Abstract
The reuniens (Re) and rhomboid (Rh) nuclei are major sources of thalamic input to hippocampus and medial prefrontal cortex. We compared effects of lesions in ReRh and other parts of the midline-intralaminar complex on tasks affected by lesions in terminal fields innervated by these nuclei, including: visuospatial reaction time (VSRT), a measure of sensory guided responding; serial VSRT, a measure of action sequence learning; and win/shift radial arm maze (RAM) measures of spatial memory. ReRh lesions affected RAM, but not VSRT or serial VSRT performance. The effects of caudal intralaminar lesions were doubly dissociated from ReRh lesions, affecting VSRT, but not RAM or serial VSRT performance. Rostral intralaminar lesions did not produce significant impairments, other than a subgroup with larger lesions that were impaired performing a delayed RAM task. Combined lesions damaging all three sites produced RAM deficits comparable to ReRh lesions and VSRT deficits comparable to caudal intralaminar lesions. Thus there was no indication that deficits produced by lesions in one site were exacerbated significantly by the cumulative effect of damage in other parts of the midline-intralaminar complex. The effects of ReRh lesions provide evidence that these nuclei affect memory functions of hippocampus and medial prefrontal cortex. The double dissociation observed between the effects of ReRh and caudal intralaminar nuclei provides evidence that different nuclei within the midline-intralaminar complex affect distinct aspects of cognition consistent with the effects of lesions in the terminal fields they innervate.
Keywords: diencephalic amnesia, midline and intralaminar thalamic nuclei, visuospatial reaction time, motor sequence learning
The midline and intralaminar thalamic nuclei comprise a complex with common inputs from visceral and marousal-related brainstem nuclei and regionally specific combinations of afferent and efferent connections related to distinct neural circuits (Groenewegen and Berendse, 1994; Krout, et al., 2002; Van der Werf, et al., 2002). The reuniens (Re) and rhomboid (Rh) nuclei appear organized to influence the flow of information between hippocampus and prefrontal cortex (PFC) (Vertes, et al., 2007). Re projects densely to PFC as well as to CA1, subiculum, and entorhinal and perirhinal cortex. Rh innervates these areas and additionally has extensive connections with sensorimotor cortex, ventral striatum, and the basolateral and basomedial nuclei of amygdala (Herkenham, 1978; Su and Bentivoglio, 1990; Vertes, et al., 2006). ReRh stimulation excites neurons directly in CA1, entorhinal cortex, amygdala, and hippocampal projection areas in medial PFC (Dolleman-van der Weel, et al. 1997; Bertram and Zhang, 1999; Zhang and Bertram, 2002; Viana Di Prisco and Vertes, 2006).
Two recent reports provide conflicting evidence about the role of Re in water maze tasks. Reversible inactivation with tetracaine was found to have broad effects on working and reference memory tasks, although anatomical controls were not included to confirm the localization of these effects in Re (Davoodi, et al., 2009). By contrast excitotoxic Re lesions were found to spare acquisition of a reference memory task but to affect probe trial performance in what was interpreted as a nonmnemonic deficit (Dolleman-van der Weel, et al. 2009). Hippocampal system lesions have well-established effects on reference memory water maze tasks that are not observed with PFC lesions (Sloan, et al., 2006) unless task parameters are manipulated to place demands on prefrontal function (de Bruin, et al., 2001; Jo, et al., 2007). These tasks may thus be insensitive to the effects of ReRh lesions on memory functions that depend on interactions between hippocampus and PFC. Here we examined the effects of ReRh lesions on win-shift radial arm maze (RAM) tasks that require memory for trial-specific information about multiple arm entries and are affected by hippocampal and prefrontal lesions (Mair, et al., 1998; McDonald and White, 1993; Porter & Mair, 1997).
To determine whether the effects of ReRh lesions are anatomically specific we compared lesions damaging rostral and caudal intralaminar nuclei. We included an additional group with lesions in all three sites to test for interactions between them. To determine whether impairments were behaviorally specific we compared effects of lesions on visuospatial reaction time (VSRT) and serial VSRT learning tasks. VSRT is a measure of sensory-guided responding specifically affected by lesions damaging lateral caudate-putamen and dorsal frontal cortical areas innervated by the caudal intralaminar nuclei (Bailey and Mair, 2004; Burk and Mair, 2001; Mair, et al, 2002). Serial VSRT is a measure of action sequence learning affected by lesions damaging areas of secondary motor cortex and medial caudate-putamen innervated by rostral intralaminar nuclei (Bailey and Mair, 2006, 2007).
Materials and Methods
Animals
Subjects were 40 male Long-Evans rats obtained from Charles River Breeding Laboratories (Wilmington MA) about 7 weeks of age at the beginning of behavioral training. Rats were housed individually in a temperature and humidity-controlled vivarium with a 12h light/dark cycle with all training occurring during the light cycle time. Rats received ad libitum access to food and were maintained on a water-deprivation schedule (1 hour/ day or 30 minutes when water was received during behavioral training) so that water could serve as a reinforcer. Rats were trained to criterion for the VSRT task and then were randomly assigned to one of five surgical treatments: ReRh, rostral intralaminar, caudal intralaminar, combined, or sham lesions.
Surgery
Rats were anesthetized with intramuscular injections of ketamine (80 mg/kg) and xylazine (8 mg/kg) and placed in a Stellar-type (ANY-angle) stereotaxic instrument (Stoelting, Wood Dale, IL). Lesions were produced by injecting 100 mM NMDA (at 0.2 μl/min through a 26-guage cannula) in a series of sites (in mm re: the intra aural line). For Re/Rh lesions 0.2 μl was injected at AP= 6.95, 6.45, and 5.95, DV= 2.6, and ML= 0 (0.6 μl total). For caudal intralaminar lesions 0.2 μl was injected at AP= 4.7, DV= 3.6 and 4.3, and ML = ± 1.4 (0.8 μl total). For rostral intralaminar lesions 0.1 μl was injected at AP= 6.6, DV= 4.0 and 4.8, and ML= 1.4 and 1.0; and AP= 5.8, DV= 4.4 and 3.6, and ML= 0.8 and 1.2 (0.8 μl total). Combined lesion animals received each of these three treatments (2.2 μl total). Sham controls were treated like the other groups except that their skulls were not opened and they did not receive NMDA. Each rat was then allowed two weeks of recovery time before water deprivation was re-established and post-surgical training began.
Apparatus
VSRT and serial VSRT were trained in test chambers (ENV 008) with a 5-port panel (ENV 115A) at one end and a runway (ENV 542) centered at the other (purchased from Med Associates, Georgia, VT). Each port was equipped with a stimulus light, a photocell positioned to measure nose pokes, and a well in which 0.1 ml water was delivered as a reinforcer by activation of a miniature solenoid valve (LFAA1201518H, The Lee Co., Essex, CT). The runway had a retractable lever (ENV 215A) at the far end to initiate trials and photocell (ENV-253B) at the near end to detect when rats entered the chamber after pressing the lever. The radial arm mazes (also purchased from Med Associates) had eight arms (ENV-543) attached to the octagonal-shaped hub (ENV-538). Each arm was attached to the hub with a motorized gate (ENV-540) and were equipped with wells milled into the floor at the far end where water was delivered as a reinforcer by activation of a miniature solenoid valve (LFAA1201518H, The Lee Co.) and infrared photocells (ENV-256-I) positioned to detect arm entries and nose pokes directed at the wells.
VSRT and serial VSRT training
Prior to surgery rats were trained to perform the VSRT task to criterion. VSRT trials began with the lever extending. This was retracted when pressed causing lights to turn on in all five response ports. Lights turned off in 4 (S-) ports when rats crossed the arm photocell (just before re-entering the test chamber) and remained on in the S+ port for a variable duration (0.05, 0.11, 0.26,0.58, 1.33, or 3.0 sec). The location of the S+ port and duration of the stimulus was changed randomly between trials. When rats responded first to the S+ port within 5 sec of crossing the arm photocell (the limited hold) the response was scored as correct and reinforcement (0.1 ml tap water) delivered by raising the dipper. A response was scored as an error of commission rats first responded to one of the S- ports and an error of omission when no nose poke was made within the 5 sec limited hold. The criterion for successful VSRT acquisition was 70% correct (averaged across stimulus durations) while performing all 96 trials in a 60 min session for 3 sessions.
After recovery from surgery, procedures for VSRT were re-established by 3 days of training at long stimulus durations (0.58, 1.33, or 3.0 sec). VSRT was then tested with 5 days of training with the full range of durations (0.05, 0.11, 0.26,0.58, 1.33, or 3.0 sec). After VSRT testing, training procedures were modified for the serial VSRT task (see Bailey & Mair 2006, 2007). Serial VSRT training began with a single nose poke task similar to VSRT except the S+ turned on when the runway photocell was broken after trials were initiated with a lever press and stayed on until a nose poke was made in the S+ port. Response time was measured from when the photocell was broken until the rat responded to the S+ port. After performances stabilized (3 to 5 sessions) repetition training began in which the location of the S+ port was held constant across trials. Repetition training continued for 5 sessions and was followed by one session in which the location of the S+ port varied randomly between trials (as in the initial training). Repetition learning was assessed by comparing response times for repetition sessions to the random sequence sessions that preceded and followed them.
A similar procedure was followed for action sequence learning except that rats were required to make a series of 5 nose pokes into ports indicated by stimulus lights to obtain reinforcement during a trial. For the first 4 stimuli, nose pokes in the S+ port caused that light to turn off and the light in the next S+ port in the sequence to turn on. Reinforcement (0.1 ml tap water) was delivered (and the stimulus light turned off) following a nose poke into the fifth (and final) port in a sequence. The lever was then inserted at the end of the arm to allow rats to initiate the next trial in a session. Rats were initially trained with different (randomly selected) sequences on every trial. When rats were able to perform 60 trials per session for three consecutive sessions, repetition training began. This consisted of 300 trials with a fixed sequence (3-1-3-5-3) on every trial, then 60 trials with randomly varying sequences, 300 additional trials with the fixed (3-1-3-5-3) sequence, and lastly 60 trials with randomly varying sequences. Response times were measured for each of nose poke in a sequence. The effects of sequence length and repetition on response initiation were assessed by comparing response times for the single nose pokes to the initial nose poke in the 5 poke sequences during random and repeated sessions. Repetition learning was assessed by interference effects between the last 60 trials of repeating (predictable) sequences with the subsequent 60 trials when sequences changed at random from trial to trial. The effects of repetition learning were determined separately for each of the five nose pokes in a sequence.
RAM training
RAM training began after serial VSRT training ended. Rats were adapted to the maze by allowing them to receive 3-4 reinforcements (two 0.1 ml pulses of water, 2 sec apart) in each arm for 5 sessions. Rats were next trained to perform the standard 8-arm task. Rats were placed in the hub and gates to the eight arms opened. These remained opened until rats made a total of 8 arm entries, defined by breaking the photocell indicating a response to the well at the end of an arm and then exiting the arm. If the arm had not yet been entered during that trial the response was scored as correct and reinforcement delivered. If the arm had already been entered an error was recorded and no reinforcement was delivered. After the eighth entry, all gates were lowered and the rat was trapped for a brief (10 second) period. All 8 arms were then opened and a new trial began. Rats were trained for three trials per day. After completion of the third trial the rat was removed and placed back in its home-cage. Testing was conducted for ten consecutive days.
Rats were then switched to a delayed choice RAM task. They were placed in the center of the maze with all of the gates were closed. Individual gates were opened 1 at a time, selected randomly without replacement, to control the order of the first 4 arm entries. After the fourth arm response, the open gate was shut for a 1 minute delay and then all gates were opened to allow access to the eight arms. Rats were allowed to enter 4 arms. Responses to arms not yet entered on that trial were reinforced and scored as correct. Each rat was tested for 2 trials/ day for five days with a minimum of 1 hour between trials. After this delays were increased to 5, then 15 and finally 30 minutes for 10 trials at each delay. For the 5 and 15 minute delays rats were trained for 2 trials/ day for 5 days with a minimum of 1 hour between trials. For the 30 minute delay rats were tested for 1 trial/ day for 10 days.
Histology
Rats were sacrificed under deep anesthesia by transcardiac perfusion with physiological saline and followed by 5% neutral buffered formalin. The brain was removed and placed in a 10% glycerin/ 4% neutral buffered formalin solution. Tissue was blocked in the plane by Paxinos and Watson (1998), frozen, and cut in the coronal plane at 50μm. Every slice was mounted in the area of the lesion and stained with cresyl violet.
Statistical Analyses
Behavioral performances were analyzed with mixed model ANOVA's with lesion group as between-subject and task parameters as within-subject variables. Given the distinct predictions for different lesion groups analyses of lesion effects were based on planned comparisons using the Bonferonni-Dunn procedure for a priori nonorthogonal contrasts with overall α = .05 (see Kirk, 1982). Adjusted based on 4 comparisons between each of the lesion groups and controls) the critical value for p is < .0125. To assess the effects of lesion size an observer (RGM) blind to behavioral performance divided each lesion group into large and small lesion subgroups. Large lesions were estimated to involve 50% or more of the intended target. Small lesions produced bilateral damage to the intended targets but were estimated to damage less than 50% of this target. These were then compared with sham operated controls in a mixed model ANOVA using planned comparisons (Bonferonni-Dunn, α = .05) to determine whether there were differences between the large and small lesion groups and between each of these lesion groups and the controls. For lesion size analyses for α = .05 the critical value of p was adjusted to < .0167.
Results
Histological observations
All rats recovered uneventfully from surgery. Lesions tended to involve tissue in the immediate vicinity of the injection sites and the tracks of cannulae used to inject the NMDA solution (Figure 1). Lesions showed characteristic signs of an excitotoxic reaction, including loss of tissue volume, loss of neurons, proliferation of microglial cells, pyknotic nuclei, and accumulation of hemosiderin (Figures 2 to 4). The ReRh lesions (Figures 1A, 2) produced damage to both sides of Re and Rh in 7/8 cases although there was only limited dorsal damage to Re in two of these cases. The ventral limit of ReRh lesions varied from 0 to 0.8 mm (mean = 0.41 mm) above the ventral portion of the third ventricle at the midpoint of the lesion (AP= 6.45 mm re: IA). Figure 2 shows an example of a ReRh lesion that ended dorsal to the third ventricle (A, C) and on that extended to the third ventricle (B, D). Overlying areas of the central medial nucleus were involved in all cases and unilateral damage was consistently observed in overlying areas of the mediodorsal thalamic nucleus, habenula, hippocampus and cortex in sections that were penetrated by cannulae used to produce lesions. In 7/8 cases ReRh lesions affected only one side of the central medial nucleus. In 5 of these cases central medial damage was confined to the immediate vicinity of the cannulae track. The rostral intralaminar lesions were associated with bilateral damage to the centrolateral nucleus in 8/8 and to the paracentral nucleus in 6/8 cases as well as damage in adjacent paralaminar areas of the mediodorsal, laterodorsal, and anterior nuclei and habenula (Figures 1B, 3). Caudal intralaminar lesions resulted in bilateral damage to lateral portions of the parafascicular nucleus in 7/8 animals and unintended damage to adjacent areas of the posterior and lateral posterior nuclei (Figures 1C, 3). All rats in the combined group exhibited signs of bilateral damage in each of the three sites (Figure 4). The individual rats with unilateral sparing in the ReRh and caudal intralaminar groups were eliminated from statistical analyses of behavioral results.
Figure 1. Histological analyses of lesions.
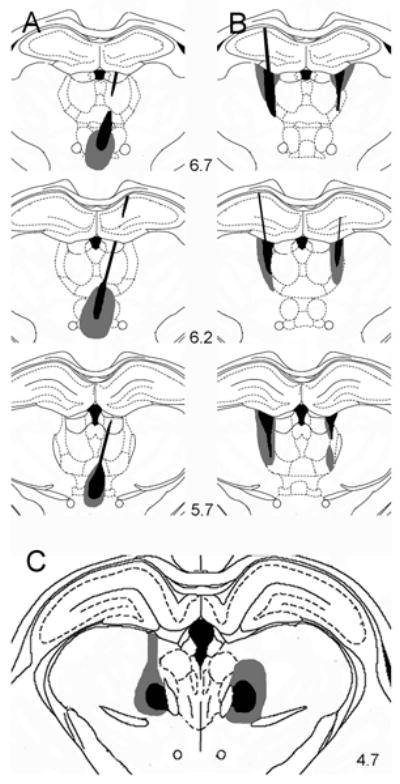
Drawings depicting damage observed with one of the largest (gray) and smallest (black) ReRh (A), rostral intralaminar (B), and caudal intralaminar (C) lesions. Drawings of ReRh (A) and rostral intralaminar (B) lesions, which were produced by NMDA infusions at multiple AP levels, are shown in coronal sections 6.7, 6.2, and 5.7 mm anterior to the interaural line (-2.3, -2.8, and -3.3 mm from bregma). Caudal intralaminar lesions (C) were made with infusions at one AP level and are shown in a section 4.7 mm anterior to the interaural line. Combined lesions produced bilateral damage in each of these sites comparable to lesions made separately in individual rats. Areas damaged by lesions were reconstructed on the basis of tissue spared in serial sections and were drawn on templates derived from Paxinos and Watson (1998).
Figure 2.
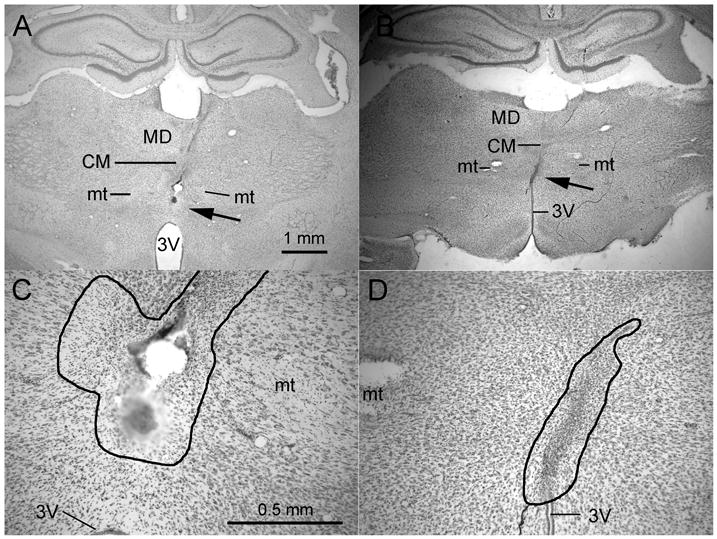
Photomicrographs showing representative ReRh lesions through 2× (A, B) and 10× (B, D) objectives. The arrows in A and B indicate the location of the lesion shown at higher power in B & D. The thin black lines in B and D outline the area of damage characterized by loss of neurons, proliferation of microglial cells, pyknotic nuclei, and accumulation of hemosiderin. The lesion shown in A and C extended to about 0.5 mm above 3V (just visible at the bottom of the figure) while the lesion in B and D extended to the roof of 3V. Abbreviations: third ventricle (3V), mediodorsal (MD), central medial (CM), and mammillothalamic tract (mt). A calibration mark for 2× objectives is shown in A and for 10× objectives in C.
Figure 4.
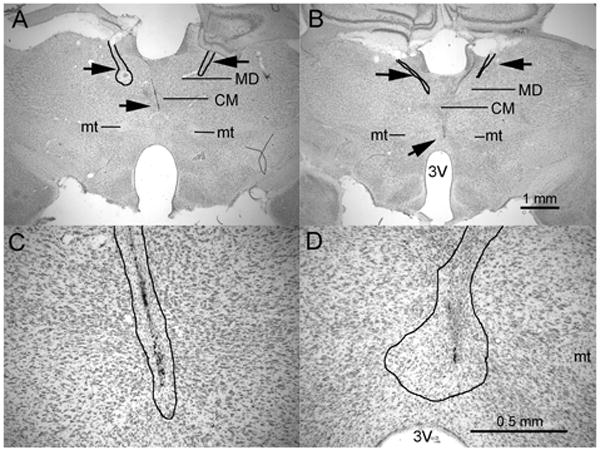
Photomicrographs showing representative combined lesions at levels showing damage to the rostral intralaminar and ReRh sites taken through 2× (A & B) and 10× (C & D) objectives. Calibrations for the two powers of magnification are shown in B & D. The thin black lines in A, B, C, and D outline areas of damage characterized by loss of neurons, proliferation of microglial cells, pyknotic nuclei, and accumulation of hemosiderin. Abbreviations: third ventricle (3V), mediodorsal (MD), central medial (CM), and mammillothalamic tract (mt).
Figure 3.
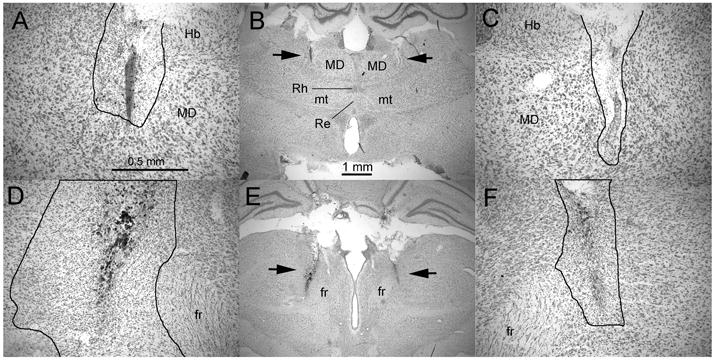
Photomicrographs showing representative rostral (A to C) and caudal intralaminar (D to F) lesions through 2× (B & E) and 10× (A, C, D, & F) objectives. The arrows in B and E indicate the locations of lesions shown at higher power in adjacent photomicrographs. The thin black lines in A, C, D, and F outline areas of damage characterized by loss of neurons, proliferation of microglial cells, pyknotic nuclei, and accumulation of hemosiderin. Calibrations for the two powers of magnification are shown in A and B. Abbreviations: mediodorsal (MD), rhomboid (Rh), reuniens (Re), mammillothalamic tract (mt), habenula (Hb), and fasciculus retroflexus (fr).
Visually-guided responding- VSRT Performance
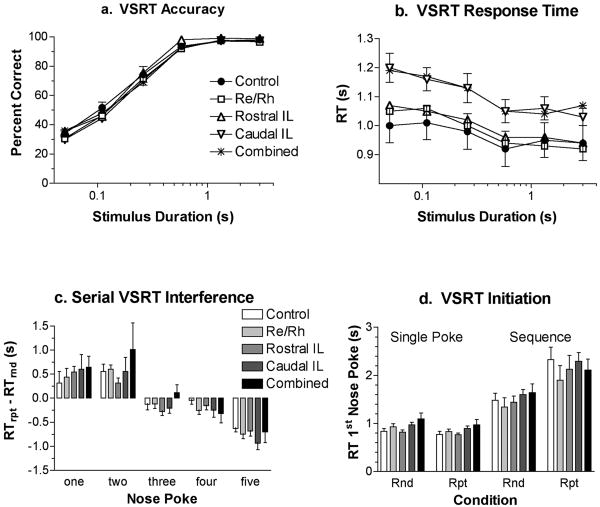
VSRT performance was assessed by response accuracy (percent correct) and speed (response time) for runway and choice responses (Figure 5 A & B). Runway response time was measured from when the lever was pressed to initiate a trial until the arm photocell was broken, a response performed without modification on every trial. Choice response time was measured from when a photocell was broken until a nose poke was made at a port, a response that had to be modified by trial-specific sensory information. Response times were analyzed as the median for correct responses in each session.
Figure 5.
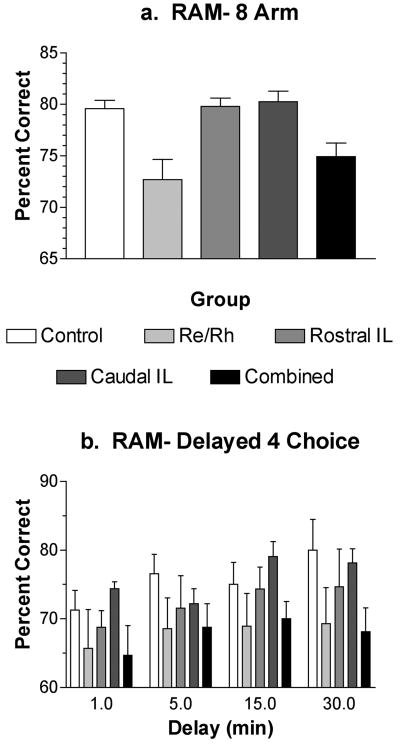
Effects of lesions on win-shift radial arm maze (RAM) tasks. RAM performance was measured as percent correct for the first 8 arm entries in the 8-arm task (a) and the first 4 arm entries following the delay in the delayed choice task (b). ReRh and combined lesions produced comparable deficits for both RAM tasks. Performances tended to improved with practice for the delayed choice task even though longer delays were tested during later training sessions. Lesion effects did not interact with this improvement. Error bars represent standard error of the mean.
The caudal intralaminar and combined lesion groups showed comparable increases in choice response time across all stimulus durations. An ANOVA showed a significant effect of stimulus duration, F5, 165 = 66.427, p < .0001 that did not interact with lesion effects, F20, 165 = 1.222, p= .242. Planned comparisons (Bonferonni-Dunn, α = .05) with the control group revealed significant effects of the caudal intralaminar (p=. 0008) and combined (p=. 0001) lesions, but not of rostral intralaminar or ReRh lesions (p's> .29). None of the lesions affected runway response times (p's> .15). VSRT accuracy varied from chance-level to errorless as stimulus duration increased from .05 to 3.0 s, F5, 165= 735.665, p < .0001, and was unaffected by the interaction between duration and lesion group, F< 1. Planned comparisons did not reveal any differences between individual lesion groups and controls for VSRT accuracy (p's> .61).
Action sequence learning- Serial VSRT performance
Reaction time studies suggest that repetition learning should have two distinct effects on sequential responding (Bailey & Mair, 2006, 2007). Learning to perform a series of independent actions as a coherent sequence (or chunk) requires organizational processes prior to execution that should increase response time for the first elements of a practiced sequence (Kennerley et al., 2004). Conversely habit learning has benefits that should gradually reduce overall response time as sequences are practiced, particularly for later elements in the sequence (Bailey & Mair, 2006). To examine these effects we measured response times separately for each of the nose pokes in the sequence and examined interference effects when training shifted from the repeated to random sequences at the end of serial VSRT training. As predicted, these analyses revealed positive interference effects (longer response times for repeated than random sequences) for the first two pokes in the sequence and negative interference effects (shorter response times for repeated than random sequences) for later pokes in the sequences (Figure 5 c). None of the lesions substantially affected these interference effects. A two-factor mixed model ANOVA revealed a significant difference between interference effects for different pokes (F4, 116 = 31.466, p < .0001) but no interaction between group and nose poke ordinal position (Fs < 1). Planned comparisons did not reveal any significant differences in interference effects between treatment groups and controls for the overall sequence (p's> .42) or for any of the pokes making up the sequence (p's> .12).
A second analysis was carried out to compare response time to initiate single nose pokes vs. nose poke sequences and to examine the effects of repetition learning on these measures (Figure 5 d). Again these manipulations had predicted effects on response time, but these were not affected by any of the lesions. Thus response times were shorter for single nose pokes than nose pokes that began sequences and repetition learning increased response time to initiate sequences but not single nose poke responses. Analyses of time to initiate responding (Figure 5 D) showed an increase in initial response times for sequences compared to single nose poke responses (F1, 30 = 104.268, p < .0001). As in earlier studies (Bailey and Mair, 2006, 2007) repetition training exacerbated this difference, substantially increasing initial response time for sequential responding and decreasing response time slightly for single nose poke responses. These trends were confirmed by a significant overall effect of repetition (F1, 39 = 65.856, p < .0001) that interacted significantly with sequence length (1 vs. 5 nose pokes) (F1, 39 = 113.599, p < .0001). None of the lesions had an apparent effect on initial response time. The interactions between group and sequence length and repetition were not significant (p's > .36). Planned comparisons did not reveal significant differences between any of the lesion groups and controls (p's> .56).
Spatial memory- win-shift RAM tasks
The ReRh and combined lesion groups exhibited significant deficits for both RAM tasks. For the 8-arm task (Figure 6A), planned comparisons (Bonferonni-Dunn, α = .05) showed a significant deficit for the ReRh (p = .0021), and combined (p= .0062) lesions, and no apparent effect of the rostral or caudal intralaminar lesions (p's> .74). For the delayed choice task (Figure 6B), planned comparisons revealed significant effects of ReRh (p= .0033) and combined (p= .0025) lesions, but not for rostral or caudal intralaminar lesions (p's> .18). There was a significant effect of memory delay (F3, 99 = 3.893, p= .0112) that did not interact with lesion effects (F< 1). Here performance improved from 68.9 % correct at the 1 minute delay to 74.1 % correct at the 30 minute delay. It should be noted that delays were increased across sessions and thus this improvement could simply reflect improvement across time as rats gained more experience with the delayed choice task.
Figure 6. Effects of lesions on visuspatial reaction time (VSRT) and serial VSRT tasks.
Visuospatial response accuracy (a) and reaction time (b) are shown as a function of stimulus duration. Accuracy varied from chance level to errorless performance across the durations tested and was unaffected by any of the lesions. Reaction time was increased comparably by caudal intralaminar and combined lesions at all durations. Motor skill learning was measured by serial VSRT interference (c), the difference in reaction time for nose poke responses when training switched from repeated (rpt) to randomly changing (rnd) sequences. Repeated (learned) sequences were associated with increased reaction times for initial nose pokes and decreased reaction times for later nose pokes completing sequences (c). Analyses of reaction times for initial nose pokes revealed an increase for sequences compared to single nose poke responses for both random (Rnd5 vs. Rnd1) and repeated (Rpt5 vs. Rpt 1) stimuli (d). Repetition learning was associated with an increase in initial reaction time for sequences (Rpt 5 vs. Rnd 5) but not single nose poke responses (Rpt1 vs. Rnd1) (d). All these effects are consistent with previous studies of serial VSRT learning (Bailey and Mair, 2006, 2007) and were unaffected by any of the lesions. Error bars represent standard error of the mean.
Effects of lesion size
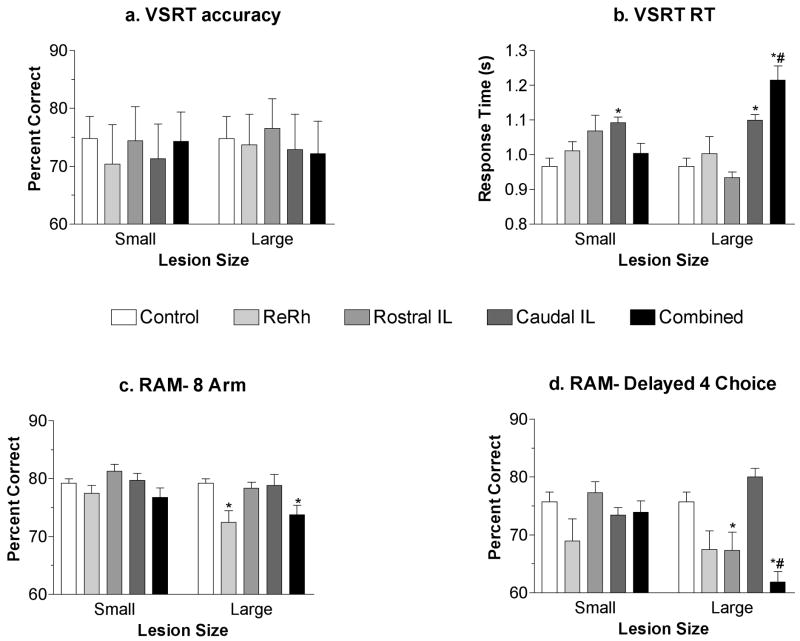
Histological analyses categorized 3 ReRh lesions as small and 4 as large, 4 caudal intralaminar lesions as small and 3 as large, 4 rostral intralaminar lesions as small and 4 as large, and 4 combined lesions as small and 4 as large. Large lesions were generally associated with larger deficits. None of the lesions, large or small, had a significant effect on VSRT accuracy (Figure 7A). Planned comparisons (Bonferonni-Dunn, α = .05) revealed comparable and significant effects of large (p= .0022) and small (p= .0013) caudal intralaminar lesions on VSRT. Large combined lesions had significant effects on response time for VSRT compared to controls (p< .0001) and to rats with small combined lesions (p< .0001). For the 8-arm RAM task (Figure 7C), there were significant effects of large ReRh (p=. 0002) and combined (p=. 0021) lesions. Small ReRh and combined lesion groups exhibited an intermediate level of performance for the 8-arm task that did not differ from controls or rats with large lesions. For the delayed choice RAM task (Figure 7D) planned comparisons showed significant differences between large rostral intralaminar lesions and both small rostral lesion (p= .0066) and control (p= .0086) groups, as well as between the large combined lesion and both small combined lesion (p= .0002) and control (p < .0001) groups. The significant impairment of the large rostral intralaminar group is the one lesion effect revealed by lesion size analyses that wasn't apparent for analyses of lesion groups as a whole (above). Both the large and small ReRh lesion groups tended to be impaired compared to controls, however neither of these effects reached statistical significance in this analysis. Examination of Figure 7 suggests that large combined lesions may have had a greater effect on VSRT response time than large caudal intralaminar lesions and a greater effect on the delayed RAM task than large ReRh or rostral intralaminar lesions. Post hoc analyses (Bonferonni-Dunn, α = .05) did not reveal a significant difference for any of these comparisons. Thus lesion size analyses did not provide evidence of a significant interaction in which a deficit produced by a lesion targeting one part of the midline-intralaminar complex was exacerbated significantly by lesions damaging other parts of this complex.
Figure 7.
Effects of lesion size. Lesion size was determined by an observer blind to behavioral performances of individual rats. Large lesions were estimated to involve 50% or more of the intended target. Small lesions produced bilateral damage to the intended targets but were estimated to damage less than 50% of the intended target. Lesion size had no effect on VSRT accuracy (a). VSRT was comparably increased by small and large caudal intralaminar lesions while large combined lesions were associated with a larger increase than for controls or rats with small combined lesions (b). Large ReRh and combined lesions were associated with significant impairment compared to controls (c). For the delayed choice RAM task, rats with large rostral intralaminar lesions were impaired compared to controls while large combined lesions were impaired compared to controls or to rats with small combined lesions (d). Although there was a significant overall effect of ReRh lesions compared to controls, these differences were not statistically significant when the ReRh group was subdivided into large and small lesion groups (d). Error bars represent standard error of the mean. * = significantly different form controls; # = significant difference between large and small lesion groups; based on planned comparisons (Bonferonni-Dunn, α = .05),
Discussion
Our results provide evidence of spatial memory impairment following ReRh lesions that is doubly dissociated with the effects of caudal intralaminar lesions. ReRh lesions produced significant deficits affecting win-shift RAM that spared VSRT responding. Caudal intralaminar lesions increased response time for VSRT without affecting RAM performance. This double dissociation provides evidence of functional specificity consistent with effects of lesions in terminal fields innervated by these nuclei. Thus lesions of ReRh projection areas in hippocampus and ventral medial PFC affect win-shift RAM tasks and hippocampal lesions spare VSRT, while lesions of caudal intralaminar projection areas in lateral caudate putamen or dorsolateral frontal cortex increase response time for VSRT while sparing RAM measures of working memory (Burk and Mair, 2001; Mair, et al., 1998; Mair, et al., 2002; Bailey and Mair, 2004). Rats with combined lesions damaging ReRh, rostral intralaminar, and caudal intralaminar nuclei exhibited RAM deficits comparable to ReRh lesions and increased response time for VSRT comparable to caudal intralaminar lesions. They were unimpaired for the other behavioral measures (Figures 5 and 6). Thus there were no signs of an overall interaction between the effects of the different lesions: RAM deficits produced by ReRh lesions and VSRT deficits produced by caudal intralaminar lesions were not significantly exacerbated by damage in other areas of the midline-intralaminar complex. None of the lesions affected serial VSRT measures of action sequence learning. Thus ReRh lesions sufficient to affect RAM measures of spatial memory did not affect procedural memory required for sequence learning in the serial VSRT task. Similarly, caudal intralaminar lesions sufficient to increase response for simple VSRT responding did not affect serial VSRT learning.
As a group, rats with rostral intralaminar lesions were not significantly impaired for any of the behavioral measures. This result may reflect a limited role of these nuclei in tasks studied or it may reflect the limited extent of damage produced by rostral intralaminar lesions in the present study. Consistent with the first possibility, Mitchell & Dalyrmple-Alford (2006) observed a double dissociation between the effects of rostral intralaminar and anterior thalamic nucleus lesions that demonstrated an effect of rostral intralaminar lesions on an egocentric delayed match to sample task that spared an 8-arm RAM task. Consistent with the second possibility, more extensive rostral intralaminar lesions have been found to affect both VSRT (Burk and Mair, 2001) and the present RAM tasks (Mair, et al., 1998). Here lesions were made with smaller amounts of NMDA injected in more dorsal and lateral sites to avoid overlap with areas damaged by ReRh lesions. Histological analyses confirmed that the present lesions spared the central medial nucleus and ventral areas of the paracentral nucleus that were damaged consistently in the earlier studies where VSRT and RAM deficits were observed (Burk and Mair, 2001; Mair, et al., 1998). The importance of lesion size in the present study was supported here by evidence that rats with relatively large rostral intralaminar lesions were impaired for the delayed choice RAM task compared to controls or to rats with relatively small rostral intralaminar lesions (Figure 7D). Bailey and Mair (2005) also found that rostral intralaminar lesions restricted to the centrolateral and dorsal paracentral nuclei do not impair performance for a delayed matching to position task that was affected by larger rostral intralaminar lesions that involved the central medial nucleus. Other studies have shown that lesions specifically damaging the mediodorsal nucleus or the central medial and dorsal midline nuclei cannot account for the effects of larger rostral intralaminar lesions on spatial memory tasks (Bailey and Mair, 2005; Burk and Mair, 1998; Mair and Lacourse, 1992; Mair, et al., 1992). The available evidence has thus failed to identify a more discrete site of pathology that can account for memory impairments associated with large rostral intralaminar lesions.
Caudal intralaminar lesions increased choice response time for VSRT without affecting serial VSRT or RAM performance. This pattern of impairment is consistent with effects of lesions damaging striatal or cortical areas that receive prominent projections from these nuclei. Thus lesions in dorsolateral sensorimotor areas of striatum (Mair, et al., 2002; Bailey & Mair, 2006) or dorsolateral sensorimotor areas of frontal cortex (Bailey & Mair, 2004, 2007) have been found to increase choice (but not runway) response time for the VSRT task without affecting RAM delayed nonmatching tasks. Runway response time measures the time taken to turn and traverse the runway after pressing the lever to initiate a trial. The comparable runway response times of lesioned (0.93 sec) and control (1.09 sec) rats demonstrates that caudal intralaminar lesions did not generally reduce response speed. Choice responses involved a similar travel distance but additionally required rats to respond to the port indicated by a stimulus light that changed randomly on each trial. The increase in choice but not runway reesponse time is consistent with evidence that sensorimotor areas of cortex and striatum specifically affect that ability to select or guide responses based on trial-specific stimulus information (Brown and Robbins, 1989; Brown and Marsden, 1998).
Effects of ReRh lesions
The present results indicate that discrete lesions damaging Re and Rh can produce significant spatial memory impairment. There is ambiguity in the localization of Re and Rh that makes it difficult to definitively determine the extent to which each of these nuclei were damaged in the present study. Here stereotaxic coordinates were based on Paxinos and Watson (1998) and confirmed by reference to Kruger, et al. (1995) and König and Klippel (1963). By these atlas coordinates 5/7 ReRh lesions extended through the dorsal-ventral extent of Re and Rh. However, analyses of connections with hippocampus and prefrontal cortex suggest that Re extends below what is indicated in these atlases to the roof of the third ventricle (Dolleman-Van der Weel & Witter, 1996; Bokor, et al, 2002; Vertes, 2002; McKenna & Vertes, 2004; Vertes, et al., 2006). By these analyses only 2/7 ReRh lesions extended throughout the dorsal-ventral extent of these nuclei. Cannulae were lowered at an angle to make these lesions (Figures 1A and 2) to avoid bilateral damage to overlying tissue. With the exception of one animal with extensive damage to the central medial nucleus, incidental damage to overlying areas of the intralaminar and mediodorsal nuclei, hippocampus, and cortex did not cross the midline. The effects of the ReRh lesion on both RAM tasks remained significant when the one animal with extensive central medial damage was eliminated from the analyses. Unilateral inactivation of medial thalamus does not affect spatial memory tasks impaired by bilateral inactivation, unless paired with damage or inactivation of anatomically related structures in the opposite hemisphere (Floresco et al, 1999; Porter et al., 2001). Similarly, discrete lesions damaging the intralaminar or the mediodorsal nuclei unilaterally do not produce spatial memory impairments comparable to bilateral lesions (Bailey & Mair, 2005). Thus it seems unlikely that unilateral damage of overlying areas of thalamus can account for the effects of ReRh lesions on the RAM tasks.
These results stand in contrast to a recent report that complete Re lesions do not affect acquisition of a water maze reference memory task (Dolleman-van der Weel, et al. 2009). There are three important differences between the studies that might explain this discrepancy. First, ventral midline lesions here targeted Re and Rh. Both Re and Rh provide dense projections to CA1, subiculum, perirhinal cortex, and infralimbic and prelimbic areas in PFC (Vertes, et al., 2006). It is possible that lesions damaging Re alone are insufficient to disrupt functions mediated by these terminal field areas. It should be noted, however, that Re lesions in Dolleman-van der Weel, et al. (2009) extended dorsally into Rh in most cases. Second, the stereotaxic coordinates in Dolleman-van der Weel, et al. (2009) targeted more anterior areas of Re (-1.80 and -2.3 mm from bregma in the plane of Paxinos and Watson) while the present study targeted sites throughout the anterior-posterior extent of Re and Rh (-2.05, -2.55, and -3.05 mm from bregma in the same plane). However Dolleman-van der Weel, et al. (2009) observed more extensive damage to Re than in the present study, with complete destruction of Re in 11/12 cases and 90% destruction in the remaining case.
The water maze task used by in Dolleman-van der Weel, et al. (2009) measured reference memory for a single spatial location repeated for 18 trials over 3 days. Here, RAM tasks required rats to remember multiple spatial locations that changed from trial to trial. A third possibility is that the water maze task did not place sufficient demands on memory to reveal functional impairments produced by ventral midline thalamic lesions. Dolleman-van der Weel et al. (2009) observed a consistent effect of Re lesions in probe trials when the hidden platform was removed. Re lesioned rats swam directly to the location of the platform during training trials but did not stop and search locally to the extent that controls did. Dolleman-van der Weel et al. (2009) argued that this might reflect a nonmnemonic deficit producing “very flexible/ impulsive behaviour” related to prefrontal dysfunction. Alternatively the failure to stop and search locally might also result from a decreased tendency to rely on spatial memory to generate a new search strategy following failure to find the platform in its training location. Such a decrease in the salience of spatial memory is consistent evidence that Re influences interactions between PFC, an area critical for executive functions, and hippocampus, an area critical for spatial memory (Vertes, et al., 2007). This interpretation is also consistent with the present findings that lesions damaging Re affect RAM measures of spatial memory known to depend on both hippocampus and PFC.
To our knowledge, this is the first report that ventral midline thalamic lesions can specifically disrupt memory function while sparing other aspects of cognition. There are two possible explanations for this effect. First, lesions damaging Re and Rh may produce a dysfunctional state in the terminal fields they innervate and thus interfere with tasks like win-shift RAM that depend on them (Mair, et al., 1998; McDonald and White, 1993; Porter & Mair, 1997). Electrical stimulation of Re and Rh has strong excitatory effects on hippocampus, medial PFC, and related cortical and subcortical areas (Bertram and Zhang, 1999; Viana Di Prisco and Vertes, 2006; Dolleman-van der Weel, et al. 1997; Herkenham, 1978; Su and Bentivoglio, 1990; Vertes, et al., 2006; Zhang and Bertram, 2002). However, if ReRh lesions simply disrupt hippocampal and medial prefrontal function it is not clear why they did not also affect VSRT or serial VSRT tasks that depend on dorsomedial areas of PFC also driven by ReRh stimulation (Viana Di Prisco and Vertes, 2006) or why lesions destroying Re and involving Rh would not affect water maze measures known to depend on hippocampus (Dolleman-van der Weel, et al. 2009).
An alternative explanation is that Re and Rh may play a more specific role affecting interactions between hippocampus and PFC, activating them in concert and thus serving more of a gating function (Vertes, 2006; Vertes, et al., 2007). By this hypothesis Re and Rh are critical for tasks that require coordinated activation of prefrontal cortex and the hippocampal system, for instance executive functions like working memory or temporal integration, that may require information represented in the hippocampal system or prefrontal input to hippocampus, for instance retrieval of memory from hippocampus under conditions that require top-down cognitive control (Jo, et al., 2007). This hypothesis predicts that ReRh lesions should have robust effects on win-shift RAM tasks that are significantly affected by both PFC and hippocampal lesions but not on tasks like VSRT that depend on PFC but not hippocampus or acquisition reference memory water maze tasks that depend on hippocampus, but not PFC. By either account, the present results provide direct evidence that the ventral midline nuclei are a site of pathology that can potentially contribute or account for similar patterns of impairment associated with medial temporal lobe and thalamic amnesia (Gold and Squire, 2006).
Acknowledgments
Grant Sponsor: National Institute of Neurological Disorders and Stroke Grant Number: NS26855
References
- Bailey KR, Mair RG. Dissociable effects of frontal cortical lesions on measures of visuospatial attention and spatial working memory in the rat. Cereb Cortex. 2004;14:974–985. doi: 10.1093/cercor/bhh058. [DOI] [PubMed] [Google Scholar]
- Bailey KR, Mair RG. Lesions of specific and nonspecific thalamic nuclei affect prefrontal cortex-dependent aspects of spatial working memory. Behav Neurosci. 2005;119:410–419. doi: 10.1037/0735-7044.119.2.410. [DOI] [PubMed] [Google Scholar]
- Bailey KR, Mair RG. The role of striatum in initiation and execution of learned action sequences in rats. J Neurosci. 2006;26:1016–1025. doi: 10.1523/JNEUROSCI.3883-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey KR, Mair RG. Effects of frontal cortex lesions on action sequence learning in the rat. Eur J Neurosci. 2007;25:2905–2915. doi: 10.1111/j.1460-9568.2007.05492.x. [DOI] [PubMed] [Google Scholar]
- Bertram EH, Zhang DX. Thalamic excitation of hippocampal CA1 neurons: a comparison with the effects of CA3 stimulation. Neurosci. 1999;92:15–26. doi: 10.1016/s0306-4522(98)00712-x. [DOI] [PubMed] [Google Scholar]
- Bokor H, Csáki A, Kocsis K, Kiss J. Cellular architecture of the nucleus reuniens thalami and its putative aspartatergic/glutamatergic projection to the hippocampus and medial septum in the rat. Eur J Neurosci. 2002;16:1227–1239. doi: 10.1046/j.1460-9568.2002.02189.x. [DOI] [PubMed] [Google Scholar]
- Brown P, Marsden CD. What do the basal ganglia do? Lancet. 1998;351:1801–1804. doi: 10.1016/s0140-6736(97)11225-9. [DOI] [PubMed] [Google Scholar]
- Brown VJ, Robbins TW. Elementary processes of response selection mediated by distinct regions of the striatum. J Neurosci. 1989;9:3760–3765. doi: 10.1523/JNEUROSCI.09-11-03760.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burk JA, Mair RG. Effects of intralaminar thalamic lesions on sensory attention and motor intention in the rat: a comparison with lesions involving frontal cortex and hippocampus. Behav Brain Res. 2001;123:49–63. doi: 10.1016/s0166-4328(01)00202-9. [DOI] [PubMed] [Google Scholar]
- Davoodi FG, Motamedi F, Naghdi N, Akbari E. Effect of reversible inactivation of the reuniens nucleus on spatial learning and memory in rats using Morris water maze task. Behav Brain Res. 2009;198:130–135. doi: 10.1016/j.bbr.2008.10.037. [DOI] [PubMed] [Google Scholar]
- de Bruin JPC, Moita MP, de Brabander HM, Joosten RNJMA. Place and response learning of rats in a Morris water maze: Differential effects of fimbria fornix and medial prefrontal cortex lesions. Neurobiol Learn Mem. 2001;75:164–178. doi: 10.1006/nlme.2000.3962. [DOI] [PubMed] [Google Scholar]
- Dolleman-Van der Weel MJ, Lopes da Silva FH, Witter MP. Nucleus reuniens thalami modulates activity in hippocampal field C1 through excitatory and inhibitory mechanisms. J Neurosci. 1997;17:5640–5650. doi: 10.1523/JNEUROSCI.17-14-05640.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolleman-van der Weel M, Morris RG, Witter MP. Neurotoxic lesions of the thalamic reuniens or mediodorsal nucleus in rats affect non-mnemonic aspects of watermaze learning. Brain Struct Funct. 2009;213:329–342. doi: 10.1007/s00429-008-0200-6. [DOI] [PubMed] [Google Scholar]
- Dolleman-van der Weel JL, Witter MP. Projections from the nucleus reuniens thalami to the entorhinal cortex, hippocampal field CA1, and the subiculum in the rat arise from different populations of neurons. J Comp Neurol. 1996;364:637–650. doi: 10.1002/(SICI)1096-9861(19960122)364:4<637::AID-CNE3>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Braaksma DN, Phillips AG. Thalamic-cortical-striatal circuitry subserves working memory during delayed responding on a radial arm maze. J Neurosci. 1999;19:11061–71. doi: 10.1523/JNEUROSCI.19-24-11061.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JJ, Squire LR. The anatomy of amnesia: Neurohistological analysis of three new cases. Learn Mem. 2006;13:699–710. doi: 10.1101/lm.357406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewegen HJ, Berendse HW. The specificity of the ‘nonspecific’ midline and intralaminar thalamic nuclei. Trends Neurosci. 1994;17:52–57. doi: 10.1016/0166-2236(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Herkenham M. The connections of the nucleus reuniens thalami: evidence for a direct thalamo-hippocampal pathway in the rat. J Comp Neurol. 1978;177:589–610. doi: 10.1002/cne.901770405. [DOI] [PubMed] [Google Scholar]
- Jo YS, Park EH, Kim IH, Park SK, Kim H, Kim HT, Choi JS. The medial prefrontal cortex is involved in spatial memory retrieval under partial-cue conditions. J Neurosci. 2007;27:13567–13578. doi: 10.1523/JNEUROSCI.3589-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennerley SW, Sakai K, Rushworth MFS. Organization of action sequences and the role of the pre-SMA. J Neurophysiol. 2004;91:978–993. doi: 10.1152/jn.00651.2003. [DOI] [PubMed] [Google Scholar]
- Kirk RE. Experimental Design: Procedures for the Behavioral Sciences. second. Belmont, CA: Wadsworth; 1982. pp. 106–109. [Google Scholar]
- König JFR, Klippel RA. The Rat Brain: A stereotaxic atlas of the forebrain and lower parts of the brain stem. Baltimore: Williams and Wilkins; 1963. [Google Scholar]
- Krout KE, Belzer RE, Loewy AD. Brainstem projections to midline and intralaminar thalamic nuclei of the rat. J Comp Neurol. 2002;448:53–101. doi: 10.1002/cne.10236. [DOI] [PubMed] [Google Scholar]
- Kruger L, Swanson LW, Saporta S. Photographic Atlas of the Rat Brain: The Cell and Fiber Architecture Illustrated in Three Planes with Stereotaxic Coordinates. Cambridge, U.K.: Cambridge Univ Press; 1995. [Google Scholar]
- Mair RG, Burk JA, Porter MC. Lesions of the frontal cortex, hippocampus, and intralaminar thalamic nuclei have distinct effects on remembering in rats. Behav Neurosci. 1998;112:772–792. doi: 10.1037//0735-7044.112.4.772. [DOI] [PubMed] [Google Scholar]
- Mair RG, Koch JK, Newman JB, Howard JR, Burk JA. A double dissociation in striatum between serial reaction time and radial maze delayed nonmatching performance in rats. J Neurosci. 2002;22:6756–6765. doi: 10.1523/JNEUROSCI.22-15-06756.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mair RG, Lacourse DM. Radiofrequency lesions of thalamus produce delayed non-matching to sample impairments comparable to pyrithiamine-induced thiamine deficiency. Behav Neurosci. 1992;106:634–645. doi: 10.1037//0735-7044.106.4.634. [DOI] [PubMed] [Google Scholar]
- Mair RG, Robinson JK, Koger SM, Fox GD, Zhang YP. Delayed non-matching to sample is impaired by extensive, but not by limited lesions of thalamus in the rat. Behav Neurosci. 1992;106:646–656. doi: 10.1037//0735-7044.106.4.646. [DOI] [PubMed] [Google Scholar]
- McDonald RJ, White NM. A triple dissociation of memory systems: Hippocampus, amygdala, and dorsal striatum. Behav Neurosci. 1993;107:3–22. doi: 10.1037//0735-7044.107.1.3. [DOI] [PubMed] [Google Scholar]
- McKenna JT, Vertes RP. Afferent projections to nucleus reuniens of the thalamus. J Comp Neurol. 2004;480:115–142. doi: 10.1002/cne.20342. [DOI] [PubMed] [Google Scholar]
- Mitchell AS, Dalrymple-Alford JC. Lateral and anterior thalamic lesions impair independent memory systems. Learn Mem. 2006;13:388–396. doi: 10.1101/lm.122206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain In Stereotaxic Coordinates. fourth. San Diego: Academic Press; 1998. [Google Scholar]
- Porter MC, Koch J, Mair RG. Effects of reversible inactivation of thalamostriatal circuitry on delayed matching trained with retractable levers. Behav Brain Res. 2001;119:61–69. doi: 10.1016/s0166-4328(00)00331-4. [DOI] [PubMed] [Google Scholar]
- Porter MC, Mair RG. The effects of frontal cortical lesions on remembering depend on the procedural demands of tasks performed in the radial arm maze. Behav Brain Res. 1997;87:115–125. doi: 10.1016/s0166-4328(96)02272-3. [DOI] [PubMed] [Google Scholar]
- Sloan HL, Good M, Dunnett SB. Double dissociation between hippocampal and prefrontal lesions on an operant delayed matching task and a water maze reference memory task. Behav Brain Res. 2006;171:116–126. doi: 10.1016/j.bbr.2006.03.030. [DOI] [PubMed] [Google Scholar]
- Su HS, Bentivoglio M. Thalamic midline cell populations projecting to the nucleus accumbens, amygdala, and hippocampus in the rat. J Comp Neurol. 1990;297:582–593. doi: 10.1002/cne.902970410. [DOI] [PubMed] [Google Scholar]
- Van der Werf YD, Witter MP, Groenewegen HJ. The intralaminar and midline nuclei of the thalamus. Anatomical and functional evidence for participation in processes of arousal and awareness. Brain Res Brain Res Rev. 2002;39:107–140. doi: 10.1016/s0165-0173(02)00181-9. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Analysis of projections from the medial prefrontal cortex to the thalamus in the rat, with emphasis on nucleus reuniens. J Comp Neurol. 2002;442:163–187. doi: 10.1002/cne.10083. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Interactions among the medial prefrontal cortex, hippocampus and midline thalamus in emotional and cognitive processing in the rat. Neuroscience. 2006;142:1–20. doi: 10.1016/j.neuroscience.2006.06.027. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Hoover WB, Do Valle AC, Sherman A, Rodriguez JJ. Efferent projections of reuniens and rhomboid nuclei of the thalamus in the rat. J Comp Neurol. 2006;499:768–796. doi: 10.1002/cne.21135. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Hoover WB, Szigeti-Buck K, Leranth C. Nucleus reuniens of the midline thalamus:link between the medial prefrontal cortex and the hippocampus. Brain Res Bull. 2007;71:601–609. doi: 10.1016/j.brainresbull.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viana Di Prisco G, Vertes RP. Excitatory actions of the ventral midline thalamus (Rhomboid/Reuniens) on the medial prefrontal cortex in the rat. Synapse. 2006;60:45–55. doi: 10.1002/syn.20271. [DOI] [PubMed] [Google Scholar]
- Zhang DX, Bertram EH. Midline thalamic region: widespread excitatory input to the entorhinal cortex and amygdala. J Neurosci. 2002;22:3277–3284. doi: 10.1523/JNEUROSCI.22-08-03277.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]