Abstract
Background
Previous studies show the shape of the femur in developmental dislocation of the hip (DDH) becomes more abnormal with increasing subluxation. Two kinds of high dislocations associated with DDH have been observed in clinical practice, one with (Type C1) and one without (Type C2) a false acetabulum. The presence or absence of a false acetabulum in high dislocated hips is associated with different loading patterns and could influence the development and shape of the proximal femur.
Questions/purposes
We therefore determined whether (1) the proximal femoral shape and dimension in Type C1 and Type C2 hips differ from each other, and (2) the femur dislocated with the same height in Types C1 and C2 hips.
Patients and Methods
We examined the following variables on 54 proximal femurs from 54 patients with high DDH (28 Type C1 hips and 26 Type C2 hips) on AP and lateral radiographs; the ML widths of the cortical and medullary canals, height of the femoral head, height of dislocation, and height of the greater trochanter. Reproducibility of the measurements was tested by two researchers with high interobserver and intraobserver agreement.
Results
The proximal femur in Type C2 hips was narrower and stovepipe shaped, with a smaller flare index (2.7 ± 0.6), compared with Type C1 hips (3.5 ± 1.2). The proximal femur migrated an average of 18 mm more superiorly in Type C2 than in Type C1 hips.
Conclusions
Our data confirm distinctions in the shape of the proximal femur in the presence and absence of a false acetabulum.
Clinical Relevance
Owing to the abnormal shapes, special implants of different geometries or modular stems may be needed for reconstruction Type C2 high dislocations.
Introduction
Prosthetic replacements of high dislocations associated with DDH sometimes are challenging to reconstruct. In one study with a minimum of 7 years followup, the total revision rate for these hips was approximately 22% [5]. The failure rate of femoral components in these hips may be as much as 16% [5]. Complications occur with relatively high frequency: femoral nerve palsy in 0% to 18%, intraoperative femoral fracture in 3.6% to 6.5%, postoperative dislocation in 0% to 9%, and fibrous union of the greater trochanter in 0% to 7.1% [3, 4, 9–11, 15].
Three types of congenital hip disease in adults have been distinguished according to the classification system of Hartofilakidis et al. [7]: dysplasia (Type A): the femoral head is contained in the original acetabulum despite the degree of subluxation or proximal femoral migration; low dislocation (Type B): the femoral head articulates with a false acetabulum that partially covers the true acetabulum to a varying degree; high dislocation (Type C): the femoral head is completely out of the true acetabulum and migrated superiorly and posteriorly to a varying degree. Two kinds of high dislocations are commonly observed: (1) one with a false acetabulum that may be contiguous with the true acetabulum or that may lie at a distant site and (2) the other with no false acetabulum and with a femoral head that may be close to the true acetabulum or may be high-riding in the gluteal musculature. Hartofilakidis et al. classified these two kinds of high dislocations as Types C1 and C2 hips [6, 7] (Fig. 1). The presence and absence of a false acetabulum in high dislocated hips are associated with different loading patterns and different soft tissue conditions.
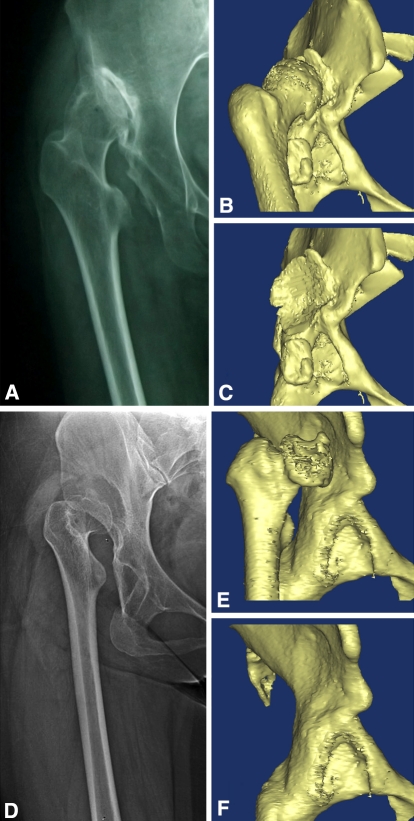
Fig. 1A–F.
The high developmental dislocations of the hips were classified into two subtypes. In Type C1 hips, (A) the femoral head is completely out of the true acetabulum, the ilium contacting the femoral head is sclerotic, and it articulates with the iliac wing; (B) there is osteophyte formation; and (C) a pseudoacetabulum is present. In Type C2 hips, (D) the femoral head is completely out of the true acetabulum, the femur is stovepipe shaped; (E) the femoral head has no direct contact with iliac wing; and (F) there is no pseudoacetabulum.
Earlier studies document various morphologic abnormalities with high dislocations: a straight, narrow femur [6], small femoral head size, a short neck length, and an anteverted femoral neck [1, 13, 14, 16]. According to Wolf’s law, mechanical loads can affect bone architecture in living beings. It therefore is reasonable to presume the different loading patterns and soft tissue conditions in Types C1 and C2 femurs may result in different morphologic features of the femur [7].
Optimal fit and fill in the proximal medullary canal are a prerequisite for primary stability and thus long-term biologic fixation of a femoral stem. Canal width and the flare index can be obtained readily from AP radiographs and are clinically relevant measurements that can be used to select proper implants and to properly ream the proximal and distal parts of the proximal femur intraoperatively.
We conducted this study to identify the morphologic distinctions between proximal femurs in Type C1 versus Type C2 hips. We asked whether (1) the proximal femoral shape and dimension in Types C1 and C2 hips differ from each other, with different canal width and flare index, and (2) the femur dislocated with the same height in Types C1 and C2 hips.
Patients and Methods
We retrospectively examined the radiographs of all 80 patients with high hip dislocations admitted to our hospital between October 2000 and September 2008 for hip reconstruction. We excluded 26 patients for the following reasons: seven for prior hip surgery, nine with a dislocation attributable to a nondevelopmental etiology (infection or trauma), and 10 for incomplete or poor-quality radiographs. These exclusions left 54 patients. In these patients, six had bilateral high dislocations. As the two hips in a patient were not independent observations, only one hip was randomly selected from each of the six patients. There were 48 female and six male patients. The average age of the patients was 43.8 ± 11.75 years; the average body mass index was 22.70 ± 2.85.
We retrospectively obtained preoperative AP and ML radiographs for every patient. The quality of the radiographs was checked to ensure any two obturator foramens were the same size, the tip of each coccyx was approximately 4 cm above the upper level of the pubic symphysis, and the lesser trochanter could be seen.
All of the dislocated hips were classified according to the system of Hartofilakidis et al. [7]. Group 1 consisted of 28 Type C1 hips, which have a pseudoacetabulum that may be contiguous with the true acetabulum or may lie at a distant site. Group 2 consisted of 26 Type C2 hips, which have no false acetabulum and a femoral head that is free-floating under the gluteal musculature.
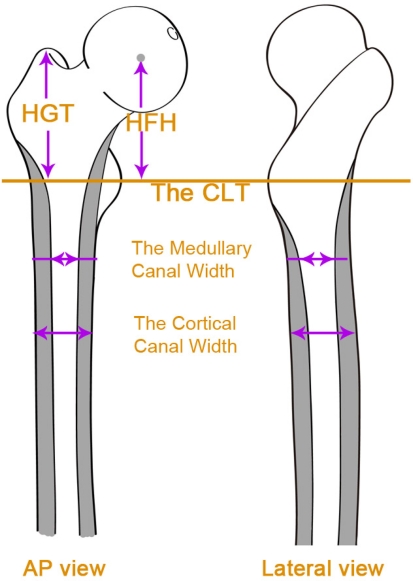
One of us (XHJ) made the following measurements for each femur from AP and ML radiographs. The ML widths of the cortical and medullary canals were measured at 1-cm intervals, from 2 cm above the center of the lesser trochanter (CLT) to 10 cm below the CLT on the AP radiographs [1, 14]. The AP width of the cortical and medullary canals was calculated on the lateral view at 2 cm above the CLT and 2 cm, 4 cm, and 10 cm below the CLT. The canal flare index was calculated as the ratio between the medullary widths of the two levels: 2 cm proximal to the lesser trochanter and at the canal isthmus [8]. The height of dislocation was calculated as the distance from the head-neck junction to the lower edge of the teardrop [2]. The height of the greater trochanter was calculated as the vertical distance from the tip of the greater trochanter to the CLT [14]. The height of the femoral head was calculated as the center of the femoral head to the CLT. Height discrepancies between the femoral head center and the tip of the greater trochanter were calculated [14] (Fig. 2).
Fig. 2.
Measurements were obtained from the AP and lateral views. ML and AP widths were measured at several levels. HGT = height of the greater trochanter; HFH = height of the femoral head.
To verify the reproducibility of the measurements, we randomly selected 20 study subjects and had an independent researcher (YXC) obtain the same measurements. Measurements were repeated at an interval of at least 20 days to verify intraobserver agreement. Intraclass correlation analysis revealed a correlation coefficient of 0.955 for interobserver agreement (p = 0.003) and 0.983 for intraobserver agreement (p = 0.001), showing the measurements were reproducible.
The following variables were normally distributed and differences between the two types determined with a Student’s t test: cortical width from 2 CLT to −5 CLT, medullary width from the CLT to −10 CLT on the AP view, height of dislocation, femoral height, height of the greater trochanter, discrepancy between height of the greater trochanter and femoral head center, cortical and medullary width at the levels AP 2 to AP 4 on the lateral view. For nonnormally distributed variables (cortical width from −6 CLT to −10 CLT, cortical and medullary width at the level AP 10), or normally distributed variables with different variances (medullary width at levels 2 CLT and 1 CLT on the AP view, and medullary width at the level AP 10), we used the Kruskal-Wallis ANOVA. As the variables of the measurements in 13 levels (from 2 CLT to −10 CLT) of the femur were multiple dependent variables, the significance level was adjusted accordingly. The p = 0.05/13 = 0.004 was regarded as the significance level for the series of 13 multiple dependent variables. Statistical analysis was performed using SPSS® software (Version 16.0, SPSS Inc, Chicago, IL).
Results
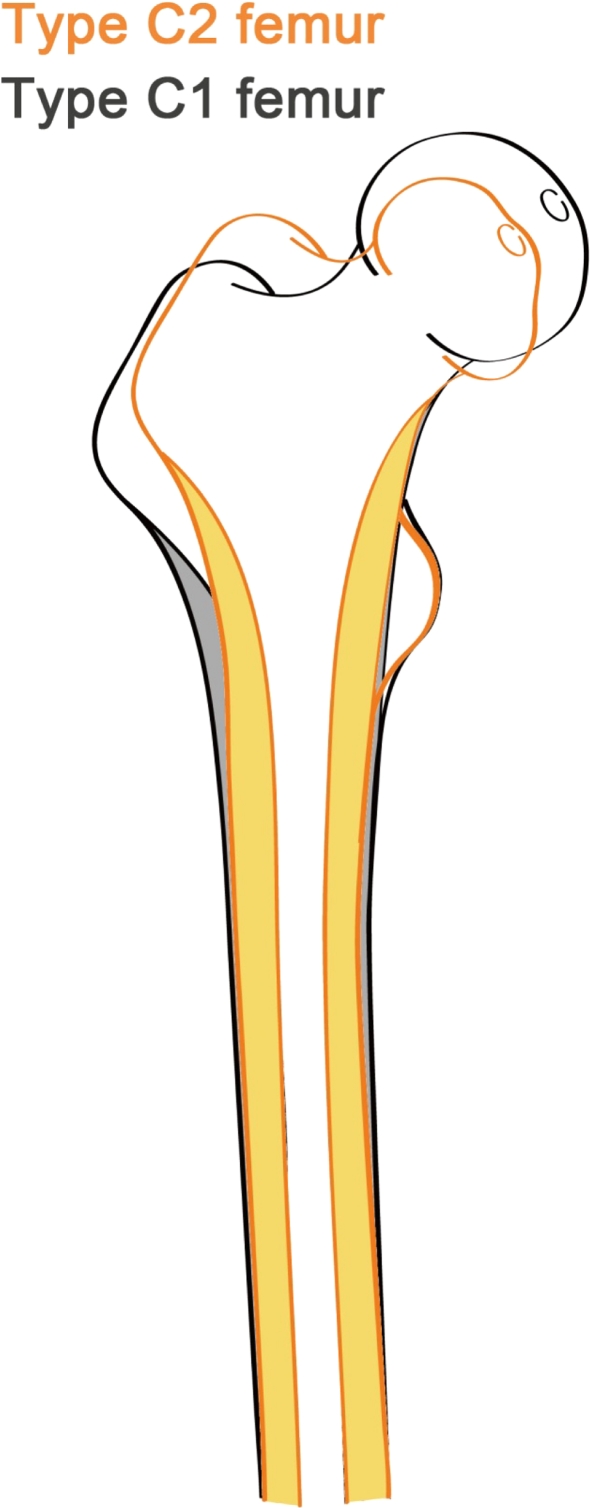
The proximal femur of the C2 high dislocated hips was narrow and stovepipelike (canal flare index < 3) (Fig. 3) as depicted by the differences in dimensions (Table 1). In the coronal plane, the Type C2 femur was narrower than the Type C1 femur in the proximal part, whereas the distal part showed no difference. The medullary width of Type C2 femurs, in levels superior to −2 CLT, was less than that of Type C1 femurs. The cortical width of Type C2 femurs also was less in levels superior to the CLT level. The canal flare index in Type C1 femurs was 3.5 ± 1.2, larger than that of Type C2 femurs (2.7 ± 0.6) (p = 0.002) (Table 1; Fig. 3). This indicated the Type C2 femur was more stovepipe-shaped than the Type C1 femur. In the sagittal plane, we found no difference in any section in terms of femoral AP width between Types C1 and C2 hips (Table 2).
Fig. 3.
This figure shows the morphologic femoral variations of Types C1 and C2 in high DDH.
Table 1.
Cortical and medullary canal widths of femurs according to classification of Hartofilakidis et al.*
| Level | Cortical width (mm) | Medullary width (mm) | ||||
|---|---|---|---|---|---|---|
| C1 Mean ± SD (mm) | C2 Mean ± SD (mm) | p Value | C1 Mean ± SD (mm) | C2 Mean ± SD (mm) | p Value | |
| 2 | 41.7 ± 8.5 | 33.4 ± 6.3 | < 0.001 | 36.2 ± 8.7 | 25.2 ± 4.8 | < 0.001 |
| 1 | 36.0 ± 6.2 | 29.2 ± 4.2 | < 0.001 | 28.1 ± 6.6 | 21.2 ± 3.8 | < 0.001 |
| CLT | 31.2 ± 4.6 | 26.6 ± 3.8 | < 0.001 | 22.8 ± 4.8 | 17.4 ± 3.8 | < 0.001 |
| −1 | 28.4 ± 4.0 | 25.8 ± 3.6 | 0.017 | 19.4 ± 4.0 | 14.9 ± 3.6 | < 0.001 |
| −2 | 26.9 ± 3.2 | 24.3 ± 3.8 | 0.010 | 17.1 ± 3.3 | 13.2 ± 3.4 | < 0.001 |
| −3 | 25.1 ± 2.7 | 23.7 ± 3.1 | 0.085 | 14.3 ± 2.8 | 11.8 ± 3.4 | 0.005 |
| −4 | 24.0 ± 2.7 | 23.1 ± 3.0 | 0.265 | 13.1 ± 2.5 | 11.3 ± 3.1 | 0.023 |
| −5 | 23.6 ± 2.5 | 22.8 ± 2.8 | 0.259 | 12.3 ± 2.4 | 10.8 ± 2.8 | 0.051 |
| -6 | 23.1 ± 2.5 | 22.3 ± 2.5 | 0.268 | 11.7 ± 2.1 | 10.5 ± 2.8 | 0.082 |
| −7 | 22.9 ± 2.5 | 22.2 ± 2.5 | 0.346 | 11.3 ± 2.2 | 10.3 ± 2.6 | 0.176 |
| −8 | 22.5 ± 2.6 | 22.3 ± 2.4 | 0.773 | 10.8 ± 2.2 | 10.0 ± 2.5 | 0.223 |
| −9 | 22.4 ± 2.6 | 22.2 ± 2.2 | 0.714 | 10.5 ± 2.1 | 9.8 ± 2.4 | 0.281 |
| −10 | 22.5 ± 2.5 | 22.3 ± 2.3 | 0.741 | 10.8 ± 2.1 | 9.7 ± 2.3 | 0.080 |
| Isthmus | 22.2 ± 2.8 | 22.0 ± 2.3 | 0.882 | 10.2 ± 1.9 | 9.1 ± 2.4 | 0.129 |
* Types C1 and C2 (AP view); values are expressed as mean ± SD; CLT = center of the lesser trochanter.
Table 2.
Cortical and medullary canal widths of femurs according to classification of Hartofilakidis et al.*
| Level | Cortical width (mm) | Medullary width (mm) | ||||
|---|---|---|---|---|---|---|
| C1 (mean ± SD, mm) | C2 (mean ± SD, mm) | p Value | C1 (mean ± SD, mm) | C2 (mean ± SD, mm) | p Value | |
| AP 2 | 35.0 ± 5.1 | 36.0 ± 5.5 | 0.651 | 28.8 ± 5.2 | 32.3 ± 5.2 | 0.119 |
| AP CLT | 30.2 ± 4.2 | 30.2 ± 3.1 | 0.988 | 20.8 ± 4.6 | 21.6 ± 4.0 | 0.609 |
| AP −2 | 26.6 ± 2.8 | 26.6 ± 2.7 | 0.940 | 16.5 ± 2.6 | 17.1 ± 3.5 | 0.593 |
| AP −4 | 24.9 ± 2.8 | 26.1 ± 2.7 | 0.244 | 14.2 ± 2.3 | 14.7 ± 2.6 | 0.588 |
| AP −10 | 25.9 ± 1.8 | 26.9 ± 3.2 | 0.441 | 13.1 ± 2.4 | 14.0 ± 3.6 | 0.575 |
* Types C1 and C2 hips (lateral view); values are expressed as mean ± SD; CLT = center of the lesser trochanter.
Femoral heads migrated 19.1 mm (45%) more superiorly (p < 0.001) in Type C2 hips (62.0 ± 17.1 mm) than in Type C1 hips (42.9 ± 12.0 mm). The greater trochanter was approximately 8 mm higher (p < 0.001) in Type C2 hips than in Type C1 hips. According to height discrepancy between the greater trochanter and femoral head center, when referring to the femoral head center, the greater trochanter tips in Type C2 hips were 8.2 mm higher (p < 0.001) than in Type C1 hips (Table 3; Fig. 3).
Table 3.
Measurements of femurs according to classification of Hartofilakidis et al.*
| Parameter | C1 Mean ± SD (mm) | C2 Mean ± SD (mm) | p Value |
|---|---|---|---|
| Height of dislocation (mm) | 42.9 ± 12.0 | 62.0 ± 17.1 | < 0.001 |
| Femoral height (mm) | 46.0 ± 8.9 | 45.2 ± 8.8 | 0.746 |
| Height of greater trochanter (mm) | 50.4 ± 6.9 | 58.3 ± 5.1 | < 0.001 |
| Discrepancy between height of greater trochanter and femoral head center* (mm) | 5.0 ± 12.0 | 13.2 ± 11.3 | 0.020 |
* Types C1 and C2 hips (AP view).
Values are expressed as mean ± SD; *the value is positive when the tip of the greater trochanter is higher than the femoral head center.
Discussion
Two kinds of high dislocations are commonly observed and were subtyped by Hartofilakidis et al. as Type C1 and Type C2 [6, 7], according to whether a false acetabulum is present or absent, respectively. Previous studies show the shape of the femur in the hips with DDH is more abnormal with increasing subluxation. We asked whether (1) the proximal femoral shape and dimension in Type C1 and Type C2 hips differ from each other, with different canal width and flare index, and (2) the femur dislocated with the same height in Types C1 and C2 hips.
We note limitations of our study. First, all measurements were based on two-dimensional plain radiographs. Because the rotational position of the femur could influence the results of measuring the diameter of the canal, we measured AP and ML dimensions of the proximal femur. We cannot ensure, however, either of these two dimensions reflects the maximum of the widths. The cortical and medullary widths were similar in the sagittal plane and differed in the coronal plane, indicating the proximal femur was morphologically different between proximal femurs of Types C1 and C2 hips. Rotation would not influence measurements of vertical distance, such as the vertical distance between the center of the femoral head and the line connecting the teardrops [6]. Second, our study considers only the anatomy of the proximal femur and we cannot ascertain the clinical importance other than as applies to implant selection.
Our data suggest that for the distal part of the proximal femur (inferior to the –5 CLT level), the canal width remains the same in Types C1 and C2 hips, whereas in the metaphyseal region, the canals are much narrower in Type C2 hips than in Type C1 hips. Sugano et al. [16] and Noble et al. [13] compared the femurs in high dislocated hips with those of dysplastic hips and found no difference in the femoral width at 35% head center height below the CLT. The canals are much narrower in high dislocated hips than in dysplastic hips at the CLT level and at 35% head center height above the CLT. Compared with Type C1 hips, the proximal femur in Type C2 hips was narrower and more stovepipe-shaped, with a smaller flare index. If a conventional stem for a Type C2 femur is used, the surgeon may find, intraoperatively, the proximal part of the stem is too big for the distal medullary canal, which has been narrowed to fit the chosen stem size. Based on our clinical experience, the risk of fracture in the proximal part of the femur may be greater in Type C2 hips. The variation of canal geometry between Types C1 and C2 hips also requires the proximal femurs in these hips be reconstructed using prosthetic stems with different geometries or proximodistal modular stems.
We found the proximal femur migrated more superiorly in Type C2 hips. Accurate restoration of leg length and balance of soft tissue tension around hips in THA is complicated by substantial superior migration of the proximal femur. Nagoya et al. [12] compared the radiographs of two groups of patients with Crowe Grade IV high dislocated hips: one group of 10 patients had iliofemoral osteoarthritis (similar to Type C1 of Hartofilakidis et al.), the other group of 10 patients had a complete intramuscular dislocation without osteoarthritis (similar to Type C2). They found that mean distalization of the greater trochanter (similar to height of dislocation in our study) in patients without iliofemoral osteoarthritis was 67.8 mm (range, 40 mm–85 mm), and in patients with iliofemoral osteoarthritis was a mean of 36.3 mm (range, 12 mm–50 mm). The discrepancy of mean distalization of the greater trochanter was 31.5 mm. Our research results support the findings of Nagoya et al. [12]. The distalization of the greater trochanter can be regarded as the height of dislocation plus the discrepancy between height of the greater trochanter and femoral height. After calculation, distalization of the greater trochanter in Type C2 femurs was 27 mm higher than in Type C1 femurs. According to our clinical experience, more thorough release of soft tissue or more frequent use of subtrochanteric osteotomy may be needed to reduce the hip, if the metal shell is going to be placed in the original acetabulum in THA for Type C2 hips. The more superior migration of the femur there is, the more substantial the leg length discrepancy. In addition, a free-floating femoral head, as occurs in Type C2 hips, may cause further deterioration of abductor function. All of these factors lead to poorer gait in patients with Type C2 hips.
We speculate that the morphologic variation may be attributable to the load distribution difference between Types C1 and C2 hips. In Type C1 hips, the femoral heads articulate with the iliac wing to form a pseudarthrosis. Even though the stress distribution pattern differs from that of normal hips, body weight is still transmitted through the femoral head, femoral neck, metaphysis, and then the proximal femur. However, in Type C2 hips, the femoral heads are floating in the gluteal musculature, and body weight may be partially directly bypassed by soft tissue to the proximal femur. The femoral head, femoral neck, and metaphyseal region are stress-shielded and thus less developed in Type C2 hips than in Type C1 hips.
There are substantial morphologic differences in proximal femurs between Types C1 and C2 hips. This morphologic variation may require surgeons to use different surgical techniques and prostheses of different shapes and sizes, either monoblock or modular, to reconstruct the proximal femur in a THA.
Acknowledgments
We thank Dr. X.C. Yang for assistance in doing this study. We also thank Katharine O’ Moore-Klopf, ELS, of East Setauket, NY, for providing editorial assistance.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Argenson JN, Ryembault E, Flecher X, Brassart N, Parratte S, Aubaniac JM. Three-dimensional anatomy of the hip in osteoarthritis after developmental dysplasia. J Bone Joint Surg Br. 2005;87:1192–1196. doi: 10.1302/0301-620X.87B9.15928. [DOI] [PubMed] [Google Scholar]
- 2.Crowe JF, Mani VJ, Ranawat CS. Total hip replacement in congenital dislocation and dysplasia of the hip. J Bone Joint Surg Am. 1979;61:15–23. [PubMed] [Google Scholar]
- 3.Erdemli B, Yilmaz C, Atalar H, Guzel B, Cetin I. Total hip arthroplasty in developmental high dislocation of the hip. J Arthroplasty. 2005;20:1021–1028. doi: 10.1016/j.arth.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 4.Eskelinen A, Helenius I, Remes V, Ylinen P, Tallroth K, Paavilainen T. Cementless total hip arthroplasty in patients with high congenital hip dislocation. J Bone Joint Surg Am. 2006;88:80–91. doi: 10.2106/JBJS.E.00037. [DOI] [PubMed] [Google Scholar]
- 5.Hartofilakidis G, Karachalios T. Total hip arthroplasty for congenital hip disease. J Bone Joint Surg Am. 2004;86:242–250. doi: 10.2106/00004623-200402000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Hartofilakidis G, Stamos K, Ioannidis TT. Low friction arthroplasty for old untreated congenital dislocation of the hip. J Bone Joint Surg Br. 1988;70:182–186. doi: 10.1302/0301-620X.70B2.3346284. [DOI] [PubMed] [Google Scholar]
- 7.Hartofilakidis G, Yiannakopoulos CK, Babis GC. The morphologic variations of low and high hip dislocation. Clin Orthop Relat Res. 2008;466:820–824. doi: 10.1007/s11999-008-0131-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khang G, Choi K, Kim CS, Yang JS, Bae TS. A study of Korean femoral geometry. Clin Orthop Relat Res. 2003;406:116–122. doi: 10.1097/00003086-200301000-00019. [DOI] [PubMed] [Google Scholar]
- 9.Kim YH, Kim JS. Total hip arthroplasty in adult patients who had developmental dysplasia of the hip. J Arthroplasty. 2005;20:1029–1036. doi: 10.1016/j.arth.2004.06.033. [DOI] [PubMed] [Google Scholar]
- 10.Lai K, Shen WJ, Huang LW, Chen MY. Cementless total hip arthroplasty and limb-length equalization in patients with unilateral Crowe type-IV hip dislocation. J Bone Joint Surg Am. 2005;87:339–345. doi: 10.2106/JBJS.D.02097. [DOI] [PubMed] [Google Scholar]
- 11.Makita H, Inaba Y, Hirakawa K, Saito T. Results on total hip arthroplasties with femoral shortening for Crowe’s group IV dislocated hips. J Arthroplasty. 2007;22:32–38. doi: 10.1016/j.arth.2006.02.157. [DOI] [PubMed] [Google Scholar]
- 12.Nagoya S, Kaya M, Sasaki M, Tateda K, Kosukegawa I, Yamashita T. Cementless total hip replacement with subtrochanteric femoral shortening for severe developmental dysplasia of the hip. J Bone Joint Surg Br. 2009;91:1142–1147. doi: 10.1302/0301-620X.91B9.21736. [DOI] [PubMed] [Google Scholar]
- 13.Noble PC, Kamaric E, Sugano N, Matsubara M, Harada Y, Ohzono K, Paravic V. Three-dimensional shape of the dysplastic femur: implications for THR. Clin Orthop Relat Res. 2003;417:27–40. [PubMed] [Google Scholar]
- 14.Robertson DD, Essinger JR, Imura S, Kuroki Y, Sakamaki T, Shimizu T, Tanaka S. Femoral deformity in adults with developmental hip dysplasia. Clin Orthop Relat Res. 1996;327:196–206. doi: 10.1097/00003086-199606000-00025. [DOI] [PubMed] [Google Scholar]
- 15.Sener N, Tozun IR, Asik M. Femoral shortening and cementless arthroplasty in high congenital dislocation of the hip. J Arthroplasty. 2002;17:41–48. doi: 10.1054/arth.2002.27672. [DOI] [PubMed] [Google Scholar]
- 16.Sugano N, Noble PC, Kamaric E, Salama JK, Ochi T, Tullos HS. The morphology of the femur in developmental dysplasia of the hip. J Bone Joint Surg Br. 1998;80:711–719. doi: 10.1302/0301-620X.80B4.8319. [DOI] [PubMed] [Google Scholar]