Abstract
Functionalized alginate and PEG polymers were used to generate covalently linked alginate-PEG (XAlgPEG) microbeads of high stability. The cell-compatible Staudinger ligation scheme was used to chemoselectively cross-link phosphine-terminated poly(ethylene glycol) (PEG) to azide-functionalized alginate, resulting in XAlgPEG hydrogels. XAlgPEG microbeads were formed by co-incubation of the two polymers, followed by ionic cross-linking of the alginate using barium ions. The enhanced stability and gel properties of the resulting XAlgPEG microbeads, as well as the compatibility of these polymers for the encapsulation of islets and beta cells lines, were investigated. Our data show that XAlgPEG microbeads exhibit superior resistance to osmotic swelling compared to traditional barium cross-linked alginate (Ba-Alg) beads, with a 5-fold reduction in observed swelling, as well as resistance to dissolution via chelation solution. Diffusion and porosity studies found XAlgPEG beads to exhibit properties comparable to standard Ba-Alg. Our data found XAlgPEG microbeads to be highly cell compatible with insulinoma cell lines, as well as rat and human pancreatic islets, where the viability and functional assessment of cells within XAlgPEG were comparable to Ba-Alg controls. The remarkable improved stability, as well as demonstrated cellular compatibility, of XAlgPEG hydrogels makes them an appealing option for a wide variety of tissue engineering applications.
Keywords: alginate, cross-linking, islet encapsulation, bioartificial pancreas, Staudinger ligation
1. Introduction
Microencapsulation of cells has been an extensively investigated approach for the immunoisolation of transplanted cells, where the goal is to protect foreign cells from the host immune system using biocompatible biomaterials. This technique has been applied to treat a wide variety of diseases, including the encapsulation of dopamine-producing adrenal chormaffin cells for Parkinson’s disease [1] and parathyroid cells for hypocalcemia [2]. Much attention has been directed to microencapsulation of insulin-producing cells for treatment of Type 1 Diabetes Mellitus (T1DM). Multiple studies have shown reversal of diabetes upon the transplantation of microencapsulated islets, illustrating the promise of this technique for retaining graft function in the presence of decreased immunosuppressive therapy [3-8].
Alginate has been the most widely used material for microbead formation. Alginate is a collective term for a family of polysaccharides derived primarily from brown algae [9]. Alginate molecules are linear binary co-polymers of β-D-mannuronic (M) and α-L-guluronic (G) acids, with a variation of sequential arrangement and composition depending on their source [9, 10]. The gelation of alginate beads is commonly achieved via the presence of divalent cations (typically Ca2+ or Ba2+); however, an ionically bonded alginate hydrogel commonly lacks the stability to withstand the mechanical and chemical strains associated with implantation, where microbead degradation and rupture commonly develops due to the slow exchange of cations with sodium ions under physiological conditions [11, 12].
The development of methods for the formation of covalent bonds within the encapsulation polymer is an appealing option for improving microbead stability. Published reports have shown alginate modification, commonly via carboxylic groups on the alginate backbone, with functional groups capable of forming cross-links results in increased stability and reduced swelling of alginate-based hydrogels [13-18]. The introduction of photopolymerizable groups, such as a methacrylate group, onto the alginate chain to permit covalent bond formation via photo-crosslinking is the most common method [15-17, 19-26]. Resulting hydrogels were shown to possess high stability and increased mechanical strength; however, photoinitiator solutions, as well as the free radical generation following initiation of bond formation, can lead to significant cell toxicity, thereby reducing the ease in translation to cell based systems, particularly for cell types inherently sensitive to free radical stress [27-30].
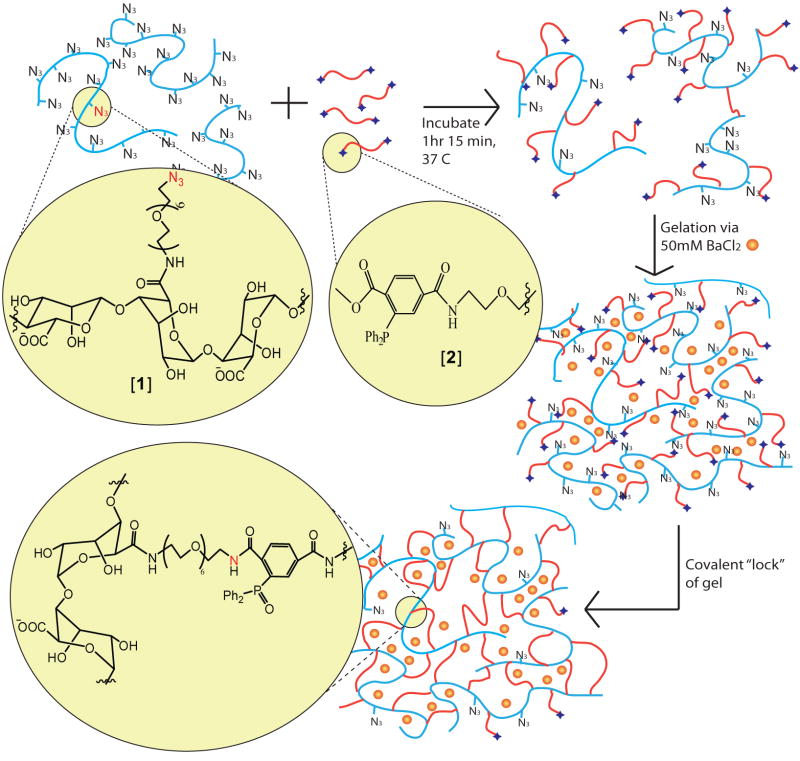
With a focus on the development of covalent ligation schemes with high cell compatibility and chemo-selectivity, we explored the utility of the Staudinger ligation scheme as a novel method for the formation of stable alginate-based hydrogels. Cell compatible, efficient, chemo-selective, and catalyst-free, Staudinger ligation has been used for numerous biological applications, from cell surface modification to protein detection, both in vitro and in vivo [31-35]. The reacting groups include an azide and a phosphine (e.g. triphenylphosphine) moiety with an electrophilic trap. To achieve hydrogel formation via covalent cross-linking, we recently developed an alginate functionalized with azide groups, termed N3-alginate or [1], and a high molecular weight poly(ethylene glycol) (PEG) terminated with complimentary phosphine groups, termed MDT-PEG or [2], as shown in Figure 1. Poly(ethylene glycol) was selected as the cross-linker polymer given its ease of functionalization, demonstrated cell compatibility, and established use in islet encapsulation [36, 37]. In a previous report, we demonstrated the capacity of these polymers to spontaneously form stable hydrogels after several hours [38]. Furthermore, instant gel formation and fabrication of microbeads were attained via incubation with divalent cations. In the latter case, once the azide-functionalized alginate and MDT terminated PEG mixture was fabricated into microbeads via ionic interactions, covalent cross-links are subsequently formed in situ via Staudinger ligation. Therefore, a “gel and lock” system was created, where ionically cross-linked alginate/PEG hydrogels were “locked” into place through the additional formation of interlocking covalent linkages (as illustrated in Figure 1) [38]. These resulting covalently cross-linked hydrogels are termed XAlgPEG.
Figure 1.
Schematic representation of XAlgPEG microbead fabrication. Azide-functionalized alginate polymer, [1], is pre-mixed with MDT PEG polymer, [2] for 1hr 15min prior to cellular incorporation and instantaneous gel formation via subsequent exposure to divalent cations (Ba2+). Additional cross-linking of gel is achieved via Staudinger ligation.
In this study, we sought to evaluate the potential of these XAlgPEG microbeads for cellular encapsulation, specifically for insulin secreting cells. We compared the stability, diffusional properties, and porosity of the resulting gels in comparison to standard barium ion cross-linked alginate, termed Ba-Alg. High guluronic alginate and barium ion cross-linking was selected for comparison, given that this alginate type and gelation ion commonly results in a highly stable ionically linked microbead [39]. The viability and function of insulin secreting cells, both cell lines and islets, within these microbeads were evaluated and compared to standard Ba-Alg microbeads. The significance of this novel alginate-PEG hydrogel in tissue engineering and particularly in the development of a bioartificial pancreas is discussed.
2. Materials and Methods
2.1 General Reagents
Sodium alginate (PRONOVA UP MVG, Mw = 300 kDa, Mw/Mn= 1.87, DPn of 28, Batch # FP-504-03) was purchased from NovaMatrix. The content and distribution of M and G unit were determined by 1H NMR spectroscopy and provided by the manufacturer. The fraction of G units (FG) was 0.6875. The fraction of diads FGG, FMG/GM, and FMM were 0.58, 0.11, and 0.20, respectively. The fraction of triads FGGG, FMGG/GGM, FMGM were 0.54, 0.043, and 0.066, respectively, with a NG>1 of 14.52. The 1-ethyl-(dimethylaminopropyl) carbodiimide hydrochloride (EDC) was from Advanced Chemtech. Other solvents and chemical reagents were purchased from Sigma-Aldrich at the highest purity available.
2.2 Polymer Synthesis
Alginate-PEG-N3, termed N3-Alg or [1], was fabricated as previously described [38]. In brief, 50 mg sodium alginate was dissolved with 14 mg N-hydroxysuccinimide (NHS) and 62 mg 2-(N- morpholino)ethanesulfonic acid in 5 mL de-ionized water. A 200 μL solution of 377 mM N3-PEG- NH2 (Mw 372 g/mol) was added, followed by 116 mg 1-ethyl-(dimethylaminopropyl)carbodiimide hydrochloride (116 mg, 0.60 mmol), 25 min stirring, and 55 μL of 6 M NaOH. Purification was achieved via 4 days dialysis (10,000 MWCO membrane) against 600 mL water, which was changed three times a day. During the first 3 days, NaOH (10 μL of 6 M) and NaCl (400 μL of 4.26 M) were added twice daily to the alginate-containing solution. The remaining solution was filtered through a 0.45 μm membrane and lyophilized.
Di-phosphine Poly(ethylene glycol) (PEG), termed MDT-PEG or [2], was fabricated as previously described [38]. In brief, 0.145 mmol N,N’-Diisopropylcarbodiimide (DIC) was added to a solution of 52.8 mg 1-methyl-2-diphenylphosphino-terephthalate (MDT) [35] and 17.2 mg N-hydroxysuccinimide (NHS) in 1.2 mL anhydrous/Ar-flushed N,N-dimethylformamide (DMF). The following agents were added at the time intervals indicated: 200 mg NH2-PEG-NH2 (Mw 3,513 g/mol) at 30 min and 14.8 mg 4-dimethylaminopyridine at 1 h. After reacting for 3 h under Ar atmosphere, the product was precipitated with 12 mL cold diethyl ether (Et2O) and collected by centrifugation at 0 °C. It was “crystallized” once in 10 mL 200 proof ethanol (warmed to 37 °C to dissolve, cooled in ice-water bath to precipitate, collected precipitate by centrifugation at 0 °C). The precipitate was washed once with 12 mL Et2O at 0 °C and dried under reduced pressure.
Purity and functionalization of both polymer [1] and [2] were assessed using ATR-FT-IR, mass spectroscopy, and 1H-NMR spectroscopy. Results from this assessment are published elsewhere [38]. In brief, the purity of [1] was a minimum of 97 %, with an average azide modification of 11% (percent modification of alginate caboxylate groups) or 149 N3 per alginate mol ratio. The purity of [2] was a minimum of 99 %, with > 97 % conversion of end groups to MDT.
2.3 Cell Culture
MIN6 cells (subclone C3, courtesy of Dr. Valerie Lilla and Alejandra Tomas) [40] were cultured as monolayers in T-flasks and fed every 2-3 days with fresh medium comprised of Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10 % fetal bovine serum (FBS; Sigma), 1 % penicillin-streptomycin (P/S), 1 % L-glutamine and 0.001 % (v/v) β-mercaptoethanol. Rat pancreatic islets were isolated from male Sprague-Dawley (SD) rats using methods described elsewhere [41]. Animal procedures were conducted according to the guidelines of the Committee on Care and Use of Laboratory Animals, Institute of Laboratory Animal Resources (National Research Council, Washington DC). Human pancreatic islets of Langerhans were obtained from the NIH ICR Consortium. Following isolation, islets were cultured in CMRL 1066 (Mediatech) for 48 h (rat islets) or MM1 (Mediatech) for 24 h following arrival (human islets) in a humidified 37 °C, 5 % CO2 / 95 % air incubator prior to encapsulation. CMRL 1066 and MM1 media was supplemented with 10 % fetal bovine serum (FBS; Sigma), 1 % penicillin-streptomycin (Sigma), and 1 % L-glutamine (Sigma).
2.4 Microbead formation and cellular encapsulation
Standard Ba-Alg and XAlgPEG microbeads were fabricated using a modification of the protocol originally developed by Sun [42]. Ba-Alg gels were fabricated using 2.5 % (wt/v) alginate in phosphate-buffered solution (PBS, Mediatech) solution. XAlgPEG gels consisted of 2.5 % (wt/v) Azide-Alg [1] and 4.7 % (wt/v) di-MDT PEG [2]. The solution of [1] and [2] were generated by first dissolving [1] and [2] in PBS in separate vials. Following complete dissolution of the polymers in PBS, the solutions were mixed at 1:1.9 ratio. The solution of [1] and [2] was sterile filtered and gently agitated for 1hr15min prior to microbead fabrication. Microbeads were prepared via needle extrusion (blunt tipped, 21 gauge) of the standard alginate or [1] / [2] solution into a gelling basin of 50 mM BaCl2 and 0.025 % (v/v) Tween 20 solution. The size of the droplets was controlled by a parallel airflow generator (10 kPa pressure of air; 1 inch distance from the needle to gelation solution) and manual force applied to the syringe. The beads were exposed to the gelation solution for 5 min, aspirated, and then rinsed with PBS (150 mL) four times to remove excess barium. The average bead diameter for alginate microbeads was 460±13 μm and 520±17 μm for Ba-Alg and XAlgPEG microbeads, respectively. Microbeads were incubated overnight in either PBS or fully supplemented CMRL 1066 culture media prior to bead swelling and/or chelation assays.
For microbeads containing cells, MIN6 or islet cells were mixed in either polymer solution immediately prior to needle extrusion. MIN6 cells were harvested from monolayer cultures using trypsin-EDTA (Sigma, St. Louis, MO) and suspended in either polymer solution at a density of 1 × 107 cells/mL polymer solution. Viable cell counts were performed via trypan blue (Sigma, St. Louis, MO) exclusion method. Islet cells were pelleted, media removed, and suspended in polymer solution at a 1.5 % (v/v) loading density. Islet cell counts were performed prior to encapsulation using the islet equivalence (IEQ) method. Following the homogeneous distribution of the cells within either the standard alginate or [1] / [2] solutions, the cell/polymer suspension was immediately extruded through the microencapsulation generator and microbeads were fabricated. Following an 8 min exposure to the barium gelation solution and PBS rinse (4 times, 150 mL), the cell-containing microbeads were washed in the appropriate culture media for the cell type. The beads were cultured in non-cell culture treated T75 flasks (Falcon) and rocked on an orbital plate in a humidified 37 °C, 5 % CO2 / 95 % air incubator for 7-8 days.
2.5 Swelling and Chelation Studies
The stability of the microbeads was tested using a dimensional stability assay [43]. Microbeads were tested 24 hrs following fabrication. Ten Ba-Alg beads or XAlgPEG beads (cell-free) were placed in 1 mL of ion free water and observed for 70 min. The stability of the beads was monitored against time by an inverted light microscope and dimensional change was recorded at 10 min intervals. To evaluate the chemical stability of the beads, Ba-Alg and XAlgPEG were exposed to 2 mL of 200 mM EDTA solution over the course of 70 min, to leach out barium ions.
2.6 Glucose diffusivity measurements
Glucose diffusivity was assessed for four different microbead fabrications: I) standard barium cross-linked alginate; II) barium cross-linked azide-alginate [1]; III) XAlgPEG; and IV) XAlgPEG with barium ions removed. D(+)-glucose to a final concentration of 81 mg/mL was added to all polymer solutions prior to fabrication. The MOPS buffer solution used in the diffusion experiments consisted of 10 mM 3-(N-morpholino)propanesulfonic acid (MOPS), 2.68 mM KCl, and NaCl. The concentration of NaCl in the buffer was 137, 62, or 12 mM for buffer with glucose, BaCl2 + glucose, or EDTA + glucose containing buffer, respectively. All solutions used prior to the start of the diffusion experiment contained a final concentration of 81 mg/mL of D(+)-glucose. The pH of all buffers was adjusted to 7.3 with HCl or NaOH. For ease of fabrication and reduction of scale, microbeads were generated using a micro-pipet into 100 mL of 50 mM BaCl2 + glucose MOPS buffer solution and incubated 8 min. For all microbeads, following BaCl2 incubation, beads were washed with glucose supplemented MOPS buffer and kept overnight at 4 °C. A subset of the XAlgPEG microbeads were washed three times, 30 min each, in 50 mM EDTA + glucose MOPS buffer solution to remove all barium cations (microbead group IV). At the start of the diffusion experiment, microbeads (10 beads/sample) were divided into wells of a 12-well plate (n = 3 per group). The glucose-supplemented buffer was removed, solution on surface of beads was removed with kimwipes, and 2 mL of glucose-free MOPS buffer was quickly added, marking time zero. Plates were placed on a plate shaker (250 rpm, Heidolph Titramax 100) for mixing and tests were performed at room temperature. For glucose measurements, 200 μL samples were collected, while 200 μL of glucose-free buffer was added, in order to maintain a constant volume, from each well at intervals of 1 min during 0-10 min, 5 min during 10-40 min, and 10 min during 40-90 min. For each run, a sample was collected after 24 h and compared to the previously collected sample to verify equilibrium. Bead diameters for all microbeads per well were record at the end of the experiment to calculate total microbead volume within each well. Glucose levels in the samples were measured via Cobas 6000 analyzer, Roche. Diffusion coefficients for each sample set was determined by fitting resulting data to models for diffusion release in three-dimensional spheres[44, 45], assuming constant diffusion coefficient, uniform concentration of the solute in solution, and partition coefficient of 1. Experimental data was fit to theoretical models using least squares fit.
2.7 Gel Permeability Study
Microbeads were fabricated using methods identical to that outlined for glucose diffusion experiments, with the exception of glucose supplements in all solutions. Twenty-four hours after microbead fabrication, beads were transferred and submerged into FITC-dextran solutions (0.15 mg/mL) of different average molecular weights. After incubating for 1 h, the beads on glass slides were imaged via scanning confocal microscope (LSM 510, Zeiss, Germany).
2.8 Cellular Viability Assessment
Visual assessment of cell viability was achieved via live/dead staining of cells within microcapsules. Cells cultured in microbeads were stained with 1-4 μM Calcein AM (green dye) and 2 μM EthD-1 (red dye) (Live/Dead Invitrogen) in DPBS for 45 min prior to observation using fluorescent scanning confocal microscope (LSM 510, Zeiss, Germany). For MIN6, cross-sections images were collected. For islets, three-dimensional (3D) images were reconstructed from 7 – 20 μm slices using the projection function on LSM image browser (Zeiss, Germany) software.
For quantitative assessment of metabolic activity within the microbeads, CellTiter 96 MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide) assay (Promega, Madison, WI) was used. One hundred microbeads from each group were collected, washed twice in the appropriate media, and conditioned in medium with 0.5 mg/ml MTT (Promega) for 1 h 15 min at 37 °C. Beads were then collected, crushed using microvortex, the stop/solubilization solution was added, and the beads were incubated overnight within humidified 37 °C, 5 % CO2 / 95 % air incubator to completely dissolve the formazan crystals. From each well, triplicates of 120 μL of solution were transferred to a 96-well plate and the optical densities were measured at 570 nm in a microplate reader (Molecular Devices). For all cells, Day 0 measurements were made prior to encapsulation. For islets, Day 0 measurements were assessed on 150 islets prior to encapsulation.
2.9 Measurement of Insulin Secretion
Functional assessment of encapsulated islets was tested via static glucose-stimulated insulin release (GSIR) assay. Ten islet encapsulated microbeads were incubated within cell inserts (Millipore) in triplicates in a 24-well plate with a series of different glucose concentrations, as described elsewhere [46]. Briefly, microbeads were incubated for 1 h in a basal glucose solution, 2.8 mM glucose buffered in Krebs-bicarbonate buffer (KRBB: 99 mM NaCl2, 5 mM KCl, 1.2 mM KH2PO4, 1.2 mM MgSO4, 2.6 mM CaCl2, 26 mM NaHCO3, and 0.2 wt% BSA), following by washing with glucose basal solution. The inserts were then transferred to a new well in the 24-well plate and incubated for 1 h in 800 μl of the 2.8 mM basal glucose solution, labeled as Basal 1. After 1 h incubation, the inserts were completely drained, and placed in a 200 mM stimulated glucose solution for 1 h, labeled as Stimulated 1. After an additional wash step, the islets were then placed in 2.8 mM basal glucose solution for an additional hour, labeled as Basal 2. Samples were collected at the end of each incubation period and frozen until ELISA (Rat or Human kits, Mercodia) analysis. Insulin concentration within the samples was quantified by following the manufacturer’s instructions. GSIR results were expressed as insulin stimulation index (SI), which is the ratio of insulin released after during Stimulated 1 to the level of insulin released during Basal 1.
2.10 Statistical Analysis
All statistical analysis performed throughout this study utilized a paired student t-test with significant differences represented by a p-value less than 0.05.
3. Results
3.1 XAlgPEG microbead fabrication
In this work, ionic and covalent in situ cross-linking of alginate/PEG polymers resulted in a stable hydrogel capable of forming microbeads. Stable microbeads were fabricated using N3-alginate [1] and MDT-PEG [2] polymers. To prevent leakage of MDT-PEG during barium cross-linking of the N3-Alginate, the two polymers were pre-incubated in solution for 1 hr 15 min. This pre-incubation period permitted the initiation of covalent linkages between N3-alginate and MDT-PEG (see Figure 1). Shorter incubation times resulted in significant leakage of MDT-PEG into the barium gelation solution and resulted in less stable XAlgPEG beads (data not shown). Longer incubation times resulted in significant increases in the solution viscosity, thereby restricting the ability to form microbeads via microneedle extrusion. A ratio of 1:1.9 w/w of N3-Alginate to MDT-PEG (ratio of N3 to MDT groups of 0.54) was found to be optimal, based on extensive preliminary screening of resulting microbeads. Lowering ratio of N3-Alginate to MDT-PEG resulted in decreased cross-linking within the beads and less resistance to swelling during chelation experiments, partially due to the diffusion of MDT-PEG from the beads following barium ion gelation. Increasing the ratio of N3-Alginate to MDT-PEG did not result in enhanced stability of the microbeads, based on our experimental study, primarily due to phase separation of the two polymers at higher ratios.
3.2 Swelling and chelation of cross-linked microbeads
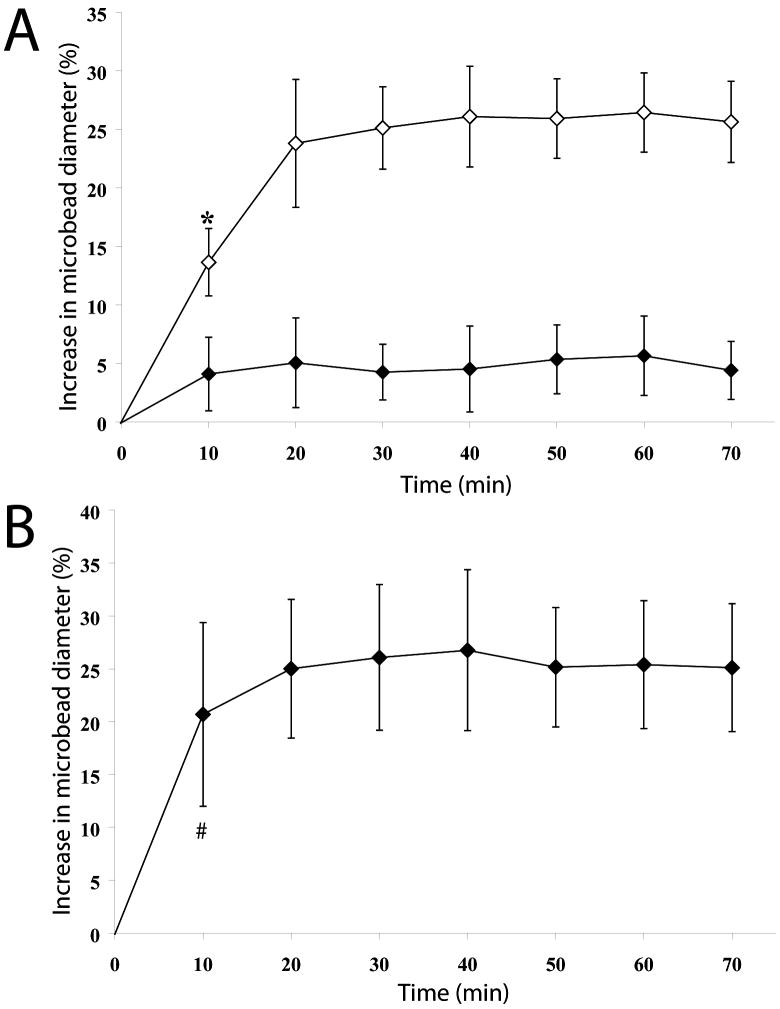
Osmotic swelling of the microbeads following incubation in ion free water was evaluated by observing dimensional changes of the beads, as shown in Figure 2A. Traditional Ba-Alg beads were used as controls. If polymers N3-Alginate and MDT-PEG were able to form covalent linkages following barium gelation, it was expected that the XAlgPEG beads would exhibit a significant decrease in swelling when compared to the Ba-Alg beads. Ba-Alg beads swelled to a maximum of 24 % in bead diameter within the first twenty minutes before stabilizing, which is in correlation with published literature reports for barium cross-linked, high guluronic acid alginate microbeads [39, 43]. In contrast, XAlgPEG microbeads exhibited a maximum 5% increase in bead diameter. A statistically significant decrease in swelling was found for XAlgPEG microbeads when compared to the standard Ba-Alginate beads, after 10 min in ion free water. Control beads fabricated using identical methods as the XAlgPEG, with the exception of using a methyl terminated PEG polymer (without phosphine functionalized groups), exhibited swelling behavior statistically identical to Ba-Alg controls (data not shown). Swelling studies performed using microbeads incubated overnight in either fully supplemented media or PBS following microbead formation were statistically identical (only PBS data shown).
Figure 2.
(A) Comparison of osmotic swelling of Ba-Alg (open diamonds) and XAlgPEG (solid diamonds) microbeads when incubated within ion free water, as expressed by percentage increase in microbead diameter as a function of time. Data points ± SD represent the average of five independent measurements. (B) Stability of XAlgPEG microbeads (solid diamonds) in a 200mM EDTA solution as expressed by percentage increase in microbead diameter as a function of time. Data points ± SD represent the average of five independent measurements. *Significant differences (p<0.05) between XAlgPEG and Ba-Alg microbeads. #Measurement of Ba-Alg microbeads was not feasible due to dissolution of gel 2 min after EDTA addition.
Verification of covalent bond formation between N3-Alginate and MDT-PEG was attained by removal of the barium ions using a concentrated chelating solution. Microbead stability following ion removal was evaluated by observing diameter changes for XAlgPEG beads, as shown in Figure 2B. XAlgPEG microbeads swelled to a maximum of 25 % increase in bead diameter after 20 min incubation in the chelation solution. In contrast, complete dissolution of the Ba-Alg beads was observed within 2 min following EDTA exposure. Chelation studies performed using microbeads incubated overnight in either fully supplemented media or PBS following microbead formation were statistically identical (only PBS data shown). These studies confirm the ability of the N3-Alginate and MDT-PEG polymers for form covalent cross-links following barium ion gelation, even in the presence of full culture media.
3.3 Effective diffusivity and permeability assessment of cross-linked microbeads
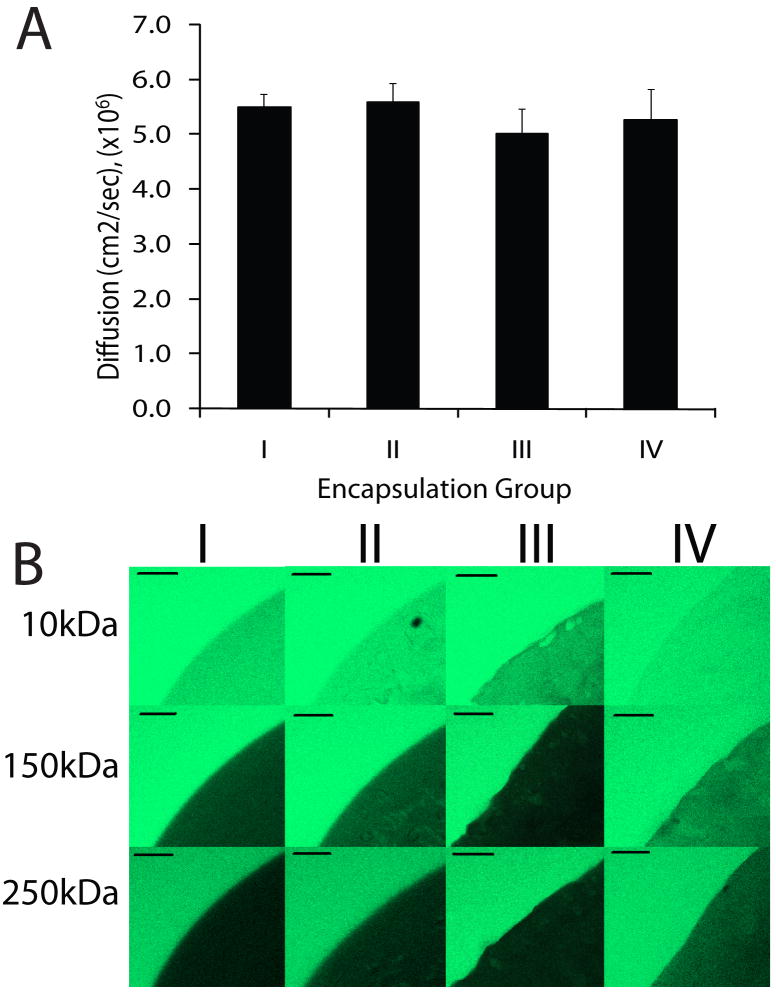
To evaluate the effects of the cross-linking within the XAlgPEG microbeads on solute diffusion, the effective diffusivity of glucose out of the microbeads was quantified and compared to Ba-Alg. Four microbead groups were fabricated (as outlined in Methods section): I) standard barium cross-linked alginate; II) barium cross-linked N3-alginate [1]; III) XAlgPEG; and IV) XAlgPEG with barium ions removed. The effective diffusion of glucose for each microbead set was calculated by fitting resulting data to models for diffusion release in three-dimensional spheres [44]. The bathing solution remained constant during the glucose experiments due to replacement with blank buffer. Therefore, glucose concentrations were corrected at each experimental data point during the analysis to account for these effects. Bead diameters were measured for each group and accounted for within the theoretical results. The resulting effective diffusion of glucose out of the microbeads is shown in Figure 3A. Effective diffusion values varied from 5.00×10-6 to 5.58×10-6 cm2/sec, with no statistical difference observed between any of the microbead formulations tested. These values are comparable with published reports for 2-3 % high guluronic alginate microbeads [47-50]. In comparison with the glucose diffusion of water at 22 °C (6.13×10-6 cm2/sec) [47, 51], the glucose diffusion out of the various alginate formulations was: 90±4 % for I, 91±6 % for II, 82±8 % for III, and 86±10 % for VI.
Figure 3.
Diffusion and permeability assessment of Ba2+ cross-linked alginate microbeads (I), Ba2+ cross-linked azide-functionalized alginate microbeads (II), XAlgPEG microbeads (III), and XAlgPEG microbeads after exposure to EDTA (IV). (A) Values of effective diffusion of glucose out of microbeads. Error bars ± standard deviation from the average (n = 3). (B) Representative confocal images of microbeads (gelation groups I, II, III and IV) incubated in 0.15 mg/mL solution of FITC-dextran of molecular weights varying from 10 to 250kDa (as indicated at left) for 1 hr prior to imaging. Scale bar = 100 μm
The diffusion of fluorescein isothiocyanate (FITC)-labeled dextrans of different average molecular weights into beads was investigated to evaluate the effects of cross-linking on the permeability of the resulting microbeads. All four microbeads groups fabricated were tested: I) standard barium cross-linked alginate; II) barium cross-linked N3-alginate [1]; III) XAlgPEG; and IV) XAlgPEG with barium ions removed. Figure 3B shows representative confocal microscope cross-sections of microbeads, illustrating the permeability of FITC-dextran solutions at the various molecular weights tested. Images illustrate high permeability of 10 kDa FITC-dextran for all groups. The permeability of 150 kDa FITC-dextran was significantly decreased for all gels. The fluorescence within the group IV gels was qualitatively observed to be higher in comparison to the other groups. This increased permeability could be attributed to the increased swelling of the XAlgPEG gels after removal of Ba2+ from the hydrogel network (see Figure 2B). A slight decrease in the permeability of dextran is observed for group III beads, likely due to the presence of both barium and covalent cross-links within this gel. Negligible fluorescence was detected within all beads submerged in the 250 kDa FITC-dextran solutions. Observationally, it appears the functionalization of the alginate and the covalent cross-linking formed within the beads did not significantly alter hydrogel permeability.
3.4 Growth/viability of cells in alginate-PEG beads
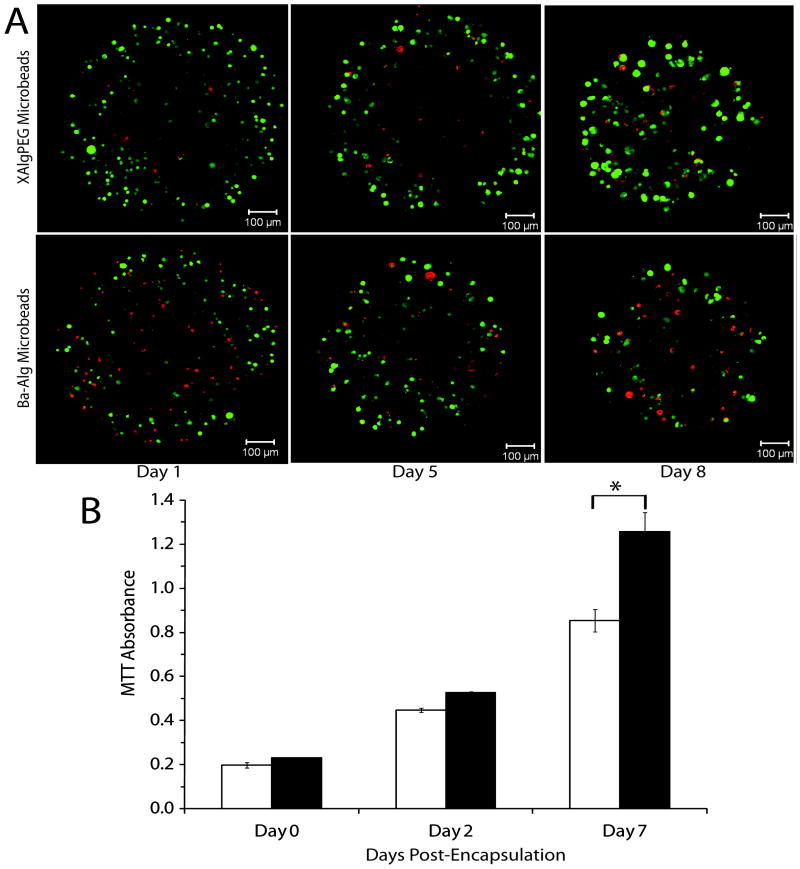
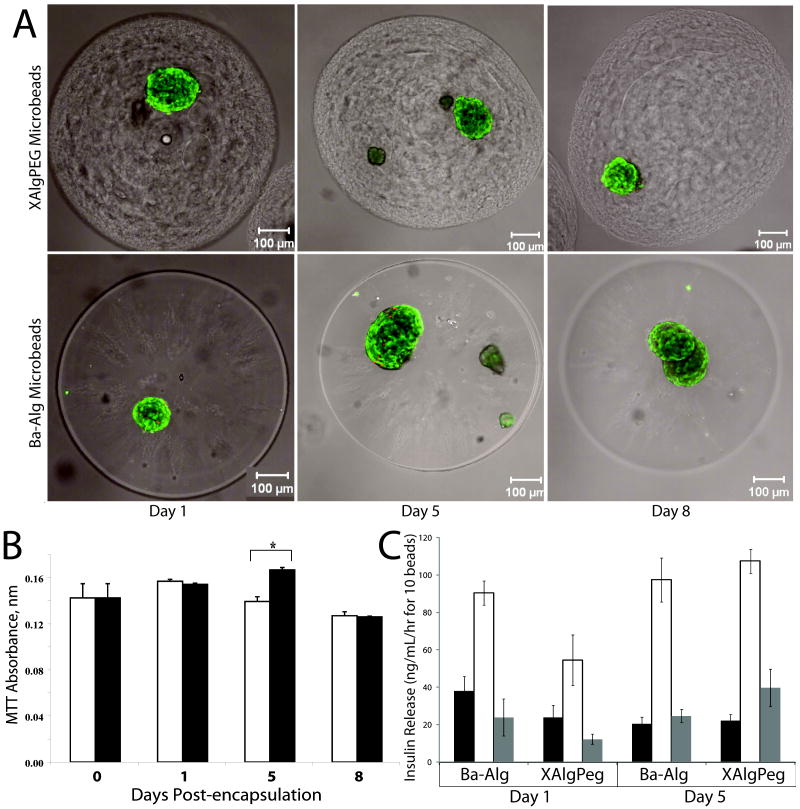
The cellular response following XAlgPEG encapsulation was evaluated and compared to Ba-Alg microbeads. Initial experiments evaluated the growth and viability of MIN6 cells, a beta cell line, following microencapsulation over the course of 7 days. Figure 4A illustrates microbead cross sections of MIN6 cells stained using the live/dead assay, where live cells and dead cells fluoresce green and red, respectively. Both microbead formulations show a high percentage of viable cells, as well as an increase in the size of the cell clusters over time, indicative of growth in culture. Cellular metabolic activity was quantified by measuring mitochondrial metabolic activity of the encapsulated MIN6 cells using the MTT assay. Figure 4B illustrates the changes in absorbance readings in comparison to Day 0 values. An significant increase in metabolic activity was observed for cells within both XAlgPEG and Ba-Alg beads, with a 2.38 and 1.91 fold increase in absorbance from Day 2 to Day 7, respectively, indicating an increase in cell number over the culture period. At Day 7, there was a statistically significant difference (p < 0.05) in the metabolic activity of cells within the XAlgPEG microbeads when compared to Ba-Alg.
Figure 4.
(A) Representative confocal images, single cross-sections, of encapsulated MIN6 cells stained using Live/Dead fluorescent kit where green and red fluorescence indicates live or dead cells, respectively. (B) MTT absorbance of MIN6 cells encapsulated within Ba-Alg (open bars) or XAlgPEG (solid bars) microbeads. Day 0 measurements were made prior to encapuslation. Data points represent the mean ± SD, for three independent assessments. *Significant difference (p<0.05).
Lewis rat islets were adequately encapsulated by the two gel solutions with ~1-2 islets per bead, as illustrated by the representative confocal images (Figure 5A). Confocal images of live/dead stained cells illustrate high percentage of viable cells for all observed time points and polymeric configurations, with no discernable difference in cell viability observed. Metabolic activity of rodent islets was quantified using MTT assay, where absorbance readings found negligible changes in viability over time and between groups, as shown in Figure 5B. Assessment of insulin stimulation index during glucose stimulated insulin secretion (GSIS) was found to be consistent throughout the culture time period. As illustrated in Figure 5C, both alginate types exhibit glucose responsiveness, where Stimulated levels were significantly higher than Basal 1 and Basal 2 levels are significantly lower than Stimulated. The index of insulin stimulation from Basal 1 to Stimulated glucose levels for rat islets was 5.41 and 7.56 ± 1.50 on Day 1 and 7.24 ±1.25 and 8.56 ± 0.41 on Day 5 for Ba-Alg and XAlgPEG gels, respectively. No statistically significant differences were found for MTT cell viability or insulin stimulation between polymer groups.
Figure 5.
(A) Representative confocal images, 7 slice projections, of encapsulated Lewis rat islets stained using Live/Dead fluorescent kit where green and red fluorescence indicates live or dead cells, respectively. (B) Changes in MTT absorbance readings as a measurement of islet viability following encapsulation with Ba-Alg (open bars) or XAlgPEG (solid bars). Day 0 assessment was performed prior to encapsulation. (C) Glucose stimulated insulin secretion results, measured as insulin release, on Day 1 and Day 5 for Lewis rat islets following encapsulation in Ba-Alg or XAlgPEG (as indicated on horizontal axis). Insulin release following 1 hr incubation in Basal 1 (solid bars), Stimulated (empty bars), and Basal 2 (grey bars) solutions is shown. Data points represent the mean ± SD, for a minimum of three independent measurements at each time point (except the Day 1 for Insulin measurements where Ba-Alg were only duplicates).
Human pancreatic islets were adequately encapsulated by the two solutions with ~1-2 islets per bead, as shown by the confocal images in Figure 6A. Confocal microscopy of live/dead stained cells exhibited highly viable islets, with no discernable difference in observed viability between polymer groups. Human islets entrapped by XAlgPEG and Ba-Alg polymers exhibited comparable metabolic activity following encapsulation, as calculated by MTT (Figure 6B). With the exception of Day 5 when a statistically significant decrease in absorbance was recorded for XAlgPEG beads, the metabolic activity between groups was comparable. Figure 6C illustrates glucose-stimulated insulin release for encapsulated human islets. Insulin release during glucose changes followed the expected pattern, with significant increase in release from Basal 1 to Stimulated, followed by a return to basal levels during Basal 2. The insulin stimulation index (SI) for the encapsulated human islets was 2.36 and 2.29 for XAlgPEG and Alginate-Barium on Day 1, and 4.16 and 4.57 for XAlgPEG and Ba-Alg polymers on Day 5, respectively. While the insulin stimulation index on Day 1 was low, there were no statistical differences observed between the polymeric configurations.
Figure 6.
(A) Representative confocal images, 7 slice projections, of encapsulated human islets stained using Live/Dead fluorescent kit where green and red fluorescence indicates live or dead cells, respectively. (B) Changes in MTT absorbance readings as a measurement of islet viability following encapsulation with Ba-Alg (open bars) or XAlgPEG (solid bars). Day 0 assessment was performed prior to encapsulation. (C) Glucose stimulated insulin secretion results, measured as insulin release, on Day 1 and Day 5 for human islets following encapsulation in Ba-Alg or XAlgPEG (as indicated on horizontal axis). Insulin release following 1 hr incubation in Basal 1 (solid bars), Stimulated (empty bars), and Basal 2 (grey bars) solutions is shown. Data points represent the mean ± SD, for a minimum of three independent measurements at each time point.
4. Discussion
In this study, the potential of a novel, covalently cross-linked alginate and poly(ethylene glycol) hydrogel for use in cellular microencapsulation was evaluated. Covalent crosslinking within the microcapsule was achieved by modification of the carboxylic groups, residing on the alginate chain, with azides; the azides were bound, with a stable amide linkage, to MDT-PEG polymers via the Staudinger ligation. Co-incubation of these two polymers (N3-Alginate and MDT-PEG) results in stable gel formation, independent of ionic interactions, within hours. While this covalent reaction is efficient when compared to alternative, catalyst-free ligation schemes, it is recognized that this delay might not be desirable for cellular encapsulation. To bypass this potential problem, we capitalized on the unique ability of the functionalized alginate polymer, N3-Alginate, to form gels via both ionic and covalent interactions. The superiority of utilizing this method is the ability to instantaneously form a gel using standard ionic methods, followed by the “locking” of the gel form via the formation of covalent linkages between the N3-Alginate and the MDT-PEG over time. Furthermore, the Staudinger ligation method is of particular advantage for cellular encapsulation in that the chemoselectivity of the reaction allows it to spontaneously occur in full media. This is in stark contrast with conventional techniques that commonly require initiators or catalysts, pH or temperature changes, and/or extended incubation in protein-free buffers [16, 25, 26, 52]. Protocol optimization of bead formulations was performed by promoting covalent linkages between alginate and PEG polymers via incubation prior to capsule formation via cationic solution.
The overall stability of the microbeads was improved with the implementation of covalent linkages. The osmotic swelling of the XAlgPEG beads was significantly less than traditional barium cross-linked alginate beads. Minimization of swelling is critically important for cell encapsulation, where hydrogel stability may impose less stress on the encapsulated cells, resulting in improved graft function and viability [53]. The incorporation of covalent linkages within the capsule also prevents the slow degradation of capsule integrity during the inevitable leakage of divalent cations from the alginate hydrogel. XAlgPEG covalently linked beads remained completely intact, exhibiting exceptional stability, upon exposure to a strong chelating solution, while the ionically bound beads showed complete dissolution within a few minutes. These results establish not only the formation of covalent linkages between the N3-Alginate and MDT-PEG polymers, but also show that these linkages are of adequate density to maintain the integrity of the hydrogel. Assessment of the effective diffusion coefficient and permeability of the microbeads indicates insignificant effects of the cross-linking scheme on the resulting hydrogel characteristics. While a moderate decrease in permeability may be inferred from dextran studies, no statistical difference in glucose diffusion and insulin release (via GSIR for rat and human islets) was observed. Therefore, through the ligation scheme utilized, we have developed mechanically superior beads, while retaining the desirable diffusional and permeability characteristics of alginate hydrogels.
Detrimental effects on cellular viability or function with the introduction of covalent interactions was not evident. Cells entrapped within the gel matrix retained their viability and function when in culture. The proliferating MIN6 cells were able to grow within the matrix, as demonstrated by the MTT results. Islets encapsulated in the XAlgPEG beads were comparable to the standard microbeads, as evident in viability and functional assessment assays.
Overall, our data indicate that the highly chemoselective ligation scheme used for the covalent linkage of these two polymers results in minimum to no detrimental side effects on cellular function and viability. This is in contrast to previously developed chemo-ligation schemes, such as photopolymerization, which commonly exhibit problems in cellular cytotoxicity due to the photoinitiating solutions and the generation of free radicals and high temperatures [27-30]. Furthermore, removal of residual catalysts and/or by-products of the reaction are not required. For instance, photoinitiated cross-linked alginate microbeads resistant to saline swelling and EDTA dissolution were recently developed by Rokstad et al [15]. While these microbeads exhibited similar properties to those reported here, extensive cell death was initially observed following encapsulation and photoinitated cross-linking. Avoiding cell toxicity required a 50% reduction in the molar concentration of the photoinitating agents and the use of decreased temperatures, thereby illustrating the need for care in the use of these agents for cellular encapsulation. Cu(I)-catalyzed and strain-promoted azide-alkyne cycloaddition reactions, commonly grouped as “click” reactions, are emerging as promising alternatives to Staudinger ligation for the fabrication of cross-linked gels, given their increased reaction efficiency [54-56]. The use of copper catalyzed reactions for the encapsulation of cells, however, has limited feasibility due to toxicity. A recent report on the use of copper-free click chemistry for three-dimensional cellular encapsulation illustrates the future applications of this chemo-selective, catalyst-free scheme [57]. In addition, native chemical ligation (NCL) has been recently used for hydrogel formation and cellular encapsulation, with promising results [58, 59]. These studies illustrate the potential of chemoselective schemes to not only provide greater control over gelation properties, but also permit selective presentation of bioactive agents, such as cytokine-inhibitory peptides [59]. Overall, the simplicity and chemoselectivity of the ligation scheme described in this study highlights its tremendous potential not only in the area of diabetes, but for a broad range of tissue engineering applications.
5. Conclusions
In this study, we have engineered polymers with the capacity to form spontaneous, covalent linkages that are highly cell compatible. To our knowledge, this is the first report of the use of polymers capable of cross-linking via Staudinger ligation for cellular encapsulation. The resulting microbeads, formed via a combination of ionic and covalent interactions, exhibit significant enhancement in hydrogel stability compared to standard alginate controls. These cross-linked microbeads retain diffusional and permeability properties similar to standard barium cross-linked alginate and exhibit undetectable cytotoxicity. The novel cross-linked alginate/PEG hydrogel developed in this study can find applications not only in the area of bioartificial pancreas development, but in broad biomedical applications from tissue engineering to drug delivery.
Acknowledgments
This research was supported by the Diabetes Research Institute Foundation (www.diabetesresearch.org), the Juvenile Diabetes Research Center for Islet Transplantation at the University of Miami – Diabetes Research Institute (4-2004-361), the Department of Defense Somatic Cell Processing Facility at the DRI (N00244-07-C-1529), and the National Institutes of Health through the Type 1 Diabetes Pathfinder Award Program (1DP2 DK08309601).
We thank the NIDDK- and JDRF-supported Islet Cell Resource Center for providing the majority of the human islets used in these studies. We thank the Diabetes Research Institute Preclinical and Translational Models Core for providing the rodent islets used in this study. We thank Dr. Alessia Fornoni for her assistance in MIN6 culture protocols. We also thank Drs Antonello Pileggi and Fotios Andreopoulos for their helpful advice and comments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shoichet MS, Winn SR. Cell delivery to the central nervous system. Adv Drug Deliv Rev. 2000;42:81. doi: 10.1016/s0169-409x(00)00055-7. [DOI] [PubMed] [Google Scholar]
- 2.Hasse C, Bohrer T, Barth P, Stinner B, Cohen R, Cramer H, Zimmermann U, Rothmund M. Parathyroid xenotransplantation without immunosuppression in experimental hypoparathyroidism: long-term in vivo function following microencapsulation with a clinically suitable alginate. World J Surg. 2000;24:1361. doi: 10.1007/s002680010225. [DOI] [PubMed] [Google Scholar]
- 3.Lanza R, Ecker D, Kuhtreiber W, Marsh J, Ringeling J, Chick W. Transplantation of islets using microencapsulation: studies in diabetic rodents and dogs. J Mol Med. 1999;77:206. doi: 10.1007/s001090050337. [DOI] [PubMed] [Google Scholar]
- 4.Lanza RP, Chick WL. Transplantation of encapsulated cells and tissues. Surgery. 1997;121:1. doi: 10.1016/s0039-6060(97)90175-6. [DOI] [PubMed] [Google Scholar]
- 5.Soon-Shiong P, Feldman E, Nelson R, Heintz T, Yao Q, Yao Z. Long-term reversal of diabetes by the injection of immunoprotected islets. Proc Natl Acad Sci. 1993;90:5843. doi: 10.1073/pnas.90.12.5843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soon-Shiong P, Heintz RE, Merideth N, Yao QX, Yao Z, Zheng T, Murphy M, Moloney MK, Schmehl M, Harris M, et al. Insulin independence in a type 1 diabetic patient after encapsulated islet transplantation. Lancet. 1994;343:950. doi: 10.1016/s0140-6736(94)90067-1. [DOI] [PubMed] [Google Scholar]
- 7.Thanos CG, Elliott RB. Encapsulated porcine islet transplantation: an evolving therapy for the treatment of type I diabetes. Expert Opin Biol Ther. 2009;9:29. doi: 10.1517/14712590802630666. [DOI] [PubMed] [Google Scholar]
- 8.Elliott RB, Escobar L, Tan PL, Muzina M, Zwain S, Buchanan C. Live encapsulated porcine islets from a type 1 diabetic patient 9.5 yr after xenotransplantation. Xenotransplantation. 2007;14:157. doi: 10.1111/j.1399-3089.2007.00384.x. [DOI] [PubMed] [Google Scholar]
- 9.McDowell RH. Properties of Alginates. London: Alginate Industries Ltd; 1977. [Google Scholar]
- 10.Haug A, Larsen B, Smidsrød O. Uronic acid sequence in alginate from different sources. Carbohydrate Research. 1974;32:217. [Google Scholar]
- 11.Thu B, Bruheim P, Espevik T, Smidsrod O, Soon-Shiong P, Skjak-Braek G. Alginate polycation microcapsules. II. Some functional properties. Biomaterials. 1996;17:1069. doi: 10.1016/0142-9612(96)85907-2. [DOI] [PubMed] [Google Scholar]
- 12.Benson JP, Papas KK, Constantinidis I, Sambanis A. Towards the development of a bioartificial pancreas: effects of poly-L-lysine on alginate beads with BTC3 cells. Cell Transplant. 1997;6:395. doi: 10.1177/096368979700600406. [DOI] [PubMed] [Google Scholar]
- 13.Kuo CK, Ma PX. Ionically crosslinked alginate hydrogels as scaffolds for tissue engineering: part 1. Structure, gelation rate and mechanical properties. Biomaterials. 2001;22:511. doi: 10.1016/s0142-9612(00)00201-5. [DOI] [PubMed] [Google Scholar]
- 14.Chandy T, Mooradian DL, Rao GH. Evaluation of modified alginate-chitosan-polyethylene glycol microcapsules for cell encapsulation. Artif Organs. 1999;23:894. doi: 10.1046/j.1525-1594.1999.06244.x. [DOI] [PubMed] [Google Scholar]
- 15.Rokstad AM, Donati I, Borgogna M, Oberholzer J, Strand BL, Espevik T, Skjak-Braek G. Cell-compatible covalently reinforced beads obtained from a chemoenzymatically engineered alginate. Biomaterials. 2006;27:4726. doi: 10.1016/j.biomaterials.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 16.Jeon O, Bouhadir KH, Mansour JM, Alsberg E. Photocrosslinked alginate hydrogels with tunable biodegradation rates and mechanical properties. Biomaterials. 2009;30:2724. doi: 10.1016/j.biomaterials.2009.01.034. [DOI] [PubMed] [Google Scholar]
- 17.Dusseault J, Leblond FA, Robitaille R, Jourdan G, Tessier J, Menard M, Henley N, Halle JP. Microencapsulation of living cells in semi-permeable membranes with covalently cross-linked layers. Biomaterials. 2005;26:1515. doi: 10.1016/j.biomaterials.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 18.Sakai S, Kawakami K. Synthesis and characterization of both ionically and enzymatically cross-linkable alginate. Acta Biomaterialia. 2007;3:495. doi: 10.1016/j.actbio.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 19.Lu MZ, Lan HL, Wang FF, Chang SJ, Wang YJ. Cell encapsulation with alginate and alpha-phenoxycinnamylidene-acetylated poly(allylamine) Biotechnol Bioeng. 2000;70:479. doi: 10.1002/1097-0290(20001205)70:5<479::aid-bit1>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 20.Desai NP, Sojomihardjo A, Yao Z, Ron N, Soon-Shiong P. Interpenetrating polymer networks of alginate and polyethylene glycol for encapsulation of islets of Langerhans. J Microencapsul. 2000;17:677. doi: 10.1080/02652040050161675. [DOI] [PubMed] [Google Scholar]
- 21.Chen H, Ouyang W, Jones M, Metz T, Martoni C, Haque T, Cohen R, Lawuyi B, Prakash S. Preparation and characterization of novel polymeric microcapsules for live cell encapsulation and therapy. Cell Biochem Biophys. 2007;47:159. doi: 10.1385/cbb:47:1:159. [DOI] [PubMed] [Google Scholar]
- 22.Breguet V, Gugerli R, Pernetti M, von Stockar U, Marison IW. Formation of microcapsules from polyelectrolyte and covalent interactions. Langmuir. 2005;21:9764. doi: 10.1021/la0512796. [DOI] [PubMed] [Google Scholar]
- 23.Wang MS, Childs RF, Chang PL. A novel method to enhance the stability of alginate-poly-L-lysine-alginate microcapsules. J Biomater Sci Polym Ed. 2005;16:91. doi: 10.1163/1568562052843302. [DOI] [PubMed] [Google Scholar]
- 24.Birnbaum S, Pendleton R, Larsson P-O, Mosbach K. Covalent stabilization of alginate gel for the entrapment of living whole cells. Biotechnol Letters. 1981;3:393. [Google Scholar]
- 25.Eiselt P, Lee KY, Mooney DJ. Rigidity of Two-Component Hydrogels Prepared from Alginate and Poly(ethylene glycol) - Diamines. Macromolecules. 1999;32:5561. [Google Scholar]
- 26.Lee KY, Rowley JA, Eiselt P, Moy EM, Bouhadir KH, Mooney DJ. Controlling Mechanical and Swelling Properties of Alginate Hydrogels Independently by Cross-Linker Type and Cross-Linking Density. Macromolecules. 2000;33:4291. [Google Scholar]
- 27.Cruise GM, Hegre OD, Scharp DS, Hubbell JA. A sensitivity study of the key parameters in the interfacial photopolymerization of poly(ethylene glycol) diacrylate upon porcine islets. Biotechnol Bioeng. 1998;57:655. doi: 10.1002/(sici)1097-0290(19980320)57:6<655::aid-bit3>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 28.Williams CG, Malik AN, Kim TK, Manson PN, Elisseeff JH. Variable cytocompatibility of six cell lines with photoinitiators used for polymerizing hydrogels and cell encapsulation. Biomaterials. 2005;26:1211. doi: 10.1016/j.biomaterials.2004.04.024. [DOI] [PubMed] [Google Scholar]
- 29.Hu X, Gao C. Photoinitiating polymerization to prepare biocompatible chitosan hydrogels. J Ap Poly Sci. 2008;110:1059. [Google Scholar]
- 30.Wilson JT, Chaikof EL. Challenges and emerging technologies in the immunoisolation of cells and tissues. Adv Drug Deliv Rev. 2008;60:124. doi: 10.1016/j.addr.2007.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kiick K, Saxon E, Tirrell D, Bertozzi C. Incorporation of azides into recombinant proteins for chemoselective modification by the Staudinger ligation. PNAS. 2002;99:19. doi: 10.1073/pnas.012583299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prescher J, Dube D, Bertozzi C. Chemical remodeling of cell surfaces in living animals. Nature. 2004;430:873. doi: 10.1038/nature02791. [DOI] [PubMed] [Google Scholar]
- 33.Lemieuz G, de Graffenried C, Bertozzi C. A fluoreogenic dye activated by the Staudinger ligation. J Am Chem Soc. 2003;125:4708. doi: 10.1021/ja029013y. [DOI] [PubMed] [Google Scholar]
- 34.Stabler CL, Sun XL, Cui W, Wilson JT, Haller CA, Chaikof EL. Surface re-engineering of pancreatic islets with recombinant azido-thrombomodulin. Bioconjug Chem. 2007;18:1713. doi: 10.1021/bc7002814. [DOI] [PubMed] [Google Scholar]
- 35.Saxon E, Bertozzi CR. Cell surface engineering by a modified Staudinger reaction. Science. 2000;287:2007. doi: 10.1126/science.287.5460.2007. [DOI] [PubMed] [Google Scholar]
- 36.Cruise GM, Hegre OD, Lamberti FV, Hager SR, Hill R, Scharp DS, Hubbell JA. In vitro and in vivo performance of porcine islets encapsulated in interfacially photopolymerized poly(ethylene glycol) diacrylate membranes. Cell Transplant. 1999;8:293. doi: 10.1177/096368979900800310. [DOI] [PubMed] [Google Scholar]
- 37.Weber LM, He J, Bradley B, Haskins K, Anseth KS. PEG-based hydrogels as an in vitro encapsulation platform for testing controlled beta-cell microenvironments. Acta Biomater. 2006;2:1. doi: 10.1016/j.actbio.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 38.Gattas-Asfura KM, Stabler CL. Chemoselective cross-linking and functionalization of alginate via Staudinger ligation. Biomacromolecules. 2009;10:3122. doi: 10.1021/bm900789a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Darrabie MD, Kendall WF, Opara EC. Effect of alginate composition and gelling cation on microbead swelling. J Microencapsul. 2006;23:613. doi: 10.1080/02652040600687621. [DOI] [PubMed] [Google Scholar]
- 40.Lilla V, Webb G, Rickenbach K, Maturana A, Steiner DF, Halban PA, Irminger J-C. Differential Gene Expression in Well-Regulated and Dysregulated Pancreatic {beta}-Cell (MIN6) Sublines. 2003;144:1368. doi: 10.1210/en.2002-220916. [DOI] [PubMed] [Google Scholar]
- 41.Pileggi A, Molano RD, Ricordi C, Zahr E, Collins J, Valdes R, Inverardi L. Reversal of diabetes by pancreatic islet transplantation into a subcutaneous, neovascularized device. Transplantation. 2006;81:1318. doi: 10.1097/01.tp.0000203858.41105.88. [DOI] [PubMed] [Google Scholar]
- 42.Lim F, Sun A. Microencapsulated islets as bioartificial endocrine pancreas. Science. 1980;210:908. doi: 10.1126/science.6776628. [DOI] [PubMed] [Google Scholar]
- 43.Strand BL, Morch YA, Syvertsen KR, Espevik T, Skjak-Braek G. Microcapsules made by enzymatically tailored alginate. J Biomed Mater Res A. 2003;64:540. doi: 10.1002/jbm.a.10337. [DOI] [PubMed] [Google Scholar]
- 44.Crank J. The mathematics of diffusion: JW Arrowsmith Ltd. 1975 [Google Scholar]
- 45.Carslaw H, Jaeger J. Conduction of Heat in Solids: Clarendon. 1959 [Google Scholar]
- 46.Ranuncoli A, Cautero N, Ricordi C, Masetti M, Molano RD, Inverardi L, Alejandro R, Kenyon NS. Islet cell transplantation: in vivo and in vitro functional assessment of nonhuman primate pancreatic islets. Cell Transplant. 2000;9:409. [PubMed] [Google Scholar]
- 47.Tanaka P, Matsumura M, V IA. Diffusion characteristics of substrates in ca-alginate gel beads. Biotechnol Bioeng. 1984;26:53. doi: 10.1002/bit.260260111. [DOI] [PubMed] [Google Scholar]
- 48.Merchant F, Margaritis A, Wallace J. A Novel Technique for Measuring Solute Diffusivity in Entrapment Matrices Used in Immobilization. Biotechnol Bioeng. 1987;30:936. doi: 10.1002/bit.260300804. [DOI] [PubMed] [Google Scholar]
- 49.Hannoun B, Stephanopoulos G. Diffusion coefficients of glucose and ethanol in cell-free and cell-occupired calcium alginate membranes. Biotechnol Bioeng. 1986;28:829. doi: 10.1002/bit.260280609. [DOI] [PubMed] [Google Scholar]
- 50.Estape D, Godia F, Sola C. Determination of glucose and ethanol effective diffusion coefficients in Ca-alginate gel. Enzyme Microb Technol. 1992;14:396. doi: 10.1016/0141-0229(92)90009-d. [DOI] [PubMed] [Google Scholar]
- 51.Longsworth L. Diffusion Measurements at 25C of Aqueous Solutions of Amino Acids, Peptides and Sugars. J Am Chem Soc. 1953;75:5705. [Google Scholar]
- 52.Weber LM, Cheung CY, Anseth KS. Multifunctional pancreatic islet encapsulation barriers achieved via multilayer PEG hydrogels. Cell Transplant. 2008;16:1049. [PubMed] [Google Scholar]
- 53.De Vos P, De Haan B, Pater J, Van Schilfgaarde R. Association Between Capsule Diameter, Adequacy of Encapsulation, and Survival of Microencapsulated Rat Islet Allografts1. Transplantation. 1996;62:893. doi: 10.1097/00007890-199610150-00004. [DOI] [PubMed] [Google Scholar]
- 54.Crescenzi V, Cornelio L, Di Meo C, Nardecchia S, Lamanna R. Novel Hydrogels via Click Chemistry: Synthesis and Potential Biomedical Applications. Biomacromolecules. 2007;8:1844. doi: 10.1021/bm0700800. [DOI] [PubMed] [Google Scholar]
- 55.Antoni P, Nystrom D, Hawker CJ, Hult A, Malkoch M. A chemoselective approach for the accelerated synthesis of well-defined dendritic architectures. Chem Commun. 2007:2249. doi: 10.1039/b703547k. [DOI] [PubMed] [Google Scholar]
- 56.Ossipov DA, Hilborn J. Poly(vinyl alcohol)-Based Hydrogels Formed by “Click Chemistry”. Macromolecules. 2006;39:1709. [Google Scholar]
- 57.DeForest CA, Polizzotti BD, Anseth KS. Sequential click reactions for synthesizing and patterning three-dimensional cell microenvironments. Nat Mater. 2009;8:659. doi: 10.1038/nmat2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hu B-H, Su J, Messersmith PB. Hydrogels Cross-Linked by Native Chemical Ligation. Biomacromolecules. 2009;10:2194. doi: 10.1021/bm900366e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Su J, Hu B-H, Lowe WL, Jr, Kaufman DB, Messersmith PB. Anti-inflammatory peptide-functionalized hydrogels for insulin-secreting cell encapsulation. Biomaterials. 2010;31:308. doi: 10.1016/j.biomaterials.2009.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]