcinoma in 36 patients with human immunodeficiency virus infection. Cancer. 2000;88:563–69. doi: 10.1002/(sici)1097-0142(20000201)88:3<563::aid-cncr11>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 28.Vyzula R, Remick SC. Lung cancer in patients with HIV-infection. Lung Cancer (Amsterdam, Netherlands) 1996;15:325–39. doi: 10.1016/0169-5002(95)00596-x. [DOI] [PubMed] [Google Scholar]