Abstract
Background
Little is known about intraarticular pathology following THA prior to the radiographic appearance of osteolysis, primarily due to imaging limitations. MRI has recently been applied to imaging the postarthroplasty hip with the ability to detect periarticular bony and soft tissue pathology; specifically, it is able to detect abnormal synovial patterns and focal bone loss well before the radiographic appearance of osteolysis.
Questions/purposes
We therefore used MRI to determine the incidence of early reactive synovitis and osteolysis in asymptomatic patients after THA, and whether there is an association between these MRI findings and clinical outcomes or radiographic wear measurements at this early stage.
Methods
We recruited 31 patients (33 hips) who underwent routine noncemented THA with one of three types of bearing surfaces: metal-on-cross-linked polyethylene (n = 7), ceramic-on-ceramic (n = 12), and ceramic-on-cross-linked polyethylene (n = 14). Patients underwent specialized MRI at a minimum of 12 months (mean, 23 months; range, 12–37 months) after surgery. MR images were analyzed for the presence of synovitis or osteolysis. WOMAC scores, patient assessment questionnaires, and radiographic wear measurements were correlated with MRI findings.
Results
Reactive synovitis was observed in 13 of 33 patients (39%) and focal osteolysis in one of 33 (3%). The presence of synovitis did not correlate with pain, activity level, patient satisfaction or clinical outcome scales, nor did it correlate with radiographic wear measurements at early followup.
Conclusions
Our observations suggest reactive synovitis is common yet asymptomatic at short-term followup. We do not know either the etiology or the long-term implications of these observations.
Introduction
Wear-induced particle disease and subsequent osteolysis have been called the dominant problem in THA [20, 45], and are among the leading causes of revision surgery [6]. Our understanding of this biologic process is improving, but there is still much that is unknown about intraarticular pathology prior to the radiographic appearance of osteolysis. Historically, the study of intraarticular hip pathology has been hampered by a lack of sensitive methods of detection. Radiographs underestimate bone loss due to osteolysis, with sensitivity for lesion detection ranging from 15–72% depending on size and location [5]. They are also limited by poor interobserver reliability [14, 44]. Although there have been advances in the radiographic analysis of wear rates [16, 31], these methods still provide indirect evidence of biological reactions and intracapsular pathology. CT offers improved sensitivity and accuracy in the detection of osteolytic lesions [30, 40], but due to limited soft tissue contrast, it is generally unable to detect synovitis. CT is also accompanied with the disadvantage of ionizing radiation, and methods to reduce the beam-hardening artifact generated by the components require increased doses of radiation.
MRI is a relatively new technology for imaging total joint arthroplasty. In recent years, there have been numerous studies examining its role in imaging THA [3, 7, 38, 39, 46, 50–52]. MRI is effective for detecting periarticular bony and soft-tissue abnormalities in patients with a postarthroplasty hip [3, 39, 46, 52], as well as determining the source of enigmatic hip following THA [7]. Due to its direct multiplanar capabilities and superior soft-tissue contrast, MRI is able to visualize intracapsular synovitis [7, 39, 52] and is more sensitive in the detection of osteolytic lesions than radiographs or CT [50, 51]. Specifically, the ability of optimized MRI to detect wear-induced particle disease in the postarthroplasty hip has been documented in two studies that have corroborated this MRI finding with tissue pathology [39, 52]. Given osteolysis involves inflammation, by the time osteolysis is visible radiographically, considerable inflammation has occurred over a period of time. When this inflammation begins is unknown.
We therefore used MRI to determine (1) the incidence of early reactive synovitis and osteolysis in asymptomatic patients after THA; (2) if there is an association between MRI-detected synovitis and early functional scores; and (3) if there is an association between synovitis and implant wear.
Patients and Methods
We retrospectively reviewed prospectively acquired data. Patient enrollment began in December 2006, from a group of 349 patients who had undergone primary THA from September 1, 2004 through August 31, 2006 performed by one of two surgeons (ASR, CSR). One hundred eighty of these 349 patients were between 40 and 65 years old at the time of surgery. We chose a younger population than the average THA population, as particle disease is of greater clinical concern in patients who are more active and expect a longer survival of their prosthesis. Enrollment was offered to all patients in three separate mailings; 31 patients (17%) with 33 THAs elected to participate in this study. The remaining 149 patients either declined to participate or did not respond to enrollment attempts. Fourteen patients were men and 19 were women. The average age was 56.1 years (range 43.4–64.9) at the time of surgery. All patients had a preoperative diagnosis of degenerative osteoarthritis. They were asked to undergo MRI 1 to 3 years after their index procedure, to evaluate an early period well in advance of the time when radiographic osteolysis and wear disease are commonly believed to occur [20]. The mean time between surgery and the MRI was 23 months (range, 12–37 months). Prior to patient enrollment, approval for this study was obtained from the Institutional Review Boards at both institutions involved in the research. All patients gave informed consent to participate in the study.
Surgeries were performed using a standardized protocol throughout the preoperative, perioperative, and postoperative course. A posterior approach to the hip was utilized, and fixation was obtained using a noncemented technique. All patients were implanted with a single implant design (Accolade TMZF stem and Trident acetabular shell; Stryker Orthopaedics, Mahwah, NJ); however, the choice of bearing interface was individualized according to surgeon or patient preference. Over the study period, three combinations of bearing surfaces were used: (1) metal-on-polyethylene (Crossfire® ultra-high molecular weight polyethylene; Stryker Orthopaedics, Mahwah, NJ); (2) ceramic-on-ceramic (Trident Alumina Ceramic; Stryker Orthopaedics, Mahwah, NJ); and (3) ceramic-on-polyethylene (BIOLOX Delta ceramic; CeramTec AG, Plochingen, Germany, on X3 polyethylene; Stryker Orthopaedics, Mahwah, NJ). The different types of highly cross-linked polyethylenes used reflect a change in the available technology over the study period.
Patients were administered clinical outcome questionnaires, including a Patient Assessment Questionnaire (PAQ) [41] and a Western Ontario and McMaster Universities Osteoarthritis (WOMAC) [1] Index, at the time of their enrollment into the study. The WOMAC is a validated clinical outcome measure [1] commonly used in patients who have undergone THA. WOMAC scores were transformed to a 0 to 100 point scale (100 being the best score). The PAQ is a clinical outcome assessment designed specifically to obtain better insight into the quality of patient results after total joint arthroplasty, with an emphasis on patient function and satisfaction; the PAQ is an unvalidated score.
Patients were followed up in the office at regular intervals of 6 weeks, 3 months, 1 year, and annually thereafter. At each followup visit, a physical examination was performed documenting range of motion and muscle strength around the affected hip. Radiographs performed at each postoperative visit included a low anteroposterior (AP) pelvis radiograph, as well as AP and false profile radiographs of the affected hip.
Two independent observers (ASR, CSR) examined the most recent radiographs for the presence of osteolysis. Two observers were used to account for the large degree of interobserver variability known to occur when examining plain radiographs for the presence of osteolytic lesions, when, at best, surgeons agree on the presence of lesions in 57% of zones, with κ coefficients ranging between 0.28 and 0.44 [14]. In our study, there was no variability between the observations of these two surgeons in the detection of osteolytic lesions (κ = 1.0).
MRIs were performed at a specialty center on a clinical 1.5 Tesla unit (HDx, General Electric Healthcare, Milwaukee, WI). Initial images were obtained with a body coil utilizing an initial coronal fast inversion recovery sequence with field of view 35 cm, repetition time (TR) 4500–5000 msec/echo time (TE) 17 msec (effective), inversion time (TI) 150 msec, receiver bandwidth 62.5 kHz (over entire frequency range), and slice thickness 5 mm with no interslice gap. Additional optimized coronal, sagittal and axial fast spin echo sequences (Fast spin echo XL, General Electric Healthcare, Milwaukee WI) were obtained using a four channel phased array receive-only shoulder coil (Med Rad phased array, Indianola, PA), with TR 3000 to 5000msec/TE 30 to 36 msec, with a wider receiver bandwidth 62.5 to 100 kHz over the entire frequency direction. Field of view ranged between 17 to 20 cm, slice thickness was 3 to 4 mm with no gap, and the matrix was 512 × 320 to 384 at 4 to 6 excitations, yielding a maximum in-plane resolution of 332 μ. Total imaging time ranged between 35 to 40 minutes, depending upon patient size and the need for repetition of pulse sequences due to involuntary motion.
Following image acquisition, two of us (HGP, LFF) examined MR images for the presence of synovial reaction, as well as the presence of osteolysis around the prosthetic components. Implants were evaluated in three planes and the presence of osteolysis and/or synovitis was confirmed in at least two planes of imaging. MRI is well-documented in its ability to discern abnormal synovial patterns [8, 42] and is an accepted imaging modality for this purpose. Intermediate to decreased signal intensity in the synovial lining with distension of the joint capsule was indicative of reactive synovitis [7, 39]. These patients showed distention of the normally thin, hypointense pseudocapsule by coarse synovitis which had signal characteristics similar to those of the osteolytic material replacing the bone. Osteolysis was demonstrated by areas of marrow replacement of intermediate intraosseous signal intensity, with or without a peripheral rim of lower signal intensity [7, 39]. These focal areas of intermediate signal intensity contrast with the high signal intensity of the medullary fat on moderate echo time sequences and the low signal of suppressed fatty marrow on the fast short tau inversion recovery sequences. If either synovitis or osteolysis were present, one of us (HGP) manually segmented the intraarticular amount of synovitis and/or the volume of bone loss and recorded it on a dedicated work station using commercial software (Functool, Advantage Windows, General Electric Healthcare, Milwaukee, WI), as in prior studies [7, 39]. The accuracy of these methods has been previously established based on direct measurements, [50] where MRI was noted to have a 95.4% sensitivity and a 97.9% specificity for the detection and measurement of simulated osteolysis.
We attempted to correlate the volume of reactive synovitis or osteolysis with an established radiographic method of wear detection. The roentgen monographic analysis (Roman) method [43] is a computer-assisted method, designed to measure penetration of the femoral head into the acetabular component, that is the most precise and user-friendly computer-assisted method (Martell Hip Analysis Suite, Rogan HyperOrtho, Rogan View Pro-X, and Roman) of radiographic wear detection [16].
The incidence and average volume of reactive synovitis and osteolysis were calculated for the overall cohort and for each of the three groups of bearing surfaces. Pearson’s product-moment correlation coefficient was used to assess any correlation between the volume of reactive synovitis or osteolysis and WOMAC scores, as well as pain, stiffness, and function subscores. Spearman’s rank order correlation coefficient and Pearson’s product-moment correlation coefficient were used to asses for any correlation between the volume of synovitis or osteolysis and ordinal and interval PAQ items, respectively. The cohort was then divided into two groups based on the presence or absence of reactive synovitis. Independent means t-tests were used to examine for any clinical differences between these groups based on WOMAC scores. A chi-squared test of independence was used to detect any differences between the groups with regard to nominal data, a Mann-Whitney U test was used to detect differences between the groups with regard to ordinal data, and an independent means t-test was used to examine for any differences in scale data on the PAQ. Pearson’s product-moment correlation coefficient was used to assess for any correlation between the Roman method of radiographic wear detection and the volume of particle disease detected on MRI. Subgroup analysis based on the type of bearing surface used was not performed to avoid committing a Type II error with such small groups.
Results
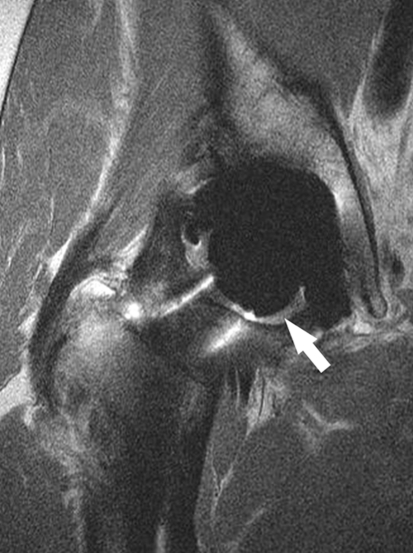
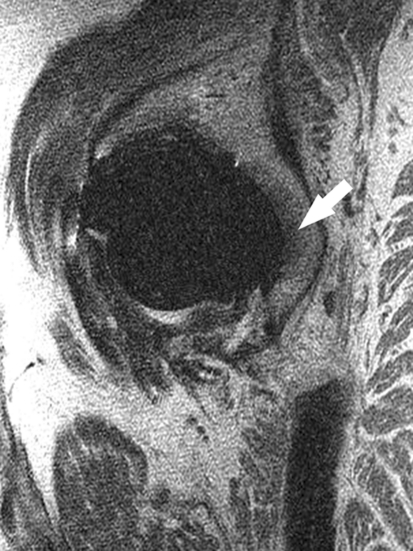
At 1 to 3 years, MR imaging consistent with reactive synovitis (Fig. 1) was seen in 13 of the 33 patients, for an overall incidence of 39% (Table 1). The average volume of this synovitis across the study cohort was 806 mm3 (± 1526 mm3), and the average volume of reactive synovitis for those in which it was present was 2046 mm3 (± 1903 mm3). One patient with a ceramic-on-ceramic hip demonstrated a minimal amount of periacetabular osteolysis (146 mm3), located in the posterior column adjacent to the acetabular shell (Fig. 2). Femoral osteolysis was not seen in any patients. The overall incidence of osteolysis was 3%. No radiographic evidence of femoral or periacetabular osteolysis was detected in any of the patients’ hips by either observer.
Fig. 1.
This coronal MR image of a hip arthroplasty demonstrates intermediate signal intensity within the synovial lining and a moderately distended hip joint (arrow), consistent with synovitis.
Table 1.
Particle-induced synovitis in the subgroups
| Bearing surface | Number of hips | Hips with synovitis | Percentage | Average volume (± standard deviation) |
|---|---|---|---|---|
| Metal-on-polyethylene | 7 | 2 | 29% | 1038 mm3 (± 2610 mm3) |
| Ceramic-on-ceramic | 12 | 4 | 33% | 805 mm3 (± 1379 mm3) |
| Ceramic-on-polyethylene | 14 | 7 | 50% | 691 mm3 (± 1041 mm3) |
| Total | 33 | 13 | 39% | 806 mm3 (± 1546 mm3) |
Fig. 2.
This sagittal MR image of a hip arthroplasty demonstrates a small amount of bone resorption around the acetabular component (arrow) in the posterior column of the acetabulum, without cortical penetration into the pelvis
The volume of reactive synovitis was not associated with any of the clinical outcome measurements at this early postoperative period. The mean WOMAC score for all patients was 93.0 (± 4.9, range 83–100) out of 100. There was no correlation between the volume of reactive synovitis and WOMAC or PAQ scores (Table 2). When the cohort was divided based on the presence or absence of reactive synovitis, we also failed to detect a difference in clinical outcomes. The overall WOMAC score (p = 0.76) as well as the pain (p = 0.99), stiffness (p = 0.54), and function (p = 0.60) subscores were similar between those with reactive synovitis and those without. Likewise, PAQ scores failed to detect any differences between the groups with regard to the presence of pain (p = 0.73), severity of pain (p = 0.28), frequency of pain (p = 0.44), frequency of limp (p = 0.14), difficulty with shoes or socks (p = 0.43), difficulty with personal care (p = 0.37), ability to perform household activities (p = 0.45), difficulty getting in or out of a car (p = 0.17), type of assistive device used, if any (p = 0.40), walking distance (p = 0.28), degree to which the hip affects performance (p = 0.45), degree to which the hip influences social activity (p = 0.42), degree to which the hip affects patient well-being (p = 0.16), and overall patient satisfaction (p = 0.42).
Table 2.
Correlations between the volume of reactive synovitis seen on MRI and the clinical outcome measurements
| Outcome measurement | Correlation coefficient | p-value |
|---|---|---|
| WOMAC | ||
| Overall Score | r = 0.104 | 0.60 |
| Pain Sub-Score | r = 0.015 | 0.94 |
| Stiffness Sub-Score | r = 0.153 | 0.44 |
| Function Sub-Score | r = 0.087 | 0.66 |
| Patient assessment questionnaire | ||
| Severity of pain | r = −0.197 | 0.31 |
| Frequency of pain | ρ = −0.150 | 0.45 |
| Frequency of limp | ρ = −0.269 | 0.17 |
| Difficulty with socks and shoes | ρ = 0.229 | 0.24 |
| Difficulty with personal care | ρ = −0.086 | 0.66 |
| Ability to perform household activities | ρ = −0.055 | 0.78 |
| Difficulty getting in or out of a car | ρ = −0.005 | 0.98 |
| Use of assistive devices | ρ = −0.149 | 0.45 |
| Walking distance | ρ = 0.175 | 0.37 |
| Degree to which hip affects performance | ρ = 0.022 | 0.91 |
| Degree to which hip influences social activity | ρ = −0.110 | 0.58 |
| Degree to which hip affects well-being | ρ = −0.169 | 0.39 |
| Overall patient satisfaction | r = 0.207 | 0.29 |
We observed no correlation (r = 0.180; p = 0.45) between femoral head penetration and the volume of reactive synovitis among the 33 hips.
Discussion
Particle disease with subsequent osteolysis is one of the major limitations of modern THA and among the leading causes of late failure. Despite its clinical and economic importance, little is known about intraarticular pathology prior to the radiographic appearance of osteolysis, primarily due to limitations in the ability to detect the early biologic response to wear particles. MRI is an emerging technology for imaging of patients with total joint arthroplasties that can visualize synovitis and osteolysis [39, 52]. The aims of this study were to use MRI to identify the early incidence of reactive synovitis and osteolysis in asymptomatic patients following THA, and to determine if there is an association between these MRI findings and either clinical outcomes or radiographic wear measurements.
We note several limitations. First, in the absence of tissue samples, we are unable to confirm whether the synovitis seen on MRI represents particle-induced synovitis. MRI can differentiate among different synovial patterns [39], and although the reactive synovitis seen in this study shared identical signal characteristics to what has been documented as particle reaction in two previous studies [39, 52], there are other potential causes for synovitis that could share these same signal characteristics. That none of the patients had a diagnosis of inflammatory arthritis makes it unlikely that this synovitis represents longstanding disease. Furthermore, since none of these patients had clinical symptoms of instability, synovitis was unlikely to be caused by micromotion. Some studies have raised concern that wear particles from highly cross-linked polyethylene may be more bioreactive despite lower wear rates [13, 22], which could potentially explain this reaction. Second, we had short-term followup. Midterm MRIs might demonstrate a greater degree of synovitis and osteolysis, which might then better correlate with clinical results or radiographic methods of wear detection. Third, performing an early radiographic method of wear detection might lack the necessary precision to provide meaningful measurements. Fourth is the small numbers in each of the bearing surface subgroups that prevent appropriate statistical analysis to detect any differences between these groups. Fifth, we have no data for other types of hard-on-hard bearing surfaces, such as metal-on-metal or ceramic-on-metal articulations. Although these limitations affect how broadly we can interpret our findings, they do not jeopardize the conclusions that we have made based on our data.
Our observations demonstrate early reactive synovitis is common, with 39% of patients showing evidence of synovitis on MRI, while osteolysis was essentially nonexistent. Apart from literature on early metal hypersensitivity reactions and aseptic lymphocyte-dominated vasculitis-associated lesions associated with metal-on-metal bearings [28, 35, 36, 53], there have been no studies published on early intraarticular pathology such as the reactive synovitis in this study. Studies of early wear disease are also limited, probably due to difficulty in detection using established methods, and are restricted to those that use computerized methods of radiographic wear detection [9, 15, 17, 26, 27]. Our finding that osteolysis is rare is consistent with the literature examining these same newer-generation bearing surfaces (Table 3) [2, 4, 11, 15, 19, 23, 25, 29, 34]. These studies failed to detect osteolysis on radiographs; the only study from this group that identified osteolysis did so using CT [29], echoing the point that these lesions are small and can easily be missed on radiographs.
Table 3.
A comparison of our findings regarding the incidence of osteolysis with other studies examining similar types of new generation bearing surfaces
| Study | Bearing(s)* | Followup time (years) | Method of detection | Incidence of osteolysis |
|---|---|---|---|---|
| Chang et al. [4] | C-C | 5.4 | XR | 0% |
| Kido et al. [23] | M-XLPE, M-M | 2.3 | XR | 0% |
| Greene et al. [19] | C-C | 4.2 | XR | 0% |
| Kim et al. [25] | C-C, C-XLPE | 5.6 | XR | 0% |
| Dorr et al. [11] | M-XLPE | 5.0 | XR | 0% |
| Bragdon et al. [2] | M-XLPE | 3.0 | XR | 0% |
| Leung et al. [29] | M-XLPE | 5.0 | CT | 8% |
| Garvin et al. [15] | C-XLPE | 2.5 | XR | 0% |
| McCalden et al. [34] | M-XLPE | 6.8 | XR | 0% |
| Cooper et al. [current study] | M-XLPE, C-C, C-XLPE | 1.9 | MRI | 3% |
* C-C = ceramic on ceramic, M-XLPE = metal on highly cross-linked polyethylene, M-M = metal on metal, C-XLPE = ceramic on highly cross-linked polyethylene.
Reactive synovitis, while common, is also asymptomatic at early followup. We found high functional scores and no differences between the patients with and without these MRI findings. Periprosthetic osteolysis has been described by Marshall et al. [32] as “a silent disease that can progress without symptoms until catastrophic structural failure or mechanical loosening of the implant components occur,” and this claim has been substantiated in other studies [12, 18, 21]. If this reactive synovitis does represent early particle disease, it should not be unexpected that we failed to associate its presence to clinical outcomes at this early stage.
Although we failed to demonstrate an association between MRI findings and the radiographic wear measurements, this should not be surprising given the time at which these measurements were made. Studies examining radiographic wear rates detected by two-dimensional manual techniques, two- and three-dimensional computer-assisted techniques, and RSA are typically performed at a longer average followup (ie, 5 to 20 or more years) [4, 10, 11, 16, 24, 25, 30, 33, 49]. These methods may lack the precision necessary to yield meaningful wear measurements for short-term followup in hips with small amounts of wear [31]. Early wear measurements are also complicated by a “bedding-in phenomenon”, generally thought to account for a substantial amount of measurable head penetration in the early postoperative interval, that results from a combination of settling of the modular liner and creep of the polyethylene [37, 47, 48]. For these reasons, there are scant data in the literature examining wear rates at a similar postoperative time; studies by Digas et al. [9] and Garvin et al. [15], both of which used a similar postoperative interval of two years, were able to demonstrate measurable amounts of wear.
While we found osteolysis was rare at early followup we observed an unexpectedly high incidence of reactive synovitis occurring soon after elective THA. It is clear synovitis can occur in the absence of symptoms and is not correlated with radiographic wear measurements at this early postoperative interval. Questions that must be raised going forward are the etiology of this reactive synovitis and whether it will become clinically important over time.
Footnotes
One of more of the authors (HJC, ASR, HGP, LFF, CSR) have received funding from The Hip Society for this project.
Each author certifies that his or her institution approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at Lenox Hill Hospital, New York, NY, and the Hospital for Special Surgery, New York, NY.
Aided by a grant from the Hip Society.
References
- 1.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: A health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833–1840. [PubMed] [Google Scholar]
- 2.Bragdon CR, Greene ME, Freiberg AA, Harris WH, Malchau H. Radiostereometric analysis comparison of wear of highly cross-linked polyethylene against 36- vs 28-mm femoral heads. J Arthroplasty. 2007;22:125–129. doi: 10.1016/j.arth.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 3.Cahir JG, Toms AP, Marshall TJ, Wimhurst J, Nolan J. CT and MRI of hip arthroplasty. Clin Radiol. 2007;62:1163–1171. doi: 10.1016/j.crad.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 4.Chang JD, Kamdar R, Yoo JH, Hur M, Lee SS. Third-generation ceramic-on-ceramic bearing surfaces in revision total hip arthroplasty. J Arthroplasty. 2009;24:1231–1235. doi: 10.1016/j.arth.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 5.Claus AM, Engh CA, Jr, Sychterz CJ, Xenos JS, Orishimo KF, Engh CA., Sr Radiographic definition of pelvic osteolysis following total hip arthroplasty. J Bone Joint Surg Am. 2003;85:1519–1526. doi: 10.2106/00004623-200308000-00013. [DOI] [PubMed] [Google Scholar]
- 6.Clohisy JC, Calvert G, Tull F, McDonald D, Maloney WJ. Reasons for revision hip surgery: a retrospective review. Clin Orthop Relat Res. 2004;429:188–192. doi: 10.1097/01.blo.0000150126.73024.42. [DOI] [PubMed] [Google Scholar]
- 7.Cooper HJ, Ranawat AS, Potter HG, Foo LF, Jawetz ST, Ranawat CS. Magnetic resonance imaging in the diagnosis and management of hip pain after total hip arthroplasty. J Arthroplasty. 2009;24:661–667. doi: 10.1016/j.arth.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 8.Coumbaras M, Le Hir P, Sautet A, Jomaah N, Tubiana JM, Arrivé L. Reactive synovitis: MRI features with arthroscopic correlation. J Radiol. 2005;86:481–486. doi: 10.1016/S0221-0363(05)81393-9. [DOI] [PubMed] [Google Scholar]
- 9.Digas G, Kärrholm J, Thanner J, Malchau H, Herberts P. The Otto Aufranc Award: Highly cross-linked polyethylene in total hip arthroplasty. Randomized evaluation of penetration rate in cemented and uncemented sockets using radiostereometric analysis. Clin Orthop Relat Res. 2004;429:6–16. doi: 10.1097/01.blo.0000150314.70919.e3. [DOI] [PubMed] [Google Scholar]
- 10.D’Antonio JA, Manley MT, Capello WN, Bierbaum BE, Ramakrishnan R, Naughton M, Sutton K. Five-year experience with Crossfire® highly cross-linked polyethylene. Clin Orthop Relat Res. 2005;441:143–150. doi: 10.1097/00003086-200512000-00024. [DOI] [PubMed] [Google Scholar]
- 11.Dorr LD, Wan Z, Shahrdar C, Sirianni L, Boutary M, Yun A. Clinical performance of a Durasul highly crosslinked polyethylene acetabular liner for total hip arthroplasty at five years. J Bone Joint Surg Am. 2005;87:1816–1821. doi: 10.2106/JBJS.D.01915. [DOI] [PubMed] [Google Scholar]
- 12.Duffy P, Sher JL, Partington PF. Premature wear and osteolysis in an HA-coated, uncemented total hip arthroplasty. J Bone Joint Surg Br. 2004;86:34–38. [PubMed] [Google Scholar]
- 13.Endo M, Tipper JL, Barton DC, Stone MH, Ingham E, Fisher J. Comparison of wear, wear debris and functional biological activity of moderately crosslinked and non-crosslinked polyethylenes in hip prostheses. Proc Inst Mech Eng H. 2002;216:111–122. doi: 10.1243/0954411021536333. [DOI] [PubMed] [Google Scholar]
- 14.Engh CA, Jr, Sychterz CJ, Young AM, Pollock DC, Toomey SD, Engh CA., Sr Interobserver and intraobserver variability in radiographic assessment of osteolysis. J Arthroplasty. 2002;17:752–759. doi: 10.1054/arth.2002.33554. [DOI] [PubMed] [Google Scholar]
- 15.Garvin KL, Hartman CW, Mangla J, Murdoch N, Martell JM. Wear analysis in THA utilizing oxidized zirconium and crosslinked polyethylene. Clin Orthop Relat Res. 2009;467:141–145. doi: 10.1007/s11999-008-0544-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geerdink CH, Grimm B, Vencken W, Heyligers IC, Tonino AJ. The determination of linear and angular penetration of the femoral head into the acetabular component as an assessment of wear in total hip replacement. J Bone Joint Surg Br. 2008;90:839–846. doi: 10.1302/0301-620X.90B7.20305. [DOI] [PubMed] [Google Scholar]
- 17.Geller JA, Malchau H, Bragdon C, Greene M, Harris WH, Freiberg AA. Large diameter femoral heads on highly cross-linked polyethylene: minimum 3-year results. Clin Orthop Relat Res. 2006;447:53–59. doi: 10.1097/01.blo.0000218742.61624.80. [DOI] [PubMed] [Google Scholar]
- 18.Goosen JHM, Castelein RM, Verheyen CCPM. Silent osteolysis associated with an uncemented acetabular component: A monitoring and treatment algorithm. Curr Orthop. 2005;19:288–293. doi: 10.1016/j.cuor.2005.02.014. [DOI] [Google Scholar]
- 19.Greene JW, Malkani AL, Kolisek FR, Jessup NM, Baker DL. Ceramic-on-ceramic total hip arthroplasty. J Arthroplasty. 2009;24:15–18. doi: 10.1016/j.arth.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 20.Harris WH. The problem is osteolysis. Clin Orthop Relat Res. 1995;311:46–53. [PubMed] [Google Scholar]
- 21.Hozack WJ, Mesa JJ, Carey C, Rothman RH. Relationship between polyethylene wear, pelvic osteolysis, and clinical symptomatology in patients with cementless acetabular components: a framework for decision making. J Arthroplasty. 1996;11:769–772. doi: 10.1016/S0883-5403(96)80175-6. [DOI] [PubMed] [Google Scholar]
- 22.Ingram JH, Stone M, Fisher J, Ingham E. The influence of molecular weight, crosslinking and counter roughness on TNF-Alpha production by macrophages in response to ultra high molecular weight polyethylene particles. Biomaterials. 2004;25:3511–3522. doi: 10.1016/j.biomaterials.2003.10.054. [DOI] [PubMed] [Google Scholar]
- 23.Kido K, Fujioka M, Takahashi K, Ueshima K, Goto T, Kubo T. Short-term results of the S-ROM-A femoral prosthesis operative strategies for Asian patients with osteoarthritis. J Arthroplasty. 2009;24:1193–1199. doi: 10.1016/j.arth.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 24.Kim YH. Comparison of polyethylene wear associated with cobalt-chromium and zirconia heads after total hip replacement: a prospective, randomized study. J Bone Joint Surg Am. 2005;87:1769–1776. doi: 10.2106/JBJS.D.02572. [DOI] [PubMed] [Google Scholar]
- 25.Kim YH, Kim JS, Choi YW, Kwon OR. Intermediate results of simultaneous alumina-on-alumina bearing and alumina-on-highly cross-linked polyethylene bearing total hip arthroplasties. J Arthroplasty. 2009;24:885–891. doi: 10.1016/j.arth.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 26.Kraay MJ, Thomas RD, Rimnac CM, Fitzgerald SJ, Goldberg VM. Zirconia versus Co-Cr femoral heads in total hip arthroplasty: Early assessment of wear. Clin Orthop Relat Res. 2006;453:86–90. doi: 10.1097/01.blo.0000246544.95316.1f. [DOI] [PubMed] [Google Scholar]
- 27.Krushell RJ, Fingeroth RJ, Cushing MC. Early femoral head penetration of a highly cross-linked polyethylene liner vs a conventional polyethylene liner: a case-controlled study. J Arthroplasty. 2005;20:73–76. doi: 10.1016/j.arth.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 28.Langton DJ, Jameson SS, Joyce TJ, Hallab NJ, Natu S, Nargol AVF. Early failure of metal-on-metal bearings in hip resurfacing and large-diameter total hip replacement: A consequence of excess wear. J Bone Joint Surg Br. 2010;92:38–46. doi: 10.1302/0301-620X.92B1.22770. [DOI] [PubMed] [Google Scholar]
- 29.Leung SB, Egawa H, Stepniewski A, Beykirch S, Engh CA, Jr, Engh CA., Sr Incidence and volume of pelvic osteolysis at early follow-up with highly cross-linked and noncross-linked polyethylene. J Arthroplasty. 2007;22:134–139. doi: 10.1016/j.arth.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 30.Looney RJ, Boyd A, Totterman S, Seo GS, Tamez-Pena J, Campbell D, Novotmy L, Olcott C, Martell J, Hayes FA, O’Keefe RJ, Schwartz EM. Volumetric computerized tomography as a measurement of periprosthetic acetabular osteolysis and its correlation with wear. Arthritis Res. 2001;4:59–63. doi: 10.1186/ar384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malchau H, Potter HG. How are wear-related problems diagnosed and what forms of surveillance are necessary? J Am Acad Orthop Surg. 2008;16(suppl):s14–s19. doi: 10.5435/00124635-200800001-00005. [DOI] [PubMed] [Google Scholar]
- 32.Marshall A, Ries MD, Paprosky W. How prevalent are implant wear and osteolysis, and how has the scope of osteolysis changed since 2000? J Am Acad Orthop Surg. 2008;16(suppl):S1–S6. doi: 10.5435/00124635-200800001-00003. [DOI] [PubMed] [Google Scholar]
- 33.Martell JM, Berkson E, Berger R, Jacobs J. Comparison of two and three-dimensional computerized polyethylene wear analysis after total hip arthroplasty. J Bone Joint Surg Am. 2003;85:1111–1117. doi: 10.2106/00004623-200306000-00020. [DOI] [PubMed] [Google Scholar]
- 34.McCalden RW, MacDonald SJ, Rorabeck CH, Bourne RB, Chess DG, Charron KD. Wear rate of highly cross-linked polyethylene in total hip arthroplasty. A randomized controlled trial. J Bone Joint Surg Am. 2009;91:773–782. doi: 10.2106/JBJS.H.00244. [DOI] [PubMed] [Google Scholar]
- 35.Ollivere B, Darran C, Barker T, Nolan J, Porteous MJ. Early clinical failure of the Birmingham metal-on-metal hip resurfacing is associated with metallosis and soft tissue necrosis. J Bone Joint Surg Br. 2009;91:1025–1030. doi: 10.1302/0301-620X.91B8.21701. [DOI] [PubMed] [Google Scholar]
- 36.Pandit H, Glyn-Jones S, McLardy-Smith P, Gundle R, Whitwell D, Gibbons CLM, Ostlere S, Athanasou N, Gill HS, Murray DW. Pseudotumours associated with metal-on-metal hip resurfacings. J Bone Joint Surg Br. 2008;90:847–851. doi: 10.1302/0301-620X.90B7.20213. [DOI] [PubMed] [Google Scholar]
- 37.Pedersen DR, Brown TD, Hillis SL, Callaghan JJ. Prediction of long-term polyethylene wear in total hip arthroplasty, based on early wear measurements made using digital image analysis. J Orthop Res. 1998;16:557–563. doi: 10.1002/jor.1100160506. [DOI] [PubMed] [Google Scholar]
- 38.Potter HG, Montgomery KD, Padgett DE, Salvati EA, Helfet DL. Magnetic resonance imaging of the pelvis: new orthopaedic applications. Clin Orthop Relat Res. 1995;319:223–231. [PubMed] [Google Scholar]
- 39.Potter HG, Nestor BJ, Sofka CM, Ho ST, Peters LE, Salvati EA. Magnetic resonance imaging after total hip arthroplasty: evaluation of periprosthetic soft tissue. J Bone Joint Surg Am. 2004;86:1947–1954. doi: 10.2106/00004623-200409000-00013. [DOI] [PubMed] [Google Scholar]
- 40.Puri L, Wixson RL, Stern SH, Kohli J, Hendrix RW, Stulberg SD. Use of helical computed tomography for the assessment of acetabular osteolysis after total hip arthroplasty. J Bone Joint Surg Am. 2002;84:609–614. doi: 10.2106/00004623-200204000-00016. [DOI] [PubMed] [Google Scholar]
- 41.Rasquina VJ, Ranawat CS, Cervieri CL, Rodríguez JA. The press-fit modular total knee system with a posterior-cruciate substituting design: a concise followup of a previous report. J Bone Joint Surg Am. 2006;88:1006–1010. doi: 10.2106/JBJS.C.01104. [DOI] [PubMed] [Google Scholar]
- 42.Rhodes LA, Keenan AM, Grainger AJ, Emery P, McGonagle D, Conaghan PG. The relationship between limited MRI section analyses and volumetric assessment of synovitis in knee osteoarthritis. Clin Radiol. 2005;60:1295–1299. doi: 10.1016/j.crad.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 43.Roman free to share software version V1.70. The Institute of Orthopaedics Web site. Available at: http://www.Keele.ac.uk/depts/rjah/. Accessed October 28, 2009.
- 44.Saleh KJ, Holtzman J, Gafni A, Saleh L, Davis A, Resig S, Gross AE. Reliability and intraoperative validity of preoperative assessment of standardized plain radiographs in predicting bone loss at revision hip surgery. J Bone Joint Surg Am. 2001;83:1040–1046. doi: 10.2106/00004623-200107000-00009. [DOI] [PubMed] [Google Scholar]
- 45.Schmalzried TP, Callaghan JJ. Wear in total hip and knee replacements. J Bone Joint Surg Am. 1999;81:115–136. doi: 10.2106/00004623-199901000-00016. [DOI] [PubMed] [Google Scholar]
- 46.Sugimoto H, Hirose I, Miyaoka E, Fujita A, Kinebuchi Y, Yamamoto W, Itoh Y. Low-field-strength MR imaging of failed hip arthroplasty: association of femoral periprosthetic signal intensity with radiographic, surgical, and pathologic findings. Radiology. 2003;229:718–723. doi: 10.1148/radiol.2293021061. [DOI] [PubMed] [Google Scholar]
- 47.Sychterz CJ, Engh CA, Jr, Yang A, Engh CA. Analysis of temporal wear patterns of porous-coated acetabular components: distinguishing between true wear and so-called bedding-in. J Bone Joint Surg Am. 1999;81:821–830. doi: 10.1302/0301-620X.81B5.9383. [DOI] [PubMed] [Google Scholar]
- 48.Sychterz CJ, Yang AM, McAuley JP, Engh CA. Two-dimensional versus threedimensional radiographic measurements of polyethylene wear. Clin Orthop Relat Res. 1999;365:117–123. doi: 10.1097/00003086-199908000-00016. [DOI] [PubMed] [Google Scholar]
- 49.Urban JA, Garvin KL, Boese CK, Bryson L, Pedersen DR, Callaghan JJ, Miller RK. Ceramic-on-polyethylene bearing surfaces in total hip arthroplasty: seventeen to twenty-one-year results. J Bone Joint Surg Am. 2001;83:1688–1694. doi: 10.2106/00004623-200111000-00011. [DOI] [PubMed] [Google Scholar]
- 50.Walde TA, Weiland DE, Leung SB, Kitamura N, Sychterz CJ, Engh CA, Jr, Claus AM, Potter HG, Engh CA. Sr. Comparison of CT, MRI, and radiographs in assessing pelvic osteolysis: a cadaveric study. Clin Orthop Relat Res. 2005;437:138–144. doi: 10.1097/01.blo.0000164028.14504.46. [DOI] [PubMed] [Google Scholar]
- 51.Weiland DE, Walde TA, Leung SB, Sychterz CJ, Ho S, Engh CA, Potter HG. Magnetic resonance imaging in the evaluation of periprosthetic acetabular osteolysis: a cadaveric study. J Orthop Res. 2005;23:713–719. doi: 10.1016/j.orthres.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 52.White LM, Kim JK, Mehta M, Merchant N, Schweitzre ME, Morrison WB, Hutchison CR, Gross AE. Complications of Total Hip Arthroplasty: MR Imaging – Initial Experience. Radiology. 2000;215:254–262. doi: 10.1148/radiology.215.1.r00ap11254. [DOI] [PubMed] [Google Scholar]
- 53.Willert HG, Buchhorn GH, Fayyazi A, Flury R, Windler M, Koster G, Lohmann CH. Metal-on-metal bearings and hypersensitivity in patients with artificial hip joints: a clinical and histomorphological study. J Bone Joint Surg Am. 2005;87:28–36. doi: 10.2106/JBJS.A.02039pp. [DOI] [PubMed] [Google Scholar]