Abstract
Background
The sensitivity and specificity to detect periprosthetic infection of the different methods have been questioned, and no single laboratory test accurately detects infection before revision arthroplasty.
Questions/purposes
We asked whether preoperative C-reactive protein (CRP) and interleukin-6 (IL-6) could lead to similar sensitivity, specificity, and predictive values as our previous results obtained with intraoperative frozen section (FS) in revision total hip arthroplasty (THA).
Methods
We prospectively followed 69 patients who had undergone revision THA for failure of a primary THA. The definitive diagnosis of an infection was determined on the basis of positive histopathologic evidence of infection or growth of bacteria on culture.
Results
Eleven of the 69 hips were infected. The combination of an elevated CRP and IL-6 was correlated with deep infection in all the cases and showed a sensitivity of 0.57 (0.13–1.00), a specificity of 1.00 (0.99–1.00), a positive predictive value of 1.00 (0.87–1.00), and a negative predictive value of 0.94 (0.87–1.00). FS showed a sensitivity of 0.81 (0.54–1.00), a specificity of 0.98 (0.94–1.00), a positive predictive value of 0.90 (0.66–1.00), and a negative predictive value of 0.96 (0.91–1.00). Combining CRP and IL-6 provided similar sensitivity, specificity, and positive predictive values as the FSs.
Conclusions
Our data suggest the combination of CRP and IL-6 would be a useful serologic tool to complement others when diagnosing periprosthetic infection.
Level of Evidence
Level I, diagnostic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
Periprosthetic joint infection is one of the most challenging complications of total joint arthroplasty. In a study of 51,345 revision THAs performed between 2005 and 2006, this complication was the third most common cause of revision THA in the United States after dislocation and mechanical aseptic loosening [5]. However, the distinction between aseptic loosening and infection is often difficult but crucial, because the treatments are different. The diagnosis of infection is typically based on the history, clinical findings, radiographs, scintigraphy, laboratory studies, and sometimes aspiration. However, the sensitivity and specificity of the different methods have been questioned, and no single laboratory test accurately detects infection before revision arthroplasty [2, 3, 8, 12, 17, 18, 22–27]. The sensitivities and specificities for single tests in these studies range from 37 to 100% and from 83 to 100% respectively [4, 7, 20, 26, 29].
Intraoperative analysis of frozen sections (FSs) is commonly used to diagnose periprosthetic infection [1, 2, 6, 9, 10, 16, 18, 19]. We previously reported FSs for infection that was in agreement with the observations on standard histology in 134 of 136 cases [20]. However, FS is a technically demanding procedure and not a universally accepted method.
Interleukin-6 (IL-6) is a cytokine produced by monocytes and macrophages that induces the production of major acute-phase proteins, including C-reactive protein (CRP), and may be a valuable marker of infection after major surgery [11, 13, 30, 31]. With a normal serum IL-6 level defined as less than 10 pg/mL, the serum IL-6 test reportedly had a sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of 1.0, 0.95, 0.89, 1.0, and 97%, respectively, in 58 patients undergoing revision hip and knee arthroplasty [7].
We therefore asked whether preoperative CRP and IL-6 could lead to similar sensitivity, specificity, and predictive values as our previous results obtained with intraoperative FS in revision THA.
Patients and Methods
We prospectively followed 69 patients with a THA needing a reoperation for failure between February 2007 and July 2008. We excluded patients with chronic inflammatory diseases (two patients), Paget’s disease (three patients), and immunodeficiency syndromes (one patient), because the IL-6 level is reportedly elevated in these conditions [14, 28]. The study group included 19 men and 50 women with a mean age of 68 years (range, 38–91 years). The study was approved by our Institutional Review Board, and the patients gave informed consent.
The definitive diagnosis of an infection was determined on the basis of positive histopathologic evidence of infection (defined by the presence of five or more polymorphonuclear leukocytes per high-power field on postoperative histologic examination of pathologic specimens) or growth of bacteria on culture [18–20].
Blood samples were obtained within 2 hours before surgery to determine erythrocyte sedimentation rate (ESR) level, CRP level, and IL-6 level. Approximately 3 mL of blood was centrifuged in the hospital clinical laboratory for 10 minutes at 4° C; the separated serum was stored at −80° C and was later thawed and used to determine ESR, CRP, and IL-6 levels in our laboratory. The ESR was measured using the Westergren method. The CRP level was determined with use of a high-sensitivity turbidimetric Synchron LX20 Pro (Beckman Coulter, Fullerton, CA). IL-6 levels were determined with use of an immunoenzymetric assay for the in vitro quantitative measurement of human IL-6 in serum (BioSource IL-6-EASIA Kit; BioSource Europe, Nivelles, Belgium).
The BioSource IL-6-EASIA is a solid-phase enzyme amplified sensitivity immunoassay performed on a microtiter plate. The assay uses monoclonal antibodies (MAbs) directed against distinct epitopes of IL-6. Calibrators and samples react with the capture monoclonal antibody (MAb 1) coated on a microtiter well and with a monoclonal antibody (MAb 2) labeled with horseradish peroxidase (HRP). After an incubation period allowing the formation of a sandwich-coated MAb 1–human IL-6–MAb 2–HRP, the microtiter plate is washed to remove unbound enzyme-labeled antibody. Bound enzyme-labeled antibody is measured through a chromogenic reaction. Chromogenic solution (TMB) is added and incubated. The reaction is stopped with the addition of stop solution and the microtiter plate is then read at the appropriate wavelength. The amount of substrate turnover is determined colorimetrically by measuring the absorbance, which is proportional to the IL-6 concentration. A calibration curve is plotted and IL-6 concentration in samples is determined by interpolation from the calibration curve. The use of the EASIA reader (linearity up to 3 OD units) and a sophisticated data reduction method (polychromatic data reduction) result in a high sensitivity in the low range and in an extended calibration range.
The threshold laboratory value to be positive for infection was assigned for the CRP level (10 mg/L) and the IL-6 level (10 pg/mL) to determine the sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of each test [7].
Three to five samples of tissues to be analyzed were taken during surgery from the pseudocapsule, the cement-bone interface of the femur and acetabulum, and any other tissues involved according to the surgeon’s judgment. All samples were referred for frozen sections and to be processed on a routine basis. Samples for cultures were taken from the same areas and were packed into a sterile surgical container to avoid contamination. No patient received antibiotic therapy for the previous 3 weeks before the samples were obtained, and all the surgical procedures were supervised by one of the authors (MB). All samples were processed and analyzed by the pathology and bacteriology units of our hospital. Histologic analysis was performed on all material. Smear tissue staining was hematoxylin-phloxine. Serial sections (4 mm thick) were processed in a freezing chamber and stained with hematoxylin-phloxine and toluidine blue. The material for standard processing was fixed in 10% formol and paraffin and embedded in an automatic tissue processor. Six microsections stained with hematoxylin and eosin and Mason’s trichrome were observed with an optical microscope under high magnification (total magnification ×400) and a polarized-light lens (Axiostar; Carl Zeiss MicroImaging, Inc, Thornwood, NY).
All samples were analyzed by two pathologists (AM, HGR) who counted the number of cells in 10 fields. Neutrophils, lymphocytes, plasma cells, macrophages, multinuclear giant cells, acrylic material, and polystyrene particles were included. For the specific purposes of this study, only neutrophils were considered. We considered a sample infected if there were five or more polymorphonuclear leukocytes [19, 20]. Bacteriologic analyses were performed in at least five samples from the same sites as those that were taken for histopathologic examination. The samples for aerobic and anaerobic cultures were plated on chocolate agar, blood agar, and different enriched culture media. They were incubated for 5 days and subcultured daily for aerobic and anaerobic bacteria. Cultures were considered negative when there was no growth of bacteria in 14 days, no growth of fungus in 4 weeks, and no growth of mycobacteria in 8 weeks. Cultures were considered positive if there was growth of at least one colony.
The sensitivity, specificity, positive predictive value (PPV), negative predictive value, and accuracy of each test were calculated. Receiver operating characteristic curves were used to examine the relationship between sensitivity and the false-positive rate (1-specificity). Fisher’s exact test was used to determine direct statistical comparison between the tests.
Results
Eleven of the 69 hips were infected (Table 1). The combination of an elevated CRP and IL-6 was correlated with deep infection in all the cases and showed a sensitivity of 0.57 (0.13–1.00), a specificity of 1.00 (0.99–1.00), a positive predictive value of 1.00 (0.87–1.00), and a negative predictive value of 0.94 (0.87–1.00).
Table 1.
Culture results
| Pathogen | Number of cases |
|---|---|
| MRSA | 3 |
| Staphylococcus viridans | 1 |
| Staphylococcus epidermidis | 2 |
| CNS | 4 |
| Enterococcus faecalis | 1 |
MRSA = methicillin-resistant Staphylococcus aureus; CNS = coagulase-negative Staphylococcus.
The combination of CRP and IL-6 identified all patients with deep infection of the implant and showed a sensitivity of 0.57 (0.13–1.00), a specificity of 1.00 (0.99–1.00), a positive predictive value of 1.00 (0.87–1.00), and a negative predictive value of 0.94 (0.87–1.00) (Table 2) (Fig. 1).
Table 2.
Results and confidence intervals according to the different tests
| Test | Ss | Sp | PPV | NPV | P value |
|---|---|---|---|---|---|
| ESR | 72% (0.41–1.00) | 86% (0.76–0.95) | 50% (0.22–0.77), | 94% (0.84–1.00) | nc |
| CRP | 72% (0.41–1.00) | 91% (0.83–0.99) | 61% (0.31–0.91) | 94% (0.87–1.00) | < 0.001 |
| FS | 81% (0.54–1.00) | 98% (0.94–1.00) | 90% (0.66–1.00) | 96% (0.91–1.00) | < 0.001 |
| IL-6 | 36% (0.30–0.69) | 94% (0.88–1.00) | 57% (0.13–1.00) | 88% (0.80–0.97) | 0.01 |
| CRP + IL-6 | 57% (0.13–1.00) | 100% (0.99–1.00) | 100% (0.87–1.00) | 94% (0.87–1.00) | < 0.001 |
Ss = specificity; Sp = specificity; PPV = positive predictive value; NPV = negative predictive value; ESR = erythrocyte sedimentation rate; CRP = C-reactive protein; FS = frozen section; IL-6 = interleukin 6; nc = not calculated.
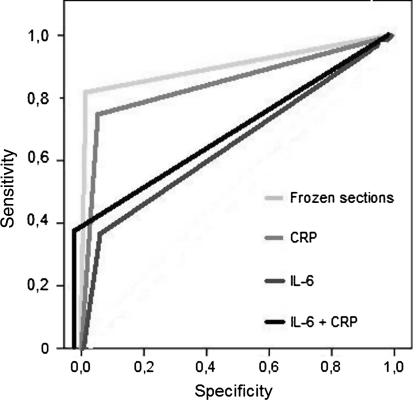
Fig. 1.
Receiver operator curves (ROC) demonstrating sensitivity and specificity for C-reactive protein, frozen sections, interleukin-6 and the combination between C-reactive protein and interleukin-6 are shown. The combination of C-reactive protein and interleukin-6 provided similar sensitivity, specificity, and positive predictive values as the frozen sections.
Discussion
The sensitivity and specificity to detect periprosthetic hip infection of the different methods have been questioned, and no single laboratory test accurately detects infection before revision arthroplasty (Table 3). That led us to ask ourselves whether preoperative CRP and IL-6 could lead to similar sensitivity, specificity, and predictive values as our historical controls obtained with intraoperative FS in revision THA.
Table 3.
Analysis of diagnostic parameters according to different authors
| Author | Diagnostic parameter | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|
| Schinsky et al. [26] | ESR and CRP | 90 | 91 | 95 | 82 |
| Schinsky et al. [26] | Aspiration | 82 | 83 | 69 | 90 |
| Tohtz et al. [29] | Soft tissue culture | 37 | 91 | 67 | 77 |
| Nunez et al. [20] | Frozen sections | 98 | 99 | 98 | 99 |
| Di Cesare et al. [7] | IL-6 | 100 | 95 | 89 | 100 |
| Bottner et al. [4] | IL-6 and CRP | 100 | 86 | 72 | 100 |
| Buttaro et al. [current study] | IL-6 and CRP | 57 | 100 | 100 | 94 |
PPV = positive predictive value; NPV = negative predictive value; ESR = erythrocyte sedimentation rate; CRP = C-reactive protein; IL-6 = interleukin-6.
We acknowledge limitations of our study. First is the relatively small number of patients that limits the statistical power of our conclusions. We found a low sensitivity (0.57) of the combination of CRP and IL-6 with a large confidence interval (0.13 to 1.00) is also a limitation. We observed high positive and negative predictive values combining CRP and IL-6. However, these values should be taken cautiously, because they depend on the prevalence of the disease in the sample being studied. There were 11 patients with infections in among our 69 patients, which is not the prevalence of infection in the general population. Second is the absence of a “gold standard” test. We used either growth of bacteria on culture or positive histopathologic evidence of infection as our gold standard. While positive intraoperative cultures have been considered by some as the gold standard for diagnosing infection at the site of a THA, with only the culture as an end point, the PPV of a positive intraoperative culture was only 2.4% [21]. This suggests cultures alone are an unreliable predictor of infection at the time of revision surgery [21]. Furthermore, intraoperative cultures are subject to various limitations such as errors in culturing technique, delays in plating cultures, failure to use all appropriate media, and a potential for antibiotics to have been given preoperatively without the surgeon’s awareness or to have been placed in the irrigant, and contaminants. Third, FS analysis may be related to more errors than a preoperative laboratory test. One error could be made by the surgeon by not taking samples from the areas of greatest suspicion. Another error might be made by the pathologist when handling the samples. There is a chance that the area chosen is not infected, and the availability of a specialized musculoskeletal pathologist is not guaranteed at any time during revision surgeries.
Limitations of the IL-6 diagnostic method include high costs and that a high number of cases are needed to perform all the tests at the same procedure. The disadvantages of the serum tests are that they are nonspecific and may increase in response to several diseases with acute inflammatory reactions. On the other hand, this test is readily available and can be serially monitored, although it may be difficult to use it routinely in public institutions. Furthermore, advances in laboratory procedures may reduce the costs for each test, making it economically justifiable to use IL-6 for screening purposes after surgery. Until these advances arrive, IL-6 may be used in combination with CRP in the cases in which infection cannot be ruled out with the use of ESR and CRP. The ideal test for infection diagnosis should be able to be carried out before the operation and should involve a minimum number of tests, which in combination should be accurate, convenient to the patient, cause minimal morbidity, and be cost-effective. Determination of CRP has gained increased acceptance for diagnosis of an infected THA. Serial CRP measurements are useful not only as a diagnostic tool for infection, but also for monitoring the effect of treatment and for the early detection of any relapse. After successful treatment of an infected THA, the normalization time for CRP is much shorter than the time needed for the ESR to drop to normal [15].
We found the IL-6 serum level was less specific than CRP (0.87 versus 0.96) and combining CRP and IL-6 identified all patients with deep infection of the implant. However, our data suggest the combination of CRP and IL-6 is as useful as FS for the detection of periprosthetic infection.
Acknowledgments
We thank A. Morandi, MD, and H. García Rivello, MD, for their collaboration on the histologic evaluation.
Footnotes
Each author certifies that he has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his institution has approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Athanasou NA, Pandey R, Steiger R, Crook D, Smith PM. Diagnosis of infection by frozen section during revision arthroplasty. J Bone Joint Surg Br. 1995;77:28–33. [PubMed] [Google Scholar]
- 2.Banit DM, Kaufer H, Hartford JM. Intraoperative frozen section analysis in revision total joint arthroplasty. Clin Orthop Relat Res. 2002;401:230–238. doi: 10.1097/00003086-200208000-00026. [DOI] [PubMed] [Google Scholar]
- 3.Barrack RL, Harris WH. The value of aspiration of the hip before total hip arthroplasty. J Bone Joint Surg Am. 1993;75:66–76. doi: 10.2106/00004623-199301000-00010. [DOI] [PubMed] [Google Scholar]
- 4.Bottner M, Wagner A, Winkelmann W, Becker K, Erren M, Götze C. Interleukin-6, procalcitonin and TNF-alpha: markers of peri-prosthetic infection following total joint replacement. J Bone Joint Surg Br. 2007;89:94–99. doi: 10.1302/0301-620X.89B1.17485. [DOI] [PubMed] [Google Scholar]
- 5.Bozic KJ, Kurtz SM, Lau E, Ong K, Vail TP, Berry DJ. The epidemiology of revision total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91:128–133. doi: 10.2106/JBJS.H.00155. [DOI] [PubMed] [Google Scholar]
- 6.Charosky CB, Bullough PG, Wilson PD., Jr Total hip replacement failures: a histological evaluation. J Bone Joint Surg Am. 1973;55:49–58. [PubMed] [Google Scholar]
- 7.Di Cesare PE, Chang E, Preston CF, Liu CJ. Serum interleukin-6 as a marker of periprosthetic infection following total hip and knee arthroplasty. J Bone Joint Surg Am. 2005;87:1921–1927. doi: 10.2106/JBJS.D.01803. [DOI] [PubMed] [Google Scholar]
- 8.Fehring TK, Cohen B. Aspiration as a guide to sepsis in revision total hip arthroplasty. J Arthroplasty. 1996;11:543–547. doi: 10.1016/S0883-5403(96)80107-0. [DOI] [PubMed] [Google Scholar]
- 9.Fehring TK, McAlister JA., Jr Frozen histologic section as a guide to sepsis in revision joint arthroplasty. Clin Orthop Relat Res. 1994;304:229–237. [PubMed] [Google Scholar]
- 10.Feldman DS, Lonner JH, Desai P, Zuckerman JD. The role of intraoperative frozen sections in revision total joint arthroplasty. J Bone Joint Surg Am. 1995;77:1807–1813. doi: 10.2106/00004623-199512000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Hack CE, Groot ER, Felt-Bersma RJ, Nuijens JH, Strack Van Schijndel RJ, Eerenberg-Belmer AJ, Thijs LG, Aarden LA. Increased plasma levels of interleukin-6 in sepsis. Blood. 1989;74:1704–1710. [PubMed] [Google Scholar]
- 12.Hanssen AD, Rand JA. Evaluation and treatment of infection at the site of a total hip or knee arthroplasty. Instr Course Lect. 1999;48:111–122. [PubMed] [Google Scholar]
- 13.Heinrich PC, Castell JV, Andus T. Interleukin-6 and the acute phase response. Biochem J. 1990;265:621–636. doi: 10.1042/bj2650621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Honda M, Kitamura K, Mizutani Y, Oishi M, Arai M, Okura T, Igarahi K, Yasukawa K, Hirano T, Kishimoto T, Mitsuyasu R, Chermann JC, Tokunaga T. Quantitative analysis of serum IL-6 and its correlation with increased levels of serum IL-2R in HIV-induced diseases. J Immunol. 1990;145:4059–4064. [PubMed] [Google Scholar]
- 15.Larsson S, Thelander U, Friberg S. C-reactive protein (CRP) levels after elective orthopedic surgery. Clin Orthop Relat Res. 1992;275:237–242. [PubMed] [Google Scholar]
- 16.Lonner JH, Desai P, DiCesare PE, Steiner G, Zuckerman JD. The reliability of analysis of intraoperative frozen sections for identifying active infection during revision hip and knee arthroplasty. J Bone Joint Surg Am. 1996;78:1553–1558. doi: 10.2106/00004623-199610000-00014. [DOI] [PubMed] [Google Scholar]
- 17.Merkel KD, Brown ML, Dewangee MK, Fitzgerald RH., Jr Comparison of indium-labeled leukocyte imaging with sequential technetium-gallium scanning in the diagnosis of low grade musculoskeletal sepsis: a prospective study. J Bone Joint Surg Am. 1985;67:465–476. [PubMed] [Google Scholar]
- 18.Mirra JM, Amstutz HC, Matos M, Gold R. The pathology of joint tissue and its clinical relevance in prosthesis failure. Clin Orthop Relat Res. 1976;117:221–240. [PubMed] [Google Scholar]
- 19.Mirra JM, Marder RA, Amstutz HC. Pathology of failed total joint arthroplasty. Clin Orthop Relat Res. 1982;170:175–183. [PubMed] [Google Scholar]
- 20.Nuñez LV, Buttaro MA, Morandi A, Pusso R, Piccaluga F. Frozen sections of samples taken intraoperatively for diagnosis of infection in revision hip surgery. Acta Orthop. 2007;78:226–230. doi: 10.1080/17453670710013726. [DOI] [PubMed] [Google Scholar]
- 21.Padgett DE, Silverman A, Sachjowicz F, Simpson RB, Rosenberg AG, Galante JO. Efficacy of intraoperative cultures obtained during revision total hip arthroplasty. J Arthroplasty. 1995;10:420–426. doi: 10.1016/S0883-5403(05)80140-8. [DOI] [PubMed] [Google Scholar]
- 22.Phillips WC, Kattapuram SV. Efficacy of preoperative hip aspiration performed in the radiology department. Clin Orthop Relat Res. 1983;179:141–146. doi: 10.1097/00003086-198310000-00020. [DOI] [PubMed] [Google Scholar]
- 23.Rand JA, Brown ML. The value of indium-111 leukocyte scanning in the evaluation of painful or infected total knee arthroplasties. Clin Orthop Relat Res. 1990;259:179–182. [PubMed] [Google Scholar]
- 24.Rand JA, Fitzgerald RH., Jr Diagnosis and management of the infected total knee arthroplasty. Orthop Clin North Am. 1989;20:201–210. [PubMed] [Google Scholar]
- 25.Roberts P, Walters AJ, McMinn DJ. Diagnosing infection in hip replacements. The use of fine-needle aspiration and radiometric culture. J Bone Joint Surg Br. 1992;74:265–269. doi: 10.1302/0301-620X.74B2.1544966. [DOI] [PubMed] [Google Scholar]
- 26.Schinsky MF, Della Valle CJ, Sporer SM, Paprosky WG. Perioperative testing for joint infection in patients undergoing revision total hip arthroplasty. J Bone Joint Surg Am. 2008;90:1869–1875. doi: 10.2106/JBJS.G.01255. [DOI] [PubMed] [Google Scholar]
- 27.Spangehl MJ, Masri BA, O’Connell JX, Duncan CP. Prospective analysis of preoperative and intraoperative investigations for the diagnosis of infection at the sites of two hundred and two revision total hip arthroplasties. J Bone Joint Surg Am. 1999;81:672–683. doi: 10.2106/00004623-199905000-00008. [DOI] [PubMed] [Google Scholar]
- 28.Tigges S, Stiles RG, Meli RJ, Roberson JR. Hip aspiration: a cost-effective and accurate method of evaluating the potentially infected hip prosthesis. Radiology. 1993;189:485–488. doi: 10.1148/radiology.189.2.8210377. [DOI] [PubMed] [Google Scholar]
- 29.Tohtz SW, Müller M, Morawietz L, Winkler T, Perka C. Validity of frozen sections for analysis of periprosthetic loosening membranes. Clin Orthop Relat Res. 2010;468:762–768. doi: 10.1007/s11999-009-1102-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vreugdenhil G, Lindemans J, Eijk HG, Swaak AJ. Elevated serum transcobalamin levels in anaemia of rheumatoid arthritis: correlation with disease activity but not with serum tumour necrosis factor alpha and interleukin 6. J Intern Med. 1992;231:547–550. doi: 10.1111/j.1365-2796.1992.tb00972.x. [DOI] [PubMed] [Google Scholar]
- 31.Wirtz DC, Heller KD, Miltner O, Zilkens KW, Wolff JM. Interleukin-6: a potential inflammatory marker after total joint replacement. Int Orthop. 2000;24:194–196. doi: 10.1007/s002640000136. [DOI] [PMC free article] [PubMed] [Google Scholar]