Opinion statement
More attention has been paid to the mitral valve (MV) than the tricuspid valve (TV), and this relative paucity of data has led to confusion regarding the timing of TV surgery. We review the American College of Cardiology/American Heart Association and European Society of Cardiology guidelines to identify areas of concordance (severe tricuspid regurgitation [TR] in a patient undergoing mitral valve surgery); discordance (less than severe TR but with markers for late TR recurrence such as pulmonary hypertension, a dilated TV annulus, atrial fibrillation, permanent transtricuspid pacing wires and others); and disagreement (surgery for primary TR). We provide our perspective from Northwestern University on these issues and where the guidelines are silent (TR in patients undergoing non-mitral valve operations). Finally, we review recent publications on the results of TV repair and replacement. Although there have been scant publications in the past, there have been more useful publications in recent years to guide our decision making.
Introduction
The tricuspid valve (TV) has not received as much attention as the aortic valve (AV) or mitral valve (MV), and hence has been referred to as the “forgotten valve.” Significant tricuspid regurgitation (TR) may be clinically silent for a prolonged period, during which time progressive right ventricle (RV) dilatation and dysfunction may develop, similar to changes that can occur with asymptomatic mitral regurgitation (MR) and its’ effect on LV function. Eventually the TR patient may be managed with diuretics for symptoms and only considered for surgery after advanced RV dysfunction, and even liver dysfunction or cirrhosis, have developed. It should be no surprise, therefore, that results from the Society of Thoracic Surgeons (STS) Database indicate that TV surgery is the most high-risk valve operation in terms of morbidity and mortality [1]. Although MV surgery has evolved over the past few decades toward progressively earlier intervention, even in selected asymptomatic patients, no such evolution has occurred in TV surgery yet [2, 3••]. The relative paucity of articles on the TV compared with AV and MV, and no randomized trials, contribute to this problem. In this article, we review and compare the US and European Guidelines regarding TV surgery, data supporting these guidelines, recent outcome data for TV surgery and repair results, and draw parallels and contrasts to the approach to MV surgery.
US and European guidelines on the management of valvular heart disease
Both the American College of Cardiology/American Heart Association (ACC/AHA) and the European Society of Cardiology (ESC) have produced extensive documents on the management of heart valve disease, including separate sections on TV surgery [2, 3••]. There are some areas of concurrence and others of disagreement (Table 1). Both list a class I indication to perform TV repair for severe TR in patients who are undergoing MV surgery, and that reflects the standard of practice for most surgeons. Both guidelines are silent about performing TV surgery when a surgeon is there for coronary bypass, aortic valve replacement (AVR), or other cardiac surgery. At Northwestern, we would typically perform TV repair in these situations. The ESC Guidelines also list TV surgery as a Class I indication in the case of severe primary TR (or tricuspid stenosis) with symptoms despite medical therapy, without severe RV dysfunction. Somewhat surprisingly, the US guidelines only list this as “reasonable” and a class IIa indication. Perhaps this reflects the reluctance to operate on some of these patients who have already developed irreversible RV dysfunction as the natural history of TR has progressed, but it is one of the rare times that valve disease with symptoms is not listed as a Class I indication for surgery in the guidelines.
Table 1.
Comparison of guidelines for tricuspid valve surgery
| ACC/AHA guidelines [3]a | ESC guidelines [2]b |
|---|---|
| Class I | Class IC |
| 1. TV repair is beneficial for severe TR in patients with MV disease requiring MV surgery (Level of Evidence: B) | Severe TR in a patient undergoing left-sided valve surgery |
| Severe primary TR and symptoms despite medical therapy without severe right ventricular dysfunctionc | |
| Severe TS (± TR), with symptoms despite medical therapy (Percutaneous technique can be attempted as a first approach if TS is isolated) | |
| Severe TS (± TR) in a patient undergoing left-sided valve intervention (Percutaneous technique can be attempted as a first approach if TS is isolated) | |
| Class IIa | Class IIaC |
| 1. TV replacement or annuloplasty is reasonable for severe primary TR when symptomatic. (Level of Evidence: C)c | Moderate organic TR in a patient undergoing left-sided valve surgery |
| 2. TV replacement is reasonable for severe TR secondary to diseased/abnormal tricuspid valve leaflets not amenable to annuloplasty or repair. (Level of Evidence: C) | Moderate secondary TR with dilated annulus (>40 mm) in a patient undergoing left-sided valve surgeryd |
| Severe TR and symptoms, after left-sided valve surgery, in the absence of left-sided myocardial, valve, or right ventricular dysfunction and without severe pulmonary hypertension (systolic pulmonary artery pressure >60 mmHg) | |
| Class IIb | Class IIbC |
| 1. Tricuspid annuloplasty may be considered for less than severe TR in patients undergoing MV surgery when there is pulmonary hypertension or tricuspid annular dilatation. (Level of Evidence: C)d | Severe isolated TR with mild or no symptoms and progressive dilatation or deterioration of right ventricular functione |
| Class III | |
| 1. TV replacement or annuloplasty is not indicated in asymptomatic patients with TR whose pulmonary artery systolic pressure is < 60 mmHg in the presence of normal MV. (Level of Evidence: C)e | |
| 2. TV replacement or annuloplasty is not indicated in patients with mild primary TR. (Level of Evidence: C) |
ACC/AHA American College of Cardiology/American Heart Association; ESC European Society of Cardiology; MV mitral valve; TR tricuspid regurgitation; TS tricuspid stenosis; TV tricuspid valve
aFrom Bonow RO, Carabello BA, Chatterjee K, et al.: 2008 Focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation 2008, 118(15):e523–661, with permission of the publisher. (Copyright © 2008, the American Heart Association)
bFrom Vahanian A, Baumgartner H, Bax J, et al.: Guidelines on the management of valvular heart disease: The Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology. European Heart Journal 2007, 28(2):230–268, with permission of the publisher. (Copyright © 2007, the European Society of Cardiology)
cDifference in approach to patients with symptomatic primary TR
dDifference in approach to patients with less than severe TR
eDifference in approach to patients with asymptomatic primary TR
Class II indications show considerable differences between the two documents. The ESC lists a IIa recommendation for severe TR and symptoms in the unfortunately too-common scenario seen after left-sided valve surgery (without left-sided valve dysfunction, RV dysfunction, or pulmonary hypertension). The US guidelines are silent in this circumstance. Although the repair rate for TV is increasing in the United States (compound annual growth rate of 16.4%) per STS data (Table 2) [1, 4], untreated TR may cause symptoms after MV surgery such that the above clinical situation is not uncommon. However, as the patient will now be a reoperation, clinicians are reticent to perform a reoperative surgery. The best strategy would be to avoid this situation by the more liberal use of TV annuloplasty (TVA) at the initial surgery, and this is reflected in the ESC guidelines; the US guidelines are more conservative, however. It is not clear if the US guidelines were meant to reflect general US practice, or whether they influence the practice and therefore TV surgery is less common here. The ESC gives a Class IIa indication for moderate secondary TR with a dilated annulus (>40 mm) in a patient undergoing left-sided valve surgery [2]. The US guidelines say TVA “may be considered” (Class IIb) when there is TV annular dilatation or pulmonary hypertension [3••].
Table 2.
Number of mitral valve and tricuspid valve proceduresa
| Year | MV repairs ± CAB, n | TV surgeries, n |
|---|---|---|
| 2000 | 4,853 | 1,786 |
| 2001 | 5,926 | 2,276 |
| 2002 | 7,776 | 3,256 |
| 2003 | 8,404 | 4,086 |
| 2004 | 8,287 | 4,466 |
| 2005 | 9,189 | 5,271 |
| 2006 | 9,930 | 5,965 |
| 2007 | 10,276 | 6,088 |
| 2008 | 11,203 | 6,684 |
| 2009 | 11,347 | 7,001 |
CAB coronary artery bypass; MV mitral valve; TV tricuspid valve
aA recent report by the Society of Thoracic Surgeons Database showed a growing number of MV and TV surgeries from 2000-2009. TV surgery procedures increased at a compound annual growth rate of 16.4%
Data from The Society of Thoracic Surgeons Adult Cardiac Surgery Database Spring 2010 Report [4]; with permission
Perhaps one of the most important differences, however is that the US guidelines give a class III indication (not indicated) in asymptomatic patients with TR whose pulmonary artery systolic pressure (PASP) is less than 60 mmHg in the presence of a normal MV [3••]. This is interesting because they now have elevated MV repair to a Class IIa indication in asymptomatic patients if there is a 90% chance of success in an experienced center. With the exception of some organic TR cases, the vast majority of TV surgeries are repair, even in the less experienced US centers. The ESC guidelines for mild or no symptoms with severe isolated TR, and progressive dilatation or deterioration of RV function, give this a IIb (may be considered) recommendation [2]. This is more reflective of the insidious natural history of TR on RV function. It also mirrors the philosophy behind earlier intervention in patients with asymptomatic severe MR to avoid the deleterious effects of inevitable progressive LV dysfunction. Both guidelines reflect a limited ability to analyze the course of functional TR due to the paucity of long-term data, difficulty quantifying the amount of TR, and because they are derived mostly from a consensus of experts, retrospective studies, or registries.
TV and other valve surgery
Data from the STS demonstrates the TR repair rate for patients undergoing isolated MV repair with preoperative 4+ TR was 80%; for those with 3+ TR it was 30%; and for those with 2+ TR it was 5%. Data from our Northwestern database show those rates for 4+ are 100% (93% repair, 7% replace), 50% for 3+ TR, and 21% for 2+ TR. For the 50% of Northwestern patients with uncorrected 3+ TR, these were generally elderly patients with previous surgeries, no history of right heart failure, and resolution of TR before surgery. Some patients with ≤2+ TR underwent repair, if there was a history of right heart failure, left ventricular ejection fraction (LVEF) <35%, pulmonary hypertension, and/or TV annular diameter >40 mm as measured in any dimension by intra-operative transesophageal echocardiography. This practice is more in keeping with the ESC guidelines. We used a rigid remodeling ring, size 26 mm or 28 mm, in 93% of patients. If the TV was severely tethered or had extensive leaflet damage, it was replaced (chord-sparing) in 7% of patients with a bioprosthetic valve. Permanent pacemakers (PPM) or implantable cardioverter-defibrillators (ICD) were removed and replaced with epicardial pacing wires or secured in the septal-posterior commissure because they are known to reduce repair durability [5].
Class II indications for surgery
Annular Size
Dreyfuss et al. [6] used tricuspid annular dilatation as the indication for TV repair, regardless of TR grade. Data from their long-term prospective series of 311 patients with no clinically significant preoperative TR in 98% (preoperative ≤1+ TR in 88%) showed that 148 patients had a large annulus diameter (≥70 mm) and were treated by TVA. Preoperative mean TR grade was 0.7 in untreated (n = 163) and 0.9 in TVA groups (n = 148). Mean follow-up was 4.8 years. TR increased by ≥2 grades in the uncorrected TR group and was more than in the TVA group (48% vs 2%; P < 0.001). The uncorrected group demonstrated significant worsening in New York Heart Association (NYHA) class (1.6 vs 1; P < 0.0001) and similar survival (85% vs 90%; P = NS) at 10 years. A similar study by Sarraj et al. [7] used the cut-off value of tricuspid diameter >21 mm/m2 (measured by four-chamber echocardiogram) to perform tricuspid repair in 17 patients with severe TR, and PASP <60 mmHg in 53%. At 2.5 years of follow-up, the authors observed a significant decrease of mean TR from 3.5 to 1.6 (P < 0.001) and substantial reduction of moderate/severe dilatation of TV annulus from 100% to 0 (P < 0.001). No operative mortality was reported and overall mortality was 6%.
Pulmonary artery hypertension and ischemic cardiomyopathy
In a study of 237 patients with preoperative symptomatic severe TR (>60%), NYHA III/IV (>50%), and mildly reduced EF, Ghanta et al. [8] observed that after tricuspid repair, patients with recurrent TR had a higher postoperative PASP compared with patients without recurrent TR (52 mmHg vs 43 mmHg). In a study of patients with low EF and pulmonary hypertension, significant ≥3+ TR was higher compared with patients with no pulmonary hypertension (37% vs 3%; P < 0.0001) during 2 years of follow-up after TV and MV repair [9•]. Absence of residual TR after TV repair in 17 patients with preoperative symptomatic severe TR was associated with significant reduction of moderate/severe pulmonary hypertension from 94% to 30% (P < 0.002) [7]. In a study of patients with predominantly nonischemic mitral disease undergoing concomitant TV repair, significant decrease of residual TR from 42% to 3% (P < 0.001) was associated with a substantial reduction of PASP from 48 mmHg to 34 mmHg (P < 0.001) [10]. These studies suggest that elevated PASP negatively impacts mid-term recurrent TR.
Matsunaga and Duran [11] reported in 70 patients with ischemic mitral regurgitation (IMR), after revascularization and MV surgery, that the incidence of TR increased from 25% at <1 year to 74% at ≥3 years, independent of whether TR was corrected or ignored. Residual significant TR was 64% and associated with the return of functional MR in 31% at last follow-up. Similarly, Bove et al. [12] reported in 78 IMR patients a prevalence of significant TR in 22% and recurrent MR was present in 9% during 2 years of follow-up after mitral repair. These studies suggest the relationship of functional TR with functional MR, and mutually related with poor LV function. Calafiore et al. [13•] have demonstrated that functional TR progression is not related to recurrent MR in patients with low EF but impacts prognosis when left untreated. MV and TV surgery was performed in 110 patients with severe IMR (EF ≤35%) and co-existent significant TR. Lower residual TR was observed in the TV repair group compared with the uncorrected group (5% vs 40%; P < 0.001). Both groups had similar recurrent MR at 5%. Five-year survival rate was 75% in TV repair group versus 45% in the no-repair group (P = 0.004).
Primary TV surgery
The ACC/AHA practice guidelines appear to be inconsistent in interpreting studies concerning asymptomatic or mild primary TR with apparently preserved RV hemodynamics and no annular dilatation. TV surgery is not indicated in asymptomatic and mild primary TR patients with PASP <60 mmHg in the absence of MV disease according to the established guidelines (Table 1) [3••]. As explained in the guidelines, moderate or severe TR may be present without classic clinical features. Likewise, they state “Patients with severe TR of any cause have a poor long-term outcome because of RV dysfunction and/or systemic venous congestion” [3••]. A study from the Mayo Clinic examined 26 asymptomatic TR patients (NYHA functional class I and no history of heart failure) caused by tricuspid flail leaflets [14]. By 10 years, cumulative high event rates were observed in 75% of patients with symptoms of heart failure, atrial fibrillation (AF), surgical intervention, or death (Fig. 1). TV repair was performed in 82% of patients with a low mortality (3%) and marked symptomatic improvement in 88% of patients. Conversely, excess mortality and high morbidity were observed in the unoperated group. The authors suggest that surgical intervention should be considered early in the course of the disease before the occurrence of irreversible consequences. ACC/AHA guidelines suggest this is not indicated (Class III) but note that if left untreated, it will lead to a “poor long-term outcome.” At Northwestern, we believe early surgery is justified, as this is an insidious disease with a definite mortality and morbidity risk. Asymptomatic patients may have clinically silent severe RV enlargement with apparently normal RV pressure, and no pulmonary hypertension and mitral disease. Messika-Zeitoun et al. [14] initially found only 27% with severe right-sided chamber enlargement and the rest had normal right-sided chamber dimension. However, by 5 years, patients had developed severe right-sided chamber enlargement, even in those who did not have any chamber enlargement at initial presentation (39% no enlargement vs 87% with enlargement; P < 0.01). This prolonged latent period led to a high 15-year event rate (69% with symptoms of heart failure, AF, cardiac surgery, or death). This is particularly important because patients with post-traumatic lesions are usually young when complications occur. The outcome was characterized by excess mortality (36% at 10 years or 3.8% yearly) [14]. Being “asymptomatic” does not guarantee that the heart has not undergone TR-related ventricular changes. Therefore, like asymptomatic MR with flail leaflets, early surgery should be favored, particularly because successful repair is highly likely.
Figure 1.
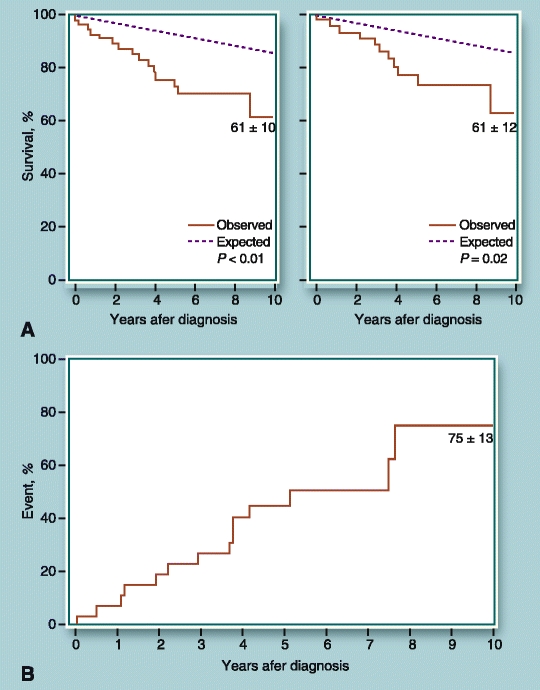
Outcomes of Mayo Clinic patients with primary tricuspid regurgitation (TR) caused by flail tricuspid valve (TV) leaflets. A Observed survival compared with expected survival for a matched population in the United States. Left panel shows excess mortality observed in patients with tricuspid flail leaflets with or without associated disease. Right panel shows high mortality of patients with symptomatic and asymptomatic primary TR with exclusion of cohort analyzed from left panel of TR patients with associated disease. B Incidences of combined endpoint of symptoms, heart failure, new atrial fibrillation, cardiac surgery, or death in asymptomatic TR patients. (Reprinted from Messika-Zeitoun D, Thomson H, Bellamy M, et al. Medical and surgical outcome of tricuspid regurgitation caused by flail leaflets. Journal of Thoracic and Cardiovascular Surgery 2004, 128(2):296–302, with permission from Elsevier. Copyright © 2003, the American Association for Thoracic Surgery.).
Factors in the development of recurrent TR
Atrial fibrillation
Data from a long-term study in 90 MV surgery patients with preoperative AF and no TR demonstrated that severe TR developed in 7.5% and was associated with an event-free survival rate of 71% at 13 years [15]. In another study from that group, significant reduction of recurrent TR was achieved in 38% of MV patients undergoing concomitant Maze procedure compared with no-Maze patients (15% vs 40%; P = 0.005) with an average follow-up of 7.6 years [16]. Similarly, a case-matched study by Stulak et al. [17•] from the Mayo Clinic found that in mitral repair patients undergoing concomitant Maze, a much lower recurrent TR rate was observed compared with the untreated AF group (9% vs 45%; P < 0.001) at last follow-up. Left atrial size continued to increased significantly in patients with persistent AF during follow-up when compared with Maze patients who converted to normal sinus rhythm (P = 0.05) [17•]. Another study by Stulak et al. [18•] found that patients with preoperative moderate TR and no history of AF may develop late-onset AF (5 years, 11%; 10 years, 23%) with a reduced survival of 62%, even after successful correction of MR and TR.
Permanent pacemaker or ICD leads
Lin et al. [19] reviewed their series of TV surgery due to symptomatic severe TR caused by previously placed PPM or implanted ICD leads in 41 patients. Mean time from PPM or ICD placement to reoperation was 6 years. The cause of TR was impingement of the lead on the TV leaflets in 40%, leaflet perforation, entanglement of the TV apparatus, and adhesion of the lead to the TV leaflet. Half of the patients underwent TV repair in the absence of extensive TV leaflet damage. Otherwise, the lead was removed or repositioned away from the affected leaflet, secured in the recess of either the posteroseptal or anteroposterior commissure, or the defect in the leaflet was suture-repaired. The rest of the patients underwent TV replacement because of extensive leaflet damage, and the pacing lead was positioned external to the sewing ring of the prosthesis. Symptomatic improvement was observed during an 8-year follow-up. McCarthy et al. [5] showed that 5 years after TV repair, 42% of patients with preoperative PPM had 3+ or 4+ TR, almost double the incidence of those without a pacemaker (P = 0.04). Similarly, Navia et al. [20•] from a later Cleveland Clinic series found in the 15% of patients with preoperative PPM, significant TR was present in 45% 5 years after TV repair. These studies suggest removing PPM and replacing with epicardial leads at the time of surgery may be the best solution [5].
TV tethering, ventricular dilatation and dysfunction
Fukuda et al. [21] showed that measuring distance and area of TV tethering is a reliable diagnostic criterion to predict the severity of early residual TR after TV repair. Later, Fukuda et al. [22] reported in patients with preoperative tethering height greater than 1.0 cm, repair failure was 55% immediately after repair, suggesting that tethering height is also a predictive factor for greater residual TR. A recent study used TV leaflet tethering distance of >8 mm or a tethering area of >16 mm2 as the threshold for performing an adjunctive procedure (pericardial patch to anterior leaflet) to lessen leaflet tethering [23•]. Low recurrent TR of 2% and 8% of patients was realized at 1 month and 1 year, respectively. In another study, Fukuda et al. [24] found a significant correlation of higher RV pressures and TR severity in 39 patients after TV repair. Patients with early postoperative LV dysfunction had sustained higher RV pressures after follow-up compared with patients with preserved LV function. de Bonis et al. [9•] reported outcomes after mitral repair in 78 patients with dilated cardiomyopathy (EF ≤31%) and co-existent TR ≤2+ and RV dysfunction. Uncorrected TR progressed to TR severity ≥2 in 18%.
Summary
In summary, there is a significant risk for residual TR if it is left untreated in patients with a dilated annulus, ischemic cardiomyopathy, and pulmonary hypertension. These are patients that we approach more aggressively at Northwestern, similar to ESC guidelines. There is a significant risk for recurrent TR after TVA for patients with permanent pacing wires, a history of AF, and RV dysfunction with dilatation and leaflet tethering. All these factors need to be properly weighed in regards to the decision whether or not to perform TV surgery and how to perform TVA.
Treatment
Operative Techniques and Outcomes
Repair Techniques: Ring and Suture
The goal of tricuspid repair is to reduce annular dilatation and lessen TV leaflet tethering, thereby stabilizing the annulus and increasing leaflet coaptation.
Techniques broadly include 1) rings, such as flexible rings and bands, and rigid remodeling rings [5, 10, 20•, 22, 24, 25]; 2) Suture annuloplasties, such as bicuspidization [8], partial purse-string (De Vega), modified De Vega; and 3) pericardial annuloplasty [5, 20•]. Other techniques may include 1) edge-to-edge “Clover” technique by suturing the free margins of the tricuspid leaflets in conjunction with ring annuloplasty [26]; 2) anterior tricuspid leaflet augmentation to increase leaflet coaptation and relief of tethered leaflets [27•]; and 3) a very preliminary RV reduction technique used to plicate the RV wall by placement of two strips of felts on the epicardial surface. This method reduced the RV cavity and approximated the papillary muscles [28].
Matsuyama et al. [29] reported by 3 years, 3+4+ TR redeveloped in 6% of patients in the ring group compared with 45% in the DeVega group. Navia et al. [20•] reported by 3 months that 3+4+ TR was present in 11% of patients. By 5 years, significant TR redeveloped in 10% to 16% in the ring group; however, a trend for higher incidence of TR was observed in the suture group (19% for Kay and 24% for De Vega). Ghanta et al. [8] found TR recurrence was 31% in the ring group compared with 25% in the “bicuspidization” group at average follow-up of 3 years. In another study, a newly developed suture pericardial strip annuloplasty was compared with conventional suture annuloplasties [30]. By 3 years, 3+4+ TR redeveloped in 10% in the pericardial strip group versus 16% in the conventional group. Long-term studies by Tang et al. [31] demonstrated significantly better freedom from recurrent TR in the ring than suture repairs (83% vs 39%; P = 0.003) at 15 years. Despite significant TV and RV dysfunction, ring repairs proved to be more durable than suture repairs. Ring annuloplasty predicted better long-term and event-free survival. Mid-term studies of ring, suture, and pericardial annuloplasties have an 8% to 14% prevalence of rapid 3+4+ TR recurrence within a week of surgery [5, 8, 20•, 22, 30]. Risk factors for repair failure included higher preoperative TR [5, 8, 20•, 30], higher PASP [8], larger ring size, MV replacement rather than repair, worse LV dysfunction [5], increased LV remodeling [20•], suture [30] and Peri-Guard annuloplasties), and presence of pacemakers [5].
Anterior tricuspid leaflet augmentation: early studies
Dreyfuss et al. [27•] reported 15 patients with dilated cardiomyopathy and post-heart transplantation undergoing isolated tricuspid repair due to severe TR, annular dilatation (≥40 mm), and tethering (height ≥than 8 mm). Leaflet augmentation concomitant with ring annuloplasty was performed. Mean follow-up was 6 to 20 months. Results of pre-discharge and last follow-up echocardiography reported no residual or recurrent TR. Coaptation length of at least 5 mm was successful in all patients. The tethering height was unchanged with better leaflet coaptation. Available data in 5 patients were reported to be in class I or II. Roshanali et al. [23•] reported 210 patients undergoing concomitant MV surgeries. Half of the patients received ring and suture De Vega annuloplasties. High preoperative tethering index (distance >8 mm or area >16 mm2 measured by echocardiography) was used as an indication to perform concomitant pericardial patch augmentation either with ring or suture, and patients were equally distributed. By 1 year, higher recurrent TR was present in increasing group order: ring/patch 8%, suture/patch 10%, isolated ring 14%, and isolated suture 28%. The investigators conclude that pericardial patch augmentation technique appears to relieve severe TV tethering; however, this repair technique may be unsuitable in reoperations and multiple valve surgeries. Careful patient selection and accurate assessment of preoperative TV tethering are necessary to prevent repair failure.
TV replacement
In patients with primary TV disease, TV repair is associated with better early, mid-term, and event-free survival than TV replacement (5 years at 90% vs 63%; 10 years at 76% vs 55%; P < 0.001) [32]. Moderate to severe RV dysfunction was significantly lower in the TV repair group (repair, 9%; replacement, 28%). However, in patients with combined TV and MV disease, Moraca et al. [33•] found no difference in survival benefits between TV repair and replacement. In the propensity-matched study, operative mortality was similar (both high) for TV repair and replacement (18% vs 13%), and late survival was similar (5 years at 72% vs 79%; 10 years at 66% vs 49%). Other investigations also demonstrated no difference between procedures, despite a higher incidence of preoperative TR severity and risk factors in the TV replacement group [34, 35]. The incidence of redo TV surgeries was not significantly different between groups [35].
Conclusions
Reassessment of the current practice guidelines is essential, as we develop more information on the poor natural history of TR and the risk factors for late TR with untreated, or inadequately treated, TR during an earlier surgery. Determining which of the current repair procedures provide the best long-term outcomes are necessary, in particular for those patients with severe tethering. Investigations of adjunctive or other repair techniques are warranted or the use of chord-sparing TV replacement.
Acknowledgments
Disclosure
Patrick M. McCarthy is a consultant to Edwards Lifesciences and Inventor of the
Edwards MC3 Tricuspid Repair Ring. Virna L. Sales reports no potential conflict of interest relevant to this article.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Rankin JS, Hammill BG, Ferguson TB, Jr, et al. Determinants of operative mortality in valvular heart surgery. J Thorac Cardiovasc Surg. 2006;131:547–557. doi: 10.1016/j.jtcvs.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 2.Vahanian A, Baumgartner H, Bax J, et al. Guidelines on the management of valvular heart disease: The Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology. Eur Heart J. 2007;28:230–268. doi: 10.1093/eurheartj/ehl428. [DOI] [PubMed] [Google Scholar]
- 3.Bonow RO, Carabello BA, Chatterjee K, et al. Focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation. 2008;118:e523–e661. doi: 10.1161/CIRCULATIONAHA.108.190748. [DOI] [PubMed] [Google Scholar]
- 4.The Society of Thoracic Surgeons Adult Cardiac Surgery Database Spring 2010 Report. Available at http://www.sts.org/documents/pdf/ndb2010/1stHarvestExecutiveSummary%5B1%5D.pdf. Accessed August 14, 2010.
- 5.McCarthy PM, Bhudia SK, Rajeswaran J, et al. Tricuspid valve repair: durability and risk factors for failure. J Thorac Cardiovasc Surg. 2004;127:674–685. doi: 10.1016/j.jtcvs.2003.11.019. [DOI] [PubMed] [Google Scholar]
- 6.Dreyfus GD, Corbi PJ, Chan KM, Bahrami T. Secondary tricuspid regurgitation or dilatation: which should be the criteria for surgical repair? Ann Thorac Surg. 2005;79:127–132. doi: 10.1016/j.athoracsur.2004.06.057. [DOI] [PubMed] [Google Scholar]
- 7.Sarraj A, Nuche JM, Dominguez L, et al. Adjustable segmental tricuspid annuloplasty: technical advantages and midterm results. Ann Thorac Surg. 2009;87:1148–1153. doi: 10.1016/j.athoracsur.2009.01.030. [DOI] [PubMed] [Google Scholar]
- 8.Ghanta RK, Chen R, Narayanasamy N, et al. Suture bicuspidization of the tricuspid valve versus ring annuloplasty for repair of functional tricuspid regurgitation: midterm results of 237 consecutive patients. J Thorac Cardiovasc Surg. 2007;133:117–126. doi: 10.1016/j.jtcvs.2006.08.068. [DOI] [PubMed] [Google Scholar]
- 9.De Bonis M, Lapenna E, Sorrentino F, et al. Evolution of tricuspid regurgitation after mitral valve repair for functional mitral regurgitation in dilated cardiomyopathy. Eur J Cardiothorac Surg. 2008;33:600–606. doi: 10.1016/j.ejcts.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 10.Jeong DS, Kim KH. Tricuspid annuloplasty using the MC3 ring for functional tricuspid regurgitation. Circ J. 2010;74:278–283. doi: 10.1253/circj.CJ-09-0231. [DOI] [PubMed] [Google Scholar]
- 11.Matsunaga A, Duran CM. Progression of tricuspid regurgitation after repaired functional ischemic mitral regurgitation. Circulation. 2005;112(9 Suppl):I453–I457. doi: 10.1161/CIRCULATIONAHA.104.524421. [DOI] [PubMed] [Google Scholar]
- 12.Bove T, Van Belleghem Y, Vandenplas G, et al. Short-term systolic and diastolic ventricular performance after surgical ventricular restoration for dilated ischemic cardiomyopathy. Eur J Cardiothorac Surg. 2009;35:995–1003. doi: 10.1016/j.ejcts.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 13.Calafiore AM, Gallina S, Iaco AL, et al. Mitral valve surgery for functional mitral regurgitation: should moderate-or-more tricuspid regurgitation be treated? a propensity score analysis. Ann Thorac Surg. 2009;87:698–703. doi: 10.1016/j.athoracsur.2008.11.028. [DOI] [PubMed] [Google Scholar]
- 14.Messika-Zeitoun D, Thomson H, Bellamy M, et al. Medical and surgical outcome of tricuspid regurgitation caused by flail leaflets. J Thorac Cardiovasc Surg. 2004;128(2):296–302. doi: 10.1016/j.jtcvs.2004.01.035. [DOI] [PubMed] [Google Scholar]
- 15.Kwak JJ, Kim YJ, Kim MK, et al. Development of tricuspid regurgitation late after left-sided valve surgery: a single-center experience with long-term echocardiographic examinations. Am Heart J. 2008;155:732–737. doi: 10.1016/j.ahj.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 16.Kim HK, Kim YJ, Kim KI, et al. Impact of the Maze operation combined with left-sided valve surgery on the change in tricuspid regurgitation over time. Circulation. 2005;112(9 Suppl):I14–I19. doi: 10.1161/CIRCULATIONAHA.104.524496. [DOI] [PubMed] [Google Scholar]
- 17.Stulak JM, Schaff HV, Dearani JA, et al. Restoration of sinus rhythm by the Maze procedure halts progression of tricuspid regurgitation after mitral surgery. Ann Thorac Surg. 2008;86:40–44. doi: 10.1016/j.athoracsur.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 18.Stulak JM, Suri RM, Dearani JA, et al. When should prophylactic maze procedure be considered in patients undergoing mitral valve surgery? Ann Thorac Surg. 2010;89:1395–1401. doi: 10.1016/j.athoracsur.2010.02.018. [DOI] [PubMed] [Google Scholar]
- 19.Lin G, Nishimura RA, Connolly HM, et al. Severe symptomatic tricuspid valve regurgitation due to permanent pacemaker or implantable cardioverter-defibrillator leads. J Am Coll Cardiol. 2005;45:1672–1675. doi: 10.1016/j.jacc.2005.02.037. [DOI] [PubMed] [Google Scholar]
- 20.Navia JL, Nowicki ER, Blackstone EH, et al. Surgical management of secondary tricuspid valve regurgitation: annulus, commissure, or leaflet procedure? J Thorac Cardiovasc Surg. 2010;139:1473–1482. doi: 10.1016/j.jtcvs.2010.02.046. [DOI] [PubMed] [Google Scholar]
- 21.Fukuda S, Song JM, Gillinov AM, et al. Tricuspid valve tethering predicts residual tricuspid regurgitation after tricuspid annuloplasty. Circulation. 2005;111:975–979. doi: 10.1161/01.CIR.0000156449.49998.51. [DOI] [PubMed] [Google Scholar]
- 22.Fukuda S, Gillinov AM, McCarthy PM, et al. Echocardiographic follow-up of tricuspid annuloplasty with a new three-dimensional ring in patients with functional tricuspid regurgitation. J Am Soc Echocardiogr. 2007;20:1236–1242. doi: 10.1016/j.echo.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 23.Roshanali F, Saidi B, Mandegar MH, et al. Echocardiographic approach to the decision-making process for tricuspid valve repair. J Thorac Cardiovasc Surg. 2010;139:1483–1487. doi: 10.1016/j.jtcvs.2009.08.035. [DOI] [PubMed] [Google Scholar]
- 24.Fukuda S, Gillinov AM, McCarthy PM, et al. Determinants of recurrent or residual functional tricuspid regurgitation after tricuspid annuloplasty. Circulation. 2006;114(1 Suppl):I582–I587. doi: 10.1161/CIRCULATIONAHA.105.001305. [DOI] [PubMed] [Google Scholar]
- 25.Filsoufi F, Salzberg SP, Coutu M, Adams DH. A three-dimensional ring annuloplasty for the treatment of tricuspid regurgitation. Ann Thorac Surg. 2006;81:2273–2277. doi: 10.1016/j.athoracsur.2005.12.044. [DOI] [PubMed] [Google Scholar]
- 26.Lapenna E, De Bonis M, Verzini A, et al. The clover technique for the treatment of complex tricuspid valve insufficiency: midterm clinical and echocardiographic results in 66 patients. Eur J Cardiothorac Surg. 2010;37:1297–1303. doi: 10.1016/j.ejcts.2009.12.020. [DOI] [PubMed] [Google Scholar]
- 27.Dreyfus GD, Raja SG, John Chan KM. Tricuspid leaflet augmentation to address severe tethering in functional tricuspid regurgitation. Eur J Cardiothorac Surg. 2008;34:908–910. doi: 10.1016/j.ejcts.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 28.Kappert U, Tugtekin SM, Ouda A, et al. Right ventricular reduction as an adjunct procedure in tricuspid valve repair. Ann Thorac Surg. 2008;85:e27–e29. doi: 10.1016/j.athoracsur.2008.01.083. [DOI] [PubMed] [Google Scholar]
- 29.Matsuyama K, Matsumoto M, Sugita T, et al. De Vega annuloplasty and Carpentier-Edwards ring annuloplasty for secondary tricuspid regurgitation. J Heart Valve Dis. 2001;10:520–524. [PubMed] [Google Scholar]
- 30.Chang BC, Song SW, Lee S, et al. Eight-year outcomes of tricuspid annuloplasty using autologous pericardial strip for functional tricuspid regurgitation. Ann Thorac Surg. 2008;86:1485–1492. doi: 10.1016/j.athoracsur.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 31.Tang GH, David TE, Singh SK, et al. Tricuspid valve repair with an annuloplasty ring results in improved long-term outcomes. Circulation. 2006;114(1 Suppl):I577–I581. doi: 10.1161/CIRCULATIONAHA.105.001263. [DOI] [PubMed] [Google Scholar]
- 32.Singh SK, Tang GH, Maganti MD, et al. Midterm outcomes of tricuspid valve repair versus replacement for organic tricuspid disease. Ann Thorac Surg. 2006;82:1735–1741. doi: 10.1016/j.athoracsur.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 33.Moraca RJ, Moon MR, Lawton JS, et al. Outcomes of tricuspid valve repair and replacement: a propensity analysis. Ann Thorac Surg. 2009;87:83–88. doi: 10.1016/j.athoracsur.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 34.Park CK, Park PW, Sung K, et al. Early and midterm outcomes for tricuspid valve surgery after left-sided valve surgery. Ann Thorac Surg. 2009;88:1216–1223. doi: 10.1016/j.athoracsur.2009.04.121. [DOI] [PubMed] [Google Scholar]
- 35.Guenther T, Noebauer C, Mazzitelli D, et al. Tricuspid valve surgery: a thirty-year assessment of early and late outcome. Eur J Cardiothorac Surg. 2008;34:402–409. doi: 10.1016/j.ejcts.2008.05.006. [DOI] [PubMed] [Google Scholar]


