Abstract
Background
HIV-positive patients at HELP/PSI, Inc., an in-patient drug rehabilitation center, had a high baseline prevalence of S. aureus colonization (49%) and incidence of infection (17%) in a previous year long study.
Methods
A randomized, double-blinded, placebo-controlled study was conducted to determine whether repeated nasal application of mupirocin ointment would decrease the odds of S. aureus nasal colonization in 100 HELP/PSI patients over an eight-month period. A five-day course of study drug was given monthly and colonization was assessed at baseline and one month after each treatment. S. aureus infection was a secondary outcome.
Results
In repeated-measures analysis, mupirocin reduced the odds of monthly S. aureus nasal colonization by 83% compared to placebo (ORadj=0.17; P<0.0001). Subjects colonized at study entry had a 91% reduction in subsequent colonization (ORadj=0.09; P<0.0001). Mupirocin also suppressed S. aureus colonization in subjects not colonized at baseline (ORadj=0.23; P=0.006). There was no difference in infection rates between the mupirocin and placebo groups (HR=0.49, P=0.29).
Conclusions
Monthly application of nasal mupirocin significantly decreased S. aureus colonization in HIV patients in residential drug rehabilitation. Monthly mupirocin application has a potential role in long-term care settings or in HIV-positive patients with high rates of S. aureus colonization and infection.
Keywords: mupirocin, Staphylococcus aureus, colonization, MRSA, HIV, AIDS
Introduction
Staphylococcus aureus is a ubiquitous pathogen capable of causing infections in both community and healthcare settings.1 S. aureus colonization of the anterior nares often serves as a reservoir for subsequent infection.2, 3 Several studies have shown that drug users and people with HIV infection appear to be at increased risk for both S. aureus colonization and infection.4–11 Specific studies have shown that HIV-infected individuals have a higher risk of S. aureus infections including bacteremias,12 endocarditis,13 and recurrent MRSA skin infections compared to HIV-negative persons.14, 15
Previously, we prospectively followed the cases of 75 patients with AIDS in a residential drug treatment facility HELP/PSI, Inc. and screened monthly for S. aureus nasal colonization and infection. Forty-nine percent of subjects were colonized at baseline and 81% were colonized at least once during the yearlong study. Thirteen subjects (17%) experienced 17 infections over the study period.16
Given the high incidence of infection, there is a clear need to examine interventions for reducing S. aureus colonization and infection in high-risk populations such as HIV-infected drug users. To our knowledge, only one other randomized, placebo-controlled controlled trial of mupirocin has been conducted in HIV-infected patients.17 Although a one-time treatment course of mupirocin lowered colonization rates, the treatment effect waned with time. We were interested in determining if repeated use of mupirocin in a high-risk population would reduce colonization and/or infection with S. aureus. Therefore, we conducted a randomized, placebo-controlled study to examine the efficacy of monthly nasal decolonization with mupirocin in the high-risk HELP/PSI population over an eight-month period.
Methods
A double-blind, randomized, placebo-controlled clinical trial (DBRPCT) of intranasal mupirocin was conducted at HELP/PSI, Inc., an inpatient drug rehabilitation facility in Bronx, New York for people with HIV-infection. The mean length of stay was approximately 18 months. The study was conducted from September 2003 to April 2006. Patients were randomized to receive either 0.25 g/nostril of 2% mupirocin calcium or 0.25 g/nostril of placebo twice daily for five days. Treatment was repeated monthly for a maximum of eight treatments. S. aureus nasal colonization status was assessed one month after each treatment. The primary outcome was a repeated-measures analysis of monthly S. aureus colonization. Prevention of S. aureus infection was the secondary outcome measure. The Columbia University and the HELP/PSI Institutional Review Boards approved this study. The study was conducted in accordance with the ethical standards of these boards and with the Helsinki Declaration of 1975, as revised in 1983. This study was registered at clinicaltrials.gov (#NCT00801879).
Inclusion and Exclusion Criteria
All current and new residents at HELP/PSI were potentially eligible for inclusion in the study regardless of colonization status. Residents were invited to participate by their caregiver at a routine medical appointment. The study nurse determined the eligibility of interested parties and obtained their written informed consent. Exclusion criteria included hypersensitivity to mupirocin or glycerol, pregnancy, lactation, expected discharge within a month, and treatment with intranasal mupirocin within two months.
Randomization
Study identification numbers were pre-randomized 1:1 in blocks of four to receive either mupirocin or placebo by the Columbia University Research Pharmacy (CURP). Subjects were assigned consecutive study identification numbers as they were enrolled. The randomization sequence was concealed from all investigators during the study.
Study Drug
GlaxoSmithKline provided 2% mupirocin calcium ointment and CURP formulated the placebo. CURP packaged and labeled identical tubes with study drug. Nursing staff at HELP/PSI supervised administration of the study drug. Two strips of ointment with a length corresponding to 0.25 g were applied to an index card and then applied to each nostril with a cotton swab.
Study Procedures
Baseline cultures of the anterior nares were performed at study entry and subjects commenced treatment on the Monday following randomization. At the next monthly medical appointment at the on-site clinic, a repeat nasal culture was performed and the next treatment course was prescribed. This process was repeated for a maximum of eight treatments. A follow-up culture was taken one month after the final treatment or upon discharge, if it occurred earlier. Adverse events were recorded. Upon leaving HELP/PSI, subjects were no longer eligible to remain in the study.
Data Collection
A study physician or nurse collected medical record data from monthly clinic visits (a standard encounter form was used), standard admission and quarterly forms, doctor’s orders, nursing records, and laboratory reports. Data were collected on demographics, details of HIV disease, co-morbidities, drug relapse, infections, hospital admissions, skin conditions, ear, nose, and throat conditions, and medication usage (including antimicrobials, antiretrovirals, allergy/cold medicines, nasal sprays, skin treatments, and immunosuppresives). HIV viral load, CD4 count, and urine toxicology were performed routinely and recorded. Cultures were not routinely obtained for non-invasive S. aureus infections. Therefore, infections usually caused by S. aureus, such as folliculitis and abscesses, were categorized as S. aureus infections. Data were directly recorded into a password-protected computer program.
Microbiologic Data
Samples were collected from the anterior nares of each subject with a rayon-tipped swab (Becton Dickinson Culturette Systems). Culturettes were used to inoculate 1 ml of THB and samples were incubated for 3 hours at 37°C to increase culture yield. 100 uL were plated onto Mannitol salt agar (Becton Dickinson), grown for 24 hours at 37°C, and one yellow colony was selected. S. aureus was confirmed using Staphaurex (Remel).
S. aureus isolates underwent antibiotic susceptibility testing by Kirby-Bauer disk diffusion according to CLSI guidelines.18 Mupirocin susceptibility was established by Etest (AB Biodisk).
Isolates underwent typing by PFGE using SmaI digestion as described elsewhere.19 PFGE images were captured, archived, and analyzed with Bionumerics software coupled with the GelDec 1000 system (Bio-Rad). Dendrograms were constructed by the Dice coefficient method using 0.5 optimization and 1% tolerance.
Statistical analyses
Using data from an earlier study,17 we estimated that the mupirocin treatment group would have a 60% or greater reduction in colonization when compared to the placebo group one month after the first treatment. If at least 40 subjects remained in each group after one month, there would be 82% power to detect a 60% reduction in colonization using a two-sided chi-square test with alpha at 0.05. Fifty patients were enrolled in each study arm to account for potential loss to follow-up. This power calculation did not take into account the repeated-measures outcome (colonization) and likely underestimated the ability to detect group differences.
Chi-squared or Fisher’s exact test were used to compare categorical variables and Student’s t-test or Wilcoxon rank sum were used to compare continuous variables. For the primary outcome measure, a robust repeated-measures analysis was performed in the following way: a logistic regression model using generalized estimating equations (GLIMMIX procedure) was used to construct odds ratios comparing monthly colonization status between the mupirocin and placebo groups while controlling for possible confounders within the preceding month (or within the previous quarter for variables such as CD4 count and HIV viral load). Therefore, possible predictors of colonization status (apart from treatment group and demographics) were also measured at multiple timepoints and coupled with the subsequent outcome measure.20 Potential confounders were identified by backwards selection and kept in the model at P<0.05. Two planned subgroup analyses were performed in those colonized and those not colonized at baseline. P values were adjusted for repeated-measures analyses. The secondary outcome, S. aureus infection, was analyzed using a Cox proportional hazards model. All analyses were intent-to-treat and were performed using SAS 9.1.3 software.
Results
S. aureus nasal colonization
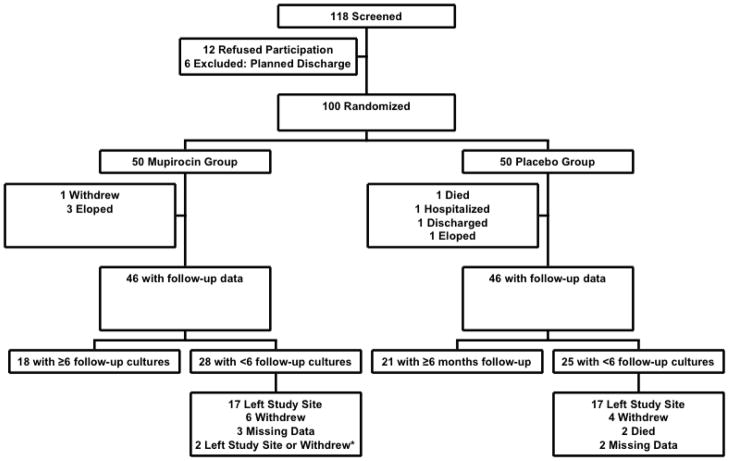
One hundred subjects were enrolled in the study and randomized to either mupirocin (n=50) or placebo (n=50) [figure 1]. Four subjects in each group did not have any post-treatment cultures and could not be evaluated. Eighteen subjects in the mupirocin [M] group and 21 in the placebo [P] group had at least 6 follow-up cultures. Most of the remaining subjects had fewer treatments because they left the study site prior to finishing the study. The reasons for study incompletion were comparable and the number of follow-up cultures was not statistically different between treatment groups.
Figure 1. Patient Screening, Exclusions, Randomization and Follow-up.
Figure 1 describes the flow of people through the study from screening through follow-up.
*For two subjects, it was unclear whether they first left the study site or withdrew from the study.
Colonization status at baseline was similar between treatment groups (43% M group vs. 48% P group; P=0.68). Subjects in each group had similar demographics and medical conditions (table 1). Subjects receiving mupirocin knew about their HIV diagnosis for longer and were more likely to have AIDS. Nonetheless, there were no statistical differences in CD4 count, HIV viral load, and number of subjects receiving antiretrovirals between the groups at baseline.
Table 1.
Baseline Characteristics of the Mupirocin vs. Placebo Group
| Mupirocin (n=46) | Placebo (n=46) | P value | |
|---|---|---|---|
| Characteristic | N (%) | N (%) | |
| S. aureus nasal colonization | 20 (43) | 22 (48) | 0.68 |
| Previous S. aureus infection ^ | 8 (17) | 4 (9) | 0.36 |
| Demographics | |||
| Gender: | |||
| Male | 38 (83) | 37 (80) | 0.79 |
| Female | 8 (17) | 9 (20) | |
| Age (mean ±SD) | 43.0 ± 6.7 | 42.7 ± 7.5 | 0.84 |
| Race: | 0.25 | ||
| Black--not hispanic | 33 (72) | 26 (57) | |
| Hispanic | 8 (17) | 15 (33) | |
| White | 4 (9) | 5 (11) | |
| No Response | 1 (2) | 0 (0) | |
| Education: | 0.97 | ||
| < High school | 22 (48) | 21 (46) | |
| Completed high school | 12 (26) | 13 (28) | |
| > High school | 12 (26) | 12 (26) | |
| Medical History (baseline) | |||
| AIDS diagnosis | 46 (100) | 40 (87) | 0.0113 |
| Years since tested HIV-positive (mean ±SD) | 12.5 ± 5.2 | 9.8 ± 5.4 | 0.0152 |
| CD4 count | 0.30 | ||
| <200 | 23 (50) | 30 (65) | |
| 201–350 | 12 (26) | 7 (15) | |
| >350 | 11 (24) | 9 (20) | |
| HIV viral load | 0.93 | ||
| undetectable* | 11 (24) | 13 (28) | |
| 50–1000 | 11 (24) | 9 (20) | |
| 1001–50000 | 14 (30) | 13 (28) | |
| >50000 | 10 (22) | 11 (24) | |
| Receiving HAART | 25 (54) | 25 (54) | 1.00 |
| Viral hepatitis | 24 (52) | 25 (54) | 0.83 |
| Diabetes | 1 (2) | 5 (11) | 0.09 |
Infection at or within one month prior to randomization
undetectable defined as VL <50 or <400 depending on year
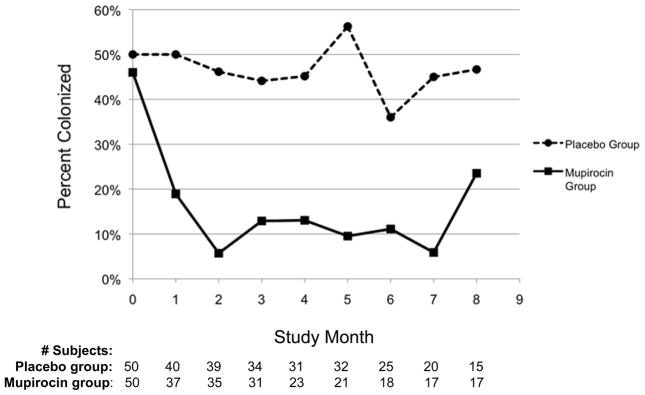
In the primary repeated-measures analysis, the odds of being colonized during the study were 81% lower in the M vs. P group (OR=0.19; P<0.0001, 95% CI 0.09–0.39). Figure 2 compares percent colonized in the treatment groups at each month of follow-up.
Figure 2. Effect of mupirocin on S. aureus nasal colonization.
Figure 2 shows the percentage of the mupirocin and placebo groups nasally colonized with S. aureus at baseline and at each month of the subjects’ follow-up. The number of subjects cultured at each follow-up month is shown.
Various other characteristics and their possible association with colonization status were investigated. Use of medications for nose and throat conditions and medications other than antibiotics were not associated with colonization status. Higher HIV viral load and lower CD4 counts were associated with colonization in univariate analysis, but were not statistically significant in multivariate analysis.
Drug/alcohol use relapse and antibiotic use in the preceding month were independently associated with monthly S. aureus colonization. Controlling for these variables, the odds of colonization were 83% lower in the M vs. P group (OR 0.17; P<0.0001, 95% CI 0.08–0.33); table 2.
Table 2.
Results of the Primary Repeated-Measures Analyses: Monthly Colonization with S. aureus in the Mupirocin vs. Placebo Group
| Analysis | OR for SA colonization | 95% CI | P value |
|---|---|---|---|
| M vs. P group, unadjusted | 0.19 | 0.095–0.387 | <0.0001 |
| M vs. P group, adjusted* | 0.17 | 0.08–0.33 | <0.0001 |
| Subgroup Analyses (M vs. P)* | |||
| Colonized at baseline | 0.09 | 0.04–0.24 | <0.0001 |
| Not colonized at baseline | 0.23 | 0.09–0.62 | 0.006 |
| Independent predictors of SA colonization | |||
| Relapse with drug or alcohol use within previous month | 4.40 | 1.60–12.12 | 0.004 |
| Antibiotic use within previous month | 1.94 | 1.15–3.25 | 0.013 |
analysis adjusted for relapse of drug or alcohol use and antibiotic use within the previous month
In the planned subgroup analysis among subjects colonized at baseline, the odds of colonization during follow-up were significantly lower in the M group (OR=0.09; P<0.0001, 95% CI 0.04–0.24) in multivariate analysis. M also suppressed colonization in those who were not colonized at baseline (OR=0.23; P=0.0056, 95% CI 0.09–0.62), (table 2).
S. aureus infections
Twenty-eight probable S. aureus infections occurred in 23 people. Twelve subjects were infected at or within 30 days prior to enrollment and were censored (two had multiple infections). Two other subjects had multiple infections, but only the initial infection was included in further analysis. With these restrictions, three (6.5%) subjects in the M group experienced a S. aureus infection compared to eight (19%) in the P group. All infections in the M group and six infections in the P group were minor skin and soft tissue infections. However, there was a case of phlebitis and a case of methicillin-resistant S. aureus (MRSA) bacteremia complicated by visceral abscesses and death in the P group. The risk of infection was not statistically significantly related to treatment group (HR=0.49, P=0.29, 95% CI 0.13–1.87). No variables were significantly related to infection status when examined in the Cox proportional hazards model. The one exception was colonization status, but because this variable is a mediator in the treatment group--infection causal pathway, it could not be included in the model.
Adverse Events
No serious adverse events were attributed to mupirocin. Three deaths occurred in the study and all were in the placebo arm—one death was associated with MRSA bacteremia complicated by abscesses. Four people in the placebo arm reported minor adverse effects.
Biological Data
One hundred baseline cultures and 470 follow-up cultures of the anterior nares were collected. Of these, 192 (34%) S. aureus isolates were obtained. Antibiotic susceptibility data are presented in table 3. As in our previous study,16 resistance to trimethoprim-sulfamethoxazole was very high (90%).
Table 3.
Antibiotic Resistance Data for 191~ S. aureus Isolates
Key: PEN, penicillin; FOX, cefoxitin; Ox, oxacillin; LVX, levofloxacin; GEN, gentamicin; ERY, erythromycin; CLIN, clindamycin; TET, tetracycline; RIF, rifampin; SXT, trimethoprim-sulfamethoxazole; VAN, vancomycin
One isolate was missing and could not be tested
Isolates classified as intermediate by Kirby-Bauer disk diffusion were categorized as resistant
Data differs from Cefoxitin data, in part, because not all samples were tested with a FOX disk
80 isolates expressed inducible resistance (positive D-test). These were categorized as resistant.
MICs to M were determined for one hundred thirty (68%) of the isolates. MICs ranged from 0.25 to 1.00 mg/mL (sensitive) with the exception of two isolates with high-level resistance (MIC >1024 mg/mL). Both isolates were obtained from the same person in the placebo group. The isolates were identical by PFGE and were present at baseline and one month later.
All 192 isolates underwent PFGE. Overall, the isolates had a similarity index (SI) of 44%. The isolates from the M group (n=52) had a SI of 44% and the isolates from the P group (n=140) had a SI of 52%. A cluster of 29 isolates (12 and 17 isolates from the M and P groups, respectively) was related to a USA500 reference strain (SI of 82%) supplied courtesy of Bo Shopsin.
Discussion
This nine-month DBRPCT demonstrates that repeated monthly mupirocin application can substantially decrease nasal colonization with S. aureus over time in HIV-infected individuals. The monthly risk of colonization was reduced 83% overall and 91% among those colonized at baseline. After one month, the proportion of colonized subjects was substantially lower in the mupirocin group compared to the placebo group and this difference was sustained. Mupirocin also decreased the odds of colonization in subjects who were not colonized at baseline by 75%. This is important because our previous study suggested that up to 63% of uncolonized subjects would eventually become colonized during one year of follow-up.16
Mupirocin has been studied and used in various populations including surgical, dialysis, and intensive-care unit patients with the goal of reducing S. aureus colonization and infection.21–23 However, the use of mupirocin for routine infection prophylaxis, was not supported by the authors of a large review.24 Another review was limited to randomized-controlled trials of mupirocin in colonized subjects where S. aureus infection was the primary outcome.25 This study showed a significant reduction in infections (RR 0.55, 95% CI 0.43–0.70), but did not include trials performed in HIV-positive subjects.
Martin et al.17 conducted a DBRPCT to examine the effect of mupirocin on colonization in 76 HIV-infected patients with stable S. aureus nasal carriage. After one 5-day treatment course, significantly more subjects in the mupirocin group than the placebo group remained persistently negative during follow-up, but subjects became re-colonized with time. Another DBRPCT demonstrated a reduction in S. aureus colonization among nursing home patients; however, persistently colonized patients were only treated once and by six months, there were no significant differences in colonization between treatment groups.26 Frequent re-colonization in these populations provides a rationale for repeated decolonization in high-risk people.
Our study also demonstrates that antibiotic use and drug/alcohol use relapse within the preceding month increased the risk of S. aureus nasal colonization. Other studies have shown that recent antibiotic use predisposes HIV-infected individuals to staphylococcal colonization, including MRSA.8, 27, 28 These results are also consistent with an investigation that demonstrated a higher prevalence of MRSA carriage among current drug addicts compared to non-addict patients and healthy controls.29 Several other potential risk factors such as skin disorders and severity of HIV disease were examined, but were not associated with colonization in multivariate analysis.
The development of mupirocin resistance was not detected in this study and has infrequently developed during clinical trials.30 The subject with a mupirocin resistant strain was colonized with it at study entry. Nonetheless, mupirocin resistance is a potential concern. It has been detected in several clinical settings31–33 as well as in a multidrug resistant USA300 MRSA strain that may be more common in men who have sex with men in Boston and San Francisco.34 Therefore, mupirocin may be ineffective against particular MRSA strains and in certain populations. Those who frequently administer mupirocin should maintain a high index of suspicion for resistance.
There was a high degree of resistance to trimethoprim-sulfamethoxazole possibly due to high trimethoprim-sulfamethoxazole exposure in our study population. There was also as a high degree of strain similarity among S. aureus isolates. Several subjects were also colonized with USA500, an MRSA clone which has an association with HIV-infected individuals for unclear reasons.7, 16, 35 These results are consistent with our previous study at HELP/PSI.16
The infection rate was not statistically different between treatment groups although there was a trend towards fewer infections in the mupirocin group (mupirocin reduced the infection rate by 51%). A retrospective study by Rahimian et al.,36 which included HIV-positive and HIV-negative subjects with recurrent skin and soft tissue infections (SSTIs) had similar findings. Because infection was a secondary outcome in our study, the study was not powered sufficiently to detect a difference in infection risk. However, S. aureus colonization was significantly associated with infection risk and was highly correlated with treatment group. Therefore, the results suggest that mupirocin may decrease the risk of S. aureus infection. A DBRPCT by Raz et al.37 supports this hypothesis. They demonstrated that among immnocompetent staphylococcal carriers experiencing recurrent SSTIs, monthly mupirocin application significantly decreased both colonization and SSTIs. They also found a strong correlation between colonization and SSTIs. Additional, sufficiently powered DBRCT studies are needed to determine if monthly mupirocin application can prevent staphylococcal infections in HIV-infected individuals.
There were several limitations to this study. A substantial proportion of subjects did not complete eight study treatments, usually because they were discharged or eloped. However, loss to follow-up was not associated with treatment group and therefore was unlikely to overestimate reductions associated with the mupirocin intervention. As the odds of colonization were significantly decreased by M (P<0.0001,) loss to follow-up did not impair our ability to detect an effect of mupirocin treatment.
Occasionally subjects missed treatments or it was difficult to confirm that they were treated. Therefore, we used an intent-to-treat analysis assuming that all subjects received equal treatment. This analysis may have led to an underestimation of the impact of mupirocin since some subjects were not exposed to the treatment.
Lastly, we were unable to definitively confirm that all of the infections that occurred in the study were due to S. aureus due to the lack of culture data. Cultures were often unavailable or could not be performed (i.e. cellulitis). This also limited our ability to assess the clonality of infection isolates and determine the role of nasal colonization in the development of infection in our population. A past study at the same institution, however, demonstrated that infections were usually caused by the person’s own commensal flora.16
Mupirocin is used in many settings and its effect on S. aureus nasal colonization and infection is not always clear. This study is unique in several ways. The effect of monthly mupirocin use was examined over time. The study was also conducted in a high-risk population: HIV-infected individuals in residential drug rehabilitation. The results suggest that a five-day course of mupirocin applied on a monthly basis can suppress S. aureus colonization over an extended period of time in high-risk individuals. Although this study suggests that mupirocin may reduce the risk of staphylococcal infection, additional studies are needed in select populations. By suppressing colonization, monthly mupirocin use may have a role in skilled nursing facilities, HIV-infected individuals, drug users and other populations with high rates of S. aureus infections.
Acknowledgments
Sources of Support: GlaxoSmithKline sponsored this study. R. Gordon has received support from NIH Grant number 1K08A1072043-01A1 and NIH Grant number 5 T32 AI0149821-04. F. Lowy has received support from NIH Grant number DA015018.
We are grateful to Allison Aiello, PhD and Abagail Zuger, MD for their critical reviews of the manuscript.
Footnotes
An abstract of preliminary results (poster #B083) was presented at the 2008 joint ICAAC/IDSA meeting.
Conflicts of Interest
None of the authors report any conflicts of interest.
References
- 1.Lowy FD. Staphylococcus aureus infections. N Engl J Med. 1998 Aug 20;339(8):520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 2.Wertheim HF, Melles DC, Vos MC, et al. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect Dis. 2005 Dec;5(12):751– 762. doi: 10.1016/S1473-3099(05)70295-4. [DOI] [PubMed] [Google Scholar]
- 3.Kluytmans J, van Belkum A, Verbrugh H. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin Microbiol Rev. 1997;10(3):505–520. doi: 10.1128/cmr.10.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nguyen MH, Kauffman CA, Goodman RP, et al. Nasal carriage of and infection with Staphylococcus aureus in HIV-infected patients. Ann Intern Med. 1999 Feb 2;130(3):221–225. doi: 10.7326/0003-4819-130-3-199902020-00026. [DOI] [PubMed] [Google Scholar]
- 5.Miller M, Cespedes C, Vavagiakis P, Klein RS, Lowy FD. Staphylococcus aureus colonization in a community sample of HIV-infected and HIV-uninfected drug users. Eur J Clin Microbiol Infect Dis. 2003 Aug;22(8):463–469. doi: 10.1007/s10096-003-0969-4. [DOI] [PubMed] [Google Scholar]
- 6.Tuazon CU, Sheagren JN. Increased rate of carriage of Staphylococcus aureus among narcotic addicts. J Infect Dis. 1974;129(6):725–727. doi: 10.1093/infdis/129.6.725. [DOI] [PubMed] [Google Scholar]
- 7.Miller M, Cespedes C, Bhat M, Vavagiakis P, Klein RS, Lowy FD. Incidence and persistence of Staphylococcus aureus nasal colonization in a community sample of HIV-infected and -uninfected drug users. Clin Infect Dis. 2007 Aug 1;45(3):343–346. doi: 10.1086/519429. [DOI] [PubMed] [Google Scholar]
- 8.Shet A, Mathema B, Mediavilla JR, et al. Colonization and Subsequent Skin and Soft Tissue Infection Due to Methicillin-Resistant Staphylococcus aureus in a Cohort of Otherwise Healthy Adults Infected with HIV Type 1. J Infect Dis. 2009 May 22; doi: 10.1086/599315. [DOI] [PubMed] [Google Scholar]
- 9.Seybold U, Supthut-Schroder B, Draenert R, Hogardt M, Bogner JR. Prevalence and risk factors of nasal colonization with Staphylococcus aureus - association with HIV infection in older patients. Scand J Infect Dis. 2009;41(1):63–66. doi: 10.1080/00365540802460000. [DOI] [PubMed] [Google Scholar]
- 10.Sissolak D, Geusau A, Heinze G, Witte W, Rotter ML. Risk factors for nasal carriage of Staphylococcus aureus in infectious disease patients, including patients infected with HIV, and molecular typing of colonizing strains. Eur J Clin Microbiol Infect Dis. 2002 Feb;21(2):88–96. doi: 10.1007/s10096-001-0666-0. [DOI] [PubMed] [Google Scholar]
- 11.Popovich KJ, Weinstein RA, Aroutcheva A, Rice T, Hota B. Community-associated methicillin-resistant Staphylococcus aureus and HIV: intersecting epidemics. Clin Infect Dis. Apr 1;50(7):979–987. doi: 10.1086/651076. [DOI] [PubMed] [Google Scholar]
- 12.Laupland KB, Ross T, Gregson DB. Staphylococcus aureus bloodstream infections: risk factors, outcomes, and the influence of methicillin resistance in Calgary, Canada, 2000–2006. J Infect Dis. 2008 Aug 1;198(3):336–343. doi: 10.1086/589717. [DOI] [PubMed] [Google Scholar]
- 13.Wilson LE, Thomas DL, Astemborski J, Freedman TL, Vlahov D. Prospective study of infective endocarditis among injection drug users. J Infect Dis. 2002 Jun 15;185(12):1761–1766. doi: 10.1086/340827. [DOI] [PubMed] [Google Scholar]
- 14.Crum-Cianflone N, Weekes J, Bavaro M. Recurrent community-associated methicillin-resistant Staphylococcus aureus infections among HIV-infected persons: incidence and risk factors. AIDS Patient Care STDS. 2009 Jul;23(7):499–502. doi: 10.1089/apc.2008.0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crum-Cianflone NF, Burgi AA, Hale BR. Increasing rates of community-acquired methicillin-resistant Staphylococcus aureus infections among HIV-infected persons. Int J STD AIDS. 2007 Aug;18(8):521–526. doi: 10.1258/095646207781439702. [DOI] [PubMed] [Google Scholar]
- 16.Gordon RJ, Quagliarello B, Cespedes C, et al. A molecular epidemiological analysis of 2 Staphylococcus aureus clonal types colonizing and infecting patients with AIDS. Clin Infect Dis. 2005 Apr 1;40(7):1028–1036. doi: 10.1086/428612. [DOI] [PubMed] [Google Scholar]
- 17.Martin JN, Perdreau-Remington F, Kartalija M, et al. A randomized clinical trial of mupirocin in the eradication of Staphylococcus aureus nasal carriage in human immunodeficiency virus disease. J Infect Dis. 1999;180(3):896–899. doi: 10.1086/314949. [DOI] [PubMed] [Google Scholar]
- 18.Clinical and Laboratory Standards Institute. M100-S16. Wayne, PA: 2006. Performance Standards for Antimicrobial Disk Susceptibility Tests. [Google Scholar]
- 19.Cespedes C, Miller M, Quagliarello B, Vavagiakis P, Klein RS, Lowy FD. Differences between Staphylococcus aureus isolates from medical and nonmedical hospital personnel. J Clin Microbiol. 2002 Jul;40(7):2594–2597. doi: 10.1128/JCM.40.7.2594-2597.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liang KY, Zeger SL. Longitudinal data analysis using generalized linar models. Biometrika. 1986;73:13–22. [Google Scholar]
- 21.Perl TM, Cullen JJ, Wenzel RP, et al. Intranasal mupirocin to prevent postoperative Staphylococcus aureus infections. N Engl J Med. 2002 Jun 13;346(24):1871–1877. doi: 10.1056/NEJMoa003069. [DOI] [PubMed] [Google Scholar]
- 22.Nasal mupirocin prevents Staphylococcus aureus exit-site infection during peritoneal dialysis. Mupirocin Study Group. J Am Soc Nephrol. 1996 Nov;7(11):2403–2408. doi: 10.1681/ASN.V7112403. [DOI] [PubMed] [Google Scholar]
- 23.Nardi G, Di Silvestre AD, De Monte A, et al. Reduction in gram-positive pneumonia and antibiotic consumption following the use of a SDD protocol including nasal and oral mupirocin. Eur J Emerg Med. 2001 Sep;8(3):203–214. doi: 10.1097/00063110-200109000-00008. [DOI] [PubMed] [Google Scholar]
- 24.Laupland KB, Conly JM. Treatment of Staphylococcus aureus colonization and prophylaxis for infection with topical intranasal mupirocin: an evidence-based review. Clin Infect Dis. 2003 Oct 1;37(7):933–938. doi: 10.1086/377735. [DOI] [PubMed] [Google Scholar]
- 25.van Rijen M, Bonten M, Wenzel R, Kluytmans J. Mupirocin ointment for preventing Staphylococcus aureus infections in nasal carriers. Cochrane Database of Systematic Reviews. 2008;(Issue 4) doi: 10.1002/14651858.CD006216.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mody L, Kauffman CA, McNeil SA, Galecki AT, Bradley SF. Mupirocin-based decolonization of Staphylococcus aureus carriers in residents of 2 long-term care facilities: a randomized, double-blind, placebo-controlled trial. Clin Infect Dis. 2003 Dec 1;37(11):1467–1474. doi: 10.1086/379325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Onorato M, Borucki MJ, Baillargeon G, et al. Risk factors for colonization or infection due to methicillin-resistant Staphylococcus aureus in HIV-positive patients: a retrospective case-control study. Infect Control Hosp Epidemiol. 1999 Jan;20(1):26–30. doi: 10.1086/501556. [DOI] [PubMed] [Google Scholar]
- 28.Villacian JS, Barkham T, Earnest A, Paton NI. Prevalence of and risk factors for nasal colonization with Staphylococcus aureus among human immunodeficiency virus-positive outpatients in Singapore. Infect Control Hosp Epidemiol. 2004 May;25(5):438–440. doi: 10.1086/502420. [DOI] [PubMed] [Google Scholar]
- 29.El-Sharif A, Ashour HM. Community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) colonization and infection in intravenous and inhalational opiate drug abusers. Exp Biol Med (Maywood) 2008 Jul;233(7):874–880. doi: 10.3181/0711-RM-294. [DOI] [PubMed] [Google Scholar]
- 30.Ammerlaan HS, Kluytmans JA, Wertheim HF, Nouwen JL, Bonten MJ. Eradication of methicillin-resistant Staphylococcus aureus carriage: a systematic review. Clin Infect Dis. 2009 Apr 1;48(7):922–930. doi: 10.1086/597291. [DOI] [PubMed] [Google Scholar]
- 31.Eltringham I. Mupirocin resistance and methicillin-resistant Staphylococcus aureus (MRSA) J Hosp Infect. 1997 Jan;35(1):1–8. doi: 10.1016/s0195-6701(97)90162-6. [DOI] [PubMed] [Google Scholar]
- 32.Rahman M, Noble WC, Cookson B, Baird D, Coia J. Mupirocin-Resistant Staphylococcus aureus. Lancet. 1987;330(8555):387–388. [PubMed] [Google Scholar]
- 33.Patel JB, Gorwitz RJ, Jernigan JA. Mupirocin resistance. Clin Infect Dis. 2009 Sep 15;49(6):935–941. doi: 10.1086/605495. [DOI] [PubMed] [Google Scholar]
- 34.Diep BA, Chambers HF, Graber CJ, et al. Emergence of multidrug-resistant, community-associated, methicillin-resistant Staphylococcus aureus clone USA300 in men who have sex with men. Ann Intern Med. 2008 Feb 19;148(4):249–257. doi: 10.7326/0003-4819-148-4-200802190-00204. [DOI] [PubMed] [Google Scholar]
- 35.Roberts RB, Chung M, de Lencastre H, et al. Distribution of methicillin-resistant Staphylococcus aureus clones among health care facilities in Connecticut, New Jersey, and Pennsylvania. Tri-State MRSA Collaborative Study Group. Microb Drug Resist. 2000;6(3):245–251. doi: 10.1089/mdr.2000.6.245. [DOI] [PubMed] [Google Scholar]
- 36.Rahimian J, Khan R, LaScalea KA. Does nasal colonization or mupirocin treatment affect recurrence of methicillin-resistant Staphylococcus aureus skin and skin structure infections? Infect Control Hosp Epidemiol. 2007 Dec;28(12):1415–1416. doi: 10.1086/523273. [DOI] [PubMed] [Google Scholar]
- 37.Raz R, Miron D, Colodner R, Staler Z, Samara Z, Keness Y. A 1-year trial of nasal mupirocin in the prevention of recurrent staphylococcal nasal colonization and skin infection. Arch Intern Med. 1996 May 27;156(10):1109–1112. [PubMed] [Google Scholar]