Abstract
The apical junctional complex (AJC) encompassing the tight junction (TJ) and adherens junction (AJ) plays a pivotal role in regulating epithelial barrier function and epithelial cell proliferative processes through signaling events that remain poorly characterized. A potential regulator of AJC protein expression is Glycogen Synthase Kinase-3 (GSK-3). GSK-3 is a constitutively active kinase that is repressed during epithelial-mesenchymal transition (EMT). In the present study, we report that GSK-3 activity regulates the structure and function of the AJC in polarized model intestinal (SK-CO15) and kidney (Madin-Darby Canine Kidney (MDCK)) epithelial cells. Reduction of GSK-3 activity, either by small molecule inhibitors or siRNA targeting GSK-3 alpha and beta mRNA, resulted in increased permeability to both ions and bulk solutes. Immunofluorescence labeling and immunoblot analyses revealed that the barrier defects correlated with decreased protein expression of AJC transmembrane proteins Occludin, Claudin-1 and E-cadherin without influencing other TJ proteins, Zonula Occludens-1 (ZO-1) and Junctional Adhesion Molecule A (JAM-A). The decrease in Occludin and E-cadherin protein expression correlated with downregulation of the corresponding mRNA levels for these respective proteins following GSK-3 inhibition. These observations implicate an important role of GSK-3 in the regulation of the structure and function of the AJC that is mediated by differential modulation of mRNA transcription of key AJC proteins, Occludin, Claudin-1 and E-cadherin.
Keywords: Glycogen Synthase Kinase 3 (GSK-3), Occludin, Claudin-1, Epithelial-Mesenchymal Transition (EMT), Apical Junctional Complex (AJC), Paracellular Permeability
Introduction
The molecular architecture and selective barrier function of epithelia is maintained by intercellular contacts. The tight junction (TJ) and adherens junction (AJ) constitute apical intercellular contacts, and are collectively referred to as the apical junctional complex (AJC). AJC transmembrane and cytoplasmic scaffold proteins mediate cell-cell adhesion and regulate paracellular diffusion of ions and small molecules across epithelial barriers [1,2]. Key transmembrane proteins in the AJC are Occludin, the Claudin protein family and Junctional Adhesion Molecules (JAM), which are all localized in the TJ, as well as E-cadherin, which is confined to the AJ. Cytoplasmic plaque proteins include the Zonula Occludins (ZO) proteins. Disruption of the AJC is a common feature of many inflammatory diseases [3], and loss of specific AJC proteins has been observed in epithelial cancers [4,5,6]. More specifically, decreased expressions of Occludin, Claudins, and E-cadherin has been associated with epithelial-mesenchymal transition (EMT) [7]. Epithelial-mesenchymal transition (EMT) is a process that occurs during normal embryonic development. During this process, embryonic epithelial cells lose polarity, dissociate and become increasingly motile[8]. EMT in mature epithelial cells has been observed in cancer development and metastasis[9]. During normal and aberrant EMT, the AJC is disrupted through the downregulation of AJ and TJ proteins. Loss of E-cadherin is a hallmark feature of EMT[10]. Additionally, downregulation of Occludin has been shown to correlate with staging, invasiveness, metastatic potential of epithelial cancers and has been associated with EMT [4,5,6,7]. Epithelial cells utilize signaling pathways to maintain TJ and AJ protein expression during epithelial cell differentiation [11]. Bachelder et al. reported that glycogen synthase kinase-3 activity (GSK-3) is essential for preserving the epithelial structure by maintaining E-cadherin expression to inhibit EMT. GSK-3 is a constitutively active serine-threonine kinase and is widely expressed as two isoforms referred to as GSK-3 alpha and GSK-3 beta in mammalian tissues [12]. While a role of GSK-3 in regulating E-cadherin has been reported, its influence on other key AJC proteins, Occludin, JAM-A and ZO-1 has not been explored.
In addition to the known role of GSK-3 in preventing EMT our results now show that endogenous GSK-3 activity is required for maintenance of the AJC and therefore epithelial barrier function by regulating the expression of transmembrane proteins, Claudin-1 and Occludin. Using two model polarized epithelial cell lines, SK-CO15 and Madin-Darby Canine Kidney (MDCK) we observed that downregulation/inhibition of GSK-3 alpha and GSK-3 beta activity resulted in increased epithelial paracellular permeability to small ions and bulk solutes. Such decrease in epithelial barrier function was associated with reduced mRNA and protein expression of Occludin, Claudin-1 and E-cadherin without influencing JAM-A and ZO-1, two other key AJC proteins. This study emphasizes the importance of GSK-3 in regulating expression of AJC proteins and epithelial barrier function.
Experimental Procedures
Reagents and Antibodies
Rabbit anti-GSK-3α/β antibody was obtained from Santa Cruz. Other antibodies included the following: mouse anti-Occludin(Invitrogen), mouse anti-ZO-1 (Invitrogen), mouse anti-E-cadherin (BD Biosciences), rat anti-E-cadherin (Invitrogen), rabbit anti-JAM-A (produced in-house), R-actin (Sigma), R-Claudin-1 (Sigma), horseradish peroxidase-conjugated secondary antibodies (Jackson ImmunoResearch) and fluorescently labeled secondary antibodies (Molecular Probes). SiRNA were ordered from Qiagen to target GSK-3 alpha and GSK-3 beta individually, along with a scramble siRNA control. The GSK-3 inhibitor SB415286 was obtained from Tocris Bioscience.
MDCK Cell Culture and Inhibitor Treatment
MDCK cells (ATCC CRL-2286) were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10 % fetal bovine serum, 2 mM L-glutamine, 100 IU of penicillin, 100 μg/ml streptomycin, 15 mM HEPES, and 1% nonessential amino acids (Cellgro; Mediatech) at 37 °C in an humidified atmosphere of 95% air 3.75 × 104 and 5% CO2 (v/v). For filter based studies cells were plated at a density of cells/0.33cm2 and for non-filter based studies cells were plated at a density of 7.5×105 cells/25cm2. The GSK-3 inhibitor SB415286 was dissolved in DMSO and added at 20 nM final concentration at the time that cells were plated. Treatments were performed for 48 hours and cells were then harvested. At the time of cell lysis or fixation, the cell monolayers were confluent. An equal concentration of DMSO (0.1% v/v) was used as a control for SB415286 treatment.
SK-CO15 Cell Culture and siRNA treatment
SKCO-15 human colonic epithelial cells were plated and grown as described for the MDCK cells. All siRNA transfections were performed using Hiperfect(Qiagen) in Opti-MEM I medium, according to the manufacturer’s protocol with a final siRNA concentration of 50 nM. SiRNA targets were as follows; GSK-3A target 1: 5′-CAGGGAACTAGTCGCCATCAA-3′; GSK-3B target 1: 5′-CCCAAATGTCAAACTACCAAA-3′.
Measurement of TER and Paracellular Permeability
Transepithelial electrical resistance (TER) was measured across cells grown on 0.33cm2 polycarbonate filters with 0.4uM pores using an epithelial voltmeter (EVOM) (Precision World Instruments). TER values were normalized for the area of the filter and were obtained by subtracting the contribution of the filter and bathing solution. Paracellular permeability to fluorescent dextran (FD-3; MW 4,000) and fluorescent Rhodamine (MW 40,000 and 70,000) was assessed as previously described [13]. Numerical values from individual experiments were pooled and expressed as mean ± standard error of the mean. Data shown are representative of at least three independent experiments.
Immunofluorescence labeling and confocal microscopy
MDCK or SK-CO15 cells were grown on permeable transwell filters that were fixed and permeabilized in 100% ethanol for 20 min at −20°C followed by blocking in Phospho-Buffered Saline (PBS) containing 1% bovine serum albumin (blocking buffer) for 1hr at room temperature. The primary antibodies were diluted in blocking buffer, and incubated with cells for 1hr at room temperature. The cells were washed twice in PBS and then incubated with fluorescently labeled secondary antibodies diluted 1:1000 in blocking buffer for 1hr at room temperature. The cells were washed twice with PBS and mounted in p-diphenylethylamine. Cells were analyzed using a Zeiss LSM510 laser scanning confocal microscope (Zeiss Microimaging) coupled to a Zeiss Axioplan with a 100x Pan-Apochromat oil lens.
Immunoblots
Cells were homogenized in RIPA buffer containing a protease inhibitor cocktail (1:100; Sigma-Aldrich). Protein concentrations were determined by Pierce BCA assay. Lysates were then immediately boiled in SDS sample buffer. SDS-PAGE and immunoblots were performed using standard methodology. Immunoblots were probed for actin to ensure equal protein loading.
RNA Extraction and primers for PCR
Total RNA was extracted using Trizol (Sigma), followed by a cleaning step using the RNeasy mini-kit (Qiagen). RNA was quantified using a NanoDrop-1000 machine (Thermo Scientific).
Real-time PCR
Quantitative real-time PCR was performed to determine relative mRNA levels. RT-PCR was performed using the iScrpit One-Step RT-PCR kit with SYBR green according to the manufacturer’s instructions (Bio-Rad). PCR amplification was performed using the GeneAmp 5700 Sequence Detection System (PE Applied Biosystems). PCR was run using the following protocol: initial activation at 94°C for 3 min, 40 cycles of 94°C for 15 s, 52°C for 30s, and 72°C for 30s. Results of the real-time PCR data were represented as Ct values, where Ct was defined as the threshold cycle of PCR at which amplified product was first detected. For the RNA internal control, a Ct was derived using GAPDH mRNA expression. Fold increase was determined by applying the formula 2DD Ct, where DCt = Ct of target gene − Ct of endogenous control gene (GAPDH or Signal Recognition Peptide (SRP)), and DDCt = DCt of experimental sample for target gene − DCt of the control sample for the target gene. Human primers are listed 5′ to 3′as follows: ZO-1.F TGCTGAGTCCTTTGGTGATG, ZO-1.R ATTTTGGATCTCCGGGAAGAC, Occludin.F GCAAAGTGAATGACAAGCGG, Occludin.R CACAGGCGAAGTTAATGGAAG, E-caherin.F CCCAATAGATCTCCCTTCACAG, E-cadherin.R CCACCTCTAAGGCCATCTTTG, Claudin-1.F TTGACTCCTTGCTGAATCTGAG, Claudin-1.R TTCTGCACCTCATCGTCTTC, GSK-3 alpha.F AGGAGTTAGTGAGGGTAGGTG, GSK-3 alpha.R TCTCAACGCCATTCTCATCC, GSK-3 beta.F GGTCTATCTTAATCTGGTGCTGG, GSK-3 beta.R TGGATATAGGCTAAACTTCGGAAC, GAPDH.F CAACAGCGACACCCACTCCT, GAPDH.R CACCCTGTTGCTGTAGCCAAA. Canine Primers are listed 5′ to 3′as follows: E-cadherin.F AAATCACATCCTACACCGCC, E-cadherin.R ATTAACCTCCAGCCAACCG, Occludin.F CCCCTTCTGACTATGTGGAG, Occludin.R CCGCTTGTCATTCACTTTACC, Claudin-1.F ATGGAAGACGATGAGGTGC, Claudin-1.R GCAACTAAAACAGCCAGACC, ZO-1.F ACAGTATCCACTCTGCTAATGC, ZO-1.R GAAGGCTCTGATCGTTGGTC, JAM-A.F CCTATAGCCTGGATCACAAGAC, JAM-A.R ACATTCAACTTCCGCAGCTTC, GSK-3 alpha.F TCTCCAGGACAAAAGGTTCAAG, GSK-3 alpha.R ACGTATTCCAGCACCAGATTTAG, GSK-3 beta.F CTGTCAAGTAATCCACCTCTAGC, GSK-3 beta.R AGCATTGTTCGTCTGTCCAC, SRP.F GACCAGACTCTTCCAGAAGTG, SRP.R CCCTCTACAGAACCTTTCCTTG. Each sample was analyzed in triplicate for each experiment. Data reported is from three separate experiments.
Statistics
All statistics for paired data sets were performed using Student’s t-test. For experiments with multiple sample groups ANOVA with post-hoc analysis was used with p<0.05 indicating a statistically significant difference.
Results
GSK-3 activity regulates epithelial barrier function
Previous reports indicate that GSK-3 kinase activity negatively regulates the expression of the adherens junction protein, E-cadherin [11] thereby influencing barrier function and inhibiting epithelial-mesenchymal transition (EMT)[14,15]. To further understand the mechanism by which GSK-3 activity regulates epithelial barrier function we used a GSK-3 loss of function approach followed by analysis of Apical Junctional Complex (AJC) proteins. GSK-3 activity was decreased using two complimentary approaches that included downregulation of GSK-3 protein in model epithelial cells (SK-CO15). GSK-3 protein levels were downregulated in SK-CO15 model epithelial cells using an RNAi approach. Epithelial cells were transfected with siRNA targeting both GSK-3 alpha and GSK-3 beta. To control for non-specific siRNA effects, cells were transfected with a scramble siRNA control and a no siRNA (mock) control. To compliment the above approach, we utilized a model kidney epithelial cell line, Madin-Darby Canine Kidney Cells (MDCK), and a small molecule GSK-3 kinase inhibitor, SB415286 or DMSO vehicle as a control. SB415286 is a small molecule inhibitor of the kinase domain of GSK-3[16] that inhibits both the alpha and beta paralogs of GSK-3.
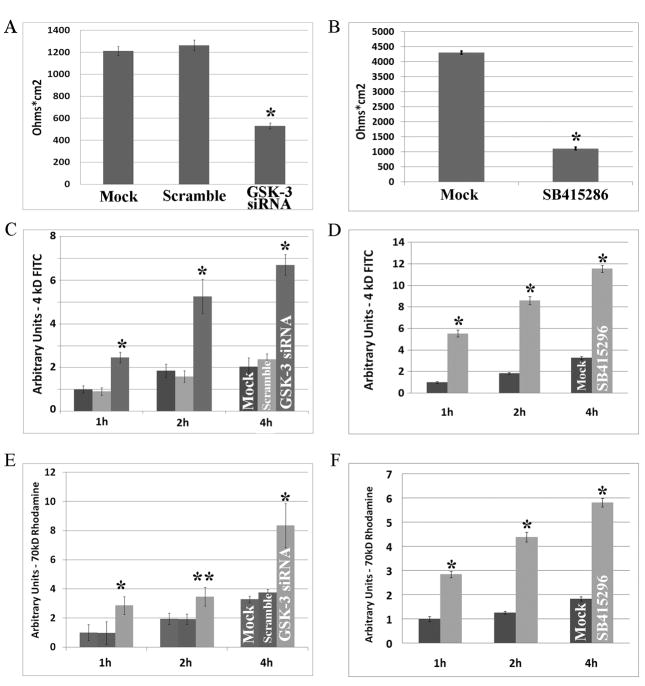
Confluent monolayers of SK-CO15 cells were grown on permeable supports for 24 hours and transfected with siRNA targeting both GSK-3 alpha and GSK-3 beta. Barrier function was measured after 72 hours by transepithelial resistance (TER) to passive ion flow. Control monolayers treated with mock or scramble siRNA displayed high, stable TER (Mock, 1.2kΩ cm2 and Scramble, 1.25 kΩ cm2), while monolayers incubated with the GSK-3 siRNA for 72 hours had decreased TER (0.5kΩ cm2) (Figure 1A, p<0.01). Although the epithelial barrier was decreased, a TER of 0.5kΩ cm2 suggests that the cell monolayer was maintained. MDCK cells treated with SB415286 to inhibit the GSK-3 kinase domain also had reduced TER (Figure 1B).
Figure 1. GSK-3 activity decreases paracellular permeability.
(A, C, E) After siRNA-mediated downregulation of GSK-3 in SK-CO15 cells, transepithelial resistance (TER) is decreased from ~1.2 kΩ cm2 to 0.5 kΩ cm2. Paracellular permeability to bulk solute is also decreased after downregulation of GSK-3. For 4kd-FITC-dextran, the increase in permeability is significant at 1, 2 and 4 hours (p<0.01). For 70kD-Rhodamine-dextran, the increase in permeability is significant at 1 ( p<0.01), 2 (p<0.05) and 4 (p<0.01) hours. As can be seen there are no significant differences between Mock (no siRNA) and Scramble siRNA treatment. These data are from three separate experiments. (B, D, F) After chemical inhibition of the GSK-3 kinase domain with SB415286 in MDCK cells, transepithelial resistance (TER) is decreased from ~4.3 kΩ cm2 to 1.2 kΩ cm2. (D, G) Paracellular permeability to bulk solute is also decreased after downregulation of GSK-3. Permeability is significantly increased to both 4kD-FITC dextran and 40kD-Rhodamine-dextran at 1, 2 and 4 hours (p<0.001). These data are from three separate experiments.
Given that siRNA mediated protein downregulation or chemical inhibition of GSK-3 activity decreased TER, we analyzed paracellular permeability to bulk solutes. 3kD FITC-dextran (FD-3) and 70kD Rhodamine-dextran (RD-70) flux across SK-CO15 epithelial monolayers was measured 72 hours after transfection with GSK-3 siRNA. Cells treated with GSK-3 siRNA showed significantly increased paracellular flux of FD-3 and RD-70 when compared to Mock and Scramble treated control cells at 1(p<0.001), 2(p<0.05) and 4 hours (p<0.001) (Figure 1C, 1E). Similar results were observed in MDCK cells treated with SB415286 (Figure 1D,1F). These findings suggest that GSK-3 activity regulates the paracellular diffusion of macromolecules and passive ion flow and supports a role of GSK-3 activity in regulation of epithelial barrier function.
GSK-3 activity modulates Occludin, Claudin-1 and E-cadherin expression
Since GSK-3 activity influenced epithelial barrier properties and the Apical Junctional complex (AJC) regulates paracellular permeability, we first analyzed the distribution of key AJC proteins in cells following downregulation of GSK-3 activity. SK-CO15 cells were grown on permeable filters for 24 hours and transfected with GSK-3 alpha and beta siRNA. After 72 hours localization of AJC proteins Occludin, ZO-1, E-cadherin, and JAM-A were evaluated by immunofluorescence labeling and confocal microscopy (Figure 2A). While AJC proteins were identified in the lateral membrane of epithelial cells under all experimental conditions, the overall intensity of Occludin and E-cadherin staining was decreased following siRNA induced downregulation of GSK-3 or inhibitor treatment when compared to the corresponding control cells (Figure 2). Similar results were observed in MDCK cells treated with SB415286. Additionally, we observed that MDCK cells treated with SB415286 had decreased Claudin-1 staining when compared to DMSO controls. Interestingly, we noted an increase in the apparent diameter of cells treated with SB415286 when compared to Mock treated cells. It is likely that this increase in cell diameter is a side effect of the SB415286 treatment, as it was not observed after downregulating GSK-3 protein with siRNA treatment.
Figure 2. GSK-3 activity increases Occludin, E-cadherin and Claudin-1 expression at cell-cell contacts.
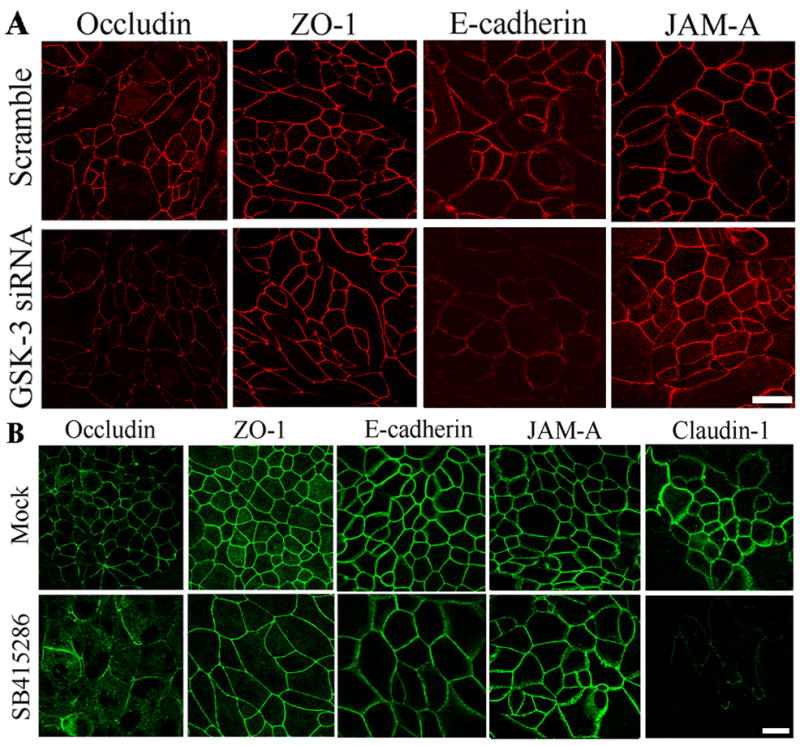
(A) The expression of various AJC proteins is shown by immunofluorescence (IF) labeling/confocal microscopy in SK-CO15 cells treated with GSK-3 siRNA. There are no substantial differences between the Mock and Scramble siRNA treated samples, however, incubation with GSK-3 siRNA decreased Occludin and E-cadherin protein levels. There is no change in JAM-A or ZO-1 expression and none of these proteins have altered cellular localization. Bar, 20 μm. (B) Treatment with GSK-3 siRNA decreased Occludin, Claudin-1 and E-cadherin protein levels. There is no change in JAM-A or ZO-1 expression. For A and B representative images of multiple experiments are shown. Bar, 20 μm.
Since we observed a differential decrease in labeling of key AJC proteins following GSK-3 inhibition, we analyzed AJC protein expression by immunoblotting. Immunoblots from both SK-CO15 cells treated with GSK-3 siRNA and MDCK cells incubated with SB415286 for 48 hours revealed a corresponding decrease in Occludin, Claudin-1 and E-cadherin protein levels (Figure 3). In contrast to Occludin, Claudin-1 and E-cadherin, we did not observe any consistent changes in the protein levels of JAM-A and ZO-1. Importantly, the immunoblots confirmed downregulation of total GSK-3 by the GSK-3 alpha and beta siRNA treatment.
Figure 3. Downregulation of GSK-3 alpha and beta decreases Occludin, Claudin-1 and E-cadherin expression.
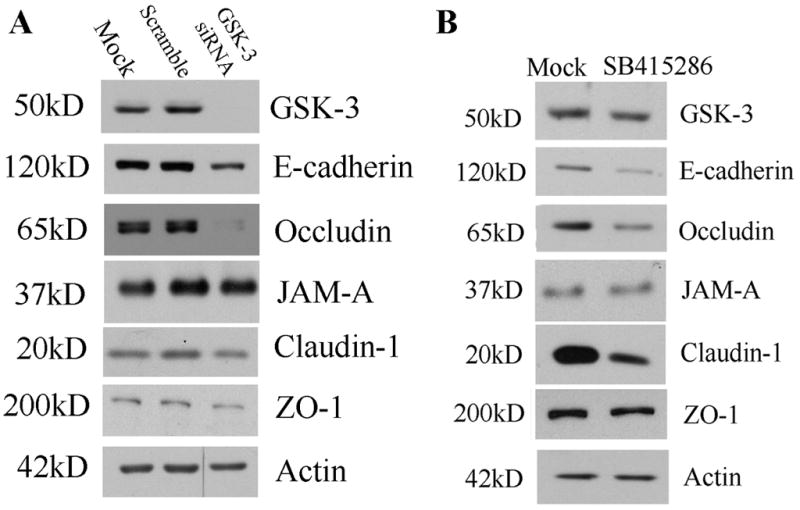
(A) Immunoblots were performed on lysates from SK-CO15 cells treated with GSK-3 alpha and beta siRNA, Scramble siRNA or Mock treatment. Compared to controls, GSK-3 siRNA treatment reduced protein levels of GSK-3, E-cadherin, Occludin and Claudin-1. JAM-A and ZO-1 expression was not consistently changed. (B) Chemical inhibition of GSK-3 kinase activity decreases Occludin, Claudin-1 and E-cadherin expression. Immunoblots were performed on lysates from MDCK cells treated SB415286 or Mock treatment. Compared to controls, SB415286 treatment reduced protein levels of E-cadherin, Occludin and Claudin-1. For A&B, Actin blots are shown as a loading control. Immunoblots are representative of at least three experiments.
GSK-3 activity influences Occludin, Claudin-1 and E-cadherin mRNA expression
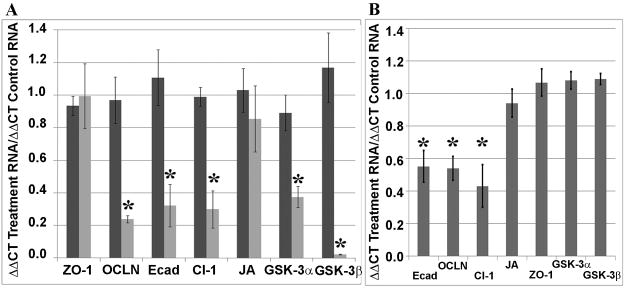
To determine if GSK-3 activity/protein expression modulates Occludin, Claudin-1 and E-cadherin protein expression, we analyzed the relative mRNA levels using qRT-PCR. Relative amounts of E-cadherin, Occludin, Claudin-1, ZO-1, JAM-A, GSK-3 alpha and GSK-3 beta RNA were determined in SKCO-15 cells incubated with GSK-3 alpha and beta siRNA. Analogous to the immunoblotting results, E-cadherin, Claudin-1, Occludin, GSK-3 alpha and GSK-3 beta mRNA levels were substantially decreased in cells with downregulated GSK-3 using the RNAi approach (p<0.01). Additionally, we did not observe significant changes in the JAM-A and ZO-1 mRNA following GSK-3 downregulation (Figure 4A). In parallel, MDCK cells treated with SB415286, Occludin, Claudin-1 and E-cadherin mRNA levels were decreased (p<0.01) without a change in the levels of ZO-1, JAM-A, GSK-3 alpha and GSK-3 beta mRNA (Figure 4B).
Figure 4. GSK-3 expression regulates Occludin, Claudin-1 and E-cadherin mRNA level.
(A) Real-time PCR was used to compare Mock treatment with Scramble or GSK-3 siRNA treatment. There were no significant differences in RNA levels between Mock and Scramble siRNA treated samples. The primers are listed in the methods. GSK-3 siRNA incubation decreased levels of Occludin (OCLN), E-cadherin (Ecad), Claudin-1 (Cl-1), GSK-3 alpha (GSK-3A) and GSK-3 beta (GSK-3B) (p<0.01, n=6). There was no significant difference in RNA levels of JAM-A (JA) or ZO-1 (n=6). (B) Real-time PCR was used to compare Mock treatment with SB415286 treatment. The primers are listed in the methods. GSK-3 siRNA resulted in decreased levels of Occludin (OCLN), E-cadherin (Ecad), Claudin-1 (Cl-1), GSK-3 alpha (GSK-3A) and GSK-3 beta (GSK-3B) (p<0.01, n=6). There was no significant difference in RNA levels of JAM-A (JA) or ZO-1 (n=6).
Discussion
GSK-3 is a ubiquitously expressed protein serine kinase that regulates numerous cellular processes such as glucose metabolism, protein synthesis, cell proliferation, microtubule dynamics, cell motility and Wnt signaling [12]. Recently, the activity of GSK-3 has been implicated in the maintenance of the epithelial architecture and prevention of Epithelial-mesenchymal Transition (EMT) [17]. Pharmacological inhibition of GSK-3 activity or decreased expression of GSK-3 in normal breast and skin epithelial cells decreased expression of E-cadherin by inhibiting degradation of the zinc finger transcriptional factor Snail [11]. While previous studies have reported an influence of GSK-3 on E-cadherin expression, epithelial polarity and EMT [17], these studies have not addressed the impact of GSK-3 on the fate of other epithelial AJC proteins. In addition to effects in epithelial cells, GSK-3 has been implicated in mediating increased endothelial barrier function after treatment with hepatocyte growth factor [18].
To examine the consequences of decreased GSK-3 activity, both siRNA mediated downregulation and small molecule inhibitors were used in this study. SiRNA treatment alone can result in off-target effects on other genes that could account for phenotypic effects observed, while small molecule inhibitors alone are insufficient to evaluate the effects of decreased GSK-3 activity as they potentially inhibit other kinases. The advantage of using these complimentary approaches is that off-target siRNA effects are unlikely to overlap with off-target kinase inhibitor effects. Thus, phenotypes observed after both chemical inhibition of GSK-3 and siRNA mediated GSK-3 downregulation are more likely to be secondary to decreased GSK-3 activity, and not secondary to side effects of the treatment modality.
Using the above complimentary approaches, the current study demonstrates an important role of GSK-3 activity in regulation of epithelial barrier function. We report that in both model human intestinal (SK-CO15) and kidney (MDCK) epithelial cells a decrease in GSK-3 activity perturbed epithelial cell-cell junctions thereby enhancing paracellular permeability. These effects on barrier function can in part be attributed to decreased Occludin, Claudin-1and E-cadherin mRNA and protein expression. Furthermore, the downregulation of Occludin and Claudin-1 proteins is secondary to decreased mRNA levels, similar to the reported mechanism of GSK-3 mediated E-cadherin regulation[11]. Previous reports have indicated that E-cadherin mRNA levels are regulated by GSK-3 via the transcription factor Snail[19]. Most likely, transcription of Occludin and Claudin-1 is similarly influenced by GSK-3 downregulation and their mRNA transcription is known to be inhibited by Snail expression[20]. These effects on Occludin, Claudin-1 and E-cadherin are specific since levels of JAM-A and ZO-1 protein and mRNA were unaffected by inhibition of GSK-3. . Another potential regulator of Occludin transcription is Slug, a close homologue of Snail whose expression has previously been shown to decrease levels of Occludin mRNA [21]. Additionally, the phosphorylation sites that are necessary for the GSK-3 regulation of Snail[11] are conserved in Slug. We therefore hypothesize that GSK-3 regulates Occludin and Claudin-1 levels via stabilization and nuclear targeting of Snail and/or Slug, and these studies will require further investigation.
This study emphasizes the physiological importance of GSK-3 activity in regulation of the normal epithelial barrier and implicate an active role of GSK-3 in not only controlling E-cadherin expression, but also in selective regulation of other proteins in the TJ. In particular, our results support a critical role of GSK-3 activity in controlling expression of the AJC proteins Occludin, Claudin-1 and E-cadherin as well as regulation of downstream signaling events essential for maintaining epithelial barrier and homeostatic functions.
Acknowledgments
We thank Susan Voss for tissue culture expertise and assistance. This study was supported by National Institutes of Health grants DK55679 and DK59888 (to A.N.) as well as DK72564, DK61379, and DK79392 (to C.P.).
Abbreviations
- GSK-3
Glycogen Synthase Kinase 3
- EMT
Epithelial-Mesenchymal Transition
- AJC
Apical Junctional Complex
- TJ
tight junction
- AJ
adherens junction
- JAM
Junctional Adhesion Molecules
- ZO
Zonula Occludins
- MDCK
Madin-Darby Canine Kidney cells
- TER
transepithelial resistance
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wong V, Gumbiner BM. A synthetic peptide corresponding to the extracellular domain of occludin perturbs the tight junction permeability barrier. J Cell Biol. 1997;136:399–409. doi: 10.1083/jcb.136.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gumbiner B. Structure, biochemistry, and assembly of epithelial tight junctions. Am J Physiol. 1987;253:C749–758. doi: 10.1152/ajpcell.1987.253.6.C749. [DOI] [PubMed] [Google Scholar]
- 3.Schmitz H, Barmeyer C, Fromm M, Runkel N, Foss HD, Bentzel CJ, Riecken EO, Schulzke JD. Altered tight junction structure contributes to the impaired epithelial barrier function in ulcerative colitis. Gastroenterology. 1999;116:301–309. doi: 10.1016/s0016-5085(99)70126-5. [DOI] [PubMed] [Google Scholar]
- 4.Hoover KB, Liao SY, Bryant PJ. Loss of the tight junction MAGUK ZO-1 in breast cancer: relationship to glandular differentiation and loss of heterozygosity. Am J Pathol. 1998;153:1767–1773. doi: 10.1016/S0002-9440(10)65691-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tobioka H, Isomura H, Kokai Y, Tokunaga Y, Yamaguchi J, Sawada N. Occludin expression decreases with the progression of human endometrial carcinoma. Hum Pathol. 2004;35:159–164. doi: 10.1016/j.humpath.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 6.Dhawan P, Singh AB, Deane NG, No Y, Shiou SR, Schmidt C, Neff J, Washington MK, Beauchamp RD. Claudin-1 regulates cellular transformation and metastatic behavior in colon cancer. J Clin Invest. 2005;115:1765–1776. doi: 10.1172/JCI24543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Z, Mandell KJ, Parkos CA, Mrsny RJ, Nusrat A. The second loop of occludin is required for suppression of Raf1-induced tumor growth. Oncogene. 2005;24:4412–4420. doi: 10.1038/sj.onc.1208634. [DOI] [PubMed] [Google Scholar]
- 8.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 9.Hay ED. An overview of epithelio-mesenchymal transformation. Acta Anat (Basel) 1995;154:8–20. doi: 10.1159/000147748. [DOI] [PubMed] [Google Scholar]
- 10.Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, Portillo F, Nieto MA. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2:76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- 11.Bachelder RE, Yoon SO, Franci C, de Herreros AG, Mercurio AM. Glycogen synthase kinase-3 is an endogenous inhibitor of Snail transcription: implications for the epithelial-mesenchymal transition. J Cell Biol. 2005;168:29–33. doi: 10.1083/jcb.200409067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frame S, Cohen P. GSK3 takes centre stage more than 20 years after its discovery. Biochem J. 2001;359:1–16. doi: 10.1042/0264-6021:3590001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanders SE, Madara JL, McGuirk DK, Gelman DS, Colgan SP. Assessment of inflammatory events in epithelial permeability: a rapid screening method using fluorescein dextrans. Epithelial Cell Biol. 1995;4:25–34. [PubMed] [Google Scholar]
- 14.Zhou BP, Deng J, Xia W, Xu J, Li YM, Gunduz M, Hung MC. Dual regulation of Snail by GSK-3beta-mediated phosphorylation in control of epithelial-mesenchymal transition. Nat Cell Biol. 2004;6:931–940. doi: 10.1038/ncb1173. [DOI] [PubMed] [Google Scholar]
- 15.Grande M, Franzen A, Karlsson JO, Ericson LE, Heldin NE, Nilsson M. Transforming growth factor-beta and epidermal growth factor synergistically stimulate epithelial to mesenchymal transition (EMT) through a MEK-dependent mechanism in primary cultured pig thyrocytes. J Cell Sci. 2002;115:4227–4236. doi: 10.1242/jcs.00091. [DOI] [PubMed] [Google Scholar]
- 16.Coghlan MP, Culbert AA, Cross DA, Corcoran SL, Yates JW, Pearce NJ, Rausch OL, Murphy GJ, Carter PS, Roxbee Cox L, Mills D, Brown MJ, Haigh D, Ward RW, Smith DG, Murray KJ, Reith AD, Holder JC. Selective small molecule inhibitors of glycogen synthase kinase-3 modulate glycogen metabolism and gene transcription. Chem Biol. 2000;7:793–803. doi: 10.1016/s1074-5521(00)00025-9. [DOI] [PubMed] [Google Scholar]
- 17.Doble BW, Woodgett JR. Role of glycogen synthase kinase-3 in cell fate and epithelial-mesenchymal transitions. Cells Tissues Organs. 2007;185:73–84. doi: 10.1159/000101306. [DOI] [PubMed] [Google Scholar]
- 18.Liu F, Schaphorst KL, Verin AD, Jacobs K, Birukova A, Day RM, Bogatcheva N, Bottaro DP, Garcia JG. Hepatocyte growth factor enhances endothelial cell barrier function and cortical cytoskeletal rearrangement: potential role of glycogen synthase kinase-3beta. FASEB J. 2002;16:950–962. doi: 10.1096/fj.01-0870com. [DOI] [PubMed] [Google Scholar]
- 19.Batlle E, Sancho E, Franci C, Dominguez D, Monfar M, Baulida J, Garcia De Herreros A. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol. 2000;2:84–89. doi: 10.1038/35000034. [DOI] [PubMed] [Google Scholar]
- 20.Ikenouchi J, Matsuda M, Furuse M, Tsukita S. Regulation of tight junctions during the epithelium-mesenchyme transition: direct repression of the gene expression of claudins/occludin by Snail. J Cell Sci. 2003;116:1959–1967. doi: 10.1242/jcs.00389. [DOI] [PubMed] [Google Scholar]
- 21.Wang Z, Wade P, Mandell KJ, Akyildiz A, Parkos CA, Mrsny RJ, Nusrat A. Raf 1 represses expression of the tight junction protein occludin via activation of the zinc-finger transcription factor slug. Oncogene. 2007;26:1222–1230. doi: 10.1038/sj.onc.1209902. [DOI] [PubMed] [Google Scholar]