Abstract
In homozygous mutants of Drosophila lethal-2-giant larvae (lgl), tissues lose apico-basal cell polarity and exhibit ectopic proliferation. Here, we use clonal analysis in the developing eye to investigate the effect of lgl null mutations in the context of surrounding wild-type tissue. lgl− clones in the larval eye disc exhibit ectopic expression of the G1-S regulator, Cyclin E, and ectopic proliferation, but do not lose apico-basal cell polarity. Decreasing the perdurance of Lgl protein in larval eye disc clones, by forcing extra proliferation of lgl− tissue (using a Minute background), leads to a loss in cell polarity and to more extreme ectopic cell proliferation. Later in development at the pupal stage, lgl mutant photoreceptor cells show aberrant apico-basal cell polarity, but this is not associated with ectopic proliferation, presumably because cells are differentiated. Thus in a clonal context, the ectopic proliferation and cell polarity defects of lgl− mutants are separable. Furthermore, lgl− mosaic eye discs have alterations in the normal patterns of apoptosis: in larval discs some lgl− and wild-type cells at the clonal boundary undergo apoptosis and are excluded from the epithelia, but apoptosis is decreased elsewhere in the disc, and in pupal retinas lgl− tissue shows less apoptosis.
Keywords: Drosophila, eye development, proliferation, apico-basal cell polarity, apoptosis, lgl, cyclin E
Introduction
In a multicellular animal, tissue architecture is important for limiting cell proliferation (reviewed by Bilder, 2004; Humbert et al., 2003). Cell-cell contact and adhesion to the extracellular matrix regulate signalling pathways that control epithelial cell proliferation (reviewed by Walker et al., 2005). Apico-basal cell polarity (cell shape and localization of cellular junction complexes to specific zones along the apico-basal axis (reviewed by Tepass et al., 2001)) is thought to be important for these controls to occur. Indeed, loss of cell polarity by mutation of the key apico-basal cell polarity genes, discs large (dlg), scribble (scrib) or lethal-2-giant larvae (lgl) in Drosophila, is associated with a misregulation of cell proliferation and neoplastic overgrowth (Bilder et al., 2000; Brumby and Richardson, 2003). Because of this phenotype, Scrib, Dlg and Lgl have been termed neoplastic tumour suppressors and have been shown to function in a common genetic pathway controlling apico-basal cell polarity in the embryo (Bilder et al., 2000).
Drosophila epithelial apico-basal cell polarity is characterized by the subdivision of the lateral membrane by specialised junctional structures, the adherens junctions and septate junctions (reviewed by Tepass et al., 2001). Apico-basal cell polarity involves the interplay of three evolutionarily conserved membrane-associated complexes; the Bazooka (Baz) complex (Baz, Par6, and atypical protein kinase C (aPKC)), the Crumbs complex (Crumbs (Crb), Stardust (Sdt) and DPatj), and the Dlg complex (Dlg, Scrib and Lgl) (Bilder et al., 2003; Tanentzapf and Tepass, 2003). The Baz complex and the Crb complex are located apical to the adherens junctions in the subapical region, and mutants in Crb or Baz complex components result in defects in apico-basal polarity and adherens junction localization (reviewed by Tepass et al., 2001). The Dlg complex comprises Dlg (a Membrane associated guanylate kinase (MAGUK) protein), Scrib (a leucine rich repeat PDZ domain protein) and Lgl (a WD40 domain-containing protein) (reviewed by Humbert et al., 2003). Dlg and Scrib are localized at the septate junctions, beneath the adherens junction, while Lgl, although not specifically located at septate junctions, is concentrated around septate junctions and genetically interacts with Scrib and Dlg (Bilder et al., 2000). Lgl can form a complex with aPKC and Par6 in Drosophila and mammalian cells, and phosphorylation of Lgl by aPKC at the apical region is important in restricting 3 the cortical localization of Lgl to more basal domains (Betschinger et al., 2003; Plant et al., 2003). Furthermore, the Crb complex acts antagonistically to the Dlg complex in cell polarity control (Bilder et al., 2003; Tanentzapf and Tepass, 2003). In lgl, scrib or dlg mutants apical polarity determinants, such as Crb, are mislocalized around the cortex and adherens junctions are fragmented (Bilder et al., 2000).
Of the cell polarity proteins, Dlg, Scrib and Lgl are unique in also acting to negatively regulate cell proliferation (reviewed by Bilder, 2004; Humbert et al., 2003). This raises the question of whether the loss-of-cell-proliferation control could be indirect, due to loss of polarity and disruption of junctions, thereby preventing the normal curb to cell proliferation that occurs upon cell-cell contact, or alternatively, due to a more direct role of these genes on regulators of cell proliferation. In all eukaryotes, cell proliferation is driven by the Cyclin-dependent protein kinases (Cdks), which are regulated by Cyclins. Cyclin E/Cdk2 is at the hub of cell cycle regulation, controlling G1 to S-phase progression via phosphorylation of key substrates involved in DNA replication initiation, transcription and centrosomal duplication (reviewed by Ekholm and Reed, 2000). In Drosophila, cyclin E is essential and rate-limiting for S-phase entry and null mutants lead to embryonic lethality (Knoblich et al., 1994; Richardson et al., 1995). However, a cyclin E hypomorphic allele, DmcycEJP, is viable and fertile, but exhibits a rough eye phenotype due to reduced S-phases (Secombe et al., 1998). We have used the DmcycEJP rough eye phenotype as the basis of a dominant modifier screen in order to uncover novel genes controlling G1-S progression (Brumby et al., 2004). Amongst the genes identified as dominant suppressors in this screen, were scrib, dlg and lgl, suggesting that these genes are rate-limiting negative regulators of S-phase progression (Brumby et al., 2004). Consistent with this, scrib− clones in the eye imaginal disc exhibit ectopic Cyclin E expression (Brumby and Richardson, 2003). These data provide a link between scrib, dlg and lgl and the cell cycle machinery.
In this study, we investigate the effect of lgl null alleles on apico-basal cell polarity and cell proliferation during eye development using clonal analysis. We also investigate the effect of lgl− clones on differentiation and apoptosis in larval and pupal mosaic eye discs. This study reveals for the first time that upon depletion of Lgl function, ectopic cell proliferation occurs without loss of apico-basal cell polarity in the larval eye disc. Furthermore, in lgl− mosaic developing eyes alterations in the normal pattern of apoptosis occur.
Materials and Methods
Fly strains, conditions of culture, overexpression analysis and clonal analysis
lgl mutants examined in this study were lgl23S9, lgl27S3, lglE2S31, and lglE6S2 (isolated in the cyclin E genetic screen (Brumby et al., 2004)), and lgl4 (a null allele (Ohshiro et al., 2000); the cleaned-up stock containing FRT40A from D. Bilder). Other fly stocks used were: scrib1 (Zeitler et al., 2004), UAS:lgl (Betschinger et al., 2003), UAS:crbintra38.1.2b (Wodarz et al., 1995).
Mitotic eye clones (Xu and Rubin, 1993) and MARCM clones (Lee and Luo, 2001) were generated as previously described (Brumby and Richardson, 2003). To generate lgl− clones in a Minute background, the ey:FLP; M(l)2 FRT40A arm-lacZ stock was crossed to lgl27S3 FRT40A.
All flies were raised on a standard cornmeal agar food at 25°C. Pupae were staged from white pre-pupae (pupation = 0% p.d.) at 25°C [100% pupal development (p.d.) corresponds to 103 hours)]. Eye discs were dissected at the times indicated in the text.
Southern analysis
Genomic DNA prepared from lgl homozygous or transheterozygous mutant larvae was digested with BanII (NEB) and probed with the following cDNA probes: BDGP-DGC: LD06034 (lgl), BDGP-DGC:RH43162 (CG31973) and BDGP-DGC:LD31354 (CG10275), under standard hybridization conditions.
Immunohistochemistry
For analysis of eye-antennal discs, third instar larvae or pupal eye discs were dissected, fixed and stained as previously described (Brumby and Richardson, 2003; Grzeschik and Knust, 2005). Antibodies used were rat anti-Cyclin E ((Crack et al., 2002) at 1:400; or obtained from H. McNeill, CRUK, at 1:250), mouse anti-BrdU (Becton-Dickinson, at 1:50), mouse anti-Elav (Developmental Studies Hybridoma Bank (DSHB), at 1:20), rabbit anti-phosphohistone H3 (Santa Cruz, at 1:400), rabbit or mouse anti-β-galactosidase (Rockland, at 1:500), mouse anti-Dlg (DSHB, at 1:10), rabbit anti-Lgl antibody ((Ohshiro et al., 2000) at 1:1000), rat anti-E-Cad (obtained from T. Uemura (Oda et al., 1994) and from DSHB, at 1:50), rabbit anti-human aPKCζ (Santa Cruz, at 1:400), rabbit anti-Patj (formally known as Discs lost (Bhat et al., 1999) at 1:400), rabbit anti-Baz (Baz N terminus 1–297 (Wodarz et al., 1999), at 1:500), rat anti-Sdt (Sdt-MPDZ from E. Knust, at 1:500), and mouse anti-β-Integrin (DSHB, at 1:15). Secondary antibodies, conjugated to biotin, Alexa 568, 647 or 488, and Streptavidin conjugated to Alexa 568, 647 or 488 (Jackson Labs and Molecular probes) were used at 1:400.
F-actin was detected with phalloidin-tetramethylrhodamine isothiocyanate (TRITC; Sigma, 0.3 mM) or phalloidin-fluorescein isothiocyanate (FITC; Sigma, 0.3 mM), and DNA was detected by staining with TO-PRO (Invitrogen). S-phase cells were detected by BrdU labeling as previously described (Brumby and Richardson, 2003). Apoptotic cells were detected by TUNEL staining using the in situ cell death detection kit, TMR Red (Roche).
Fluorescent labeled samples were mounted in 80% glycerol and analyzed by Confocal microscopy (Bio-Rad MRC1000 or Olympus FV1000) and images were processed using Confocal AssistantR, Adobe Photoshop 7.0R and Adobe Illustrator 10R for image assembly. Transverse images of larval eye discs were derived from Z stacks using the Olympus FV1000 and Fluorview viewer software, except for in Fig. 3 where larval eye discs were side-mounted and imaged with the Bio-Rad MRC1000.
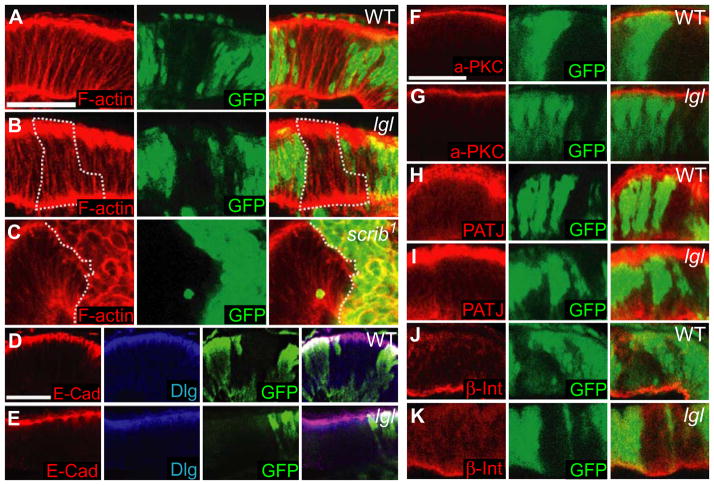
Figure 3. lgl− mosaic tissue in larval eye discs shows a regular apico-basal polarity and localization of polarity determinants.
Side mounts of third instar lgl− mosaic eye discs. Apical is to the top. scrib 1 clones are positively marked by GFP (green), control and lgl− clones are marked by its absence.
(A–C) Mosaic eye discs stained for F-actin (red). Control (A) and lgl− mosaic (B) cells show columnar morphology. (C) scrib 1 clones contain multi-layered, rounded cells. Examples of clones are outlined.
(D–K) Localization of E-Cad (red, D, E), Dlg (blue, D, E), aPKC (red, F, G), Patj (red, H, I) and β-Integrin (red J, K) are identical in control (D, F, H, J) and lgl− mosaics (E, G, I, K).
White bar = 25 μm.
Adult eye sections, scanning electron microscopy and light microscopy of adult eyes
Sectioning of Drosophila adult eyes and scanning electron microscopy was carried out as previously described (Richardson et al., 1995). Light microscopy images of adult eyes were taken on an Olympus dissection microscope using an Olympus DP11 camera.
Results
lgl− larval eye disc clones exhibit ectopic cell proliferation
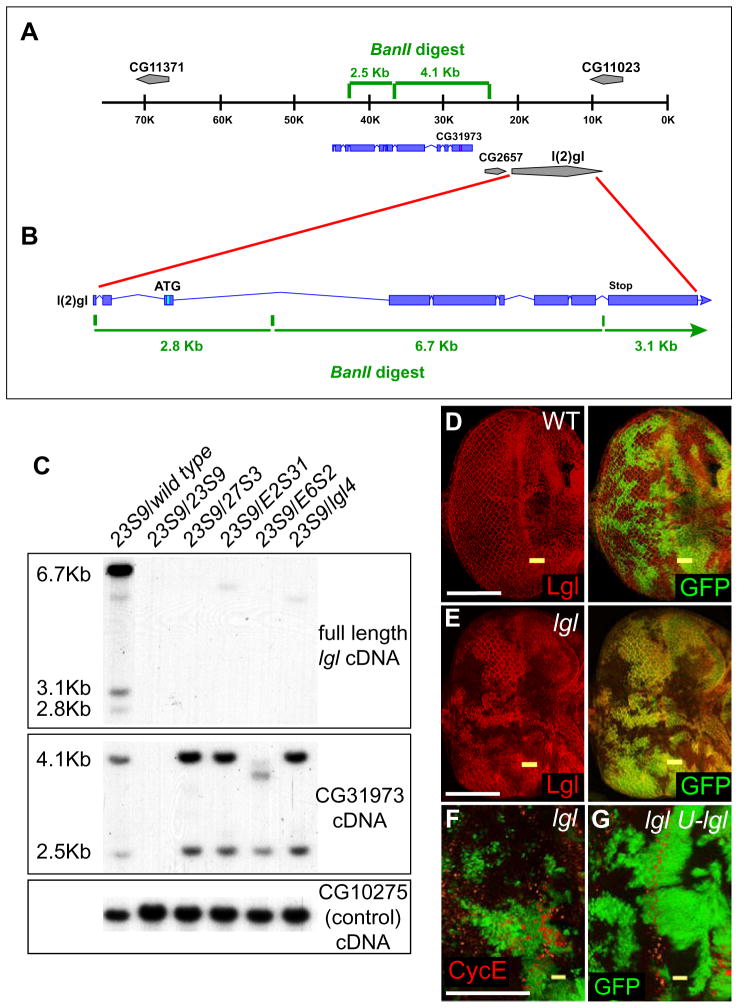
We identified four alleles (two X-ray and two EMS-generated alleles) of lgl (Su(DmcycEJP )2-1) in a genetic screen for dominant suppressors of the hypomorphic allele of cyclin E, DmcycEJP (Brumby et al., 2004). To determine the molecular lesions of these alleles in the lgl gene, we carried out Southern analysis (Fig. 1A–C). Both X-ray alleles and both EMS alleles contained complete deletions of the lgl locus, as did a previously characterized allele, lgl4 (Mechler et al., 1985) (Fig. 1A–C). While the large deletions exhibited by the EMS-generated alleles were unexpected, the lgl locus has been well documented to be prone to spontaneous deletions and there is high incidence of 2L terminal chromosome deficiencies occurring in natural Drosophila populations (Green and Shepherd, 1979; Walter et al., 1995). In confirmation of the Southern analysis, Lgl protein was undetectable in Su(DmcycEJP )2-1 mutant eye disc clones when compared with surrounding normal tissue and with control mosaic eye discs (Fig. 1D, E). Southern analysis also revealed that the deletions of all four Su(DmcycEJP )2-1 alleles, as well as lgl4, remove CG11023 at the distal tip of 2L (data not shown). However, the removal of this gene appears to have no effect, since the defects of Su(DmcycEJP )2-1 allele mutant clones could be fully rescued by expression of UAS:lgl in these mutant clones, by using the MARCM system (Lee and Luo, 2001) (Fig. 1F, G; and data not shown; see below).
Figure 1. Su(DmcycEJP )2-1 alleles have deletions of the lgl gene.
(A) Map of the lgl region at 21A on 2L. Expected BanII digest fragment sizes are indicated for the region covering CG31973. 0K indicates the distal end of 2L.
(B) A schematic of the lgl gene. The blue rectangles represent the full-length lgl cDNA (the ATG and Stop codons are indicated). Expected BanII digest fragment sizes are indicated for the region covering lgl and CG31973 (A). At the distal tip of 2L lies CG11023, followed by lgl, CG2657, CG31973 and CG11371.
(C) Southern blot analysis showing the genetic lesions of the lgl alleles. lglE2S31 and lglE6S2 were generated by EMS mutagenesis, while lgl23S9 and lgl27S3 by X-ray mutagenesis. Genomic DNA from wild-type or transheterozygous lgl alleles was probed with a full-length lgl cDNA probe, with the neighbouring gene, CG31973 or with the control CG10275 (localized at 36E6 on 2L). Expected fragment sizes are indicated. For lgl23S9, lgl27S3 and lglE6S2 no bands were detected with the lgl probe. lglE2S31 and lgl4 show faint bands with the lgl probe that are likely to be background bands, because these bands are lost when probed with individual PCR-generated lgl exon probes (data not shown). When probed with the neighbouring gene CG31973, lgl27S3, lglE2S31 and lgl4 showed the expected bands, whereas lgl23S9 gave no bands and lglE6S2 exhibited loss of the 4.1 Kb band replaced by a novel band.
(D, E) ey:FLP generated clones in third instar eye imaginal discs stained for Lgl (red) and the clonal marker, GFP (green). (D) Control clones showing uniform cytoplasmic staining. (E) Clones of Su(DmcycEJP )2-127S3 (lgl27S3 ) show no detectable Lgl protein in lgl− tissue (GFP-negative). Similar results are observed with lglE2S31, lgl23S9, lglE6S2 and lgl4 mosaic eye discs (data not shown).
(F, G) Expression of UAS:lgl using the ey:FLP MARCM system in lgl27S3 clones restores Cyclin E expression to normal. Mutant clones are positively marked with GFP (green) and stained for Cyclin E (red). (F) lgl27S3 clones show ectopic Cyclin E (red) expression. (G) Expression of UAS:lgl in lgl27S3 clones (lgl; U-lgl), prevents the ectopic Cyclin E expression seen in lgl− clones (compare with Fig. 2B).
Yellow bars indicate the morphogenetic furrow (MF). White bar = 50 μm.
To determine whether lgl− clones exhibited cell cycle defects, we used ey:FLP/FRT recombination (Xu and Rubin, 1993) to generate lgl− mosaic eye discs (which are composed of patches of lgl− and wild-type tissue) and examined S-phase by bromodeoxyuridine (BrdU) labelling and Cyclin E expression. For this analysis we used the lgl27S3 allele, since it contained the smallest deletion spanning the lgl locus, but similar results were observed for all other lgl2.1 alleles and lgl4 (using the cleaned-up stock from D. Bilder).
In wild-type 3rd instar larval eye discs, cells in the anterior region cycle asynchronously, whereas in the morphogenetic furrow (MF) cells are arrested in G1, and posterior to this, a subset of cells undergo a synchronous S-phase and then mitosis termed the second mitotic wave (SMW), and then most cells exit the cell cycle. In control eye discs, Cyclin E is expressed immediately posterior to the MF (Fig. 2A) in the region where the band of synchronous S-phases (measured by BrdU labelling) occurs (Fig. 2C), however Cyclin E is less abundant in cells undergoing S-phase than in G1-arrested photoreceptor pre-cluster cells that have begun differentiation (data not shown, Firth and Baker, 2005). In wild-type eye discs, very few S-phase cells are observed posterior to the SMW (Fig. 2C). By contrast, lgl− clones showed ectopic Cyclin E expression and ectopic S-phases in the posterior region of the eye disc (Fig. 2B, D compared with 2A, C). Moreover, ectopic expression of the G2/M-phase cyclins, Cyclin A and Cyclin B, and mitoses (scored by phospho-histone H3 staining) were observed in lgl− clones posterior to the MF (data not shown), consistent with cells proceeding through the entire cell cycle. Thus, consistent with the hyperplasia observed in homozygous lgl− brain and imaginal tissue (Bilder et al., 2000; Gateff, 1978), lgl− clones show upregulation of Cyclin E and ectopic cell proliferation. Interestingly, despite this increase in proliferation of cells in the posterior region of the eye disc the overall clone size of the lgl− tissue did not appear to be over-represented compared with the wild-type clones (see discussion).
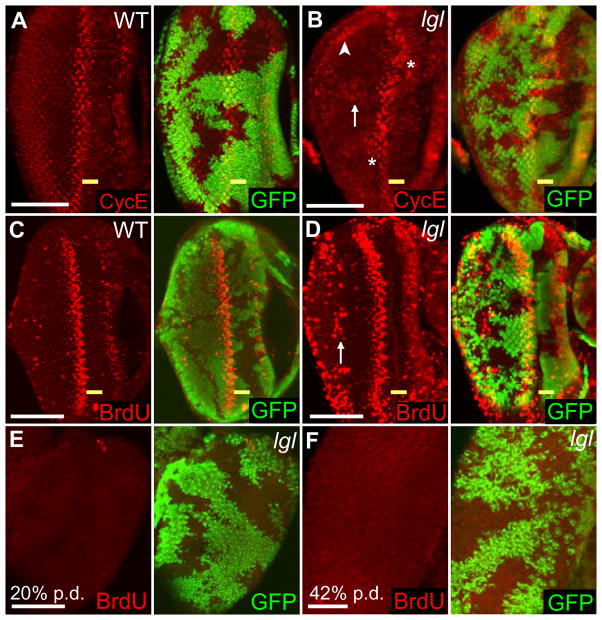
Figure 2. lgl− clones exhibit ectopic Cyclin E and increased S-phase cells in larval eye discs but not in pupal eye discs.
Third instar lgl− mosaic eye discs. Posterior is to the left. Wild-type clones are marked with GFP (green).
(A, C) Control discs stained with Cyclin E (A, red) or labelled with BrdU (C, red).
(B, D) lgl− clones show ectopic Cyclin E (B, red) and incorporation of BrdU (D, red). The asterisk indicates ectopic Cyclin E directly posterior or anterior of the SMW (B). An arrow indicates ectopic Cyclin E (B) or ectopic BrdU incorporation (D) in the posterior region. An arrowhead indicates a cross-section where basally-localized nuclei express ectopic Cyclin E (B).
(E, F) Confocal images of pupal mosaic eye discs labelled with BrdU (red) and lgl− tissue is marked by the absence GFP. In wild-type tissue, S-phases are largely complete by 20% p.d. (E) At 20% p.d., a few BrdU labelled cells (red) are observed. (F) At 42% p.d. ectopic S-phases (red) are not detected in lgl− mosaic eye discs.
(A–D) The yellow bar indicates the MF. White bar = 50 μm.
Some differences were observed in the coincidence of ectopic Cyclin E expression and ectopic S-phases in lgl− clones. Firstly, Cyclin E expression was expanded anteriorly from its normal band of expression in the SMW into the G1-arrested band in the MF in lgl− clones (Fig. 2B), but ectopic S-phases were not observed within the MF (Fig. 2D). Thus, in lgl− clones ectopic Cyclin E is not sufficient to induce ectopic S-phases in the MF. However, when expressed via the heat shock driver, ectopic Cyclin E expression can induce S-phase within the MF (Crack et al., 2002). Therefore in lgl− clones, negative regulatory controls within the MF must prevail. Secondly, while Cyclin E ectopic expression was expanded posteriorly from its normal band of expression in the SMW, ectopic S-phases were not observed in cells immediately posterior to the SMW in lgl− clones (Fig. 2B, D). Cells immediately posterior to the SMW in lgl− clones expressing Cyclin E may be unable to enter S-phase since most of these cells are differentiating photoreceptor cells (PRCs) that express high level of the Cyclin E/Cdk2 inhibitor, Dacapo (de Nooij et al., 1996). Strong ectopic expression of Cyclin E, generated by heat shock induction of a Cyclin E transgene, is able to drive many of these cells into S-phase (Crack et al., 2002), however the lower level of ectopic Cyclin E expression seen in lgl− clones appears to be insufficient to drive these differentiating cells into S-phase. In addition, some of the unspecified cells in this region may be refractory to S-phase induction by Cyclin E since many of these cells are arrested in G2 (reviewed by Baker, 2001).
The ectopic Cyclin E expressing cells associated with ectopic S-phases in the more posterior region of the eye disc in the lgl− clones are likely to be unspecified cells, since confocal sections showed that in the more posterior clones the nuclei ectopically expressing Cyclin E are basally localized (Fig. 2B, arrowhead), whereas the nuclei of differentiating PRCs that express the Elav differentiation marker are normally located apically (see Fig. 5C). Furthermore, co-staining with Cyclin E and Elav showed that these posterior basally-localized ectopic Cyclin E-expressing cells in lgl− clones do not express Elav (data not shown). Taken together, these data show that in lgl− clones, many cells ectopically express Cyclin E and in the more posterior region of the larval eye disc some cells undergo ectopic S-phases. Moreover, it should be noted that the ectopic Cyclin E and S-phases were confined to the lgl− clones, showing that the effect of lgl loss-of-function on cell proliferation is cell autonomous.
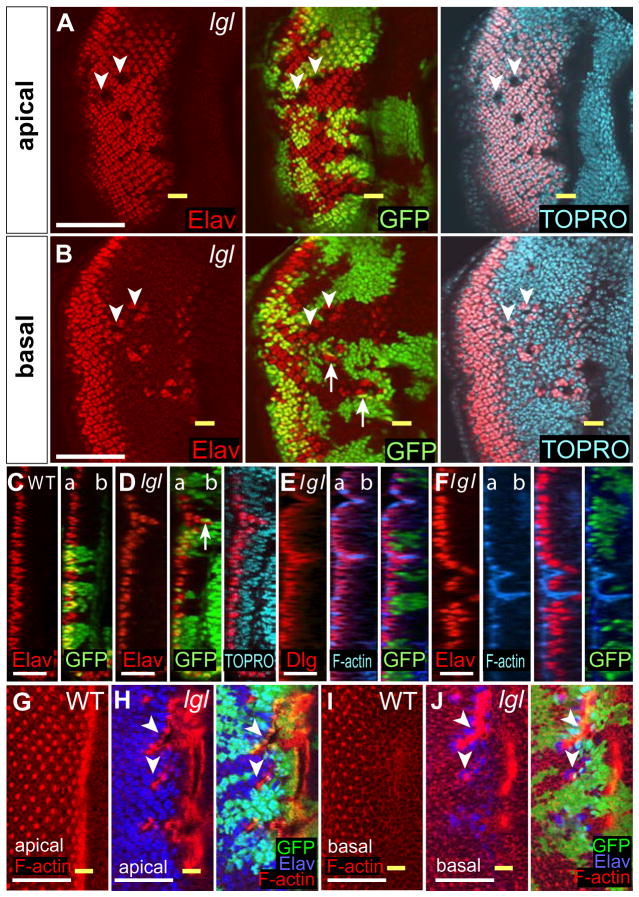
Figure 5. Some nuclei of PRCs at the boundary of lgl− clones in larval eye discs are basally-localised.
(A, B, G–I) Planar and (C–F) transverse sections of third instar mosaic eye discs. Control
(C, G, I) and lgl− clones are marked by the absence of GFP (green). In planar sections, posterior is to the left. In transverse sections apical is to the left.
(A–D) Elav (red) marks nuclei of differentiated PRCs, TOP-RO (blue) marks all nuclei.
In planar sections of an lgl− mosaic disc (A, B) PRCs that are missing in the apical region (A) are located baso-laterally (B, arrowheads). Some adjacent wild-type cells (GFP-positive) also relocate basally (B, arrows). Transverse sections (D, compare with wild-type in C) confirm the relocation of lgl− and wild-type cells at the clonal boundary (arrow). “a” = apical and “b” = basal.
(E–J) The relocation of PRC nuclei is associated with an accumulation of F-actin but polarity is unaffected. Transverse sections stained for Dlg (red), F-actin (blue) and GFP (E) and Elav (red), F-actin (blue) and GFP (F).
Apical and basal planar sections stained for F-actin (red) in a wild-type disc (G, I) and Factin (red), Elav (blue) and GFP in an lgl− mosaic disc (H, J). Arrowheads indicate “holes” (H, J).
The yellow bar indicates the MF. White bar = 50 μm (A, B) and 25 μm (C–J).
lgl− clones in the larval eye disc do not show cell polarity defects
Apico-basal cell polarity is characterized by columnar shape and the localization of polarity determinants and cellular junction complexes to specific zones along the apico-basal axis (see introduction). Previous studies have shown that lgl mutants exhibit loss of apico-basal cell polarity in homozygous mutant neuroblasts and epithelial cells, ovarian follicle cell clones, and maternal-zygotic null mutant embryonic epithelial and neuroblast cells, resulting in the mislocalization of polarity determinants (Bilder et al., 2000; Merz et al., 1990; Peng et al., 2000; Ryerse and Nagel, 1984). To test if apico-basal cell polarity was also disrupted in lgl− larval eye disc clones, we examined cell morphology and the localization of key proteins of the adherens junctions, septate junctions and subapical complexes and a basal determinant in ey:FLP-generated mosaic eye discs. Surprisingly, lgl− clones in the larval eye disc showed normal columnar epithelial cell morphology, as revealed by Phalloidin staining of F-actin (Fig. 3B compared with 3A). By contrast, ey:FLP scrib− clones showed loss of apico-basal cell polarity, with rounded cells and tissue multi-layering (Fig. 3C). Moreover, in lgl− clones the localization of E-Cadherin (E-Cad, adherens junction), Dlg (septate junction), aPKC and Patj (subapical region) and β-integrin (β-int, basal region) was equivalent to the adjacent wild-type tissue (Fig. 3D–K). Thus, while lgl mutants result in disruption of cell polarity in other situations (see above), lgl− clones in the eye disc do not disrupt apico-basal cell polarity. Therefore, we conclude that the effect of lgl loss-of-function on ectopic cell proliferation occurs without a disruption of apico-basal cell polarity in lgl− larval eye disc clones.
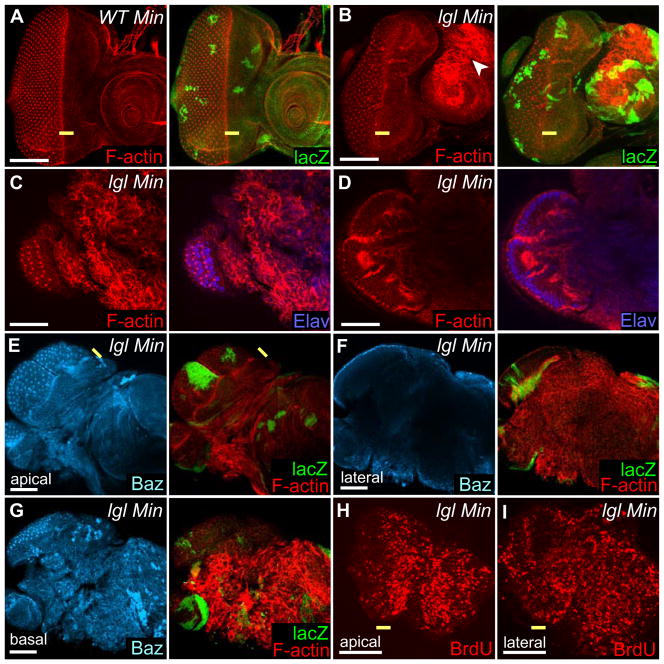
lgl− larval eye disc clones in a Minute background lose polarity and exhibit a more severe ectopic cell proliferation phenotype
Since homozygous lgl− larval tissues lose polarity, the ectopic cell proliferation without cell polarity defects observed in lgl− eye disc clones may be due to the perdurance of Lgl protein in the ey:FLP induced lgl− clones, despite the fact that we could not detect any Lgl protein by antibody staining of third instar larval lgl− mosaic eye discs (Fig. 1E). To test this possibility, we generated clones using ey:FLP in a Minute background where the Minute clones have a proliferative disadvantage and the lgl− tissue is required to proliferate more in order to produce the required number of cells in the tissue. In this situation, due to the increased numbers of cell divisions, maternally-supplied and zygotic Lgl protein produced before the generation of the lgl− clone would be expected to be further depleted. In 5 day-old instar ey:FLP-generated lgl− Minute mosaic eye-antennal discs, where the majority of the tissue was lgl−, F-actin staining revealed that the eye disc maintained polarity, but regions of the antennal disc had lost polarity (Fig. 4B compared with 4A). However, lgl− Minute mosaic third instar larvae undergo an extended larval stage to form giant larvae. In 11 day-old larvae, most of the lgl− cells showed loss of apico-basal polarity, although as seen by F-actin and Elav-staining (Fig. 4C, D) or F-actin and Baz staining (Fig. 4E–G) the posterior differentiated region maintained polarity. Thus, when forced to undergo further cell divisions, where perduring Lgl protein would be expected to be further depleted, most of the lgl− tissue in the eye disc shows loss of apico-basal cell polarity. The exception being the already differentiated (Elav-positive) tissue, which due to its differentiated state and established tissue architecture, appeared to be unaffected by the depletion of Lgl.
Figure 4. lgl− clones in a Minute background show a loss of polarity and more extreme ectoptic cell proliferation in larval eye-antennal imaginal discs.
Confocal images of third instar ey:FLP Minute mosaic eye discs. Posterior is to the left.
(A, B) lgl− Minute and control discs from 5 day old third instar larvae, stained for F-actin (red) and Minute clones are marked with arm-LacZ (green). (A) Minute control clones (green) have a proliferative disadvantage. (B) An lgl− Minute mosaic eye disc shows that most of the eye-antennal disc is comprised of lgl− tissue (LacZ-negative), but only the antennal disc shows loss of cell polarity as detected by rounded cells and high F-actin staining (arrowhead). (C) Apical and (D) basal focal planes of an lgl− Minute mosaic eye disc from an 11 day old giant larva stained for F-actin (red) and Elav (blue). Most of the eye-antenna disc is overgrown, except for the region of differentiated photoreceptor cells (Elav-positive) that maintain apical-basal cell polarity. (E) Apical, (F) middle (lateral) and (G) basal focal planes of an lgl− Minute mosaic eye disc from an 10 day old giant larva stained for F-actin (red), Baz (blue) and arm-LacZ (green), showing that differentiation and tissue structure is maintained in the posterior region of the eye disc. (H, I) BrdU labelling (red) of lgl− Minute mosaic eye disc from a 6 day old third instar larva; (H) apical and (I) lateral views showing excessive numbers of S-phase cells in anterior regions where apico-basal polarity is lost.
Yellow bar = morphogenetic furrow. White bar = 50 μm.
To determine how cell proliferation correlated with the loss of apico-basal cell polarity in lgl− Minute mosaic third instar eye discs, we then carried out BrdU labelling. This experiment revealed that in the regions where cell polarity is lost, but are not already differentiated, ectopic cell proliferation was more extreme than in regions where polarity was maintained (Fig. 4H, I and data not shown). Apical and lateral views of a 6 day-old larval lgl− Minute mosaic eye disc showed that there were more BrdU labelling cells where cells lose polarity in the antennal disc and in the anterior region of the eye disc, compared with in the posterior region where polarity was maintained (Fig. 4H, I; and data not shown). Taken together these results suggest that as Lgl protein becomes depleted, ectopic cell proliferation occurs before the loss of apico-basal cell polarity, however upon the disruption of cell polarity a more severe ectopic cell proliferation phenotype manifests.
Some cells at lgl− clonal boundaries in larval eye discs are more basally localized
Although, lgl− clones in a wild-type background did not lose polarity, we noticed defects in the regular pattern of PRCs at the clonal boundary of lgl− tissue and wild-type tissue. In wild-type eye discs, PRC nuclei, as revealed by staining with the differentiation marker, Elav, are located apically (Fig. 5C). Confocal sections through lgl mosaic eye discs stained for Elav, and for DNA revealed that the nuclei of some cells at clonal boundaries were localized more basally (Fig. 5A, B). This was confirmed by transverse sections of lgl− clones co-stained for DNA and Elav (Fig. 5D compared with control clones stained for Elav, Fig. 5C), for F-actin and Dlg (Fig. 5E) or for F-actin and Elav (Fig. 5F). In some cases, nuclei were missing in basal sections (Fig. 5B), suggesting that cells in the centre may have been eliminated by apoptosis (see below). Double staining with GFP also revealed that not just lgl− nuclei were affected, but also some adjacent wild-type cells (GFP + ) were basally localized (Fig. 5D). These defects were found in and posterior to the MF, but never anterior to it, and were, in all cases, located at the clonal borders. Planar confocal section of lgl− mosaics stained for F-actin (Fig. 5H, J compared with control clones, Fig. 5G, I) revealed high concentration of F-actin outlining the “holes” in apical sections (Fig. 5H) and most prominently at the centre of the “hole” in basal sections (Fig. 5J). F-actin staining also revealed distortions to the MF in lgl− mosaic discs (Fig. 5H, J), however Elav staining showed that differentiation was not delayed in lgl− clones relative to surrounding wild-type clones, but many Elav-expressing cells at the clonal boundaries were more basally localized (Fig. 5A, B). It should be noted, in terms of the relationship of these basally localised cells at the clonal boundaries to the ectopic cell proliferation observed, that these “dropped-out” cells are mainly Elav positive PRCs, while the cells that show ectopic Cyclin E and undergo ectopic S-phase are most likely unspecified cells and are observed throughout the lgl− clones (Fig. 2B, D; see above). Moreover, these “dropped-out” cells can not be resulting in the ectopic proliferation by a compensatory mechanism, since compensatory proliferation is non-cell autonomous (reviewed by Brumby and Richardson, 2005; Vidal and Cagan, 2006), and ectopic Cyclin E expression and S-phases are highly restricted to the lgl− clones. Thus, we have shown here that some cells at the boundary of lgl− clones become more basally localized, which we refer to as the “drop-out” phenotype. In these basally-localized cells, F-actin and Dlg remain localised to the apical side, suggesting that although they have become shorter they have not lost apical-basal cell polarity. The fact the “drop-out” phenotype occurred at the boundary of lgl and wild-type clones and was both cell-autonomous and non-cell autonomous, suggests that it may be related to morphogenic apoptosis, a phenomenon where cells exhibiting discontinuities in signalling pathways are sensed and eliminated by apoptosis (reviewed by Adachi-Yamada and O’Connor, 2004) (see discussion).
Cells at the borders of lgl− larval eye disc clones undergo apoptosis, but cell death within lgl− clones is reduced, leading to increased numbers of IOCs at the pupal stage
In order to determine whether the “drop-out” phenotype was associated with apoptosis, we labelled lgl− mosaic eye discs using in situ TdT-mediated dUTP-Nick-End Labeling (TUNEL) to detect fragmented DNA in dying cells (Chen et al., 1996). In control discs, some TUNEL positive cells were observed immediately anterior to the MF and a band in the posterior part of the eye disc, representing the normal pattern of developmental cell death in the larval eye disc (Fig. 6A). In lgl− mosaic discs, TUNEL-positive cells were observed only along the borders of clones in both lgl− and wild-type cells (Fig. 6B). More TUNEL positive cells were observed than “dropped-out cells” (Fig. 6B compared with 5A), suggesting that cell death may begin before the morphogenetic changes become apparent. Interestingly, cell death was not observed in the central region of lgl− clones and the normal bands of developmental cell death in the eye discs were not evident in the lgl− mosaic discs.
Figure 6. Cell death is increased at the borders of lgl− clones in larval eye discs, but cell death is inhibited in pupal development leading to additional IOCs.
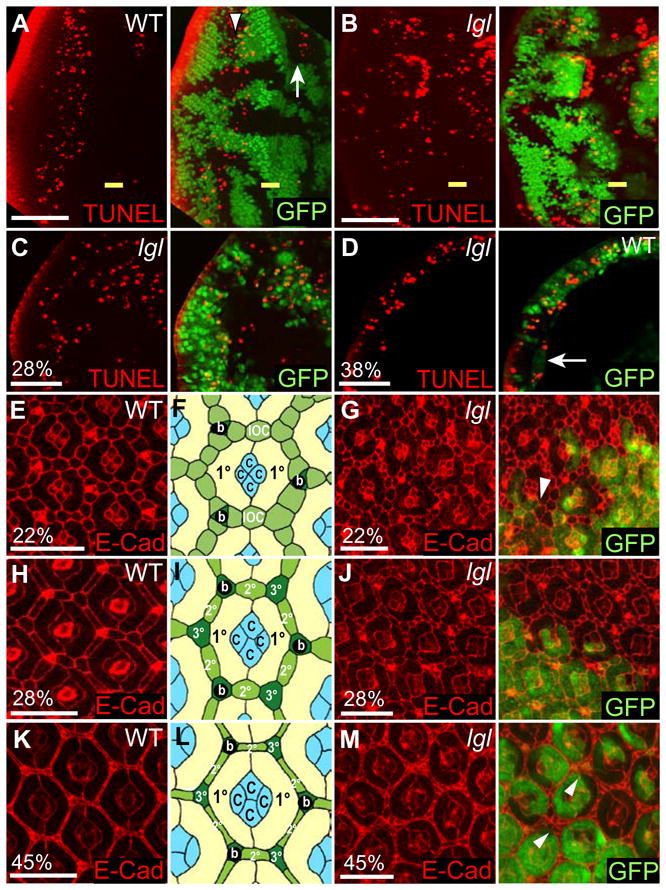
(A, B) Planar sections of third instar larval mosaic eye discs. Posterior is to the left. (C-M) Planar sections of pupal mosaic eye discs. Control and lgl− clones are marked by the absence of GFP (green) and cell death is detected by TUNEL (red).
(A) In control mosaic discs, TUNEL positive cells are detected anterior (arrow) and posterior (arrowhead) to the MF.
(B) In lgl− mosaic discs, many TUNEL positive cells are seen at the clonal boundaries in both lgl− and wild-type cells.
(C, D) Cell death in lgl− mosaic pupal eye discs at 28% (C) or 38% p.d (D), marked by TUNEL (red). In lgl− mosaic pupal eye discs, cell death of IOCs (C, 28% p.d.) and perimeter clusters (D, 38% p.d.) marked by TUNEL (red) is suppressed (arrow).
(E–M) Pupal eye discs stained for E-Cadherin (red) and schematics. (E, H, K) Pupal control (with schematics (B, E, H), c = cone cells, b = bristles, 1° = primary, 2° = secondary and 3° = tertiary pigment cells.) at 22% (E, F), 28% (H, I) and 45% p.d. (K, L). (G, J, M) lgl− mosaic discs, at 22% (G), 28% (J) and 45% p.d. (M). lgl− tissue shows additional IOCs. Arrowheads mark IOC clusters that are not removed by cell death.
White bar = 50 μm (A, B), 20 μm (C, D) and 10μm (E, G, H, J, K, M).
Next, we examined whether lgl mutant clones showed defects in cell death at the pupal stage. Between 16%–22% of pupal development (p.d.), interommatidial cells (IOCs) are rearranged from a random and multilayered pattern to form a single layer around the photoreceptor clusters aligned head-to-tail, separating the ommatidial clusters from each other (termed “cell sorting”) (reviewed by Brachmann and Cagan, 2003). Associated with this process, excess IOCs are eliminated by developmental cell death, which is initiated between 23%–28% pupal development (p.d.) and reaches its peak at ~30% p.d. By TUNEL staining, we found that in lgl− mosaic discs at 28% p.d. lgl− tissue had decreased numbers of dying cells compared with the surrounding wild-type tissue (Fig. 6C). Moreover, apoptosis of perimeter cluster cells (Fig. 6D), which normally occurs independent of cell sorting (reviewed by Brachmann and Cagan, 2003), was also suppressed in lgl− clones. This data shows that Lgl function is important for the normal developmental cell death in pupal eyes.
We then examined the effect on IOC number in lgl− mosaic discs at the pupal stage, by outlining cells using E-Cadherin (E-Cad) staining. In wild-type discs at 22% p.d. the IOCs have been sorted to form a single layer surrounding each ommatidial cluster (Fig. 6E, F). At 28% p.d., excess cells are eliminated by apoptosis (Fig. 6H, I) and by 45% p.d. the remaining IOCs have been arranged into the final regular hexagonal pattern around the PRC clusters (Fig. 6K, L). In early lgl− mosaic pupal eye discs, before programmed cell death occurs (22% p.d.), excess cell numbers were present in lgl− clones and cell-sorting defects were observed (Fig. 6G). These defects were particularly evident at vertices where single tertiary pigment cells should be localized or around bristles. At later stages (28% and 45%), lgl− clones still contained sorted and unsorted surplus IOCs, many of which were smaller than normal (Fig. 6J, M). In some cases more severe defects were observed, with large clusters of IOCs remaining between the ommatidial clusters (Fig. 7B, D, F compared with A, C, E). Thus, the ectopic proliferation combined with sorting defects and the decrease in cell death, leads to excess IOCs at the pupal stage and abnormalities in the arrangement of PRC clusters.
Figure 7. Some lgl− mutant clones show excessive numbers of IOCs in pupal retinas.
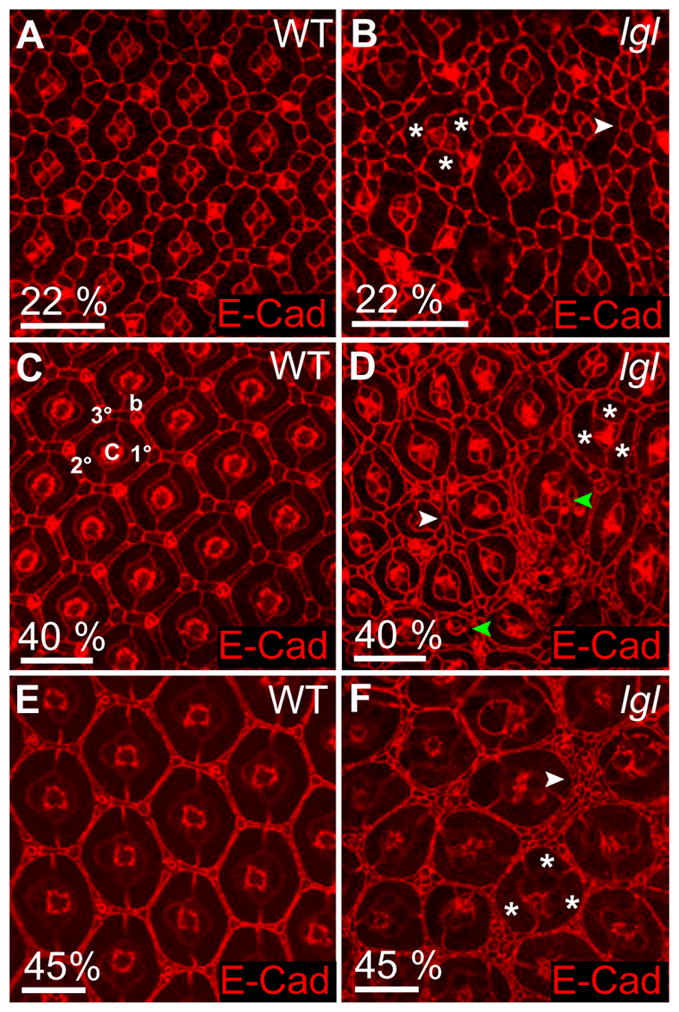
Confocal images of pupal mosaic eye discs stained for E-Cad (red).
(A, C, E) Control discs at 22% p.d. (A), 40% p.d. (C) and 45% p.d. (E). (c = cone cells, b = bristles, 1° = primary, 2° = secondary and 3° = tertiary pigment cells. Compare with schematics in Fig. 6).
(B, D, F) lgl− mosaic pupal eye discs showing that some lgl− clones have excessive numbers of IOCs. Cells are not properly rearranged (B) and none of the surplus cells are eliminated (D, F); Some ommatidia show a variable number of cone cells (green arrowheads in D; number of cones ranges from 1 to 6) and occasionally three primary pigment cells (indicated by *).
White bar = 10 μm.
lgl− PRCs lose polarity in pupal development without showing ectopic cell proliferation
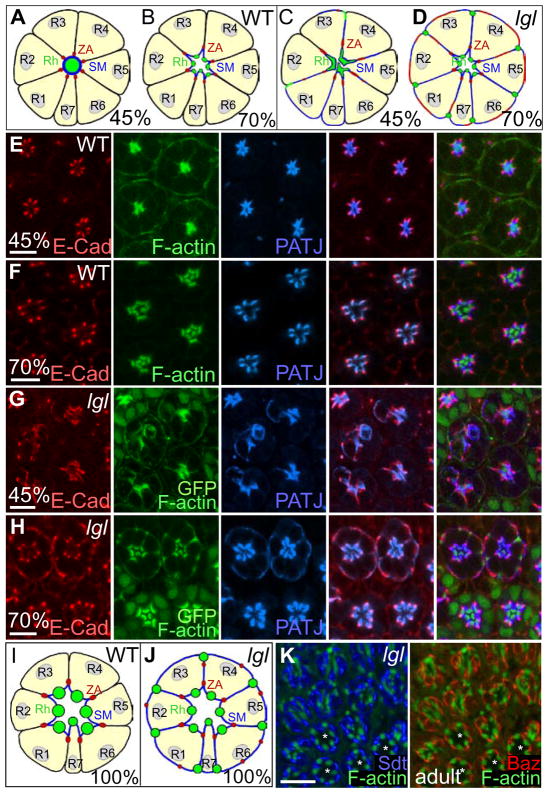
Since no loss of cell polarity occurred in third instar larval lgl− mosaic eye discs in a wild-type background, but did when we forced the lgl− tissue to proliferate further in a Minute background (Figs 2, 3), we suspected that the perdurance of Lgl protein provided sufficient cell polarity function in larval eye disc tissue. It was therefore of interest to determine whether defects in cell polarity could be observed later in development in lgl− mosaic eye discs in a wild-type background, where the perdurance of Lgl protein should be less. Indeed, staining for the localization of cell polarity markers in lgl− mosaic pupal retinas and adult eyes revealed that PRCs showed defects in the localisation of polarity determinants (Figs 8–10). In wild-type PRCs at 45% p.d., Patj localizes with F-actin at the apical membrane (Fig. 8A, E), and by 70% p.d. the apical region separates into apical rhabdomere (where F-actin is concentrated) and the stalk membrane (where Patj is localized) (Fig. 8B, F). E-Cad marks the zonula adherens, which is localized laterally to Patj at 45% and 70% p.d. (Fig. 8A, B, E, F). lgl− mosaic PRCs at 45% p.d., showed a lateral expansion of E-Cad, Patj and F-actin (Fig. 8C, G compared with 8A, E and cross-sections in Fig. 9A, B). The adherens junctions of the IOCs, however, were not disrupted (see Fig. 9A–C, F, G). In lgl− mosaic PRCs at 70% p.d., the mislocalization of Patj, E-Cad and F-actin was even more pronounced with high levels observed on lateral and basal cell membranes (Fig. 8D, H compared with 8B, F). This mislocalization of Patj and E-Cad persists through late pupal development and into the adult (Figs 8J, 10A–C compared with 8I, 10D; and data not shown). Other polarity determinants, Sdt (Crb complex) and Baz and aPKC (Baz complex) were also mislocalized to the baso-lateral membranes in lgl− PRCs in late pupal and adult eyes (Fig. 8J, K compared with 8I; and data not shown). The mislocalization of these polarity determinants was similar to that observed in pupal PRCs when Crumbs is overexpressed (Izaddoost et al., 2002). Since lgl− pupal PRCs show mislocalization of apical cell polarity complex components and the adherens junction protein E-Cad to the baso-lateral membrane, we conclude that these cells show aberrant apico-basal cell polarity.
Figure 8. lgl− PRCs show aberrant apico-basal polarity in pupal development.
(A, B, E, F) Schematics and confocal images of wild-type PRCs (R1-7) and lgl− mosaics (C, D, G, H) at 45% and 70% p.d. lgl− PRCs are marked by the absence of GFP (green) in PRC nuclei.
In a control retina at 45% p.d. (A, E) the components of the future rhabdomeres (Rh), marked by F-actin (green) and future stalk membrane (SM), marked by Patj (blue) co-localize. By 70% p.d. (B, F) the apical membrane is separated into an apical rhabdomere and a subapical stalk membrane. E-Cad (red) marks the zonula adherens (ZA).
In lgl− PRCs around 45% p.d. (C, G) Patj (blue) becomes mislocalized baso-laterally and speckles of E-Cad (red) and F-actin (green) are seen laterally. At 70% p.d. (D, H) E-Cad and Patj are both baso-lateral. Ectopic F-actin accumulates at the baso-lateral corners of PRCs to form ectopic rhabdomere-like structures.
(I, J) Schematics of wild-type (I) and lgl− (J) mosaic retinas at 100% p.d..
(K) In wild-type adult PRCs (*) Sdt (blue), Baz (red) are localized to the SM. In adult lgl− PRCs (no *) Sdt (blue) and Baz (red) are mislocalized baso-laterally. F-actin (green) marks rhabdomeres and rhabdomere-like structures. White bar = 5 μm (E–H) and 10 μm (K).
Figure 10. In pupal and adult retinas lgl− ommatidia show defects in F-actin organisation and form ectopic rhabdomeres.
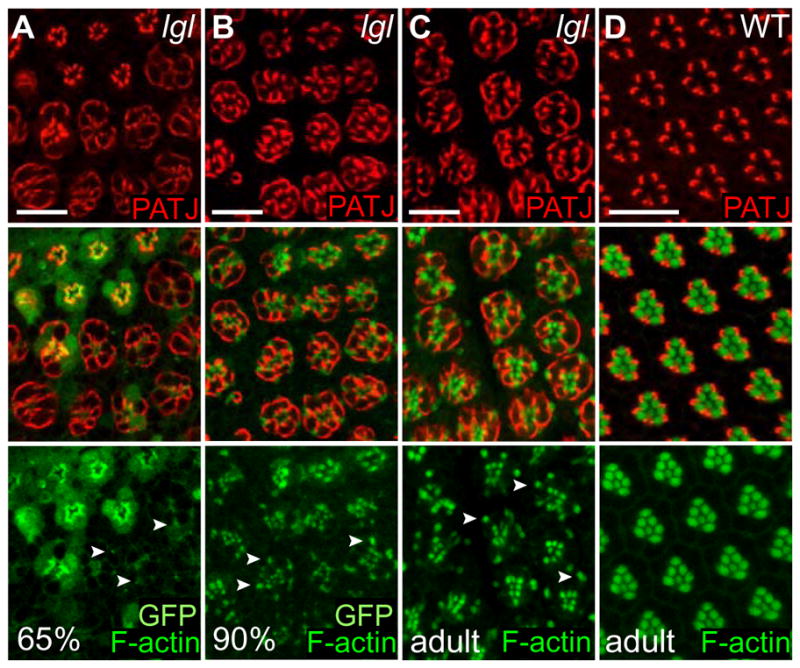
Confocal images of PRCs at 65%, 90% and 100% p.d. (adult) in lgl− mosaic (A–C) and wild-type adult (D) retinas, stained for Patj (red) and F-actin (green). Wild-type tissue is marked by nuclear GFP (A–C).
(A–C) Mislocalization of F-actin results in smaller rhabdomeres and rhabdomere-like structures (A, 65% p.d.). Arrangement and actin polymerization recover in later stages (B) 90% p.d. and (C) adult, but rhabdomeres and ectopic rhabdomeres remain smaller than in wild-type (see schematic Fig. 8J).
(D) In wild-type adult PRCs Patj (red) is localized to the SM (see schematic Fig. 8I).
Arrowheads point to examples of ectopic rhabdomere-like structures.
White bar = 10 μm.
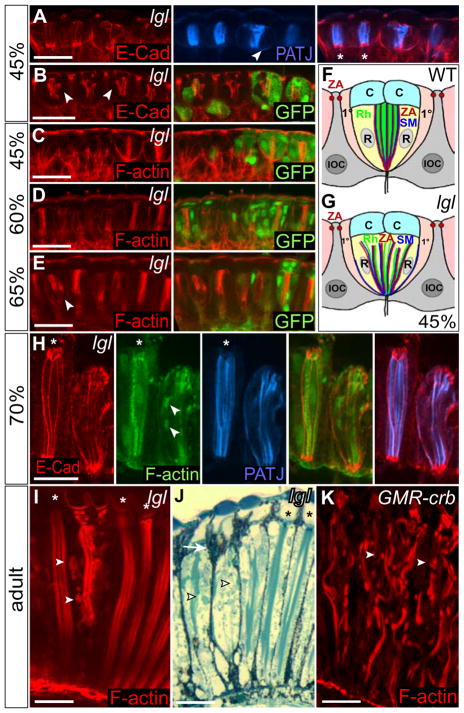
Figure 9. lgl− ommatidia show defects in rhabdomere morphology and elongation in pupal and adult retinas.
(A–E, H) Longitudinal sections of pupal lgl− mosaic discs and (F, G) schematics at 45%, and (I, J) adult lgl− mosaic and (K) GMR-crb eyes. Wild-type tissue is marked by GFP or (*).
(A, B) lgl− mosaic retinas at 45% p.d. stained for E-Cad (red) and Patj (blue, A). E-Cad (red) marks the zonula adherens (ZA) of pigment and PRCs (see F). The ZA is not affected in lgl− IOCs, but is disorganized in PRCs (arrowheads, see G). Patj (A, blue) is also affected and detected basally (arrowhead).
(C–E) lgl− mosaic retinas at 45% (C), 60% (D) and 65% p.d. (E) stained for F-actin (red). At 45% p.d. F-actin outlines wild-type ommatidia and is concentrated centrally, where future rhabdomeres are formed and later marks the growing rhabdomeres. In lgl− tissue at 45% and 60% p.d. F-actin is mislocalized, but by 65% p.d. the rhabdomeres show recovery (E, arrowhead).
(F, G) Schematic of a longitudinal section through wild-type (F) and lgl− (G) ommatidium at 45% p.d. ZA = Zonula adherens of IOCs and PRCs (R); Rh = rhabdomeres; SM = stalk membrane; C = cone cells, 1° = primary pigment cells.
(H) Stainings for E-Cad (red), Patj (blue) and F-actin (green) at 70% p.d. show further recovery of the lgl− rhabdomeres and formation of ectopic rhabdomere-like structures (arrowheads), but lgl− ommatidia remain shorter than their wild-type neighbours (*).
(I, J) Longitudinal sections through adult lgl− mosaic retinas, stained for F-actin (I, red) and with Toluidine Blue (J), show fragmentation of lgl− rhabdomeres, ectopic rhabdomere-like structures (arrowheads), and increased numbers of pigment cells (J, arrow).
(K) Longitudinal section through a GMR-crb adult eye, stained for F-actin (red). The rhabdomeres, marked by F-actin, are fragmented and F-actin is observed in the periphery (arrowheads).
White bar = 10 μm.
To examine whether these defects in cell polarity also result in ectopic cell proliferation, we carried out BrdU labelling of pupal eye discs (Fig. 2E, F). In wild-type pupal retinas, cell proliferation of the bristle cells occurs during the early pupal stage, but has ceased by ~18% p.d. (Cagan and Ready, 1989). In lgl− mosaic pupal retinas at ~20% p.d., no ectopic S-phases were observed in lgl− cells (Fig. 2E, F) and the lgl− tissue was not overgrown relative to wild-type tissue during the pupal stage or adult (see Fig. 11D). Collectively, these data show that during pupal development, lgl− clones show cell polarity defects in the PRCs without ectopic cell proliferation. This result was unexpected, considering the results of the lgl−/Minute experiment, where regions of the larval eye discs that lose cell polarity show excessive cell proliferation, but may be explained by the differentiation state of the tissue (see discussion).
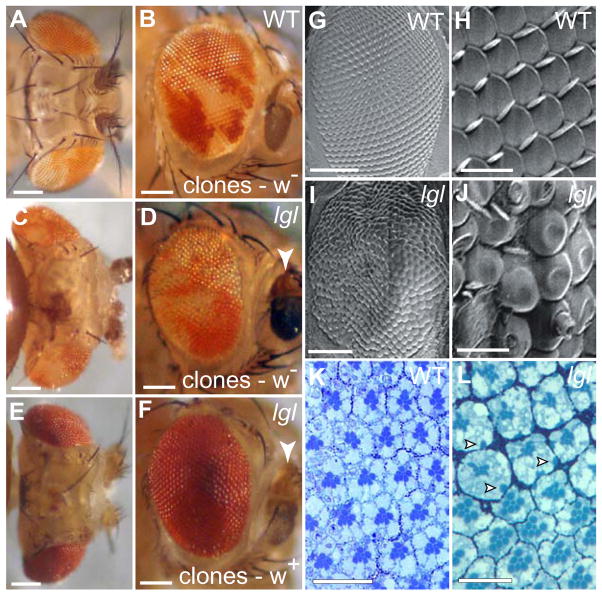
Figure 11. lgl− mosaics result in a rough adult eye where the clonal tissue is not over-represented.
(A–F) Light microscope images, (G–J) scanning electron microscope (SEM) images and (K–L) semi-thin cross-sections of adult control wild-type and lgl− mosaic eyes. Fly eyes are orientated with posterior to the left and dorsal to the top.
(A–B) Adult control eyes show approximately equal representation of wild-type orange clones to wild-type white clones.
(C–F) Induction of lgl− mutant clones in a white− (C, D) or white + background (E, F) leads to the formation of rough, disorganized eyes. In a white− background, the eyes do not show an over-representation of mutant (white) clones relative to wild-type (orange) clones. Side-views of the mosaic eyes (C, E) show that they are distorted but are not larger than wild-type mosaic eyes (A). lgl− mosaic flies often exhibit darkened, necrotic antennae (D, F, arrowheads).
(G, H) SEM images of control eyes show a regular, hexagonal arrangement of ommatidia with a bristle at every alternate vertex of array. (H) Close-up of (G).
(I, J) SEM images of an lgl− mosaic eye reveal a rough, undulated appearance. (J) Close-up of (I).
(K, L) Semi-thin sections through adult control eyes (K) and lgl− mosaic eyes (L) stained with Toluidine Blue. (K) Control mosaic eyes generated in a white− background show white− and white + tissue (detected by presence of pigment granules). (L) Clones of lgl− mutants, generated in a white + background, thus all pigment cells show pigment granules. The lgl− regions show more pigment cells between ommatidia; ommatidia are larger in diameter, but contain smaller rhabdomeres in their centre and ectopic rhabdomere-like structures at the basal side of the PRCs (arrowheads).
White bar = 100 μm (A–F, G, I) and 20 μm (H, J–L).
lgl− PRCs show defects in F-actin localization, rhabdomere organization and elongation in the pupal and adult eye
To examine the consequence of lgl− defects on PRC morphology, we examined the localization of cell polarity markers and F-actin in later stages of pupal development and in adult eyes (Figs 8–10). In wild-type pupal retinas, after the hexagonal pattern of the eye has been established and refined at mid-pupal development (around 45–50% p.d.) the ommatidia start to elongate from around 10–15μm with a final length of >100μm. At 45% p.d., at the beginning of elongation, F-actin outlines the ommatidia and is also highly concentrated in the middle of each ommatidium where the future rhabdomeres form (GFP-positive cells in Fig. 9C, and summarised in Fig. 9F). This concentration was not observed in lgl− clones, where F-actin appeared less organized or even absent (GFP-negative cells in Figs 9C, 8G). However, at later stages in lgl− tissue (60–70% p.d., Figs 9D–H, 8H) F-actin organisation showed progressive improvement and by 70% p.d., rhabdomeres were clearly identifiable, even though their proper morphogenesis was still affected; PRCs were more rounded than in wild-type, ommatidial elongation was reduced, F-actin was clustered, and E-Cad and Patj localization was aberrant (Fig. 9H). lgl− mosaic eyes at 90% p.d. and adult stages (Fig. 10B, C compared with 10D) showed progressive improvement in F-actin polymerisation relative to 65% p.d. (Fig. 10A) to produce rhabdomeres, although these were smaller than normal, as well as ectopic rhabdomere-like structures at the basal side of the PRCs. However, longitudinal sections of adult lgl− mosaic eyes revealed that there were still severe defects in F-actin organization, including fragmented rhabdomeres and rhabdomere-like structures located at the basal side of PRCs (Fig. 9I). Similar defects were evident in semi-thin longitudinal sections and planar sections through the lgl− mosaic adult eye stained with Toluidine Blue (Figs 9J, 11L compared with wild-type, 11K). The effect of lgl− clones on the organization of F-actin and the morphology of the rhabdomeres is reminiscent of that caused by the overexpression of the intracellular domain of crb (crbintra38.1.2b ) via the GMR-Gal4 driver, (expressed in all cells in the differentiating region of the eye disc at the larval-pupal stage), which resulted in severe disorganization of the rhabdomeres, where pieces of ectopic rhabdomere material were found in the periphery of the ommatidia (Fig. 9K) (Fan et al., 2003). The similarity of the crb overexpression phenotype to lgl− defects is consistent with the antagonistic interactions observed between the Lgl/Scrib/Dlg and the Crb complex in the embryo (Bilder et al., 2003; Tanentzapf and Tepass, 2003). Collectively, these results show that lgl− PRCs show aberrant cell morphology, consistent with the mislocalization of apico-basal cell polarity determinants (Fig. 8).
Ultimately, the induction of lgl− clones results in a disorganized adult eye (Fig. 11C–F, I, J compared with 11A, B, G, H). In lgl− mosaic adult eyes, sections revealed that the normal hexagonal organization of the ommaditial array is almost completely lost and the space between single ommatidia is often larger than in wild-type (Fig. 11L compared with 11K), consistent with the survival of surplus pigment cells in pupal discs (Figs 6, 7). Furthermore, scanning electronmicrographs revealed that the lenses have morphological defects and the organized pattern of bristles at every alternate vertex is disturbed (Fig. 11J compared with 11H). However, despite the increased proliferation in lgl− tissue at the larval stage and the fact that excessive IOCs are not eliminated at the pupal stage (Figs 6, 7), lgl− tissue is not over-represented in the adult eye, although the eye is distorted (Fig. 11C, D compared with 11A, B) (see discussion). Taken together, our analysis of the lgl− mosaic eyes during larval and pupal development has shown that the defects in larval cell proliferation and cell death, and pupal cell death, PRC polarity and F-actin organization, combine to produce a disorganized adult eye.
Discussion
Here we have analysed the requirement of Lgl for cell proliferation and apico-basal cell polarity in the developing Drosophila eye. We have shown for the first time that the effects of a lgl mutant on cell proliferation and apico-basal cell polarity can be separated; in the larval eye disc ectopic expression of Cyclin E and cell proliferation occurs without a loss of cell polarity, whereas in the pupal eye disc PRCs show aberrant polarity without undergoing ectopic cell proliferation. Interestingly, we also found that the normal pattern of apoptosis was affected in lgl− mosaic eye discs.
Lgl and cell death regulation
In this study we observed that lgl− mosaic eye discs have alterations in the normal patterns of apoptosis: (1) in larval lgl− mosaic eye discs at the clonal boundaries, some lgl− and wild-type cells undergo apoptosis and are excluded from the epithelia, (2) in lgl− larval mosaic eye discs, developmental cell death was suppressed throughout the disc, and (3) in the pupal retina apoptosis is inhibited in lgl− tissue relative to the surrounding wild-type tissue.
The cell death that occurs at the boundary of lgl− clones, which is associated with the “drop-out” phenotype, is similar to the process of morphogenic apoptosis, which is due to discontinuities in morphogen gradients (Adachi-Yamada and O’Connor, 2004). Here perturbation in the Wingless, Hedgehog and Dpp pathway lead to upregulation of the JNK pathway and cell death (Adachi-Yamada and O’Connor, 2002). Interestingly, upregulation of Src kinase signaling can also result in cells being extruded from the wing disc epithelia, where they undergo invasive migration and apoptosis (Vidal et al., 2006). How these pathways relate to the lgl− clonal border cell death and “drop-out” phenotype requires further investigation.
The inhibition of the normal bands of developmental cell death observed in larval lgl− mosaic eye discs could be a secondary result of the increased cell death at the clonal borders, or to a more specific effect of the lgl− tissue. How this occurs is unclear, but one possible mechanism is suggested by a previous study showing that in wing discs lgl− cells have reduced secretion of Dpp (Arquier et al., 2001). However, it is unlikely that a decrease in Dpp secretion, if indeed this occurs in lgl− mosaic eye discs, accounts for the suppression of developmental cell death, since previous studies have shown that perturbations in Dpp gradients leads to morphological apoptosis (see above), rather than leading to the inhibition of cell death. However, given this analogy and Lgl’s potential role in directed exocytosis (reviewed by Vasioukhin, 2006; Wirtz-Peitz and Knoblich, 2006), it is possible that decreased secretion in lgl− clones of an unknown extracellular factor(s) may be responsible for this inhibition on developmental cell death throughout the eye disc. Likewise, during pupal eye development the inhibition of developmental cell death of the IOCs and perimeter clusters in lgl− clones may also occur via modulation of signalling pathways that are required for pupal cell death. However, in this case, the factor appears to acts only cell autonomously, inhibiting cell death only in the lgl− tissue. Determining which signalling pathways are misregulated in lgl− cells will help elucidate how depletion of Lgl affects cell polarity, proliferation and apoptosis.
Relationship between cell death and cell proliferation, and clonal size in lgl mutant mosaics
Since increases in cell death can be compensated for by ectopic cell proliferation, so-called “compensatory proliferation” (Huh et al., 2004; Kuranaga and Miura, 2007; Perez- Garijo et al., 2004; Ryoo et al., 2004), this raised the possibility that the increase in apoptosis at the boundary of lgl− clones, might lead to the ectopic cell proliferation observed. However, it is highly unlikely that this is occurring in lgl mutant mosaic eye discs, since compensatory cell proliferation occurs non-cell autonomously (Huh et al., 2004; Perez-Garijo et al., 2004; Ryoo et al., 2004), whereas the upregulation of Cyclin E and ectopic S-phases were confined to the lgl− tissue (Fig. 2B, D). Thus, we conclude that the ectopic Cyclin E expression and S-phases observed in lgl− cells is most likely a direct effect of loss of Lgl function on the cell cycle machinery.
The balance between cell proliferation and cell death controls the size of a tissue, and since lgl− clones show ectopic cell proliferation and less cell death it may have been expected that lgl− tissue would be more represented compared with wild-type tissue in the developing mosaic eye. However, despite the increased cell proliferation, and overall decreased cell death of lgl− tissue, the clonal size of lgl− tissue was not significantly increased compared with the wild-type clones at larval, pupal or adult stages. The reason for this during larval development is likely to be due to the increased cell death at the clonal borders, which although involving both lgl− and wild-type cells, may show a greater effect on the lgl− cells. In addition, unlike other situations that promote the proliferation of a tissue, such as overexpression of Myc in clones (de la Cova et al., 2004), there is not increased cell death of the surrounding wild-type tissue in lgl− mosaics, so the lgl− clones do not obtain a competitive advantage by this mechanism.
During pupal development, although there was less cell death of the lgl− IOCs compared with surrounding wild-type clones, and additional IOCs were observed at pupal and adult eyes, these IOCs appeared significantly smaller than in the surrounding wild-type tissue. Furthermore, the majority of these IOCs were sorted correctly around the PRC clusters, and therefore, due to tight packing and the smaller size of the additional IOCs, they occupy less space than would have been expected. In addition, the distortion of the late pupal and adult eyes by the loss of apico-basal cell polarity of the PRCs may decrease the area occupied by the lgl− tissue at the surface of the adult eye. Thus, due to these additional effects, lgl− mosaic eye discs and adult eyes appear different to other mutants that promote cell proliferation and inhibit apoptosis, such as those of the Hippo/Warts/Salvador/Mats pathway, which result in increased representation of the mutant clones at larval, pupal and adult stages (reviewed by Harvey and Tapon, 2007).
The role of Lgl in cell proliferation and apico-basal cell polarity
Our results show that lgl depletion results in ectopic Cyclin E and proliferation in larval eye disc mosaics without disruption to apico-basal cell polarity. While cell polarity was not lost in lgl− mosaic larval eye discs, when forced to undergo additional cell proliferation (by using a Minute background) cell polarity was lost in undifferentiated cells. This suggests that the perdurance of maternal and pre-clonal zygotic Lgl protein in lgl− clones in a wild-type background generates a threshold level of Lgl function that is sufficient for cell polarity function, but insufficient for inhibiting cell proliferation. Thus, we propose that high levels of Lgl are required to negatively regulate proliferation, while lower levels are needed for the maintenance of apico-basal cell polarity. By contrast, in pupal eye discs, where perduring Lgl protein would be expected to be significantly less, loss of lgl in clones resulted in aberrant apico-basal cell polarity in PRCs, as evidenced by the baso-lateral mislocalization of apical polarity determinants and adherens junction components and cell morphology changes, without ectopic cell proliferation. In this situation, the differentiated state of the cells may prevent the expression of critical cell cycle regulators and/or the induction of cell proliferation upon Lgl depletion. The differentiation state of the cells may also explain the observation that in lgl, scrib or dlg maternal-zygotic null embryos and follicle cell clones, apico-basal cell polarity defects apparently occur without excessive cell proliferation (Bilder et al., 2000).
The differentiation state and the established architecture of the Elav-positive cells in lgl− clones in a Minute background, and of the IOCs in the lgl pupal eye disc may also enable these tissues to maintain cell shape despite the depletion of Lgl function. Ectopic expression of crb throughout the differentiating region of the eye disc (via GMR) also results in loss of polarity in the PRCs, but not the IOCs, where the zonula adherens was disorganized but still apically localized (Fan et al., 2003; Grzeschik and Knust, 2005). The fact that only PRCs are affected in lgl mutants and by overexpression of crb, could relate to the specialised morphological changes that occur at the apical membranes of these cells for the formation of rhabdomeres, and moreover, since lgl mutants and Crb overexpression have a similar phenotype, this implicates the importance of the mutual antagonism between Lgl and Crb function (Bilder et al., 2003; Tanentzapf and Tepass, 2003) in this process. Furthermore, this greater requirement for Lgl function in specific cell types is consistent with previous studies, where temperature shift experiments of a temperature sensitive allele of lgl suggested that Lgl plays an important role in the establishment of epithelial cell polarity during embryogenesis, but not in its maintenance (Manfruelli et al., 1996). Therefore, Lgl may play a more important role in apico-basal cell polarity regulation in cells undergoing morphogenetic remodelling events, but be less important in cells where the apical domain is static.
Cyclin E was observed to be upregulated in all lgl− clones in larval eye discs, but only those in the more posterior region of the eye disc underwent ectopic S-phases. The fact that Cyclin E is upregulated independent of entry into S-phase in some regions of the eye disc, suggests that Cyclin E is a key target of the Lgl pathway in its role in cell proliferation control. This result is consistent with our identification of mutants of lgl, scrib and dlg as dominant suppressors of the cyclin E hypomorphic allele (Brumby et al., 2004). Since Dlg, Lgl and Scrib function in a common pathway to control apico-basal cell polarity in the embryo (Bilder et al., 2003; Tanentzapf and Tepass, 2003), we predict that they will also function in a common pathway to regulate Cyclin E.
Previous studies of dlg and scrib mutants have also suggested that a particular threshold level of protein function is required for cell polarity regulation, while a higher level is required for cell proliferation control (Hough et al., 1997; Woods et al., 1996; Zeitler et al., 2004), however the results were not as clear as our studies with lgl− mosaics. One study of homozygous dlg mutants suggested that the SH3 and GUK domain are important for cell proliferation control (Woods et al., 1996), whereas another study showed that the PDZ2 and PDZ3 domains are required (Hough et al., 1997). These results may be reconciled if a particular threshold level of Dlg function is required for cell polarity regulation, while a higher level is required for cell proliferation control, similar to what we have shown with lgl− mosaic eye discs. A similar situation may occur with Scrib, where it was found that deletion of the PDZ domains, leads to only mild polarity defects but to moderate overproliferation (Zeitler et al., 2004). Thus, a higher level of Scrib function may be needed to inhibit cell proliferation than is needed for the cell polarity function.
The role of Lgl/Scrib/Dlg function in mammalian cell proliferation and apical-basal cell polarity is still unclear. While overexpression of these genes can inhibit cell proliferation (reviewed by Humbert et al., 2003), the knockdown studies are less clear. Knockdown of human Scrib by RNAi in one study in Caco-2 cells revealed increased cell proliferation (Nagasaka et al., 2006), while in another study in human MCF10A epithelial cells increased cell proliferation was not observed and cells exited the cell cycle appropriately upon serum withdrawal, however defects in polarized cell migration occurred (Dow et al., 2006). These different outcomes could be due to the level of knockdown or to differences in the cell lines. Knockout of one of the two mouse homologs of lgl, lgl1, results in hyperproliferation of the neural-epithelial cells of the mouse embryo, which is most likely due to the failure to asymmetrically localize Numb resulting in symmetric divisions and the inability to appropriately differentiate (Klezovitch et al., 2004). However, perhaps due to redundancy with Lgl2, other tissues in the embryo appear to appropriately exit the cell cycle and differentiate and have normal tissue architecture. Studies of a hypomorphic mutation in one of the four mouse Dlg homologs dlg1gt, have also suggested that Dlg1 plays a role in the negative regulation of cell proliferation in the developing lens epithelia (Nguyen et al., 2003). In addition, this study has provided evidence that cell proliferation and apico-basal cell polarity defects may also be separable in mutants of Lgl/Dlg/Scrib in mammalian cells, since homozygous dlg1gt mouse embryos showed inappropriate cell proliferation in the developing lens epithelia, without apparent defects in tissue structure (Nguyen et al., 2003).
Concluding remarks
Here we have shown that depletion of lgl in the developing Drosophila eye results in a loss-of-cell-proliferation control without loss of apico-basal cell polarity, providing evidence for a threshold effect of Lgl on cell proliferation control and apico-basal cell polarity maintenance. This observation has relevance to mammalian development and disease, since lgl1 knockout mice show neural hyperplasia (Klezovitch et al., 2004) and lgl1 is downregulated or mislocalized in several types of human cancer (Grifoni et al., 2007; Grifoni et al., 2004; Kuphal et al., 2006; Schimanski et al., 2005). Moreover, human dlg genes and hscrib are downregulated in many cancers and the proteins are targeted for degradation by various DNA tumour virus oncoproteins, including the high-risk human papilloma virus E6 oncoprotein (reviewed by Humbert et al., 2003). Our studies are also relevant to mechanisms of tumourigenesis, where cancers need to acquire new properties as they progress towards the malignant state, and our results suggest that decreasing function in Lgl/Dlg/Scrib could promote tumourigenesis by first promoting hyperplasia, and secondly resulting in a loss of cell polarity, thereby reducing cell-cell adhesion and allowing more aggressive overgrowth and invasive behaviour (reviewed by Brumby and Richardson, 2005).
Acknowledgments
We thank Helen McNeill for the gift of the Cyclin E antibody, Elisabeth Knust for helpful discussions, and Drs Bilder, Knoblich, Knust, Mechler, Oda, Ohshiro, and Wodarz, and the Bloomington Drosophila Stock Centre and the Developmental Studies Hybridoma Bank for supplying Drosophila stocks or antibodies. We also thank Helen Irving-Rodgers, University of Adelaide, for carrying out the sections of the adult eyes. Thanks to Kieran Harvey, Patrick Humbert, Linda Parsons and Sarah Russell for comments on the manuscript. This work was supported by grants from the Australian National Health and Medical Research Foundation, the Association of International Cancer Research Foundation (UK) and from the NIH (USA) #1R21CA098997-01. N.A. and J.S. were supported by Australian Postgraduate Awards, and H.E.R. was supported by Wellcome and NHMRC Senior Research Fellowships.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adachi-Yamada T, O’Connor MB. Morphogenetic apoptosis: a mechanism for correcting discontinuities in morphogen gradients. Dev Biol. 2002;251:74–90. doi: 10.1006/dbio.2002.0821. [DOI] [PubMed] [Google Scholar]
- Adachi-Yamada T, O’Connor MB. Mechanisms for removal of developmentally abnormal cells: cell competition and morphogenetic apoptosis. J Biochem (Tokyo) 2004;136:13–7. doi: 10.1093/jb/mvh099. [DOI] [PubMed] [Google Scholar]
- Arquier N, Perrin L, Manfruelli P, Semeriva M. The Drosophila tumor suppressor gene lethal(2)giant larvae is required for the emission of the Decapentaplegic signal. Development. 2001;128:2209–20. doi: 10.1242/dev.128.12.2209. [DOI] [PubMed] [Google Scholar]
- Baker NE. Cell proliferation, survival, and death in the Drosophila eye. Semin Cell Dev Biol. 2001;12:499–507. doi: 10.1006/scdb.2001.0274. [DOI] [PubMed] [Google Scholar]
- Betschinger J, Mechtler K, Knoblich JA. The Par complex directs asymmetric cell division by phosphorylating the cytoskeletal protein Lgl. Nature. 2003;422:326–30. doi: 10.1038/nature01486. [DOI] [PubMed] [Google Scholar]
- Bhat MA, Izaddoost S, Lu Y, Cho KO, Choi KW, Bellen HJ. Discs Lost, a novel multi-PDZ domain protein, establishes and maintains epithelial polarity. Cell. 1999;96:833–45. doi: 10.1016/s0092-8674(00)80593-0. [DOI] [PubMed] [Google Scholar]
- Bilder D. Epithelial polarity and proliferation control: links from the Drosophila neoplastic tumor suppressors. Genes Dev. 2004;18:1909–25. doi: 10.1101/gad.1211604. [DOI] [PubMed] [Google Scholar]
- Bilder D, Li M, Perrimon N. Cooperative regulation of cell polarity and growth by Drosophila tumor suppressors. Science. 2000;289:113–6. doi: 10.1126/science.289.5476.113. [DOI] [PubMed] [Google Scholar]
- Bilder D, Schober M, Perrimon N. Integrated activity of PDZ protein complexes regulates epithelial polarity. Nat Cell Biol. 2003;5:53–8. doi: 10.1038/ncb897. [DOI] [PubMed] [Google Scholar]
- Brachmann CB, Cagan RL. Patterning the fly eye: the role of apoptosis. Trends Genet. 2003;19:91–6. doi: 10.1016/S0168-9525(02)00041-0. [DOI] [PubMed] [Google Scholar]
- Brumby A, Secombe J, Horsfield J, Coombe M, Amin N, Coates D, Saint R, Richardson H. A genetic screen for dominant modifiers of a cyclin E hypomorphic mutation identifies novel regulators of S-phase entry in Drosophila. Genetics. 2004;168:227–51. doi: 10.1534/genetics.104.026617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumby AM, Richardson HE. scribble mutants cooperate with oncogenic Ras or Notch to cause neoplastic overgrowth in Drosophila. Embo J. 2003;22:5769–79. doi: 10.1093/emboj/cdg548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumby AM, Richardson HE. Using Drosophila melanogaster to map human cancer pathways. Nat Rev Cancer. 2005;5:626–39. doi: 10.1038/nrc1671. [DOI] [PubMed] [Google Scholar]
- Cagan RL, Ready DF. The emergence of order in the Drosophila pupal retina. Dev Biol. 1989;136:346–62. doi: 10.1016/0012-1606(89)90261-3. [DOI] [PubMed] [Google Scholar]
- Chen P, Nordstrom W, Gish B, Abrams JM. grim, a novel cell death gene in Drosophila. Genes Dev. 1996;10:1773–82. doi: 10.1101/gad.10.14.1773. [DOI] [PubMed] [Google Scholar]
- Crack D, Secombe J, Coombe M, Brumby A, Saint R, Richardson H. Analysis of Drosophila cyclin EI and II function during development: identification of an inhibitory zone within the morphogenetic furrow of the eye imaginal disc that blocks the function of cyclin EI but not cyclin EII. Dev Biol. 2002;241:157–71. doi: 10.1006/dbio.2001.0496. [DOI] [PubMed] [Google Scholar]
- de la Cova C, Abril M, Bellosta P, Gallant P, Johnston LA. Drosophila myc regulates organ size by inducing cell competition. Cell. 2004;117:107–16. doi: 10.1016/s0092-8674(04)00214-4. [DOI] [PubMed] [Google Scholar]
- de Nooij JC, Letendre MA, Hariharan IK. A cyclin-dependent kinase inhibitor, Dacapo, is necessary for timely exit from the cell cycle during Drosophila embryogenesis. Cell. 1996;87:1237–47. doi: 10.1016/s0092-8674(00)81819-x. [DOI] [PubMed] [Google Scholar]
- Dow LE, Kauffman JS, Caddy J, Peterson AS, Jane SM, Russell SM, Humbert PO. The tumour-suppressor Scribble dictates cell polarity during directed epithelial migration: regulation of Rho GTPase recruitment to the leading edge. Oncogene. 2006 doi: 10.1038/sj.onc.1210016. [DOI] [PubMed] [Google Scholar]
- Ekholm SV, Reed SI. Regulation of G(1) cyclin-dependent kinases in the mammalian cell cycle. Curr Opin Cell Biol. 2000;12:676–84. doi: 10.1016/s0955-0674(00)00151-4. [DOI] [PubMed] [Google Scholar]
- Fan SS, Chen MS, Lin JF, Chao WT, Yang VC. Use of gain-of-function study to delineate the roles of crumbs in Drosophila eye development. J Biomed Sci. 2003;10:766–73. doi: 10.1159/000073964. [DOI] [PubMed] [Google Scholar]
- Firth LC, Baker NE. Extracellular signals responsible for spatially regulated proliferation in the differentiating Drosophila eye. Dev Cell. 2005;8:541–51. doi: 10.1016/j.devcel.2005.01.017. [DOI] [PubMed] [Google Scholar]
- Gateff E. The genetics and epigenetics of neoplasms in Drosophila. Biol Rev Camb Philos Soc. 1978;53:123–68. doi: 10.1111/j.1469-185x.1978.tb00994.x. [DOI] [PubMed] [Google Scholar]
- Green MM, Shepherd SH. Genetic instability in Drosophila melanogaster: the induction of specific chromosome 2 deletions by MR elements. Genetics. 1979;92:823–32. doi: 10.1093/genetics/92.3.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grifoni D, Garoia F, Bellosta P, Parisi F, De Biase D, Collina G, Strand D, Cavicchi S, Pession A. aPKCzeta cortical loading is associated with Lgl cytoplasmic release and tumor growth in Drosophila and human epithelia. Oncogene. 2007 doi: 10.1038/sj.onc.1210389. [DOI] [PubMed] [Google Scholar]
- Grifoni D, Garoia F, Schimanski CC, Schmitz G, Laurenti E, Galle PR, Pession A, Cavicchi S, Strand D. The human protein Hugl-1 substitutes for Drosophila lethal giant larvae tumour suppressor function in vivo. Oncogene. 2004;23:8688–94. doi: 10.1038/sj.onc.1208023. [DOI] [PubMed] [Google Scholar]
- Grzeschik NA, Knust E. IrreC/rst-mediated cell sorting during Drosophila pupal eye development depends on proper localisation of DE-cadherin. Development. 2005;132:2035–45. doi: 10.1242/dev.01800. [DOI] [PubMed] [Google Scholar]
- Harvey K, Tapon N. The Salvador-Warts-Hippo pathway- an emerging tumour-suppressor network. Nat Rev Cancer. 2007;7:182–91. doi: 10.1038/nrc2070. [DOI] [PubMed] [Google Scholar]
- Hough CD, Woods DF, Park S, Bryant PJ. Organizing a functional junctional complex requires specific domains of the Drosophila MAGUK Discs large. Genes Dev. 1997;11:3242–53. doi: 10.1101/gad.11.23.3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh JR, Guo M, Hay BA. Compensatory proliferation induced by cell death in the Drosophila wing disc requires activity of the apical cell death caspase Dronc in a nonapoptotic role. Curr Biol. 2004;14:1262–6. doi: 10.1016/j.cub.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Humbert P, Russell S, Richardson H. Dlg, Scribble and Lgl in cell polarity, cell proliferation and cancer. Bioessays. 2003;25:542–53. doi: 10.1002/bies.10286. [DOI] [PubMed] [Google Scholar]
- Izaddoost S, Nam SC, Bhat MA, Bellen HJ, Choi KW. Drosophila Crumbs is a positional cue in photoreceptor adherens junctions and rhabdomeres. Nature. 2002;416:178–83. doi: 10.1038/nature720. [DOI] [PubMed] [Google Scholar]
- Klezovitch O, Fernandez TE, Tapscott SJ, Vasioukhin V. Loss of cell polarity causes severe brain dysplasia in Lgl1 knockout mice. Genes Dev. 2004;18:559– 71. doi: 10.1101/gad.1178004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoblich JA, Sauer K, Jones L, Richardson H, Saint R, Lehner CF. Cyclin E controls S phase progression and its down-regulation during Drosophila embryogenesis is required for the arrest of cell proliferation. Cell. 1994;77:107–20. doi: 10.1016/0092-8674(94)90239-9. [DOI] [PubMed] [Google Scholar]
- Kuphal S, Wallner S, Schimanski CC, Bataille F, Hofer P, Strand S, Strand D, Bosserhoff AK. Expression of Hugl-1 is strongly reduced in malignant melanoma. Oncogene. 2006;25:103–10. doi: 10.1038/sj.onc.1209008. [DOI] [PubMed] [Google Scholar]
- Kuranaga E, Miura M. Nonapoptotic functions of caspases: caspases as regulatory molecules for immunity and cell-fate determination. Trends Cell Biol. 2007;17:135–44. doi: 10.1016/j.tcb.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Lee T, Luo L. Mosaic analysis with a repressible cell marker (MARCM) for Drosophila neural development. Trends Neurosci. 2001;24:251–4. doi: 10.1016/s0166-2236(00)01791-4. [DOI] [PubMed] [Google Scholar]
- Manfruelli P, Arquier N, Hanratty WP, Semeriva M. The tumor suppressor gene, lethal(2)giant larvae (1(2)g1), is required for cell shape change of epithelial cells during Drosophila development. Development. 1996;122:2283–94. doi: 10.1242/dev.122.7.2283. [DOI] [PubMed] [Google Scholar]
- Mechler BM, McGinnis W, Gehring WJ. Molecular cloning of lethal(2)giant larvae, a recessive oncogene of Drosophila melanogaster. Embo J. 1985;4:1551–7. doi: 10.1002/j.1460-2075.1985.tb03816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz R, Schmidt M, Torok I, Protin U, Schuler G, Walther HP, Krieg F, Gross M, Strand D, Mechler BM. Molecular action of the l(2)gl tumor suppressor gene of Drosophila melanogaster. Environ Health Perspect. 1990;88:163–7. doi: 10.1289/ehp.9088163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasaka K, Nakagawa S, Yano T, Takizawa S, Matsumoto Y, Tsuruga T, Nakagawa K, Minaguchi T, Oda K, Hiraike-Wada O, Ooishi H, Yasugi T, Taketani Y. Human homolog of Drosophila tumor suppressor Scribble negatively regulates cell-cycle progression from G1 to S phase by localizing at the basolateral membrane in epithelial cells. Cancer Sci. 2006;97:1217–25. doi: 10.1111/j.1349-7006.2006.00315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen MM, Nguyen ML, Caruana G, Bernstein A, Lambert PF, Griep AE. Requirement of PDZ-containing proteins for cell cycle regulation and differentiation in the mouse lens epithelium. Mol Cell Biol. 2003;23:8970–81. doi: 10.1128/MCB.23.24.8970-8981.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda H, Uemura T, Harada Y, Iwai Y, Takeichi M. A Drosophila homolog of cadherin associated with armadillo and essential for embryonic cell-cell adhesion. Dev Biol. 1994;165:716–26. doi: 10.1006/dbio.1994.1287. [DOI] [PubMed] [Google Scholar]
- Ohshiro T, Yagami T, Zhang C, Matsuzaki F. Role of cortical tumour- suppressor proteins in asymmetric division of Drosophila neuroblast. Nature. 2000;408:593–6. doi: 10.1038/35046087. [DOI] [PubMed] [Google Scholar]
- Peng CY, Manning L, Albertson R, Doe CQ. The tumour-suppressor genes lgl and dlg regulate basal protein targeting in Drosophila neuroblasts. Nature. 2000;408:596–600. doi: 10.1038/35046094. [DOI] [PubMed] [Google Scholar]
- Perez-Garijo A, Martin FA, Morata G. Caspase inhibition during apoptosis causes abnormal signalling and developmental aberrations in Drosophila. Development. 2004;131:5591–8. doi: 10.1242/dev.01432. [DOI] [PubMed] [Google Scholar]
- Plant PJ, Fawcett JP, Lin DC, Holdorf AD, Binns K, Kulkarni S, Pawson T. A polarity complex of mPar-6 and atypical PKC binds, phosphorylates and regulates mammalian Lgl. Nat Cell Biol. 2003;5:301–8. doi: 10.1038/ncb948. [DOI] [PubMed] [Google Scholar]
- Richardson H, O’Keefe LV, Marty T, Saint R. Ectopic cyclin E expression induces premature entry into S phase and disrupts pattern formation in the Drosophila eye imaginal disc. Development. 1995;121:3371–9. doi: 10.1242/dev.121.10.3371. [DOI] [PubMed] [Google Scholar]
- Ryerse JS, Nagel BA. Gap junction distribution in the Drosophila wing disc mutants vg, l(2)gd, l(3)c43hs1, and l(2)gl4. Dev Biol. 1984;105:396–403. doi: 10.1016/0012-1606(84)90296-3. [DOI] [PubMed] [Google Scholar]
- Ryoo HD, Gorenc T, Steller H. Apoptotic cells can induce compensatory cell proliferation through the JNK and the Wingless signaling pathways. Dev Cell. 2004;7:491–501. doi: 10.1016/j.devcel.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Schimanski CC, Schmitz G, Kashyap A, Bosserhoff AK, Bataille F, Schafer SC, Lehr HA, Berger MR, Galle PR, Strand S, Strand D. Reduced expression of Hugl-1, the human homologue of Drosophila tumour suppressor gene lgl, contributes to progression of colorectal cancer. Oncogene. 2005;24:3100–9. doi: 10.1038/sj.onc.1208520. [DOI] [PubMed] [Google Scholar]
- Secombe J, Pispa J, Saint R, Richardson H. Analysis of a Drosophila cyclin E hypomorphic mutation suggests a novel role for cyclin E in cell proliferation control during eye imaginal disc development. Genetics. 1998;149:1867– 82. doi: 10.1093/genetics/149.4.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanentzapf G, Tepass U. Interactions between the crumbs, lethal giant larvae and bazooka pathways in epithelial polarization. Nat Cell Biol. 2003;5:46–52. doi: 10.1038/ncb896. [DOI] [PubMed] [Google Scholar]
- Tepass U, Tanentzapf G, Ward R, Fehon R. Epithelial cell polarity and cell junctions in Drosophila. Annu Rev Genet. 2001;35:747–84. doi: 10.1146/annurev.genet.35.102401.091415. [DOI] [PubMed] [Google Scholar]
- Vasioukhin V. Lethal giant puzzle of Lgl. Dev Neurosci. 2006;28:13–24. doi: 10.1159/000090749. [DOI] [PubMed] [Google Scholar]
- Vidal M, Cagan RL. Drosophila models for cancer research. Curr Opin Genet Dev. 2006;16:10–6. doi: 10.1016/j.gde.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Vidal M, Larson DE, Cagan RL. Csk-deficient boundary cells are eliminated from normal Drosophila epithelia by exclusion, migration, and apoptosis. Dev Cell. 2006;10:33–44. doi: 10.1016/j.devcel.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Walker JL, Fournier AK, Assoian RK. Regulation of growth factor signaling and cell cycle progression by cell adhesion and adhesion-dependent changes in cellular tension. Cytokine Growth Factor Rev. 2005;16:395–405. doi: 10.1016/j.cytogfr.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Walter MF, Jang C, Kasravi B, Donath J, Mechler BM, Mason JM, Biessmann H. DNA organization and polymorphism of a wild-type Drosophila telomere region. Chromosoma. 1995;104:229–41. doi: 10.1007/BF00352254. [DOI] [PubMed] [Google Scholar]
- Wirtz-Peitz F, Knoblich JA. Lethal giant larvae take on a life of their own. Trends Cell Biol. 2006;16:234–41. doi: 10.1016/j.tcb.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Wodarz A, Hinz U, Engelbert M, Knust E. Expression of crumbs confers apical character on plasma membrane domains of ectodermal epithelia of Drosophila. Cell. 1995;82:67–76. doi: 10.1016/0092-8674(95)90053-5. [DOI] [PubMed] [Google Scholar]
- Wodarz A, Ramrath A, Kuchinke U, Knust E. Bazooka provides an apical cue for Inscuteable localization in Drosophila neuroblasts. Nature. 1999;402:544–7. doi: 10.1038/990128. [DOI] [PubMed] [Google Scholar]
- Woods DF, Hough CD, Peel D, Callaini G, Bryant PJ. Dlg protein is required for junction structure, cell polarity, and proliferation control in Drosophila epithelia. J Cell Biol. 1996;134:1469–82. doi: 10.1083/jcb.134.6.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Rubin GM. Analysis of genetic mosaics in developing and adult Drosophila tissues. Development. 1993;117:1223–37. doi: 10.1242/dev.117.4.1223. [DOI] [PubMed] [Google Scholar]
- Zeitler J, Hsu CP, Dionne H, Bilder D. Domains controlling cell polarity and proliferation in the Drosophila tumor suppressor Scribble. J Cell Biol. 2004;167:1137–46. doi: 10.1083/jcb.200407158. [DOI] [PMC free article] [PubMed] [Google Scholar]