Abstract
The majority of breast cancer and prostate cancer patients with metastatic disease will go on to develop bone metastases, which contribute largely to patient morbidity and mortality. Numerous small animal models of cancer metastasis to bone have been developed in order to study tumor-induced bone destruction, but the advancement of imaging modalities utilized for these models has lagged significantly behind clinical imaging. Therefore, there is a significant need for improvements to live small animal imaging, particularly when obtaining high resolution images for longitudinal quantitative analyses. Recently, live animal micro-Computed Tomography (μCT) has gained popularity due to the ability to obtain high resolution, 3-dimensional images. However, the utility of μCT in bone metastasis models has been limited to end-point analyses due to off-target radiation effects on tumor cells. We hypothesized that live animal in vivo μCT can be utilized to perform reproducible and quantitative longitudinal analyses of bone volume in tumor bearing mice, particularly in a drug treatment model of breast cancer metastasis to bone. To test this hypothesis we utilized the MDA-MB-231 osteolytic breast cancer model in which the tumor cells are inoculated directly into the tibia of athymic nude mice and imaged mice weekly by Faxitron (radiography), Imtek μCT (in vivo), and Maestro (GFP-imaging). Ex-vivo μCT and histology were performed at end-point for validation. After establishing a high resolution scanning protocol for the Imtek CT, we determined whether clear, measurable differences in bone volume were detectable in mice undergoing bisphosphonate drug treatments. We found that in vivo μCT can be used to obtain quantifiable and longitudinal images of the progression of bone destruction over time without altering tumor cell growth. Additionally, we found that we could detect lesions as early as week one and that this approach could be used to monitor the effect of drug treatment on bone. Taken together, these data indicate in vivo μCT is an effective and reproducible method for longitudinal monitoring of tumor-associated bone destruction in mouse models of tumor-induced bone disease.
Keywords: microCT, faxitron, radiography, imaging, osteolysis, bone destruction
1. Background
Nearly 70% of breast cancer and 90% of prostate cancer patients with metastatic disease will develop bone metastases [1]. In order to investigate breast and prostate tumor effects on bone, several small animal models have been developed that are capable of producing pathogenesis strikingly similar to the clinical condition in both tumor burden and bone disease [2–6]. While these animal models accurately reflect the bone disease portion of the clinical disease, small animal imaging has lagged significantly behind clinical imaging. This has hindered pre-clinical rodent models in which drug treatments typically commence upon visual evidence of bone disease and is particularly limiting due to the difficulty of detecting tumors in animal bone as early as can be detected in human patients. In addition, the lack of precise small animal imaging has limited quantitative longitudinal analyses of animals in drug treatment studies. Therefore, significant improvements to small animal imaging modalities are needed.
Over the past 20 years the primary methods for monitoring cancer bone disease in small animal models have relied heavily upon radiography using Faxitron analyses [7]. While this approach has been very successful, in our experience it is difficult to accurately view the entire skeleton and small, early stage lesions are undetectable by radiography. In recent years micro-Computed Tomography (μCT) analyses have gained popularity due to the ability to obtain high resolution 3-dimensional images and the degree of accuracy exhibited by this imaging modality. While the highest resolution images can be obtained by ex-vivo scanners, such as the Scanco μCT, the utility of these scanners is obviously limited to endpoint analyses. Recent reports combine μCT evaluation with other imaging techniques such as magnetic resonance imaging (MRI) and fluorescence stereomicroscopy to generate a more complete evaluation of the bone microenvironment in metastatic cancer [8], but the limitation of end-point analyses persists. Our group has previously used live animal μCT successfully for imaging bone, but found that high resolution scans killed the tumor cells (unpublished data), limiting its utility to endpoint analyses. In addition, it was recently reported that certain doses of CT radiation may enhance metastasis to specific sites in bone [9].
Missbach-Guetner et al. addressed several of these issues utilizing a 3-dimensional flat-panel detector-based Volume Computed Tomography (fpVCT); however, the detector in use is fairly uncommon, and the majority of quantification performed was primarily 2-dimensional measurements. Quantitative analysis of 3-dimensional bone volume was only reported in one mouse, lacking statistical significance, and the method used for quantification was unclear [10].
In this study, we set out to determine if live animal μCT can be utilized to perform reproducible and quantitative longitudinal analyses of bone volume in tumor bearing mice, particularly in a drug treatment model of breast cancer metastasis to bone. To do this we utilized the MDA-MB-231 osteolytic breast cancer model in which the tumor cells are inoculated directly into the tibia of athymic nude mice and imaged mice weekly by Faxitron (radiography), Imtek μCT (in vivo), and Maestro (GFP-imaging). Scanco μCT (ex-vivo) and histology were performed at end-point for validation. After establishing a high resolution scanning protocol for the Imtek CT, we determined whether clear, measurable differences in bone volume were detectable in mice undergoing bisphosphonate drug treatments. Bisphosphonates are clinically utilized in breast and prostate cancer metastasis to bone in order to palliate tumor-associated bone pain and prevent the recurrence of skeletal related events by binding to the surface of bone and inducing osteoclast apoptosis [11]. We found that in vivo μCT can be used to obtain quantifiable and longitudinal images of the progression of bone destruction over time without altering tumor cell growth. Additionally, we found that we could detect lesions as early as week one and that this approach could be used to monitor the effect of drug treatment on bone.
2. Methods
2.1 Single Group Longitudinal Study
Preliminary studies were first performed on 16 mice to determine the effect of weekly irradiation on this cell line. Mice were broken down into four groups, and two different μCT protocols were performed using the Imtek MicroCAT II, one high resolution (Bin-2), and one lower resolution (Bin-4), with imaging being performed 1 (n=4), 2 (n=6), or 3 time(s) (n=3 Bin-2, n=3 Bin-4) during the four week study, with the group scanned one time receiving the CT scan only at sacrifice. The Bin-2 protocol used 80 kVp, 500 μA with 900 msec per projection and 600 projections over 360° for a total scan time of approximately 20 minutes, while the Bin-4 protocol was acquired with 80kVp, 500μA with 600msec per projection and 300 projections over 360° for a total scan time of approximately 10 minutes. All images were reconstructed to 512 × 512 × 512 voxels, with Bin-2 having 0.1 × 0.1 × 0.05 mm3 voxel size and Bin-4 having 0.159 × 0.159 × 0.062 mm3 voxel size. The radiation dose from Bin 2 and Bin 4 were estimated to be 148.3 mGy and 49.4 mGy respectively. Both protocols were reconstructed with the same conebeam filtered back projection algorithm with a Hamming filter.
2.2 Two-Group Longitudinal Study
MDA-MB-231 tibia-injected mice were imaged using the Imtek MicroCAT II weekly for four weeks. Sixteen mice were divided into two groups, one treatment group (n=8) and one control group (n=8). All mice were injected in one tibia with tumor cells at week 0, and the treatment group received one tail-vein injection of 0.1mg/mouse of the bisphosphonate zoledronic acid on day 6 after injection. Images were acquired at weeks 1, 2, 3, and 4 post injection for all 16 mice, using the same Bin-2 protocol as was performed in the preliminary study, and were reconstructed to have 512 × 512 × 512 voxels, each 0.15 × 0.15 × 0.212 mm3 in size.
2.3 Longitudinal CT Quantification Procedure
Image quantification of bone volume was performed using a threshold method based on Hounsfield Units (HU). Due to unstable detector performance and differences between intensity scales in each image, all images being quantified were first converted to Hounsfield Units (HU) to give them all the same intensity scale and to allow for reasonable week to week comparisons. In each image, the average value of ROIs drawn in air, bed (plastic), and bone were fitted to −1000, 0 and 1700 respectively; the resulting linear fit was used to convert all voxel values to HU. All image analysis was done using Amira 5.2 (Visage Imaging, Inc).
Following conversion to HU, each image was roughly cropped into two parts: the tibia region of the lesion limb, and the contra-lateral tibia region for use as a control limb. The lesion limb or control limb images were registered to the first time point using Amira’s Affine Registration function using the Correlation metric with a Quasi Newton optimizer step. Each limb was then carefully cropped to extend from just below the patella, but above the growth plate, to the point where the tibia and fibula join, with the fibula being removed as well. It was determined that a threshold of 1000 HU was most sensitive to changes in bone volume (data not shown) and subsequently applied to each image. The number of voxels that were above the threshold were then summed and converted to a volume measure of the tibia.
2.4 Tumor Cell Intratibial Inoculation & Histological Processing
Confluent MDA-MB-231 human breast cancer cells were trypsinized, washed and re-suspended in PBS for injection into the right tibia of anesthetized 4-week-old female athymic nude mice (Harlan Sprague Dawley, Inc.) at 2.5 × 105 cells per mouse. Contralateral intratibial injections of PBS were used as an internal control for each mouse. Animals were euthanized four weeks after injection. Hind limb specimens (tibia and femora) were removed during autopsy and fixed in 10% neutral buffered formalin (Fisher Scientific) for 48 hours at room temperature. Bone specimens were decalcified in 10% EDTA for 2 weeks and embedded in paraffin. Bone sections were stained with hematoxylin, eosin, orange G and phloxine. Histomorphometry was used to analyze tumor burden in the tibia and femurs using Metamorph software (Molecular Devices, Inc.). Specifically, using the drawing tool in metamorph the region between the cortices directly below the growth plate was selected and calculated by the software as the total area in centimeters2. The tumor, as determined by H+E staining, was selected using the same approach. Tumor burden was calculated as a percentage of tumor area over total tissue area. Multiple levels of bone sections were stained and imaged, and all statistical analyses were quantified at the same histomorphometric level. All statistical analyses were performed using InStat version 3.03 software (GraphPad Software Inc.).
2.5 Radiographs of Mice
Animals were anesthetized deeply and laid down in a prone position on the platform of the Faxitron LX-60. Images were acquired at 35 kVp for 8 seconds. Lesion area and lesion number were determined using quantitative image analysis software (Metamorph, Molecular Devices).
2.6 Fluorescent Imaging (MAESTRO)
GFP tagged tumor cell growth was measured and quantified using the CRi MAESTRO system. Mice were anesthetized using isoflurane and then placed in the MAESTRO imaging equipment. After the image was obtained, it was spectrally unmixed to remove the background fluorescence. Images were quantified using region of interest analysis (ROI) software that is supplied with the MAESTRO system.
2.7 Ex-vivo μCT Analysis
Tibias were analyzed using the Scanco μCT 40. Specifically, 100 slices from the proximal tibia were scanned at 12 micron resolution. Images were analyzed using the Scanco Medical Imaging software to determine the VOX-Bone Volume.
2.8 Statistical Analyses
For statistical analysis of the two-group longitudinal μCT data, we fitted a linear mixed model to the data and included random subject effect to account for the correlation of the longitudinal measurements from the same subject. Slope analysis for each group was performed and compared to zero. To adjust for multiple comparisons, we used 0.05/8=0.006 as the cut off point for p-values being statistically significant. The slope of the tibia volume as a function of time in the untreated control limb group was found to be significantly less than zero (p<0.0001) while the slope of the treated control limb was found to be significantly greater (p<0.0001). The statistical differences between the slopes of groups were determined. The treated and untreated lesion limb slopes were significantly different (p<0.0001), along with the untreated lesion limb and untreated control limb (p<0.0001). There was no statistical difference between the slopes of the treated and untreated control limb slopes (p=0.0132) or between the treated lesion limb and treated control limb slopes (p=0.0443). Also for the untreated group, it was determined that week 3 and week 4 volumes had significant mean differences between the lesion and control limbs with p=0.0031 and p<0.0001 respectively.
3. Results
3.1 Longitudinal μCT does not alter tumor growth
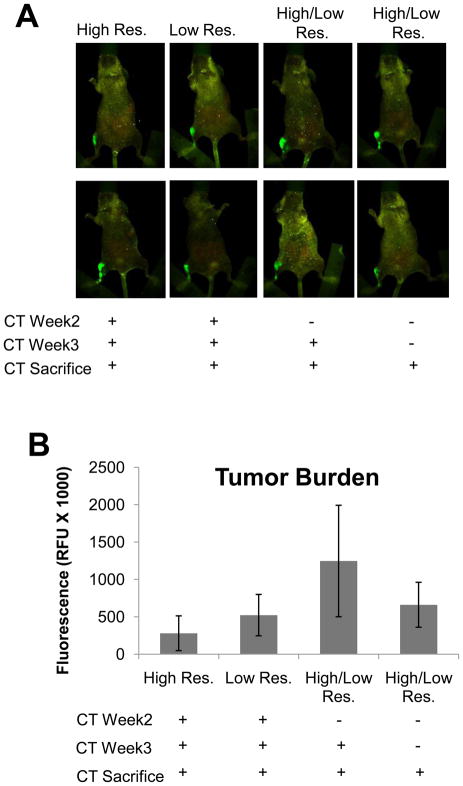
In order to determine if μCT protocols could be developed that would allow for longitudinal CT analyses of bone, we inoculated athymic nude mice with the osteolytic breast cancer cell line MDA-MB-231. We chose to use an intratibial model for this study, since there is less variability in this model than the intracardiac model, which allowed us to more easily validate the technology. After tumor cell inoculation we utilized two different imaging protocols, both with similar radiation doses to those previously reported [10,9], but at different resolutions. Animals were either imaged three times (weeks 2, 3 and at sacrifice), twice (week 3 and at sacrifice), or once (only at sacrifice). We found by fluorescence imaging of the GFP tumor cells (Maestro) that while there was a slight reduction in the relative fluorescent units (RFU) there were no significant changes in the overall RFUs between the groups (Figure 1A&B), suggesting no effect on the tumor cell growth. This observation was verified by histology, which showed no changes in tumor volume (Figure 1C&D).
Figure 1. Longitudinal μCT does not affect tumor burden in vivo.
Athymic nude mice were inoculated with MDA-MB-231 cells and imaged by μCT utilizing a Bin-2 (high resolution) or Bin-4 (low resolution) protocol. (A) Mice were monitored for tumor burden by tumor cell fluorescence utilizing CRi MAESTRO. Mice scanned at three time points by μCT at high (far left, n=3) or low resolutions (middle left, n=3) exhibited no change in tumor burden when compared to mice scanned twice (n=6) or once (n=4) by μCT at sacrifice. (B) Quantification of tumor burden by relative fluorescent units in mice scanned by μCT. (C&D) Histomorphometric analysis demonstrated no change in tumor volume in mice scanned at multiple time points by μCT when compared to mice scanned only upon sacrifice. Black bars represent a length of 500μM. Columns indicate average group values, and error bars represent standard error (SE). Statistics demonstrated non-significance across all groups and are therefore not visually represented. Statistical significance is considered p<0.05.
3.2 Longitudinal μCT illustrates bone loss over time
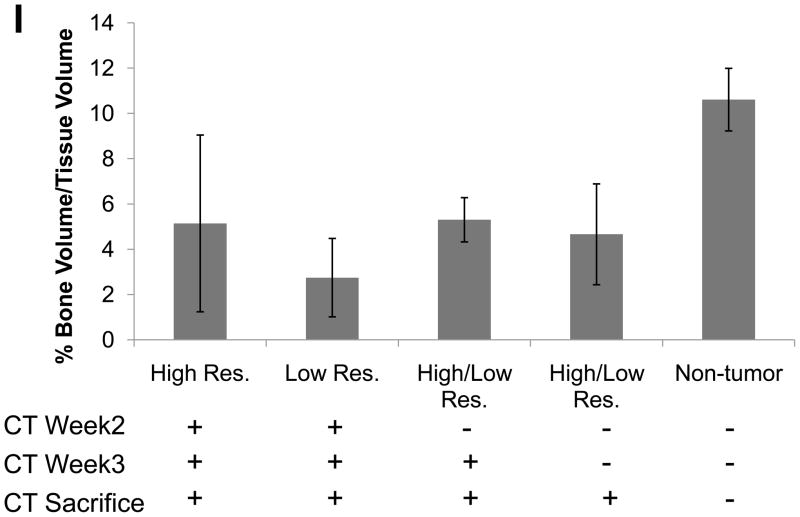
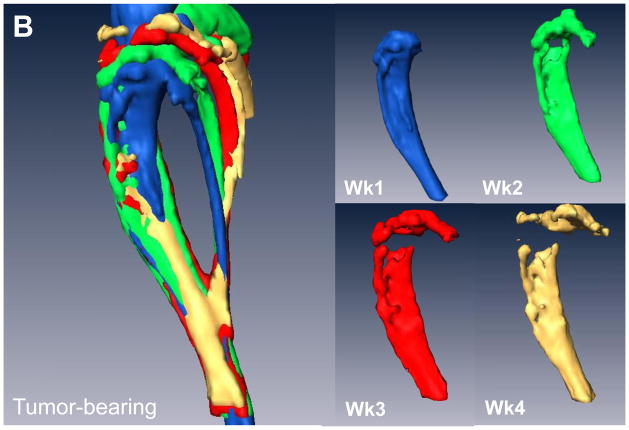
Weekly μCTs acquired during the course of the experiment were registered and over-laid into a single image so that we could visually see the loss in bone over time in tumor-bearing mice versus controls (Figure 2A&E). Individual scans were rendered at each week and can be visualized in Figure 2B–D and F–H. The representative image illustrates the loss in bone during the 3 weeks of image analysis, clearly showing a gradual loss of bone over time. Importantly, we were able to generate high resolution images and data such as these for each mouse. In addition, we quantified the changes in percent of bone volume/tissue volume (BV/TV) histologically in order to validate the μCT data (Figure 2I). By histology, we found that there were clear differences between the tumor leg and the control leg.
Figure 2. Longitudinal in vivo μCT demonstrates bone loss over time in individual mice.
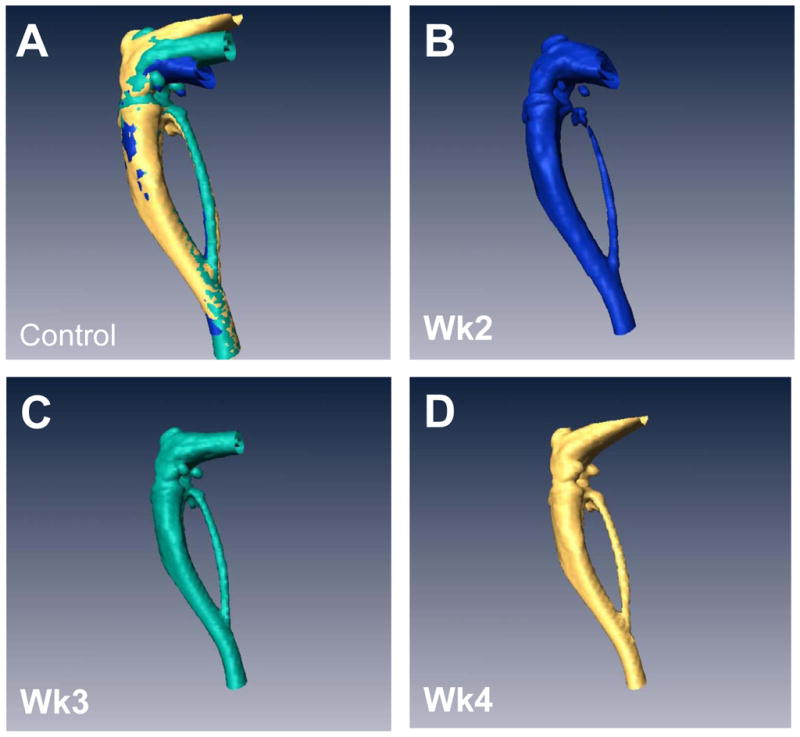
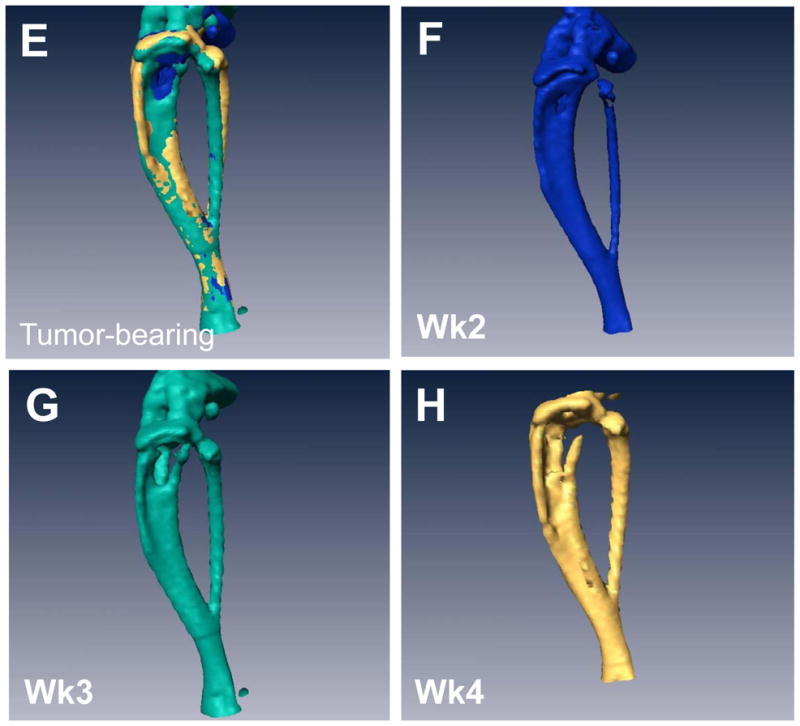
(A) Overlay of registered isosurface volume images from three individual time points of longitudinal in vivo μCT scans of control hind limb. (B–D) Weekly isosurface volume renderings of week 2, 3, and 4 (sacrifice) of the control hind limb of the same mouse using high resolution μCT. (E) Overlay of registered isosurface volume images from three individual time points of longitudinal in vivo μCT scans of tumor-bearing hind limb. (F–H) Weekly isosurface volume renderings of week 2, 3, and 4 (sacrifice) of the tumor-bearing hind limb of the same mouse using high resolution μCT. All rendering thresholds were set to 1000 HU. (I) Histomorphometric analyses indicate no significant difference in percent of bone volume/tissue volume, regardless of the number of μCT scans. Columns indicate average group values, and error bars represent standard error (SE). Statistics demonstrated non-significance across all groups and are therefore not visually represented.
3.3 In vivo μCT renders quantifiable longitudinal images of tumor-induced bone destruction
These data illustrate that longitudinal μCT is a valid option for imaging tumor-induced bone disease over time, which we previously thought was not possible in animal models. However, the most useful application for this imaging tool is in drug treatment models in which investigators need to detect lesions at early stages to determine the best time-points for drug treatment. Therefore, we wanted to find methods to quantify the in vivo μCT data similar to quantification used in the ex-vivo μCT system and histology. In order to do this we treated mice with zoledronic acid one week after tumor cell inoculation, when small osteolytic lesions were visible. We chose this model since it is well-established to reduce bone destruction in animal models and human patients [11], and would therefore provide clear differences in bone volume for quantification.
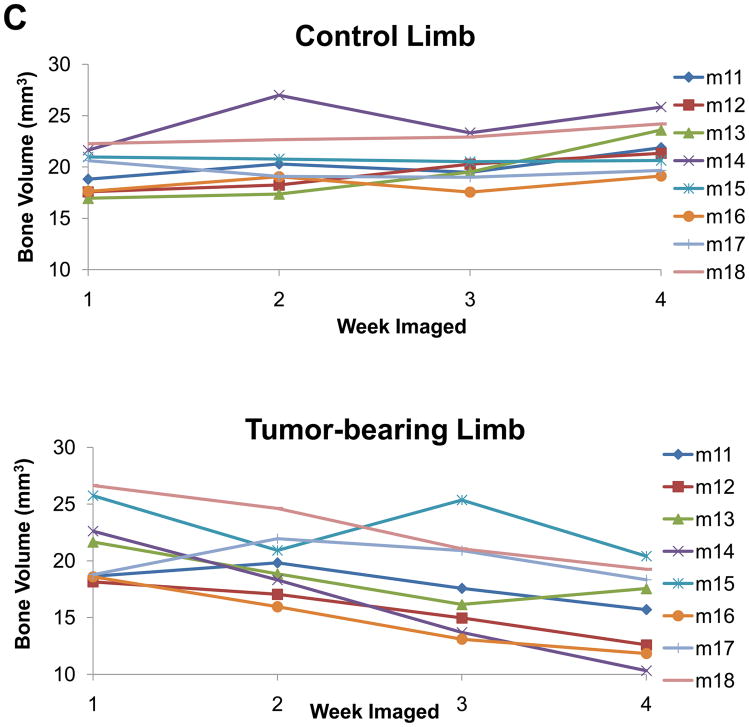
Initially, we examined the non-treated groups in this study and compared the control versus the tumor leg. Similar to the previous study we cropped and then registered the weekly acquired images (Figure 3A&B). We drew ROIs covering regions of empty space, bed (plastic) and bone and generated a calibration curve, which was used to convert each image’s intensity values to HU. Using this approach we found that we could accurately measure the volume of bone destruction over time. These results are displayed per mouse in Figure 3C. In addition, the quantification results were able to statistically distinguish at a group level between the control limb and the lesion limb groups (p<0.0001).
Figure 3. In vivo μCT allows for quantifiable longitudinal images of tumor-induced bone destruction.
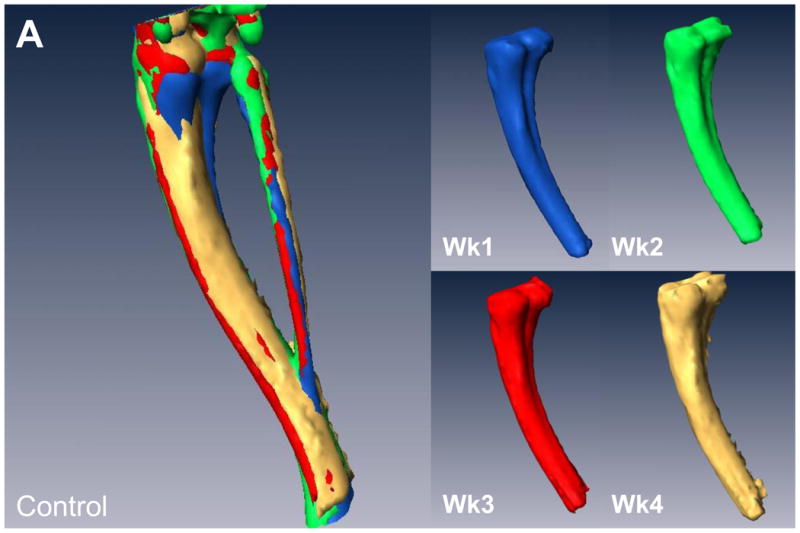
(A) Isosurface volume renderings of the control limb from a representative mouse at week 1 (blue), 2 (green), 3 (red), and 4 (tan), post-intratibial sham PBS injection. (B) Isosurface volume renderings of the tumor-bearing limb from the same representative mouse at week 1, 2, 3, and 4, post-intratibial inoculation of MDA-MB-231 cells. Additionally, a registered rendering is shown for both the control (A, large panel) and tumor-bearing (B, large panel) limbs combining all four time-points. All rendering thresholds were set to 1000 HU. (C) Bone volume was determined for each mouse at each time point (week 1, 2, 3, and 4) in both the control and tumor-bearing limbs.
3.4 In vivo μCT can be utilized to track changes in a drug treatment model
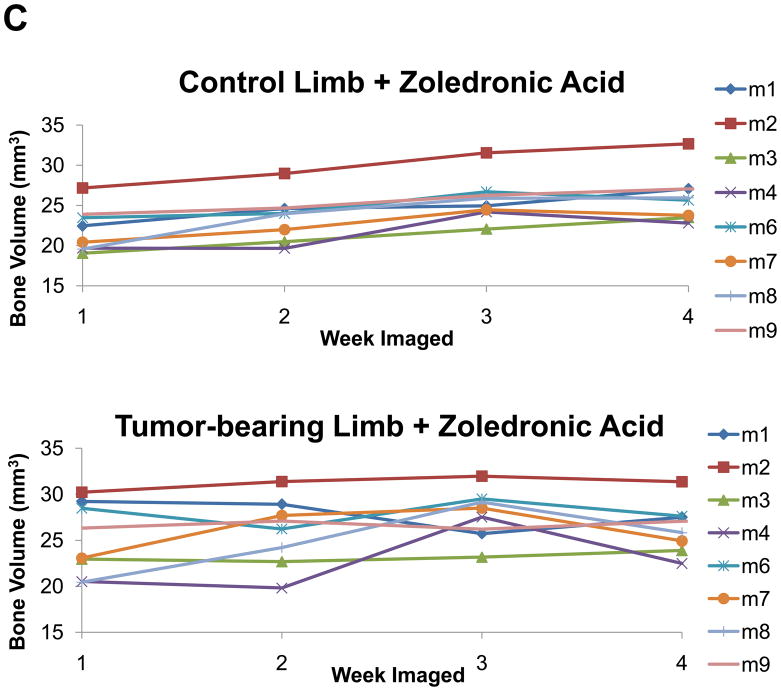
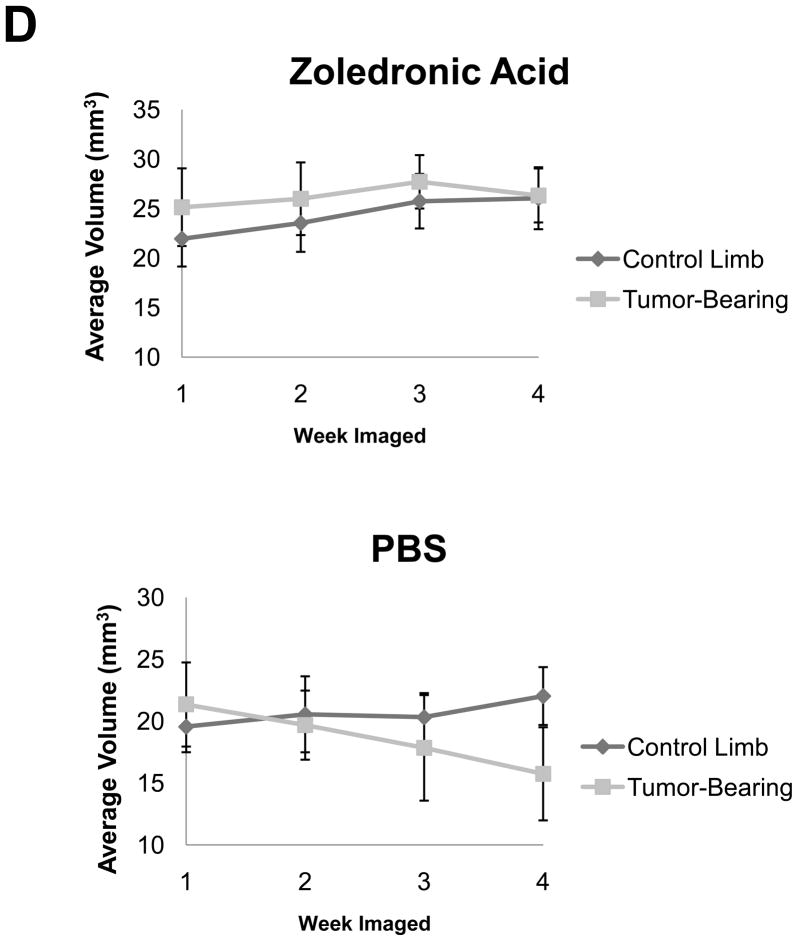
As described above, we treated mice with zoledronic acid starting at week 1 (when lesions were detectable) and imaged the mice weekly. With this approach we found that we could determine bone volumes for the treatment and control limbs in all mice, as well as quantify changes between the treated versus untreated groups by in vivo μCT (Figure 4A–D). Figure 4A and 4B demonstrate the ability to register weekly scans at end-point and determine average bone volume for treatment groups, as graphed per mouse and by groups in Figure 4C and 4D. There was no statistical significance (p=0.0443) between the mean bone volume of zoledronic acid treated tumor and non-tumor limbs, but there was a significant difference between the treated and untreated lesion limbs (p<0.0001). Interestingly, with this approach we could also see a statistical increase in the mean bone volume of the non-tumor limb when treated with zoledronic acid (p<0.0001).
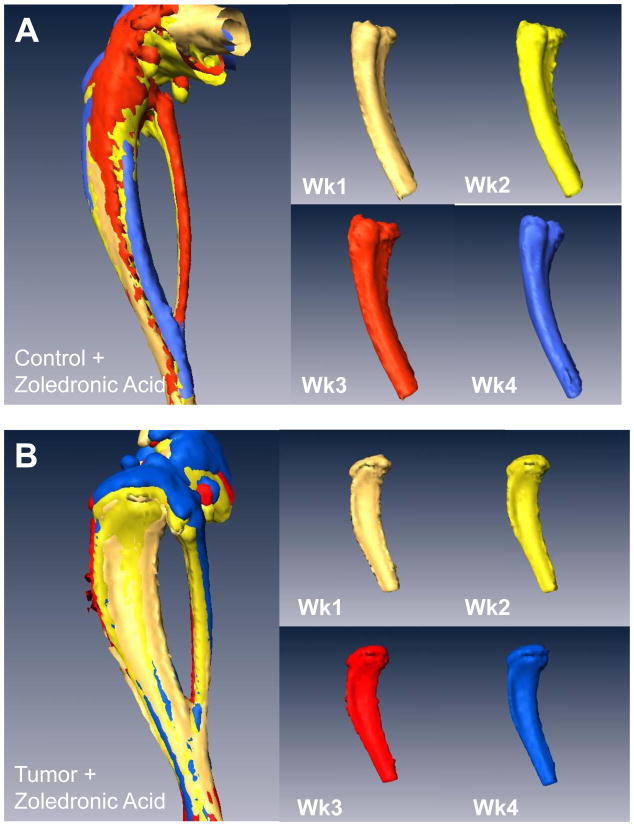
Figure 4. In vivo μCT monitors changes in bone volume when mice are treated with zoledronic acid.
(A) Isosurface volume renderings of the zoledronic acid treatment group at week 1 (tan), 2 (yellow), 3 (red), and 4 (blue), post-intratibial sham PBS injection. (B) Isosurface volume renderings of the zoledronic acid treatment group at week 1, 2, 3, and 4, post-intratibial inoculation of MDA-MB-231 cells. Additionally, a registered rendering is shown for both the control (A, large panel) and tumor-bearing (B, large panel) limbs at week 1, 2, 3, and 4. All rendering thresholds were set to 1000 HU. (C) Bone volume was determined for each mouse at each time point (week 1, 2, 3, and 4) in both the control and tumor-bearing limbs, as well as (D) the mean group volume and standard deviation over time for both the tumor-bearing and control limb of the zoledronic acid and control groups.
3.5 Conventional imaging methods demonstrate comparable bone volume to in vivo μCT
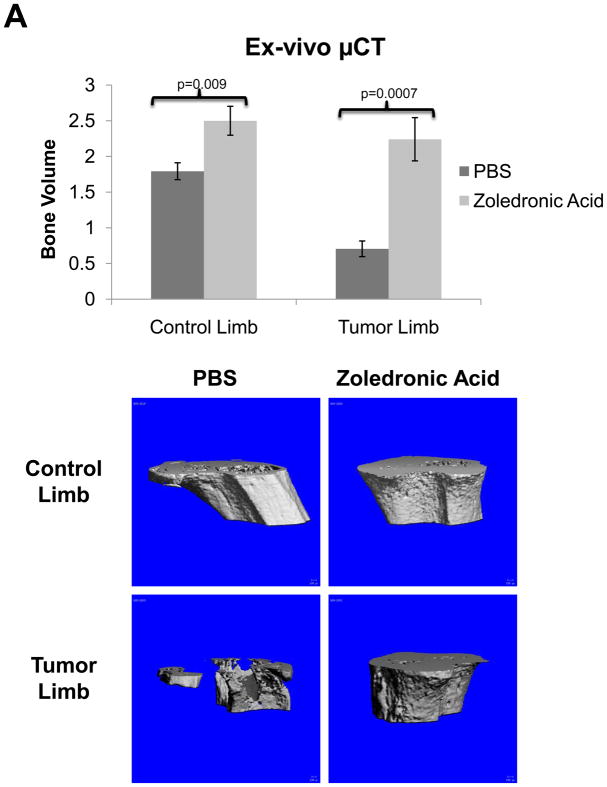
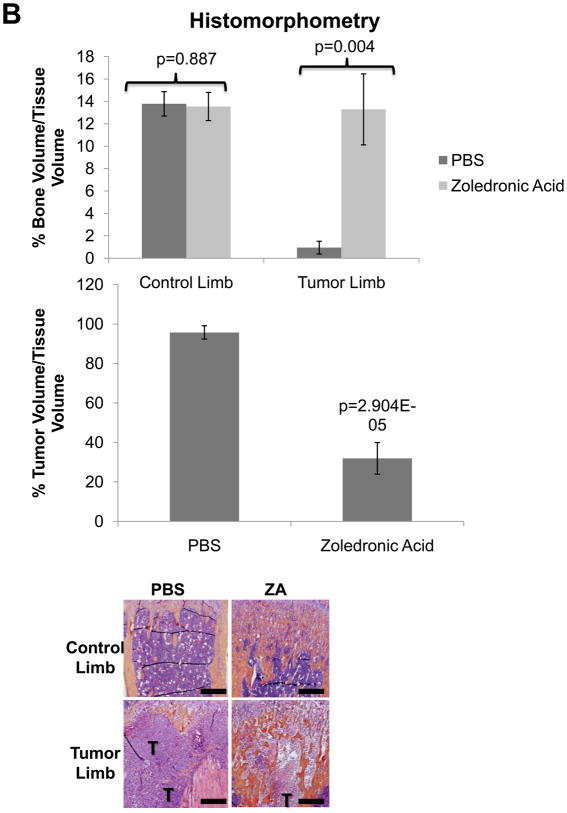
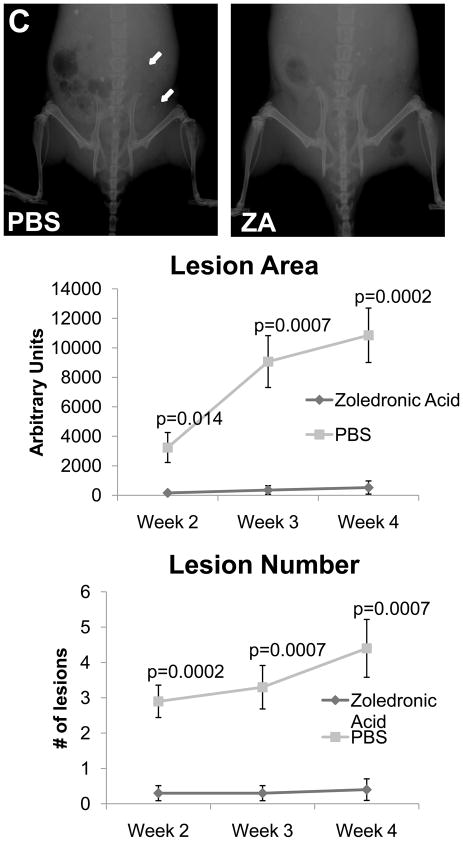
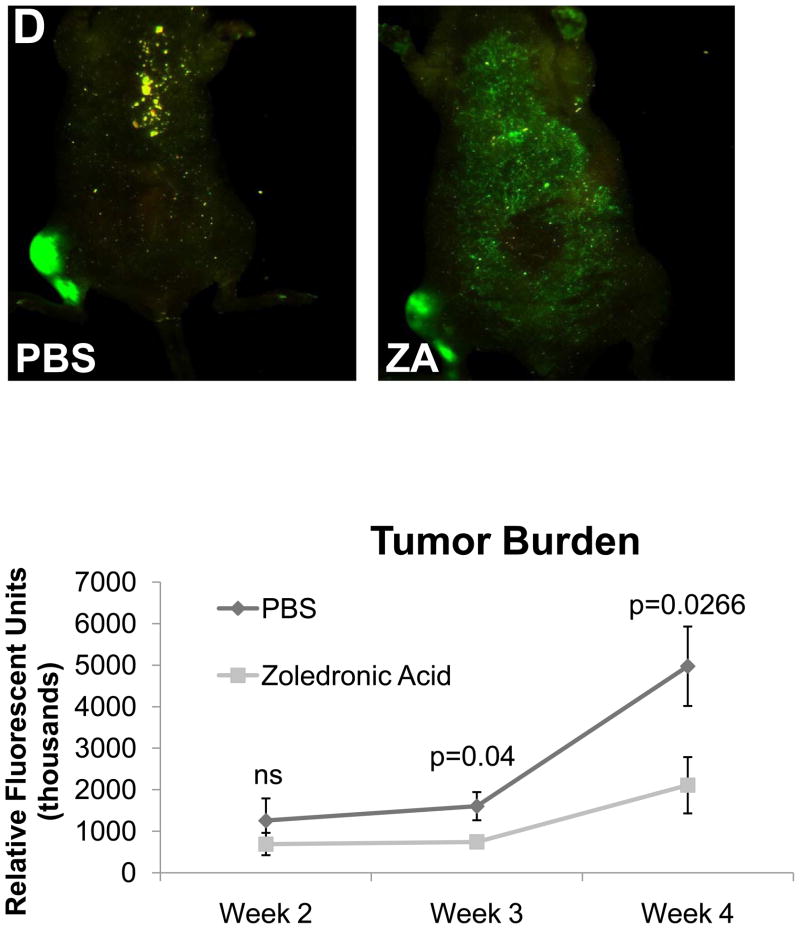
In order to validate the in vivo μCT quantification method we directly compared these results to ex-vivo μCT (Figure 5A) and BV/TV results calculated from histological analyses (Figure 5B), and we found that the quantification of bone volume by μCT and histomorphometry was comparable to in vivo μCT. We also found that while histological analyses were higher resolution than the longitudinal CT, we could obtain comparable results with lower resolution scans, and more importantly we could examine the progression of lesions in a single mouse over time with in vivo μCT. In vivo μCT bone volume computations were comparable to Faxitron data longitudinal analysis of lesion area and number (Figure 5C), and we confirmed that tumor cell fluorescence persisted throughout the duration of the study (Figure 5D).
Figure 5. Conventional imaging methods are comparable to in vivo μCT bone volume analysis of zoledronic acid treatment mice.
(A) Ex-vivo μCT analysis demonstrates a significant difference in bone volume between the treated and un-treated tumor-bearing limbs, similar to the group analysis by in vivo μCT in panel 4C. Representative images of control and tumor-inoculated hind limbs with and without zoledronic acid treatment demonstrate the rescue of bone volume in tumor-bearing limbs with bisphosphonate treatment. (B) Histological analyses indicate a significant increase in bone volume in tumor-bearing limbs of mice treated with zoledronic acid, further validating the data generated by in vivo μCT. Similar to the in vivo μCT, histomorphometry did not indicate any difference in bone volume in the control, non-tumor limbs of mice treated with zoledronic acid or PBS. Black bars represent a length of 500μM. (C) Faxitron images demonstrate tumor-associated osteolysis at week 4 in tumor-bearing mice treated with either PBS (left panel) or the zoledronic acid (right panel). White arrows indicate representative osteolytic lesions in bone. X-ray analyses indicate a decrease in lesion area in mice treated with zoledronic acid versus PBS, consistent with both ex-vivo and in vivo μCT (bottom, left graph). Lesion number by faxitron analyses is also decreased in mice treated with zoledronic acid versus PBS (bottom, right graph). (D) Tumor burden was monitored by tumor cell fluorescence utilizing Cri MAESTRO throughout the drug treatment study. Mice treated with zoledronic acid exhibited significantly smaller tumors at week 4 than control mice treated with PBS. Columns indicate average group values, and error bars represent standard error (SE). P-values are represented respectively for each graph, and statistical significance is considered p< 0.05.
4. Discussion
Investigators are commonly forced to combine multiple imaging modalities in order to achieve longitudinal and endpoint analyses, such as MRI and ex-vivo μCT [8]. More recent imaging methods have included 18F-fluoride μPET to monitor osteolytic bone destruction in the PC3 model of prostate cancer metastasis to bone, although the potential affect of radiation from multiple scans requires further investigation [12], and similar investigations are underway using SPECT imaging in breast cancer metastasis to bone [13]. We found that our method of in vivo μCT can be utilized for longitudinal tracking of osteolysis in a model of breast cancer metastasis to bone, without altering the course of tumor growth. Our group has previously found that the radiation dose required for high-resolution analyses by μCT can result in tumor cell killing, and is therefore only effective for end-point analyses (unpublished data). Therefore, a high-resolution method for monitoring cancer bone disease without killing the tumor cells in a longitudinal study is a key finding, although this method will need to be validated and possibly refined for individual cell lines, tumor models, and/or imaging protocols. In addition, we found that this imaging technique can detect significant changes in bone volume when utilized with a pre-clinical drug treatment model aimed at slowing bone resorption.
Previous studies found that longitudinal fpVCT renders 3-dimensional high-resolution images for monitoring bone metastases [10]; however, these studies utilized a cardiac-injection model causing unpredictable tumor locations, and therefore lacked overall group statistical significance. Furthermore, final analyses of fpVCT were primarily focused on determining areas of bone loss, with only a minimal investigation of bone volume [10]. By applying HU linear conversion to all images there is less variation of volumes in the same animal, allowing for more repeatable quantification. Additionally, the authors of that paper indicate that live animal μCT has better spatial resolution than their fpVCT system [10]. Volumetric analyses are crucial for translational analysis of tumor effects on bone, since bone area may change dramatically depending on the analysis plane. Our method allows tracking of bone volume in a longitudinal manner, and can determine significant changes in bone volume in a pre-clinical drug treatment model of bone metastasis.
Bisphosphonates are clinically utilized to slow bone resorption, ease bone pain, and decrease the number of skeletal related events commonly affecting bone metastasis patients [11]. Specifically, zoledronic acid has been shown to slow bone degradation at the site of osteolytic tumor in patients [14]. In vivo bisphosphonate treatment has been demonstrated to inhibit bone resorption in mouse models of cancer metastasis to bone [15]; therefore, we utilized this pre-clinical drug treatment model to determine whether in vivo μCT could distinguish between mice treated with zoledronic acid or sham drug. We were encouraged by the ability of this method to identify new bone growth in the bisphosphonate drug model, particularly for its implications in other bone metastasis models. It should be addressed that the mice used in our studies were 3–4 weeks old upon initial treatment with zoledronic acid, indicating that these mice will continue to develop new bone and increase bone volume over the course of the study as a result of normal bone maturation, which explains the lack of significance in bone volume between treated and untreated control limbs at end point; however, we did find a significant decrease in bone volume in the tumor limb of mice receiving the sham drug injection.
Although we focused our studies on a model of breast cancer metastasis to bone, our method of live animal μCT may be very beneficial for models of prostate cancer metastasis to bone, for which there are no quantitative methods for the detection of new bone. When prostate cancer metastasizes to bone, it frequently causes phases of bone resorption and bone formation, but usually causes a net increase in bone volume [16]. Since we were able to quantitatively detect an overall increase in bone volume in the zoledronic acid model, this holds great promise for the detection of newly ossified bone in blastic prostate cancer models. We are currently exploring the ability of in vivo μCT to specifically detect new bone growth, as it commonly occurs in blastic prostate cancer models of bone metastasis.
A potential future use for the high-resolution scans generated by in vivo μCT may be for early detection of bone lesions in mouse tumor metastasis models. Many patients who present with negative bone scans develop bone metastases within the first 12–18 months following initial diagnosis [17], indicating the importance of high resolution and sensitive imaging techniques. Accordingly, our method for in vivo μCT may potentially be tuned for detecting mouse bone metastases early in the disease pathogenesis and offer a more sensitive mechanism of detecting tumor-associated bone destruction than radiography. This is supported by the ability of our live animal μCT system to visually detect bone lesions at week 1 following tumor cell inoculation, and statistically differentiate lesions at week 3 more effectively than radiography.
In a model of breast cancer metastasis to bone, we found that in vivo μCT is a useful method for quantifying 3-dimensional longitudinal osteolysis without killing tumor cells, and is comparable to conventional methods of bone analysis such as ex-vivo μCT, faxitron analysis, and histomorphometry. In addition, we found that this method of in vivo μCT is reproducible and can detect significant changes in bone volume in a pre-clinical drug treatment model, suggesting its potential utility in blastic models of prostate cancer metastasis to bone. Taken together, these data indicate that in vivo μCT is an effective and reproducible method for longitudinal monitoring of tumor-associated bone destruction in mouse models of tumor-induced bone disease.
Acknowledgments
The authors wish to thank Ms. Alyssa Merkel for her technical expertise, Mr. Josh Johnson for histological processing and sectioning, and the following NIH funding sources: PO1CA040035 (GRM); U54CA126595 (GRM); 5T32CA009592-23 (LMM).
Abbreviations
- μCT
micro-Computed Tomography
- MRI
Magnetic Resonance Imaging
- fpVCT
flat-panel detector-based Volume Computed Tomography
- GFP
Green Fluorescent Protein
- PBS
Phosphate Buffered Saline
- HU
Hounsfield Units
- PET
Positron Emission Tomography
- SPECT
Single Photon Emission Computed Tomography
- RFU
Relative Fluorescent Units
- EDTA
Ethylenediaminetetraacetic Acid
- BV/TV
Bone Volume/Tissue Volume
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Coleman RE. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev. 2001;27(3):165–176. doi: 10.1053/ctrv.2000.0210. [DOI] [PubMed] [Google Scholar]
- 2.Yoneda T, Michigami T, Yi B, Williams PJ, Niewolna M, Hiraga T. Use of bisphosphonates for the treatment of bone metastasis in experimental animal models. Cancer Treat Rev. 1999;25(5):293–299. doi: 10.1053/ctrv.1999.0133. [DOI] [PubMed] [Google Scholar]
- 3.Yin JJ, Selander K, Chirgwin JM, Dallas M, Grubbs BG, Wieser R, Massague J, Mundy GR, Guise TA. TGF-β signaling blockade inhibits PTHrP secretion by breast cancer cells and bone metastases development. J Clin Invest. 1999;103:197–206. doi: 10.1172/JCI3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gallwitz WE, Guise TA, Mundy GR. Guanosine nucleotides inhibit different syndromes of PTHrP excess caused by human cancers in vivo. J Clin Invest. 2002;110:1559–72. doi: 10.1172/JCI11936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fisher JL, Schmitt JF, Howard ML, Mackie PS, Choong PF, Risbridger GP. An in vivo model of prostate carcinoma growth and invasion in bone. Cell Tissue Res. 2002;307(3):337–45. doi: 10.1007/s00441-001-0503-x. [DOI] [PubMed] [Google Scholar]
- 6.Rosol TJ, Tannehill-Gregg SH, LeRoy BE, Mandl S, Contag CH. Animal models of bone metastasis. Cancer. 2003;97(Suppl 3):748–57. doi: 10.1002/cncr.11150. [DOI] [PubMed] [Google Scholar]
- 7.Rajarubendra N, Bolton D, Lawrentschuk N. Diagnosis of Bone Metastases in Urological Malignancies-An Update. Urology. 2010 Mar 24; doi: 10.1016/j.urology.2009.12.050. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 8.Bussard KM, Mastro AM. Ex-vivo analysis of the bone microenvironment in bone metastatic breast cancer. J Mammary Gland Biol Neoplasia. 2009;14(4):387–95. doi: 10.1007/s10911-009-9159-z. [DOI] [PubMed] [Google Scholar]
- 9.Cowey S, Szafran AA, Kappes J, Zinn KR, Siegal GP, Desmond RA, Kim H, Evans L, Hardy RW. Breast cancer metastasis to bone: evaluation of bioluminescent imaging and microSPECT/CT for detecting bone metastasis in immunodeficient mice. Clin Exp Metastasis. 2007;24:389–401. doi: 10.1007/s10585-007-9076-8. [DOI] [PubMed] [Google Scholar]
- 10.Missbach-Guentner J, Dullin C, Zientkowska M, Domeyer-Missbach M, Kimmina S, Obenauery S, Kauerb F, Stuhmerz W, Grabbey E, Vogel WF, Alves F. Flat-Panel Detector–Based Volume Computed Tomography: A Novel 3D Imaging Technique to Monitor Osteolytic Bone Lesions in a Mouse Tumor Metastasis Model. Neoplasia. 2007;9(9):755–65. doi: 10.1593/neo.07466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Costa L, Major PP. Effect of bisphosphonates on pain and quality of life in patients with bone metastases. Nat Clin Pract Oncol. 2009;6(3):163–74. doi: 10.1038/ncponc1323. [DOI] [PubMed] [Google Scholar]
- 12.Virk MS, Petrigliano FA, Liu NQ, Chatziioannou AF, Stout D, Kang CO, Dougall WC, Lieberman JR. Influence of simultaneous targeting of the bone morphogenetic protein pathway and RANK/RANKL axis in osteolytic prostate cancer lesion in bone. Bone. 2009 Jan;44(1):160–7. doi: 10.1016/j.bone.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ben-Haim S, Israel O. Breast cancer: role of SPECT and PET in imaging bone metastases. Semin Nucl Med. 2009 Nov;39(6):408–15. doi: 10.1053/j.semnuclmed.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 14.Polascik TJ. Bisphosphonates in oncology: evidence for the prevention of skeletal events in patients with bone metastases. Drug Des Devel Ther. 2009;3:27–40. [PMC free article] [PubMed] [Google Scholar]
- 15.Labrinidis A, Hay S, Liapis V, Ponomarev V, Findlay DM, Evdokiou A. Zoledronic acid inhibits both the osteolytic and osteoblastic components of osteosarcoma lesions in a mouse model. Clin Cancer Res. 2009;15(10):3451–61. doi: 10.1158/1078-0432.CCR-08-1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guise TA, Mundy GR. Cancer and Bone. Endocr Rev. 1998;19(1):18–54. doi: 10.1210/edrv.19.1.0323. [DOI] [PubMed] [Google Scholar]
- 17.McNeil BJ. Rationale for the use of bone scans in selected metastatic and primary bone tumors. Semin Nucl Med. 1978;8(4):336–45. doi: 10.1016/s0001-2998(78)80019-1. [DOI] [PubMed] [Google Scholar]