Abstract
Amino acid balance across skeletal muscle and across subcutaneous adipose tissue plus skin of the forearm has been quantified in postabsorptive man before and after insulin infusion into the brachial artery.
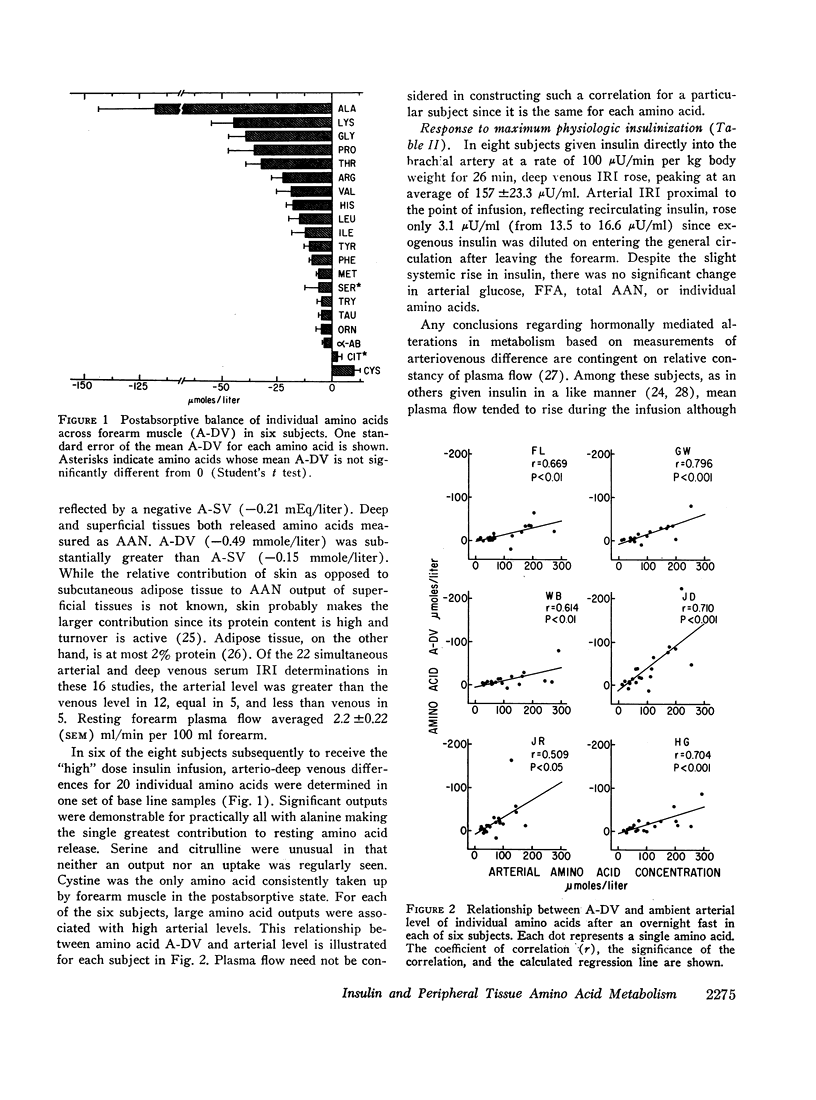
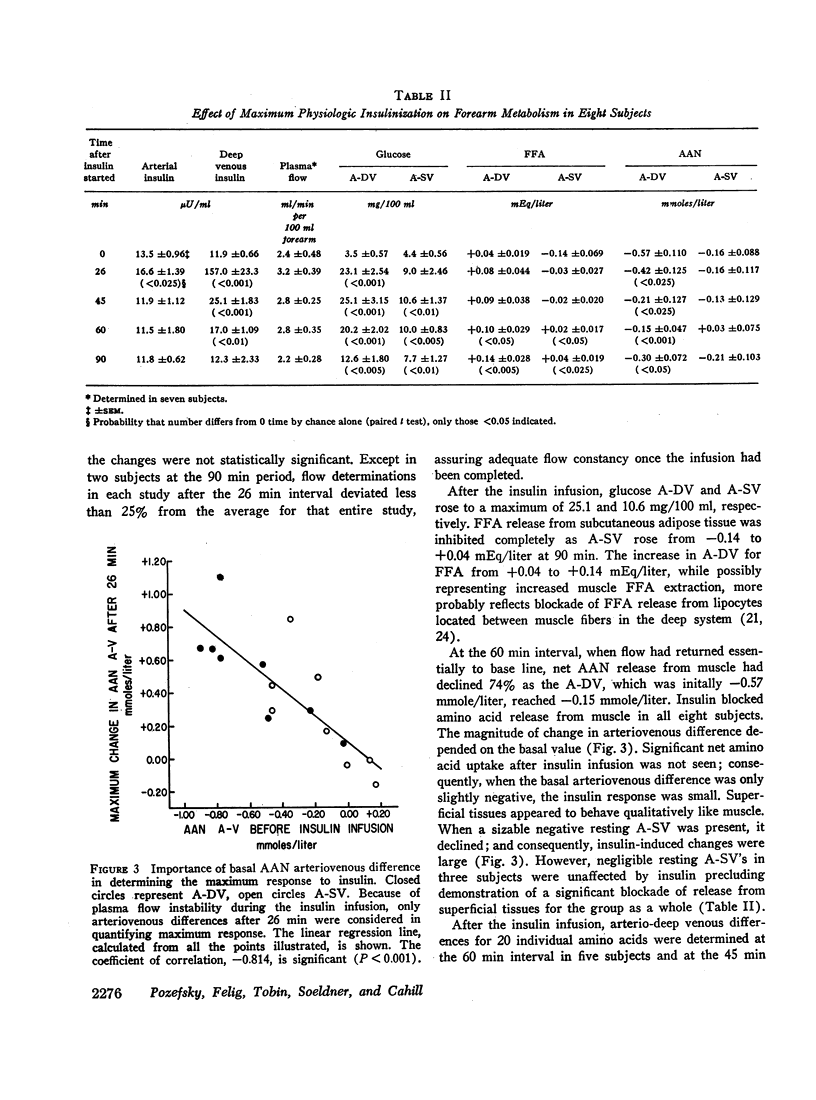
Skeletal muscle released significant amounts of alpha amino nitrogen after an overnight fast. Most individual amino acids were released. Alanine output was by far the greatest. The pattern of release probably reflects both the composition of muscle protein undergoing degradation and de novo synthesis of alanine by transamination. A significant correlation was observed between the extent of release of each amino acid and its ambient arterial concentration.
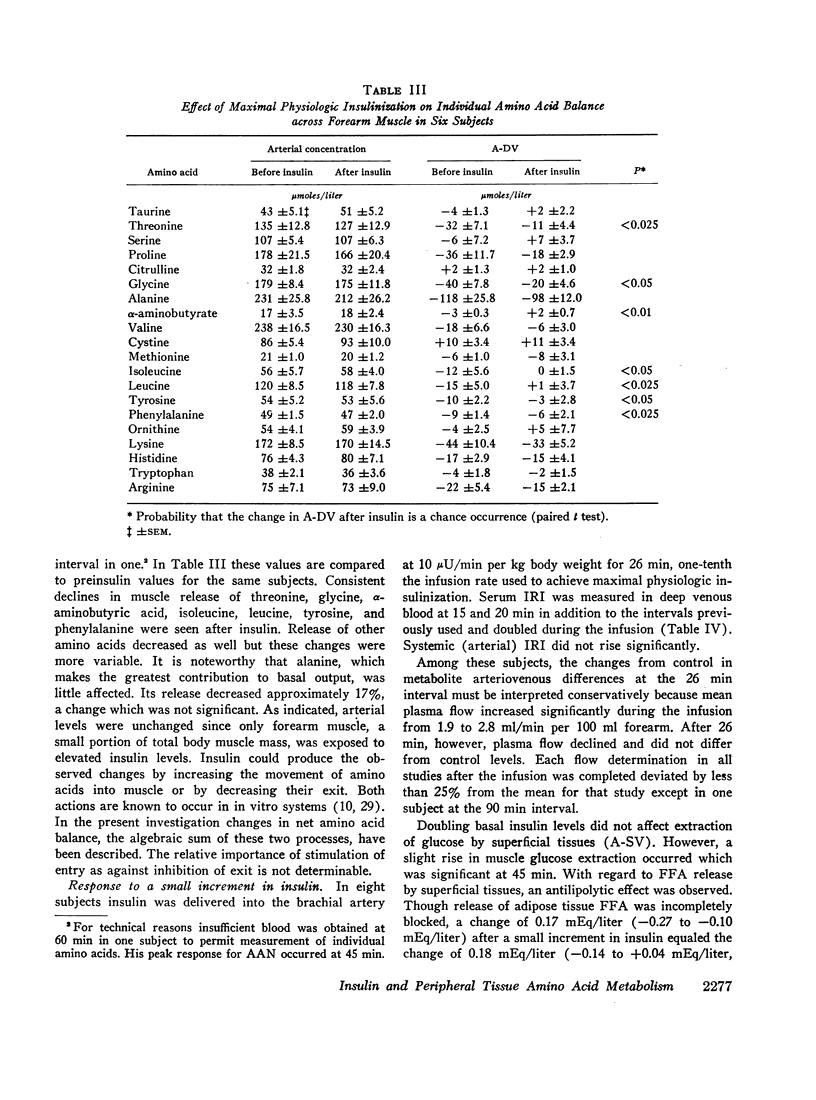
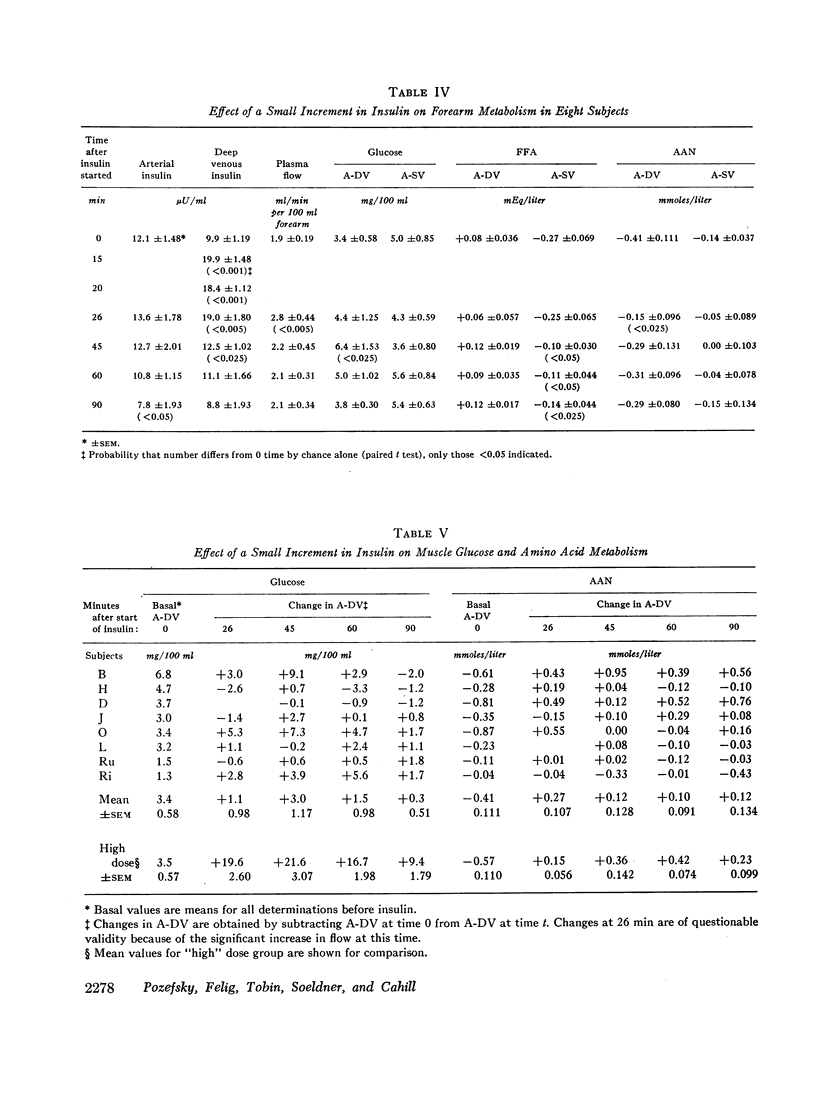
Elevation of forearm insulin in eight subjects from postabsorptive (12 μU/ml) to high physiologic levels (157 μU/ml) in addition to stimulating muscle glucose uptake blocked muscle alpha amino nitrogen release by 74%. Consistent declines in output were seen for leucine, isoleucine, tyrosine, phenylalanine, threonine, glycine, and α-aminobutyric acid. Alanine output was insignificantly affected. Doubling forearm insulin levels (from 10 to 20 μU/ml) in eight subjects increased muscle glucose uptake in three and blocked alpha amino nitrogen output in two although both effects were seen concurrently in only one subject. Changes in net amino acid balance after insulin could be accounted for by increased transport of amino acids into muscle cells or retardation of their exit.
It is likely that ambient arterial amino acid concentrations are established and maintained primarily by the extent of muscle amino acid release. The individual amino acids whose outputs from forearm muscle decline after forearm insulinization correspond well with those whose levels fall systematically after systemic insulinization. This suggests that declines in amino acid levels after systemic insulinization are due to inhibition of muscle release. Doubling basal insulin approaches the threshold both for blockade of muscle amino acid output and stimulation of glucose uptake, effects which appear to occur independently.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AKEDO H., CHRISTENSEN H. N. Nature of insulin action on amino acid uptake by the isolated diaphragm. J Biol Chem. 1962 Jan;237:118–122. [PubMed] [Google Scholar]
- ANDRES R., BALTZAN M. A., CADER G., ZIERLER K. L. Effect of insulin on carbohydrate metabolism and on potassium in the forearm of man. J Clin Invest. 1962 Jan;41:108–115. doi: 10.1172/JCI104452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANDRES R., CADER G., ZIERLER K. L. The quantitatively minor role of carbohydrate in oxidative metabolism by skeletal muscle in intact man in the basal state; measurements of oxygen and glucose uptake and carbon dioxide and lactate production in the forearm. J Clin Invest. 1956 Jun;35(6):671–682. doi: 10.1172/JCI103324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANDRES R., ZIERLER K. L., ANDERSON H. M., STAINSBY W. N., CADER G., GHRAYYIB A. S., LILIENTHAL J. L., Jr Measurement of blood flow and volume in the forearm of man; with notes on the theory of indicator-dilution and on production of turbulence, hemolysis, and vasodilatation by intra-vascular injection. J Clin Invest. 1954 Apr;33(4):482–504. doi: 10.1172/JCI102919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BALTZAN M. A., ANDRES R., CADER G., ZIERLER K. L. Heterogeneity of forearm metabolism with special reference to free fatty acids. J Clin Invest. 1962 Jan;41:116–125. doi: 10.1172/JCI104453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEARN A. G., BILLING B. H., SHERLOCK S. Hepatic glucose output and hepatic insulin sensitivity in diabetes mellitus. Lancet. 1951 Oct 20;2(6686):698–701. doi: 10.1016/s0140-6736(51)91476-6. [DOI] [PubMed] [Google Scholar]
- BOLLMAN J. L., FLOCK E. V., GRINDLAY J. H., MANN F. C., BLOCK M. A. Action of glucose and insulin on free amino acids of the dehepatized dog. Am J Physiol. 1953 Sep;174(3):467–470. doi: 10.1152/ajplegacy.1953.174.3.467. [DOI] [PubMed] [Google Scholar]
- Bondy P. K., James D. F., Farrar B. W. STUDIES OF THE ROLE OF THE LIVER IN HUMAN CARBOHYDRATE METABOLISM BY THE VENOUS CATHETER TECHNIC. I. NORMAL SUBJECTS UNDER FASTING CONDITIONS AND FOLLOWING THE INJECTION OF GLUCOSE. J Clin Invest. 1949 Mar;28(2):238–244. doi: 10.1172/JCI102065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CROFFORD O. B., FELTS P. W., LACY W. W. EFFECT OF GLUCOSE INFUSION ON THE INDIVIDUAL PLASMA FREE AMINO ACIDS IN MAN. Proc Soc Exp Biol Med. 1964 Oct;117:11–14. doi: 10.3181/00379727-117-29483. [DOI] [PubMed] [Google Scholar]
- Cahill G. F., Jr, Herrera M. G., Morgan A. P., Soeldner J. S., Steinke J., Levy P. L., Reichard G. A., Jr, Kipnis D. M. Hormone-fuel interrelationships during fasting. J Clin Invest. 1966 Nov;45(11):1751–1769. doi: 10.1172/JCI105481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsten A., Hallgren B., Jagenburg R., Svanborg A., Werkö L. Amino acids and free fatty acids in plasma in diabetes. I. The effect of insulin on the arterial levels. Acta Med Scand. 1966 Mar;179(3):361–370. doi: 10.1111/j.0954-6820.1966.tb05471.x. [DOI] [PubMed] [Google Scholar]
- Carlsten A., Hallgren B., Jagenburg R., Svanborg A., Werkö L. Arterio-hepatic venous differences of free fatty acids and amino acids. Studies in patients with diabetes or essential hypercholesterolemia, and in healthy individuals. Acta Med Scand. 1967 Feb;181(2):199–207. doi: 10.1111/j.0954-6820.1967.tb07246.x. [DOI] [PubMed] [Google Scholar]
- FORKER L. L., CHAIKOFF I. L., ENTENMAN C., TRAVER H. Formation of muscle protein in diabetic dogs, studies with S35 methionine. J Biol Chem. 1951 Jan;188(1):37–48. [PubMed] [Google Scholar]
- Felig P., Owen O. E., Wahren J., Cahill G. F., Jr Amino acid metabolism during prolonged starvation. J Clin Invest. 1969 Mar;48(3):584–594. doi: 10.1172/JCI106017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd J. C., Jr, Fajans S. S., Conn J. W., Knopf R. F., Rull J. Insulin secretion in response to protein ingestion. J Clin Invest. 1966 Sep;45(9):1479–1486. doi: 10.1172/JCI105455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gries F. A., Berger M., Oberdisse K. Untersuchungen zum antilipolytischen Effekt des Insulins am menschlichen Fettgewebe in vitro. Diabetologia. 1968 Nov;4(5):262–267. doi: 10.1007/BF01309898. [DOI] [PubMed] [Google Scholar]
- HERRERA M. G., RENOLD A. E. Hormonal effects on glycine metabolism in rat epididymal adipose tissue. Biochim Biophys Acta. 1960 Oct 21;44:165–167. doi: 10.1016/0006-3002(60)91536-5. [DOI] [PubMed] [Google Scholar]
- KOMINZ D. R., HOUGH A., SYMONDS P., LAKI K. The amino acid composition of action, myosin, tropomyosin and the meromyosins. Arch Biochem Biophys. 1954 May;50(1):148–159. doi: 10.1016/0003-9861(54)90017-x. [DOI] [PubMed] [Google Scholar]
- KRAHL M. E. Incorporation of C14-amino acids into glutathione and protein fractions of normal and diabetic rat tissues. J Biol Chem. 1953 Jan;200(1):99–109. [PubMed] [Google Scholar]
- KRAHL M. E. STIMULATION OF PEPTIDE SYNTHESIS IN ADIPOSE TISSUE BY INSULIN WITHOUT GLUCOSE. Am J Physiol. 1964 Mar;206:618–620. doi: 10.1152/ajplegacy.1964.206.3.618. [DOI] [PubMed] [Google Scholar]
- LASSEN N. A. MUSCLE BLOOD FLOW IN NORMAL MAN AND IN PATIENTS WITH INTERMITTENT CLAUDICATION EVALUATED BY SIMULTANEOUS XE-133 AND NA24 CLEARANCES. J Clin Invest. 1964 Sep;43:1805–1812. doi: 10.1172/JCI105054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen O. A., Lassen N. A., Quaade F. Blood flow through human adipose tissue determined with radioactive xenon. Acta Physiol Scand. 1966 Mar;66(3):337–345. doi: 10.1111/j.1748-1716.1966.tb03208.x. [DOI] [PubMed] [Google Scholar]
- MANCHESTER K. L. Insulin and incorporation of amino acids into protein of muscle. Cellular amino acid levels and aminoisobutyric acid uptake. Biochem J. 1961 Oct;81:135–147. doi: 10.1042/bj0810135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANCHESTER K. L., WOOL I. G. INSULIN AND INCORPORATION OF AMINO ACIDS INTO PROTEIN OF MUSCLE. 1. ACCUMULATION AND INCORPORATION STUDIES WITH THE PERFUSED RAT HEART. Biochem J. 1963 Nov;89:202–209. doi: 10.1042/bj0890202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOORE S., STEIN W. H. A modified ninhydrin reagent for the photometric determination of amino acids and related compounds. J Biol Chem. 1954 Dec;211(2):907–913. [PubMed] [Google Scholar]
- MYERS J. D. Net splanchnic glucose production in normal man and in various disease states. J Clin Invest. 1950 Nov;29(11):1421–1429. doi: 10.1172/JCI102380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OWEN E. E., ROBINSON R. R. Amino acid extraction and ammonia metabolism by the human kidney during the prolonged administration of ammonium chloride. J Clin Invest. 1963 Feb;42:263–276. doi: 10.1172/JCI104713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozefsky T., Felig P., Soeldner J. S., Cahill G. F., Jr Insulin blockade of amino acid release by human forearm tissues. Trans Assoc Am Physicians. 1968;81:258–265. [PubMed] [Google Scholar]
- RABINOWITZ D., ZIERLER K. L. Forearm metabolism in obesity and its response to intra-arterial insulin. Characterization of insulin resistance and evidence for adaptive hyperinsulinism. J Clin Invest. 1962 Dec;41:2173–2181. doi: 10.1172/JCI104676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RABINOWITZ D., ZIERLER K. L. Role of free fatty acids in forearm metabolism in man, quantitated by use of insulin. J Clin Invest. 1962 Dec;41:2191–2197. doi: 10.1172/JCI104678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross B. D., Hems R., Krebs H. A. The rate of gluconeogenesis from various precursors in the perfused rat liver. Biochem J. 1967 Mar;102(3):942–951. doi: 10.1042/bj1020942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SINEX F. M., MACMULLEN J., HASTINGS A. B. The effect of insulin on the incorporation of C14 into the protein of rat diaphragm. J Biol Chem. 1952 Oct;198(2):615–619. [PubMed] [Google Scholar]
- Soeldner J. S., Slone D. Critical variables in the radioimmunoassay of serum insulin using the double antibody technic. Diabetes. 1965 Dec;14(12):771–779. doi: 10.2337/diab.14.12.771. [DOI] [PubMed] [Google Scholar]
- THOMAS L. W. The chemical composition of adipose tissue of man and mice. Q J Exp Physiol Cogn Med Sci. 1962 Apr;47:179–188. doi: 10.1113/expphysiol.1962.sp001589. [DOI] [PubMed] [Google Scholar]
- TROUT D. L., ESTES E. H., Jr, FRIEDBERG S. J. Titration of free fatty acids of plasma: a study of current methods and a new modification. J Lipid Res. 1960 Apr;1:199–202. [PubMed] [Google Scholar]
- VALLANCE-OWEN J., WRIGHT P. H. Assay of insulin in blood. Physiol Rev. 1960 Apr;40:219–244. doi: 10.1152/physrev.1960.40.2.219. [DOI] [PubMed] [Google Scholar]
- WERK E. E., Jr, McPHERSON H. T., HAMRICK L. W., Jr, MYERS J. D., ENGEL F. L. Studies on ketone metabolism in man. I. A method for the quantitative estimation of splanchnic ketone production. J Clin Invest. 1955 Aug;34(8):1256–1267. doi: 10.1172/JCI103172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterlow J. C., Stephen J. M. Adaptation of the rat to a low-protein diet: the effect of a reduced protein intake on the pattern of incorporation of L-[14C] lysine. Br J Nutr. 1966;20(3):461–484. doi: 10.1079/bjn19660047. [DOI] [PubMed] [Google Scholar]
- Weisenfeld S., Podolsky S., Goldsmith L., Ziff L. Adsorption of insulin to infusion bottles and tubing. Diabetes. 1968 Dec;17(12):766–771. doi: 10.2337/diab.17.12.766. [DOI] [PubMed] [Google Scholar]
- Wilson H. E. Studies on the physiology of protein retention. J Physiol. 1931 Jul 6;72(3):327–343. doi: 10.1113/jphysiol.1931.sp002777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZIERLER K. L., RABINOWITZ D. EFFECT OF VERY SMALL CONCENTRATIONS OF INSULIN ON FOREARM METABOLISM. PERSISTENCE OF ITS ACTION ON POTASSIUM AND FREE FATTY ACIDS WITHOUT ITS EFFECT ON GLUCOSE. J Clin Invest. 1964 May;43:950–962. doi: 10.1172/JCI104981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zierler K. L. THEORY OF THE USE OF ARTERIOVENOUS CONCENTRATION DIFFERENCES FOR MEASURING METABOLISM IN STEADY AND NON-STEADY STATES. J Clin Invest. 1961 Dec;40(12):2111–2125. doi: 10.1172/JCI104437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinneman H. H., Nuttall F. Q., Goetz F. C. Effect of endogenous insulin on human amino acid metabolism. Diabetes. 1966 Jan;15(1):5–8. doi: 10.2337/diab.15.1.5. [DOI] [PubMed] [Google Scholar]


