Abstract
BACKGROUND
Previously, we reported that the complement cleavage fragments C3a and C5a are important modulators of trafficking of hematopoietic stem/progenitor cells (HSPC). The aim of this study was to examine a possible role for C1q in HSPC migration.
STUDY DESIGN AND METHODS
CD34+ HSPC isolated from cord blood (CB), bone marrow (BM) and G-CSF-mobilized peripheral blood (mPB) were evaluated for the expression of C1q and its receptor C1qRp using RT-PCR, Western blot and FACS. Chemotactic responses and chemo-invasiveness towards stromal cell-derived factor (SDF)-1, and expression of matrix metalloproteinase (MMP)-9 were also examined after C1q stimulation. Moreover, G-CSF- and zymosan-induced mobilization was evaluated in C1q-deficient mice.
RESULTS
C1q was expressed in CD34+ cells from mPB, but not from CB or steady-state BM; however, stimulation of the latter with G-CSF induced C1q expression. C1qRp receptor was found on BM, CB and mPB CD34+ cells and more mature ex vivo-expanded myeloid and megakaryocytic precursors. Although C1q itself was not a chemoattractant for HSPC, it primed/enhanced the chemotactic response of CD34+ cells to a low SDF-1 gradient and their chemoinvasion across the reconstituted basement membrane Matrigel, and increased secretion of MMP-9 by these cells. Moreover, in in vivo studies C1q-deficient mice were found to be easy GCSF mobilizers compared to wild-type mice and normal zymosan mobilizers.
CONCLUSION
We demonstrated that C1q primes the responses of CD34+ HSPC to an SDF-1 gradient, which may enhance their ability to stay within BM niches, suggesting that the C1q/C1qRp axis contributes to HSPC homing/retention in BM.
Keywords: Complement, C1q, HSPC homing, SDF-1, MMP-9
INTRODUCTION
The complement (C) system is a powerful effector of the innate immune system which provides critical protection against microbial infections and unwanted host molecules.1 It can be activated in a cascade-like fashion through the classical, alternative or lectin pathways leading to the generation of bioactive peptides such as C3a and C5a (termed anaphylatoxins) and also the formation of the membrane attack complex.2-4 Upstream of C3a and C5a is component C1, the initiator of the classical pathway, which consists of C1q, the recognition subunit of the C1 complex, and two other catalytic subunits. Most complement proteins are of liver origin, but C1q is predominantly produced by macrophages and dendritic cells. C1q has been shown to interact with B-cells, eosinophils, neutrophils, monocytes, fibroblasts, endothelial cells and platelets.5 Binding of C1q to C1qRp (receptor for complement component 1, subcomponent q phagocytosis, also known as human CD93) on these different cell types induces a variety of cellular responses including enhancement of phagocytosis by macrophages,6 production of interleukin (IL)-6, IL-8 and monocyte chemoattractant peptide-1 by endothelial cells7 and gingival and periodontal ligament fibroblasts,8 induction of interferon-γ-producing antigen-specific T cells,9 calcium signaling in microglial cells triggering the release of IL-6 and tumor necrosis factor-α,10 and inhibition of platelet adhesion and spreading.11 Absence of C1q leads to recurrent infections and spontaneous autoimmune disorders associated with cell destruction and the appearance of multiple apoptotic bodies.5 Complement has been implicated in several developmental pathways, organ and tissue regeneration and non-inflammatory processes.
Recently, we have shown that the complement cascade (CC) becomes activated in the bone marrow (BM) during granulocyte-colony stimulating factor (G-CSF)- and zymosan-induced mobilization, and the resulting complement C3 and C5 component cleavage fragments, C3a and C5a, differentially modulate stem cell-trafficking.12-14 While C3a enhances (primes) responsiveness of hematopoietic stem/progenitor cells (HSPC) to a gradient of stromal cell-derived factor (SDF)-1, activation of C5 promotes the mobilization of HSPC by increasing the recruitment and egress of granulocytes. Based on this, we became interested in the role of other complement components in HSPC trafficking and in this study we focused on C1q, an initiator of the classical pathway of complement activation.
Homing of HSPC to the BM and their engraftment is mediated by chemotactic factors, the most potent being SDF-1, which is constitutively produced in BM by stromal cells and osteoblasts and whose functions are mediated by CXCR4, its seven-span transmembrane G-protein coupled receptor. Since the migration capacity of CXCR4null HSPC is impaired, but not totally abrogated,15 it has been suggested that other chemoattractant factors are likely involved in the HSPC homing. As it has been shown that, aside from mature cells, HSPC also express C1qRp,16 we sought to investigate the possible role of C1q in trafficking of HSPC.
MATERIALS AND METHODS
Cells and cultures
Normal BM was obtained from unrelated donors with their informed consent and cord blood (CB) was obtained with the mother's informed consent. Mobilized peripheral blood (mPB) was obtained from patients undergoing leukapheresis using the Cobe Spectra Apheresis System (COBE, Lakewood, CO) following mobilization regimen with G-CSF (Filgastrim, 5 μg/kg BID, Amgen, Thousand Oaks, CA). The protocols used for sample acquisition were approved by the Health Research Ethics Board of the University of Alberta. Light-density mononuclear cells (MNC) were separated from the BM, CB and mPB samples by density gradient centrifugation over 60% Percoll (GE Healthcare, Baie D'Urfe, PQ) and enriched for CD34+ cells by immunoaffinity selection with MACS paramagnetic beads and LS separation columns (Miltenyi Biotec, Auburn, CA) as described previously.17 The purity of the isolated CD34+ cells from mPB was greater than 95%, as determined by fluorescence activated cell sorter (FACS) analysis.
Erythroid, myeloid and megakaryocytic progenitors were expanded ex vivo from CD34+ cells as described previously.18 Briefly, CD34+ cells were suspended in Dulbecco's modified Eagle medium (Invitrogen, Burlington, ON) supplemented with 25% artificial serum. Growth of colony forming unit-granulocyte/macrophage (CFU-GM) cells was stimulated with recombinant human (rh) IL-3, (10 ng/mL) and rh granulocyte/macrophage-colony stimulating factor (GMCSF, 5 ng/mL); burst forming unit-erythroid (BFU-E) cells with rh erythropoietin (2 IU/mL) and rh kit ligand (10 ng/mL); and CFU-megakaryocyte (Meg) with rh thrombopoietin (50 ng/mL) and rh IL-3 (10 ng/mL). Cytokines and growth factors were obtained from Peprotech Inc. (Rocky Hill, NJ). Cultures were incubated at 37°C in a fully humidified atmosphere supplemented with 5% CO2. The cells were stained for C1qRp on days 3 and 11 of expansion and on day 11 for glycophorin A (erythroid), CD33 (myeloid) and CD41 (megakaryocytic) lineage markers and analyzed by flow cytometry as described previously.19
RT-PCR and Western blotting
Expression of mRNA for C1q and GAPDH was evaluated in CD34+ cells isolated from BM, CB and mPB. RNA was isolated using TRIZOL (Gibco-BRL, Gaithersburg, MD). RT-PCR reactions were carried out using primer sequences for human GAPDH (housekeeping gene) as described previously.19 Sequences for C1q were obtained from GenBank (Los Alamos, NM) and used to design the following primer pairs: 5’-CCCAGGGATAAAAGGAGAGAAAGG -3’ sense primer and 5’-GAGATGATGAAGTGGATGGTGCGG -3’ anti-sense primer. Thermocycling was performed with an Eppendorf Mastercycler (Westbury, NY) and the PCR products were electrophoresed on a 2% agarose gel containing ethidium bromide. Gels were visualized under UV light and photographed using the Alpha Innotech Imaging System (San Leandro, CA, USA).
Cell lysates were collected and analyzed for protein expression of C1q by Western blot as previously described by us.19 The membrane was probed with C1q monoclonal antibody (mouse anti-human C1q, Quidel Corp., San Diego, CA) and with a secondary antibody (Immunopure goat anti-mouse, peroxidase-conjugated immunoglobulin (IgG, Pierce Biotechnology, Rockford, IL). Chemiluminescence was detected using the Supersignal West Pico Chemiluminescence system (Pierce).
FACS analysis
For detection of C1q on BM CD34+ cells, BM leukocytes (treated or not with G-CSF) were incubated with isotypic mouse IgG (Dako, Mississauga, ON) and with mouse anti-human C1q (Quidel) for 45 min on ice, then washed and incubated with AlexaFluor 488 goat anti-mouse antibody (Invitrogen) for 30 min on ice. The cells were then incubated with mouse IgG for 15 min followed by labeling with anti-mouse CD34-PE (Beckman Coulter, Mississauga, ON) for 30 min. The C1q receptor, C1qRp, was evaluated using an anti-C1qRp monoclonal antibody (mAb), clone no. 273107 (R &D Systems, Minneapolis, MN), and AlexaFluor 488 goat anti-mouse antibody (Invitrogen). CD34+ cells from mPB, CB and BM, and ex vivo expanded myeloid, megakaryocytic and erythroid progenitors were incubated with mouse IgG for 15 min followed by labeling with lineage markers. After the final wash, cells were fixed in 1% paraformaldehyde and subjected to flow cytometric analysis using a FACScan (Becton Dickinson, San Jose, CA).
Chemotaxis and trans-Matrigel migration assay
Chemotaxis was evaluated using modified Boyden chambers (Neuro Probe Inc., Gaithersburg, MD) as we described in detail previously.20 Pre-warmed serum-free Iscove's modified Dulbecco's medium (IMDM, supplemented with 0.1% bovine serum albumin) containing no chemoattractant (control), C1q (1 μg/mL, Quidel), or SDF-1α(10 ng/mL or 200 ng/mL, Biomedical Research Centre, University of British Columbia, Vancouver, BC) was added to the lower compartments. Aliquots of CD34+ suspension (1.5 × 105 cells/100 μL) pre-incubated or not with C1q (1 μg/mL) were loaded onto the upper compartments and incubated (at 37°C, 95% humidity, 5% CO2) for 3 h. The percentage migration was calculated from the ratio of the number of cells recovered from the lower compartment to the total number of cells loaded. For trans-Matrigel chemo-invasion assay, polycarbonate filters (Nucleopore, Toronto, ON) were coated with reconstituted basement membrane Matrigel (25 μg/filter, Collaborative Biomedical Products, Bedford, MA). In some experiments, CD34+ cells were pre-incubated with mouse anti-human C1q (Quidel) and the rest of the chemotaxis assay was carried out as before. Each experiment was performed using at least three chambers for each condition and counts were performed in duplicate.
Zymography
In order to evaluate whether matrix-degrading enzymes contribute to migration across reconstituted basement membrane, zymography was performed on cell-conditioned media as described previously.17 Briefly, cells were incubated in serum-free IMDM at a concentration of 2 × 106 cells/ml with or without (control) C1q (1 μg/mL) for 24 h at 37°C, 5% CO2. The cell-conditioned media (supernates) were collected after centrifugation and electrophoresed on a 12% sodium dodecyl sulfate-polyacrylamide gel containing 1.5 mg/ml gelatin (Sigma). Medium conditioned by the fibrosarcoma cell line HT-1080 was used as a standard and a clear band at 92 kDa against a Coomassie blue background indicated the presence of MMP-9.
In vivo C1q-/- mice studies
The pathogen-free, 4- to 6-week-old C1q-/- mice (a kind gift from Dr. M. Botto, Imperial College, London, UK) were used in the experiments at age 6 to 8 weeks. The mice were of the same genetic background as C57B1/6. Both mutants (homozygotes) and wild type C57B1/6 mice were bred and maintained for several generations in our laboratory. These animal studies were approved by the Animal Care and Use Committee of the University of Louisville. For G-CSF-induced mobilization mice were injected subcutaneously with 250 μg/kg of human G-CSF (Amgen) daily for 6 days and for zymosan-induced mobilization, injected intravenously with 0.5 mg/mouse of zymosan (Sigma). Six hours after the last G-CSF injection or at 1 h after zymosan injection, mice were bled from the retro-orbital plexus for white blood cell (WBC) count. Fifty μL of PB collected into microvette ethylenediaminetetraacetic acid (EDTA)-coated tubes (Sarstedt Inc., Newton, NC) were run within 2 h of collection on a HemaVet 950 (Drew Scientific Inc., Oxford, CT). In addition, 450 μL of PB was obtained from the vena cava with a 25-gauge needle and 1 mL syringe containing 50 μL of 100 mM EDTA. Red blood cells were lysed with BD Pharm Lyse buffer (BD Biosciences, San Jose, CA) and nucleated cells were washed twice and used for CFU-GM colonies. Briefly, cells were resuspended in human methylcellulose base media (R&D Systems, Inc., Minneapolis, MN) supplemented with 25 ng/mL recombinant murine (rm) GM-CSF and 10 ng/mL rm IL-3 (Millipore, Billerica, MA). Cultures were incubated for 7 days, at which time they were scored for number of CFU-GM colonies under an inverted microscope.
Statistical analysis
Arithmetic means and standard deviations were calculated and statistical significance was defined as p < 0.05 using Student's t test.
RESULTS
C1q is not expressed by CD34+ cells from steady-state BM but G-CSF induces its expression
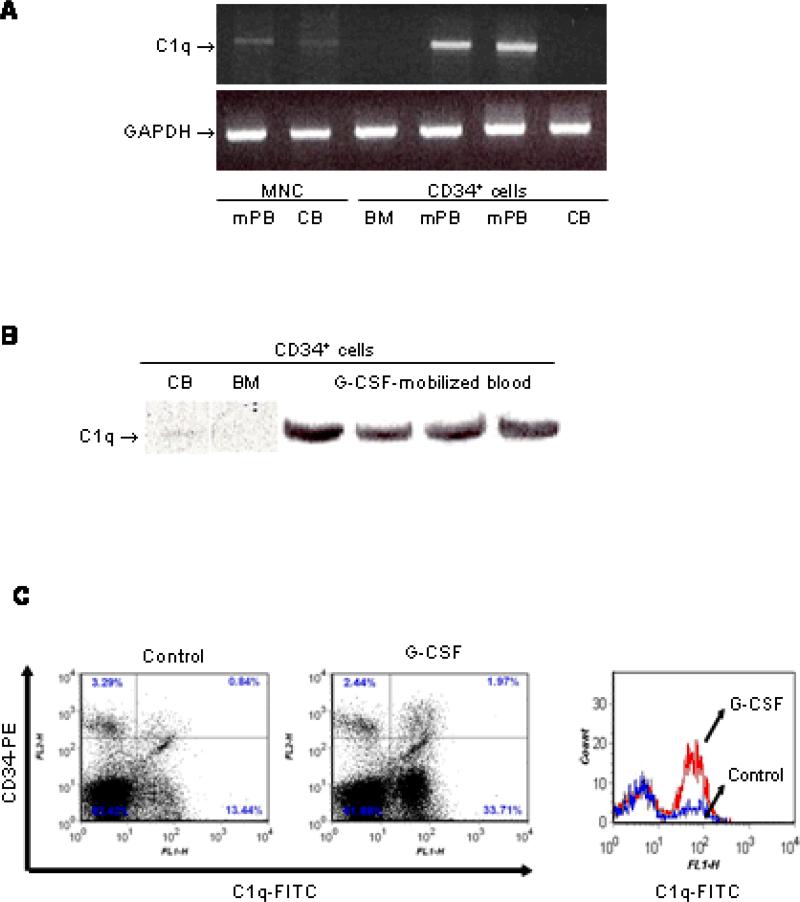
Using semi-quantitative gel-based RT-PCR we found that C1q is not expressed at the mRNA level in CD34+ cells from steady-state BM or CB, but is strongly expressed in CD34+ cells from G-CSF-mobilized PB. To verify that the C1q transcript in CD34+ cells from mPB was not due to contaminating CD34-negative cells, we also performed RT-PCR on mPB mononuclear cells and found only a very weak mRNA expression (Fig. 1A). C1q expression was confirmed at the protein level using Western blot and more CD34+ samples from G-CSF-mobilized patients (n = 4), which demonstrated strong expression of C1q (Fig. 1B). We previously reported that complement is activated in the BM microenvironment during G-CSF-mobilization.21 To determine further whether G-CSF stimulation in vitro induces C1q expression on steady-state BM CD34+ cells, we stimulated BM leukocytes with 200 ng/ml G-CSF (for 5 days). We examined the expression of C1q on the BM CD34+ cells and found that G-CSF induced expression of C1q antigen (Fig. 1C).
Figure 1. C1q is expressed on mobilized (m)PB CD34+ cells but not on steady-state BM CD34+ cells, but G-CSF induces its expression in the latter.
(A) RT-PCR analysis of mRNA transcripts for C1q in CD34+ cells from BM, CB and mPB, and in mononuclear cells (MNC) from mPB and CB. GAPDH was used as a housekeeping gene to ensure equivalence of loading. (B) Western blot for C1q in lysates from normal steady-state BM, mPB and CB CD34+ cells. An equal amount of protein, as measured by Bradford protein assay (30 μg/sample), was loaded in each lane. (C) Steady-state BM leukocytes from healthy donors (n = 2) were stimulated (with 200 ng/mL G-CSF) or not (control) for 5 days. Cells were stained as described in the Materials and Methods. The left and middle histograms represent the expression of C1q on unstimulated (control) and G-CSF-stimulated cells, respectively. In the right panel, BM cells gated based on the expression of the CD34 antigen were analyzed for C1q expression. The lower line shows unstimulated BM CD34+ cells (control) and the upper line G-CSF-stimulated cells.
C1qRp is expressed on HSPC and more mature progenitor cells of granulocytic and megakaryopoietic lineages
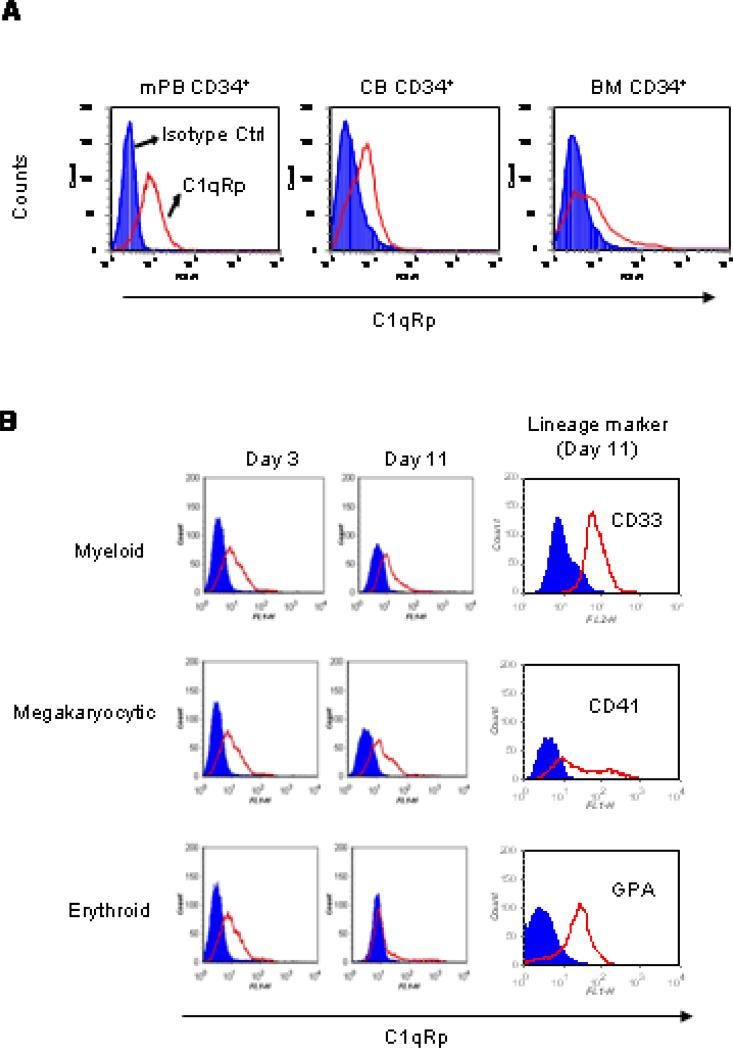
Next, we examined the expression of the C1q receptor, C1qRp, on CD34+ cells from BM, CB and mPB and observed that cells from all three sources express this antigen (Fig. 2A). Furthermore, C1qRp was expressed on ex vivo-expanded progenitor cells evaluated on days 3 and 11 of expansion. C1qRp expression remained high on CFU-GM and CFU-Meg progenitors during their maturation for 11 days, but not on BFU-E precursors (Fig. 2B).
Figure 2. C1qRp is expressed on HSPC and ex vivo-expanded progenitor cells.
(A) CD34+ cells from mPB, CB and BM were labeled with mouse isotype IgG control or with mouse anti-human C1qRp and AlexaFluor 488-conjugated goat anti-mouse antibody. (B) Similar staining was carried out on purified CB CD34+ cells expanded towards granulocytic, megakaryocytic and erythroid lineages on days 3 and 11 of expansion. Expression of surface lineage markers were evaluated on day 11 of expansion. Shaded histograms show isotype IgG control.
C1q increases/primes chemotaxis and chemoinvasion of CD34+ cells towards an SDF-1 gradient
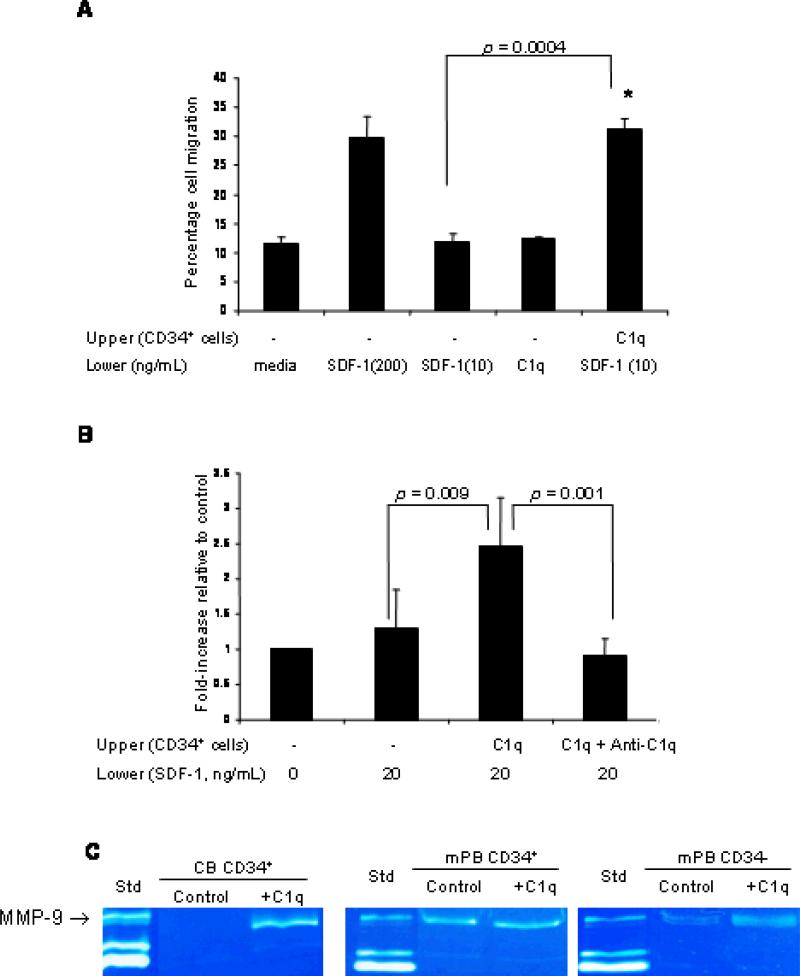
As immune functions involving C1q are considered to be mediated by C1q receptors present on the effector cell surface, we then evaluated the functionality of C1qRp in the chemotactic response of CD34+ cells towards an SDF-1 gradient. Although C1q (1 μg/mL) by itself did not chemoattract CD34+ cells (Fig. 3A), we found that CD34+ cells pre-incubated with C1q protein displayed an increased chemotactic response to a low, “threshold” dose of SDF-1 (10 ng/mL) to the extent that it approximated response to a high SDF-1 gradient (200 ng/mL) (Fig. 3A). Since the SDF-1–CXCR4 axis plays an essential role in the retention and release of HSPC from the BM,22 we then investigated whether C1q can modulate functions that are critical to the homing and engraftment of HSPC. Given that the ability of cells to cross basement membranes and extracellular matrix (ECM) Matrigel is required for their homing to BM niches, we performed the migration assay using reconstituted basement membrane Matrigel. We found that CD34+ cells stimulated with C1q significantly increased trans-Matrigel migration towards a low dose of SDF-1 (20 ng/mL) and this priming effect was inhibited by the neutralizing anti-C1q antibody (Fig. 3B). To determine whether MMPs, which are capable of degrading ECM components, contribute to increased trans-Matrigel migration, CD34+ cells were pre-incubated with C1q and the secretion of MMP-9 was evaluated by zymography. We found that C1q induced MMP-9 secretion by CB CD34+ cells, and stimulated only weakly MMP-secretion in CD34+ cells from G-CSF mobilized blood (Fig. 3C), probably because the CD34+ cells from the latter have already been exposed to complement activation during the mobilization process.
Figure 3. Priming effect of C1q on chemotaxis and trans-Matrigel migration of CD34+ cells towards SDF-1.
(A) Chemotaxis of CB CD34+ cells pre-incubated or not with C1q (1 μg/mL) was evaluated towards media alone, low SDF-1 gradient (10 ng/mL), high SDF-1 gradient (200 ng/mL) or C1q (1 μg/mL). Data are pooled from at least triplicate samples from 3 independent experiments. (B) CD34+ cells, pre-incubated or not with C1q and anti-C1q antibody, were allowed to migrate across Matrigel (reconstituted basement membrane) towards media alone (0 ng/mL SDF-1) or towards a low SDF-1 gradient (20 ng/mL). Each experiment was performed using at least 3 chambers for each set of conditions and cell counts were done in duplicate. (C) Zymograms of media conditioned by CD34+ cells from cord blood (CB) or mobilized peripheral blood (mPB) in the presence or absence (control) of C1q. Media conditioned by HT-1080 cells was used as standard (Std) to indicate the position of MMP-9 in the gel.
C1q-deficient mice are good G-CSF mobilizers and normal zymosan mobilizers
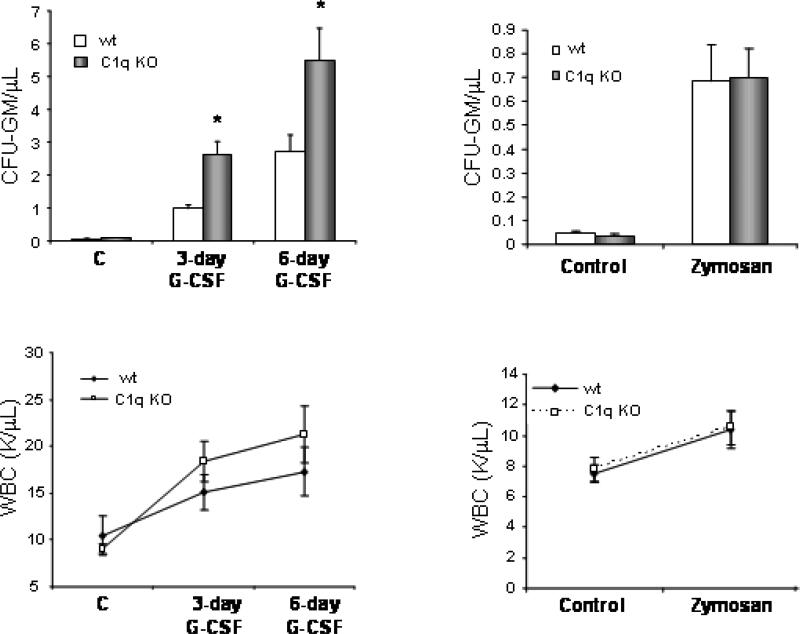
Previously, we reported that while G-CSF activates the classical C1q-dependent CC pathway, zymosan activates the alternative C1q-independent pathway. To shed more light on the role of C1q in HSPC trafficking, we performed mobilization studies in C1q-/- mice and wt mice. Mice were mobilized by employing either G-CSF, which activates the classical C1q-initiated complement activation pathway, or zymosan, which activates the C1q-independent alternative complement activation pathway. We found that C1q-/- mice are good/easy G-CSF mobilizers (greater mobilization than wt mice) but normal zymosan-induced mobilizers (mobilization equal to that in wt mice) (Fig. 4). Accordingly, the number of circulating CFU-GM progenitors and WBC in PB was higher in C1q-/- mice at days 3 and 6 of G-CSF administration as compared to wild type (wt) animals, but did not differ in wt mice after zymosan-induced mobilization.
Figure 4. C1q-deficient mice are easy mobilizers but normal zymosan mobilizers.
C1q-/-mice (C1q KO) as well as age- and sex-matched C57BL/6 mice (wt) were mobilized for 3 or 6 days with G-CSF (250 μg/kg s.c. per day; n=5 mice per group; left panels) or 1 h with zymosan (0.5 mg/kg i.v.; n=5 mice per group; right panels). The number of circulating CFU-GM progenitors (A), and white blood cells (WBC) (B) per microliter of PB are shown. * p< 0.05 as compared with wt mice. Data show representative results from three independent experiments.
DISCUSSION
Since the immune and hematopoietic systems occupy similar compartments in the BM, it is reasonable to predict that complement may modulate the signaling responses involved in HSPC trafficking. In fact, we have already shown that innate immunity and the CC proteins are not only activated during HSPC mobilization21 and myeloablative conditioning for transplantation,23 but also play important roles in regulating these processes. Moreover, it has become apparent that various components of the CC play different roles in the mobilization or retention of HSPC in BM.12,24-26 Specifically, we reported that C3- and C3aR-deficient mice are easy mobilizers; in contrast, mobilization is impaired in C5-deficient mice. We also described that the CC is activated in the BM microenvironment differently in response to various external stress-related factors. Accordingly, while myeloablative conditioning before transplant or G-CSF-induced mobilization triggers the classical C1q-dependent CC activation pathway, zymosan activates CC by an alternative C1q-independent pathway.
To investigate the potential contribution of other CC proteins, we turned our attention to C1q, the first subcomponent of the C1 complex of the classical pathway of complement activation. C1q is unusual for a complement protein in that it is synthesized by tissue macrophages but not by hepatocytes.27 C1q is reportedly not detectable in monocytes at the protein or mRNA levels; however, monocytes synthesize C1q upon their differentiation to macrophages or dendritic cells.28-29 It has been shown that C1q is of hematopoietic origin as C1q production was restored in irradiated C1q-/- mice transplanted with wt BM cells and, when irradiated wt mice received BM from C1q-/- mice, C1q production was not detected.30 In fact, these investigators suggested that transplantation of HSPC might be a potential treatment for patients with hereditary C1q deficiency. Drawing upon this observation, we first determined the expression of C1q in human CD34+ cells in steady-state and compared it during G-CSF-induced mobilization. We found that CD34+ cells from CB and steady-state BM do not express C1q, while CD34+ cells from G-CSF-mobilized PB strongly expressed it. Moreover, in vitro stimulation of BM cells with G-CSF induced the expression of C1q in the CD34+ cell population.
Several functions have been attributed to C1q which are considered to be mediated by C1q receptors present on the effector cell surface. A C1q receptor that modulates monocyte phagocytosis (C1qRp) has been identified and was reported to be selectively expressed in cells of myeloid origin, endothelial cells and platelets.31 It has also been suggested that C1qRp can be a human stem cell marker that defines the most primitive population of human BM-repopulating cells.16 Here we confirmed that C1qRp is present on HSPC from human BM, mPB and CB CD34+ cells and observed its expression on myeloid, megakaryocytic and erythroid progenitors. Having established the expression of C1q and C1qRp in CD34+ cells, we next investigated their role in HSPC trafficking. C1q has been implicated in the stimulation of Ca2+-activated K+ channels and initiation of chemotaxis in mouse fibroblasts.32 Moreover, C1q chemoattracts immature dendritic cells from blood to inflammatory tissues and enhances the migration of mature dendritic cells to the chemokine CCL19.33 C1q has also been reported to induce chemoattraction of neutrophils,34 monocytes but not macrophages, and also of mast cells.2 Here we demonstrated that C1q by itself is not a chemoattractant for CD34+ HSPC, but it potentiates/primes the SDF-1-dependent chemotaxis of CD34+ cells. It also primes SDF-1-dependent trans-Matrigel migration, which mimics the physiologic process of cell migration across the subendothelial basement membrane barrier towards an SDF-1 gradient. These results indicate that the presence of functional C1qRp on human CD34+ cells may increase their ability to respond to the SDF-1 produced by BM stromal cells and be retained within BM niches. At the same time CD34+ cells stimulated with recombinant C1q secrete more of the matrix-degrading enzyme MMP-9, providing further evidence of its involvement in modulating the trafficking of HSPC.
Having shown that C1q enhances the responsiveness of CD34+ cells to SDF-1 in vitro, we subsequently used a C1q-deficient mouse HSPC mobilization model to further elucidate its role in vivo. We found that C1q-deficient mice are more sensitive to mobilization induced by GCSF than wt mice, suggesting that C1q is involved in the retention of HSPC in the BM niches and that perturbation of this axis may facilitate egress into PB.
This is in agreement with our previous observations that activation of the C1q-triggered classical pathway by G-CSF leads to cleavage of C3 and generation of C3a, desArgC3a and iC3b, which play an important role in the homing/retention of HSPC in the BM.21 Further supporting this is our finding that C3 cleavage fragments, similar to C1q, increase the responsiveness of HSPC to an SDF-1 gradient.21-25 This explains why animals deficient in C3 or C3aR, which display impaired responses of HSPC to SDF-1 homing signals, engraft poorly after transplantation of wt BM cells, and at the same time are easy G-CSF-induced mobilizers.13 We envision that C3 cleavage fragments that increase the responsiveness of HSPC to an SDF-1 gradient are in the BM microenvironment as a “last line of defense” that prevents uncontrolled egress of HSPC into PB when the SDF-1−CXCR4 axis becomes attenuated after the administration of a mobilizing agent. Since lack of C1q leads to defective activation of the classical complement pathway, then C3 cleavage by C1q cannot occur. Our observation that C1q-deficient mice, similarly to C3-deficient mice, are easy G-CSF-induced mobilizers supports the notion that activation of upstream elements of the classical pathway of the CC that leads to cleavage of C3 promotes homing and retention of HSPC in the BM microenvironment (13, 21, 23, 25) whereas, activation of downstream elements and cleavage of C5 promotes their egress into PB (14, 35). Thus, C1q-/- mice that cannot activate the C1q-dependent steps of the CC, and as a result cannot cleave C3, are like C3-deficient mice, easy mobilizers. However, as we have shown in this study, they mobilize normally after administration of zymosan, which activates the CC directly at the C3 level.
It is known that mPB-derived HSPC engraft faster after transplantation than those derived from BM. Because the complement system is activated during mobilization and leukapheresis, it is likely that mPB CD34+ cells are exposed to C1q, whose priming effect could increase their homing and engraftment potential after transplantation. Previously, we have shown that mice deficient in complement C3 displayed a significant delay in hematopoietic recovery after transplantation of wt HSPC and we suggested that it may be possible to use C3a for ex vivo priming of HSPC as a strategy to increase the responsiveness of HSPC to SDF-1.23 Furthermore, during myeloablative conditioning before transplantation, when the CC becomes activated in the BM microenvironment, both C1q (via C1qR) and C3a (via C3aR) may increase responsiveness of HSPC to SDF-1 in the BM and facilitate HSPC engraftment. In support of this notion, we have already demonstrated that C3aR deficiency on HSPC leads to defective homing and engraftment,13 and we plan to perform similar experiments with C1qR-deficient HSPC. The results of our studies could be particularly important for improving the efficacy of CB transplantation in adults, where the number of HSPC available for transplantation is limited and hematopoietic engraftment is delayed. Thus, our studies could have an important translational aspect and we propose that C1q could be employed as a potential novel agent to prime HSPC ex vivo before transplantation.
Acknowledgments
This work was supported by grants from Canadian Blood Services (CBS) R & D/Canadian Institutes of Health Research (CIHR), Blood Utilization and Conservation Initiative to AJ-W and a CBS Postdoctoral Fellowship award to AJ and NIH grant R01 DK074720 to MZR. We thank Jencet Montaño and April Xu for technical help.
This work was supported by a postdoctoral research fellowship to AJ, an operating grant from the Canadian Blood Services/Canadian Institutes for Health Research to AJW and a National Institute of Health grant (R01 DK074720) to MZR.
ABBREVIATIONS
- BFU-E
blast forming unit-erythroid
- BM
bone marrow
- C1qRp
receptor for complement component 1, subcomponent q, phagocytosis
- CB
cord blood
- CC
complement cascade
- CFU-GM
colony forming unit-granulocyte macrophage
- FACS
fluorescence activated cell sorting
- G-CSF
granulocyte-colony stimulating factor
- HSPC
hematopoietic stem/progenitor cells
- IgG
immunoglobulin G
- IL
interleukin
- IMDM
Iscove's modified Dulbecco's medium
- MNC
mononuclear cells
- mPB
mobilized peripheral blood
- SDF
stromal-cell derived factor
Footnotes
The authors declare that they have no conflicts of interest relevant to the manuscript submitted to TRANSFUSION.
REFERENCES
- 1.Gasque P. Complement: a unique innate immune sensor for danger signals. Mol Immunol. 2004;41:1089–98. doi: 10.1016/j.molimm.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 2.Lu J, Teh BK, Wang L, Wang Y, Tan YS, Lai MC, Reid KBM. The classical and regulatory functions of C1q in immunity and autoimmunity. Cell Mol Immunol. 2008;5:9–21. doi: 10.1038/cmi.2008.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu J, Wu X. The BK. The regulatory roles of C1q. Immunobiol. 2007;212:245–52. doi: 10.1016/j.imbio.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 4.Sjoberg AP, Trouw LA, Blom AM. Complement activation and inhibition: a delicate balance. Trends Immunol. 2009;30:83–90. doi: 10.1016/j.it.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 5.Kishore U, Reid KBM. C1q: structure, function, and receptors. Immunopharmacol. 2000;49:159–70. doi: 10.1016/s0162-3109(00)80301-x. [DOI] [PubMed] [Google Scholar]
- 6.Bobak DA, Gaither TG, Frank MM, Tenner AJ. Modulation of FCr function by complement: subcomponent C1q enhances the phagocytosis of IgG-opsonized targets by human monocytes and culture-derived macrophages. J Immunol. 1987;138:1150–6. [PubMed] [Google Scholar]
- 7.van den Berg RH, Faber-Krol MC, Sim RB, Daha MR. The first subcomponent of complement, C1q, triggers the production of IL-8, IL-6, and monocyte chemoattractant peptide-1 by human umbilical vein endothelial cells. J Immunol. 1998;161:6924–30. [PubMed] [Google Scholar]
- 8.Verardi S, Page RC, Ammons WF, Bordin S. Differential chemokine response of fibroblast subtypes to complement C1q. J Periodont Res. 2007;42:62–8. doi: 10.1111/j.1600-0765.2006.00916.x. [DOI] [PubMed] [Google Scholar]
- 9.Baruah P, Dumitriu IE, Malik TH, Cook HT, Dyson J, Scott D, Simpson E, Botto M. C1q enhances IFN-γ production by antigen-specific T cells via the CD40 costimulatory pathway on dendritic cells. Blood. 2009;113:3485–93. doi: 10.1182/blood-2008-06-164392. [DOI] [PubMed] [Google Scholar]
- 10.Farber K, Cheung G, Mitchell D, Wallis R, Weihe E, Schwaeble W, Kettenmann H. C1q, the recognition subcomponent of the classical pathway of complement, drives microglial activation. J Neurosci Res. 2009;87:644–52. doi: 10.1002/jnr.21875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Skoglund C, Wettero J, Skogh T, Sjowall C, Tengvall P, Bengtsson T. C-reactive protein and C1q regulate platelet adhesion and activation on adsorbed immunoglobulin G and albumin. Immunol Cell Biol. 2008;86:466–74. doi: 10.1038/icb.2008.9. [DOI] [PubMed] [Google Scholar]
- 12.Lee HM, Ratajczak MZ. Innate immunity: a key player in the mobilization of hematopoietic stem/progenitor cells. Arch Immunol Ther Exp. 2009;57:1–9. doi: 10.1007/s00005-009-0037-6. [DOI] [PubMed] [Google Scholar]
- 13.Wysoczynski M, Reca R, Lee H, Wu W, Ratajczak J, Ratajczak MZ. Defective engraftment of C3aR-/- hematopoietic stem progenitor cells shows a novel role of the C3a–C3aR axis in bone marrow homing. Leukemia. 2009;23:1455–61. doi: 10.1038/leu.2009.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee HM, Wu W, Wysoczynski M, Liu R, Zuba-Surma EK, Kucia M, Ratajczak J, Ratajczak MZ. Impaired mobilization of hematopoietic stem/progenitor cells in C5-deficient mice supports the pivotal involvement of innate immunity in this process and reveals novel promobilization effects of granulocytes. Leukemia. 2009;23:2052–62. doi: 10.1038/leu.2009.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lapidot T, Kollet O. The essential roles of the chemokine SDF-1 and its receptor CXCR4 in human stem cell homing and repopulation of transplanted immune-deficient NOD/SCID and NOD/SCID/B2mnull mice. Leukemia. 2002;16:1992–2003. doi: 10.1038/sj.leu.2402684. [DOI] [PubMed] [Google Scholar]
- 16.Danet GH, Luongo JL, Butler G, Lu MM, Tenner AJ, Simon MC, Bonnet DA. C1qRp defines a new human stem cell population with hematopoietic and hepatic potential. Proc Natl Acad Sci. 2002;99:10441–5. doi: 10.1073/pnas.162104799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Janowska-Wieczorek A, Marquez LA, Nabholtz JM, Cabuhat ML, Montaño J, Chang H, Rozmus J, Russell JA, Edwards DR, Turner AR. Growth factors and cytokines upregulate gelatinase expression in bone marrow CD34+ cells and their transmigration through reconstituted basement membrane. Blood. 1999;93:3379–90. [PubMed] [Google Scholar]
- 18.Majka M, Janowska-Wieczorek A, Ratajczak J, Ehrenman K, Pietrzkowski Z, Kowalska MA, Gewirtz AM, Emerson SG, Ratajczak MZ. Numerous growth factors, cytokines, and chemokines are secreted by human CD34+ cells, myeloblasts, erythroblasts, and megakaryoblasts and regulate normal hematopoiesis in an autocrine/paracrine manner. Blood. 2001;97:3075–85. doi: 10.1182/blood.v97.10.3075. [DOI] [PubMed] [Google Scholar]
- 19.Son B-R, Marquez-Curtis LA, Kucia M, Wysoczynski M, Turner AR, Ratajczak J, Ratajczak MZ, Janowska-Wieczorek A. Migration of bone marrow and cord blood mesenchymal stem cells in vitro is regulated by stromal-derived factor-1-CXCR4 and hepatocyte growth factor-c-met axes and involves matrix metalloproteinases. Stem Cells. 2006;24:1254–64. doi: 10.1634/stemcells.2005-0271. [DOI] [PubMed] [Google Scholar]
- 20.Janowska-Wieczorek A, Marquez LA, Dobrowsky A, Ratajczak MZ, Cabuhat ML. Differential MMP and TIMP production by human marrow and peripheral blood CD34+ cells in response to chemokines. Exp Hematol. 2000;28:1274–85. doi: 10.1016/s0301-472x(00)00532-4. [DOI] [PubMed] [Google Scholar]
- 21.Ratajczak J, Reca R, Kucia M, Majka M, Allendorf DJ, Baran JT, Janowska-Wieczorek A, Wetsel RA, Ross GD, Ratajczak MZ. Mobilization studies in mice deficient in either C3 or C3a receptor (C3aR) reveal a novel role for complement in retention of hematopoietic stem/progenitor cells in bone marrow. Blood. 2004;103:2071–8. doi: 10.1182/blood-2003-06-2099. [DOI] [PubMed] [Google Scholar]
- 22.Aiuti A, Webb IJ, Bleul C, Springer T, Gutierrez-Ramos JC. The chemokine SDF-1 is a chemoattractant for human CD34(+) hematopoietic progenitor cells and provides a new mechanism to explain the mobilization of CD34(+) progenitors to peripheral blood. J Exp Med. 1997;185:111–20. doi: 10.1084/jem.185.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ratajczak MZ, Reca R, Wysoczynski M, Kucia M, Baran JT, Allendorf DJ, Ratajczak J, Ross GD. Transplantation studies in C3-deficient animals reveal a novel role of the third complement component (C3) in engraftment of bone marrow cells. Leukemia. 2004;18:1482–90. doi: 10.1038/sj.leu.2403446. [DOI] [PubMed] [Google Scholar]
- 24.Reca R, Cramer D, Yan J, Laughlin MJ, Janowska-Wieczorek A, Ratajczak J, Ratajczak MZ. A novel role of complement in mobilization: immunodeficient mice are poor granulocyte-colony stimulating factor mobilizers because they lack complement-activating immunoglobulins. Stem Cells. 2007;25:3093–100. doi: 10.1634/stemcells.2007-0525. [DOI] [PubMed] [Google Scholar]
- 25.Ratajczak MZ, Reca R, Wysoczynski M, Yan J, Ratajczak J. Modulation of the SDF-1–CXCR4 axis by third complement component (C3) – Implications for trafficking of CXCR4+ stem cells. Exp Hematol. 2006;34:986–95. doi: 10.1016/j.exphem.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 26.Reca R, Mastellos D, Majka M, Marquez L, Ratajczak J, Franchini S, Glodek A, Honczarenko M, Spruce LA, Janowska-Wieczorek A, Lambris JD, Ratajczak MZ. Functional receptor for C3a anaphylatoxin is expressed by normal hematopoietic stem/progenitor cells, and C3a enhances their homing-related responses to SDF-1. Blood. 2003;101:3784–93. doi: 10.1182/blood-2002-10-3233. [DOI] [PubMed] [Google Scholar]
- 27.Muller W, Hanauske-Abel H, Loos M. Biosynthesis of the first component of complement by human and guinea pig peritoneal macrophages: evidence for an independent production of the C1 subunits. J Immunol. 1978;121:1578–84. [PubMed] [Google Scholar]
- 28.Lu J, Le Y, Kon OL, Chan J, Lee SH. Biosynthesis of human ficolin, an Escherichia coli-binding protein, by monocytes: comparison with the synthesis of two macrophage-specific proteins, C1q and the mannose receptor. Immunol. 1996:89289–94. doi: 10.1046/j.1365-2567.1996.d01-732.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cao W, Bobryshev YV, Lord RSA, Oakley REI, Lee SH, Lu J. Dendritic cells in the arterial wall express C1q: potential significance in atherogenesis. Cardiovasc Res. 2003;60:175–86. doi: 10.1016/s0008-6363(03)00345-6. [DOI] [PubMed] [Google Scholar]
- 30.Petry F, Botto M, Holtappels R, Walport MJ, Loos M. Reconstitution of the complement function in C1q-deficient (C1q-/-) mice with wild-type bone marrow cells. J Immunol. 2001;167:4033–7. doi: 10.4049/jimmunol.167.7.4033. [DOI] [PubMed] [Google Scholar]
- 31.Nepomuceno RR, Tenner AJ. C1qRp, the C1q receptor that enhances phagocytosis, is detected specifically in human cells of myeloid lineage, endothelial cells, and platelets. J Immunol. 1998;160:1929–35. [PubMed] [Google Scholar]
- 32.Oiki S, Okada Y. C1q induces chemotaxis and K+ conductance activation coupled to increased cytosolic Ca2+ in mouse fibroblasts. J Immunol. 1988;141:3177–85. [PubMed] [Google Scholar]
- 33.Liu S, Wu J, Zhang T, Qian B, Wu P, Li L, Yu Y, Cao X. Complement C1q chemoattracts human dendritic cells and enhances migration of mature dendritic cells to CCL19 via activation of AKT and MAPK pathways. Mol Immunol. 2008;46:242–9. doi: 10.1016/j.molimm.2008.08.279. [DOI] [PubMed] [Google Scholar]
- 34.Leigh LE, Ghebrehiwet B, Perera TP, Bird IN, Strong P, Kishore UK, Reid B, Eggleton P. C1q-mediated chemoattraction by human neutrophils: involvement of gC1qR and G-protein signalling mechanisms. Biochem J. 1998;330:274–54. doi: 10.1042/bj3300247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jalili A, Shirvaikar N, Marquez-Curtis L, Qiu Y, Korol C, Lee H, Turner AR, Ratajczak MZ, Janowska-Wieczorek A. Fifth complement cascade protein (C5) cleavage fragments disrupt the SDF-1/CXCR4 axis: Further evidence that innate immunity orchestrates the mobilization of hematopoietic stem/progenitor cells. Exp Hematol. 2010 Feb 11; doi: 10.1016/j.exphem.2010.02.002. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]