Abstract
Background
Methamphetamine (MA) use among pregnant women is a world-wide problem, but little is known of its impact on exposed infants.
Design
The prospective, controlled longitudinal Infant Development, Environment and Lifestyle (IDEAL) study of prenatal MA exposure from birth to 36 months was conducted in the US and NZ. The US cohort has 183 exposed and 196 comparison infants; the NZ cohort has 85 exposed and 95 comparison infants. Exposure was determined by self-report and meconium assay with alcohol, marijuana, and tobacco exposures present in both groups. The NICU Neurobehavior Scale (NNNS) was administered within 5 days of life. NNNS summary scores were analyzed for exposure including heavy exposure and frequency of use by trimester and dose-response relationship with the amphetamine analyte.
Results
MA Exposure was associated with poorer quality of movement, more total stress/abstinence, physiological stress, and CNS stress with more nonoptimal reflexes in NZ but not in the USA. Heavy MA exposure was associated with lower arousal and excitability. First trimester MA use predicted more stress and third trimester use more lethargy and hypotonicity. Dose-response effects were observed between amphetamine concentration in meconium and CNS stress.
Conclusion
Across cultures, prenatal MA exposure was associated with a similar neurobehavioral pattern of under arousal, low tone, poorer quality of movement and increased stress.
Keywords: Prenatal exposure, Methamphetamine, Neurodevelopment, Meconium
1. Introduction
Methamphetamine (MA) use is a world-wide public health problem with recent reports of approximately 250 million users, exceeding to cocaine and heroin combined [4]. In the United States (US), MA use is predominantly in the west and midwest regions, but is steadily moving eastward [52]. Based on the 2008 National Survey on Drug use and Health (NSDUH), the Substance Abuse and Mental Health Services Administration (SAMSHA) reported 5% of persons aged 12 and older used MA at least once in their lifetimes. Further, 5.1% of pregnant women used illicit drugs during pregnancy [3]. In 2000, Vince Smeriglio, Ph.D. of the National Institute on Drug Abuse (NIDA) recognized the rise in MA use was reminiscent of the crack cocaine epidemic that occurred in the US in the 1980s. To avoid similar unsubstantiated claims of harm to MA exposed infants as occurred with cocaine-exposed infants [34,41,58], Dr. Smeriglio and NIDA convened a meeting for researchers in the prenatal exposure field and clinicians who worked with MA exposed children [38]. This meeting led to a Request for Applications (RFA) by NIDA in 2001 to conduct a longitudinal study of MA exposed children in diverse populations and geographic locations. The RFA required the formation of a Community Research Network that brought together experienced researchers and clinicians in communities where MA use was prevalent. Our own Infant Development, Environment, and Lifestyle study (IDEAL-US) was funded under this initiative to follow a cohort of MA exposed children in Iowa, Oklahoma, California, and Hawaii. In these regions, we found 5.5% of women reporting MA use during pregnancy [7].
A similar problem occurred in NZ [1]. Based on the 2003 New Zealand Health Behaviours Survey-Drug Use [2], amphetamines were the second most widely used recreational drug in NZ, with approximately 6.8% of New Zealanders overall and 5.3% of females using at least once in their lifetime. By 2008, 9.3% of New Zealanders used amphetamines and 2.5% reported using MA [56]. While there are no national statistics available regarding MA use in pregnant women in NZ, the National Women’s Health Services, the obstetrical unit in Auckland Hospital and one venue of the present study, reported that 55% of referrals to the hospital’s Alcohol Drug and Pregnancy Team (ADAPT) were using MA during pregnancy, an increase from 10% in 2001 [57]. The increase in MA use in pregnant women in NZ was thought to be due, in part, to the advent of “P”. P stands for “pure” and refers to 85% MA purity in NZ as compared to the variable (20 – 90%) purity on the west coast of the US.
Due again to Dr. Smeriglio’s encouragement, we established a collaboration with Auckland, NZ, resulting in IDEAL-NZ, which replicated the US study design from birth to 36 months. NZ has a unique population and society that can improve our understanding particularly of long-term effects of MA. The NZ healthcare system provides free pre- and postnatal care, as well as, free doctor visits during early childhood. Further, NZ provides ongoing financial support when needed. These differences suggest a more homogeneous society with less extreme poverty and free access to healthcare. Further, NZ does not have mandatory reporting statutes for illicit drug use during pregnancy which means that mothers may be more likely to report their drug use and seek prenatal care because they are less likely to have their children removed by child protection services. These cultural differences may not impact newborn neurobehavior but could improve outcomes of MA exposed children during childhood and beyond compared to exposed children in the US. On the other hand, the supply of MA is thought to be of higher purity in NZ compared to the US (2002–2005 recruitment) and, therefore, there is potential for greater neurobehavioral effects from prenatal exposure.
MA is frequently compared to cocaine as both are sympathomimetic agents; however, MA’s neurotoxic effects may be greater due to its longer half-life and multiple mechanisms of action. Similar to cocaine, MA blocks the reuptake of dopamine and other catecholamines [25], but MA also increases dopamine and norepinephrine release [54]. The mechanism of action of MA most likely occurs by increasing the synaptic concentrations of the neurotransmitters dopamine and norepinephrine [24]. MA may enhance synaptic catecholamine levels by inhibiting monoamine oxidase, the enzyme responsible for the oxidation of norepinephrine and serotonin [8]. MA like cocaine also has vasoconstrictive effects, resulting in decreased uteroplacental blood flow and fetal hypoxia [39,50].
There is support for the neurotoxic effect of MA from pre-clinical studies. MA toxicity to dopaminergic and sertonergic neurons was found in rodents [20,43]. In pregnant mice, MA caused dopaminergic nerve terminal degeneration and long term motor deficits in offspring [29]. In the rat model, neurotoxic effects of prenatal exposure to MA [5,20,43] and cocaine [23,39,40] include both structural and functional changes to circuitry subserving functions such as arousal, regulation and reactivity to stress. Human studies generally find subtle neurobehavioral effects of prenatal exposure. Smith and colleagues who published the first report of newborn neurobehavior from the IDEAL US cohort (N=74 exposed; N=92 not exposed) found MA exposed infants were under aroused, with poorer quality of movement and increased total stress/abstinence signs. The only other longitudinal study of amphetamine exposure was a study by Billings and colleagues in Sweden, who also reported drowsiness during the first few months of life [9], although there were methodological limitations such as small sample size (N = 66), no control group and confounding with polydrug use. The Maternal Lifestyle Study, a large study of prenatal cocaine study (N=460 exposed; N=751 not exposed) also using the same neurobehavioral measure as the IDEAL study, reported underarousal, poorer quality of movement, regulation and reflexes, more excitability and hypertonia in cocaine exposed infants at 1 month of age [36].
The current study is the largest to date of MA exposed infants, including the full US-IDEAL cohort and the NZ cohort. Taken together there are 268 MA exposed and 291 not exposed infants. Thus this study has increased power to detect subtle neurobehavioral deficits than either study alone. Further to our knowledge, this is the first study of prenatal exposure and behavior to examine cross-cultural generalizability of exposure effects. This study is possible due to the common protocol in the US and NZ IDEAL studies. During the postpartum period, the NICU Network Neurobehavioral Scale (NNNS) is administered to neonates in both the US and NZ population. The NNNS is a standardized neurobehavioral exam for the healthy and at-risk neonates [35] that has been used in studies of prenatal exposure to cocaine [36,42], methamphetamine [46,48], opiates [14,30], marijuana [16] and tobacco [32,51]. Recent evidence shows that NNNS profiles predict medical and behavioral outcomes during childhood [37]. Summary scores are described in Table 1.
Table 1.
Description of NNNS summary scores
| Description | |
|---|---|
| Habituation | Response decrement to repeated auditory and visual stimuli; mean of items (3 items; range:1–9) |
| Attention | Response to animate and inanimate auditory and visual stimuli: mean of items (7 items; range: 1–9) |
| Arousal | Level of arousal including state and motor activity during the examination; mean of items recoded for high arousal (7 items; range: 1–9) |
| Regulation | Capacity to organize motor activity, physiology, and state during the examination and to respond to cuddling, consoling, and negative stimuli; mean of items recoded for good regulation (15 items; range: 1–9) |
| Handling | Handling strategies used during attention to maintain alert state; mean number of strategies used (8 items; range: 0–1) |
| Quality of movement | Measure of motor control including smoothness, maturity, lack of startles and tremors; mean of items recoded for good motor control (6 items; range: 1–9) |
| Excitability | Measure of high levels of motor, state, and physiologic reactivity: sum of items recoded for excitable behavior (15 items; range: 0–15) |
| Lethargy | Measure of low levels of motor, state, and physiologic reactivity: sum of items recoded for lethargic behavior (15 items; range: 0–15) |
| Nonoptimal reflexes | Any nonoptimal response to reflex elicitation; sum of items recoded for nonoptimal reflexes (15 items; range: 0–15) |
| Asymmetric. reflexes | Any asymmetric response to reflex elicitation; sum of items recoded for asymmetric response (16 items; range: 0–16) |
| Hypertonicity | Hypertonic response in arms, legs, or trunk or in general tone; sum of items recoded for hypertonic indicators (10 items; range: 0–10) |
| Hypotonicity | Hypotonic response in arms, legs, or trunk or in general tone; sum of items recoded for hypotonic indicators (10 items; range: 0–10) |
| Stress/abstinence | Amount of stress and abstinence signs observed during examination; mean number of observed stress signs (50 items; range: 0–1) |
Given that the pharmacology and mechanisms of action of MA are the same in NZ and US, we expect similar neurobehavioral effects associated with MA exposure across cohorts. But due to differences in the purity of methamphetamine, the NZ infants could show greater deficits than US infants. Further, exposure to heavy levels of MA is likely to show the greater deficits than less or no MA exposure. We also examine the association between trimester of MA use and neurobehavioral deficits as previously reported by Smith and colleagues in a smaller sample [48]. Finally, we identify neurobehavioral differences between the countries due in part to racial and ethnic composition, legal and health care systems, and financial entitlements that could impact future development.
2. Methods
The US and NZ cohorts were enrolled postpartum. In the US, recruitment also took place postpartum at seven hospitals at four sites (Los Angeles, CA; Des Moines, IA; Tulsa, OK; and Honolulu, HI). In NZ, recruitment took place during pregnancy based on referral from midwives in most cases. Infants were born at three regional/local hospitals (Auckland City Hospital, North Shore Hospital, and Waitakere Hospital). In both cohorts, all data collection was postpartum.
In the US the study was approved by the Institutional Review Boards at all participating sites. A NIDA Certificate of Confidentiality was obtained to assure confidentiality of information regarding participants’ drug use, superseding mandatory reporting of illegal substance use, but not evidence of abuse and neglect. Mothers were contacted postpartum, and if interested and eligible, written informed consent was obtained, followed by interviews concerning prenatal drug use with the Substance Use Inventory [17] and sociodemographic information with the Lifestyle Interview. Complete details of recruitment are reported elsewhere [7,17].
The NZ site first received approval for the study from the Auckland District Health Board (DHB) and Waitemata DHB. Final approval was received through the Northern Regional Ethics Committee, administered by the NZ Ministry of Health. As the Maori population was included, the study required consultation with local Iwi (tribes) and Maori Health care agencies. Approval of the project was granted from the Maori Research Committees at both Auckland DHB and Waitemata DHB. There are no statutes for mandatory reporting of mothers who use illegal substances during pregnancy; consequently there is very little child removal due to prenatal substance use. Recruitment in NZ took place during pregnancy. NZ offers universal health care and therefore, care during pregnancy and childbirth is free. Most women receive care throughout their pregnancy, at the birth and postnatally from independent midwives; however, some women select a midwife employed by maternity services in regional hospitals. All potential subjects were first referred to the study by one of these midwives during the prenatal period. Hence, the first step toward recruitment in NZ involved informing the midwives about the study and referral procedure. Once the mother agreed to speak with study staff they met with her to explain the study in detail and obtain written consent to participate. When the infant was born, study staff visited the mother at the hospital, reviewed the study protocol, and conducted the Lifestyle Interview and the Substance Use Inventory as in the US. In both cohorts, interview questions were read to the mother to ensure standardization. Meconium specimens were collected for analysis of drug metabolites.
Maternal exclusion criteria were lysergic acid diethylamide, phencyclidine and/or other hallucinogens use during the current pregnancy; age < 18 years (age of consent in the USA) or < 17.5 years at the infant’s birth (age of consent in NZ); history of institutionalization for retardation or emotional disorders; low cognitive functioning; overt psychotic behavior or a documented history of psychosis; and unable to speak English (except Maori in NZ). Exclusion criteria for children were being critically ill at birth and unlikely to survive; multiple birth; major life threatening congenital anomaly; documented chromosomal abnormality associated with mental or neurologic deficiency; overt TORCH infection; and/or sibling previously enrolled in the study. There was concern that many mothers who used MA during pregnancy also used cocaine in the US or opiates in NZ. In the US, 18 MA-using mothers who also used cocaine were accepted in the exposed group. In NZ, 12 MA-using mothers who also used opiates were accepted in the exposed group.
Meconium was shipped to a central laboratory (United States Drug Testing Laboratory in Des Plaines, IL) for analysis of the amphetamines, cocaine, cannabinoids, opiates and nicotine metabolites. Specimens were initially screened with enzyme multiplied immunoassays (EMIT II; Date-Behring, Cupertino, CA). If positive results were obtained, the specific drug analyte or metabolite was confirmed by gas chromatography-mass spectrometry (GC/MS). In the US, meconium was shipped within 2 days of collection while in NZ, meconium specimens were frozen at −20° C and shipped every six months. In the US, consented participants were not enrolled if there were positive results for opiates. Mothers identified as a MA user by self-report or positive MA results and who also reported cocaine use or had positive results for cocaine were enrolled in the exposed group. However, cocaine use was an exclusion criterion in the comparison group. Given the long delay for meconium results in NZ, participants were enrolled based on self-report. Opiates use by self-report or positive meconium results was allowed in the exposed group, but not in the comparison group. Participants with results indicating the presence of opiates (if no MA use was reported and negative results for MA) were reassigned to a non-study group to continue in the study as required by the Ethics Agreement, but not considered part of the IDEAL-NZ. If positive for MA but denied use, the participant was reassigned to the exposed group; this occurred in 8 participants in the US and 2 participants in NZ.
In both cohorts, MA exposure was defined as maternal report of MA use during the pregnancy based on the hospital interviews or positive GC/MS confirmation of amphetamine and/or metabolites in infant meconium. Inclusion in the comparison group required denial of MA use during this pregnancy and a negative meconium screen for MA.
The Substance Use Inventory [17] is a detailed questionnaire of frequency and quantity of drugs of abuse used during the three months before pregnancy and each trimester thereafter. A calendar covering the previous year, with holidays and personal events annotated served as the anchor for memory. A history of maternal alcohol, marijuana, and tobacco use during the pregnancy was considered as background variables in both the exposed and comparison groups. Targeted medical data were obtained from the mothers’ and infants’ charts. Health Insurance Portability and Accountability Act (HIPAA) authorization was required in the US, but not in NZ.
2.1. Participants
All exposed infants and mothers were enrolled in a longitudinal follow-up from birth to 36 month. Comparison neonates within the four US sites and NZ were matched based on race, birth weight category (<1500 g, 1500–2500 g, >2500 g) private versus public insurance in the US only as health insurance for prenatal care or maternity services is not required in NZ, education (high school education completed versus not completed in the US and < 5th form certificate achieved in NZ, which is the closest analogue to completing high school in the US). Typically in the US, the first consented subject to match an exposed subject was enrolled with a one to one ratio. For patterns of subject characteristics that were difficult to match, a few comparison subjects were enrolled in advance of identification of exposed subjects, leading to uneven group sizes. As the referral process in NZ limited the pool of potential comparison subjects (unlike the US), the matching process was geared toward group matching rather than one-to one matching. At this time, self-identified ethnicity does not differ by exposure group, but the exposed group in NZ was less likely to have obtained the 5th form certificate than the comparison group.
The NZ cohort continues to enroll subjects, while the US cohort has completed enrollment. To date there are no infants born less than 36 weeks gestational age in NZ. To avoid prematurity affecting one cohort and not the other, we excluded 33 US subjects (N=21 exp, N=12 comp), who were ≤35 weeks gestational age. In the US cohort there are 183 subjects in the exposed and 196 in the comparison group, and 85 subjects in the exposed and 95 in the comparison group in NZ. Overall, there are 268 infants with MA exposed and 291 comparison infants.
2.2. NNNS
By protocol, the NNNS exam was administered within the first 5 days of life by examiners who were masked to exposure status and trained and certified on the NNNS by gold standard reviewers. To maintain reliability, examiners were periodically rechecked during the course of recruitment. The NNNS provides an assessment of neurologic, behavior, and stress/abstinence neurobehavioral function [35]. The neurologic component includes active and passive tone, primitive reflexes, and items that reflect the integrity of the central nervous system and maturity of the infant. The behavior component is based on items from the Neonatal Behavior Assessment Scale (NBAS) [10], modified to be sensitive to putative drug effects. The stress/abstinence component is a checklist of ‘yes’ or ‘no’ items organized by organ system primarily based on the work of Finnegan [18]. The NNNS follows a relatively invariant sequence of administration that starts with a pre-examination observation, followed by the neurologic and behavioral components. The stress/abstinence scale is based on signs observed throughout the examination. The NNNS items are summarized into the following summary scores [33], as described in Table 1: habituation, attention, arousal, regulation, handling, quality of movement, excitability, lethargy, nonoptimal reflexes, asymmetric reflexes, hypertonicity, hypotonicity, and total stress/abstinence. Coefficient alpha was used to evaluate the psychometric properties of the scale and ranged from 0.56 to 0.85 [36]. Consistent with the early IDEAL study of neurobehavioral outcomes [48], we included the subscales of the total stress/abstinence summary score [33].
The actual sequence of administration and means used by the examiner to maintain an infant’s participation in the examination are recorded. The examination was administered in a quiet room, midway between feedings with the infant initially asleep and covered if possible. Habituation was not analyzed as too few subjects were sleeping at the start of the exam.
2.3. Statistical analysis
Analysis of variance (ANOVA) and Chi-square statistics were used to compare the MA and comparison groups in each country on medical and demographic characteristics. The neurobehavioral summary scores were tested by general linear models (GLM) for MA exposure (exposed/comparison or heavy, some, no MA exposure), country (US/NZ), and the interaction between exposure and country. These analyses were conducted with and without covariates (described below). Cohen’s d effect sizes were calculated based on unadjusted mean differences. GLM (type III sum of squares) calculates the sums of squares of an effect adjusted for other effects that do not contain it (main effects) and orthogonal to any effects that contain it (interactions). The method is invariant with respect to cell frequencies as long as the general form of estimability remains constant, which is appropriate for an unbalanced model with no missing cells. Consistent with published papers of MA exposure [17,48] and cocaine exposure [36], heavy use is defined as average use of MA ≥ 3 days per week across pregnancy, some use is defined as MA use less < 3 days per week. Significant level of use and interaction effects were further tested by contrast tests using the lmatrix command, followed by step down Bonferroni correction of P values [55] to minimize type I errors (false positives) due to multiple comparison tests. All parametric values in the tables are reported as unadjusted means and standard deviations. All nonparametric values are reported as absolute number and percentages.
The association between NNNS summary scores and frequency of MA use (days per week) during each trimester was tested by a forced entry regression analysis including country; MA use by trimester, birth weight in 100g, average quantities of alcohol, tobacco and/or marijuana use across pregnancy, SES, and first born. Significant interactions of country by trimester of MA use were evaluated by separate regression analysis for each country. Finally, correlation analyses tested the dose-response association between GC/MS amphetamine concentrations in meconium and NNNS summary scores. The concentration values were log transformed to normalize the distribution for analysis, but raw values (ng/g) are shown in Figure 1. There were 48 cases in the US with concentrations available, but only 10 in NZ in part due to the delay in processing meconium. Thus, only the US data are used in these analyses. Significance was accepted at P<0.05 in all analyses.
Fig. 1.
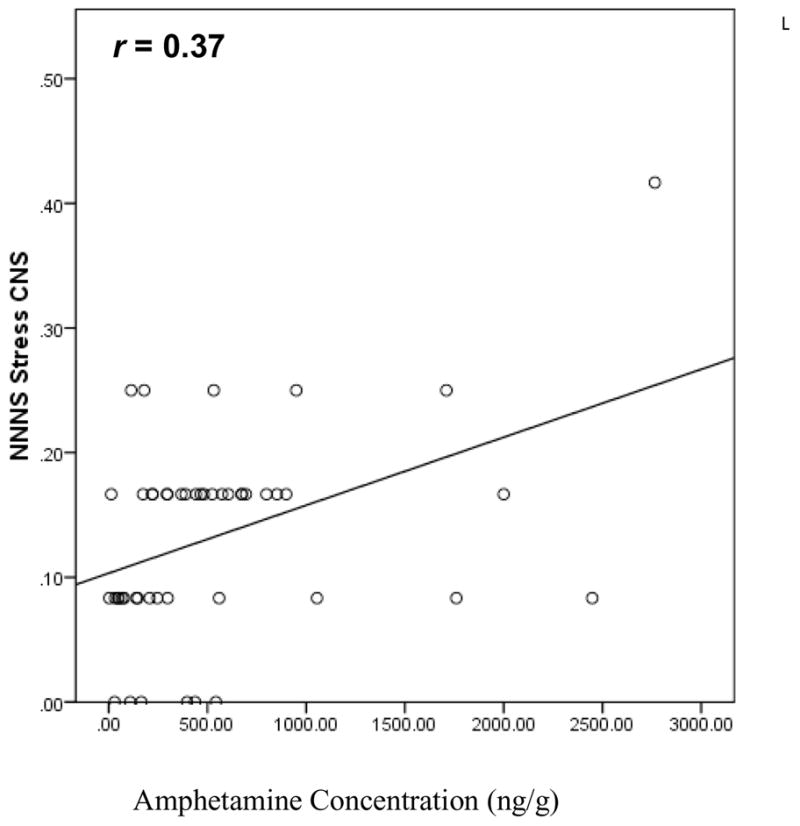
Correlation between NNNS CNS stress and amphetamine concentration (US N=58, P<0.05).
2.4 Standard covariate set
Variables in Tables 2 and 3 were examined for possible inclusion as covariates. Covariates were selected based on conceptual reasons, published literature, and characteristics from Tables 2 and 3 that differed between groups in either country if not highly correlated with other covariates. The covariates included birth weight, socioeconomic status (SES), and 3-level alcohol, tobacco, and marijuana use (heavy/some/no use), and first born. Education and occupation information was collected to calculate the four-factor Hollingshead Index which has been adapted to single parent and non-nuclear families [26,31]. The continuous SES measure, index of social prestige, was used in analysis, but for presentation purposes, the categorical low SES (Hollingshead V) was reported in Table 2. Levels of drug use were based on thresholds for detecting effects that have been reported by others [15,19,27,28,36]. For alcohol, heavy use was ≥ 0.5 oz of absolute alcohol per day (1 standard drink), averaged across pregnancy. For tobacco, heavy use was defined as ≥10 cigarettes per day, averaged across pregnancy. For marijuana, heavy use was defined as ≥0.5 joints per day, averaged across pregnancy. All other uses were defined as some use. Reported quantity of standard drinks of alcohol in NZ and US was converted to absolute alcohol based on conventions for each country. First born was a dichotomized [yes/no] variable. SES and birth weight were continuous variables. Summary statistics of these covariates by MA exposure are shown in Tables 2 and 3.
Table 2.
Maternal characteristics of methamphetamine-exposed and comparison groups in US and NZ
| US | NZ | |||
|---|---|---|---|---|
| N(%) or Mean (SD) | Exposed (N=183) | Comparison (N=196) | Exposed (N=85) | Comparison (N=95) |
| Race/Ethnicity | ||||
| White | 66 (36%) | 77 (39%) | 51 (60%) | 43 (45%) |
| Maori | 29 (34%) | 34 (36%) | ||
| Hawaiian/Pacific Islander | 35 (19%) | 32 (16%) | 4 (5%) | 14 (15%) |
| Hispanic | 42 (23%) | 43 (22%) | ||
| Asian | 25 (14%) | 28 (14%) | 1 (1%) | 3 (3%) |
| Black | 9 (5%) | 12 (6%) | ||
| American Indian | 6 (3%) | 4 (3%) | ||
| Indian-Pakistani | 1 (1%) | |||
| Low SES (Hollingshead V) | 58 (32%)*** | 24 (12%) | 42 (49%)*** | 17 (18%) |
| Monthly income | US$ 609** | US$ 881 | NZ$ 1,877 | NZ$ 1,735 |
| No partner | 100 (55%)*** | 67 (34%) | 47 (55%)*** | 22 (23%) |
| Education < high school/< 5th form | 85 (47%) | 77 (40%) | 54 (64%)* | 45 (47%) |
| Maternal age, yr | 25.9 (5.7)** | 24.22 (5.3) | 26.8 (6.2) | 26.0 (6.9) |
| Gest. Age at 1st prenatal visit, week | 14.8 (8.1)*** | 9.5 (5.6) | 16.3 (7.3)* | 13.7 (6.0) |
| Number of prenatal visits | 11.4 (7.4)*** | 14.4 (5.4) | 15.6 (6.8) | 16.9 (6.1) |
| Prenatal tobacco use | 146 (80%)*** | 52 (26%) | 74 (87%)*** | 52 (54%) |
| Heavy tobacco use | 56 (31%)*** | 15 (8%) | 36 (42%)*** | 15 (16%) |
| # of cigarettes per day | 7.05 (8.26)*** | 1.64 (4.54) | 9.10 (7.76)*** | 3.55 (5.82) |
| Prenatal alcohol use | 71 (39%)*** | 25 (13%) | 51 (60%) | 50 (53%) |
| Heavy alcohol use | 11 (6%)*** | 0 | 11 (13%) | 7 (7%) |
| oz. of absolute alcohol per day | 0.13 (0.51)** | 0.01 (0.02) | 0.35 (0.87)** | 0.09 (.22) |
| Prenatal marijuana use | 64 (35%)*** | 7 (4%) | 54 (64%)*** | 20 (21%) |
| Heavy marijuana use | 14 (8%)** | 2 (1%) | 23 (27%)** | 8 (8%) |
| # of joints per day | 0.10 (0.26)*** | 0.01 (0.09) | 0.52 (1.07)*** | 0.15 (0.54) |
P<0.001,
P <0.01, P <0.05
Table 3.
Newborn characteristics of methamphetamine and comparison groups in US and NZ
| US (N=379) | NZ (N=180) | |||
|---|---|---|---|---|
| N(%) or Mean (SD) | Exposed (N=183) | Comparison (N=196) | Exposed (N=85) | Comparison (N=95) |
| Gestational age, week | 38.9 (1.5)* | 39.24 (1.4) | 39.2 (1.6) | 39.5 (1.3) |
| Birth weight, g | 3278 (528) | 3378 (536) | 3380 (473) | 3474 (527) |
| Length, cm | 50.3 (3.2)** | 51.3 (2.8) | 51.1 (2.2) | 51.1 (2.5) |
| Head circumference, cm | 33.9 (0.6)* | 34.3 (1.7) | 34.7 (1.7) | 35.0 (1.7) |
| Apgar, 1 min, <5,% | 8 (4%) | 2 (1%) | 0 | 3 (3%) |
| Apgar, 5 min, <5,% | 0 | 0 | 0 | 0 |
| Male, % | 98 (54%) | 104 (53%) | 43 (51%) | 50 (53%) |
| First born, % | 43 (24%) | 76 (39%)** | 36 (43%) | 44 (46%) |
P <0.01, P <0.05
In regression analyses, MA use frequency was defined as the average number of days using MA per week during each trimester. The same covariates were used in the regression analyses with the exception that quantities of alcohol, tobacco, and marijuana use were continuous measures not classified into heavy, some, and no use. As each MA exposed participant was matched with a comparison within each site, site effects in the US cohort were not tested in this study. No partner, gestational age at first prenatal visit, monthly income, and educational level were highly correlated with SES, and gestational age, birth length and head circumference were highly correlated with birth weight and were not included in the analyses.
Twenty-two assessments (N=11 [6%] exposed, N=11 [6%] comparison, range 6–14 days) in the US and 2 assessments in NZ (N=2 [2%] exposed, range 6–8 days) were conducted after 5 days postpartum. Preliminary analyses showed no association with NNNS summary scores or exposure status and these cases were maintained in the sample. We also examined the impact of prenatal cocaine exposure in the US exposed group (N=18) and prenatal opiate exposure in the NZ exposed group (N=12) by analyzing the effects of exposure and level of use on NNNS summary scores including and excluding these cases. There were no changes in results and these cases were retained in the study.
3. Results
3.1. Maternal and newborn characteristics
In both cohorts, the largest racial/ethnic group was White (Table 2). In NZ, 35% of the cohort was Maori and 13% were from the Pacific Islands and other populations. There was more diversity in the US cohort with 18% from the Pacific Islands, 22% Hispanic, 14% Asian, 6% Black and 3% American Indian. As expected, no differences in self-reported ethnicity were observed between the groups in both cohorts due to matching. Mothers in the exposed group were lower SES, less likely to have a partner and more likely to have their first prenatal visit during the second trimester compared to their respective comparison groups. Also, they were older and had fewer prenatal visits in the US and were less likely to obtain the 5th form certificate in NZ. Further, all mothers in NZ had at least some prenatal care, while in the US, 9 (5%) mothers in the exposed group and 4 (2%) in the comparison group had no prenatal care. In addition, we asked mothers for their monthly net income before pregnancy. In the US, mothers in the exposed group reported less income than mothers in the comparison group (P=0.007). In NZ, there were no income differences between the MA exposed and comparison groups (P=0.666), which is consistent with social policies in NZ that reduce poverty. In the US, exposed newborns were lower gestational age, shorter with a smaller head circumference and less likely to be first born than comparison infants. In NZ, there were no differences on newborn growth and first born status (Table 3).
3.2 Maternal drug use
In both the US and NZ, mothers in the MA exposed group used more tobacco and marijuana than their respective comparison groups (Table 2). A similar pattern for alcohol use was observed in the US. In NZ however, there were no differences in any or heavy use of alcohol between the MA exposed and comparison groups, but the mothers in the exposed group had a higher level of absolute alcohol per day than the comparison group.
Table 4 shows the frequency of MA use by self-reporters in the exposed group of both cohorts by trimester and overall. Daily use was reported by 18% in the US and 15% in NZ in the first trimester but only 3% in the US and 1% in NZ in the third trimester. The number of women abstaining from MA use in the first trimester was 14% in the US and 6% in NZ increasing to 61% and 60% respectively in the third trimester. The declining frequency of MA use over pregnancy was similar in the US and NZ participants (F (1,256) = 1.95, P=0.164). However, given the definition of heavy use (average ≥3 days per week across pregnancy), there were more heavy users in the US than in NZ (χ2=4.35, P=0.037).
Table 4.
Frequency of self-reported methamphetamine use in US and NZ
| MA use | US (N=175) | NZ (N=83) | ||||
|---|---|---|---|---|---|---|
| Trimester | ||||||
| First | Second | Third | First | Second | Third | |
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | |
| Daily | 31 (18%) | 17 (10%) | 6 (3%) | 12 (15%) | 4 (5%) | 1 (1%) |
| 3–6 days/wk. | 50 (29%) | 23 (13%) | 14 (8%) | 19 (23%) | 7 (8%) | 4 (5%) |
| 1–2 days/wk. | 25 (14%) | 17 (10%) | 10 (6%) | 18 (22%) | 11 (13%) | 7 (9%) |
| 1–3 days/mo. | 15 (8%) | 18 (10%) | 12 (7%) | 12 (14%) | 11 (13%) | 8 (10%) |
| 1–2 days/3 mos. | 29 (17%) | 20 (11%) | 26 (15%) | 17 (20%) | 8 (10%) | 13 (15%) |
| Not at all | 25 (14%) | 80 (46%) | 107 (61%) | 5 (6%) | 42 (51%) | 50 (60%) |
| Days/week (mean, SD) | 2.64 (2.59) | 1.39 (2.26) | 0.69 (0.17) | 2.37 (2.47) | 0.99 (1.88) | 0.45 (1.19) |
| Heavy use (≥3 day/wk) | 35 (20%) * | 8 (10%) | ||||
P=0.037
3.3. Neurodevelopmental outcome on the NNNS
Analyses of exposure status by country are presented in Table 5. The unadjusted means and standard deviations of the NNNS summary scores are shown in the table with P values for results, unadjusted and adjusted for covariates. Relative to the comparison group, the exposed group had poorer quality of movement (P=0.001 unadj., P=0.001 adj.; effect size 0.31), more total stress/abstinence (P=0.012 unadj., P=0.036 adj.; effect size 0.18), more physiological stress (P=0.001 unadj., P=0.002 adj.; effect size 0.23), more CNS stress (P=0.004 unadj., P=0.026 adj.; effect size 0.12). In unadjusted analysis, the exposed group had more autonomic stress than the comparison groups (P=0.042; effect size 0.17).
Table 5.
NNNS summary scores by methamphetamine exposure status and country
| US (N=379) | NZ (N=180) | Exposure P= | Country P= | Interaction P= | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Exposed (N=183) | Comparison (N=196) | Exposed (N=85) | Comparison (N=95) | Unadj. | Adj. | Unadj. | Adj. | Unadj. | Adj. |
| Attention | 5.21 (1.08) | 5.19 (1.16) | 4.47 (1.15) | 4.39 (.99) | 0.607 | 0.248 | 0.001 | 0.001 | 0.767 | 0.636 |
| Arousal | 3.92 (0.65) | 4.11 (0.62) | 3.99 (0.73) | 4.00 (0.61) | 0.086 | 0.761 | 0.773 | 0.692 | 0.107 | 0.171 |
| Regulation | 5.46 (0.62) | 5.47 (0.73) | 5.02 (0.78) | 5.11 (0.66) | 0.433 | 0.125 | 0.001 | 0.001 | 0.578 | 0.990 |
| Handling | 0.30 (0.23) | 0.32 (0.25) | 0.37 (0.30) | 0.29 (0.28) | 0.205 | 0.070 | 0.406 | 0.441 | 0.041 | 0.117 |
| Quality of movement | 4.39 (0.57) | 4.51 (0.59) | 4.62 (0.74) | 4.95 (0.46) | 0.001 | 0.001 | 0.001 | 0.001 | 0.052 | 0.083 |
| Excitability | 2.92 (1.92) | 3.25 (1.87) | 3.85 (3.02) | 3.36 (2.30) | 0.690 | 0.113 | 0.009 | 0.021 | 0.037 | 0.098 |
| Lethargy | 4.56 (2.58) | 4.16 (2.46) | 4.66 (2.58) | 4.33 (2.34) | 0.107 | 0.525 | 0.552 | 0.979 | 0.883 | 0.604 |
| Nonoptimal reflexes | 0.33 (0.74) | 0.37 (0.74) | 1.11 (1.42) | 0.67 (0.80) | 0.014 | 0.066 | 0.001 | 0.001 | 0.004 | 0.012 |
| Asymm. Reflexes | 0.33 (0.56) | 0.27 (0.58) | 0.54 (1.04) | 0.41 (0.91) | 0.152 | 0.583 | 0.007 | 0.013 | 0.577 | 0.573 |
| Hypertonicity | 0.03 (0.16) | 0.03 (0.19) | 0.18 (0.38) | 0.17 (0.38) | 0.834 | 0.926 | 0.001 | 0.001 | 0.895 | 0.844 |
| Hypotonicity | 0.15 (0.47) | 0.10 (0.32) | 0.46 (0.73) | 0.36 (0.56) | 0.089 | 0.230 | 0.001 | 0.001 | 0.576 | 0.785 |
| Stress/abstinence | 0.094 (0.06) | 0.091 (0.05) | 0.056 (0.06) | 0.036 0 (0.03) | 0.012 | 0.036 | 0.001 | 0.001 | 0.063 | 0.125 |
| Physiological | 0.016 (0.09) | 0.003 (0.04) | 0.036 (0.15) | 0 | 0.001 | 0.002 | 0.252 | 0.284 | 0.135 | 0.446 |
| Autonomic | 0.092 (0.13) | 0.074 (0.11) | 0.125 (0.13) | 0.098 (0.12) | 0.042 | 0.203 | 0.010 | 0.096 | 0.691 | 0.702 |
| CNS | 0.138 (0.08) | 0.133 (0.08) | 0.062 (0.10) | 0.026 (0.04) | 0.004 | 0.026 | 0.001 | 0.001 | 0.031 | 0.074 |
| Skin | 0.064 (0.11) | 0.077 (0.14) | 0.016 (0.08) | 0.002 (0.02) | 0.980 | 0.691 | 0.001 | 0.001 | 0.161 | 0.225 |
| Visual | 0.092 (0.09) | 0.089 (0.09) | 0.046 (0.05) | 0.038 (0.05) | 0.488 | 0.295 | 0.001 | 0.001 | 0.726 | 0.737 |
| GI | 0.042 (0.14) | 0.051 (0.14) | 0.024 (0.09) | 0.021 (0.08) | 0.780 | 0.540 | 0.036 | 0.023 | 0.603 | 0.664 |
| State | 0.097 (0.13) | 0.093 (0.12) | 0.063 (0.11) | 0.041 (0.08) | 0.221 | 0.147 | 0.001 | 0.001 | 0.404 | 0.534 |
There were four interactions between exposure and country with post hoc results of exposure effects by country reported below. Exposed infants in NZ had more nonoptimal reflexes than the comparison group in both unadjusted (P=0.002) and adjusted (P=0.012) analyses with effect size 0.39, but not in the US (effect size 0.07). In unadjusted analyses only, exposed infants in NZ had higher scores for CNS stress (P=0.004; effect size 0. 40), but not in the US (effect size 0.13). Other interactions were not significant by country and not reported.
Table 5 also shows significant differences between US and NZ on 15 of 19 NNNS summary scores (all P values < 0.05). In order to determine whether these effects were due to averaging across the variability among the four US sites, we conducted one-way ANOVAs for each significant country effect on NNNS by five groups (including NZ as a 5th site) with Newman Keuls follow up tests. There were five NNNS summary scores where NZ was significantly different (P<0.05) from the four US sites: Increased nonoptimal reflexes (NZ 0.88 versus US range 0.21–0.58), hypotonicity (NZ 0.41 versus US range 0.05–0.18), and hypertonicity (NZ 0.17 versus US range 0–0.05), and less total stress (NZ 0.046 versus US range 0.065–0.135) including CNS stress (NZ 0.043 versus US range 0.119–0.157).
Table 6 shows the results of heavy MA use on NNNS summary scores in the US and NZ. The unadjusted means and standard deviations are shown in the table with P values for unadjusted and adjusted analyses. The follow up test for significant level of use effects examined whether heavy use was associated with greater neurobehavioral decrements over and above some and no use. Compared to the some and no use groups, the heavy use group had lower arousal (P=0.003, unadj., P=0.009 adj.; effect size 0.59), and less excitability (P=0.027, unadj. only; effect size 0.46). Other level of use effects were not significant when tested for heavy versus some and no use. Although nonoptimal reflexes showed significant interactions of level of use by country in adjusted and unadjusted analyses, separate follow up tests for heavy versus some and no use in NZ and US were not significant in either country. Country effects were the same as in Table 5 and the related text, and not repeated here or in Table 6.
Table 6.
NNNS summary scores by level of methamphetamine use and interaction with country
| US | NZ | Level of use P= | Interaction P= | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Heavy use (N=35) | Some use (N=140) | No Use (N=196) | Heavy use (N = 8) | Some use (N=75) | No use (N=95) | Unadj. | Adj. | Unadj. | Adj. |
| Attention | 5.26 (1.20) | 5.16 (1.06) | 5.19 (1.60) | 4.08 (1.33) | 4.50 (1.13) | 4.39 (0.99) | 0.790 | 0.474 | 0.554 | 0.655 |
| Arousal | 3.70 (0.69) | 3.98 (0.63) | 4.12 (0.62) | 3.52 (0.59) | 4.07 (0.72) | 4.00 (0.61) | 0.003 | 0.013 | 0.187 | 0.210 |
| Regulation | 5.52 (0.50) | 5.43 (0.65) | 5.47 (0.73) | 5.27 (0.64) | 4.98 (0.80) | 5.11 (0.66) | 0.257 | 0.157 | 0.713 | 0.877 |
| Handling | 0.31 (.22) | 0.30 (0.24) | 0.32 (0.25) | 0.29 (0.22) | 0.38 (0.31) | 0.29 (0.28) | 0.336 | 0.336 | 0.113 | 0.118 |
| Quality of Mvt. | 4.33 (0.48) | 4.40 (0.59) | 4.51 (0.59) | 4.83 (0.58) | 4.57 (0.75) | 4.95 (0.46) | 0.001 | 0.001 | 0.054 | 0.097 |
| Excitability | 2.37 (1.57) | 3.06 (1.95) | 3.25 (1.87) | 2.25 (1.75) | 4.11 (3.06) | 3.36 (2.30) | 0.015 | 0.013 | 0.057 | 0.105 |
| Lethargy | 4.91 (3.16) | 4.52 (2.48) | 4.16 (2.46) | 5.88 (3.87) | 4.44 (2.28) | 4.33 (2.34) | 0.069 | 0.196 | 0.583 | 0.549 |
| Nonopt. reflexes | 0.46 (0.92) | 0.32 (0.71) | 0.37 (0.74) | 0.88 (1.13) | 1.13 (1.47) | 0.67 (0.80) | 0.050 | 0.162 | 0.012 | 0.023 |
| Asymm. reflexes | 0.17 (0.38) | 0.39 (0.59) | 0.27 (0.58) | 0.75 (0.89) | 0.53 (1.07) | 0.41 (0.91) | 0.205 | 0.711 | 0.333 | 0.297 |
| Hypertonicity | 0 | 0.04 (0.19) | 0.03 (0.19) | 0.25 (0.46) | 0.17 (0.38) | 0.17 (0.38) | 0.855 | 0.940 | 0.582 | 0.595 |
| Hypotonicity | 0.23 (0.60) | 0.14 (0.44) | 0.10 (0.32) | 0.25 (0.46) | 0.49 (0.76) | 0.36 (0.56) | 0.167 | 0.310 | 0.234 | 0.297 |
| Stress/abstinence | 0.091 (0.06) | 0.096 (0.06) | 0.091 (0.05) | 0.046 (0.02) | 0.057 (0.06) | 0.036 (0.03) | 0.029 | 0.102 | 0.237 | 0.293 |
| Physiological | 0.014 (0.08) | 0.018 (0.09) | 0.003 (0.04) | 0.062 (0.18) | 0.034 (0.15) | 0 | 0.002 | 0.008 | 0.210 | 0.359 |
| Autonomic | 0.110 (0.15) | 0.088 (0.13) | 0.074 (0.11) | 0.063 (0.09) | 0.133 (0.13) | 0.098 (0.12) | 0.099 | 0.326 | 0.168 | 0.178 |
| CNS | 0.152 (0.08) | 0.136 (0.08) | 0.133 (0.08) | 0.063 (0.07) | 0.063 (0.10) | 0.026 (0.04) | 0.013 | 0.061 | 0.065 | 0.129 |
| Skin | 0.067 (0.14) | 0.064 (0.11) | 0.077 (0.14) | 0 | 0.018 (0.09) | 0.002 (0.02) | 0.941 | 0.762 | 0.368 | 0.334 |
| Visual | 0.070 (0.08) | 0.098 (0.10) | 0.089 (0.09) | 0.038 (0.04) | 0.046 (0.06) | 0.038 (0.05) | 0.425 | 0.444 | 0.835 | 0.858 |
| GI | 0.010 (0.06) | 0.048 (0.15) | 0.051 (0.14) | 0.042 (0.12) | 0.022 (0.08) | 0.021 (0.08) | 0.919 | 0.793 | 0.475 | 0.640 |
| State | 0.086 (0.13) | 0.102 (0.14) | 0.093 (0.12) | 0.053 (0.08) | 0.060 (0.12) | 0.041 (0.08) | 0.449 | 0.382 | 0.866 | 0.891 |
Note: main effects for country are shown in Table 5 and not repeated in this table.
Table 7 shows the results of regression analysis on the frequency of MA use by trimester after adjusting for covariates. Across the US and NZ, first trimester use was associated with greater total stress/abstinence (β=0.11, P=0.026) and physiological stress (β=0.16, P=0.003). Third trimester use was related to increased lethargy (β=0.14, P=0.006) and hypotonicity (β=0.11, P=0.032). Analysis of the significant interaction of trimester by country (P=0.024) showed that first trimester use in NZ infants was associated with greater CNS stress (β=0.21, P=0.016), but not in the US. Use of MA during the 3 trimesters was not so highly correlated as to cause multicollinearity. The correlations were 0.50 between trimesters 1 and 2, 0.27 between 1st and 3rd trimesters, and 0.51 between 2nd and 3rd trimesters.
Table 7.
Regression models predicting NNNS summary scores by trimester of MA use
| Frequency of MA use (# days per week) | Unstandardized coefficient (SE) | βa | P | R2 | |
|---|---|---|---|---|---|
| Lethargy | first trimester | 0.010 (0.063) | 0.008 | 0.878 | 0.037 |
| second trimester | 0.016 (0.095) | 0.010 | 0.868 | ||
| third trimester | 0.330 (0.121) | 0.142 | 0.006 | ||
| Hypotonicity | first trimester | 0.014 (0.012) | 0.061 | 0.255 | 0.103 |
| second trimester | −0.023 (0.019) | −0.074) | 0.209 | ||
| third trimester | 0.051 (0.024) | 0.108 | 0.032 | ||
| Stress/abstinence | first trimester | 0.003 (0.001) | 0.113 | 0.026 | 0.188 |
| second trimester | −0.004 (0.002) | −0.103 | 0.067 | ||
| third trimester | 0.001 (0.003) | −0.007 | 0.876 | ||
| Physiological stress | first trimester | 0.006 (0.002) | 0.161 | 0.003 | 0.055 |
| second trimester | −0.005 (0.003) | −0.087 | 0.149 | ||
| third trimester | 0.007 (0.004) | 0.090 | 0.082 | ||
| CNS stress (NZ only) | first trimester | 0.008 (0.003) | 0.210 | 0.016 | 0.200 |
| second trimester | −0.004 (0.005) | −0.066 | 0.455 | ||
| third trimester | −0.010 (0.007) | −0.112 | 0.170 | ||
The result of testing the dose-response relationship of the GC/MS amphetamine and neurobehavior is shown in Fig. 1. CNS stress was correlated with increasing levels of the amphetamine analyte (N=48, r = 0.37, P<0.05). The detection window of amphetamines in meconium is primarily the 3rd trimester but can extend into the 2nd trimester [21]. Consistent with the pattern of MA use in the US section of Table 4, 93% of the cases with positive GC/MS for amphetamines used in the 3rd trimester. The range of CNS stress in this subsample is 0 to 0.42, which is the same range in the full US cohort and the NZ cohort. To identify potential outliers in this small sample, we applied the Mahalanobis distance procedure to detect multivariate outliers. All values were P > 0.001, indicating no multivariate outliers. We also examined box plots and z scores to detect univariate outliers for the analyte and CNS values separately. There was one potential outlier for CNS Stress and two for the amphetamine analyte. After removing the 3 potential univariate outliers, the correlation remained significant (r = 0.35, P<0.05) and the cases were retained.
4. Discussion
Taken together, the IDEAL US and NZ cohorts comprise the only large, systematically-controlled study of MA exposed and matched comparison children with extensive measurement of polydrug use, environmental and maternal characteristic, as well as cultural and racial variation that increases generalizability of findings. Hence, this study is the largest study of prenatal MA exposure and neurobehavioral outcome in newborns. The main outcome of this study is that there are unique effects of MA on newborn neurobehavior. These effects are observed in two cultures after controlling for covariates including other drug exposure. We found that MA exposure in utero was associated with neurobehavioral patterns of poorer quality of movement and increased stress signs, in particular, physiological and CNS stress. Heavy MA use predicted lower arousal and less excitability. The frequency of MA use in the 1st trimester was associated with increased total stress signs and physiological stress in particular. The frequency of MA use during the 3rd trimester was associated with increased lethargy and hypotonicity. Analyte concentration in meconium, primarily reflecting substantial use of MA in the third trimester, was related to CNS stress. All trimester effects were adjusted for prenatal exposure to other drugs, SES, birth weight, firstborn as well as the other factors—country and interaction of country by exposure. Moreover, these effects were observed in the newborn period before postnatal environmental factors come into play. The fact that these results were observed in both cultures suggests that these MA effects are universal and robust.
Our findings of underarousal, low tone, poorer quality of movement but increased stress when aroused also were reported with the NNNS in MA exposed infants with approximately half of the final US cohort but including preterm infants[48] and in cocaine-exposed infants 1 month after birth that also included preterm infants [36]. However, there are population differences between pregnant women who use cocaine and MA that could impact long-term consequences. Most pregnant cocaine users are Black, poor and from inner city communities [22]. On the other hand, most pregnant MA users are White or Hispanic, working class, and from rural communities [52]. Moreover, the postnatal environments of MA exposed children may involve additional exposure to MA by passive inhalation or ingestion if the drug is manufactured in the home. Clandestine home labs also are dangerous due to toxic, volatile chemicals involved in the manufacturing process [6]. However, the number of small clandestine laboratories is decreasing due to constraints on purchasing precursor chemicals in the US. Now most MA in the US is smuggled into the country through the illegal drug trade.
There was a significant MA by country interaction on nonoptimal reflexes. MA exposure was associated with more nonoptimal reflexes in NZ but not in the US. This could be due to the purity of MA in NZ, which may also explain why exposure findings across countries are mostly consistent despite a lower frequency of use in NZ than in the US. There were also cross-cultural differences. Overall, infants in NZ showed more nonoptimal reflexes, poor tone, but less total stress including CNS stress than infants in the US. These effects were observed with adjustment for covariates including other drugs. There are a number of factors that could explain these cross cultural differences that we did not study such as genetic, reproductive and intrauterine influences. Cultural differences could also reflect sociodemographic and health care differences between the two countries. We will continue to monitor whether these differences represent added or decreased vulnerability to children with MA exposure.
Poorer neurobehavioral outcomes in MA exposed newborns may represent neurotoxic effects of the drug, either acute or long term, or transient symptoms in response to acute discontinuation from MA. Due to the fact that approximately 60% of mothers in both countries stopped MA ingestion before the third trimester and remaining users reduced the frequency of use, it is unlikely that the effects of MA exposure is due to discontinuation of the drug, with the possible exception of CNS stress, given the dose-response relationship with amphetamines level in meconium.
Research involving imaging provides evidence for long lasting neurotoxic effects of MA. Positron emission tomography studies in abstinent MA users demonstrated decreased dopamine transporters, suggesting persistent neuroxicity due to prior MA abuse [45,53]. Several imaging studies examined children with prenatal MA exposure. A study of MA exposed children aged 3–4 years with diffusion tensor imaging showed lower diffusion in the frontal and parietal white matter, suggesting alterations in white matter maturation in exposed children [13]. In the same sample with proton magnetic resonance spectroscopy, alteration in neuronal and glial development in white matter was associated with poorer visual motor performance [11]. Magnetic resonance imaging (MRI) was conducted in 61 children aged 5 to 15 years with prenatal MA and alcohol exposure. MA exposure was associated with striatal volume reduction but increased volume in the cingulate and inferior frontal gyrus compared to the alcohol and control groups. In the MA exposed group only, caudate volume was negatively associated with IQ scores [49]. A study of 12 MA exposed children and 14 controls aged 3 to 16 years using magnetic resonance spectroscopy found increased creatine in the striatum of the MA exposed group suggesting an abnormality in energy metabolism [47]. Using MRI for brain morphometry, these children showed smaller subcortical volumes in the putamen, globus pallidus, and hippocampus which were related to deficits in sustained attention and verbal memory [12]. Taken together, these studies suggest that prenatal MA exposure may have neurotoxic effect on regions of the brain related to executive function. We review imaging studies because they are an emerging area of research with promise for understanding the biological basis for prenatal MA exposure effects. But these studies have small sample sizes and are not able to control for confounding factors that could contribute to neurological and behavioral outcomes.
In this study, MA effects observed in newborns across cultures serve as a baseline for understanding the contribution of the postnatal environment [6]. NZ and the US share a common heritage and language with similar levels of technology. MA is illegal in both societies. However, there are important ways in which the societal and legal policies of the countries differ. Unlike NZ, many states in the US, including three of four IDEAL sites, have mandatory reporting of MA use during pregnancy leading to child removal in many cases. NZ has a free or nearly free health care system and provision of consistent need-based financial and housing support to reduce poverty. Health care in the US is limited or not available, particularly for the working poor, and financial resources to mothers are frequently conditional based on time limitations and maternal compliance. In the US, prenatal use of illicit drugs including MA is associated with poverty, less access to health care, and loss of custody after the infant’s birth. The two IDEAL cohorts provide an opportunity to untangle the effects of acute MA exposure from long term insults due to the combination of vulnerable infants and low resource environments.
There are limitations to the current study. First, the sample size for the heavy MA use group in NZ is small, which could underestimate or generate unreliable effects of heavy use contributed by NZ to the analysis. The analyses of the level of use by country interactions are particularly vulnerable. Second, the subtle differences between the exposure groups versus the more robust dose-response effect observed within the MA group suggest that MA exposed neonates are a heterogeneous group. More extensive analyses could potentially identify more affected subgroups of infants. However, due to the low number of positive MA results from NZ, dose-response effects could not be tested across countries. Thus these conclusions may not generalize across cohorts. Third, as the meconium assay for MA biomarkers was most likely to be positive if maternal drug use continued into the third trimester and exceeded once a week [21], prenatal drug use in the first and second trimester is mainly by self report. However, the reported use of other drugs is consistent with national surveillance data. Fourth, although the study involves two countries, the regions of data collection were limited to the Auckland area in NZ and four cities and their environs in the US. Thus, the generalizability may not extend to other ethnic or income groups. Fifth, based on our previous work, we anticipated that MA exposure would have mostly small effects on neurobehavior. We did not correct for the possibility of type I error in planned analyses of the multiple NNNS outcomes. Type I error rejects the null hypothesis when correct (false positives). The cost of minimizing type I error is to reduce the sensitivity of the analysis and increase the risk of type II error in which true effects are missed [44]. Our small to medium effects are consistent with other studies and important to report to the scientific community as well as treatment providers. On the other hand, follow up tests were corrected for multiple comparisons as these were additional analyses of significant main and interaction effects.
In summary, we find clear evidence of the effects of MA exposure on newborn neurobehavior across two cultures in a large, controlled prospective multisite study supporting the generalizability of these effects. It is critical to follow these children to determine potentially long-term effects and to tease out prenatal drug effects from the effects of the postnatal environment.
Acknowledgments
We are very grateful to Vince Smeriglio for his vision and encouragement of IDEAL US and NZ and even more so for his personal support to the careers of all IDEAL investigators.
This study was supported from the National Institute on Drug Abuse (LLL: R01DA021757; BML: R01DA014948) and in part from the Auckland Medical Research Foundation (TW), the National Center on Research Resources (M01RR00426; P20RR11091) and the National Institutes of Health Intramural Research Program (MAH). We thank Carolyn Ho, Jennie Rodgers, Jo Cliffe, and Heather Stewart in NZ and Matt Hinckley, Jean Twomey, Darlene Decesare, Fatima Guerra and Hai Lin in the US for their assistance on this international collaboration as well as the staff at the IDEAL-US sites.
Footnotes
Conflict of interest statement
The authors declare there are no conflicts of interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.National Drug Policy New Zealand . Methamphetamine Action Plan. 2003 http://www.ndp.govt.nz/moh.nsf/indexcm/ndp-publications-methamphetamineactionplan.
- 2.Ministry of Health. Drug Use in New Zealand: Analysis of the 2003 New Zealand Health Behaviours Survey--Drug Use. Ministry of Health; Wellington: 2007. [Google Scholar]
- 3.Substance Abuse and Mental Health Services Administration. Results from the 2008 National Survey on Drug Use and Health: National Findings, (Office of Applied Studies, NSDUH Series H-36, HHHS Publication No. SMA 09-4434) 2009. [Google Scholar]
- 4.United Nations Office on Drugs and Crime. World drug report 2007. New York: United Nations Publications; [accessed 2008 Apr 15]. 2007. Available: www.unodc.org/documents/data-and-analysis/WDR-2007-exsum.pdf. [Google Scholar]
- 5.Acuff-Smith KD, George M, Lorens SA, Vorhees CV. Preliminary evidence for methamphetamine-induced behavioral and ocular effects in rat offspring following exposure during early organogenesis. Psychopharmacology (Berl) 1992;109:255–63. doi: 10.1007/BF02245871. [DOI] [PubMed] [Google Scholar]
- 6.Altshuler SJ. Drug-endangered children need a collaborative community response. Child Welfare. 2005;84:171–90. [PubMed] [Google Scholar]
- 7.Arria AM, Derauf C, Lagasse LL, Grant P, Shah R, Smith L, Haning W, Huestis M, Strauss A, Della Grotta S, et al. Methamphetamine and other substance use during pregnancy: preliminary estimates from the Infant Development, Environment, and Lifestyle (IDEAL) study. Matern Child Health J. 2006;10:293–302. doi: 10.1007/s10995-005-0052-0. [DOI] [PubMed] [Google Scholar]
- 8.Bennett BA, Hyde CE, Pecora JR, Clodfelter JE. Differing neurotoxic potencies of methamphetamine, mazindol, and cocaine in mesencephalic cultures. J Neurochem. 1993;60:1444–52. doi: 10.1111/j.1471-4159.1993.tb03307.x. [DOI] [PubMed] [Google Scholar]
- 9.Billing L, Eriksson M, Larsson G, Zetterstrom R. Amphetamine addiction and pregnancy. III. One year follow-up of the children. Psychosocial and pediatric aspects. Acta Paediatr Scand. 1980;69:675–80. doi: 10.1111/j.1651-2227.1980.tb07342.x. [DOI] [PubMed] [Google Scholar]
- 10.Brazelton TB. Neonatal Behavioral Assessment Scale. JB Lippinicott; Philadelphia, PA: 1984. Editoin Edition. [Google Scholar]
- 11.Chang L, Cloak C, Jiang CS, Farnham S, Tokeshi B, Buchthal S, Hedemark B, Smith LM, Ernst T. Altered neurometabolites and motor integration in children exposed to methamphetamine in utero. Neuroimage. 2009;48:391–7. doi: 10.1016/j.neuroimage.2009.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang L, Smith LM, LoPresti C, Yonekura ML, Kuo J, Walot I, Ernst T. Smaller subcortical volumes and cognitive deficits in children with prenatal methamphetamine exposure. Psychiatry Res. 2004;132:95–106. doi: 10.1016/j.pscychresns.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 13.Cloak CC, Ernst T, Fujii L, Hedemark B, Chang L. Lower diffusion in white matter of children with prenatal methamphetamine exposure. Neurology. 2009;72:2068–75. doi: 10.1212/01.wnl.0000346516.49126.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coyle MG, Ferguson A, Lagasse L, Liu J, Lester B. Neurobehavioral effects of treatment for opiate withdrawal. Arch Dis Child Fetal Neonatal Ed. 2005;90:F73–4. doi: 10.1136/adc.2003.046276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davis PJ, Partridge JW, Storrs CN. Alcohol consumption in pregnancy. How much is safe? Arch Dis Child. 1982;57:940–3. doi: 10.1136/adc.57.12.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Moraes Barros MC, Guinsburg R, de Araujo Peres C, Mitsuhiro S, Chalem E, Laranjeira RR. Exposure to marijuana during pregnancy alters neurobehavior in the early neonatal period. J Pediatr. 2006;149:781–7. doi: 10.1016/j.jpeds.2006.08.046. [DOI] [PubMed] [Google Scholar]
- 17.Della Grotta S, Lagasse LL, Arria AM, Derauf C, Grant P, Smith LM, Shah R, Huestis M, Liu J, Lester BM. Patterns of Methamphetamine Use During Pregnancy: Results from the Infant Development, Environment, and Lifestyle (IDEAL) Study. Matern Child Health J. 2009 doi: 10.1007/s10995-009-0491-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Finnegan LP. Neonatal abstinence syndrome: Assessment and pharmacotherapy. In: Rubatelli FF, Granati B, editors. Neonatal Therapy and Update. Excerpta Medica; New York, NY: 1986. [Google Scholar]
- 19.Fried PA, O’Connell CM. A comparison of the effects of prenatal exposure to tobacco, alcohol, cannabis and caffeine on birth size and subsequent growth. Neurotoxicol Teratol. 1987;9:79–85. doi: 10.1016/0892-0362(87)90082-1. [DOI] [PubMed] [Google Scholar]
- 20.Fuller RW, Hemrick-Luecke SK. Further studies on the long-term depletion of striatal dopamine in iprindole-treated rats by amphetamine. Neuropharmacology. 1982;21:433–8. doi: 10.1016/0028-3908(82)90027-2. [DOI] [PubMed] [Google Scholar]
- 21.Gray TR, LaGasse LL, Smith LM, Derauf C, Grant P, Shah R, Arria AM, Della Grotta SA, Strauss A, Haning WF, et al. Identification of prenatal amphetamines exposure by maternal interview and meconium toxicology in the Infant Development, Environment and Lifestyle (IDEAL) study. Ther Drug Monit. 2009;31:769–75. doi: 10.1097/FTD.0b013e3181bb438e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hans SL. Demographic and psychosocial characteristics of substance-abusing pregnant women. Clin Perinatol. 1999;26:55–74. [PubMed] [Google Scholar]
- 23.Harvey JA. Cocaine effects on the developing brain: current status. Neurosci Biobehav Rev. 2004;27:751–64. doi: 10.1016/j.neubiorev.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 24.Heller A. Neurotoxicology and Developmental Effects of Meth and MDMA: Effects of in Utero Exposure to Methamphetamines. National Institute on Drug Abuse; Bethesda, MD: 2000. [Google Scholar]
- 25.Heller A, Bubula N, Freeney A, Won L. Elevation of fetal dopamine following exposure to methamphetamine in utero. Brain Res Dev Brain Res. 2001;130:139–42. doi: 10.1016/s0165-3806(01)00222-x. [DOI] [PubMed] [Google Scholar]
- 26.Hollingshead AA. Four-factor index of social status. Yale University, Department of Sociology; New Haven, CT: 1975. [Google Scholar]
- 27.Jacobson JL, Jacobson SW, Sokol RJ. Effects of prenatal exposure to alcohol, smoking, and illicit drugs on postpartum somatic growth. Alcohol Clin Exp Res. 1994;18:317–23. doi: 10.1111/j.1530-0277.1994.tb00020.x. [DOI] [PubMed] [Google Scholar]
- 28.Jacobson SW, Jacobson JL, Sokol RJ, Martier SS, Chiodo LM. New evidence for neurobehavioral effects of in utero cocaine exposure. J Pediatr. 1996;129:581–90. doi: 10.1016/s0022-3476(96)70124-5. [DOI] [PubMed] [Google Scholar]
- 29.Jeng W, Wong AW, Ting AKR, Wells PG. Methamphetamine-enhanced embryonic oxidative DNA damage and neurodevelopmental deficits. Free Radic Biol Med. 2005;39:317–26. doi: 10.1016/j.freeradbiomed.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 30.Johnson RE, Jones HE, Jasinski DR, Svikis DS, Haug NA, Jansson LM, Kissin WB, Alpan G, Lantz ME, Cone EJ, et al. Buprenorphine treatment of pregnant opioid--dependent women: maternal and neonatal outcomes. Drug Alcohol Depend. 2001;63:97–103. doi: 10.1016/s0376-8716(00)00194-0. [DOI] [PubMed] [Google Scholar]
- 31.LaGasse L, Seifer R, Wright LL, Lester BM, Tronick EZ, Bauer CR, Shankaran S, Bada HS, Smeriglio VL. The Maternal Lifestyle Study (MLS): The caretaking environment of infants exposed to cocaine/opiates. Pediatr Res. 1999;45:247A. [Google Scholar]
- 32.Law KL, Stroud LR, LaGasse LL, Niaura R, Liu J, Lester BM. Smoking during pregnancy and newborn neurobehavior. Pediatrics. 2003;111:1318–23. doi: 10.1542/peds.111.6.1318. [DOI] [PubMed] [Google Scholar]
- 33.Lester B, Tronick EZ. Appendix 2: National Intensive Care Unit Network Neurobehavior Scale Summary Score Definitions. Pediatrics. 2004;113:694–699. [Google Scholar]
- 34.Lester BM, LaGasse L, Freier K, Brunner S. Studies of cocaine-exposed human infants. NIDA Res Monogr. 1996;164:175–210. [PubMed] [Google Scholar]
- 35.Lester BM, Tronick EZ, Brazelton TB. The Neonatal Intensive Care Unit Network Neurobehavioral Scale procedures. Pediatrics. 2004;113:641–67. [PubMed] [Google Scholar]
- 36.Lester BM, Tronick EZ, LaGasse L, Seifer R, Bauer CR, Shankaran S, Bada HS, Wright LL, Smeriglio VL, Lu J, et al. The Maternal Lifestyle Study: effects of substance exposure during pregnancy on neurodevelopmental outcome in 1-month-old infants. Pediatrics. 2002;110:1182–92. doi: 10.1542/peds.110.6.1182. [DOI] [PubMed] [Google Scholar]
- 37.Liu J, Bann C, Lester B, Tronick E, Das A, LaGasse L, Bauer C, Shankaran S, Bada H. Neonatal neurobehavior predicts medical and behavioral outcome. Pediatrics. 2009;125:e90–8. doi: 10.1542/peds.2009-0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marwick C. NIDA seeking data on effect of fetal exposure to methamphetamine. JAMA. 2000;283:2225–6. [PubMed] [Google Scholar]
- 39.Mayes LC. A behavioral teratogenic model of the impact of prenatal cocaine exposure on arousal regulatory systems. Neurotoxicol Teratol. 2002;24:385–95. doi: 10.1016/s0892-0362(02)00200-3. [DOI] [PubMed] [Google Scholar]
- 40.Mayes LC. Developing brain and in utero cocaine exposure: effects on neural ontogeny. Dev Psychopathol. 1999;11:685–714. doi: 10.1017/s0954579499002278. [DOI] [PubMed] [Google Scholar]
- 41.Mayes LC, Granger RH, Bornstein MH, Zuckerman B. The problem of prenatal cocaine exposure. A rush to judgment. JAMA. 1992;267:406–8. [PubMed] [Google Scholar]
- 42.Napiorkowski B, Lester BM, Freier MC, Brunner S, Dietz L, Nadra A, Oh W. Effects of in utero substance exposure on infant neurobehavior. Pediatrics. 1996;98:71–5. [PubMed] [Google Scholar]
- 43.Pu C, Vorhees CV. Developmental dissociation of methamphetamine-induced depletion of dopaminergic terminals and astrocyte reaction in rat striatum. Brain Res Dev Brain Res. 1993;72:325–8. doi: 10.1016/0165-3806(93)90201-k. [DOI] [PubMed] [Google Scholar]
- 44.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1:43–6. [PubMed] [Google Scholar]
- 45.Sekine Y, Minabe Y, Ouchi Y, Takei N, Iyo M, Nakamura K, Suzuki K, Tsukada H, Okada H, Yoshikawa E, et al. Association of dopamine transporter loss in the orbitofrontal and dorsolateral prefrontal cortices with methamphetamine-related psychiatric symptoms. Am J Psychiatry. 2003;160:1699–701. doi: 10.1176/appi.ajp.160.9.1699. [DOI] [PubMed] [Google Scholar]
- 46.Singer L. MDMA Use During Pregnancy and Early Infant Outcomes. Annual meeting of the XVth biennial International Conference on Infant Studies; Kyoto, Japan. June 19 2006. [Google Scholar]
- 47.Smith LM, Chang L, Yonekura ML, Grob C, Osborn D, Ernst T. Brain proton magnetic resonance spectroscopy in children exposed to methamphetamine in utero. Neurology. 2001;57:255–60. doi: 10.1212/wnl.57.2.255. [DOI] [PubMed] [Google Scholar]
- 48.Smith LM, Lagasse LL, Derauf C, Grant P, Shah R, Arria A, Huestis M, Haning W, Strauss A, Grotta SD, et al. Prenatal methamphetamine use and neonatal neurobehavioral outcome. Neurotoxicol Teratol. 2008;30:20–8. doi: 10.1016/j.ntt.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sowell ER, Leow AD, Bookheimer SY, Smith LM, O’Connor MJ, Kan E, Rosso C, Houston S, Dinov ID, Thompson PM. Differentiating Prenatal Exposure to Methamphetamine and Alcohol versus Alcohol and Not Methamphetamine using Tensor-Based Brain Morphometry and Discriminant Analysis. J Neurosci. 30:3876–85. doi: 10.1523/JNEUROSCI.4967-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stek AM, Fisher BK, Baker RS, Lang U, Tseng CY, Clark KE. Maternal and fetal cardiovascular responses to methamphetamine in the pregnant sheep. Am J Obstet Gynecol. 1993;169:888–97. doi: 10.1016/0002-9378(93)90022-b. [DOI] [PubMed] [Google Scholar]
- 51.Stroud LR, Paster RL, Papandonatos GD, Niaura R, Salisbury AL, Battle C, Lagasse LL, Lester B. Maternal smoking during pregnancy and newborn neurobehavior: effects at 10 to 27 days. J Pediatr. 2009;154:10–6. doi: 10.1016/j.jpeds.2008.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Terplan M, Smith EJ, Kozloski MJ, Pollack HA. Methamphetamine use among pregnant women. Obstet Gynecol. 2009;113:1285–91. doi: 10.1097/AOG.0b013e3181a5ec6f. [DOI] [PubMed] [Google Scholar]
- 53.Volkow ND, Chang L, Wang GJ, Fowler JS, Leonido-Yee M, Franceschi D, Sedler MJ, Gatley SJ, Hitzemann R, Ding YS, et al. Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. Am J Psychiatry. 2001;158:377–82. doi: 10.1176/appi.ajp.158.3.377. [DOI] [PubMed] [Google Scholar]
- 54.Vorhees CV. Whole Animal Approach Effects of in Utero Exposure to Methamphetamines. National Institute on Drug Abuse; Bethesda, MD: 2000. Basic pharmacology, obstetric/gynecologic considerations, and (primarily) ontogenetic effects of methamphetamine (meth) and MDMA. [Google Scholar]
- 55.Westfall PH, Tobias RD, Rom D, Wolfinger RD, Hochberg Y. Multiple Comparisons and Multiple Tests Using the SAS System. Cary, NC: SAS Institute, Inc; 1999. [Google Scholar]
- 56.Wilkins C, Sweetsur P. Trends in population drug use in New Zealand: findings from national household surveying of drug use in 1998, 2001, 2003, and 2006. N Z Med J. 2008;121:61–71. [PubMed] [Google Scholar]
- 57.Wouldes T, LaGasse L, Sheridan J, Lester B. Maternal methamphetamine use during pregnancy and child outcome: what do we know? N Z Med J. 2004;117:U1180. [PubMed] [Google Scholar]
- 58.Zuckerman B, Frank DA. “Crack kids”: not broken. Pediatrics. 1992;89:337–9. [PubMed] [Google Scholar]


