Abstract
The effects of late pregnancy on metabolic fuels, liver composition, gluconeogenesis, and nitrogen metabolism have been examined in fed and fasted rats.
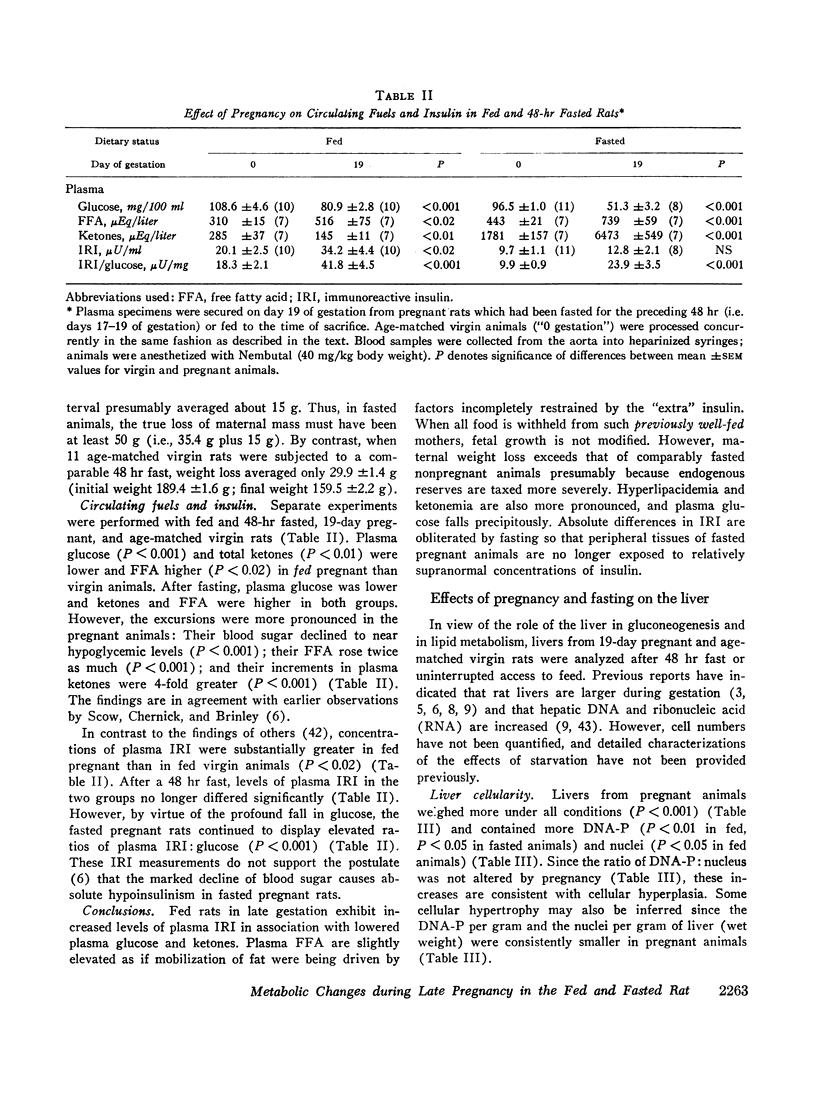
Plasma free fatty acid (FFA) and immunoreactive insulin (IRI) are greater and glucose and ketones are lower in fed 19-day pregnant than they are in agematched virgin rats. A 48 hr fast elicits greater increases in FFA and ketones and more profound reductions in glucose in the pregnant rats and obliterates the differences in IRI. Fetal weight is not modified by such fasting but maternal weight losses exceed that of the nongravid rats.
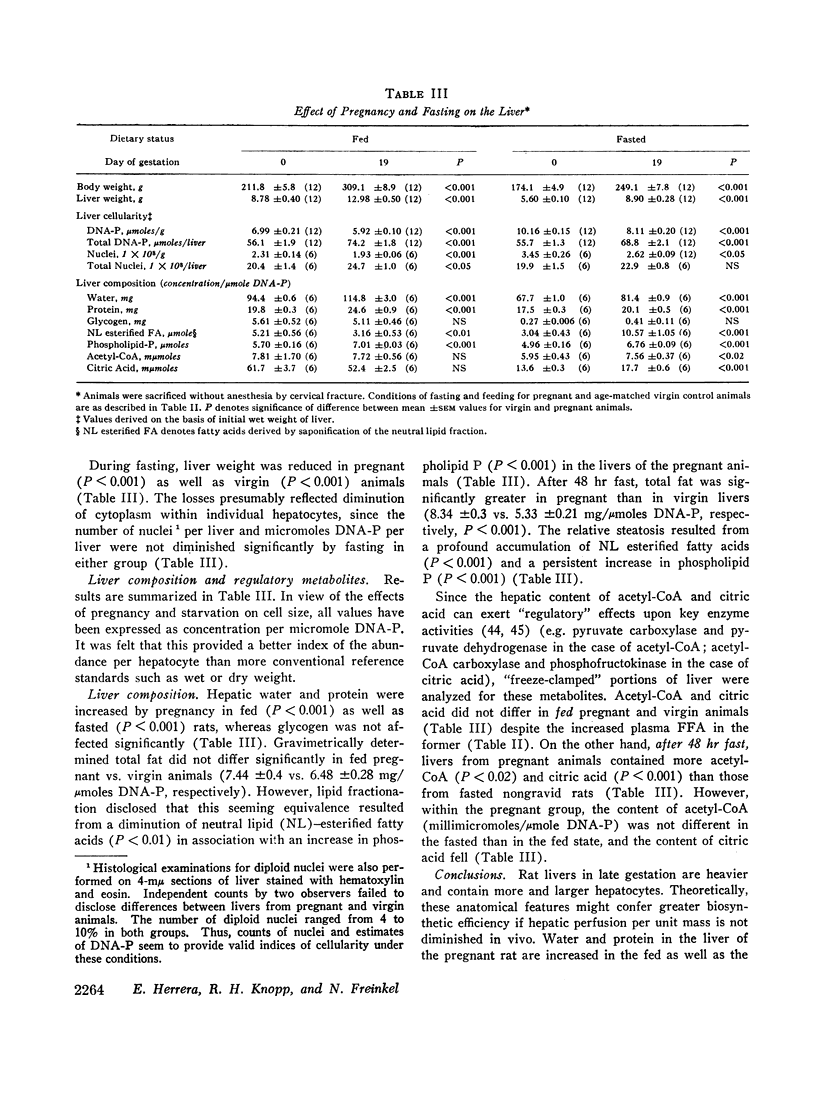
Livers from rats 19 days pregnant contain more and larger hepatocytes. Per μmole hepatic deoxyribonucleic acid (DNA)-phosphorus, water and protein are more abundant, whereas glycogen is unaffected. Livers from fed pregnant rats contain more lipid phosphorus and less neutral lipid fatty acid. After a 48 hr fast, hepatic steatosis supervenes in gravid animals due to accumulated neutral fat. The contents of hepatic acetyl-coenzyme A (CoA) and citric acid are not different in fed pregnant and virgin rats but are greater in the pregnant rats after fasting.
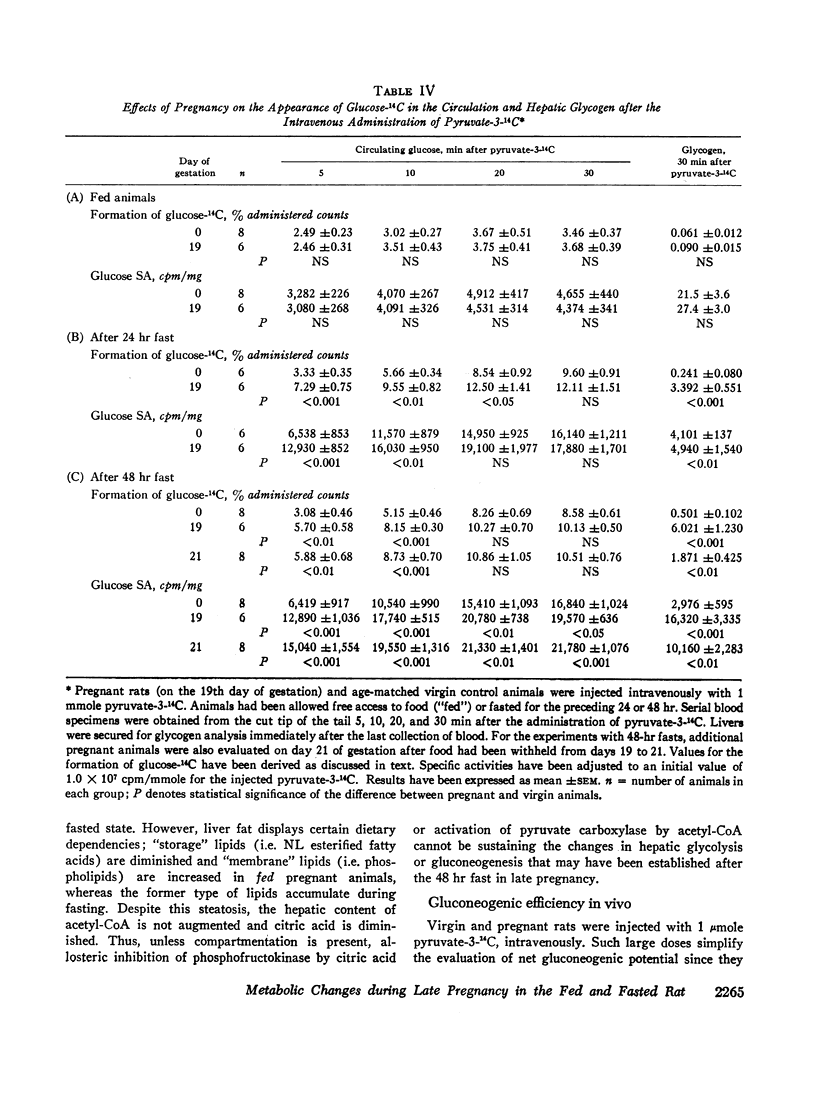
Formation of glucose-14C and glycogen-14C from administered pyruvate-14C are the same in fed pregnant and virgin rats, but greater in the pregnant ones after a 24 or 48 hr fast.
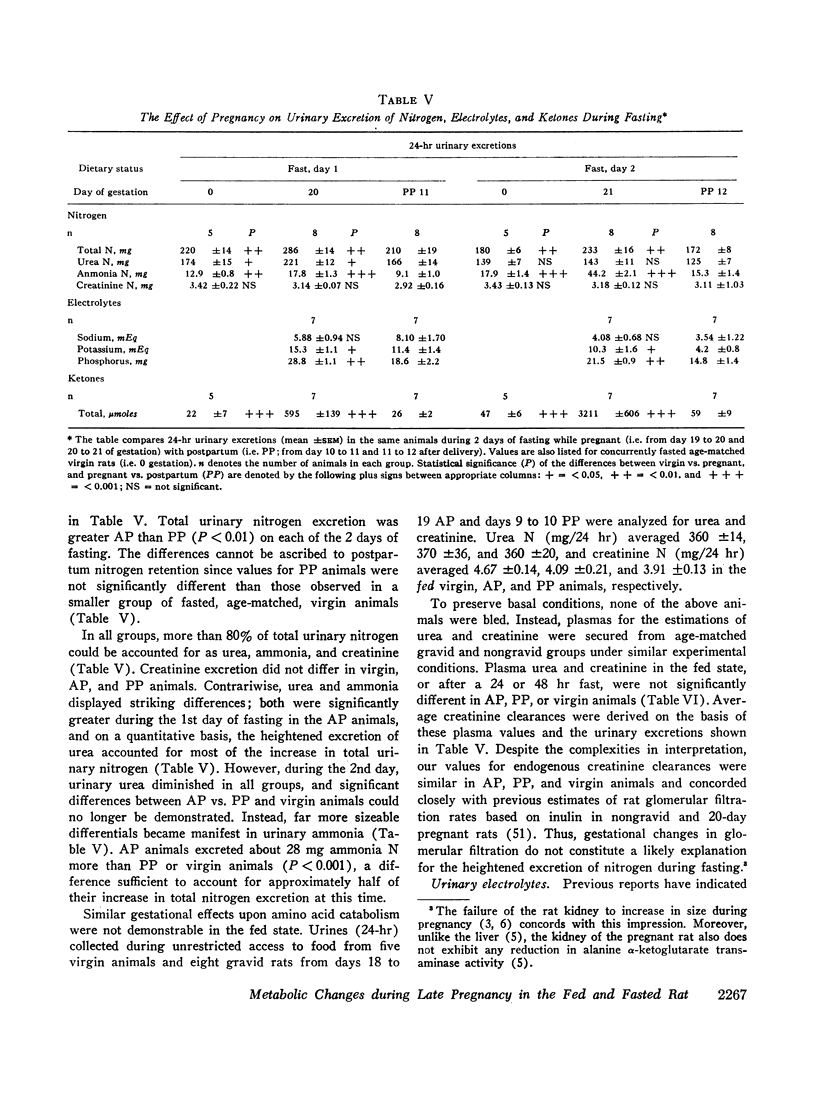
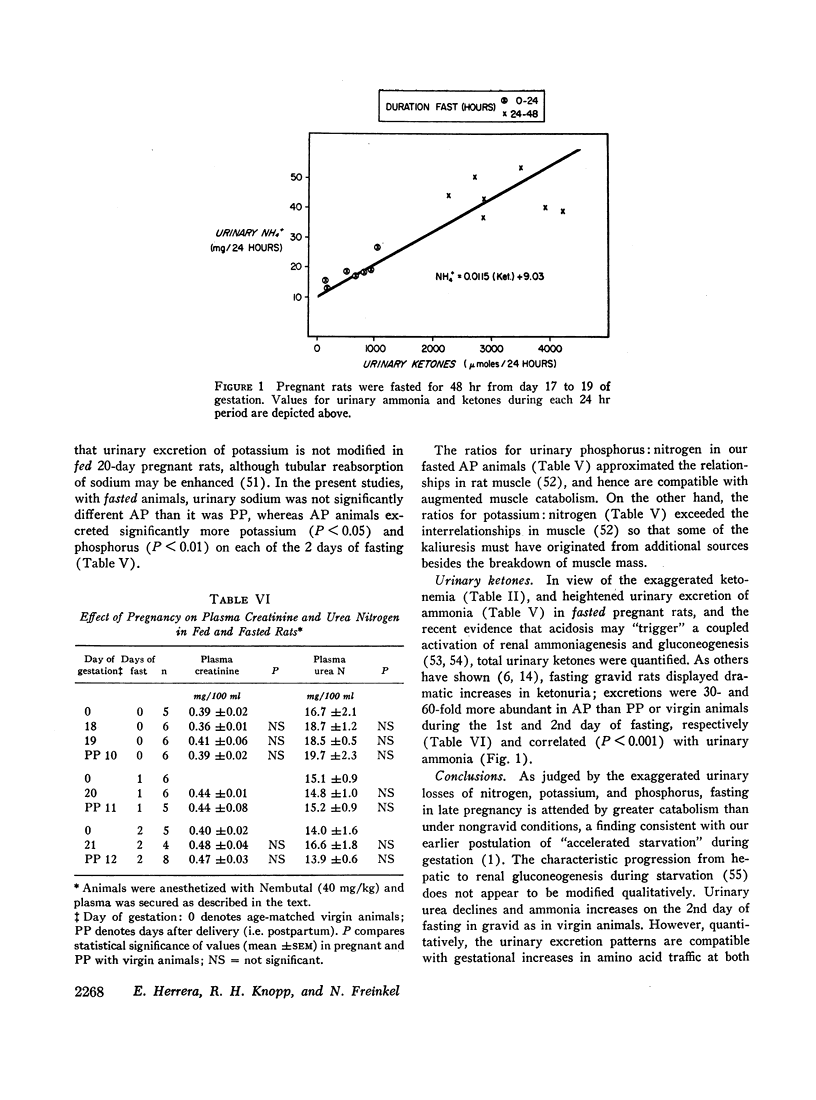
Pregnancy does not affect creatinine excretion, and urinary urea is not different in fed pregnant, virgin, and postpartum animals. Contrariwise, more nitrogen, potassium, and phosphorus are excreted by the pregnant animals during a 2 day fast. The increment in urinary nitrogen is due largely to urea on the 1st day, whereas heightened ammonia accounts for half the increase on the 2nd and correlates with the enhanced ketonuria.
Muscle catabolism, gluconeogenesis, and diversion to fat are activated more rapidly and to a greater degree when food is withheld during late gestation in the rat. These catabolic propensities are restrained in the fed state. The capacity for “accelerated starvation” may confer survival benefit upon an intermittently eating mother in the presence of a continuously feeding fetus.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEATON G. H., BEARE J., RYU M. H., McHENRY E. W. Protein metabolism in the pregnant rat. J Nutr. 1954 Oct 11;54(2):291–304. doi: 10.1093/jn/54.2.291. [DOI] [PubMed] [Google Scholar]
- BEATON G. H. Urea formation in the pregnant rat. Arch Biochem Biophys. 1957 Mar;67(1):1–9. doi: 10.1016/0003-9861(57)90240-0. [DOI] [PubMed] [Google Scholar]
- BERGMAN E. N., SELLERS A. F. Comparison of fasting ketosis in pregnant and nonpregnant guinea pigs. Am J Physiol. 1960 May;198:1083–1086. doi: 10.1152/ajplegacy.1960.198.5.1083. [DOI] [PubMed] [Google Scholar]
- BOURDEL G., JACQUOT R. Rôle du placenta dans les facultés anabolisantes des rattes gestantes. C R Hebd Seances Acad Sci. 1956 Jan 23;242(4):552–555. [PubMed] [Google Scholar]
- Basabe J. C., Chieri R. A., Foglia V. G. Action of sex hormones on the insulinemia of castrated female rats. Proc Soc Exp Biol Med. 1969 Apr;130(4):1159–1161. doi: 10.3181/00379727-130-33742. [DOI] [PubMed] [Google Scholar]
- Beck P., Daughaday W. H. Human placental lactogen: studies of its acute metabolic effects and disposition in normal man. J Clin Invest. 1967 Jan;46(1):103–110. doi: 10.1172/JCI105503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck P., Parker M. L., Daughaday W. H. Radioimmunologic measurement of human placental lactogen in plasma by a double antibody method during normal and diabetic pregnancies. J Clin Endocrinol Metab. 1965 Nov;25(11):1457–1462. doi: 10.1210/jcem-25-11-1457. [DOI] [PubMed] [Google Scholar]
- Beck P. Progestin enhancement of the plasma insulin response to glucose in Rhesus monkeys. Diabetes. 1969 Mar;18(3):146–152. doi: 10.2337/diab.18.3.146. [DOI] [PubMed] [Google Scholar]
- CAMPBELL R. M., INNES I. R., KOSTERLITZ H. W. Some dietary and hormonal effects on maternal, foetal and placental weights in the rat. J Endocrinol. 1953 Jan;9(1):68–75. doi: 10.1677/joe.0.0090068. [DOI] [PubMed] [Google Scholar]
- CAMPBELL R. M., INNES I. R., KOSTERLITZ H. W. The role of hormonal and dietary factors in the formation of excess ribonucleic acid in the livers of pregnant rats. J Endocrinol. 1953 Jan;9(1):52–67. doi: 10.1677/joe.0.0090052. [DOI] [PubMed] [Google Scholar]
- CAMPBELL R. M., KOSTERLITZ H. W. Some effects of pregnancy and lactation on the liver. J Endocrinol. 1949 Oct;6(2):171-83, pl. doi: 10.1677/joe.0.0060171. [DOI] [PubMed] [Google Scholar]
- CARTER N. W., SELDIN D. W., TENG H. C. Tissue and renal response to chronic respiratory acidosis. J Clin Invest. 1959 Jun;38(6):949–960. doi: 10.1172/JCI103878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHRISTENSEN P. J., DATE J. W., SCHONHEYDER F., VOLQVARTZ K. Amino acids in blood plasma and urine during pregnancy. Scand J Clin Lab Invest. 1957;9(1):54–61. doi: 10.3109/00365515709088114. [DOI] [PubMed] [Google Scholar]
- CURRY D. M., BEATON G. H. Cortisone resistance in pregnant rats. Endocrinology. 1958 Aug;63(2):155–161. doi: 10.1210/endo-63-2-155. [DOI] [PubMed] [Google Scholar]
- Cahill G. F., Jr, Owen O. E., Felig P. Insulin and fuel homeostasis. Physiologist. 1968 May;11(2):97–102. [PubMed] [Google Scholar]
- DOLE V. P. A relation between non-esterified fatty acids in plasma and the metabolism of glucose. J Clin Invest. 1956 Feb;35(2):150–154. doi: 10.1172/JCI103259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUNCOMBE W. G. THE COLORIMETRIC MICRO-DETERMINATION OF NON-ESTERIFIED FATTY ACIDS IN PLASMA. Clin Chim Acta. 1964 Feb;9:122–125. doi: 10.1016/0009-8981(64)90004-x. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- FREINKEL N., COHEN A. K., ARKY R. A., FOSTER A. E. ALCOHOL HYPOGLYCEMIA. II. A POSTULATED MECHANISM OF ACTION BASED ON EXPERIMENTS WITH RAT LIVER SLICES. J Clin Endocrinol Metab. 1965 Jan;25:76–94. doi: 10.1210/jcem-25-1-76. [DOI] [PubMed] [Google Scholar]
- Felig P., Owen O. E., Wahren J., Cahill G. F., Jr Amino acid metabolism during prolonged starvation. J Clin Invest. 1969 Mar;48(3):584–594. doi: 10.1172/JCI106017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari A., Catanzaro E., Russo-Alesi F. Nitrogen analysis by a continuous digestion system. Ann N Y Acad Sci. 1965 Nov 9;130(2):602–620. doi: 10.1111/j.1749-6632.1965.tb12604.x. [DOI] [PubMed] [Google Scholar]
- Fraser A. H., Godden W., Snook L. C., Thomson W. Ketonaemia in pregnant ewes and its possible relation to pregnancy disease. J Physiol. 1939 Nov 14;97(1):120–127. doi: 10.1113/jphysiol.1939.sp003794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedmann B., Goodman E. H., Jr, Weinhouse S. Effects of insulin and fatty acids on gluconeogenesis in the rat. J Biol Chem. 1967 Aug 25;242(16):3620–3627. [PubMed] [Google Scholar]
- Goodman A. D., Fuisz R. E., Cahill G. F., Jr Renal gluconeogenesis in acidosis, alkalosis, and potassium deficiency: its possible role in regulation of renal ammonia production. J Clin Invest. 1966 Apr;45(4):612–619. doi: 10.1172/JCI105375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodner C. J., Thompson D. J. Glucose metabolism in the fetus in utero: the effect of maternal fasting and glucose loading in the rat. Pediatr Res. 1967 Nov;1(6):443–451. doi: 10.1203/00006450-196711000-00003. [DOI] [PubMed] [Google Scholar]
- Goorno W. E., Rector F. C., Jr, Seldin D. W. Relation of renal gluconeogenesis to ammonia production in the dog and rat. Am J Physiol. 1967 Oct;213(4):969–974. doi: 10.1152/ajplegacy.1967.213.4.969. [DOI] [PubMed] [Google Scholar]
- Greengard O., Dewey H. K. Initiation by glucagon of the premature development of tyrosine aminotransferase, serine dehydratase, and glucose-6-phosphatase in fetal rat liver. J Biol Chem. 1967 Jun 25;242(12):2986–2991. [PubMed] [Google Scholar]
- HAGERMAN D. D. Metabolism of tissues from pregnant, diabetic rats in vitro. Endocrinology. 1962 Jan;70:88–94. doi: 10.1210/endo-70-1-88. [DOI] [PubMed] [Google Scholar]
- HUGGETT A. S., NIXON D. A. Use of glucose oxidase, peroxidase, and O-dianisidine in determination of blood and urinary glucose. Lancet. 1957 Aug 24;273(6991):368–370. doi: 10.1016/s0140-6736(57)92595-3. [DOI] [PubMed] [Google Scholar]
- Herrera E. M., Knopp R. H., Freinkel N. Urinary excretion of epinephrine and norepinephrine during fasting in late pregnancy in the rat. Endocrinology. 1969 Feb;84(2):447–450. doi: 10.1210/endo-84-2-447. [DOI] [PubMed] [Google Scholar]
- Herrera E., Freinkel N. Internal standards in the estimation of acetyl-CoA in liver extracts. J Lipid Res. 1967 Sep;8(5):515–518. [PubMed] [Google Scholar]
- Herrera E., Freinkel N. Interrelationships between liver composition, plasma glucose and ketones, and hepatic acetyl-CoA and citric acid during prolonged starvation in the male rat. Biochim Biophys Acta. 1968 Dec 23;170(2):244–253. doi: 10.1016/0304-4165(68)90004-4. [DOI] [PubMed] [Google Scholar]
- JOSIMOVICH J. B., MACLAREN J. A. Presence in the human placenta and term serum of a highly lactogenic substance immunologically related to pituitary growth hormone. Endocrinology. 1962 Aug;71:209–220. doi: 10.1210/endo-71-2-209. [DOI] [PubMed] [Google Scholar]
- Josimovich J. B. Potentiation of somatotrophic and diabetogenic effects of growth hormone by human placental lactogen (HPL). Endocrinology. 1966 Apr;78(4):707–714. doi: 10.1210/endo-78-4-707. [DOI] [PubMed] [Google Scholar]
- KAPLAN S. L., GRUMBACH M. M. IMMUNOASSAY FOR HUMAN CHORIONIC "GROWTH HORMONE-PROLACTIN" IN SERUM AND URINE. Science. 1965 Feb 12;147(3659):751–753. doi: 10.1126/science.147.3659.751. [DOI] [PubMed] [Google Scholar]
- Kerr G. R. The free amino acids of serum during development of Macaca mulatta. II. During pregnancy and fetal life. Pediatr Res. 1968 Nov;2(6):493–500. doi: 10.1203/00006450-196811000-00007. [DOI] [PubMed] [Google Scholar]
- Kumaresan P., Turner C. W. Effect of pregnancy on feed consumption and mammary gland growth in rats. Proc Soc Exp Biol Med. 1968 Dec;129(3):957–960. doi: 10.3181/00379727-129-33467. [DOI] [PubMed] [Google Scholar]
- LANDAU R. L., LUGIBIHL K. The catabolic and natriuretic effects of progesterone in man. Recent Prog Horm Res. 1961;17:249–292. [PubMed] [Google Scholar]
- LOGSDON E. E. A method for the determination of ammonia in biological materials on the autoanalyzer. Ann N Y Acad Sci. 1960 Jul 22;87:801–807. doi: 10.1111/j.1749-6632.1960.tb23237.x. [DOI] [PubMed] [Google Scholar]
- Landau R. L., Lugibihl K. The effect of progesterone on the concentration of plasma amino acids in man. Metabolism. 1967 Dec;16(12):1114–1122. doi: 10.1016/0026-0495(67)90057-1. [DOI] [PubMed] [Google Scholar]
- Leake N. H., Burt R. L. Effect of HPL and pregnancy on glucose uptake in rat adipose tissue. Am J Obstet Gynecol. 1969 Jan 1;103(1):39–43. doi: 10.1016/s0002-9378(16)34337-x. [DOI] [PubMed] [Google Scholar]
- Lichton I. J., Hugh J. E. Renal clearance of water and solutes by pregnant rats treated with spironolactone. Proc Soc Exp Biol Med. 1968 Oct;129(1):312–315. doi: 10.3181/00379727-129-33311. [DOI] [PubMed] [Google Scholar]
- MARSH W. H., FINGERHUT B., KIRSCH E. Determination of urea nitrogen with the diacetyl method and an automatic dialyzing apparatus. Am J Clin Pathol. 1957 Dec;28(6):681–688. doi: 10.1093/ajcp/28.6_ts.681. [DOI] [PubMed] [Google Scholar]
- Malaisse W. J., Malaisse-Lagae F., Picard C., Flament-Durand J. Effects of pregnancy and chorionic growth hormone upon insulin secretion. Endocrinology. 1969 Jan;84(1):41–44. doi: 10.1210/endo-84-1-41. [DOI] [PubMed] [Google Scholar]
- Martin J. M., Friesen H. Effect of human placental lactogen on the isolated islets of Langerhans in vitro. Endocrinology. 1969 Mar;84(3):619–621. doi: 10.1210/endo-84-3-619. [DOI] [PubMed] [Google Scholar]
- Matthies D. L. Studies of the luteotropic and mammotropic factor found in trophoblast and maternal peripheral blood of the rat at mid-pregnancy. Anat Rec. 1967 Sep;159(1):55–67. doi: 10.1002/ar.1091590109. [DOI] [PubMed] [Google Scholar]
- Newsholme E. A., Gevers W. Control of glycolysis and gluconeogenesis in liver and kidney cortex. Vitam Horm. 1967;25:1–87. doi: 10.1016/s0083-6729(08)60033-3. [DOI] [PubMed] [Google Scholar]
- Owen O. E., Felig P., Morgan A. P., Wahren J., Cahill G. F., Jr Liver and kidney metabolism during prolonged starvation. J Clin Invest. 1969 Mar;48(3):574–583. doi: 10.1172/JCI106016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAY E. W., AVERILL S. C., LYONS W. R., JOHNSON R. E. Rat placental hormonal activities corresponding to those of pituitary mammotropin. Endocrinology. 1955 Apr;56(4):359–373. doi: 10.1210/endo-56-4-359. [DOI] [PubMed] [Google Scholar]
- Rishi S., Golob E. K., Becker K. L., Shah N. Pancreatic insulin content of nonpregnant, pregnant and postpartum rats and the developing rat fetus. Diabetes. 1969 May;18(5):268–272. doi: 10.2337/diab.18.5.268. [DOI] [PubMed] [Google Scholar]
- SCOW R. O., CHERNICK S. S., BRINLEY M. S. HYPERLIPEMIA AND KETOSIS IN THE PREGNANT RAT. Am J Physiol. 1964 Apr;206:796–804. doi: 10.1152/ajplegacy.1964.206.4.796. [DOI] [PubMed] [Google Scholar]
- SILVERSTONE F. A., SOLOMONS E., RUBRICIUS J. The rapid intravenous glucose tolerance test in pregnancy. J Clin Invest. 1961 Dec;40:2180–2189. doi: 10.1172/JCI104444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaan N., Yen S. C., Friesen H., Pearson O. H. Serum placental lactogen levels during pregnancy and in trophoblastic disease. J Clin Endocrinol Metab. 1966 Dec;26(12):1303–1308. doi: 10.1210/jcem-26-12-1303. [DOI] [PubMed] [Google Scholar]
- Schalch D. S., Reichlin S. Plasma growth hormone concentration in the rat determined by radioimmunoassay: influence of sex, pregnancy, lactation, anesthesia, hypophysectomy and extrasellar pituitary transplants. Endocrinology. 1966 Aug;79(2):275–280. doi: 10.1210/endo-79-2-275. [DOI] [PubMed] [Google Scholar]
- Soeldner J. S., Slone D. Critical variables in the radioimmunoassay of serum insulin using the double antibody technic. Diabetes. 1965 Dec;14(12):771–779. doi: 10.2337/diab.14.12.771. [DOI] [PubMed] [Google Scholar]
- Spellacy W. N., Carlson K. L., Birk S. A. Dynamics of human placental lactogen. Am J Obstet Gynecol. 1966 Dec 15;96(8):1164–1173. doi: 10.1016/0002-9378(66)90529-1. [DOI] [PubMed] [Google Scholar]
- Spellacy W. N., Carlson K. L., Birk S. A., Schade S. L. Glucose and insulin alterations after one year of combination-type oral contraceptive treatment. Metabolism. 1968 Jun;17(6):496–501. doi: 10.1016/0026-0495(68)90041-3. [DOI] [PubMed] [Google Scholar]
- Spellacy W. N., Cohen W. D., Carlson K. L. Human placental lactogen levels as a measure of placental function. Am J Obstet Gynecol. 1967 Feb 15;97(4):560–561. doi: 10.1016/0002-9378(67)90570-4. [DOI] [PubMed] [Google Scholar]
- Turtle J. R., Kipnis D. M. The lipolytic action of human placental lactogen on isolated fat cells. Biochim Biophys Acta. 1967 Dec 5;144(3):583–593. doi: 10.1016/0005-2760(67)90047-1. [DOI] [PubMed] [Google Scholar]
- WEBER G., CANTERO A. Studies on hormonal factors influencing hepatic glucose-6-phosphatase. Endocrinology. 1957 Dec;61(6):701–712. doi: 10.1210/endo-61-6-701. [DOI] [PubMed] [Google Scholar]
- WHITE L. W., LANDAU B. R. SUGAR TRANSPORT AND FRUCTOSE METABOLISM IN HUMAN INTESTINE IN VITRO. J Clin Invest. 1965 Jul;44:1200–1213. doi: 10.1172/JCI105226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLLENBERGER A., RISTAU O., SCHOFFA G. [A simple technic for extremely rapid freezing of large pieces of tissue]. Pflugers Arch Gesamte Physiol Menschen Tiere. 1960;270:399–412. [PubMed] [Google Scholar]
- Yeung D., Stanley R. S., Oliver I. T. Development of gluconeogenesis in neonatal rat liver. Effect of triamcinolone. Biochem J. 1967 Dec;105(3):1219–1227. doi: 10.1042/bj1051219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinneman H. H., Seal U. S., Doe R. P. Urinary amino acids in pregnancy, following progesterone, and estrogen-progesterone. J Clin Endocrinol Metab. 1967 Mar;27(3):397–405. doi: 10.1210/jcem-27-3-397. [DOI] [PubMed] [Google Scholar]
- Zorzoli A., Turkenkopf I. J., Mueller V. L. Gluconeogenesis in developing rat kidney cortex. Biochem J. 1969 Jan;111(2):181–185. doi: 10.1042/bj1110181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuspan F. P., Goodrich S. Metabolic studies in normal pregnancy. I. Nitrogen metabolism. Am J Obstet Gynecol. 1968 Jan 1;100(1):7–14. doi: 10.1016/s0002-9378(15)33632-2. [DOI] [PubMed] [Google Scholar]


