Abstract
Background
Prolonged use of bisphosphonates in patients with osteoporosis reportedly induces femoral insufficiency fractures. However, the natural course of these fractures and how to treat them remain unknown.
Questions/purposes
We determined the rates of fracture displacement and subsequent operations of undisplaced insufficiency fractures of the femur in patients treated with prolonged bisphosphonate therapy.
Patients and Methods
We retrospectively collected and reviewed the clinical course of 11 patients (14 fractures) who had been diagnosed as having an insufficiency fracture of the femur after prolonged use (mean, 4.5 years; range, 3–10 years) of bisphosphonate. All patients were women with a mean age of 68 years (range, 57–82 years). The fracture site was subtrochanteric in six and femoral shaft in eight. The minimum followup was 12 months (mean, 27 months; range, 12–60 months).
Results
During the followup period, secondary displacement of the fracture occurred in five of the 14 fractures after a mean of 10 months (range, 1–19 months). Three fractures were treated with internal fixation using a compression hip screw and two with intramedullary nailing. Because five additional fractures were treated surgically owing to intractable pain, surgery was performed in 10 of 14 insufficiency fractures during the followup period. All 10 fractures healed during followup. The remaining four patients (four fractures) not undergoing any surgery had persistent pain.
Conclusions
Femoral insufficiency fractures after prolonged bisphosphonate therapy seldom healed spontaneously and most patients had surgery either for fracture displacement or persistent pain.
Level of Evidence
Level IV, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Bisphosphonates have been widely used to treat osteoporosis and reportedly reduce the risk of vertebral and nonvertebral fractures [4, 5, 7]. However, long-term use of bisphosphonates carries a potential risk of oversuppression of bone turnover and can paradoxically limit reserve capacity to repair microdamage in bones. Microdamage accumulation in bone is associated with reduction in bone toughness and increase of brittleness [1, 13, 14, 16, 17, 22].
In 2005, Odvina et al. reported nine patients who sustained spontaneous nonvertebral fractures while receiving prolonged alendronate therapy. They emphasized the need for increased awareness and monitoring for the potential development of excessive suppression of bone turnover during long-term alendronate therapy [20]. Several additional reports regarding insufficiency femoral fractures in patients with long-term medication of bisphosphonates have been published [9, 11, 15, 19]. However, two of these reports focused on the clinical manifestations, including prodromal pain in the affected limb several months before the occurrence of insufficiency fractures, and radiographic findings, including cortical hypertrophy, unicortical beak, thickening and beaking of the cortex on one side, and a thin transverse fracture line [9, 15]. None of these studies reported the likelihood of healing of insufficiency fractures of the femur or whether the patients became pain-free.
We therefore asked four questions: (1) How frequently do undisplaced insufficiency fractures of the femur become displaced? (2) How many of these fractures subsequently require surgery? (3) What is the union rate of internal fixation of these fractures? (4) Do these fractures heal spontaneously and does the pain disappear if left unoperated?
Patients and Methods
We retrospectively reviewed medical records to identify patients with insufficiency fractures of the femur that were related to prolonged use of bisphosphonates to treat osteoporosis or osteopenia. Patients who had no history of bisphosphonate treatment before the diagnosis of fracture were excluded. From January 2002 to December 2008, 12 patients were diagnosed as having insufficiency fractures of the femur related to prolonged use of bisphosphonates. Of the 12 patients, eight had bilateral femoral insufficiency fractures.
Among the eight patients with bilateral femoral fractures, four had complete displacement of the fracture at the time of fracture diagnosis of the unilateral femur and one had displacement of the bilateral femurs. These six fractures with complete displacement at the time of diagnosis of the insufficiency fracture were treated surgically immediately after the diagnosis and were excluded from the study. The remaining 11 patients with 14 fractures without displacement were included in the final analysis.
We considered a fracture as an insufficiency fracture if it was a simple, transverse fracture in areas of thickened cortices with a unicortical beak [9, 18] or in a common location for insufficiency fractures (fractures below the lesser trochanter and above the distal 1/3 of the diaphysis) [3, 9]. A subtrochanteric insufficiency fracture was defined as one in the region of the femur that extended from the lesser trochanter to the proximal 1/3 of the diaphysis. A femoral shaft fracture was defined as one below the proximal 1/3 and above the distal 1/3 of the diaphysis [3, 9]. Low-energy trauma was defined as an injury caused by the equivalent to a fall from a standing height or less [6, 15, 18]. All 11 patients were women who had little or no trauma; their mean age was 68 years (range, 57–82 years). Although all patients had mild thigh pain on initial presentation, none received any form of nonsurgical treatment with the exception of analgesics. Of the 11 patients, three had bilateral involvement and therefore we evaluated 14 fractures. Six fractures were located in the subtrochanteric area and eight were located in the femoral shaft. All patients had used or were using bisphosphonates before the diagnosis of insufficiency fracture for a mean of 4.5 years (range, 3–10 years). Nine patients (11 fractures) took alendronate for a mean of 4.6 years (range, 3–10 years). Two patients (three fractures) took pamidronate for a mean of 4.3 years (range, 3–5 years). None of patients had glucocorticoid-induced osteoporosis. Two patients had underlying diseases other than osteoporosis; one patient (Patient 1) underwent a thyroidectomy owing to cancer 12 years previously and one patient (Patient 7) underwent rectal cancer surgery 15 years previously. The design and protocol of this study were approved by the Institutional Review Board at our hospital, and all patients provided informed consent for participation in the study.
Bone mineral density (BMD) was measured within 1 week after the diagnosis of femoral insufficiency fracture by dual-energy xray absorptiometry. Five patients had a BMD consistent with osteoporosis (greater than 2.5 SDs below that of the young adult peak BMD) and six patients had a BMD consistent with osteopenia (between 1.0 SD and 2.5 SDs less than the young adult peak BMD) [12]. The bisphosphonate was discontinued after the diagnosis of the insufficiency fracture, except for four (Patients 3, 6, 9, 10) patients (Table 1).
Table 1.
Data for 11 patients with insufficiency fractures of the femur
| Patient number | Age (years) | Side | Fracture location | Bisphosphonate | Duration of bisphosphonate treatment (years) | DXA | Fracture displacement | Interval* (months) | Surgical treatment | Followup (months) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 63 | Left | Subtrochanter | Alendronate | 4 | −1.7 | No | Not done | 18 | |
| 2 | 64 | Left | Subtrochanter | Alendronate | 5 | −1.6 | No | IM nailing | 24 | |
| 3 | 75 | Left | Midshaft | Pamidronate | 5 | −2.9 | No | Plate fixation | 12 | |
| 75 | Right | Midshaft | Yes | 12 | IM nailing | 12 | ||||
| 4 | 67 | Left | Subtrochanter | Alendronate | 10 | −1.5 | Yes | 1 | CHS fixation | 12 |
| 5 | 78 | Left | Midshaft | Pamidronate | 3 | −2.2 | No | Plate fixation | 18 | |
| 6 | 65 | Left | Subtrochanter | Alendronate | 4 | −2.7 | No | IM nailing | 48 | |
| 65 | Right | Subtrochanter | Yes | 19 | CHS fixation | 48 | ||||
| 7 | 60 | Left | Subtrochanter | Alendronate | 4 | −1.7 | No | IM nailing | 36 | |
| 60 | Right | Subtrochanter | Yes | 3 | CHS fixation | 36 | ||||
| 8 | 57 | Right | Midshaft | Alendronate | 3 | −1.9 | No | Not done | 24 | |
| 9 | 68 | Right | Midshaft | Alendronate | 5 | −3.8 | No | Not done | 36 | |
| 10 | 82 | Right | Midshaft | Alendronate | 4 | −4.8 | Yes | 15 | IM nailing | 60 |
| 11 | 73 | Left | Midshaft | Alendronate | 4 | −2.9 | No | Not done | 12 |
*Time between the diagnosis of insufficiency fracture and fracture displacement; DXA = dual-energy xray absorptiometry (lowest T-score of total femur or lumbar area); IM = intramedullary; CHS = compression hip screw
Patients had been followed at 1- to 3-month intervals after diagnosis of the fracture. Two nurses and one private locator visited and obtained information regarding symptoms and walking ability from patients who had not returned for regularly scheduled visits.
Two patients (Patients 3 and 5), who underwent plate fixation of their fractures, did not return after obtaining bony union and pain relief. Their final followups were by telephone interview. Therefore, no patients were lost to followup before a minimum of 12 months from the time of fracture diagnosis. No patients were recalled specifically for this study; all data were obtained from the medical records or telephone interviews. The mean followup from the time of the fracture diagnosis was 27 months (range, 12–60 months).
Two of us (Y-CH, S-YK) determined bony union of the fracture by bridging of three of the four cortices on AP and lateral radiographs [8, 10].
Results
Five of the 14 fractures (three subtrochanteric and two midshaft) became displaced during the followup period. The mean time from the diagnosis of insufficiency fracture until displacement was 10 months (range, 1–19 months) (Table 1). All five displacements occurred after a low-energy injury such as slipping, tripping, or stumbling. Three fractures were treated with fixation using a compression hip screw and two fractures were treated with intramedullary nailing (Fig. 1). An additional five patients (five fractures) underwent internal fixation because of persistent intractable pain and/or progression of the fracture line observed at serial radiologic evaluations. After surgical treatment, their pain disappeared (Fig. 2). Collectively, during the followup of 12 to 60 months, 10 fractures (71%) were surgically treated.
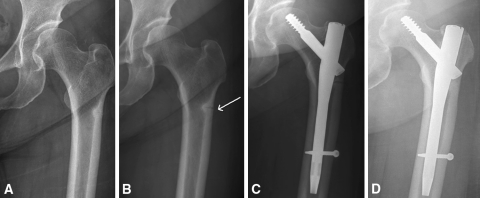
Fig. 1A–D.
Patient 4 was a 67-year-old woman who had pain in her right thigh. (A) Her radiograph shows a transverse fracture line and thickening of the lateral cortex at the subtrochanteric area. (B) Her bone scan shows hot uptake at the subtrochanteric area. (C) She experienced a slip after 1 month of followup. Her radiograph shows a completely displaced fracture. She underwent internal fixation using a compression hip screw. (D) Twelve months postoperatively, her radiograph shows complete union of the fracture.
Fig. 2A–D.
Patient 2 was a 64-year-old woman who had pain in her left thigh. (A) Her radiograph shows a transverse fracture line and thickening of the lateral cortex at the subtrochanteric area. (B) At 16 months followup, her radiograph shows progression of the fracture line at the subtrochanteric area. (C) She underwent internal fixation using an intramedullary nail as a result of intractable pain. (D) A radiograph obtained 6 months postoperatively shows complete union of the fracture.
There were no perioperative complications and all patients who underwent surgery achieved bony union during the followup period.
Pain at the fracture site disappeared a mean of 4 months (range, 3–6 months) after surgery in seven patients. All could walk without crutches after a mean of 5 months (range, 4–7 months). The remaining four patients refused surgery owing to fears about the surgery. These four patients continued to experience pain and no evidence of fracture healing was observed on their radiographs at last followup (Table 1).
Discussion
It has been suggested that long-term use of bisphosphonates can paradoxically induce femoral insufficiency fractures [8, 10, 15, 20, 21]. The potential risk is drawing attention as osteoporosis accounts for a substantial burden of disease worldwide and bisphosphonates are the primary agents for treatment of osteoporosis to prevent fractures. However, the natural history of femoral insufficiency fractures has been not determined. Thus, we performed the study to ask the unanswered questions: (1) how frequently these fractures become displaced; (2) how many of them necessitate operative treatment; (3) what the union rate is for internal fixation of these fractures; and (4) whether these fractures heal spontaneously and pain disappears if left unoperated.
We note several limitations to our study. First, our patients were not followed prospectively and two patients were interviewed by telephone; the risk for misinterpreting symptoms is associated with telephone interviews. Second, we had a small number of patients; we identified only 14 fractures at four teaching hospitals over 7 years. Bisphosphonate-induced femoral insufficiency fractures are not common and a large multicenter study would be necessary to more precisely characterize the fate of the atypical femoral fracture. The prevalence of femoral insufficiency fractures has been estimated to be less than one per 10,000 patient-years for patients taking bisphosphonates [2]. Third, the study subjects were selected retrospectively. Alendronate was approved by the Food and Drug Administration in the United States in 1995, but the association between long-term bisphosphonate therapy and bisphosphonate-induced insufficiency fractures was reported more recently [6, 9, 21]. Therefore, we might not have identified all patients with these fractures at our hospitals.
Our data suggest no patient had spontaneous healing of the insufficiency femur fractures or resolution of pain at the fracture site during the followup period. Furthermore, 36% of nondisplaced fractures progressed to fractures with secondary displacement. Consequently, 10 fractures (71%) underwent surgical treatment during the 12 to 60 months after the diagnosis of femoral insufficiency fracture. Moreover, with secondary displacement, all patients had complete fractures develop even with a low-energy injury, which actually is inevitable. The four patients who did not undergo surgery had persistent pain.
As some studies have suggested potential negative effects of bisphosphonate on the fracture healing process [9, 11, 14, 20, 21], the union rate is another important issue in our study. Odvina et al. described histomorphometric data for patients with spontaneous nonspinal fractures after alendronate treatment, showing a marked suppression of bone turnover [20]. Additionally, Sayed-Noor and Sjoden reported two patients with femoral insufficiency fractures after long-term alendronate therapy and one of these patients had delayed union even after internal fixation [21]. Visekruna et al. reported three patients with subtrochanteric insufficiency fractures, one of whom had no radiographic evidence of union at 22 months [23]. However, Capeci and Tejwani [6] reported that all femoral insufficiency fractures went on to union at an average of 4 months (range, 3–5 months) with reamed intramedullary nailing. Our findings were consistent with those of Capeci and Tejwani: all 10 fractures healed with bony union after internal fixation. This discrepancy may result from the preoperative states of bone turnover or the exposure to medications involving bone metabolism such as glucocorticoids and antiosteoporotic agents. Unfortunately, we did not measure serum or urine markers of bone metabolism. However, 10 of 14 patients in our series were not taking other medications suppressing bone turnover. Capeci and Tejwani were concerned about complete fracture through the stress site and recommended prophylactic fixation of the insufficiency fracture [6]. Our results also support prophylactic fixation of incomplete femoral insufficiency fractures, considering the high rate of persistent symptoms, progression to displaced fractures, and high union rate after internal fixation,
Many patients with femoral insufficiency fractures are likely to have osteoporosis. Among our 11 patients, five (46%) persistently had osteoporosis. Therefore, to choose antiosteoporotic agents is a critical issue after the development of insufficiency fractures. The use of a bone-forming agent such as a recombinant parathyroid hormone (PTH) can be a therapeutic option for underlying osteoporosis [13, 18]. The efficacy of a recombinant PTH has been established in patients with osteoporosis. Several studies have shown PTH may accelerate fracture healing and reduce rates of fracture nonunion [6, 7, 13]. However, it is not known whether PTH treatment is useful for patients with bisphosphonate-induced insufficiency femur fractures.
Our data suggest internal fixation should be considered as initial treatment for patients with insufficiency fractures of the femur, even for nondisplaced fractures.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at the Department of Orthopaedic Surgery, Chung-Ang University College of Medicine, Seoul, South Korea.
References
- 1.Allen MR, Iwata K, Phipps R, Burr DB. Alterations in canine vertebral bone turnover, microdamage accumulation, and biomechanical properties following 1-year treatment with clinical treatment doses of risedronate or alendronate. Bone. 2006;39:872–879. doi: 10.1016/j.bone.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 2.Black DM, Kelly MP, Genant HK, Palermo L, Eastell R, Bucci-Rechtweg C, Cauley J, Leung PC, Boonen S, Santora A, Papp A, Bauer DC. Fracture Intervention Trial Steering Committee; HORIZON Pivotal Fracture Trial Steering Committee. Bisphosphonates and fractures of the subtrochanteric or diaphyseal femur. N Engl J Med. 2010;362:1761–1771. doi: 10.1056/NEJMoa1001086. [DOI] [PubMed] [Google Scholar]
- 3.Boden BP, Osbahr DC, Jimenez C. Low-risk stress fractures. Am J Sports Med. 2001;29:100–111. doi: 10.1177/03635465010290010201. [DOI] [PubMed] [Google Scholar]
- 4.Boivin GY, Chavassieux PM, Santora AC, Yates J, Meunier PJ. Alendronate increases bone strength by increasing the mean degree of mineralization of bone tissue in osteoporotic women. Bone. 2000;27:687–694. doi: 10.1016/S8756-3282(00)00376-8. [DOI] [PubMed] [Google Scholar]
- 5.Bone HG, Hosking D, Devogelaer JP, Tucci JR, Emkey RD, Tonino RP, Rodriguez-Portales JA, Downs RW, Gupta J, Santora AC, Liberman UA. Alendronate Phase III Osteoporosis Treatment Study Group. Ten years’ experience with alendronate for osteoporosis in postmenopausal women. N Engl J Med. 2004;350:1189–1199. doi: 10.1056/NEJMoa030897. [DOI] [PubMed] [Google Scholar]
- 6.Capeci CM, Tejwani NC. Bilateral low-energy simultaneous or sequential femoral fractures in patients on long-term alendronate therapy. J Bone Joint Surg Am. 2009;91:2556–2561. doi: 10.2106/JBJS.H.01774. [DOI] [PubMed] [Google Scholar]
- 7.Eastell R, Barton I, Hannon RA, Chines A, Garnero P, Delmas PD. Relationship of early changes in bone resorption to the reduction in fracture risk with risedronate. J Bone Miner Res. 2003;18:1051–1056. doi: 10.1359/jbmr.2003.18.6.1051. [DOI] [PubMed] [Google Scholar]
- 8.Frolke JP, Patka P. Definition and classification of fracture non-unions. Injury. 2007;38(suppl 2):S19–S22. doi: 10.1016/S0020-1383(07)80005-2. [DOI] [PubMed] [Google Scholar]
- 9.Goh SK, Yang KY, Koh JS, Wong MK, Chua SY, Chua DT, Howe TS. Subtrochanteric insufficiency fractures in patients on alendronate therapy: a caution. J Bone Joint Surg Br. 2007;89:349–353. doi: 10.1302/0301-620X.89B3.18146. [DOI] [PubMed] [Google Scholar]
- 10.Heckman JD, Ryaby JP, McCabe J, Frey JJ, Kilcoyne RF. Acceleration of tibial fracture-healing by non-invasive, low-intensity pulsed ultrasound. J Bone Joint Surg Am. 1994;76:26–34. doi: 10.2106/00004623-199401000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Iwamoto J, Takeda T. Insufficiency fracture of the femoral neck during osteoporosis treatment: a case report. J Orthop Sci. 2002;7:707–712. doi: 10.1007/s007760200126. [DOI] [PubMed] [Google Scholar]
- 12.Kanis JA, Melton LJ, 3rd, Christiansen C, Johnston CC, Khaltaev N. The diagnosis of osteoporosis. J Bone Miner Res. 1994;9:1137–1141. doi: 10.1002/jbmr.5650090802. [DOI] [PubMed] [Google Scholar]
- 13.Komatsubara S, Mori S, Mashiba T, Ito M, Li J, Kaji Y, Akiyama T, Miyamoto K, Cao Y, Kawanishi J, Norimatsu H. Long-term treatment of incadronate disodium accumulates microdamage but improves the trabecular bone microarchitecture in dog vertebra. J Bone Miner Res. 2003;18:512–520. doi: 10.1359/jbmr.2003.18.3.512. [DOI] [PubMed] [Google Scholar]
- 14.Komatsubara S, Mori S, Mashiba T, Li J, Nonaka K, Kaji Y, Akiyama T, Miyamoto K, Cao Y, Kawanishi J, Norimatsu H. Suppressed bone turnover by long-term bisphosphonate treatment accumulates microdamage but maintains intrinsic material properties in cortical bone of dog rib. J Bone Miner Res. 2004;19:999–1005. doi: 10.1359/JBMR.040126. [DOI] [PubMed] [Google Scholar]
- 15.Kwek EB, Goh SK, Koh JS, Png MA, Howe TS. An emerging pattern of subtrochanteric stress fractures: a long-term complication of alendronate therapy? Injury. 2008;39:224–231. doi: 10.1016/j.injury.2007.08.036. [DOI] [PubMed] [Google Scholar]
- 16.Mashiba T, Hirano T, Turner CH, Forwood MR, Johnston CC, Burr DB. Suppressed bone turnover by bisphosphonates increases microdamage accumulation and reduces some biomechanical properties in dog rib. J Bone Miner Res. 2000;15:613–620. doi: 10.1359/jbmr.2000.15.4.613. [DOI] [PubMed] [Google Scholar]
- 17.Mashiba T, Turner CH, Hirano T, Forwood MR, Johnston CC, Burr DB. Effects of suppressed bone turnover by bisphosphonates on microdamage accumulation and biomechanical properties in clinically relevant skeletal sites in beagles. Bone. 2001;28:524–531. doi: 10.1016/S8756-3282(01)00414-8. [DOI] [PubMed] [Google Scholar]
- 18.Neviaser AS, Lane JM, Lenart BA, Edobor-Osula F, Lorich DG. Low-energy femoral shaft fractures associated with alendronate use. J Orthop Trauma. 2008;22:346–350. doi: 10.1097/BOT.0b013e318172841c. [DOI] [PubMed] [Google Scholar]
- 19.Niimi R, Hasegawa M, Sudo A, Uchida A. Unilateral stress fracture of the femoral shaft combined with contralateral insufficiency fracture of the femoral shaft after bilateral total knee arthroplasty. J Orthop Sci. 2008;13:572–575. doi: 10.1007/s00776-008-1262-2. [DOI] [PubMed] [Google Scholar]
- 20.Odvina CV, Zerwekh JE, Rao DS, Maalouf N, Gottschalk FA, Pak CY. Severely suppressed bone turnover: a potential complication of alendronate therapy. J Clin Endocrinol Metab. 2005;90:1294–1301. doi: 10.1210/jc.2004-0952. [DOI] [PubMed] [Google Scholar]
- 21.Sayed-Noor AS, Sjoden GO. Case reports: two femoral insufficiency fractures after long-term alendronate therapy. Clin Orthop Relat Res. 2009;467:1921–1926. doi: 10.1007/s11999-009-0725-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stepan JJ, Burr DB, Pavo I, Sipos A, Michalska D, Li J, Fahrleitner-Pammer A, Petto H, Westmore M, Michalsky D, Sato M, Dobnig H. Low bone mineral density is associated with bone microdamage accumulation in postmenopausal women with osteoporosis. Bone. 2007;41:378–385. doi: 10.1016/j.bone.2007.04.198. [DOI] [PubMed] [Google Scholar]
- 23.Visekruna M, Wilson D, McKiernan FE. Severely suppressed bone turnover and atypical skeletal fragility. J Clin Endocrinol Metab. 2008;93:2948–2952. doi: 10.1210/jc.2007-2803. [DOI] [PubMed] [Google Scholar]