Abstract
Background
Emerging evidence has linked the long-term use of bisphosphonates with femoral insufficiency fractures. It has been suggested that the prolonged effect on bone remodeling leads to the accumulation of microfractures and weakening of bone.
Questions/purposes
We investigated the association between bisphosphonate use and femoral insufficiency fractures.
Methods
We evaluated 100 patients with low-energy femoral shaft fractures before and after bisphosphonates became available for use. Twenty-one consecutive patients who presented between January 1995 and February 1997 were compared with 79 consecutive patients who presented between January 2007 and February 2009. The radiographs of all 100 patients were examined for evidence of preexisting insufficiency fractures. We identified insufficiency fractures by a transverse fracture line on the tension side of the femur with lateral cortical thickening immediately adjacent to the fracture. Relevant details from the history were recorded.
Results
Forty-one patients had an underlying femoral insufficiency fracture, all of whom had been receiving bisphosphonate therapy. Among the 21 patients with low-energy femoral fractures before the availability of bisphosphonates, none had insufficiency fractures. Of the 41 patients with insufficiency fractures, 29 (71%) had prodromal pain and 18 (44%) had bilateral insufficiency fractures. Bisphosphonate use was associated (odds ratio greater than 1000) with insufficiency fracture. The mean duration of bisphosphonate use in patients with insufficiency fractures was longer than in patients without fractures (7.1 versus 3.2 years).
Conclusion
Long-term bisphosphonate use is associated with insufficiency fractures of the femoral shaft, which commonly present with prodromal thigh pain and may be bilateral. These fractures were not seen before bisphosphonates became available for use.
Level of Evidence
Level III, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Bisphosphonates have been used successfully for nearly two decades for prevention of fractures in patients with osteoporosis. There is a high level of evidence to suggest it improves bone mineral density, prevents bone loss, and reduces the number of fractures in the spine and long bones [8, 9, 11, 15, 19, 38, 40, 43, 47, 50–52, 63, 66]. Although the short-term efficacy of bisphosphonates is well documented [7, 10, 28, 45, 64], there are concerns regarding the safety of long-term therapy resulting from the effect on bone physiology [33, 35, 46, 49]. Bisphosphonates inhibit osteoclastic bone resorption, and therefore bone turnover, by inducing osteoclast apoptosis [39]. However, osteoclast function is vital for bone health through its link to osteoblast function. The combined and coordinated action of resorbing damaged bone and laying down new bone is fundamental to the process of bone remodeling. If this coupling is impaired, the microdamage that occurs under physiologic conditions that normally is repaired may accumulate, resulting in a major reduction in the energy required to cause fracture [4, 5, 31, 42, 59].
The notion that bisphosphonates may have detrimental effects on bone physiology is not new. Data from animal models suggest alendronate suppresses bone turnover, causes a sevenfold increase in microdamage, and results in a 40% reduction in the energy required to cause fracture [4, 5, 27, 31, 37, 41, 42]. In addition, chronic oversuppression of bone turnover by bisphosphonates may allow secondary mineralization to continue, producing hypermineralized bone that may be more brittle [2, 13, 18, 46, 59]. Other theories to explain this association have been proposed, including those relating bone brittleness caused by long-term alendronate therapy to trabecular bone collagen cross-linking and isomerization [3, 55]. Others suggest it may be attributable to osteoclastic abnormalities or a variant form of osteoporosis [6, 65]. These effects last well beyond cessation owing to poor degradation of the drug, accumulation with use, and subsequent deposition in bone [10, 13, 14, 17, 20, 25, 32, 41, 46, 49, 53, 54, 59, 61, 65, 67].
There is emerging evidence that the accumulation of microdamage from prolonged bisphosphonate therapy may be associated with femoral insufficiency fractures [1, 16, 23, 26, 30, 33, 35, 36, 44–46, 48, 49, 57, 58, 68]. These fractures are low-energy injuries and have characteristic findings observed on femoral radiographs: a transverse fracture line originating from the lateral tension side of the cortex and lateral cortical thickening adjacent to the fracture. In addition, prodromal thigh pain from the insufficiency changes may be present. The subsequent minimal trauma that often is required to complete the fracture is characteristic, with patients often sustaining a spontaneous nontraumatic fracture during activities of daily living. Despite the current evidence supporting an association between bisphosphonates and femoral insufficiency fractures, none of the studies determined the incidence of these fractures before introduction of alendronate, nor the incidence of alendronate use among patients with low-energy femoral fractures [1, 16, 23, 26, 30, 33, 35, 36, 44–46, 48, 49, 58, 68].
We therefore asked the following questions: (1) Is there an association between bisphosphonate use and femoral insufficiency fractures? (2) Are these insufficiency fractures associated with long-term bisphosphonate use? (3) Did characteristic insufficiency fractures occur before the availability of bisphosphonates? (4) Are insufficiency fractures overrepresented by bisphosphonate users in patients presenting with low-energy femoral fractures? (5) What proportion of patients experience prodromal pain from insufficiency fractures? (6) What proportion of patients experience bilateral insufficiency fractures?
Patients and Methods
We retrospectively identified 100 patients who presented with low-energy subtrochanteric or midshaft femoral fractures from January 1995 to February 1997 and January 2007 to February 2009. These two periods coincided with the period before and after bisphosphonates became available for use. Low energy was defined as a fall from a standing height or less. We excluded patients younger than 65 years, or who had high-energy trauma, known underlying malignancy, or periprosthetic fractures. Thirty-eight patients were identified during the former period and 79 from the latter. Of the 38 patients identified from the former period, 17 (45%) did not have radiographs available for review. These patients were not included in the final analyses. Of 79 patients from the latter period, 41 (52%) were identified as having the characteristic insufficiency fracture pattern observed on plain radiographs. All but one of these 41 patients had been receiving prolonged alendronate therapy; the other had been taking risedronate. Fifty-three of the 79 patients from the latter period had a history of bisphosphonate use. The mean ages were similar between the two groups. The mean American Society of Anesthesiologists (ASA) scores also were similar between groups, suggesting both groups had comparable comorbidities. Demographics (Table 1) and clinical characteristics (Table 2) for all patients with low-energy femoral fractures are presented.
Table 1.
Demographics for 100 patients with low-energy subtrochanteric or femoral shaft fractures
| Parameter | January 2007 to February 2009 (n = 79) | January 1995 to February 1997† (n = 21) | p Value* |
|---|---|---|---|
| Mean age (years) | 75.4 (74.3–76.5) | 74.7 (71–78.4) | 0.67 |
| Gender (F, M) | 62, 17 | 16, 5 | 0.71 |
| Mean ASA score | 2.4 (2.3–2.6) | 2.4 (2.1–2.8) | 0.93 |
†January 1995 to February 1997 was the period before alendronate was introduced; ASA = American Society of Anesthesiologists score; *p values calculated using Student’s t test and Fisher’s exact test
Table 2.
Clinical characteristics of patients with low-energy femoral fractures
| Parameter | Insufficiency fracture present (n = 41) | Insufficiency fracture not present (n = 59) | p Value* |
|---|---|---|---|
| Mean age (years) | 73.7 (72.4–75.0) | 75.2 (73.5–77.0) | 0.76 |
| Mean ASA score | 2.5 (2.3–2.6) | 2.4 (2.3–2.6) | 0.74 |
| History of bisphosphonate use | 41† | 12 | – |
| Duration of bisphosphonate use (years) | 7.1 (6.6–7.6) | 3.2 (2.6–3.8) | < 0.0001 |
| Bilateral insufficiency fracture | 18 | – | – |
| Prodrome of pain | 29 | – | – |
| Spontaneous nontraumatic fracture | 9 | – | – |
ASA = American Society of Anesthesiologists score; †40 patients had been taking alendronate, one patient had been taking risedronate; *p values calculated using Student’s t test
The medical records, patients, treating physicians, and family members were consulted to gather information regarding whether bisphosphonates were used, duration of use, presence of prodromal pain, mechanism of injury, and coexisting medical conditions. During the period after bisphosphonates became available (January 2007 to February 2009), 52 patients (66%) had a history of alendronate use. Of these patients, 48 had a history of alendronate use and four had a history of risedronate use. Radiographs were examined to identify the presence of preexisting insufficiency fractures. The characteristic findings observed on radiographs were a transverse fracture line on the lateral (tension) side of the femur and lateral cortical thickening adjacent to the fracture site (Fig. 1). The radiographic outcome assessor was blinded regarding whether the patient was receiving bisphosphonate therapy.
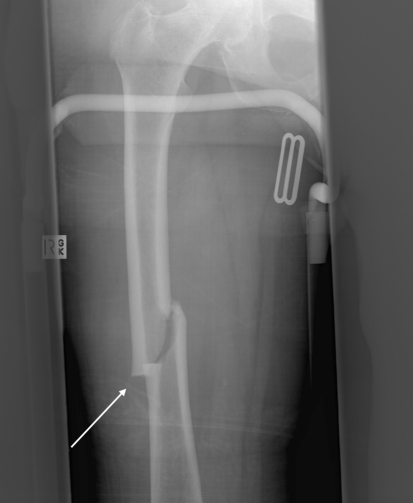
Fig. 1.
A low-energy femoral fracture in a 70-year-old woman can be seen on this radiograph. The characteristic insufficiency fracture pattern is evident with a transverse fracture line and lateral cortical thickening adjacent to the fracture (white arrow). The woman had been receiving alendronate therapy for 8 years and reported prodromal anterolateral thigh pain for 2 months.
We then identified the proportion of patients who were taking alendronate from all patients who presented with a proximal femoral fracture after a low-energy injury between January 2007 and February 2009. This allowed us to determine whether femoral insufficiency fractures were overrepresented by alendronate users compared with other fractures in a similar cohort of patients with similar mechanisms of injury.
We compared continuous variables using Student’s t test. Categorical variables were analyzed using chi square or Fisher’s exact test. Data were analyzed using Stata 8 statistical software package (College Station, TX, USA). Approval was given by the Human Research Ethics Committee.
Results
For patients presenting with low-energy femoral shaft fractures, bisphosphonate use was associated with the presence of an insufficiency fracture (Table 3): the odds ratio of an insufficiency fracture in patients taking bisphosphonates was greater than 1000. All 41 patients who were identified as having insufficiency fractures were receiving or had received bisphosphonate therapy.
Table 3.
Association between bisphosphonate use and insufficiency fracture
| History of bisphosphonate use | Insufficiency fracture present | Insufficiency fracture not present | Total number of patients |
|---|---|---|---|
| Yes | 41 | 12 | 53 |
| No | 0 | 47 | 47 |
| Totals | 41 | 59 | 100 |
Odds ratio = greater than 1000, p < 0.0001; sensitivity = 100%, specificity = 80%, positive predictive value = 77%, negative predictive value = 100%.
Femoral insufficiency fractures were associated with long-term bisphosphonate therapy (Table 2). Patients who had sustained insufficiency fractures after receiving bisphosphonate therapy had been taking the medication for an average of 7.1 years (95% confidence interval [CI], 6.6–7.6 years). The mean duration of bisphosphonate use in the 12 patients without insufficiency fractures was 3.2 years (95% CI, 2.6–3.8 years). The duration of bisphosphonate use was greater (p < 0.001) in patients with an insufficiency fracture: 7.1 versus 3.2 years, respectively. Seven of 41 (17%) patients with insufficiency fractures were not currently taking a bisphosphonate but had stopped taking the drug between 6 months and 2 years prior. These patients received the drug for an average of 7.2 years.
There were no femoral insufficiency fractures identified in the 21 patients we examined during the period before bisphosphonates became available (January 1995 to February 1997).
For patients presenting with low-energy proximal and diaphyseal femoral fractures, bisphosphonate use was overrepresented among those with insufficiency fractures. Three hundred ninety-eight patients presented with low-energy proximal femoral fractures between 2007 and 2009. Of these, only 52 (13%) were taking a bisphosphonate. For the same time period, the proportion of patients taking a bisphosphonate was greater in those presenting with femoral insufficiency fractures (98% versus 13%; p < 0.001). The proportion of patients taking a bisphosphonate in the insufficiency fracture group also was greater (p < 0.001) than the proportion of patients with subtrochanteric or femoral shaft fractures alone (98% versus 32%).
Twenty-nine (71%) of 41 patients with insufficiency fractures reported prodromal pain in the affected femur. The pain was localized to the groin, anterior thigh, lateral thigh, or knee and had been present for between 3 weeks and 6 months. Of these patients, 20 (69%) had experienced enough pain to warrant visiting their general practitioner or specialist physician at least once, and six (21%) subsequently were referred for radiographs. After obtaining radiographs, we identified femoral insufficiency fractures in all of these patients (Fig. 2).
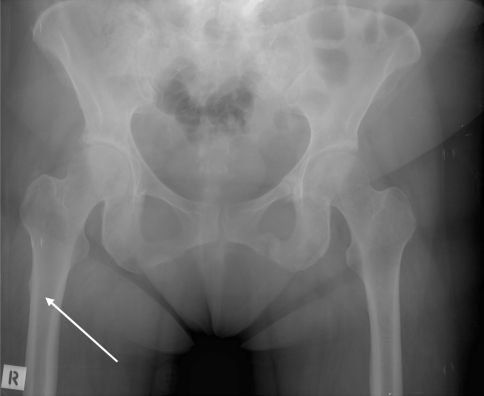
Fig. 2.
A 68-year-old woman who had received long-term alendronate therapy presented to her physician with prodromal pain in the right anterolateral thigh. This radiograph was diagnostic for an insufficiency fracture (white arrow). This was missed by the referring physician and radiologist and the woman presented to us with a low-energy subtrochanteric fracture at the site indicated by the arrow.
Eighteen (44%) of 41 patients with insufficiency fractures had bilateral femoral insufficiency fractures. Five (28%) of these patients previously had sustained a contralateral subtrochanteric fracture secondary to an underlying insufficiency fracture. Nine patients (22%) sustained spontaneous nontraumatic fractures during activities of daily living; eight of these (89%) patients were observed to have bilateral femoral insufficiency fractures (Figs. 2–4).
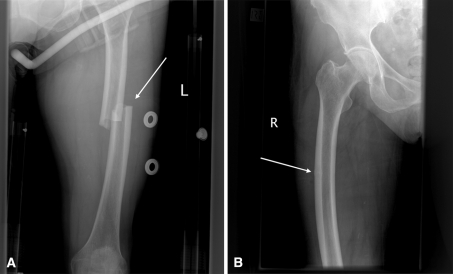
Fig. 3A–B.
A 71-year-old woman presented with (A) a spontaneous nontraumatic left femoral shaft fracture while standing and was incidentally found to have (B) a femoral shaft insufficiency fracture on the right. The woman reported a 6-month history of prodromal left anterolateral thigh pain, a 2-month history of right anterior thigh pain, and a 10-year history of alendronate use. The bilateral femoral insufficiency fracture pattern can be seen (white arrows).
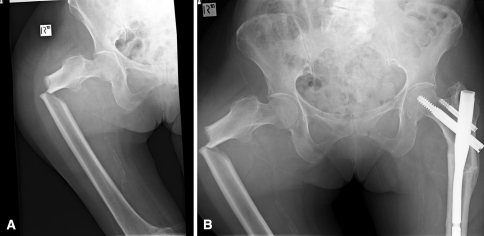
Fig. 4A–B.
Radiographs of the right hip and pelvis, respectively, show (A) a low-energy subtrochanteric fracture on the right and (B) a previous left subtrochanteric fracture in the same patient. The bilateral subtrochanteric insufficiency fracture pattern with the transverse fracture line on the tension side of the femur and lateral cortical thickening adjacent to the fracture can be seen.
Discussion
Bisphosphonates are considered the first-line therapy for postmenopausal osteoporosis. However, many clinicians do not recommend bisphosphonate use beyond 5 years [8, 10, 21, 24]. This is predominantly the result of the unknown long-tem side effects and the fact that there rarely is any added benefit beyond this time [10]. Despite the widely accepted safety profile and tolerability of bisphosphonates, there has been concern regarding the safety of long-term therapy [33, 35, 46, 49]. It is purported that the bisphosphonate-induced prolonged impairment of bone remodeling leads to the accumulation of microfractures and weakening of bone [2, 4, 5, 10, 12–14, 17, 18, 20, 25, 27, 31, 32, 37, 41, 42, 46, 49, 53, 54, 59, 61, 62, 65, 67]. The first report of insufficiency fractures in patients receiving bisphosphonate therapy was by Odvina and colleagues in 2005 [46]. They discussed nine patients who had sustained spontaneous nontraumatic fractures while receiving alendronate therapy longer than 3 years. Their patients had delayed healing and histomorphometric evidence of severely suppressed bone turnover. The bone surface was devoid of cellular elements, the bone formation rate was reduced, and matrix formation was severely impaired. The authors concluded severe suppression of bone turnover might be a complication from long-term bisphosphonate therapy. Since the initial observations by Odvina et al., there have been numerous published reports of low-energy femoral insufficiency fractures in patients receiving long-term bisphosphonate therapy [1, 16, 23, 26, 30, 33, 35, 36, 44, 45, 48, 57, 58, 68]. All describe the characteristic fracture pattern and all attribute the radiographic findings to severe suppression of bone turnover from bisphosphonates. However, the rarity of these fractures is evidenced by the fact that the largest series published before now had 19 patients [45]. We performed the current study to further investigate the association between bisphosphonates and femoral insufficiency fractures (Table 4), to examine whether these types of fractures were more common after the availability of bisphosphonates, and also to determine whether they were more common in patients taking bisphosphonates.
Table 4.
Reported clinical characteristics of patients with femoral insufficiency fractures
| Study | Femoral insufficiency fractures | Bilateral insufficiency fractures | Prodrome of pain | Mean duration of bisphosphonate use (years) | Mean age (years) | Insufficiency fractures identified with no history of bisphosphonate use |
|---|---|---|---|---|---|---|
| Odvina et al., 2005 [46] | 5 | 2 | – | 5.6 (3–8) | 60.0 (49–68) | – |
| Goh et al., 2007 [26] | 6 | 3 | 5 | 4.2 (2.5–5) | 66.9 (55–82) | 0 |
| Kwek et al., 2008 [33] | 17 | 9 | 13 | 4.4 (2–10) | 66.1 (53–82) | 0 |
| Lenart et al., 2008 [35] | 10 | 2 | – | 7.3 (5.5–9) | 70.4 (55–83) | 3 |
| Neviaser et al., 2008 [45] | 19 | – | – | 6.9 | 69.5 | 1 |
| Sayed-Noor and Sjoden, 2008 [56] | 1 | 1 | 1 | 7.0 | 72.0 | – |
| Capeci and Tejwani, 2009 [16] | 7 | 7 | 4 | 8.6 (5–13) | 61.0 (53–75) | – |
| Sayed-Noor and Sjoden, 2009 [57] | 2 | 1 | 2 | 9.0 | 66.5 | – |
| Schneider, 2009 [59] | 3 | – | 2 | 7.3 (6–9) | 63.3 (59–66) | – |
| Aspenberg, 2008 [6] | 1 | 1 | 1 | 9.0 | 57.0 | – |
| Edwards et al., 2010 [23] | 1 | 1 | 1 | 6.0 | 60.0 | – |
| Koh et al., 2010 [30] | 16 | 0 | 7 | 4.5 (2–7) | 68.0 (53–92) | – |
| Current study | 41 | 18 | 29 | 7.1 (4–11) | 73.7 (67–85) | 0 |
* The exact number in the literature is unknown owing to multiple publications from the same patient populations; numbers in parentheses denote ranges; – = data not reported.
Our study is limited by numerous factors. First, we may not have identified all cases of low-energy fractures, as the medical records could have incompletely, inaccurately, or ambiguously recorded the mechanism of injury. However, we suspect these would be a relatively small number as we examined the records of all patients with subtrochanteric and midshaft femoral fractures. Second, we may have underestimated the incidence of insufficiency fractures during the period before 1997 (before bisphosphonates were in mainstream use) because many of the radiographs were unavailable for review. Third, we defined an insufficiency fracture as one with a transverse fracture line on the lateral (tension) side of the femur and lateral cortical thickening adjacent to the fracture site. We believe this is an appropriate definition, but realize others might suggest a different definition.
Our data suggest a strong association between femoral insufficiency fractures and bisphosphonate therapy. These findings confirm those of other studies [1, 16, 23, 26, 30, 33, 35, 36, 44–46, 48, 57, 58, 68]. Although this does not establish cause and effect, it emphasizes the need for additional research into the causality and pathophysiology of these fractures. For example, identifying which people are more susceptible to insufficiency fractures would improve the safety and efficacy of bisphosphonates.
Our data also show that insufficiency fractures are associated with long-term bisphosphonate use, rather than bisphosphonate use per se. This supports the argument that longer-term bisphosphonate use results in prolonged impairment of bone remodeling, accumulation of microfractures, and weakening of bone [2, 4, 5, 10, 12–14, 17, 18, 20, 25, 27, 31, 32, 37, 41, 42, 46, 49, 53, 54, 59, 61, 62, 65, 67]. Again, more research is needed before definitive conclusions can be made.
We identified no patients with femoral insufficiency fractures during the period before bisphosphonates becoming available. This suggests that these fractures may have appeared only since the introduction of bisphosphonates, possibly being a direct result of them. However, as noted, we may have overlooked the presence of insufficiency fractures in this earlier period, because many of the radiographs were unavailable for review.
It could be argued that patients presenting with femoral insufficiency fractures originally were taking bisphosphonates because they were at greater risk of having osteoporotic fractures. However, one would expect the proportions of postmenopausal women taking bisphosphonates would have similar rates of femoral neck, intertrochanteric, and subtrochanteric fracture presentations, because all occur in similar populations through similar mechanisms of injury. In our study, however, 100% of patients who presented with femoral insufficiency fractures were taking bisphosphonates compared with only 13% of all other patients who sustained low-energy proximal femoral fractures. Similar findings have been reported elsewhere [27, 52] and highlight the overrepresentation of bisphosphonate use among those with femoral insufficiency fractures.
The high proportion of bilateral insufficiency fractures in our study has been observed by other authors [16, 26, 33, 35, 56, 57, 68] and provides more support for the argument that these changes have a systemic cause such as the oversuppression of bone turnover. Some authors have commented that suppression of bone turnover is more marked in patients receiving concurrent glucocorticoid and estrogen therapy and this may be why bilateral changes are seen in some people [46, 58]. However, we were not able to ascertain this from our study.
We do not interpret these findings to suggest alendronate is dangerous. Bisphosphonates are cost-effective treatment for management of osteoporosis and they reduce morbidity, disability, and mortality associated with fractures [1–13]. However, our observations suggest care must be taken when prescribing bisphosphonates for prolonged periods and doctors should be alerted by symptoms of thigh pain in patients taking them, because this may be a symptom of insufficiency changes in the femoral shaft. It is unclear if early detection reduces the risk of fracture progression. However, cortical thickenings have a higher risk of progressing to complete stress fractures [30].
The management of bisphosphonate-induced femoral insufficiency fractures has yet to be well-established. Until we have evidence-based recommendations, it would be reasonable to cease prescribing bisphosphonate, but continue calcium and vitamin D supplementation and follow up with dual energy xray absorptiometry and bone resorption marker measurements. Furthermore, treatment with teriparatide (recombinant human parathyroid hormone) may be beneficial by reducing the accumulation of microdamage in patients previously treated with bisphosphonates [22, 29, 60].
The unique subtrochanteric insufficiency fracture pattern that we have observed almost exclusively in bisphosphonate users is likely to represent a new type of pathologic fracture. As a result of the numbers of these patients who sustain spontaneous nontraumatic fractures during activities of daily living, it is the practice at our institution to prophylactically internally fix the fractures as soon as they are identified. Because the effects of bisphosphonates last well beyond their cessation date, we do not believe stopping the drug alone will be sufficient to prevent the progression to complete fracture.
Long-term bisphosphonate use is associated with insufficiency fractures of the femoral shaft, which commonly present with prodromal thigh pain and may be bilateral. Consideration should be given to stopping bisphosphonates after 5 years of continuous use. At the very least, patients taking bisphosphonates should be radiographically monitored and routinely questioned regarding prodromal pain.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research, and that informed consent was obtained.
References
- 1.Ahn JK, Lee J, Cha HS, Koh EM. Non-traumatic fracture of the femoral shaft in a patient taking long-term bisphosphonate therapy. Rheumatol Int. 2010 Apr 10. [Epub ahead of print]. [DOI] [PubMed]
- 2.Akkus O, Polyakova-Akkus A, Adar F, Schaffler MB. Aging of microstructural compartments in human compact bone. J Bone Miner Res. 2003;18:1012–1019. doi: 10.1359/jbmr.2003.18.6.1012. [DOI] [PubMed] [Google Scholar]
- 3.Allen MR. Skeletal accumulation of bisphosphonates: implications for osteoporosis treatment. Expert Opin Drug Metab Toxicol. 2008;4:1371–1378. doi: 10.1517/17425255.4.11.1371. [DOI] [PubMed] [Google Scholar]
- 4.Allen MR, Burr DB. Three years of alendronate treatment results in similar levels of vertebral microdamage as after one year of treatment. J Bone Miner Res. 2007;22:1759–1765. doi: 10.1359/jbmr.070720. [DOI] [PubMed] [Google Scholar]
- 5.Allen MR, Iwata K, Phipps R, Burr DB. Alterations in canine vertebral bone turnover, microdamage accumulation, and biomechanical properties following 1-year treatment with clinical treatment doses of risedronate or alendronate. Bone. 2006;39:872–879. doi: 10.1016/j.bone.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 6.Aspenberg P. Bisphosphonate-induced fractures: nature strikes back? Acta Orthop. 2008;79:459–460. doi: 10.1080/17453670710015427. [DOI] [PubMed] [Google Scholar]
- 7.Bauer DC, Black D, Ensrud K, Thompson D, Hochberg M, Nevitt M, Musliner T, Freedholm D. Upper gastrointestinal tract safety profile of alendronate: the fracture intervention trial. Arch Intern Med. 2000;160:517–525. doi: 10.1001/archinte.160.4.517. [DOI] [PubMed] [Google Scholar]
- 8.Black DM, Cummings SR, Karpf DB, Cauley JA, Thompson DE, Nevitt MC, Bauer DC, Genant HK, Haskell WL, Marcus R, Ott SM, Torner JC, Quandt SA, Reiss TF, Ensrud KE. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures Fracture Intervention Trial Research Group. Lancet. 1996;348:1535–1541. doi: 10.1016/S0140-6736(96)07088-2. [DOI] [PubMed] [Google Scholar]
- 9.Black DM, Reiss TF, Nevitt MC, Cauley J, Karpf D, Cummings SR. Design of the Fracture Intervention Trial. Osteoporos Int. 1993;3(suppl 3):S29–39. doi: 10.1007/BF01623005. [DOI] [PubMed] [Google Scholar]
- 10.Black DM, Schwartz AV, Ensrud KE, Cauley JA, Levis S, Quandt SA, Satterfield S, Wallace RB, Bauer DC, Palermo L, Wehren LE, Lombardi A, Santora AC. Cummings SR; FLEX Research Group. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA. 2006;296:2927–2938. doi: 10.1001/jama.296.24.2927. [DOI] [PubMed] [Google Scholar]
- 11.Black DM, Thompson DE, Bauer DC, Ensrud K, Musliner T, Hochberg MC, Nevitt MC, Suryawanshi S. Cummings SR; Fracture Intervention Trial Fracture risk reduction with alendronate in women with osteoporosis: the Fracture Intervention Trial. FIT Research Group. J Clin Endocrinol Metab. 2000;85:4118–4124. doi: 10.1210/jc.85.11.4118. [DOI] [PubMed] [Google Scholar]
- 12.Boivin G, Farlay D, Bala Y, Doublier A, Meunier PJ, Delmas PD. Influence of remodeling on the mineralization of bone tissue. Osteoporos Int. 2009;20:1023–1026. doi: 10.1007/s00198-009-0861-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boivin G, Meunier PJ. Changes in bone remodeling rate influence the degree of mineralization of bone. Connect Tissue Res. 2002;43:535–537. doi: 10.1080/03008200290000934. [DOI] [PubMed] [Google Scholar]
- 14.Boivin GY, Chavassieux PM, Santora AC, Yates J, Meunier PJ. Alendronate increases bone strength by increasing the mean degree of mineralization of bone tissue in osteoporotic women. Bone. 2000;27:687–694. doi: 10.1016/S8756-3282(00)00376-8. [DOI] [PubMed] [Google Scholar]
- 15.Bone HG, Hosking D, Devogelaer JP, Tucci JR, Emkey RD, Tonino RP, Rodriguez-Portales JA, Downs RW, Gupta J, Santora AC, Liberman UA, Alendronate Phase III Osteoporosis Treatment Study Group Ten years’ experience with alendronate for osteoporosis in postmenopausal women. N Engl J Med. 2004;350:1189–1199. doi: 10.1056/NEJMoa030897. [DOI] [PubMed] [Google Scholar]
- 16.Capeci CM, Tejwani NC. Bilateral low-energy simultaneous or sequential femoral fractures in patients on long-term alendronate therapy. J Bone Joint Surg Am. 2009;91:2556–2561. doi: 10.2106/JBJS.H.01774. [DOI] [PubMed] [Google Scholar]
- 17.Chavassieux P, Seeman E, Delmas PD. Insights into material and structural basis of bone fragility from diseases associated with fractures: how determinants of the biomechanical properties of bone are compromised by disease. Endocr Rev. 2007;28:151–164. doi: 10.1210/er.2006-0029. [DOI] [PubMed] [Google Scholar]
- 18.Ciarelli TE, Fyhrie DP, Parfitt AM. Effects of vertebral bone fragility and bone formation rate on the mineralization levels of cancellous bone from white females. Bone. 2003;32:311–315. doi: 10.1016/S8756-3282(02)00975-4. [DOI] [PubMed] [Google Scholar]
- 19.Cummings SR, Black DM, Thompson DE, Applegate WB, Barrett-Connor E, Musliner TA, Palermo L, Prineas R, Rubin SM, Scott JC, Vogt T, Wallace R, Yates AJ, LaCroix AZ. Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial. JAMA. 1998;280:2077–2082. doi: 10.1001/jama.280.24.2077. [DOI] [PubMed] [Google Scholar]
- 20.Currey JD. Effects of differences in mineralization on the mechanical properties of bone Philos Trans R Soc. Lond B Biol Sci. 1984;304:509–518. doi: 10.1098/rstb.1984.0042. [DOI] [PubMed] [Google Scholar]
- 21.Curtis JR, Westfall AO, Cheng H, Delzell E, Saag KG. Risk of hip fracture after bisphosphonate discontinuation: implications for a drug holiday. Osteoporos Int. 2008;19:1613–1620. doi: 10.1007/s00198-008-0604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dobnig H, Stepan JJ, Burr DB, Li J, Michalska D, Sipos A, Petto H, Fahrleitner-Pammer A, Pavo I. Teriparatide reduces bone microdamage accumulation in postmenopausal women previously treated with alendronate. J Bone Miner Res. 2009;24:1998–2006. doi: 10.1359/jbmr.090527. [DOI] [PubMed] [Google Scholar]
- 23.Edwards MH, McCrae FC, Young-Min SA. Alendronate-related femoral diaphysis fracture: what should be done to predict and prevent subsequent fracture of the contralateral side? Osteoporos Int. 2010;21:701–703. doi: 10.1007/s00198-009-0986-y. [DOI] [PubMed] [Google Scholar]
- 24.Ensrud KE, Barrett-Connor EL, Schwartz A, Santora AC, Bauer DC, Suryawanshi S, Feldstein A, Haskell WL, Hochberg MC, Torner JC, Lombardi A, Black DM, Fracture Intervention Trial Long-Term Extension Research Group Randomized trial of effect of alendronate continuation versus discontinuation in women with low BMD: results from the Fracture Intervention Trial long-term extension. J Bone Miner Res. 2004;19:1259–1269. doi: 10.1359/JBMR.040326. [DOI] [PubMed] [Google Scholar]
- 25.Garnero P, Shih WJ, Gineyts E, Karpf DB, Delmas PD. Comparison of new biochemical markers of bone turnover in late postmenopausal osteoporotic women in response to alendronate treatment. J Clin Endocrinol Metab. 1994;79:1693–1700. doi: 10.1210/jc.79.6.1693. [DOI] [PubMed] [Google Scholar]
- 26.Goh SK, Yang KY, Koh JS, Wong MK, Chua SY, Chua DT, Howe TS. Subtrochanteric insufficiency fractures in patients on alendronate therapy: a caution. J Bone Joint Surg Br. 2007;89:349–353. doi: 10.1302/0301-620X.89B3.18146. [DOI] [PubMed] [Google Scholar]
- 27.Hirano T, Turner CH, Forwood MR, Johnston CC, Burr DB. Does suppression of bone turnover impair mechanical properties by allowing microdamage accumulation? Bone. 2000;27:13–20. doi: 10.1016/S8756-3282(00)00284-2. [DOI] [PubMed] [Google Scholar]
- 28.Iwamoto J, Takeda T, Sato Y. Efficacy and safety of alendronate and risedronate for postmenopausal osteoporosis. Curr Med Res Opin. 2006;22:919–928. doi: 10.1185/030079906X100276. [DOI] [PubMed] [Google Scholar]
- 29.Jobke B, Pfeifer M, Minne HW. Teriparatide following bisphosphonates: initial and long-term effects on microarchitecture and bone remodeling at the human iliac crest. Connect Tissue Res. 2009;50:46–54. doi: 10.1080/03008200802412462. [DOI] [PubMed] [Google Scholar]
- 30.Koh JS, Goh SK, Png MA, Kwek EB, Howe TS. Femoral cortical stress lesions in long-term bisphosphonate therapy: a herald of impending fracture? J Orthop Trauma. 2010;24:75–81. doi: 10.1097/BOT.0b013e3181b6499b. [DOI] [PubMed] [Google Scholar]
- 31.Komatsubara S, Mori S, Mashiba T, Ito M, Li J, Kaji Y, Akiyama T, Miyamoto K, Cao Y, Kawanishi J, Norimatsu H. Long-term treatment of incadronate disodium accumulates microdamage but improves the trabecular bone microarchitecture in dog vertebra. J Bone Miner Res. 2003;18:512–520. doi: 10.1359/jbmr.2003.18.3.512. [DOI] [PubMed] [Google Scholar]
- 32.Komatsubara S, Mori S, Mashiba T, Li J, Nonaka K, Kaji Y, Akiyama T, Miyamoto K, Cao Y, Kawanishi J, Norimatsu H. Suppressed bone turnover by long-term bisphosphonate treatment accumulates microdamage but maintains intrinsic material properties in cortical bone of dog rib. J Bone Miner Res. 2004;19:999–1005. doi: 10.1359/JBMR.040126. [DOI] [PubMed] [Google Scholar]
- 33.Kwek EB, Goh SK, Koh JS, Png MA, Howe TS. An emerging pattern of subtrochanteric stress fractures: a long-term complication of alendronate therapy? Injury. 2008;39:224–231. doi: 10.1016/j.injury.2007.08.036. [DOI] [PubMed] [Google Scholar]
- 34.Lanza F, Sahba B, Schwartz H, Winograd S, Torosis J, Quan H, Reyes R, Musliner T, Daifotis A, Leung A. The upper GI safety and tolerability of oral alendronate at a dose of 70 milligrams once weekly: a placebo-controlled endoscopy study. Am J Gastroenterol. 2002;97:58–64. doi: 10.1111/j.1572-0241.2002.05446.x. [DOI] [PubMed] [Google Scholar]
- 35.Lenart BA, Lorich DG, Lane JM. Atypical fractures of the femoral diaphysis in postmenopausal women taking alendronate. N Engl J Med. 2008;358:1304–1306. doi: 10.1056/NEJMc0707493. [DOI] [PubMed] [Google Scholar]
- 36.Lenart BA, Neviaser AS, Lyman S, Chang CC, Edobor-Osula F, Steele B, Meulen MC, Lorich DG, Lane JM. Association of low-energy femoral fractures with prolonged bisphosphonate use: a case control study. Osteoporos Int. 2009;20:1353–1362. doi: 10.1007/s00198-008-0805-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li J, Mashiba T, Burr DB. Bisphosphonate treatment suppresses not only stochastic remodeling but also the targeted repair of microdamage. Calcif Tissue Int. 2001;69:281–286. doi: 10.1007/s002230010036. [DOI] [PubMed] [Google Scholar]
- 38.Liberman UA, Weiss SR, Broll J, Minne HW, Quan H, Bell NH, Rodriguez-Portales J, Downs RW, Jr, Dequeker J, Favus M. Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis The Alendronate Phase III Osteoporosis Treatment Study Group. N Engl J Med. 1995;333:1437–1443. doi: 10.1056/NEJM199511303332201. [DOI] [PubMed] [Google Scholar]
- 39.Luckman SP, Hughes DE, Coxon FP, Graham R, Russell G, Rogers MJ. Nitrogen-containing bisphosphonates inhibit the mevalonate pathway and prevent post-translational prenylation of GTP-binding proteins, including Ras. J Bone Miner Res. 1998;13:581–589. doi: 10.1359/jbmr.1998.13.4.581. [DOI] [PubMed] [Google Scholar]
- 40.Lufkin EG, Wahner HW, O’Fallon WM, Hodgson SF, Kotowicz MA, Lane AW, Judd HL, Caplan RH, Riggs BL. Treatment of postmenopausal osteoporosis with transdermal estrogen. Ann Intern Med. 1992;117:1–9. doi: 10.7326/0003-4819-117-1-1. [DOI] [PubMed] [Google Scholar]
- 41.Mashiba T, Hirano T, Turner CH, Forwood MR, Johnston CC, Burr DB. Suppressed bone turnover by bisphosphonates increases microdamage accumulation and reduces some biomechanical properties in dog rib. J Bone Miner Res. 2000;15:613–620. doi: 10.1359/jbmr.2000.15.4.613. [DOI] [PubMed] [Google Scholar]
- 42.Mashiba T, Turner CH, Hirano T, Forwood MR, Johnston CC, Burr DB. Effects of suppressed bone turnover by bisphosphonates on microdamage accumulation and biomechanical properties in clinically relevant skeletal sites in beagles. Bone. 2001;28:524–531. doi: 10.1016/S8756-3282(01)00414-8. [DOI] [PubMed] [Google Scholar]
- 43.McClung MR, Geusens P, Miller PD, Zippel H, Bensen WG, Roux C, Adami S, Fogelman I, Diamond T, Eastell R, Meunier PJ, Reginster JY, Hip Intervention Program Study Group Effect of risedronate on the risk of hip fracture in elderly women. Hip Intervention Program Study Group. N Engl J Med. 2001;344:333–340. doi: 10.1056/NEJM200102013440503. [DOI] [PubMed] [Google Scholar]
- 44.Napoli N, Novack D, Armamento-Villareal R. Bisphosphonate-associated femoral fracture: implications for management in patients with malignancies. Osteoporos Int. 2010;21:705–708. doi: 10.1007/s00198-009-1012-0. [DOI] [PubMed] [Google Scholar]
- 45.Neviaser AS, Lane JM, Lenart BA, Edobor-Osula F, Lorich DG. Low-energy femoral shaft fractures associated with alendronate use. J Orthop Trauma. 2008;22:346–350. doi: 10.1097/BOT.0b013e318172841c. [DOI] [PubMed] [Google Scholar]
- 46.Odvina CV, Zerwekh JE, Rao DS, Maalouf N, Gottschalk FA, Pak CY. Severely suppressed bone turnover: a potential complication of alendronate therapy. J Clin Endocrinol Metab. 2005;90:1294–1301. doi: 10.1210/jc.2004-0952. [DOI] [PubMed] [Google Scholar]
- 47.Orwoll E, Ettinger M, Weiss S, Miller P, Kendler D, Graham J, Adami S, Weber K, Lorenc R, Pietschmann P, Vandormael K, Lombardi A. Alendronate for the treatment of osteoporosis in men. N Engl J Med. 2000;343:604–610. doi: 10.1056/NEJM200008313430902. [DOI] [PubMed] [Google Scholar]
- 48.Ott SM. Atraumatic bilateral femur fracture in long-term bisphosphonate use. Orthopedics. 2020;33:468. doi: 10.3928/01477447-20100526-10. [DOI] [PubMed] [Google Scholar]
- 49.Ott SM. Long-term safety of bisphosphonates. J Clin Endocrinol Metab. 2005;90:1897–1899. doi: 10.1210/jc.2005-0057. [DOI] [PubMed] [Google Scholar]
- 50.Pak CY, Sakhaee K, Adams-Huet B, Piziak V, Peterson RD, Poindexter JR. Treatment of postmenopausal osteoporosis with slow-release sodium fluoride: final report of a randomized controlled trial. Ann Intern Med. 1995;123:401–408. doi: 10.7326/0003-4819-123-6-199509150-00001. [DOI] [PubMed] [Google Scholar]
- 51.Papapoulos SE, Quandt SA, Liberman UA, Hochberg MC, Thompson DE. Meta-analysis of the efficacy of alendronate for the prevention of hip fractures in postmenopausal women. Osteoporos Int. 2005;16:468–474. doi: 10.1007/s00198-004-1725-z. [DOI] [PubMed] [Google Scholar]
- 52.Pols HA, Felsenberg D, Hanley DA, Stepan J, Munoz-Torres M, Wilkin TJ, Qin-sheng G, Galich AM, Vandormael K, Yates AJ, Stych B. Multinational, placebo-controlled, randomized trial of the effects of alendronate on bone density and fracture risk in postmenopausal women with low bone mass: results of the FOSIT study. Fosamax International Trial Study Group. Osteoporos Int. 1999;9:461–468. doi: 10.1007/PL00004171. [DOI] [PubMed] [Google Scholar]
- 53.Roschger P, Rinnerthaler S, Yates J, Rodan GA, Fratzl P, Klaushofer K. Alendronate increases degree and uniformity of mineralization in cancellous bone and decreases the porosity in cortical bone of osteoporotic women. Bone. 2001;29:185–191. doi: 10.1016/S8756-3282(01)00485-9. [DOI] [PubMed] [Google Scholar]
- 54.Rossini M, Gatti D, Zamberlan N, Braga V, Dorizzi R, Adami S. Long-term effects of a treatment course with oral alendronate of postmenopausal osteoporosis. J Bone Miner Res. 1994;9:1833–1837. doi: 10.1002/jbmr.5650091121. [DOI] [PubMed] [Google Scholar]
- 55.Saito M, Marumo K. Collagen cross-links as a determinant of bone quality: a possible explanation for bone fragility in aging, osteoporosis, and diabetes mellitus. Osteoporos Int. 2010;21:195–214. doi: 10.1007/s00198-009-1066-z. [DOI] [PubMed] [Google Scholar]
- 56.Sayed-Noor AS, Sjoden GO. Subtrochanteric displaced insufficiency fracture after long-term alendronate therapy: a case report. Acta Orthop. 2008;79:565–567. doi: 10.1080/17453670710015580. [DOI] [PubMed] [Google Scholar]
- 57.Sayed-Noor AS, Sjoden GO. Case reports: two femoral insufficiency fractures after long-term alendronate therapy. Clin Orthop Relat Res. 2009;467:1921–1926. doi: 10.1007/s11999-009-0725-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schneider JP. Should bisphosphonates be continued indefinitely? An unusual fracture in a healthy woman on long-term alendronate. Geriatrics. 2006;61:31–33. [PubMed] [Google Scholar]
- 59.Schneider JP. Bisphosphonates and low-impact femoral fractures: current evidence on alendronate-fracture risk. Geriatrics. 2009;64:18–23. [PubMed] [Google Scholar]
- 60.Sloan AV, Martin JR, Li S, Li J. Parathyroid hormone and bisphosphonate have opposite effects on stress fracture repair. Bone. 2020;47:235–240. doi: 10.1016/j.bone.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 61.Stock JL, Bell NH, Chesnut CH, 3rd, Ensrud KE, Genant HK, Harris ST, McClung MR, Singer FR, Yood RA, Pryor-Tillotson S, Wei L, Santora AC., II Increments in bone mineral density of the lumbar spine and hip and suppression of bone turnover are maintained after discontinuation of alendronate in postmenopausal women. Am J Med. 1997;103:291–297. doi: 10.1016/S0002-9343(97)00130-7. [DOI] [PubMed] [Google Scholar]
- 62.Tang SY, Allen MR, Phipps R, Burr DB, Vashishth D. Changes in non-enzymatic glycation and its association with altered mechanical properties following 1-year treatment with risedronate or alendronate. Osteoporos Int. 2009;20:887–894. doi: 10.1007/s00198-008-0754-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tonino RP, Meunier PJ, Emkey R, Rodriguez-Portales JA, Menkes CJ, Wasnich RD, Bone HG, Santora AC, Wu M, Desai R, Ross PD. Skeletal benefits of alendronate: 7-year treatment of postmenopausal osteoporotic women Phase III Osteoporosis Treatment Study Group. J Clin Endocrinol Metab. 2000;85:3109–3115. doi: 10.1210/jc.85.9.3109. [DOI] [PubMed] [Google Scholar]
- 64.Unal E, Abaci A, Bober E, Buyukgebiz A. Efficacy and safety of oral alendronate treatment in children and adolescents with osteoporosis. J Pediatr Endocrinol Metab. 2006;19:523–528. [PubMed] [Google Scholar]
- 65.Visekruna M, Wilson D, McKiernan FE. Severely suppressed bone turnover and atypical skeletal fragility. J Clin Endocrinol Metab. 2008;93:2948–2952. doi: 10.1210/jc.2007-2803. [DOI] [PubMed] [Google Scholar]
- 66.Watts NB, Harris ST, Genant HK, Wasnich RD, Miller PD, Jackson RD, Licata AA, Ross P, Woodson GC, 3rd, Yanover MJ, et al. Intermittent cyclical etidronate treatment of postmenopausal osteoporosis. N Engl J Med. 1990;323:73–79. doi: 10.1056/NEJM199007123230201. [DOI] [PubMed] [Google Scholar]
- 67.Whyte MP, Wenkert D, Clements KL, McAlister WH, Mumm S. Bisphosphonate-induced osteopetrosis. N Engl J Med. 2003;349:457–463. doi: 10.1056/NEJMoa023110. [DOI] [PubMed] [Google Scholar]
- 68.Yoon RS, Beebe KS, Benevenia J. Prophylactic bilateral intramedullary femoral nails for bisphosphonate-associated signs of impending subtrochanteric hip fracture. Orthopedics. 2010;16:267–270. doi: 10.3928/01477447-20100225-21. [DOI] [PubMed] [Google Scholar]