Abstract
We examined longitudinal associations between ApoE4+ status and several cognitive outcomes and tested effect modification by sex. Data on 644 Non-Hispanic White adults, from the Baltimore Longitudinal Study of Aging (BLSA) were used. Dementia onset, cognitive impairment and decline were assessed longitudinally. After 27.5 years median follow-up, 113 participants developed dementia. ApoE4+ predicted dementia significantly (HR=2.89; 95% CI: 1.93–4.33), with non-significant sex differences. Taking all time points for predicting cognition, women had significantly stronger positive associations than men between ApoE4+ status and impairment or decline on the California Verbal Learning Test (CVLT-delayed recall and List A total recall) and on Verbal Fluency Test-Categories. This ApoE4×sex interaction remained significant with bonferroni correction only for CVLT-delayed recall. Taking time points prior to dementia for cognitive predictions, the positive association between impairment in CVLT-delayed recall and ApoE4+ status remained stronger among women, though only before bonferroni correction. While ApoE4+ status appears to be a sex neutral risk factor for dementia, its association with verbal memory and learning decline and impairment was stronger among women.
Keywords: Apolipoprotein E genotype, dementia, cognitive decline, cognitive impairment, aging
INTRODUCTION
A growing body of literature supports a positive association between the Apolipoprotein E ε4 allele (ApoE4+ status) and the risks of impaired cognitive performance and cognitive decline (Small, et al., 2000). Despite general consistency in the related findings, some studies found no association or an inverse one between ApoE4+ status and poor cognitive performance, while others indicated a positive association between ApoE4+ status and poor cognition only among dementia patients.(Kim, et al., 2002,Riley, et al., 2000,Small, et al., 2000,Smith, et al., 1998,Winnock, et al., 2002,Yip, et al., 2002) Moreover, it is unclear whether this excess risk ascribed to ApoE4+ status is specific to certain cognitive domains over others, and whether this association is differentially modified by sex.
Multiple studies have found associations between the ApoE4+ status and impairment or decline on episodic memory, particularly delayed recall. (Bondi, et al., 1995,Chey, et al., 2000,Hyman, et al., 1996,Jorm, et al., 2007,Lehmann, et al., 2006,Luczywek, et al., 2002,Nilsson, et al., 2006,Packard, et al., 2007) This allele may also adversely influence verbal ability, (Mortensen and Hogh, 2001) executive functioning, (Chey, et al., 2000,Romero, et al., 2002,Swan, et al., 2005) perceptual/psychomotor speed and visuo-spatial skill (Blair, et al., 2005,Jorm, et al., 2007,O'Hara, et al., 2008,Packard, et al., 2007) in addition to global cognition. (Berr, et al., 1996,Bretsky, et al., 2003,Bunce, et al., 2004,Kuller, et al., 1998,Sawyer, et al., 2008,Slooter, et al., 1998,Winnock, et al., 2002) Moreover, while a number of those studies had a cross-sectional or case-control design, among twenty-five recent longitudinal studies, twenty-one had a follow-up of less than 10 years. (Aggarwal, et al., 2005,Blair, et al., 2005,Brayne, et al., 1996,Bretsky, et al., 2003,Bunce, et al., 2004,Feskens, et al., 1994,Henderson, et al., 1995,Hoyt, et al., 2005,Hyman, et al., 1996,Jonker, et al., 1998,Lam, et al., 2006,Martinez, et al., 1998,Molero, et al., 2001,Packard, et al., 2007,Payami, et al., 1997,Qiu, et al., 2006,Romero, et al., 2002,Swan, et al., 2005,Wilson, et al., 2002,Winnock, et al., 2002,Yaffe, et al., 1997) Moreover, study populations were relatively small in the majority of these longitudinal studies with eight out of twenty-five having baseline sample sizes of less than 300 participants (Aggarwal, et al., 2005,Brayne, et al., 1996,Hoyt, et al., 2005,Lam, et al., 2006,Martinez, et al., 1998,Mortensen and Hogh, 2001,Romero, et al., 2002,Swan, et al., 2005).
Two cross-sectional and several longitudinal studies have specifically or additionally examined ApoE4+ status and sex interactions in relation to cognition or dementia risk (Hyman, et al., 1996,Lehmann, et al., 2006,Martinez, et al., 1998,Molero, et al., 2001,Mortensen and Hogh, 2001,Payami, et al., 1997,Swan, et al., 2005) and an earlier meta-analysis indicated that sex was potentially an effect modifier in the ApoE genotype-Alzheimer’s disease (AD) association. (Farrer, et al., 1997) Moreover, experimental and neurobiological evidence suggests that ApoE4+ status may play a greater role in the neurodegenerative process among women than among men. (Fleisher, et al., 2005,Juottonen, et al., 1998,Villasana, et al., 2006) Thus far, no long-term large prospective cohort study has looked specifically at the possible effect modification by sex for the association of ApoE genotypes with incident dementia, as well as decline and impairment in specific neuropsychological domains of cognition.
In the present study, we examined whether the risks of cognitive impairment, cognitive decline, and dementia in older adults were associated with the ApoE ε4 allele. In particular, we investigated effect modification by sex for the association between the ε4 allele and cognitive outcomes and whether certain domains were more likely to be affected than others by this putative interaction.
METHODS
Participants
Data were obtained from the Baltimore Longitudinal Study on Aging (BLSA). Initiated in 1958, the BLSA is an ongoing prospective open cohort study of community-dwelling, generally highly educated, upper to middle class adults with a total enrollment of 3,005 (N1) participants aged 17–97 years (60.1% men). (Shock, 1984)Participants undergo medical and psychological examinations, including neuropsychological testing, a neurological exam, and medical history and physical examination; the details and exclusionary criteria have been summarized elsewhere.(Zonderman, et al., 1995) In the present study, participants were eligible for analyses only when they had at least one visit at or beyond age 50 years and when they were at risk for dementia (N2=2,321).
ApoE genotyping was performed on consecutive series of participants selected in two phases based on funding at the time, the first selecting participants 60 years or older and the second selecting 400 participants of any age. Among the eligible sample (N2=2,321), ApoE genotypes were available for 724 eligible participants. Additionally, only non-hispanic whites (N3 = 644) were included because associations between ApoE4+ status and dementia risk or cognition may differ between whites and other ethnicities. (Farrer, et al., 1997,Sawyer, et al., 2008)The numbers of eligible participants with complete genetic and cognitive test data varied between 515 for Trails B and 640 for BVRT (median frequency of repeated measurements ranged between 14 and 15), when all time points were considered for predictions.
Beyond the last visit per individual, 237 deaths occurred (162 men and 75 women) in the Non-Hispanic white sample with complete ApoE genotypes (N3=644). Mean ages and standard deviations (SD) among participants who died (n3=237) was 86.1 years (SD= 7.8) for men and 88.6 years (SD=8.0) for women (p<0.05, based on t-test with 1 d.f).
Clinical evaluation of dementia
All participants were followed annually and were reviewed at a consensus conference if their Blessed Information Memory Concentration score (Blessed, et al., 1968) was ≥4, if their informant or subject Clinical Dementia Rating (CDR) (Morris, 1997) score was ≥0.5, or if their Dementia Questionnaire (DQ) (Kawas, et al., 1994) was abnormal. All participants, regardless of screening tests, were evaluated by case conference at the time of death or withdrawal. Dementia diagnosis was determined according to DSM-III-R (American Psychiatric Association, 1987) criteria. Year of dementia onset was estimated based on consecutive case conference findings. A diagnosis of mild cognitive impairment (MCI) was made when participants had either single domain cognitive impairment (usually memory), or cognitive impairment in multiple domains without any significant functional loss in activities of daily living (ADLs), following the Petersen algorithm. (Petersen, 2004) In our present analysis, MCI cases were retained in the “at risk for dementia” group. Diagnoses of dementia type were formulated during multidisciplinary evaluations based on prospectively collected evidence using National Institute of Neurological and Communication Disorders—Alzheimer’s Disease and Related Disorders Association criteria (McKhann, et al., 1984). Secondary analyses were also conducted in which the outcomes were incident MCI and AD.
Cognitive assessment
A battery of five cognitive tests was used, namely Mini-Mental State Exam (MMSE), (Folstein, et al., 1975) Benton Visual Retention Test (BVRT), (Benton, 1974) California Verbal Learning Test (CVLT); List A and delayed recall score, (Delis, et al., 1988) verbal fluency tests, both letter (VFT-L) (Lezak, 1983,Lezak, 1995,Spreen and Benton, 1969) and category (VFT-C), (Rosen, 1980) and Trails A and B (Reitan, 1992) (see Appendix A). Linear mixed models with a quadratic age term (to allow for nonlinear age effects) were used to predict their values at specific ages, particularly mean individual age at follow-up prior to the onset of dementia or prior to the end of follow-up, and to predict the slope for annual cognitive change at that particular age. The latter can be interpreted as the annual rate of change in the cognitive score between ages 50 and the mean age of follow-up per individual and cognitive test (See Appendix B). Using quintiles, the poorest performance (“cognitive impairment) or steepest “cognitive decline” were determined and compared to all other quintiles combined. Additionally, the continuous scores (“cognitive function”) and rates of “cognitive change” were also considered as cognitive outcomes in a separate set of analyses.
ApoE genotypes
ApoE genotype was determined by polymerase chain reaction amplification of leukocyte DNA followed by HhaI digestion and product characterization, a process described by Hixson and Vernier. (Hixson and Vernier, 1990) Participants possessing at least one ε-4 allele were labeled as ApoE4+, while those without the allele were labeled as ApoE4−.
Covariates
We examined three sets of covariates as potential confounders in the main associations of interest: (1) Socio-demographic factors, namely individual age at first-visit and mean ages of follow-up (per individual and cognitive test), sex, educational attainment (years of schooling), and one lifestyle-related factor namely smoking status (never, former or current smoker); (2) self-reported history of type 2 diabetes, hypertension, cardiovascular disease (stroke, congestive heart failure, non-fatal myocardial infarction or atrial fibrillation) and dyslipidemia at first-visit; and (3) measured first-visit body mass index (BMI in kg/m2). In addition, first-visit blood pressure (systolic and diastolic in mm Hg), plasma total and HDL-cholesterol, and fasting blood glucose (in mg/dL) were only analyzed in relation to ApoE4 status and sex for descriptive purposes, given their higher proportion with missing data compared to the self-reported conditions.
Data analysis
Means of continuous measures across categorical variables were tested using t-test and one-way ANOVA. Associations between categorical variables were examined with the chi-square test. Kaplan-Meier survival curves and log-rank tests were used to compare the number of incident dementia cases by ApoE4 status. (Freedman, 1982) In addition, dose-response to the number of ε4 allele in the ApoE genotype (none vs. one (i.e. ε2/ε4 or ε3/ε4) ; none vs. two (i.e. ε4/ε4)) was assessed using a Log-rank test with its associated p-value for trend for the total eligible population. Cox proportional hazards (PH) models were conducted to assess risk of dementia associated with ApoE4+ status, stratifying by sex and controlling for potentially confounding covariates. The dependent measure was age at onset of dementia or the last observed (censored) age of non-diagnosed participants. (Cox, 1972) Moreover, predicted values of cognitive scores and associated slopes from multivariate linear mixed models with a quadratic age term added -- controlling for selected demographic and lifestyle factors -- were obtained at mean age when cognitive tests were conducted, taking all time available time points in one predictive model and time points prior to onset of dementia in another (See Appendix B). Further, a multivariate logistic regression analysis was carried out to examine the association between ApoE4+ status and significant cognitive decline or impairment (<20th percentile of cognitive change or predicted cognitive score at mean age of follow-up, except for BVRT, Trails A and B where the cutpoint was the 80th percentile). As a sensitivity analysis, we conducted OLS multivariate regression models to assess the association between ApoE4 status and cognitive function or annual rate of cognitive change as continuous outcomes, controlling for the same covariates as for the logistic models.
A type I error of 0.05 was considered for all analyses, and p-values between 0.05 and 0.10 were considered as borderline significant. To assess interaction between ApoE4 status and sex, a separate model was conducted (either Cox PH model or logistic regression) in which sex was included among the main effects and an ApoE4×sex interaction term was examined for both direction and statistical significance. Assuming that a type I error for interaction of 0.05 is statistically significant for each of the eight tests, bonferroni correction was carried out to set that type I error to 0.05/8=0.00625. All analyses were performed using Stata version 10.0 (STATA, 2007).
RESULTS
Among the 644 eligible Non-Hispanic white participants who were at risk of dementia starting at age 50 and had available genotype data, the distribution of ApoE genotypes was ε2/2=2; ε2/3=83; ε2/4=10; ε3/3=387; ε3/4=147; ε4/4=15. There were 113 incident dementia cases with an incidence rate of 707 per 100,000 person-years (95% CI: 588–850). Median follow-up time was 27.5 years. Ninety-four of the 113 dementia cases, (i.e. 83%) had died by the end of follow-up in 2006 (60 men and 34 women). Sixty-one of the total eligible population (N=644) were diagnosed as incident MCI by the end of follow-up and were retained in the risk set in the analysis for dementia risk. Among incident dementia cases that were retained in our analysis (i.e. n=113), seventy-six were differentially diagnosed as AD.
NH white eligible study participants with complete genetic data (n=644), were generally younger (Mean±SD first-visit age: 54.7±16.0 vs. 59.2±16.3) and healthier compared to the eligible NH white BLSA sub-population without genetic data (n=1,274) in terms of first-visit current smoking status (17.7% vs. 25.6%), body mass index (24.6±3.3 vs. 25.1±3.4) and some self-reported co-morbid conditions (e.g. type 2 diabetes (1.6% vs. 3.6%) and hypertension (30.3% vs. 40.8%)) and had a higher proportion of women (42.9% vs. 28.1%); (p<0.05 based on χ2 or t -test); (data not shown).
Men and women positive for the ε4 allele (ApoE4+) had a similar socio-demographic and metabolic profile as those who were ApoE4−. Incidence proportion of dementia was significantly higher among ApoE4+ compared to ApoE4− genotypes in women (26.2% vs. 13.0%; P<0.05 based on χ2 test) but not in men. However, comparing men to women’s profile, women had a significantly higher proportion with ApoE4+ status (31.2% vs. 23.5%), they were less educated, less likely to be former or current smokers, were older at first-visit (mean age 58.1 years (SD=16.8) vs. 52.3 years (SD=20.0) among men), had a significantly lower mean first-visit BMI, but a higher proportion obese, and had a generally better metabolic profile than men in terms of DBP, HDL-C and fasting glucose levels at first-visit (Table 1).
Table 1.
Baseline characteristics (at visit 1) of study participants by sex and ApoE4 status among eligible participants with at least one visit at or after age 50 years (N=644); Baltimore Longitudinal Study on Aging
| Men | Women | |||||
|---|---|---|---|---|---|---|
| All | ApoE4+ | ApoeE4− | All | ApoE4+ | ApoE4− | |
| 374 | 88 | 286 | 270 | 84*** | 186 | |
| N (%) | (100%) | (23.5%) | (76.5%) | (100%) | (31.2%) | (68.8%) |
| Education (yr.); Mean (SD) | 17.1 (2.8) | 17.1 (2.5) | 17.1 (2.8) | 16.2 (2.5)** | 16.3 (2.6) | 16.2 (2.4) |
| Smoking status (%) | ||||||
| Never | 37.4 | 40.2 | 36.5 | 48.8** | 53.7 | 46.7 |
| Former | 41.1 | 42.5 | 40.7 | 38.8 | 40.0 | 38.3 |
| Current | 21.5 | 17.2 | 22.8 | 12.3 | 6.2 | 15.0 |
| Type 2 diabetes (%) | 1.6 | 2.3 | 1.4 | 1.5 | 1.2 | 1.6 |
| Hypertension (%) | 32.6 | 31.0 | 33.1 | 27.1 | 25.3 | 27.9 |
| Cardiovascular disease† (%) | 7.0 | 4.6 | 7.6 | 6.3 | 5.9 | 6.5 |
| Dyslipidemia (%) | 5.4 | 3.4 | 6.0 | 5.3 | 4.8 | 5.5 |
| Age at first-visit (yr.); Mean (SD)
% |
52.3 (16.8) | 54.0 (17.4) | 51.7 (16.6) | 58.1 (14.0)** | 58.7 (12.0) | 57.7 (15.0) |
| ≤20 | 0.3 | 0.0 | 0.3 | 0.0** | 0.0 | 0.0 |
| 21–29 | 9.4 | 7.0 | 10.2 | 0.4 | 0.0 | 0.5 |
| 30–39 | 19.3 | 17.2 | 20.0 | 8.6 | 4.8 | 10.4 |
| 40–49 | 22.0 | 26.4 | 20.7 | 22.6 | 19.3 | 24.0 |
| 50–59 | 11.6 | 6.9 | 13.0 | 27.1 | 34.9 | 23.5 |
| 60–69 | 16.4 | 18.4 | 15.8 | 15.8 | 20.5 | 13.7 |
| 70–79 | 15.6 | 14.9 | 15.8 | 18.0 | 13.2 | 20.2 |
| 80+ | 5.4 | 9.2 | 4.2 | 7.5 | 7.2 | 7.6 |
| BMI (kg/m2); Mean (SD)
% |
24.9 (3.1) | 24.9 (3.9) | 25.0 (2.8) | 24.2 (3.6)** | 24.1 (3.4) | 24.2 (3.7) |
| Underweight (BMI≤18.5) | 0.3 | 1.2 | 0.0 | 1.9** | 1.2 | 2.2 |
| Normal weight (18.5<BMI≤24.9) | 57.5 | 63.2 | 55.8 | 65.1 | 65.1 | 65.2 |
| Overweight (25.0≤BMI≤29.9) | 36.0 | 28.7 | 38.2 | 23.9 | 27.7 | 22.1 |
| Obese (BMI≥30) | 6.2 | 6.9 | 6.0 | 9.1 | 6.0 | 10.5 |
| Mean (SD) | ||||||
| Systolic blood pressure (mm Hg.) | 126.9 (17.4) | 127.0 (18.9) | 126.8 (17.0) | 125.1 (19.5) | 124.7 (17.6) | 125.3 (20.3) |
| Diastolic blood pressure (mm Hg.) | 80.5 (10.4) | 80.6 (11.7) | 80.4 (10.0) | 76.6 (9.5)** | 76.8 (9.0) | 76.5 (9.8) |
| Total cholesterol level (mg/dl.) | 217.8 (39.8) | 222.2 (39.5) | 216.4 (40.0) | 217.5 (38.2) | 224.0 (37.3) | 214.5 (38.4) |
| HDL-C (mg/dl.) | 43.0 (10.3) | 41.8 (8.9) | 43.4 (10.7) | 53.1 (13.5)** | 53.3 (13.5) | 53.0 (13.6) |
| Fasting plasma glucose (mg/dl.) | 101.2 (15.4) | 103.8 (20.0) | 100.4 (13.4) | 95.9 (9.4)** | 96.2 (9.4) | 95.7 (9.4) |
| Dementia (%) | 17.9 | 23.9 | 16.1 | 17.1 | 26.2 | 13.0* |
Abbreviations: ApoE4=Apolipoprotein E ε4 allele carrier status; BMI=Body Mass Index; HDL-C=High Density Lipoprotein-Cholesterol; Hg=Mercury; SD=Standard Deviation.
p<0.05 for the null hypothesis of no difference between ApoE status for means among women.
p<0.05 for null hypothesis of no difference between men and women in means or proportions based on ANOVA or χ2 test.
p<0.05 for null hypothesis of no difference between men and women in ApoE status distribution.
Reported any of the following conditions at first-visit: stroke, congestive heart failure, non-fatal myocardial infarction or atrial fibrillation.
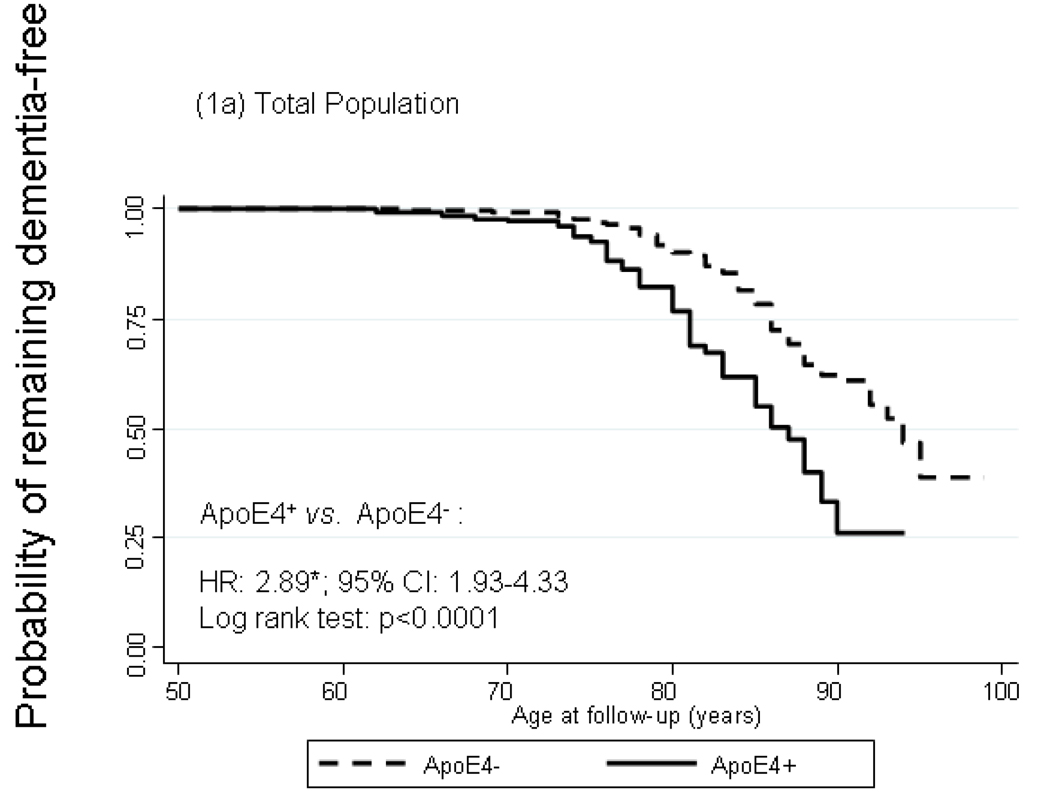
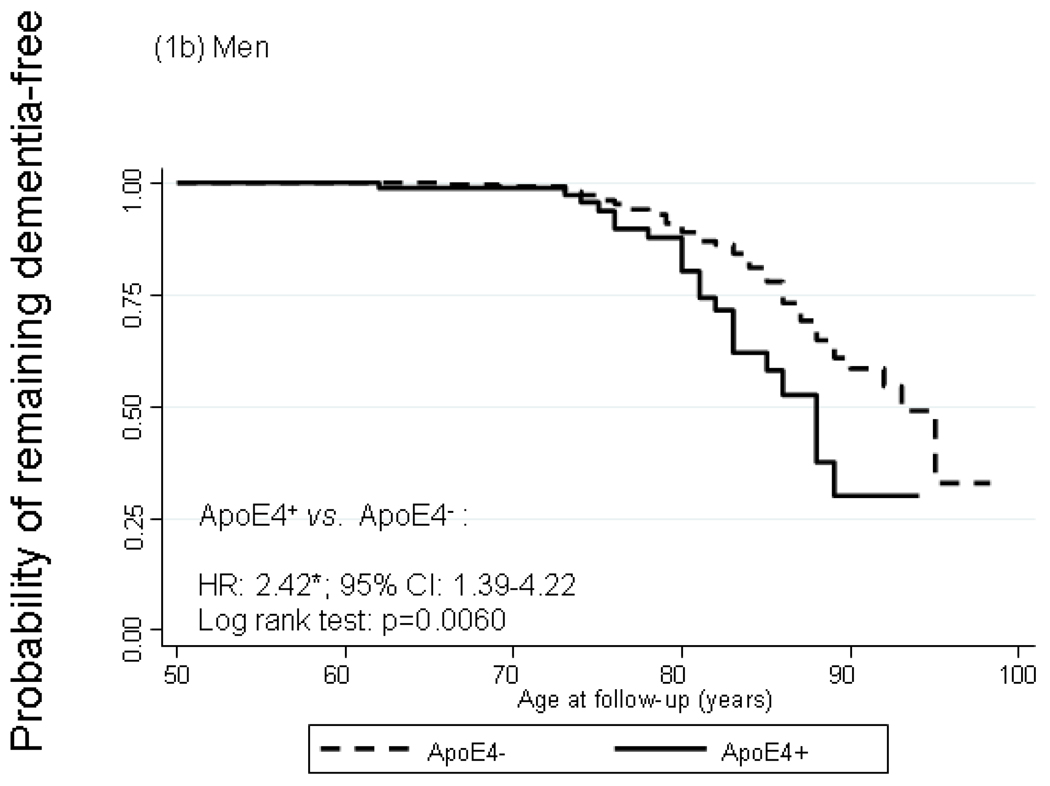
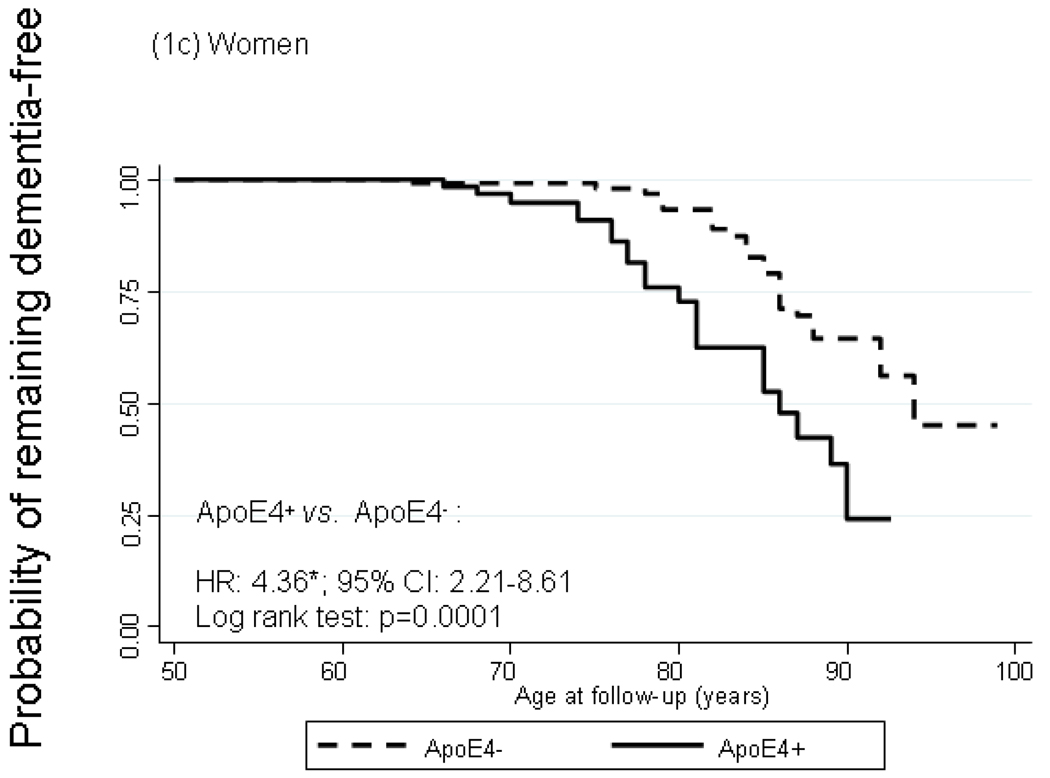
Figure 1 shows Kaplan-Meier survival curves of incident dementia by ApoE4+ status with time starting at age 50 years. In the total population, cumulative incidence of dementia was significantly higher among ApoE4+ compared to ApoE4− participants particularly by age 75. The log rank test for equality of survivor functions indicated that the divergence between the two curves was statistically significant (P<0.05). Modeling instantaneous hazard functions against ApoE4+ status and controlling for potential confounders –namely sex, first-visit age, educational attainment, smoking status, self-reported co-morbid conditions (See section on Covariates), and first-visit body mass index -- using Cox PH models yielded a hazard ratio (HR) for dementia by ApoE4+ status of 2.89 with a 95% CI of 1.93–4.33. Both survivor functions and Cox models indicated a stronger relationship with ApoE4+ status among women compared to men (HRs: 4.36 with 95% CI: 2.21–8.61 among women vs. 2.42 with 95% CI: 1.39–4.22 among men), though effect modification by sex was not statistically significant (P=0.219 for ApoE4×sex interaction term in a model including sex among main effects). A secondary analysis in which ApoE4 status was replaced with ApoE2 status (at least one ε2 allele vs. other ApoE genotypes) did not show evidence of an association between the ε2 allele and dementia risk (HR=0.99 with a 95% CI: 0.58–1.68). Another secondary analysis in which the outcome was incident AD (n=75 cases) and exposure was ApoE4+ status, showed even greater homogeneity of HRs between men and women (HR=2.53 with a 95% CI: 1.30–4.95 for men and HR=2.82 with a 95% CI: 1.33–5.94 for women) and p=0.59 for ApoE4×sex interaction. However, when incident MCI was considered as the outcome of interest, there was no significant association observed with ApoE4 status for men or women (data not shown).
FIGURE 1.
Kaplan-Meier survival curve of incident dementia by apolipoprotein E epsilon 4 allele status by sex
Abbreviations: ApoE4=Apolipoprotein E ε4 allele carrier status; CI=Confidence Interval; HR=Hazard Ratio.
Notes: Failure is defined as first incidence of dementia at or after age 50. HR is adjusted for sex, first-visit age, education, first-visit smoking status, first-visit self-reported type 2 diabetes, hypertension, dyslipidemia, cardiovascular disease and measured body mass index. *p<0.05 for null hypothesis that Loge(HR)=0. N=6,515 observations representing 631 participants (371 men and 260 women) n=107 failures after age 50 (64 men and 43 women).
Examining the number of ε4 alleles against dementia risk in the total population, the Log-rank test for trend indicated a dose-response relationship (χ2 for trend (1 df.)=21.79; p-value for trend<0.0001). In addition, Cox PH with number of ε4 alleles entered as two dummy variables (1 vs. none; 2 vs. none) yielded HRs of 2.79 (95% CI: 1.83–4.27) and 3.56 (95% CI: 1.52–8.35)), respectively, which shows a dose-response when plotting the HRs point estimates on the Loge scale with the initial point (no ε4 allele) being equal to zero. This dose-response was also observed when incident AD was considered as the outcome of interest [HR=2.57 (95% CI: 1.55–4.27) for 1 allele vs. none and HR=4.08 (95% CI:1.59–10.49) for 2 alleles vs. none]; (data not shown).
Using all cognitive data—including scores from assessments administered after some participants were diagnosed with dementia -- in one analysis to predict cognitive impairment or decline, ApoE4+ men and women were at increased risk of global cognitive decline in MMSE at mean age of follow-up (ORs were 2.47 with 95% CI: 1.17–5.21 among men and 4.92 with 95% CI: 1.79–13.56 among women). Moreover, using a similar methodology in including all time points to predict cognitive outcomes at mean age of follow-up, significant sex interaction with ApoE4+ status was found for impairment in CVLT delayed recall (p=0.005), CVLT List A (p=0.020), VFT-C (p=0.030), and for decline in CVLT delayed recall (p=0.001) and CVLT list A (p=0.022), the associations being stronger among women. After bonferroni correction, significance of ApoE4×sex interaction was retained only for decline and impairment in CVLT (delayed recall). No significant sex differences were noted for other binary cognitive outcomes, particularly after bonferroni correction for multiple comparisons. Additionally, although sex differences were noted in many continuous cognitive outcomes (i.e. using multivariate OLS regression; six out of sixteen), including CVLT, none remained significant after bonferroni correction for multiple testing (Table 2).
Table 2.
Cognitive function/impairment and cognitive change/decline (all time points) by sex and ApoE4 status; Baltimore Longitudinal Study on Aging
| Men | Women | |||||
|---|---|---|---|---|---|---|
| ApoE4+ | ApoeE4− | ApoE4+ | ApoE4− | |||
|
Predicted cognitive function/impairment at mean
age at follow-up † |
||||||
| MMSE | ||||||
| N | 76 | 243 | 69 | 142 | ||
| Mean (SD) | 28.0 (2.0) | 28.4 (1.5) | ~ | 27.7 (3.1) | 28.6 (1.4) | * |
| −0.21 | −0.84* ¶ | |||||
| Multivariate-adjusted β‡ (95% CI) | (−0.56; 0.14) | (−1.30; −0.38) | ||||
| % <20th percentile (score <27.4) | 27.6 | 16.5 | * | 23.2 | 11.3 | * |
| 2.47* | 4.92* | |||||
| Multivariate-adjusted OR§ (95% CI) | (1.17; 5.21) | (1.79; 13.56) | ||||
| BVRT | ||||||
| N | 88 | 281 | 83 | 188 | ||
| Mean (SD) | 5.4 (3.4) | 5.0 (3.0) | 6.7 (4.7) | 5.1 (3.2) | * | |
| 0.13 | 1.16* ¶ | |||||
| Multivariate-adjusted β (95% CI) | (−0.43; 0.68) | (0.43; 1.89) | ||||
| % >80th percentile (score >7.4) | 27.3 | 19.9 | 32.5 | 23.9 | ||
| 1.59 | 2.36* | |||||
| Multivariate-adjusted OR (95% CI) | (0.74; 3.42) | (1.08; 5.16) | ||||
| CVLT (List A) | ||||||
| N | 78 | 251 | 69 | 171 | ||
| Mean (SD) | 49.0 (10.5) | 48.3 (10.5) | 53.8 (12.2) | 57.0 (10.4) | * | |
| 1.30 | −3.01* ¶ | |||||
| Multivariate-adjusted β (95% CI) | (−0.97; 3.57) | (−5.42; −0.59) | ||||
| % <20th percentile (score <43.2) | 30.8 | 33.1 | 20.3 | 11.1 | ~ | |
| 0.82 | 4.60* ¶ | |||||
| Multivariate-adjusted OR (95% CI) | (0.43; 1.58) | (1.65; 12.84) | ||||
| CVLT (delayed recall) | ||||||
| N | 78 | 251 | 69 | 171 | ||
| Mean (SD) | 10.3 (2.8) | 10.0 (3.3) | 11.3 (3.4) | 12.1 (2.7) | ~ | |
| 0.41 | −0.88* ¶ | |||||
| Multivariate-adjusted β (95% CI) | (−0.29; 1.11) | (−1.57; −0.19) | ||||
| % <20th percentile (score <8.6) | 24.4 | 30.3 | 20.3 | 11.1 | ~ | |
| 0.60 | 3.62* ¶ | |||||
| Multivariate-adjusted OR (95% CI) | (0.30; 1.20) | (1.39; 9.45) | ||||
| VFT-L | ||||||
| N | 77 | 248 | 72 | 149 | ||
| Mean (SD) | 14.8 (3.9) | 14.4 (3.9) | 14.0 (3.9) | 14.6 (3.5) | ||
| 0.44 | −0.44 | |||||
| Multivariate-adjusted β (95% CI) | (−0.53; 1.42) | (−1.43; 0.55) | ||||
| % <20th percentile (score <10.9) | 11.7 | 20.6 | ~ | 16.7 | 14.1 | |
| 0.47~ | 1.13 | |||||
| Multivariate-adjusted OR (95% CI) | (0.20; 1.07) | (0.47; 2.72) | ||||
| VFT-C | ||||||
| N | 77 | 248 | 72 | 149 | ||
| Mean (SD) | 13.6 (3.5) | 14.0 (3.4) | 14.5 (3.9) | 15.5 (3.4) | ~ | |
| −0.02 | −0.89* | |||||
| Multivariate-adjusted β (95% CI) | (−0.71; 0.67) | (1.65; −0.13) | ||||
| % <20th percentile (score <11.4) | 23.4 | 21.8 | 23.6 | 12.7 | * | |
| 0.93 | 3.96* ¶ | |||||
| Multivariate-adjusted OR (95% CI) | (0.41; 2.08) | (1.32; 11.88) | ||||
| Trails A | ||||||
| N | 76 | 237 | 67 | 136 | ||
| Mean (SD) | 41.2 (15.4) | 39.2 (15.6) | 41.4 (14.6) | 41.7 (20.6) | ||
| 1.82 | 0.54 | |||||
| Multivariate-adjusted β (95% CI) | (−1.73; 5.36) | (−3.66; 4.74) | ||||
| % >80th percentile (score >52.0) | 21.0 | 12.7 | ~ | 14.9 | 20.6 | |
| 2.58* | 0.69 | |||||
| Multivariate-adjusted OR (95% CI) | (1.11; 5.97) | (0.26; 1.93) | ||||
| Trails B | ||||||
| N | 76 | 237 | 67 | 135 | ||
| Mean (SD) | 107.3 (46.9) | 98.1 (38.6) | ~ | 119.5 (56.3) | 103.7 (44.0) | * |
| 6.73 | 15.29* | |||||
| Multivariate-adjusted β (95% CI) | (−1.83; 15.30) | (5.29; 26.00) | ||||
| % >80th percentile (score >146.0) | 23.7 | 13.9 | * | 28.4 | 15.6 | * |
| 2.44* | 4.14* | |||||
| Multivariate-adjusted OR (95% CI) | (1.09; 5.48) | (1.54; 11.16) | ||||
|
Predicted cognitive annual rate change/decline at
mean age of follow-up † |
||||||
| MMSE | ||||||
| N | 76 | 243 | 69 | 142 | ||
| Mean (SD) | −0.01 (0.13) | 0.03 (0.08) | * | −0.01 (0.17) | 0.05 (0.10) | * |
| −0.03* | −0.06* | |||||
| Multivariate-adjusted β (95% CI) | (−0.05; −0.01) | (−0.09; −0.04) | ||||
| % <20th percentile (change <-0.03) | 33.8 | 20.8 | * | 23.2 | 8.4 | * |
| 2.03* | 6.58* | |||||
| Multivariate-adjusted OR (95% CI) | (1.08; 3.80) | (2.28; 18.89) | ||||
| BVRT | ||||||
| N | 88 | 281 | 83 | 188 | ||
| Mean (SD) | 0.13 (0.08) | 0.12 (0.07) | 0.14 (0.10) | 0.11 (0.07) | * | |
| 0.005 | 0.028* ¶ | |||||
| Multivariate-adjusted β (95% CI) | (−0.010; 0.020) | (0.011; 0.046) | ||||
| % >80th percentile (change >0.18) | 31.8 | 24.2 | 31.3 | 16.5 | * | |
| 1.42 | 3.14* | |||||
| Multivariate-adjusted OR (95% CI) | (0.77; 2.64) | (1.51; 6.55) | ||||
| CVLT (List A) | ||||||
| N | 78 | 251 | 69 | 171 | ||
| Mean (SD) | −0.33 (0.23) | −0.31 (0.26) | −0.34 (0.30) | −0.26 (0.23) | * | |
| −0.017 | −0.071* | |||||
| Multivariate-adjusted β (95% CI) | (−0.078; 0.043) | (−0.133; −0.008) | ||||
| % <20th percentile (change <−0.47) | 26.9 | 27.9 | 31.9 | 17.0 | * | |
| 0.96 | 3.80* ¶ | |||||
| Multivariate-adjusted OR (95% CI) | (0.50; 1.83) | (1.65; 8.72) | ||||
| CVLT (delayed recall) | ||||||
| N | 78 | 251 | 69 | 171 | ||
| Mean (SD) | −0.09 (0.05) | −0.09 (0.06) | −0.09 (0.07) | −0.07 (0.05) | * | |
| 0.006 | −0.018* ¶ | |||||
| Multivariate-adjusted β (95% CI) | (−0.007; 0.020) | (−0.032; −0.004) | ||||
| % <20th percentile (change <−0.13) | 21.8 | 27.5 | 27.5 | 12.3 | * | |
| 0.63 | 5.78* ¶ | |||||
| Multivariate-adjusted OR (95% CI) | (0.32; 1.25) | (2.29; 14.54) | ||||
| VFT-L | ||||||
| N | 77 | 248 | 72 | 149 | ||
| Mean (SD) | −0.01 (0.12) | −0.01 (0.11) | 0.01 (0.14) | 0.02 (0.11) | ||
| −0.001 | −0.009 | |||||
| Multivariate-adjusted β (95% CI) | (−0.029; 0.027) | (−0.041; 0.022) | ||||
| % <20th percentile (change <−0.08) | 23.4 | 24.6 | 19.4 | 20.1 | ||
| 0.91 | 1.00 | |||||
| Multivariate-adjusted OR (95% CI) | (0.47; 1.77) | (0.43; 2.39) | ||||
| VFT-C | ||||||
| N | 77 | 248 | 72 | 149 | ||
| Mean (SD) | −0.07 (0.15) | −0.04 (0.14) | ~ | −0.07(0.16) | −0.01 (0.13) | * |
| −0.025 | −0.057* | |||||
| Multivariate-adjusted β (95% CI) | (−0.055; 0.005) | (−0.091; −0.023) | ||||
| % <20th percentile (change <−0.13) | 32.5 | 22.9 | ~ | 26.4 | 16.7 | ~ |
| 1.81~ | 2.49* | |||||
| Multivariate-adjusted OR (95% CI) | (0.90; 3.63) | (1.05; 5.90) | ||||
| Trails A | ||||||
| N | 76 | 237 | 67 | 136 | ||
| Mean (SD) | 0.34 (0.96) | 0.12 (0.98) | ~ | 0.23 (0.95) | 0.23(0.80) | |
| 0.233 | 0.031 | |||||
| Multivariate-adjusted β (95% CI) | (−0.017; 0.483) | (−0.201; 0.263) | ||||
| % >80th percentile (change >0.85) | 21.0 | 11.8 | * | 16.4 | 16.2 | |
| 2.19* | 1.19 | |||||
| Multivariate-adjusted OR (95% CI) | (1.02; 4.70) | (0.44; 3.22) | ||||
| Trails B | ||||||
| N | 76 | 237 | 67 | 135 | ||
| Mean (SD) | 0.52 (2.21) | 0.04 (1.77) | ~ | 0.78 (2.40) | 0.13 (1.85) | * |
| 0.391 | 0.673* | |||||
| Multivariate-adjusted β (95% CI) | (−0.067; 0.848) | (0.173; 1.174) | ||||
| % >80th percentile (change >1.92) | 21.0 | 13.9 | 25.4 | 15.6 | ||
| 1.70 | 2.70* | |||||
| Multivariate-adjusted OR (95% CI) | (0.80; 3.58) | (1.09; 6.73) | ||||
Abbreviations: ApoE4=Apolipoprotein E ε4 allele carrier status; CI=Confidence Interval; BVRT=Benton Visual Retention Test; CVLT=California Verbal Learning Test; MMSE=Mini- Mental State Examination; OR=Odds Ratio; SD=Standard Deviation; VFT-C=Verbal Fluency Test, Categories or “Category Fluency” test; VFT-L=Verbal Fluency Test, Letters or “Letter Fluency” test.
p<0.10;
p<0.05 for the null hypothesis of no difference between ApoE status for means, proportions, β coefficient (OLS regression) or Loge(OR) (logistic regression) within each sex group.
Cognitive scores were predicted at mean age at follow-up prior to onset of dementia using a multivariate linear mixed model controlling for sex, race/ethnicity, education (years), and smoking status, with age added among the fixed effect variables to allow for quadratic non-linear change. The lowest quintile in the total population with data on this test was considered as “cognitive impairment” for most tests except BVRT, Trails A and B where the highest quintile was considered as impairment. The slope or annual rate of change was also predicted from these models at the mean age at follow-up (i.e. between age 50 and individual mean age of follow-up for each cognitive test). The lowest quintile of the slope or rate of change was considered as “significant cognitive decline” for most tests except for BVRT, Trails A and B where the highest quintile was considered as decline ( See Appendix B for more details).
Based on multivariate OLS regression models with outcome being cognitive function or cognitive annual rate of change and main exposure being ApoE4 status, stratified by sex. The model controlled for first-visit age, mean age at follow-up, education, first-visit smoking status, first-visit self-reported type 2 diabetes, hypertension, cardiovascular disease and body mass index.
Based on multivariate logistic regression models with outcome being cognitive impairment or cognitive decline and main exposure being ApoE4 status, stratified by sex. The model controlled for first-visit age, mean age at follow-up, education, first-visit smoking status, first-visit self-reported type 2 diabetes, hypertension, cardiovascular disease and body mass index.
p<0.05 for null hypothesis that ApoE4×sex is significant in a multivariate OLS or logistic regression model with cognitive function/change or impairment/decline as the outcomes and ApoE4 status as the main exposure, with sex entered into the main effects and control made for first-visit age, mean age at follow-up, education, first-visit smoking status, first-visit self-reported type 2 diabetes, hypertension, cardiovascular disease and body mass index.
Excluding cognitive data from assessments administered after some participants developed dementia for cognitive predictions (Table 3), ApoE4+ status had a stronger positive association with impairment in CVLT (delayed recall) among women compared to men. Bonferroni correction, however, indicated that this interaction term was not significant taking into account multiple testing. Moreover, both men and women were at significantly or borderline significant increased risk of global cognitive decline (i.e. in MMSE) with ApoE4+ compared to ApoE4−, although this positive association was significant for BVRT decline only among women, without appreciable ApoE4×sex interaction.
Table 3.
Cognitive function/impairment and cognitive change/decline (Time points prior to onset of dementia) by sex and ApoE4 status; Baltimore Longitudinal Study on Aging
| Men | Women | |||||
|---|---|---|---|---|---|---|
| ApoE4+ | ApoeE4 | ApoE4+ | ApoE4 | |||
|
Predicted cognitive function/impairment at mean
age at follow-up † |
||||||
| MMSE | ||||||
| N | 72 | 230 | 61 | 137 | ||
| Mean (SD) | 28.5 (1.3) | 28.6 (1.1) | 29.0 (0.8) | 28.9 (0.9) | ||
| −0.10 | −0.02 | |||||
| Multivariate-adjusted β (95% CI) | (−0.36; 0.17) | (−0.26; 0.22) | ||||
| % <20th percentile (score <27.9) | 18.1 | 18.7 | 11.5 | 11.7 | ||
| 1.11 | 2.20 | |||||
| Multivariate-adjusted OR‡ (95% CI) | (0.51; 2.41) | (0.69; 7.03) | ||||
| BVRT | ||||||
| N | 83 | 272 | 78 | 183 | ||
| Mean (SD) | 4.98 (2.79) | 4.99 (2.95) | 5.49 (2.90) | 5.01 (3.14) | ||
| −0.13 | 0.38 | |||||
| Multivariate-adjusted β (95% CI) | (−0.68; 0.42) | (−0.23; 0.99) | ||||
| % >80th percentile (score >7.1) | 22.9 | 22.1 | 24.4 | 25.7 | ||
| 1.09 | 1.51 | |||||
| Multivariate-adjusted OR (95% CI) | (0.52; 2.29) | (0.65; 3.53) | ||||
| CVLT (List A) | ||||||
| N | 71 | 235 | 63 | 164 | ||
| Mean (SD) | 50.9 (8.5) | 49.7 (9.6) | 56.3 (10.5) | 58.1 (9.1) | ||
| 1.76 | −1.90¶ | |||||
| Multivariate-adjusted β (95% CI) | (−0.43; 3.96) | (−4.19; 0.38) | ||||
| % <20th percentile (score <45.6) | 29.6 | 34.0 | 12.7 | 10.4 | ||
| 0.71 | 2.20 | |||||
| Multivariate-adjusted OR (95% CI) | (0.36; 1.39) | (0.74; 6.55) | ||||
| CVLT (delayed recall) | ||||||
| N | 71 | 235 | 63 | 164 | ||
| Mean (SD) | 10.8 (2.3) | 10.4 (3.0) | 12.2 (2.7) | 12.4 (2.4) | ||
| 0.52 | −0.40 | |||||
| Multivariate-adjusted β (95% CI) | (−0.15; 1.20) | (−1.04; 0.24) | ||||
| % <20th percentile (score <9.1) | 25.3 | 31.1 | 15.9 | 9.8 | ||
| 0.67 | 2.73~ ¶ | |||||
| Multivariate-adjusted OR (95% CI) | (0.34; 1.32) | (1.00; 7.50) | ||||
| VFT-L | ||||||
| N | 72 | 234 | 64 | 138 | ||
| Mean (SD) | 15.4 (3.5) | 14.7 (3.7) | 15.1 (3.4) | 15.0 (3.5) | ||
| 0.69 | 0.15 | |||||
| Multivariate-adjusted β (95% CI) | (−0.24; 1.62) | (−0.88; 1.18) | ||||
| % <20th percentile (score <11.4) | 12.5 | 20.0 | 14.1 | 13.0 | ||
| 0.60 | 1.10 | |||||
| Multivariate-adjusted OR (95% CI) | (0.26; 1.35) | (0.44; 2.71) | ||||
| VFT-C | ||||||
| N | 72 | 235 | 64 | 143 | ||
| Mean (SD) | 14.4 (2.6) | 14.4 (3.0) | 15.9 (2.7) | 15.9 (3.0) | ||
| 0.22 | −0.23 | |||||
| Multivariate-adjusted β (95% CI) | (−0.41; 0.85) | (−0.95; 0.48) | ||||
| % <20th percentile (score <12.4) | 20.8 | 25.5 | 10.9 | 12.6 | ||
| 0.60 | 1.44 | |||||
| Multivariate-adjusted OR (95% CI) | (0.27; 1.34) | (0.43; 4.78) | ||||
| Trails A | ||||||
| N | 71 | 223 | 59 | 126 | ||
| Mean (SD) | 37.1 (11.5) | 37.0 (11.4) | 36.4 (10.2) | 37.9 (14.9) | ||
| 0.61 | 0.74 | |||||
| Multivariate-adjusted β (95% CI) | (−1.93; 9.22) | (−2.40; 3.89) | ||||
| % >80th percentile (score >47.2) | 15.5 | 13.0 | 10.2 | 18.2 | ||
| 1.72 | 1.09 | |||||
| Multivariate-adjusted OR (95% CI) | (0.69; 4.28) | (0.33; 3.58) | ||||
| Trails B | ||||||
| N | 71 | 223 | 59 | 123 | ||
| Mean (SD) | 95.2 (35.0) | 93.1 (33.9) | 100.1 (41.5) | 91.5 (31.8) | ||
| 1.80 | 11.47* | |||||
| Multivariate-adjusted β (95% CI) | (−5.61; 9.22) | (2.55; 20.38) | ||||
| % >80th percentile (score >154.2) | 4.2 | 5.4 | 8.5 | 6.5 | ||
| 0.83 | 2.19 | |||||
| Multivariate-adjusted OR (95% CI) | (0.20; 3.44) | (0.56; 8.60) | ||||
|
Predicted cognitive change/decline beyond mean
age at follow-up † |
||||||
| MMSE | ||||||
| N | 72 | 230 | 61 | 137 | ||
| Mean (SD) | −0.02 (0.07) | −0.01 (0.05) | 0.01 (0.04) | 0.02 (0.04) | ||
| −0.011 | −0.010 | |||||
| Multivariate-adjusted β (95% CI) | (−0.026; 0.003) | (−0.220; 0.002) | ||||
| % <20th percentile (change <-0.04) | 31.9 | 20.4 | * | 11.5 | 8.8 | |
| 2.04* | 2.87~ | |||||
| Multivariate-adjusted OR (95% CI) | (1.08 3.84) | (0.87; 9.43) | ||||
| BVRT | ||||||
| N | 83 | 272 | 78 | 183 | ||
| Mean (SD) | 0.11 (0.06) | 0.11 (0.06) | 0.11 (0.06) | 0.10 (0.06) | ||
| 0.000 | 0.011 | |||||
| Multivariate-adjusted β (95% CI) | (−0.012; 0.013) | (−0.002; 0.024) | ||||
| % >80th percentile (change >0.15) | 26.5 | 22.8 | 25.6 | 19.1 | ||
| 1.25 | 2.11~ | |||||
| Multivariate-adjusted OR (95% CI) | (0.65; 2.41) | (1.00; 4.45) | ||||
| CVLT (List A) | ||||||
| N | 71 | 235 | 63 | 164 | ||
| Mean (SD) | −0.28 (0.17) | −0.27 (0.21) | −0.27 (0.23) | −0.24 (0.18) | ||
| −0.005 | −0.033 | |||||
| Multivariate-adjusted β (95% CI) | (−0.057; 0.048) | (−0.085; 0.020) | ||||
| % <20th percentile (change <−0.40) | 23.9 | 28.1 | 23.8 | 20.7 | ||
| 0.83 | 1.55 | |||||
| Multivariate-adjusted OR (95% CI) | (0.41; 1.65) | (0.70; 3.43) | ||||
| CVLT (delayed recall) | ||||||
| N | 71 | 235 | 63 | 164 | ||
| Mean (SD) | −0.07 (0.04) | −0.08 (0.05) | −0.06 (0.05) | −0.06 (0.04) | ||
| 0.008 | −0.007 | |||||
| Multivariate-adjusted β (95% CI) | (−0.03; 0.020) | (−0.019; 0.004) | ||||
| % <20th percentile (change <−0.11) | 22.5 | 29.8 | 15.9 | 11.0 | ||
| 0.60 | 2.50~ | |||||
| Multivariate-adjusted OR (95% CI) | (0.31; 1.18) | (0.96; 6.45) | ||||
| VFT-L | ||||||
| N | 72 | 234 | 64 | 138 | ||
| Mean (SD) | −0.02 (0.08) | −0.02 (0.08) | 0.03 (0.08) | 0.01 (0.09) | ~ | |
| −0.002 | 0.0212 | |||||
| Multivariate-adjusted β (95% CI) | (−0.022; 0.018) | (−0.002; 0.044) | ||||
| % <20th percentile (change <−0.07) | 23.6 | 27.3 | 6.2 | 17.4 | * | |
| 0.93 | 0.19* | |||||
| Multivariate-adjusted OR (95% CI) | (0.48; 1.81) | (0.05; 0.75) | ||||
| VFT-C | ||||||
| N | 72 | 235 | 64 | 143 | ||
| Mean (SD) | −0.08 (0.10) | −0.06 (0.09) | −0.04 (0.08) | −0.03 (0.09) | ||
| −0.014 | −0.012 | |||||
| Multivariate-adjusted β (95% CI) | (−0.035; 0.007) | (−0.034; 0.009) | ||||
| % <20th percentile (change <−0.12) | 30.6 | 27.2 | 12.5 | 17.5 | ||
| 1.30 | 0.94 | |||||
| Multivariate-adjusted OR (95% CI) | (0.65; 2.63) | (0.32; 2.71) | ||||
| Trails A | ||||||
| N | 71 | 223 | 59 | 126 | ||
| Mean (SD) | 0.23(0.52) | 0.14 (0.56) | 0.14 (0.49) | 0.18 (0.59) | ||
| 0.109 | 0.033 | |||||
| Multivariate-adjusted β (95% CI) | (−0.030; 0.290) | (−0.117; 0.183) | ||||
| % >80th percentile (change >0.64) | 19.7 | 15.2 | 11.9 | 13.5 | ||
| 1.71 | 1.89 | |||||
| Multivariate-adjusted OR (95% CI) | (0.77; 3.80) | (0.57; 6.22) | ||||
| Trails B | ||||||
| N | 71 | 223 | 59 | 123 | ||
| Mean (SD) | 0.27 (1.63) | 0.13 (1.50) | 0.38 (1.83) | 0.06 (1.4) | ||
| 0.147 | 0.424 | |||||
| Multivariate-adjusted β (95% CI) | (−0.234; 0.528) | (−0.178; 0.867) | ||||
| % >80th percentile (change >2.80) | 8.4 | 6.3 | 10.2 | 5.7 | ||
| 1.52 | 2.52 | |||||
| Multivariate-adjusted OR (95% CI) | (0.52; 4.48) | (0.68; 9.33) | ||||
Abbreviations: ApoE4=Apolipoprotein E ε4 allele carrier status; CI=Confidence Interval; BVRT=Benton Visual Retention Test; CVLT=California Verbal Learning Test; MMSE=Mini-Mental State Examination; OR=Odds Ratio; SD=Standard Deviation; VFT-C=Verbal Fluency Test, Categories or “Category Fluency” test; VFT-L=Verbal Fluency Test, Letters or “Letter Fluency” test.
p<0.10;
p<0.05 for the null hypothesis of no difference between ApoE status for means, proportions, β coefficient (OLS regression) or Loge(OR) (logistic regression) within each sex group.
Cognitive scores were predicted at mean age at follow-up prior to onset of dementia using a multivariate linear mixed model controlling for sex, race/ethnicity, education (years), and smoking status, with age added among the fixed effect variables to allow for quadratic non-linear change. The lowest quintile in the total population with data on this test was considered as “cognitive impairment” for most tests except BVRT, Trails A and B where the highest quintile was considered as impairment. The slope or annual rate of change was also predicted from these models at the mean age at follow-up (i.e. between age 50 and individual mean age of follow-up for each cognitive test). The lowest quintile of the slope or rate of change was considered as “significant cognitive decline” for most tests except for BVRT, Trails A and B where the highest quintile was considered as decline (See Appendix B for more details).
Based on multivariate OLS regression models with outcome being cognitive function or cognitive annual rate of change and main exposure being ApoE4 status, stratified by sex. The model controlled for first-visit age, mean age at follow-up, education, first-visit smoking status, first-visit self-reported type 2 diabetes, hypertension, cardiovascular disease and body mass index.
Based on multivariate logistic regression models with outcome being cognitive impairment or cognitive decline and main exposure being ApoE4 status, stratified by sex. The model controlled for first-visit age, mean age at follow-up, education, first-visit smoking status, first-visit self-reported type 2 diabetes, hypertension, cardiovascular disease and body mass index.
p<0.05 for null hypothesis that ApoE4×sex is significant in a multivariate OLS or logistic regression model with cognitive function/change or impairment/decline as the outcomes and ApoE4 status as the main exposure, with sex entered into the main effects and control made for first-visit age, mean age at follow-up, education, first-visit smoking status, first-visit self-reported type 2 diabetes, hypertension, cardiovascular disease and body mass index.
DISCUSSION
The current study assembles a large and long-term prospectively assessed sample to test the association between genetic factors and dementia risk as well as cognitive impairment and cognitive decline. After a median follow-up of 27.5 years between 1958 and 2006, 113 of 644 Non-Hispanic White participants with available ApoE genotype data developed dementia. ApoE4+ was an important predictor of dementia (hazard ratio, HR=2.89; 95% CI: 1.93–4.33), though sex differences were not significant (HRs: 4.36 with 95% CI: 2.21–8.61 among women vs. 2.42 with 95% CI: 1.39–4.22 among men). In the total population, there was a dose-response relationship between the number of epsilon 4 alleles and dementia risk. Taking all time points available in BLSA for predicting cognition, ApoE4+ was associated with increased risk of cognitive decline at mean follow-up age on MMSE (ORs were 2.47 (95% CI:1.17–5.21) and 4.92 (95% CI:1.79–13.56), for men and women, respectively). Women had significantly stronger positive associations than men between ApoE4+ status and impairment or decline on the California Verbal Learning Test (CVLT, delayed recall and List A total recall) and on Verbal Fluency Test-Categories. This ApoE4×sex interaction remained significant with bonferroni correction only for CVLT, delayed recall. Taking time points prior to dementia for cognitive predictions, ApoE4+ status had a stronger positive association with impairment in CVLT (delayed recall) among women compared to men. Bonferroni correction, however, indicated that this interaction term was not significant after taking into account multiple testing.
Despite the strength of the association between ApoE4+ status and dementia risk, the literature is equivocal about its relation to cognitive function, impairment or decline. This apparent inconsistency is due to several factors. Aside from their relatively smaller sample sizes (<300 participants at first-visit), their case-control or cross-sectional designs, or short follow-up periods (<10 years), a large portion of null studies (Riley, et al., 2000,Small, et al., 2000,Smith, et al., 1998,Winnock, et al., 2002,Yip, et al., 2002) have evaluated only overall cognitive status. Furthermore, unlike our study which used logistic regression models with cognitive decline and impairment as outcomes, these studies used linear statistical models for the most part.
On the other hand, a wealth of evidence supports the presence of sex differences in the associations between cognitive deficits and ApoE4+ status. For instance, Moreno et al and Payami et al found an increased risk of familial Alzheimer’s disease (AD) in women carriers of the ε4 allele but not in men carriers. (Molero, et al., 2001,Payami, et al., 1997) In a third study, women had a twofold increased risk of AD compared to men among relatives of ε4 carriers, but not among relatives of non-carriers. (Martinez, et al., 1998) In another study, ε4 carrier women but not men had steeper cognitive decline in performance IQ and three performance subtests (digit symbol, block design, and object assembly) compared to noncarriers. (Mortensen and Hogh, 2001) This finding was also replicated by another study, though the main domain of interest was the development of impairment in delayed recall task. (Hyman, et al., 1996) Swan and colleagues found an interaction between sex and ApoE4+ affecting cognitive decline in various domains such that men with ε4 had greater decline in some measures of executive function (Digit Symbol Substitution Test and Color-Word Interference performance) and verbal memory (short delay free recall and short delay cued recall) compared to those without the allele, and women with the allele showed greater decline in Trail Making test performance relative to women without the allele. (Swan, et al., 2005) In addition, a cross-sectional study by Lehmann and colleagues indicated that among women, heterozygous ApoE genotype ε3/ε4 was associated with cognitive impairment in episodic memory (OR=1.8; 95% CI:1.1–2.8), whereas among men, only homozygous genotype ε4/ε4 had a dramatic and significant effect on cognition. (Lehmann, et al., 2006) In our study, there was no statistically significant sex difference in the ApoE4 status-dementia association in contrast to that found for decline and cognitive impairment in delayed verbal memory and learning. This may be due to either of the following: (1) A lack of statistical power to detect significant interactions in the Cox PH model (2) Domains other than verbal memory and learning needed to diagnose dementia did not show sex differences in the ApoE4 status -cognitive outcome association (3) Sex differences in the ApoE4 status-cognitive outcome associations (other than dementia) were inverse to the hypothesized direction for some cognitive tests such as Trails A impairment (i.e. ApoE4+ status was positively associated with Trails A impairment only among men). Finally, most large studies reported in the literature have observed a positive association between ApoE4+ status and poorer performance on tests of overall cognitive status, episodic memory, and executive functioning. The present study shows that this association also includes verbal learning and category fluency as well as visual short-term memory and constructional ability.
ApoE genotype has been associated with a wide spectrum of neurobiological factors known to be implicated in dementia: beta-amyloid deposition, tangle formation, oxidative stress, lipid homeostasis dysregulation, synaptic plasticity loss and cholinergic dysfunction. (Cedazo-Minguez, 2007) Sex and ApoE genotypes both may affect the distribution and covariance between plasma lipids and apolipoproteins. Specifically, the heterogeneity in the plasma lipid-apolipoprotein correlation coefficients between ApoE4+ and ApoE4− groups was shown to be more significant among women than among men. (Reilly, et al., 1994) Moreover, females seem to be more efficient at using Apolipoprotein E for redistributing myelin cholesterol during nerve repair (Poirier, 1994). Additionally, both animal and human studies demonstrate that the hippocampus, a brain region involved in memory, may be differentially affected by sex and ApoE4+ status. For instance, in experimental studies on rats, females that were ApoE4+ compared to those who were ApoE4− took more trials to criterion on a passive avoidance test designed to measure hippocampal integrity. (Villasana, et al., 2006) Among humans, the hippocampal volumes of ApoE4+ early AD women were 45% smaller compared to sex matched controls, and encountered a 10% greater reduction in volume compared with early AD men. (Juottonen, et al., 1998) Furthermore, women with MCI and heterozygous for the ε4 allele had smaller hippocampal volumes compared to ApoE4− women, but similar reductions were found only in homozygous (ε4/ ε4) MCI men. (Fleisher, et al., 2005) Taken together, these findings suggest an interplay between various plasma lipids and apolipoproteins, particularly among women, may be mediating the association between ApoE genotype and cognitive decline. However, further studies are needed to confirm the mechanisms in the ApoE4×sex interactions. On the other hand, some studies suggest that sex-specific differences in longevity, perhaps involving ApoE, could determine who is available for cognitive evaluation. Thus, early ascertainment of dementia or cognitive decline (e.g. between ages 50 and 80) should be made to examine real sex differences in the effect of ApoE genotype on cognition, excluding the potential competing risk of death which is higher among men and among ApoE4+ individuals (Corder, et al., 1995). Restricting the mean age of follow-up at which cognitive function and rate of cognitive change was predicted to ≤87 years (mean age at death for men and women combined), we found that significant ApoE4×sex interaction at an alpha level of 0.05 was retained for most tests, particularly in the case of CVLT-delayed recall cognitive decline (p=0.001). This interaction remained significant even after adding the variable (status=1 if dead by end of follow-up; status=0 if alive by end of follow-up). Finally, in addition to the previous two restrictions and adjustments, we conducted a 2-stage Heckman selection model(Heckman, 1979) in which an inverse mills ratio was added to the logistic regression model to account for sample selectivity in terms of baseline socio-demographic, lifestyle and health-related variables, an approach we had applied elsewhere(Beydoun, et al., 2009). This adjustment did not change the interaction term appreciably for CVLT-delayed recall cognitive decline (data not shown).
Our study has several strengths. First, the frequency of follow-up was high compared to other studies (median frequency ranged between 13 and 15 depending on the outcome), which allowed to detect incident dementia around onset time and an accurate depiction of cognitive trajectories with aging. Moreover, we combined linear mixed models with logistic regression analyses to examine significant cognitive impairment and decline.
However, the BLSA is a sample of convenience; the cohort was not fixed, and recruitment and dropout were continuous throughout the follow-up. We used statistical modeling to mitigate the effects this might have on the final results, including survival analysis(Freedman, 1982), restriction to younger age ranges to account of competing risk of death(Corder, et al., 1995), adjustment for death status in logistic models, and a 2-stage Heckman selection model(Heckman, 1979). Furthermore, while the frequency of observations was high, first-visit age and duration between visits varied among participants making the data structure unbalanced in terms of follow-up. To this end, we used mixed models to predict cognitive scores at specific ages where a large proportion of the data were available (mean age at follow-up for each subject) and controlled for the mean age at follow-up as well as first-visit age in the statistical models we conducted. Finally, several of our positive findings such as significant interactions between ApoE4+ and sex in predicting cognitive decline or impairment in specific domains, may have been due to chance, residual confounding or selection bias, while other negative findings may have been caused by lack of adequate power (e.g. lack of sex interaction with ApoE4+ in the case of Cox PH models with dementia risk as outcome). In particular, there were no adequate data on dementia-related therapies, which may have acted as additional potential confounders in the main associations of interest. Thus, until those findings are replicated elsewhere, they should be interpreted with caution.
Our study suggests that older adults with at least one ApoE ε4 allele are at increased risk of developing dementia and are more likely to decline or have impairments in various cognitive domains compared to those without this allele. Our study also showed that the association between ApoE ε4 allele and cognitive decline in specific domains including verbal memory and learning is not sex neutral because women are at higher risk compared to men when possessing the ε4 allele. Future mechanistic studies should investigate the lack of sex neutrality in the ApoE4+ and cognitive decline or impairment associations in specific domains of cognition.
Supplementary Material
ACKNOWLEDGEMENT
We would like to thank Dr. Larry Brant and Dr. Vonetta Dotson (Laboratory of Personality and Cognition, NIA/NIH/IRP) for internally reviewing our manuscript. We also would like to thank Dr. Bethrand Ugwu (Eastern Virginia Medical School, Norfolk, VA) for his assistance with literature search and review. The authors declare no conflict of interest. This research was supported entirely by the Intramural Research Program of the NIH, National Institute on Aging.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Aggarwal NT, Wilson RS, Beck TL, Bienias JL, Berry-Kravis E, Bennett DA. The apolipoprotein E epsilon4 allele and incident Alzheimer's disease in persons with mild cognitive impairment. Neurocase. 2005;11(1):3–7. doi: 10.1080/13554790490903038. doi:K7JUV2831T8ULN37 [pii] 10.1080/13554790490903038. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Third Edition. Washington, DC: American Psychiatric Association; 1987. Revised. [Google Scholar]
- Benton AL. Revised visual retention test (fifth edition) New York: The Psychological Corportation; 1974. [Google Scholar]
- Blair CK, Folsom AR, Knopman DS, Bray MS, Mosley TH, Boerwinkle E. APOE genotype and cognitive decline in a middle-aged cohort. Neurology. 2005;64(2):268–276. doi: 10.1212/01.WNL.0000149643.91367.8A. [DOI] [PubMed] [Google Scholar]
- Blessed G, Tomlinson BE, Roth M. The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. Br J Psychiatry. 1968;114(512):797–811. doi: 10.1192/bjp.114.512.797. [DOI] [PubMed] [Google Scholar]
- Bondi MW, Salmon DP, Monsch AU, Galasko D, Butters N, Klauber MR, Thal LJ, Saitoh T. Episodic memory changes are associated with the APOE-epsilon 4 allele in nondemented older adults. Neurology. 1995;45(12):2203–2206. doi: 10.1212/wnl.45.12.2203. [DOI] [PubMed] [Google Scholar]
- Brayne C, Harrington CR, Wischik CM, Huppert FA, Chi LY, Xuereb JH, O'Connor DW, Paykel ES. Apolipoprotein E genotype in the prediction of cognitive decline and dementia in a prospectively studied elderly population. Dementia. 1996;7(3):169–174. doi: 10.1159/000106873. [DOI] [PubMed] [Google Scholar]
- Bretsky P, Guralnik JM, Launer L, Albert M, Seeman TE. The role of APOE-epsilon4 in longitudinal cognitive decline: MacArthur Studies of Successful Aging. Neurology. 2003;60(7):1077–1081. doi: 10.1212/01.wnl.0000055875.26908.24. [DOI] [PubMed] [Google Scholar]
- Bunce D, Fratiglioni L, Small BJ, Winblad B, Backman L. APOE and cognitive decline in preclinical Alzheimer disease and non-demented aging. Neurology. 2004;63(5):816–821. doi: 10.1212/01.wnl.0000137041.86153.42. doi:63/5/816 [pii] [DOI] [PubMed] [Google Scholar]
- Cedazo-Minguez A. Apolipoprotein E and Alzheimer's disease: molecular mechanisms and therapeutic opportunities. J Cell Mol Med. 2007;11(6):1227–1238. doi: 10.1111/j.1582-4934.2007.00130.x. doi:JCMM130 [pii] 10.1111/j.1582-4934.2007.00130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chey J, Kim JW, Cho HY. Effects of apolipoprotein E phenotypes on the neuropsychological functions of community-dwelling elderly individuals without dementia. Neurosci Lett. 2000;289(3):230–234. doi: 10.1016/s0304-3940(00)01288-x. doi:S030439400001288X [pii] [DOI] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Jr, Roses AD, Pericak-Vance MA, Small GW, Haines JL. The apolipoprotein E E4 allele and sex-specific risk of Alzheimer's disease. JAMA. 1995;273(5):373–374. [PubMed] [Google Scholar]
- Cox DR. Regression models and life tables (with discussion) Journal of the Royal Statistical Society B. 1972;74:187–220. [Google Scholar]
- Delis DC, Freeland J, Kramer JH, Kaplan E. Integrating clinical assessment with cognitive neuroscience: construct validation of the California Verbal Learning Test. J Consult Clin Psychol. 1988;56(1):123–130. doi: 10.1037//0022-006x.56.1.123. [DOI] [PubMed] [Google Scholar]
- Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, Myers RH, Pericak-Vance MA, Risch N, van Duijn CM. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA. 1997;278(16):1349–1356. [PubMed] [Google Scholar]
- Feskens EJ, Havekes LM, Kalmijn S, de Knijff P, Launer LJ, Kromhout D. Apolipoprotein e4 allele and cognitive decline in elderly men. BMJ. 1994;309(6963):1202–1206. doi: 10.1136/bmj.309.6963.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleisher A, Grundman M, Jack CR, Jr, Petersen RC, Taylor C, Kim HT, Schiller DH, Bagwell V, Sencakova D, Weiner MF, DeCarli C, DeKosky ST, van Dyck CH, Thal LJ. Sex, apolipoprotein E epsilon 4 status, and hippocampal volume in mild cognitive impairment. Arch Neurol. 2005;62(6):953–957. doi: 10.1001/archneur.62.6.953. doi:62/6/953 [pii] 10.1001/archneur.62.6.953. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Freedman LS. Tables of the number of patients required in clinical trials using the logrank test. Stat Med. 1982;1(2):121–129. doi: 10.1002/sim.4780010204. [DOI] [PubMed] [Google Scholar]
- Heckman JJ. Sample selection bias as a specification error. Econometrica. 1979;47:153–161. [Google Scholar]
- Henderson AS, Easteal S, Jorm AF, Mackinnon AJ, Korten AE, Christensen H, Croft L, Jacomb PA. Apolipoprotein E allele epsilon 4, dementia, and cognitive decline in a population sample. Lancet. 1995;346(8987):1387–1390. doi: 10.1016/s0140-6736(95)92405-1. [DOI] [PubMed] [Google Scholar]
- Hixson JE, Vernier DT. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. J Lipid Res. 1990;31(3):545–548. [PubMed] [Google Scholar]
- Hoyt BD, Massman PJ, Schatschneider C, Cooke N, Doody RS. Individual growth curve analysis of APOE epsilon 4-associated cognitive decline in Alzheimer disease. Arch Neurol. 2005;62(3):454–459. doi: 10.1001/archneur.62.3.454. doi:62/3/454 [pii] 10.1001/archneur.62.3.454. [DOI] [PubMed] [Google Scholar]
- Hyman BT, Gomez-Isla T, Briggs M, Chung H, Nichols S, Kohout F, Wallace R. Apolipoprotein E and cognitive change in an elderly population. Ann Neurol. 1996;40(1):55–66. doi: 10.1002/ana.410400111. doi:10.1002/ana.410400111. [DOI] [PubMed] [Google Scholar]
- Jonker C, Schmand B, Lindeboom J, Havekes LM, Launer LJ. Association between apolipoprotein E epsilon4 and the rate of cognitive decline in community-dwelling elderly individuals with and without dementia. Arch Neurol. 1998;55(8):1065–1069. doi: 10.1001/archneur.55.8.1065. [DOI] [PubMed] [Google Scholar]
- Jorm AF, Mather KA, Butterworth P, Anstey KJ, Christensen H, Easteal S. APOE genotype and cognitive functioning in a large age-stratified population sample. Neuropsychology. 2007;21(1):1–8. doi: 10.1037/0894-4105.21.1.1. doi:2006-23022-001 [pii] 10.1037/0894-4105.21.1.1. [DOI] [PubMed] [Google Scholar]
- Juottonen K, Lehtovirta M, Helisalmi S, Riekkinen PJ, Soininen H. Major decrease in the volume of the entorhinal cortex in patients with Alzheimer's disease carrying the apolipoprotein E epsilon4 allele. J Neurol Neurosurg Psychiatry. 1998;65(3):322–327. doi: 10.1136/jnnp.65.3.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawas C, Segal J, Stewart WF, Corrada M, Thal LJ. A validation study of the Dementia Questionnaire. Arch Neurol. 1994;51(9):901–906. doi: 10.1001/archneur.1994.00540210073015. [DOI] [PubMed] [Google Scholar]
- Lam LC, Tang NL, Ma SL, Lui VW, Chan AS, Leung PY, Chiu HF. Apolipoprotein epsilon-4 allele and the two-year progression of cognitive function in Chinese subjects with late-onset Alzheimer's disease. Am J Alzheimers Dis Other Demen. 2006;21(2):92–99. doi: 10.1177/153331750602100208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann DJ, Refsum H, Nurk E, Warden DR, Tell GS, Vollset SE, Engedal K, Nygaard HA, Smith AD. Apolipoprotein E epsilon4 and impaired episodic memory in community-dwelling elderly people: a marked sex difference. The Hordaland Health Study. J Neurol Neurosurg Psychiatry. 2006;77(8):902–908. doi: 10.1136/jnnp.2005.077818. doi:jnnp.2005.077818 [pii] 10.1136/jnnp.2005.077818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezak MD. Neuropsychological assessment. 2nd edition. New York: Oxford University Press; 1983. [Google Scholar]
- Lezak MD. Neuropsychological assessment. 3rd edition. New York: Oxford University Press; 1995. [Google Scholar]
- Luczywek E, Nowicka A, Pfeffer A, Czyzewski K, Styczynska M, Lalowski M, Barcikowska M. Cognitive deficits and polymorphism of apolipoprotein E in Alzheimer's disease. Dement Geriatr Cogn Disord. 2002;13(3):171–177. doi: 10.1159/000048649. doi:dem13171 [pii]. [DOI] [PubMed] [Google Scholar]
- Martinez M, Campion D, Brice A, Hannequin D, Dubois B, Didierjean O, Michon A, Thomas-Anterion C, Puel M, Frebourg T, Agid Y, Clerget-Darpoux F. Apolipoprotein E epsilon4 allele and familial aggregation of Alzheimer disease. Arch Neurol. 1998;55(6):810–816. doi: 10.1001/archneur.55.6.810. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Molero AE, Pino-Ramirez G, Maestre GE. Modulation by age and gender of risk for Alzheimer's disease and vascular dementia associated with the apolipoprotein E-epsilon4 allele in Latin Americans: findings from the Maracaibo Aging Study. Neurosci Lett. 2001;307(1):5–8. doi: 10.1016/s0304-3940(01)01911-5. doi:S0304-3940(01)01911-5 [pii]. [DOI] [PubMed] [Google Scholar]
- Morris JC. Clinical dementia rating: a reliable and valid diagnostic and staging measure for dementia of the Alzheimer type. Int Psychogeriatr. 1997;9 Suppl 1:173–176. doi: 10.1017/s1041610297004870. discussion 7–8. [DOI] [PubMed] [Google Scholar]
- Mortensen EL, Hogh P. A gender difference in the association between APOE genotype and age-related cognitive decline. Neurology. 2001;57(1):89–95. doi: 10.1212/wnl.57.1.89. [DOI] [PubMed] [Google Scholar]
- Nilsson LG, Adolfsson R, Backman L, Cruts M, Nyberg L, Small BJ, Van Broeckoven C. The influence of APOE status on episodic and semantic memory: data from a population-based study. Neuropsychology. 2006;20(6):645–657. doi: 10.1037/0894-4105.20.6.645. doi:2006-20657-003 [pii] 10.1037/0894-4105.20.6.645. [DOI] [PubMed] [Google Scholar]
- Packard CJ, Westendorp RG, Stott DJ, Caslake MJ, Murray HM, Shepherd J, Blauw GJ, Murphy MB, Bollen EL, Buckley BM, Cobbe SM, Ford I, Gaw A, Hyland M, Jukema JW, Kamper AM, Macfarlane PW, Jolles J, Perry IJ, Sweeney BJ, Twomey C. Association between apolipoprotein E4 and cognitive decline in elderly adults. J Am Geriatr Soc. 2007;55(11):1777–1785. doi: 10.1111/j.1532-5415.2007.01415.x. doi:JGS1415 [pii] 10.1111/j.1532-5415.2007.01415.x. [DOI] [PubMed] [Google Scholar]
- Payami H, Grimslid H, Oken B, Camicioli R, Sexton G, Dame A, Howieson D, Kaye J. A prospective study of cognitive health in the elderly (Oregon Brain Aging Study): effects of family history and apolipoprotein E genotype. Am J Hum Genet. 1997;60(4):948–956. [PMC free article] [PubMed] [Google Scholar]
- Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256(3):183–194. doi: 10.1111/j.1365-2796.2004.01388.x. doi:10.1111/j.1365-2796.2004.01388.x JIM1388 [pii]. [DOI] [PubMed] [Google Scholar]
- Poirier J. Apolipoprotein E in animal models of CNS injury and in Alzheimer's disease. Trends Neurosci. 1994;17(12):525–530. doi: 10.1016/0166-2236(94)90156-2. [DOI] [PubMed] [Google Scholar]
- Qiu C, Winblad B, Fratiglioni L. Cerebrovascular disease, APOE epsilon4 allele and cognitive decline in a cognitively normal population. Neurol Res. 2006;28(6):650–656. doi: 10.1179/016164106X130443. [DOI] [PubMed] [Google Scholar]
- Reilly SL, Ferrell RE, Sing CF. The gender-specific apolipoprotein E genotype influence on the distribution of plasma lipids and apolipoproteins in the population of Rochester, MN. III. Correlations and covariances. Am J Hum Genet. 1994;55(5):1001–1018. [PMC free article] [PubMed] [Google Scholar]
- Reitan R. Trail Making Test: Manual for Administration and Scoring. Tucson, AZ: Reitan Neuropsychological Laboratory; 1992. [Google Scholar]
- Riley KP, Snowdon DA, Saunders AM, Roses AD, Mortimer JA, Nanayakkara N. Cognitive function and apolipoprotein E in very old adults: findings from the Nun Study. J Gerontol B Psychol Sci Soc Sci. 2000;55(2):S69–S75. doi: 10.1093/geronb/55.2.s69. [DOI] [PubMed] [Google Scholar]
- Romero LJ, Schuyler M, Kamboh MI, Qualls C, LaRue A, Liang HC, Rhyne R. The APO E4 allele and cognition in New Mexico Hispanic elderly. Ethn Dis. 2002;12(2):235–241. [PubMed] [Google Scholar]
- Rosen W. Verbal fluency in aging and dementia. Journal of Clinical Neuropsychology. 1980;2:135–146. [Google Scholar]
- Sawyer K, Sachs-Ericsson N, Preacher KJ, Blazer DG. Racial Differences in the Influence of the APOE Epsilon 4 Allele on Cognitive Decline in a Sample of Community-Dwelling Older Adults. Gerontology. 2008 doi: 10.1159/000137666. doi:000137666 [pii] 10.1159/000137666. [DOI] [PubMed] [Google Scholar]
- Shock N, Greulich RC, Andres R, Arenberg D, Costa PT, Lakatta EG, Tobin JD. Washington, DC: US Government Printing Office; Normal Human Aging: The Baltimore Longitudinal Study of Aging. 1984
- Singer JD, Willet JB. Applied Longitudinal Data Analysis: Modeling change and event occurrence. New York: Oxford University Press; 2003. [Google Scholar]
- Small BJ, Graves AB, McEvoy CL, Crawford FC, Mullan M, Mortimer JA. Is APOE--epsilon4 a risk factor for cognitive impairment in normal aging? Neurology. 2000;54(11):2082–2088. doi: 10.1212/wnl.54.11.2082. [DOI] [PubMed] [Google Scholar]
- Smith GE, Bohac DL, Waring SC, Kokmen E, Tangalos EG, Ivnik RJ, Petersen RC. Apolipoprotein E genotype influences cognitive 'phenotype' in patients with Alzheimer's disease but not in healthy control subjects. Neurology. 1998;50(2):355–362. doi: 10.1212/wnl.50.2.355. [DOI] [PubMed] [Google Scholar]
- Spreen O, Benton AL. Neurosensory Center Comprehensive Examination for Aphasia: Manual of directions. Victoria, BC: 1969. [Google Scholar]
- STATA. Statistics/Data Analysis: Release 10.0. Texas: Stata Corporation; 2007. [Google Scholar]
- Swan GE, Lessov-Schlaggar CN, Carmelli D, Schellenberg GD, La Rue A. Apolipoprotein E epsilon4 and change in cognitive functioning in community-dwelling older adults. J Geriatr Psychiatry Neurol. 2005;18(4):196–201. doi: 10.1177/0891988705281864. doi:18/4/196 [pii] 10.1177/0891988705281864. [DOI] [PubMed] [Google Scholar]
- Villasana L, Acevedo S, Poage C, Raber J. Sex- and APOE isoform-dependent effects of radiation on cognitive function. Radiat Res. 2006;166(6):883–891. doi: 10.1667/RR0642.1. doi:RR0642 [pii] 10.1667/RR0642.1. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Schneider JA, Barnes LL, Beckett LA, Aggarwal NT, Cochran EJ, Berry-Kravis E, Bach J, Fox JH, Evans DA, Bennett DA. The apolipoprotein E epsilon 4 allele and decline in different cognitive systems during a 6-year period. Arch Neurol. 2002;59(7):1154–1160. doi: 10.1001/archneur.59.7.1154. [DOI] [PubMed] [Google Scholar]
- Winnock M, Letenneur L, Jacqmin-Gadda H, Dallongeville J, Amouyel P, Dartigues JF. Longitudinal analysis of the effect of apolipoprotein E epsilon4 and education on cognitive performance in elderly subjects: the PAQUID study. J Neurol Neurosurg Psychiatry. 2002;72(6):794–797. doi: 10.1136/jnnp.72.6.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe K, Cauley J, Sands L, Browner W. Apolipoprotein E phenotype and cognitive decline in a prospective study of elderly community women. Arch Neurol. 1997;54(9):1110–1114. doi: 10.1001/archneur.1997.00550210044011. [DOI] [PubMed] [Google Scholar]
- Yip AG, Brayne C, Easton D, Rubinsztein DC. Apolipoprotein E4 is only a weak predictor of dementia and cognitive decline in the general population. J Med Genet. 2002;39(9):639–643. doi: 10.1136/jmg.39.9.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zonderman AB, Giambra LM, Arenberg D, Resnick SM, Costa PT, Jr, Kawas CH. Changes in immediate visual memory predict cognitive impairment. Arch Clin Neuropsychol. 1995;10(2):111–123. doi:0887-6177(94)00040-9 [pii]. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.