Abstract
Prior research findings are mixed as to whether ADHD is related to illicit drug use independent of conduct problems (CP). The current study adds to this literature by investigating the association between trajectories of ADHD symptoms across childhood and adolescence and onset of illicit drug use, with and without controlling for CP. Using a longitudinal panel study of a community sample of 754 male and female youth recruited in kindergarten, the research question was examined by utilizing a combination of growth mixture modeling (to model parent-reported ADHD symptom trajectories) and survival analysis (to model youth-reported initiation of illicit drug use). Results revealed a three-class model of ADHD trajectories, with one class exhibiting no or minimal symptoms throughout childhood and adolescence, another class showing a convex shape (increase, then decrease in symptoms) across time, and a third class showing a concave shape (decrease, then slight increase in symptoms) over time. The concave-trajectory class demonstrated significantly earlier onset of illicit drug use than the minimal-problem class, with the convex-trajectory class falling between (but not significantly different from either of the other two classes). These results did not change when CP was added to the model as a covariate. Implications of findings for theory and practice are discussed.
Keywords: ADHD, Substance Use, Growth Mixture Modeling, Adolescence
A common childhood disorder, attention-deficit/hyperactivity disorder (ADHD) is diagnosed in 3–5% of children (American Psychiatric Association [APA], 2000). The core symptoms of ADHD – inattention, hyperactivity, and impulsivity – contribute to impairment in a variety of areas, such as social functioning (Hoza et al., 2005) and academic achievement (Frazier, Youngstrom, Glutting, & Watkins, 2007). As symptoms and impairment persist into adolescence and adulthood (Bagwell, Molina, Pelham, Hoza, 2001; Barkley, Fischer, Edelbrook, & Smallish, 1990), individuals with ADHD often experience increased risk for additional problematic outcomes, such as substance use. Childhood ADHD symptoms have been found to predict adolescent illicit drug use (e.g., marijuana, cocaine, heroin, methamphetamines; Barkley et al., 1990; Gittelman, Mannuzza, Shenker, & Bonagura, 1985; Molina & Pelham, 2003); however, the large overlap between ADHD and other externalizing disorders (e.g., oppositional deviant disorder [ODD] and conduct disorder [CD]), which are also strong predictors of illicit drug use, has made unclear the degree to which ADHD predicts illicit drug use. Findings from several studies have indicated that, when controlling for conduct problems (CP) – symptoms of ODD and CD – ADHD no longer predicts illicit drug use. The present study addresses this debate by examining the effect of early childhood CP on the degree to which trajectories of ADHD symptoms across childhood predict onset of illicit drug use in adolescence.
ADHD and Illicit Drug Use: The Debate
The role of CP in ADHD’s prediction of illicit drug use remains ambiguous. Three decades of research provide evidence that childhood CP is a strong risk factor for adolescent illicit drug use (Capaldi & Stoolmiller, 1999; Fergusson, Boden, & Horwood, 2008; Jessor & Jessor, 1977; Kellam, Brown, Rubin, & Ensminger, 1983; Keyes & Block, 1984; Lynskey & Fergusson, 1995; White, Loeber, Stouthamer-Loeber, & Farrington, 1999). CP and ADHD are frequently comorbid, appearing together in 30–50% of children, according to epidemiologic (Szatmari, Boyle, & Offord, 1989) and clinical samples (Barkley et al., 1990; Biederman, Newcorn, & Sprich, 1991). Given the high overlap between ADHD and CP and the strong risk for illicit drug use that CP confers, it is possible that co-occurring CP may completely account for ADHD’s prediction of illicit drug use.
However, studies that have included CP have yielded conflicting results. The predominant finding is that ADHD no longer predicts illicit drug use when CP is added (Barkley et al., 1990; Chilcoat & Breslau, 1999; Disney, Elkins, McGue, & Iacona, 1999; Gittelman et al., 1985; Greenbaum, Prange, Friedman, & Silver, 1991; Loeber, Stouthamer-Loeber, & White, 1999; Lynskey & Fergusson, 1995). For example, in a cross-sectional study using a clinical sample, Greenbaum et al. (1991) reported that the relation between ADHD and illicit drug use became non-significant once CP was included in the model. Disney et al. (1999) also found, using a cross-sectional, community sample of adolescent monozygotic twins, that CP fully accounted for the relation between ADHD and illicit drug use. Using a longitudinal, community sample, Chilcoat and Breslau (1999) found that adolescents with childhood ADHD were more likely than adolescents with no diagnosis to have used illicit drugs when current high rates of CP were also present. Finally, Barkley et al. (1990) used a longitudinal, clinical sample and found that adolescents with childhood ADHD and CP were two to five times more likely to use illicit drugs than those with ADHD alone and controls.
In contrast, a few studies have found that ADHD remains predictive of illicit drug use when CP is included in the analyses. For example, Elkins, McGue, and Iacono (2007) found in a longitudinal, community sample that severity of childhood ADHD symptoms predicted adolescent illicit drug use, when controlling for CP. In a cross-sectional study using a clinical sample, Molina and Pelham (2003) also found that adolescent inattentive symptoms contributed to onset of illicit drug use, independent of concurrent CP. In summary, given the contradictory findings regarding the degree to which ADHD predicts illicit drug use independent of CP, further research is necessary to clarify the relations between ADHD and CP in the prediction of illicit drug use. Investigating these relations with longitudinally-assessed ADHD symptoms might elucidate the inconsistencies of previous findings.
Onset of Illicit Drug Use
Little is known about how ADHD and CP relate to the onset of illicit drug use, as previous research has primarily focused on frequency of use, dependence, and abuse. Since onset of use includes experimentation that does not lead to continued use, it may have eluded investigation. Due to an increase in sensation-seeking and insufficient self-regulatory competence during adolescence, experimentation with a variety of dangerous behaviors, including illicit drug use, occurs more frequently in adolescence than other developmental periods (Crews, He, & Hodge, 2007; Steinberg & Lerner, 2004). Although not all initial illicit drug use results in abuse or dependence (Fleming, Kellam, & Brown, 1982), it is a strong predictor of dependence, where risk of addiction increases as age of first use decreases (Anthony & Petronis, 1995; Bukstein, 1995; Robins & McEvoy, 1990; Robins & Przybeck, 1985). Further, biological vulnerability may link adolescent onset of illicit drug use to more intractable addiction than onset in adulthood (Bukstein, 1995; Kandel, Davies, Karus, & Yamaguchi, 1986; Warner, Kessler, Hughes, Anthony, & Nelson, 1995), as use during adolescence, a critical period of brain development, may catalyze neurological changes that predispose adolescents to dependence (Chambers, Taylor, & Potenza, 2003; Crews et al., 2007). Given that adolescents with ADHD already display high rates of impulsivity and risk-taking, they may be more likely to initiate illicit drug use than adolescents without ADHD, leaving them more vulnerable to developing a substance use disorder.
Further, isolating onset of use may provide important information about whether both ADHD and CP confer risk for illicit drug use. According to epidemiological studies, the interplay between risk factors and illicit drug use varies at different stages of use (e.g., onset, abuse, dependence; Bukstein, 1995; Robins, Davis, & Wish, 1977). Thus, ADHD and CP may also interact differently to predict different stages of drug use. For example, CP may facilitate affiliation with deviant peers, which may lead to onset of use for adolescents with ADHD (Marshal & Molina, 2006; Marshal, Molina, & Pelham, 2003). On the other hand, adolescents with ADHD may continue to use illicit drugs to manage their symptoms, regardless of CP (e.g., self-medication hypothesis; see Khantzian, 1997; Wilens et al., 2007). The potentially distinct associations between ADHD and CP in predicting patterns of illicit drug use at different stages of use may also hold important implications for treatment. Only three studies examining whether ADHD predicts illicit drug use have included onset of use (Elkins et al., 2007; Jester al., 2009; Molina & Pelham, 2003). Using logistic regression within a clinical sample, Molina and Pelham (2003) found that ADHD inattentive symptoms and CP predicted onset of illicit drug use. Within a community sample of monozygotic twins, Elkins et al. (2007) used logistic regression and found that childhood ADHD symptoms and CP predicted onset of use at two time points when participants were 14 and 18 years of age. Jester et al. (2008) found that a growth mixture model of ADHD and aggression measured from 6 to 18 years of age resulted in four trajectory classes: healthy, only ADHD, only aggression, and comorbid ADHD and aggression. Both the ADHD-only and comorbid trajectory classes predicted significantly earlier onset of marijuana use than the healthy trajectory class (Jester et al., 2008). In Molina and Pelham (2003), age of first use ranged from 10 to 18 years of age. Within Elkins et al. (2007), onset of use was limited to analyzing use that occurred between 14 and 18 years of age. Given that risk for developing substance use disorders increases as age of onset decreases, the risk of onset of illicit drug use related to ADHD and CP warrants further investigation, and requires assessing longitudinal data.
Measurement of ADHD
Prior inconsistent findings regarding the role of CP in ADHD’s prediction of illicit drug use may stem from limitations in methodological approaches. When longitudinal designs were utilized, often only one assessment of childhood ADHD was used to predict adolescent illicit drug use (August et al., 2006; Barkley et al., 1990; Chilcoat & Breslau, 1999; Fergusson et al., 2008; Loeber et al., 1999). This approach does not account for ADHD symptoms concurrent with drug use or any change in symptoms across childhood.
Further, even when change in ADHD symptoms was included in analyses, conventional analytic techniques (e.g., ANOVA and regression) were used. These techniques present two crucial limitations in assessing change over time. First, they do not allow for the consideration of non-linear change (Curran & Muthen, 1999; Rogosa & Willett, 1985; Singer & Willett, 2003). This limitation is problematic, as it is not clear that ADHD symptoms possess a linear trajectory across childhood. For example, hyperactivity/impulsivity appears to gradually decrease across childhood, and inattention seems to maintain a consistent level of severity (e.g., Biederman, Mick, & Faraone, 2000; Hart, Lahey, Loeber, Applegate, & Frick, 1995). Symptoms may also display a curvilinear shape across childhood. Some research suggests that symptoms increase dramatically during the transition to middle school (Langberg et al., 2008). Taken together, these findings indicate that the trajectory of ADHD symptoms may be either linear or curvilinear.
Second, conventional techniques do not allow for the consideration of individual differences in symptom change across time points. Since 50–80% of childhood diagnoses persist into adolescence (Barkley et al., 1990), all children do not retain their ADHD diagnosis in adolescence, suggesting that individual differences in trajectories of ADHD may occur. In fact, heterogeneity in the course of ADHD symptoms over childhood is probable with the progression of symptoms potentially following three pathways. In a seven year, longitudinal study of a community sample of 59 boys with a mean age of 14.3 years at follow-up, Lambert, Hartsough, Sassone, and Sandoval (1987) identified three developmental patterns of ADHD symptoms. It should be noted that, as is the case for most of the few studies that have investigated the course of ADHD symptom dimensions across childhood, this study commenced prior to the DSM-III (APA, 1980). Thus, the “hyperkinetic reaction of childhood” diagnosis from the DSM-II (APA, 1968) was used instead of the current diagnosis of ADHD. Remitters, making up 20% of the sample, demonstrated few hyperactive symptoms and little functional impairment. Although residuals (37%) did not have diagnosable rates of hyperactive symptoms and were not currently receiving treatment, they still displayed behavioral and academic impairment. Persisters (43%) presented with stable rates of hyperactive symptoms, as well as CP and a variety of poor outcomes. Other studies have reported a similar pattern of symptoms, providing some support for these three trajectories (Barkley et al., 1990; Loney, 1981; Mendelson, Johnson, & Stewart, 1971; Weiss & Hechtman, 1993). Different trajectories of ADHD symptoms may differentially predict onset of illicit drug use. However, without analysis of symptom trajectories, identifying these individual differences and their meaningfulness in predicting onset of use is not possible. Thus, the analyses in this study were conducted using growth mixture modeling, which allows for the investigation of inter- and intra-individual change (e.g., trajectory) as well as the shape of trajectories (Curran & Muthen, 1999; Rogosa & Willett, 1985; Singer & Willett, 2003).
The Current Study
The current study uses innovative analytic techniques within a high-risk community sample of adolescents to investigate if trajectories of ADHD symptoms during childhood predict onset of illicit drug use, when controlling for CP. Previous research assessing the association between ADHD and CP has primarily been conducted with clinical samples. Despite some limitations, the use of community samples avoids several crucial drawbacks associated with clinical samples. Specifically, a referral bias is often present in clinical samples, where participants are disproportionately male, tend to have comorbid disorders, and display greater symptom severity than is typical in the general population (Carlson & Mann, 2000; Flory et al., 2003). This study also differs from previous research in that continuous symptom dimensions of ADHD and CP were used as opposed to discrete diagnoses. Using continuous dimensions permitted the examination of how ADHD and CP symptom severity contributes to onset of illicit drug use.
The present study addresses gaps in the literature in two ways. First, it is one of few studies to consider onset of illicit drug use, rather than frequency of use, dependence, or abuse. Second, this study implemented rigorous, novel, longitudinal analyses by using ADHD symptom trajectories with three time points across childhood to predict onset of use. Based on previous findings, we hypothesized that the longitudinal analyses would result in three trajectories of ADHD symptoms across childhood: a group with no symptoms, a group with a few symptoms that remit, and a group with symptoms that persist into adolescence. Given that the predominant finding across studies is that CP accounts for the association between ADHD and illicit drug use, we hypothesized that ADHD symptoms would not be related to onset of use when controlling for CP.
Method
Participants
Participants came from the control schools of a longitudinal, multi-site investigation of the development and prevention of conduct problems in children, the Fast Track Project (Conduct Problems Prevention Research Group [CPPRG], 1992). At each of four sites (Durham, NC; Nashville, TN; Seattle, WA; and rural, central PA), high-risk schools were selected and randomly assigned to intervention or control conditions. From among the control schools (n = 27), teachers completed ratings of child disruptive behavior in order to identify a within-site stratified sample of about 10 children within each decile of behavior problems. Across the four sites, 387 children were selected to represent the normative population of these schools. In addition, high-risk children were over-sampled in order to measure finer gradations of high risk. A multi-stage, multi-informant screening process identified three annual cohorts of kindergartners with the highest disruptive behavior scores, yielding an additional n of 367, bringing the total number of participants to 754 (see Lochman & CPPRG, 1995, for further details). Of the normative sample, 35% came from single-parent families; 23% of mothers had not graduated from high school; 50% were male; and 44% were African American, 52% European American, 2% Latino, and 4% Asian American, Native American, or other (race and ethnicity were asked of the parents in a single question). Family SES was diverse, with a mean Hollingshead continuous SES score of 26.4, SD = 13.3, Mdn = 24.5. Languages spoken in the home were not surveyed. Although the high-risk sub-sample included higher proportions of single-parent families, under-educated mothers, males, African American youth, and lower SES, weighting was used in all analyses to reflect the over-sampling of high-risk children.
Procedures
Beginning in kindergarten and lasting beyond grade twelve (i.e., twelve years after kindergarten; actual grade is not used in these analyses), annual measurements were collected from multiple sources that included the teacher, the peer group, administrative school records, the mother, the child, and interviewer ratings. Teacher, peer, and administrative-record measures were collected in the spring, and mother, child, and interviewer measures were collected in the following summer. In the present study, the grade number refers to years since kindergarten (e.g., grade 3 refers to 3 years since kindergarten) in all analyses.
Measures
The following measures used in the current study are described in detail at www.fasttrackproject.org. The principal constructs include childhood and early adolescent ADHD symptoms and later adolescent substance use, along with a measure of early childhood externalizing behaviors.
Childhood ADHD symptoms
In the summers after the child’s third-, sixth-, and ninth-grade years (i.e., three, six, and nine years after kindergarten recruitment), the parent completed the computerized version of the National Institutes of Mental Health’s Diagnostic Interview Schedule for Children (DISC; Schaffer, Fisher, Dulcan, & Davies, 1996), a structured interview designed to assess DSM-III-R (APA, 1987) psychiatric disorders and symptoms in adolescence. For each diagnosis module included, the parent responded “yes” or “no” to the presence of each symptom. A symptom count variable was created to indicate the number of diagnostic criteria a subject met for a given disorder. The current study used counts of criteria for ADHD-Inattentive subtype and ADHD-Hyperactive subtype as they had been noted in the child in the six months (for grade three) or year (for grades six and nine) prior to measure administration. These symptom counts were modeled as Poisson variables where possible.
Illicit drug use
Beginning in the summer after the youth’s eighth-grade year (i.e., eight years after kindergarten recruitment), the youth reported substance use on the Tobacco, Alcohol, and Drugs measure (CPPRG, 1995). The measure included detailed assessment of substance use; the items used in the current study asked age of first use for each of marijuana, cocaine or crack, inhalants, and “any other type of illegal drug” (including non-prescribed pills). For those youth who had not reported illicit drug use in a prior year, reporting any age of first use for any of the above was scored as initiation; the actual ages reported were not used due to substantial year-to-year inconsistency in the youth reports for these variables. Reports of early use were supplemented by reports of past-year use from earlier measures administered annually after grades four through seven.
Early externalizing behavior problems
As part of the process for screening children into the high-risk and normative samples, a parent (usually the mother) of each child in the study completed the Child Behavior Checklist (CBCL; Achenbach, 1991) in the summer following the child’s kindergarten year. This instrument comprises 112 items that each significantly differentiated clinically-referred from non-referred children. Parents rated each item of the CBCL with a 0 for “not true,” a 1 for “somewhat or sometimes true,” or a 2 for “very true or often true.” The Externalizing Behavior “broad-band” scale encompasses the thirteen Delinquent Behavior and twenty Aggressive Behavior items. We used the Externalizing Behavior T-score in the current study.
Results
Modeling Strategy
The primary hypothesis was that trajectories of ADHD symptoms would be related to year of onset of illicit substance use, with the role of externalizing behavior problems an open question. We approached this by estimating a growth mixture model of ADHD symptoms through grade nine, with the trajectory classes acting as predictors of the hazard of initiating drug use in grade eight (eight years after recruitment, and one year before the third ADHD measure) or later. Thus, the model is a combination of a growth mixture model and a survival model, following Muthén and Masyn (2005). The first stages in the modeling process were to separately estimate mixture models for ADHD growth and substance use hazards. Following that, the two models were estimated concurrently. The final stage was to incorporate the measure of early externalizing behavior as a possible confound. All models were estimated in Mplus v.5.2 (Muthén & Muthén, 2008), using sampling weights to reflect the original school populations.
A growth mixture model is an extension of the better-known latent trajectory model. In a latent trajectory, or growth, model, the growth parameters (typically intercept [level] and parameters modeling the changes over time) are latent variables indicated by the time-specific measurements. In a growth mixture model, different “clusters” of growth parameters are in turn indicators of a latent class, or mixture, model. Thus, different latent classes represent different empirically-identified subpopulations in the sample with prototypical patterns of development. The traditional latent trajectory model can accurately be thought of as the one-class version of the growth mixture. A general presentation of growth mixture models can be found in Muthén (2001).
In a survival model – specifically, the discrete-time survival analysis used herein – a hazard function is defined which models the likelihood of the first occurrence of an event (drug use) as a function of time. The hazard of initiating drug use at time T is an odds of initiation in the pool of respondents who had not initiated drug use by time T−1. Discrete-time survival analysis is an extension of logistic regression (Singer & Willett, 2003), and thus predictors of onset can be modeled and estimated in terms of hazard odds ratios (hOR), the relative odds of initiation given the predictor.
In the combination model of Muthén and Masyn (2005) used here, the latent class membership is the primary predictor of the hazard, and thus the hOR associated with class membership can be interpreted as (in the case of ratio greater than 1.0) the increased odds of initiation in any given year relative to the comparison group.
Mathematical Model
The model used herein is that of Muthén and Masyn (2005), itself an extension of the growth mixture model of Muthén and Shedden (1999). Using a specific version of the latter’s notation, the growth mixture model for a Poisson outcome is defined by Equations 1–4, below.
First, repeated measures of log-ADHD symptoms, yi1 - yi3 for individual i, are described by two random effect growth factors and one fixed effect as a function of time t,
| (1) |
Differences in the means of η0, η1, and η2, and the variances and covariance of η0 and η1, are allowed across classes. The covariates x, such as behavior problems, may influence the trajectory parameters – in our model, in a constant fashion across classes.
| (2) |
| (3) |
| (4) |
Finally, the latent class variable is predicted by x in a standard multinomial logistic regression (not shown).
In Muthén and Masyn’s (2005) work, they extended this model such that the latent class variable influences the survival part of the model. Again specific to our use, the survival part is specified in Equations 5 and 6, where subscript u indicates whether or not the event occurs in time period j and λ is a vector of logistic regression parameters.
| (5) |
| (6) |
The intercept parameters, βj, are invariant across classes; the growth class has its effect through the class-varying αk.
Descriptive Statistics
Tables 1 and 2 present frequency distributions for ADHD symptom counts and year of first illicit drug use, respectively. One can see that the mean number of ADHD symptoms decreases across time. Onset of illicit drug use has low probability through middle school but by the end of high school the cumulative probability indicates that such use, or at least experimentation, has become normative (66.7%). Marijuana was by far the most common illicit drug used, with 46% of those responding to the last-used (twelve years after recruitment) substance use measure reporting lifetime marijuana use, compared to 4% for crack or cocaine, 2% for inhalants, and 7% for other illicit drugs (e.g., amphetamines, barbiturates, hallucinogens). Table 1 also includes the weighted and unweighted means and standard deviations of the symptom counts. The weighted mean of the CBCL externalizing behavior T-score in kindergarten was 52.7, SD = 33.4; unweighted M = 57.6, SD = 10.2.
Table 1.
ADHD Symptom Count Distributions
| Symptom Count (of 18) | Grade 3 | Grade 6 | Grade 9 |
|---|---|---|---|
| 0 | 31.5 | 47.0 | 51.7 |
| 1 | 10.4 | 12.1 | 12.5 |
| 2 | 9.6 | 5.4 | 7.6 |
| 3 | 6.2 | 7.8 | 5.3 |
| 4–5 | 9.8 | 8.3 | 7.8 |
| 6–7 | 8.8 | 5.1 | 1.8 |
| 8–9 | 6.8 | 4.0 | 3.3 |
| 10–12 | 7.2 | 5.0 | 4.1 |
| 13–15 | 5.2 | 3.9 | 2.6 |
| 16–18 | 4.4 | 1.5 | 0.9 |
| Mean (unweighted) | 4.35 | 2.81 | 2.34 |
| SD (unweighted) | 4.95 | 4.12 | 3.81 |
| Mean (weighted) | 2.71 | 1.48 | 1.40 |
| SD (weighted) | 12.50 | 9.54 | 9.20 |
| % Missing | 12.8 | 16.7 | 24.7 |
N = 754. Tabled values in first 10 rows are proportions within column.
Table 2.
Drug Use Onset by Grade
| Grade | % First Use | Cumulative % Used | % Missing |
|---|---|---|---|
| 7 | 18.5 | 18.5 | 15.7 |
| 8 | 12.9 | 29.8 | 24.2 |
| 9 | 19.0 | 43.1 | 26.3 |
| 10 | 14.8 | 51.5 | 32.6 |
| 11 | 18.3 | 60.4 | 34.4 |
| 12 | 16.0 | 66.7 | 35.7 |
N = 754.
Missing Data and Attrition
Less than one percent (<1%) of data were missing for the externalizing score. Due to computational limitations, youth missing the externalizing behavior score were omitted listwise from analyses. Missing data in the outcome variables — ADHD symptoms and drug use — were accommodated with Mplus’s facility for full information maximum likelihood estimation with missing data under the assumption that the data — conditioned on race, sex, site, cohort, and externalizing behaviors — were missing at random (MAR; Little & Rubin, 1987). The models used the numeric integration capabilities of Mplus to accommodate missing data on the ADHD count variables.
Attrition in the Fast Track sample as a whole has been moderate (see http://www.fasttrackproject.org, “Data Collection”); however, there was substantial missingness on the latter collections of substance use data. To test for potential differential attrition predictable by study variables, we conducted a series of discrete-time survival analyses with attrition as the hazard. None of student race, sex, or kindergarten externalizing behavior or kindergarten family SES significantly predicted attrition, either in separate or combined models, ps > .05. Site and cohort could not be tested in these models due to sparse cross-tabulations of attrition with the effect codes. This suggests that, to a reasonable approximation, the data are missing completely at random (MCAR; Little & Rubin, 1987), and thus attrition is ignorable.
ADHD Growth Mixture Model
As described above, the growth mixture model is a generalization of the latent growth model (Muthén, 2001). The mixture element is essentially a latent class analysis in which the individual trajectory parameters (intercept and slope) are the indicators of the latent class. Due to the availability of only three measures of ADHD symptoms, we were limited to two random coefficients: an intercept and a slope. To accommodate the expected nonlinearity of the trends in ADHD symptoms, we also included a fixed quadratic effect, which was allowed to vary across latent class. ADHD symptom counts were modeled as Poisson variables (we did consider zero-inflated Poisson models; however, given that, empirically, much of the zero-inflation was drawn into a low-symptom class, we elected to retain the simpler Poisson estimation). Thus, the base model was a three-parameter curve in which the quadratic time trend was fixed within class, with a logarithmic link to the observed count data. We initially intended to model hyperactive and inattentive symptoms separately, but the symptom counts were so highly correlated at each time (rs > .57, unadjusted for skewed, discrete measurement), we concluded there was no useful distinction in this dataset and modeled the combined symptom count in order to improve stability of the outcome.
We estimated one-, two-, three-, and four-class models. In each model, the means, variances, and covariances of the intercept and linear slope latent variables, as well as the mean of the quadratic slope variable, were free to vary across class. Class membership was regressed on race, sex, site, cohort, and kindergarten SES. Within classes, the intercept, slope, and quadratic terms were regressed on the same covariates, with the regression parameters constrained to equality across classes (the quadratic terms were allowed to vary as a function of the covariates, but still were constrained to zero disturbance variance for identification purposes). The mixture models were estimated over large numbers (greater than 400) of random starting values to minimize the risk of settling on a local likelihood maximum. Information criteria (measures of relative fit) of the first three models are presented in Table 3. The four-class model did not converge on a solution replicated across starting values. The three-class model was favored over the two-class model by the sample-size-adjusted Bayesian Information Criterion (adj. BIC). The bootstrapped likelihood ratio test recommended by Nylund, Asparouhov, and Muthén (2007), could not be computed for this model; however the Vuong-Lo-Mendell-Rubin (VLMR) test did indicate that three classes were favored over two (also reported in Table 3), though it should be noted that the VLMR test did not favor two classes over one. Thus, we used the three-class model for subsequent analyses. The class structure is described with the full model, below.
Table 3.
Relative Model Fit by Number of Latent Classes of ADHD Trajectories
| Classes | Log-Likelihood | Number of Parameters | Entropy | AIC | BIC | Adj. BIC | VLMR |
|---|---|---|---|---|---|---|---|
| 1 | −3056 | 30 | n/a | 6171 | 6307 | 6212 | n/a |
| 2 | −3002 | 45 | .712 | 6093 | 6298 | 6155 | 108, p = .94 |
| 3 | −2887 | 60 | .745 | 5893 | 6166 | 6166 | 166, p < .001 |
Note. For entropy, higher numbers (up to 1.0) indicated greater certainty of classification. For AIC, BIC, and sample-size adjusted BIC, lower values indicate better fit, considering parsimony. Vuong-Lo-Mendell-Rubin (VLMR) tests that k classes are needed for better fit, vs. k-l classes being adequate.
Drug Use Survival Model
We estimated a discrete-time survival model for illicit drug use to model the hazard of initiating such use at each year, beginning in year seven, counting the relatively few youth (8.6%) who used illicit drugs before then as starting in year seven. In this model, the hazard of initiation was predicted by race, sex, site, cohort, and kindergarten SES. The hazard was essentially steady over time, with the logistic regression intercepts ranging from hazard odds = 0.35, 95% CI: 0.23 – 0.54, at year eleven to hazard odds = 0.20, 95% CI: 0.12 – 0.32, at year ten. Earlier onset of drug use was significantly predicted by being male, hOR = 1.31, 95% CI: 1.06 – 1.62, but not by race nor by SES, ps > .30.
Full Model
The full model was a straightforward combination of the two models described above. Three latent classes were indicated by the latent intercepts and slopes of trajectories of ADHD symptom counts, modeled as Poisson variables. The individual difference in proclivity for early drug use (known in the survival analysis literature as “frailty”) was allowed to vary across classes, though the residual variance within class was fixed to zero to facilitate estimation. Class membership and frailty were regressed on race, sex, site, cohort, and SES.
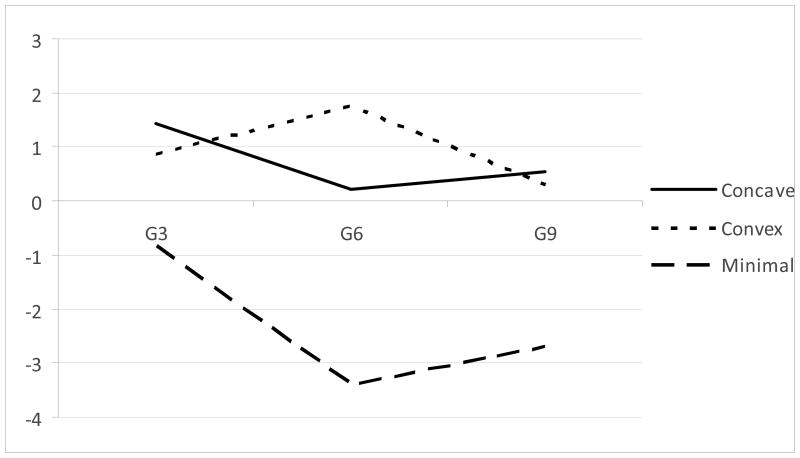
The class structure is depicted in Figure 1 (the figure depicts the estimated mean log-counts for the Poisson model, as back-transforming the non-linear logarithmic transformation would distort the relations among the model-implied values). Values are after adjusting for the within-class covariates. Classes 1 (24% of the weighted sample) and 2 (18% of the sample) showed the highest levels of ADHD symptoms at grade three, whereas Class 3 (58% of the sample) showed a significantly lower intercept, ps < .05. Class 3 was essentially a minimal-problem class (and referred to as such hereinafter); the concavity shown in Figure 1, while significant, is likely to be the result of a floor effect in symptom counts. Classes 1 and 2 differed significantly in their shape, Class 1 being significantly concave, while Class 2 was significantly convex. The three classes differed significantly in implications for drug use. The omnibus test of differences in the frailty by class membership was significant, Wald χ2 (2, N = 649) = 12.68, p = .002. Parameter estimates for this model are presented as Model 1 in the upper portion of Table 4.
Figure 1.
Prototypic trajectories of log-ADHD symptoms over time by latent class.
Table 4.
Summary of Key Model Parameters
| Class | Proportion of Population | Log-Symptom Intercept (Gr. 3) | Log-Symptom Linear Slope | Log-Symptom Quadratic Slope (fixed) | OR by Sex (Male) | OR by Ethnicity (African-American) | OR by SES(SD) | OR by Kindergarten Externalizing(T-score) | Frailty (adj.) |
|---|---|---|---|---|---|---|---|---|---|
| Model 1 (no externalizing) | |||||||||
| 1 (Concave) | .24 | 1.78 (1.20, 2.35) | −2.11 (−.71, −1.49) | 0.78 (0.49, 1.06) | 3.46 (1.52, 7.84) | 0.41 (0.17, 0.98) | 0.30 (0.04, 2.08) | -- | 0.30 (−0.43, 1.02) |
| 2 (Convex) | .17 | 0.56 (−0.01, 1.13) | 1.94 (1.23, 2.65) | −1.10 (−1.48, 0.72) | 2.30 (0.72, 7.35) | 2.46 (0.54, 11.10) | 0.30 (0.03, 3.33) | -- | −0.08 (−1.72, 0.12) |
| 3 (Minimal problem) | .59 | (−0.88 (1.25, −0.50) | (−4.37 (−6.66, −2.09) | 1.78 (0.51, 3.05) | -- | -- | -- | −0.76 (−1.34, −0.18) | |
| Model 2 (externalizing included) | |||||||||
| 1 (Concave) | .27 | 1.64 (1.30, 1.99) | (−2.62 (−3.31, −1.93) | 1.04 (0.74, 1.35) | 4.66 (1.98, 10.94) | 0.44 (0.18, 1.10) | 0.41 (0.08, 2.19) | 1.07 (1.03, 1.11) | 0.49 (−0.191.17) |
| 2 (Convex) | .16 | 0.52 (0.04, 0.99) | 1.93 (0.94, 2.93) | −0.98 (−1.38, −0.58) | 1.35 (0.55, 3.32) | 0.66 (0.15, 2.96) | 0.69 (0.07, 6.44) | 1.02 (0.97 1.07) | −0.37 (−1.25 0.52) |
| 3 (Minimal problem) | .58 | −0.80 (−1.15, −0.46) | −3.16 (−4.73, −1.59) | 1.14 (0.28, 1.99) | -- | -- | -- | -- | −0.45 (1.01,0.11) |
Note. OR = Odds Ratio, SD = Standard Deviation. For AIC, BIC, and sample-size adjusted BIC, lower values indicate better fit, considering parsimony. Vuong-Lo-Mendell-Rubin (VLMR) tests that k classes are needed for better fit, vs. k-1 classes being adequate.
Relative to the non-problem class, the concave trajectory class was disproportionately male and non-African American. The concave trajectory class did not significantly differ from the non-problem class in SES, nor did the convex trajectory class differ in race, sex, or SES. Within latent class, race, sex, and SES did not predict onset of drug use, ps > .10.
The Role of Externalizing Behavior
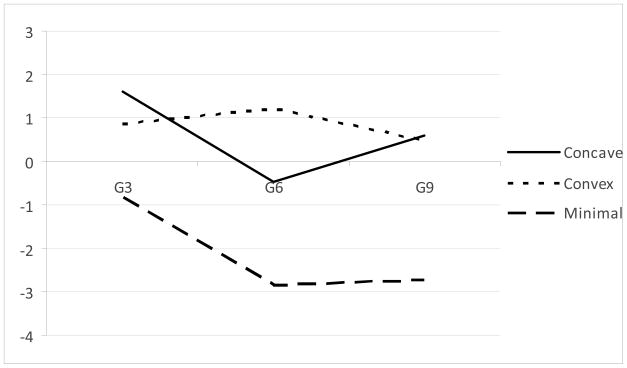
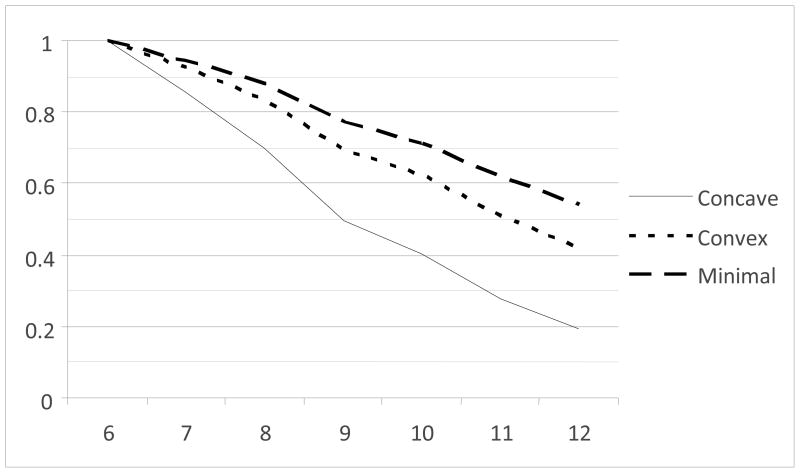
The final model estimated was identical to the previous model in all respects except that the kindergarten externalizing behavior score was included as a covariate predicting class membership, trajectory parameters, and frailty, along with the demographic and study variables. In this model, the shape of the trajectories was essentially similar (Figure 2). The test of class differences in frailty remained significant, χ2 (2, N = 642) = 6.55, p = .038. Parameter estimates are presented as Model 2 in the lower portion of Table 4. The intercept of frailty (i.e., of the logistic hazard model) for the concave trajectory class was significantly greater than the frailty for the non-problem class, Wald χ2 (1) = 5.52, p = .019. The frailty for the convex trajectory class was not significantly different from either the concave trajectory class or the non-problem class, ps > .18. The model-implied survival curves, adjusting for the covariates, are shown in Figure 3. As indicated, the non-problem Class 3 showed the latest and least illicit drug use, with the concave trajectory class showing the earliest onset of illicit drug use, with the convex trajectory class intermediate.
Figure 2.
Prototypic trajectories of log-ADHD symptoms over time by latent class, incorporating early externalizing problems as a predictor.
Figure 3.
Survival curves for illicit drug use by grade and latent class, with externalizing problems as a predictor.
Discussion
The goal of this study was to clarify the relation between trajectories of ADHD and onset of illicit drug use. We found that the concave trajectory class of ADHD predicted earliest onset of illicit drug use, when controlling for SES, gender, and race. This class of ADHD continued to predict onset of use when early conduct problems (CP) were added as a covariate.
Several methodological strengths fortify these findings. Beyond using a large sample and symptom-level assessment of ADHD, this study used highly innovative analyses by integrating growth mixture modeling and survival analysis. This study is also one of the first to use growth mixture modeling to investigate the association between ADHD and CP when predicting substance use (see Jester et al., 2008). Further, few studies on this topic have used more than two time points to assess ADHD, whereas this study used multiple time points. Using only one or two time points confines analyses to the assumption that either symptoms are static across time or that their primary impact occurs at the point of measurement. However, the use of multiple measurement periods and trajectories allows for the investigation of the quality and quantity of symptom growth and how growth in ADHD predicts onset of illicit drug use. Not only are ADHD symptoms included in this study’s analyses as trajectories, the onset of illicit drug use is also modeled as a longitudinal phenomenon. Together, these analyses provide an increased level of specificity and account for several alternate explanations for the results, both of which strengthen the detection of ADHD’s effect on the onset of illicit drug use.
Onset of Use
Our finding that ADHD symptoms predicted onset of drug use while controlling for CP is consistent with some findings from previous research (Elkins et al., 2007; Jester et al., 2008; Molina & Pelham, 2003), but conflicts with other studies, where CP entirely accounted for ADHD’s prediction of illicit drug use (Barkley et al., 1990; Disney et al., 1999; Gittelman et al., 1985; Greenbaum et al., 1991; Loeber et al., 1999; Lynskey & Fergusson, 1995). This discrepancy may have occurred for several reasons. First, the use of categorical ADHD diagnoses rather than continuous symptoms may have contributed to null findings in previous research. Using a community (vs. clinical) sample, this study found that even sub-threshold ADHD symptoms increased risk for onset of use. Thus, categorizing low and non-symptomatic individuals together may confound analyses, obscuring the degree to which ADHD predicts onset of use.
Second, the association between ADHD and CP in predicting drug use may differ according to stage of use. Many studies (Barkley et al., 1990; Disney et al., 1999; Gittelman et al., 1985; Greenbaum et al., 1991; Loeber et al., 1999; Lynskey & Fergusson, 1995) finding that CP drives the relation between ADHD symptoms and drug use assessed frequency of use, substance abuse, and dependence, rather than onset of use as we did. Third, aggregating ADHD symptoms across late childhood and early adolescence may confound ADHD’s prediction of onset of use. ADHD symptom severity varies across these developmental periods and the processes by which ADHD predicts onset of drug use may also change across developmental periods. Thus, the potency of risk that ADHD confers for onset of drug use may also change depending on the age when symptoms are measured.
ADHD Trajectories
ADHD symptoms that have plateaued between early and mid adolescence (the concave-trajectory class) may have a larger effect on onset of use than symptoms that are higher early in adolescence but decline into mid adolescence (the convex-trajectory class). ADHD symptoms may not only directly affect onset of use, but functional impairment may also mediate this association. For example, social impairment is a pervasive problem for children with ADHD. Their behavior often leads to social rejection (Mrug, Hoza, Pelham, Gnagy, & Greiner, 2007; Pelham & Bender, 1982), leaving them with few friends (Hoza, et al., 2005). Peer rejection is a known causal risk factor for affiliating with deviant peer groups (Dishion, Patterson, Stoolmiller, & Skinner, 1991). Children with ADHD also often demonstrate academic performance below their cognitive ability level (Frazier et al., 2007), due in part to difficulties with organization, homework completion, and insufficient study habits (Robin, 1998). Low academic achievement predicts both onset of illicit drug use and affiliation with deviant peers (Bachman et al., 2008; Bryant, Schulenberg, O’Malley, Bachman, & Johnston, 2003; Smith & Fogg, 1974). The association between illicit drug use and affiliation with deviant peers has been extensively replicated (Dishion, Andrew, & Crosby, 1995; Elliott, Huizinga, & Ageton, 1985; Hawkins, Catalano, & Miller, 1992; Wilks, Callan, & Austin, 1989), and appears in the majority of theories on pathways to substance use (Petraitis, Flay, & Miller, 1995). Further, recent research has shown that affiliation with deviant peers has a mediating effect on the relation between ADHD and illicit drug use (Marshal et al., 2003; Marshal & Molina, 2006). Although additional research is necessary to identify potential linkages among these factors and onset of illicit drug use for children with ADHD, symptoms that are high in childhood and lower in adolescence may engage mediating processes more proximal to onset of drug use than other patterns of symptom growth.
Thus, these findings highlight the importance of early detection and treatment of ADHD. Reducing ADHD symptoms early in childhood may be crucial for attenuating risk of initiating illicit drug use. Given that the trajectory with convex symptom growth and high symptoms in 6th grade did not predict earliest onset of use, these results also imply that simply targeting reductions in ADHD during adolescence may be insufficient for preventing the processes involved in initiating illicit drug use for youth with ADHD.
Findings also suggest that even sub-threshold ADHD symptoms may generate risk for onset of drug use. The mean symptom count for the concave trajectory class, which predicted the earliest onset of illicit drug use, was below the diagnostic cut-off for ADHD (see the DSM-IV; APA, 2000). Given that DSM-III-R criteria were used, which were less sensitive to detecting inattention symptoms, it is possible that some participants with sub-threshold symptoms actually had diagnostic levels of inattentive symptoms (Biederman et al., 1997). This possibility is important as Molina and Pelham (2003) found that inattention and not hyperactivity/impulsivity predicted onset of drug use. Nonetheless, just three ADHD symptoms have predicted significant functional impairment in prior studies (e.g., Scahill et al., 1999), demonstrating that sub-threshold ADHD symptoms can impart negative effects. Despite experiencing risk for poor outcomes, children with sub-threshold ADHD symptoms may not receive treatment, because their low symptom severity would make them ineligible for ADHD intervention programs. Untreated symptoms may lead to risk that accumulates across childhood, magnifying processes associated with onset of drug use. Thus, an important research endeavor would be to identify the threshold at which ADHD symptoms catalyze processes that put children at risk for initiating drug use in adolescence.
The general decrease in ADHD symptoms during adolescence that we saw is consistent with previous research (Barkley, 1990; Biederman et al., 2000; Molina et al., 2009; Van Lier, van der Ende, Koot, & Verhulst, 2007). ADHD has been found to remit during adolescence for 20–50% of individuals diagnosed in childhood (Barkley et al., 1990). Our finding that, despite this overall decrease, meaningful ADHD symptom severity is still present is also supported. Using latent class membership based on 36-month trajectories of symptoms, the six and eight year follow-up analyses of the Multimodal Treatment Study of Children with ADHD (MTA) study found that all three trajectory classes demonstrated decreased symptom severity. However, the average rating per item for hyperactivity/impulsivity across classes ranged from .65 to 1.12 and for inattention from 1.28 to 1.65 (Molina et al., 2009). After following participants from ages 4 to 18, Van Lier et al. (2007) used growth mixture modeling to identify trajectories of ADHD symptom as measured with the Child Behavior Checklist. The class with the highest symptoms increased in severity until the age of 11 and then decreased. At age 18, participants on average still endorsed 6 symptoms, demonstrating again that, although symptoms may decrease in adolescence, meaningful levels of ADHD symptoms generally remain.
It is important to also consider that the well-documented decline in ADHD symptoms during adolescence may not accurately represent symptom severity. Instead, this decrease may result from inadequacies of current measurement of ADHD during adolescence. An assumption of the current diagnostic system is that the behavioral expression of ADHD does not change across developmental periods (i.e., homotypic continuity; see Willoughby, 2003), but ADHD likely displays heterotypic continuity, where behavioral expressions of some symptoms change as individuals mature. For example, hyperactivity may appear as excessive running and climbing in childhood, but as restlessness in adolescence. Thus, ADHD symptoms may be equally present in adolescence, but current measurement of ADHD may lack ecological validity in adolescence.
Alternatively, parents may be less informed reporters of ADHD behaviors during their child’s adolescence than during childhood, leading to decreases in reported ADHD symptom severity. As adolescents engage in increasingly autonomous activities, the opportunities that parents have to observe their child’s ADHD behaviors decrease. Adolescents may demonstrate equivalent levels of ADHD symptoms as they did in childhood, but parents may simply no longer be capable of accurately reporting them. Thus, decreased symptoms in adolescence may represent decreased validity in parent report when children reach adolescence rather than fewer ADHD behaviors.
Limitations
This study has several limitations. One concern regarding the use of CP as a potential confound derives from theoretical work on causal inference (e.g., Pearl, 2009). In our study, it is probable that unmeasured, pre-kindergarten, confounding variables are sources of some of the relations among ADHD, CP, and illicit drug use, in an M-structure (so-called because of the appearance of the graph; Pearl, 2009). If so, CP is more properly termed a “collider,” rather than a classic confound (Shrier & Platt, 2008). In our study, in which at least one unmeasured confound links ADHD and CP, and at least one other links CP and illicit drug use, inclusion of CP in the model could increase, rather than decrease, bias in the observed ADHD-drug use relations. However, we argue that the stability of our findings of the ADHD-illicit drug use link regardless of inclusion of CP in the model is evidence for a true, underlying, predictive relation.
As a second limitation, inattention and hyperactivity/impulsivity symptoms were not considered separately within our analyses because of their strong correlation within this dataset. However, the results of several confirmatory factor analysis (CFA) studies (DuPaul, Power, Anastopoulos & Reid, 1998; Hartung, McCarthy, Milich & Martin, 2005; Pillow, Pelham, Hoza, Molina, & Stultz, 1998) indicate that inattention and hyperactivity/impulsivity are generally distinct dimensions and should be considered as independent constructs. Not using symptom dimensions also restricts the generalization of our results to sub-types of ADHD. Third, ADHD symptoms were measured with the DSM-III-R (APA, 1987), which has been superseded by the DSM-IV (APA, 2000). Notable differences include the conceptualization of ADHD as one construct (i.e., rather than three subtypes) in the DSM-III-R and poorer assessment of and sensitivity to inattentive symptoms (i.e., DSM-III-R has four fewer inattention symptoms than the DSM-IV). However, Biederman et al. (1997) found good reliability between DSM versions of ADHD diagnosis (Kappa coefficient = .73) with a 93% diagnostic overlap. Discrepant cases were predominantly inattentive type ADHD diagnoses (90%) identified by DSM-IV criteria and not the DSM-III-R.
Fourth, our survival analysis began in 7th grade, whereas 9th grade was the last time point for the ADHD symptom trajectories. The overlap of time points limits the temporal precedence of ADHD symptoms and onset of use, weakening the study’s causal claims. Fifth, the three classes of trajectories were created based on the associations in this dataset. Thus, the classes of symptoms may not reflect a true classification of symptom patterns and may not generalize to other samples. Future research should replicate our findings to provide validity for the ADHD classes and their prediction of onset of illicit drug use (Curran & Bauer, 2004).
Finally, we acknowledge that these models were difficult to estimate, particularly with the sparse collection of ADHD symptom data. We attempted variant models to address distributional concerns and alternative hypotheses, but encountered substantial and sometimes insurmountable challenges in estimating these variants. The statistical techniques we used are very recent in development and quite taxing to the available software, which may make replication of our findings more challenging.
Future Directions
Several directions for future research are indicated. First, questions remain regarding the implications of concave growth in ADHD symptoms providing the strongest prediction of onset of illicit drug use. This concave growth may be a proxy for early symptoms, whereas the convex growth may be a proxy for adolescent symptoms. Future research should identify whether childhood and adolescent symptoms differentially predict initiating illicit drug use. Second, although several mediators have been implicated, how concave rather than convex symptom growth leads to increased risk of onset of illicit drug use is unclear. Research supports the mediating influence of deviant peers on the link between ADHD symptoms and illicit drug use (Marshal et al., 2003; Marshal & Molina, 2006). Research with other populations suggests that peer rejection, school failure, emotion regulation, and tolerance of deviant behavior are risk factors for illicit drug use (Hawkins et al., 1992). Future research should identify whether these factors play a role in increasing risk for illicit drug use for children with ADHD. Fourth, the relation between ADHD and CP symptoms and their influence on drug use may change not only across developmental periods, but also across stages of drug use. Whereas one set of processes may account for the onset of use, different processes may sustain use and lead to dependence. Future research should investigate the interplay among ADHD, CP, and illicit drug use across not only developmental periods but also stages of use. Finally, our analyses considered the independent effects of ADHD and CP, but an alternate explanation is that ADHD and CP interact to predict onset of drug use. Research findings conflict regarding the interaction of ADHD and CP. Some studies suggest that CP moderates ADHD’s prediction of illicit drug use (Chilcoat & Breslau, 1999; Claude & Firestone, 1995; Flory & Lynam, 2003), whereas the interaction between ADHD and CP symptoms does not significantly predict illicit drug use in other studies (Disney et al., 1999; Fergusson et al., 2008; Molina, Smith, & Pelham, 1999). This should be further investigated.
Conclusion
This study found that the trajectory with concave ADHD symptom growth predicted the earliest age of onset of illicit drug use, when controlling for CP, gender, SES, and race. These findings suggest that ADHD independent of CP plays an important role in predicting the age of onset of illicit drug use. Future research should investigate mediating variables that account for the effect of ADHD on onset of use and if ADHD in childhood rather than adolescence are more predictive of illicit drug use.
Acknowledgments
This work was supported by National Institute on Drug Abuse (NIDA) Career Development Award K01 DA024116 to the first author. The authors gratefully acknowledge the support of the Conduct Problems Prevention Research Group for facilitating access to the Fast Track data and for helpful comments on earlier drafts of this manuscript, as well as the Prevention Science and Methodology Group, funded by NIMH grant R01 MH40859, in the development of the analytic strategies.
References
- Achenbach TM. Integrative guide for the 1991 Child Behavior Chechklist (4–18), Youth Self Report, and Teacher Report Form profiles. Burlington, VT: University of Vermont, Department of Psychiatry; 1991. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 2. Washington, DC: American Psychiatric Association; 1968. [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 3. Washington, DC: American Psychiatric Association; 1980. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 3. Washington, DC: American Psychiatric Association; 1987. Revised. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: American Psychiatric Association; 2000. Text Revision. [Google Scholar]
- Anthony JC, Petronis KR. Early-onset drug use and risk of later drug problems. Drug & Alcohol Dependence. 1995;40:9–15. doi: 10.1016/0376-8716(95)01194-3. [DOI] [PubMed] [Google Scholar]
- August GJ, Winters KC, Realmuto GM, Fahnhorst T, Botzet A, Lee S. Prospective study of adolescent drug use among community samples of ADHD and non-ADHD participants. Journal of the American Academy of Child & Adolescent Psychiatry. 2006;45:824–832. doi: 10.1097/01.chi.0000219831.16226.f8. [DOI] [PubMed] [Google Scholar]
- Bachman JG, O’Malley PM, Schulenberg JE, Johnston LD, Freedman-Doan P, Messersmith EE. The education-drug use connection: How successes and failures in school relate to adolescent smoking, drinking, drug use, and delinquency. New York, NY: Taylor & Francis Group/Lawrence Erlbaum Associates; 2008. [Google Scholar]
- Bagwell CL, Molina BSG, Pelham WE, Hoza B. Attention-Deficit Hyperactivity Disorder and problems in peer relations: Predictions from childhood to adolescence. Journal of American Academy of Child Adolescent Psychiatry. 2001;40:1285–1293. doi: 10.1097/00004583-200111000-00008. [DOI] [PubMed] [Google Scholar]
- Barkley RA, Fischer M, Edelbrock CS, Smallish L. The adolescent outcome of hyperactive children diagnosed by research criteria: I. An 8-year prospective follow-up study. Journal of the American Academy of Child & Adolescent Psychiatry. 1990;29:546–557. doi: 10.1097/00004583-199007000-00007. [DOI] [PubMed] [Google Scholar]
- Biederman J, Faraone SV, Weber W, Russell RL, Rater M, Park KS. Correspondence between DSM-III-R and DSM-IV attention deficit/hyperactivity disorder. Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36:1682–1687. doi: 10.1097/00004583-199712000-00016. [DOI] [PubMed] [Google Scholar]
- Biederman J, Mick E, Faraone SV. Age-dependent decline of symptoms of attention deficit hyperactivity disorder: Impact of remission definition and symptom type. American Journal of Psychiatry. 2000;157:816–818. doi: 10.1176/appi.ajp.157.5.816. [DOI] [PubMed] [Google Scholar]
- Biederman J, Newcorn J, Sprich S. Comorbidity of attention deficit hyperactivity disorder with conduct, depressive, anxiety, and other disorders. American Journal of Psychiatry. 1991;148:564–577. doi: 10.1176/ajp.148.5.564. [DOI] [PubMed] [Google Scholar]
- Bryant AL, Schulenberg JE, O’Malley PM, Bachman JG, Johnston LD. How Academic Achievement, Attitudes, and Behaviors Relate to the Course of Substance Use During Adolescence: A 6-Year, Multiwave National Longitudinal Study. Journal of Research on Adolescence. 2003;13:361–397. [Google Scholar]
- Bukstein OG. Adolescent substance abuse: Assessment, prevention, and treatment. Oxford, United Kingdom: John Wiley & Sons; 1995. [Google Scholar]
- Capaldi DM, Stoolmiller M. Co-occurrence of conduct problems and depressive symptoms in early adolescent boys: III. Prediction to young-adult adjustment. Development & Psychopathology. 1999;11:59–84. doi: 10.1017/s0954579499001959. [DOI] [PubMed] [Google Scholar]
- Carlson CL, Mann M. Attention-deficit/hyperactivity disorder, predominately inattentive subtype. Child & Adolescent Psychiatric Clinics of North America. 2000;9:499–510. [PubMed] [Google Scholar]
- Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: A critical period of addiction vulnerability. American Journal of Psychiatry. 2003;160:1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilcoat HD, Breslau N. Pathways from ADHD to early drug initiation. Journal of the American Academy of Child & Adolescent Psychiatry. 1999;38:1347–1354. doi: 10.1097/00004583-199911000-00008. [DOI] [PubMed] [Google Scholar]
- Claude D, Firestone P. The development of ADHD boys: A 12-year follow-up. Canadian Journal of Behavioural Science. 1995;27:226–249. [Google Scholar]
- Conduct Problems Prevention Research Group (CPPRG) A developmental and clinical model for the prevention of conduct disorder: The FAST Track Program. Development & Psychopathology. 1992;4:509–527. [Google Scholar]
- Conduct Problems Prevention Research Group. Tobacco, alcohol, and drugs. Unpublished technical report 1995 [Google Scholar]
- Crews F, He J, Hodge C. Adolescent cortical development: A critical period of vulnerability for addiction. Pharmacology, Biochemistry & Behavior. 2007;86:189–199. doi: 10.1016/j.pbb.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Bauer DJ, Curran PJ. The integration of continuous and discrete latent variable models: Potential problems and promising opportunities. Psychological Methods. 2004;9:3–29. doi: 10.1037/1082-989X.9.1.3. [DOI] [PubMed] [Google Scholar]
- Curran PJ, Muthén BO. The application of latent curve analysis to testing developmental theories in intervention research. American Journal of Community Psychology. 1999;27:567–595. doi: 10.1023/A:1022137429115. [DOI] [PubMed] [Google Scholar]
- Dishion TJ, Andrews DW, Crosby L. Antisocial boys and their friends in early adolescence: Relationship characteristics, quality, and interactional process. Child Development. 1995;66:139–151. doi: 10.1111/j.1467-8624.1995.tb00861.x. [DOI] [PubMed] [Google Scholar]
- Dishion TJ, Patterson GR, Stoolmiller M, Skinner ML. Family, school, and behavioral antecedents to early adolescent involvement with antisocial peers. Developmental Psychology. 1991;27:172–180. [Google Scholar]
- Disney ER, Elkins IJ, McGue M, Iacona WG. Effects of ADHD, conduct disorder, and gender on substance use and abuse in adolescence. American Journal of Psychiatry. 1999;156:1515–1521. doi: 10.1176/ajp.156.10.1515. [DOI] [PubMed] [Google Scholar]
- DuPaul GJ, Power TJ, Anastopoulos AD, Reid R. ADHD rating scale: Checklists, norms, and clinical interpretation. The Guilford Press; NY: 1998. [Google Scholar]
- Elkins IJ, McGue M, Iacono WG. Prospective effects of attention-deficit/hyperactivity disorder, conduct disorder, and sex on adolescent substance use and abuse. Archives of General Psychiatry. 2007;64:1145–1152. doi: 10.1001/archpsyc.64.10.1145. [DOI] [PubMed] [Google Scholar]
- Elliott DS, Huizinga D, Ageton SS. Explaining delinquency and drug use. Beverly Hills, CA: Sage; 1985. [Google Scholar]
- Fergusson DM, Boden JM, Horwood LJ. The developmental antecedents of illicit drug use: Evidence from a 25-year longitudinal study. Drug & Alcohol Dependence. 2008;96:165–177. doi: 10.1016/j.drugalcdep.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Fleming JP, Kellam SG, Brown CH. Early predictors of age at first use of alcohol, marijuana, and cigarettes. Drug & Alcohol Dependence. 1982;9:285–303. doi: 10.1016/0376-8716(82)90068-0. [DOI] [PubMed] [Google Scholar]
- Flory K, Brown TL, Lynam D, Miller J, Leukefeld C, Clayton R. A comparison of African American and Caucasian adolescents’ developmental patterns of alcohol use. Cultural Diversity & Ethnic Minority Psychology. 2006;12:740–746. doi: 10.1037/1099-9809.12.4.740. [DOI] [PubMed] [Google Scholar]
- Flory K, Lynam D. The relation between attention deficit hyperactivity disorder and substance abuse: What role does conduct disorder play? Clinical Child & Family Psychology Review. 2003;6:1–16. doi: 10.1023/a:1022260221570. [DOI] [PubMed] [Google Scholar]
- Frazier TW, Youngstrom EA, Glutting JJ, Watkins MW. ADHD and achievement: Meta-analysis of the child, adolescent, and adult literatures and a concomitant study with college students. Journal of Learning Disabilities. 2007;40:49–65. doi: 10.1177/00222194070400010401. [DOI] [PubMed] [Google Scholar]
- Gittelman R, Mannuzza S, Shenker R, Bonagura N. Hyperactive boys almost grown up. Archives of General Psychiatry. 1985;42:937–947. doi: 10.1001/archpsyc.1985.01790330017002. [DOI] [PubMed] [Google Scholar]
- Greenbaum PE, Prange ME, Friedman RM, Silver SE. Substance abuse prevalence and comorbidity with other psychiatric disorders among adolescents with severe emotional disturbances. Journal of the American Academy of Child & Adolescent Psychiatry. 1991;30:575–583. doi: 10.1097/00004583-199107000-00008. [DOI] [PubMed] [Google Scholar]
- Hart EL, Lahey BB, Loeber R, Applegate B, Green SM, Frick PJ. Developmental change in attention-deficit hyperactivity disorder in boys: A four-year longitudinal study. Journal of Abnormal Child Psychology. 1995;23:729–749. doi: 10.1007/BF01447474. [DOI] [PubMed] [Google Scholar]
- Hartung CM, McCarthy DM, Milich R, Martin CA. Parent-adolescent agreement on disruptive behavior symptoms: A multitrait-multimethod model. Journal of Psychopathology & Behavioral Assessment. 2005;27:159–168. [Google Scholar]
- Hawkins JD, Catalano RF, Miller JY. Risk and protective factors for alcohol and other drug problems in adolescence and early adulthood: Implications for substance abuse prevention. Psychological Bulletin. 1992;112:64–105. doi: 10.1037/0033-2909.112.1.64. [DOI] [PubMed] [Google Scholar]
- Hoza B, Gerdes AC, Mrug S, Hinshaw SP, Bukowski WM, Gold JA, et al. Peer-assessed outcomes in the multimodal treatment study of children with attention deficit hyperactivity disorder. Journal of Clinical Child & Adolescent Psychology. 2005;34:74–86. doi: 10.1207/s15374424jccp3401_7. [DOI] [PubMed] [Google Scholar]
- Jessor R, Jessor SL. Problem behavior and psychosocial development: A longitudinal study of youth. New York, NY: Academic Press; 1977. [Google Scholar]
- Jester JM, Nigg JT, Buu A, Puttler LI, Glass JM, Heitzeg MM, et al. Trajectories of childhood aggression and inattention/hyperactivity: Differential effects on substance abuse in adolescence. Journal of the American Academy of Child & Adolescent Psychiatry. 2008;47:1158–1165. doi: 10.1097/CHI.0b013e3181825a4e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel DB, Davies M, Karus D, Yamaguchi K. The consequences in young adulthood of adolescent drug involvement: An overview. Archives of General Psychiatry. 1986;43:746–754. doi: 10.1001/archpsyc.1986.01800080032005. [DOI] [PubMed] [Google Scholar]
- Kellam SG, Brown CH, Rubin BR, Ensminger ME. Paths leading to teenage psychiatric symptoms and substance use: Developmental epidemiological studies in Woodlawn. In: Guze SB, Earls FJ, Barnett JE, editors. Childhood psychopathology and development. New York, NY: Raven Press; 1983. pp. 17–51. [Google Scholar]
- Keyes S, Block J. Prevalence and patterns of substance use among early adolescents. Journal of Youth & Adolescence. 1984;13:1–14. doi: 10.1007/BF02088649. [DOI] [PubMed] [Google Scholar]
- Khantzian EJ. The self-medication hypothesis of substance use disorders: A reconsideration and recent applications. Harvard Review of Psychiatry. 1997;4:231–244. doi: 10.3109/10673229709030550. [DOI] [PubMed] [Google Scholar]
- Langberg JM, Epstein JN, Altaye M, Molina BSG, Arnold LE, Vitiello B. The transition to middle school is associated with changes in the developmental trajectory of ADHD symptomatology in young adolescents with ADHD. Journal of Consulting & Clinical Psychology. 2008;37:651–663. doi: 10.1080/15374410802148095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert NM, Hartsough CS, Sassone D, Sandoval J. Persistence of hyperactivity symptoms from childhood to adolescence and associated outcomes. American Journal of Orthopsychiatry. 1987;57:22–32. doi: 10.1111/j.1939-0025.1987.tb03505.x. [DOI] [PubMed] [Google Scholar]
- Little RJA, Rubin DB. Statistical Analysis with Missing Data. New York, NY: Wiley; 1987. [Google Scholar]
- Lochman JE, CPPRG Screening of child behavior problems for prevention programs at school entry. Journal of Consulting & Clinical Psychology. 1995;63:549–559. doi: 10.1037//0022-006x.63.4.549. [DOI] [PubMed] [Google Scholar]
- Loeber R, Stouthamer-Loeber M, White HR. Developmental aspects of delinquency and internalizing problems and their association with persistent juvenile substance use between ages 7 and 18. Journal of Clinical Child Psychology. 1999;28:322–332. doi: 10.1207/S15374424jccp280304. [DOI] [PubMed] [Google Scholar]
- Loney J. Hyperkinesis comes of age: What do we know and where should we go? In: Chess S, Thomas A, editors. Annual Progress in Child Psychiatry & Child Development. New York, NY: Brunner/Mazel; 1981. pp. 598–616. [Google Scholar]
- Lynskey MT, Fergusson DM. Childhood conduct problems, attention deficit behaviors, and adolescent alcohol, tobacco, and illicit drug use. Journal of Abnormal Child Psychology. 1995;23:281–302. doi: 10.1007/BF01447558. [DOI] [PubMed] [Google Scholar]
- Marshal MP, Molina BSG. Antisocial Behaviors Moderate the Deviant Peer Pathway to Substance Use in Children With ADHD. Journal of Clinical Child & Adolescent Psychology. 2006;35:216–226. doi: 10.1207/s15374424jccp3502_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshal MP, Molina BSG, Pelham WE. Childhood ADHD and Adolescent Substance Use: An Examination of Deviant Peer Group Affiliation as a Risk Factor. Psychology of Addictive Behaviors. 2003;17:293–302. doi: 10.1037/0893-164X.17.4.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson W, Johnson N, Stewart MA. Hyperactive children as teenagers: A follow-up study. Journal of Nervous and Mental Disease. 1971;153:273–279. doi: 10.1097/00005053-197110000-00005. [DOI] [PubMed] [Google Scholar]
- Molina BSG, Hinshaw SP, Swanson JM, Arnold LE, Vitiello B, Jensen PS, et al. The MTA at 8 years: Prospective follow-up of children treated for combined-type ADHD in a multi-site study. Journal of American Academy of Child & Adolescent Psychiatry. 2009;48:484–500. doi: 10.1097/CHI.0b013e31819c23d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina BSG, Pelham WE. Childhood predictors of adolescent substance use in a longitudinal study of children with ADHD. Journal of Abnormal Psychology. 2003;112:497–507. doi: 10.1037/0021-843x.112.3.497. [DOI] [PubMed] [Google Scholar]
- Molina BSG, Smith BH, Pelham WE. Interactive effects of attention deficit hyperactivity disorder and conduct disorder on early adolescent substance use. Psychology of Addictive Behaviors. 1999;13:348–358. [Google Scholar]
- Mrug S, Hoza B, Pelham WE, Gnagy EM, Greiner AR. Behavior and peer status in children with ADHD: Continuity and change. Journal of Attention Disorders. 2007;10:359–371. doi: 10.1177/1087054706288117. [DOI] [PubMed] [Google Scholar]
- Muthén B. Second-generation structural equation modeling with a combination of categorical and continuous latent variables: New opportunities for latent class-latent growth modeling. In: Collins LM, Sayer AG, editors. New methods for the analysis of change. Washington, DC: American Psychological Association; 2001. pp. 291–322. [Google Scholar]
- Muthén B, Masyn K. Discrete-time survival mixture analysis. Journal of Educational and Behavioral Statistics. 2005;30:27–58. [Google Scholar]
- Muthén LK, Muthén BO. Mplus 5.2. [Computer program] Los Angeles, CA: Muthén, & Muthén; 2008. [Google Scholar]
- Nylund KL, Asparouhov T, Muthén BO. Deciding on the number of classes in latent class analysis and growth mixture modeling: A Monte Carlo simulation study. Structural Equation Modeling. 2007;14:535–569. [Google Scholar]
- Pearl J. Causality: Models, reasoning, and inference. 2. New York: Cambridge University Press; 2009. [Google Scholar]
- Pelham WE, Bender ME. Peer relationships in hyperactive children: Description and treatment. In: Gadow KD, Bailor I, editors. Advances in learning and behavioral disabilities. Greenwich, CT: JAI Press; 1982. pp. 365–436. [Google Scholar]
- Petraitis J, Flay BR, Miller TQ. Reviewing theories of adolescent substance use: Organizing pieces in the puzzle. Psychological Bulletin. 1995;117:67–86. doi: 10.1037/0033-2909.117.1.67. [DOI] [PubMed] [Google Scholar]
- Pillow DR, Pelham WE, Hoza B, Molina BSG, Stultz CH. Confirmatory factor analyses examining attention deficit hyperactivity disorder symptoms and other childhood disruptive behaviors. Journal of Abnormal Child Psychology. 1998;26:293–309. doi: 10.1023/a:1022658618368. [DOI] [PubMed] [Google Scholar]
- Robin AL. ADHD in adolescents: Diagnosis and treatment. NY, NY: Guilford Press; 1998. [Google Scholar]
- Robins LN, Davis DH, Wish E. Detecting predictors of rare events: Demographic, family and personal deviance as predictors of stages in the progression toward narcotic addiction. In: Strauss JS, Haroutun B, Roff M, editors. The origins and course of psychopathology. New York, NY: Plenum; 1977. pp. 379–406. [Google Scholar]
- Robins LN, McEvoy L. Conduct problems as predictors of substance abuse. In: Robins LN, Rutter M, editors. Straight and devious pathways from childhood to adulthood. New York, NY: Cambridge University Press; 1990. pp. 182–204. [Google Scholar]
- Robins PN, Pryzbeck TR. Age of Onset of Drug Use as a Factor in Drug and Other Disorders. NIDA Research Monograph. 1985;56:178–192. [PubMed] [Google Scholar]
- Rogosa DR, Willett JB. Understanding correlates of change by modeling individual differences in growth. Psychometrika. 1985;50:203–228. [Google Scholar]
- Scahill L, Schwab-Stone M, Merikangas KR, Leckman JF, Zhang H, Kasl S. Psychosocial and clinical correlates of ADHD in a community sample of school-age children. Journal of the American Academy of Child & Adolescent Psychiatry. 1999;38:976–984. doi: 10.1097/00004583-199908000-00013. [DOI] [PubMed] [Google Scholar]
- Shrier I, Platt RW. Reducing bias through directed acyclic graphs. BMC Medical Research Methodology. 2008;8:70–84. doi: 10.1186/1471-2288-8-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer J, Willett J. Applied longitudinal data analysis: Modeling change and event occurrence. New York, NY: Oxford University Press; 2003. [Google Scholar]
- Shaffer D, Fisher P, Dulcan MK, Davies M. The NIMH Diagnostic Interview Schedule for Children Version 2.3 (DISC-2.3): Description, acceptability, prevalence rates, and performance in the MECA study. Journal of the American Academy of Child & Adolescent Psychiatry. 1996;35:865–877. doi: 10.1097/00004583-199607000-00012. [DOI] [PubMed] [Google Scholar]
- Smith GM, Fogg CP. Teenage drug use: A search for causes and consequences. Personality & Social Psychology Bulletin. 1974;1:426–429. [Google Scholar]
- Steinberg L, Lerner RM. The scientific study of adolescence: A brief history. The Journal of Early Adolescence. 2004;24:45–54. [Google Scholar]
- Szatmari P, Boyle MH, Offord DR. ADDH and conduct disorder: Degree of diagnostic overlap and differences among correlates. Journal of the American Academy of Child & Adolescent Psychiatry. 1989;28:865–872. doi: 10.1097/00004583-198911000-00010. [DOI] [PubMed] [Google Scholar]
- Van Lier PAC, van der Ende J, Koot HM, Verhulst FC. Which better predicts conduct problems? The relationship of trajectories of conduct problems with ODD and ADHD symptoms from childhood into adolescence. Journal of Child Psychology & Psychiatry. 2007;48:601–608. doi: 10.1111/j.1469-7610.2006.01724.x. [DOI] [PubMed] [Google Scholar]
- Warner LA, Kessler RC, Hughes M, Anthony JC. Prevalence and correlates of drug use and dependence in the United States: Results from the National Comorbidity Survey. Archives of General Psychiatry. 1995;52:219–229. doi: 10.1001/archpsyc.1995.03950150051010. [DOI] [PubMed] [Google Scholar]
- Weiss G, Hechtman LT. Hyperactive children grown up: ADHD in children, adolescents, and adults. 2. New York, NY: Guilford Press; 1993. [Google Scholar]
- White HR, Loeber R, Stouthamer-Loeber M, Farrington DP. Developmental associations between substance use and violence. Development & Psychopathology. 1999;11:785–803. doi: 10.1017/s0954579499002321. [DOI] [PubMed] [Google Scholar]
- Wilens TE, Adamson J, Sgambati S, Whitley J, Santry A, Monuteaux MC, et al. Do individuals with ADHD self-medicate with cigarettes and substances of abuse? Results from a controlled family study of ADHD. The American Journal on Addictions. 2007;16:14–23. doi: 10.1080/10550490601082742. [DOI] [PubMed] [Google Scholar]
- Willoughby MT. Developmental course of ADHD symptomatology during the transition from childhood to adolescence: A review with recommendations. Journal of Child Psychology & Psychiatry. 2003;44:88–106. doi: 10.1111/1469-7610.t01-1-00104. [DOI] [PubMed] [Google Scholar]
- Wilks J, Callan VJ, Austin DA. Parent, peer and personal determinants of adolescent drinking. British Journal of Addiction. 1989;84:619–630. doi: 10.1111/j.1360-0443.1989.tb03477.x. [DOI] [PubMed] [Google Scholar]
- Zentall SS. Research on the educational implications of attention deficit hyperactivity disorder. Exceptional Children. 1993;60:143–153. [Google Scholar]