Abstract
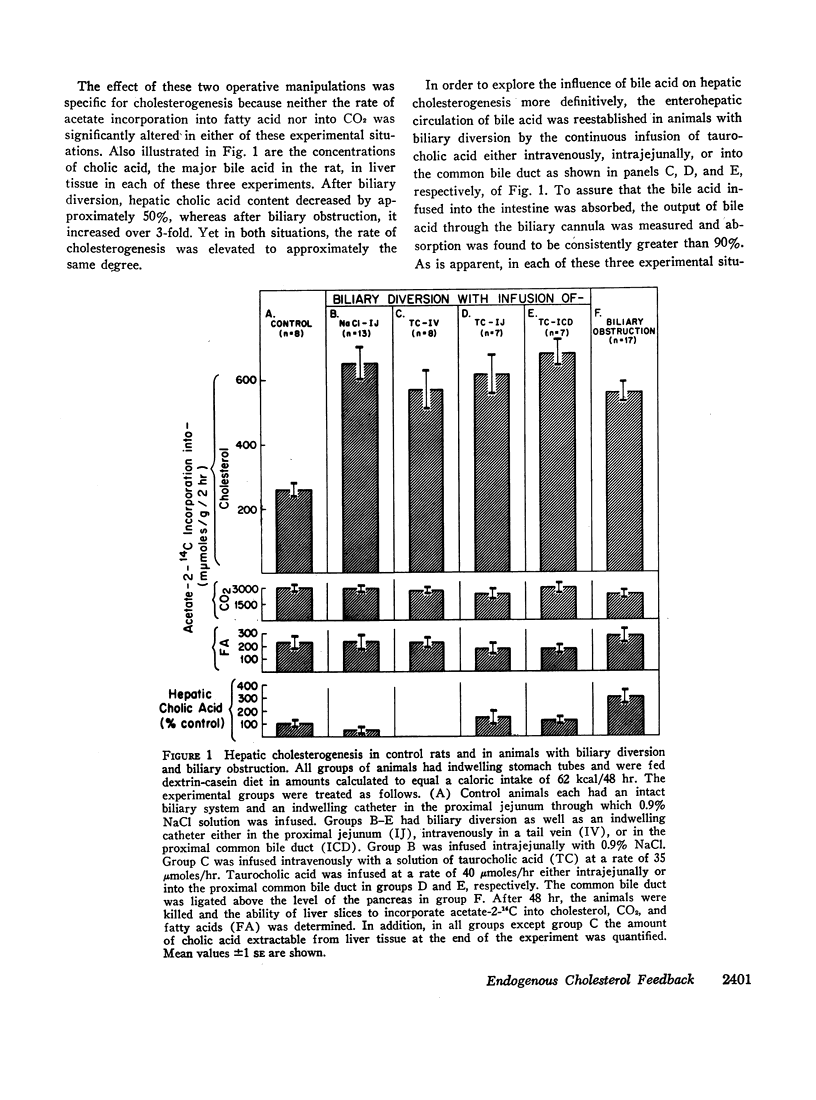
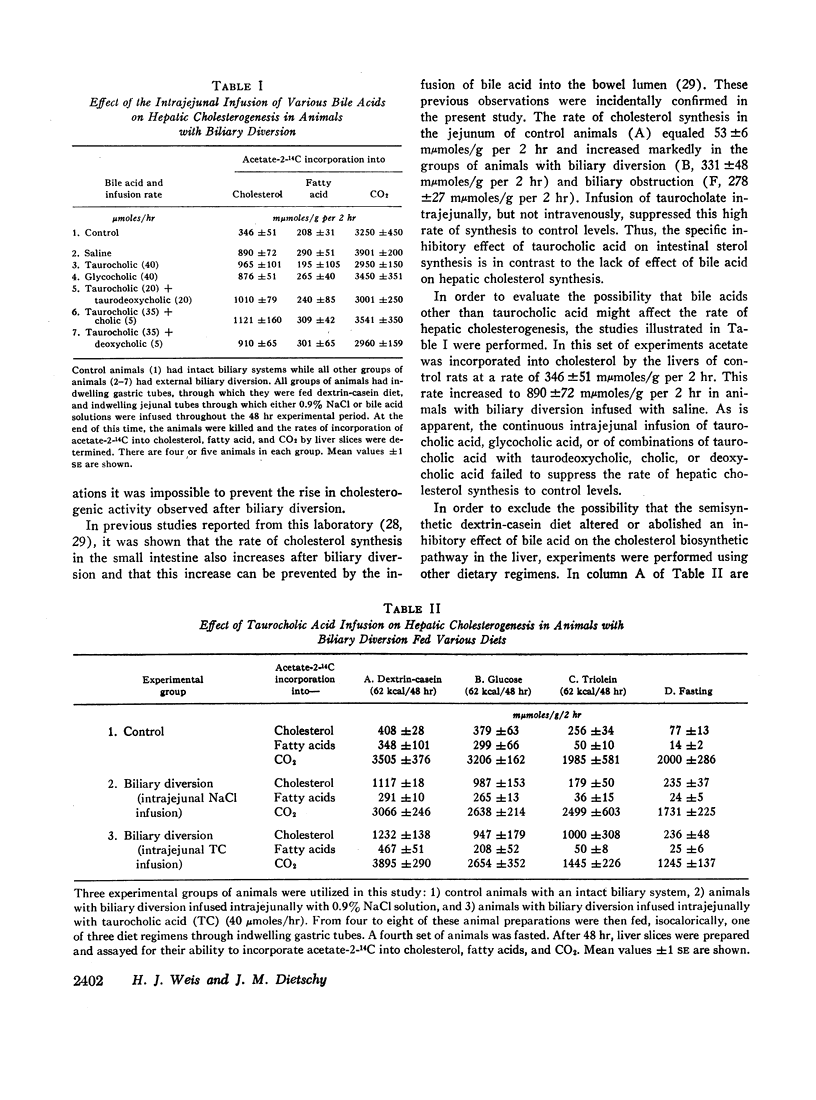
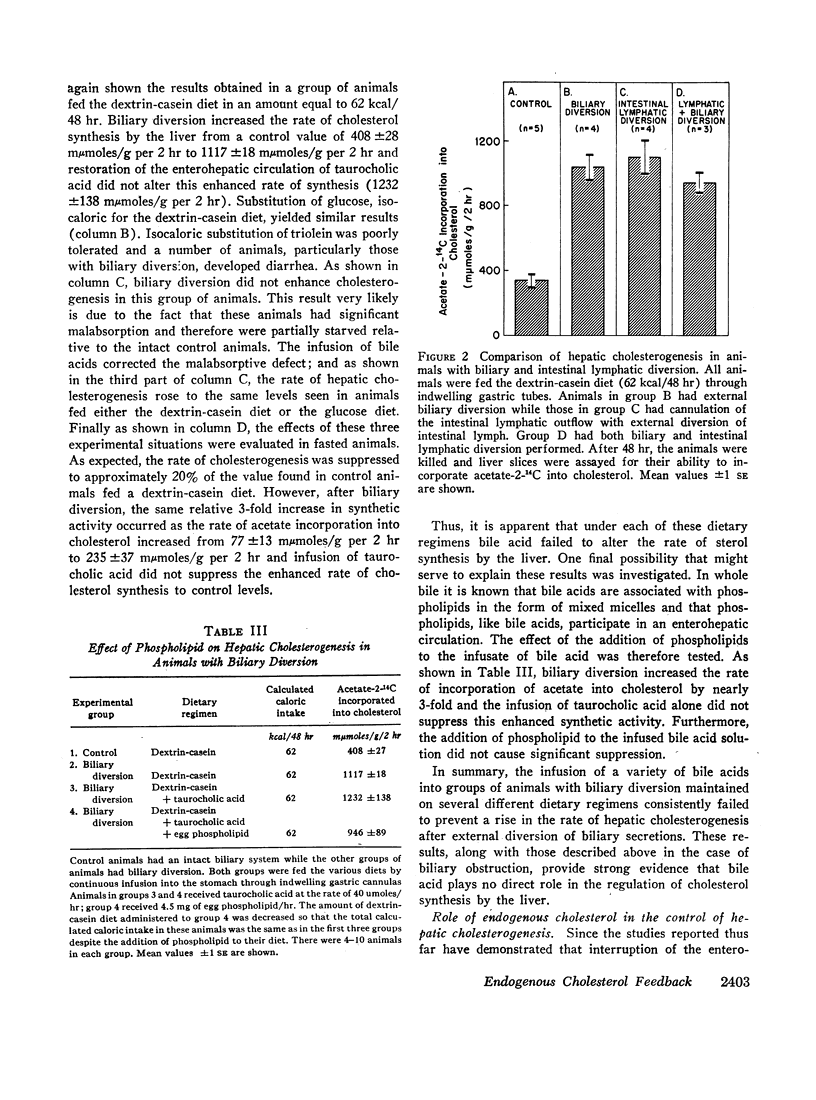
Studies were undertaken to define the role of bile acids in the control of hepatic cholesterogenesis from acetate. Both biliary diversion and biliary obstruction increase the rate of sterol synthesis by the liver 2.5- to 3-fold. After biliary diversion, however, the bile acid content of the liver is decreased, whereas after biliary obstruction, it is markedly increased. Thus, there is no relationship between the tissue content of bile acid and the rate of hepatic cholesterol synthesis. Furthermore, restoration of the enterohepatic circulation of bile acid in animals with biliary diversion fails to prevent the rise in synthetic activity seen after this manipulation. These data indicate that bile acid plays no direct inhibitory role in the regulation of cholesterol synthesis by the liver.
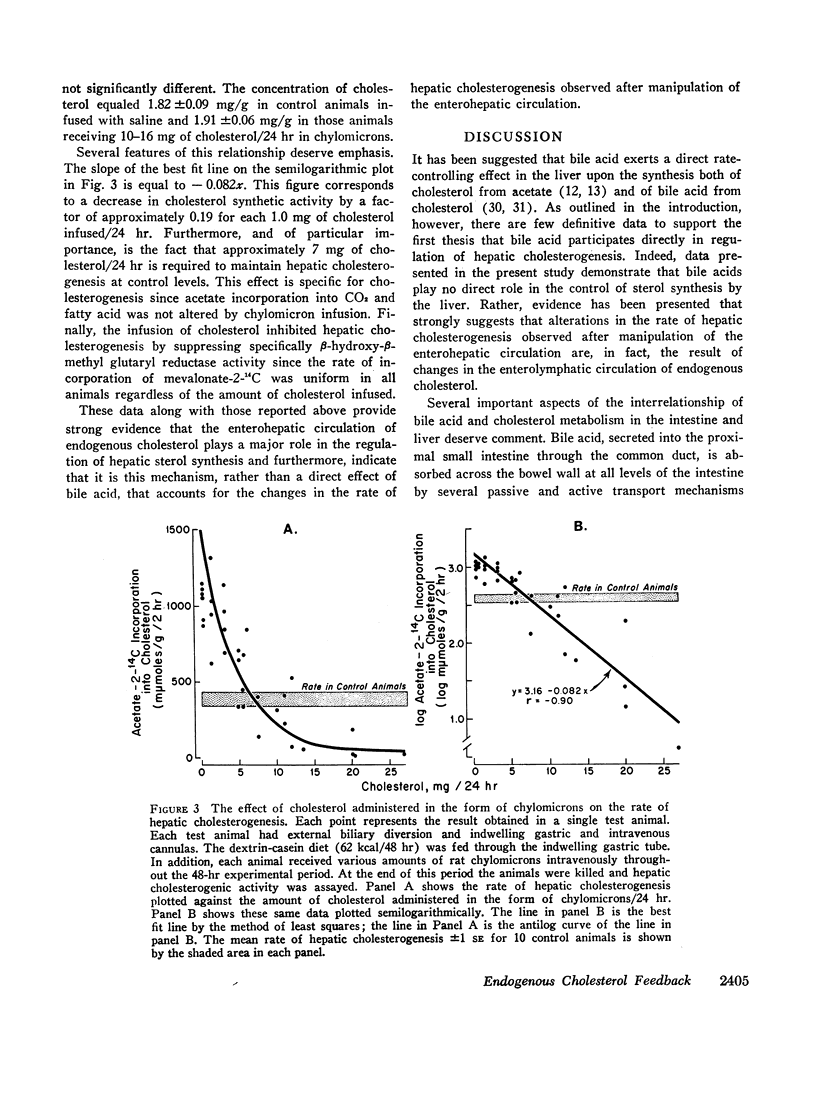
Other experiments were therefore undertaken to evaluate the possibility that changes in cholesterogenic activity observed after manipulation of the enterohepatic circulation of bile acid actually are the result of changes in the enterolymphatic circulation of cholesterol. In support of this thesis it was found that intestinal lymphatic diversion causes the same specific enhancement of cholesterol synthetic activity as biliary diversion and that both of these operative procedures increase enzymatic activity at the step mediated by β-hydroxy-β-methyl glutaryl reductase. Furthermore, the increase in the rate of sterol synthesis by the liver seen in animals with biliary diversion can be prevented by the infusion of approximately 7 mg of cholesterol/24 hr in the form of chylomicrons. This is an amount of cholesterol circulating normally in the enterolymphatic circulation of the intact rat.
These results indicate that bile acid plays no direct role in the control of hepatic cholesterogenesis, but rather, it is the enterohepatic circulation of endogenous cholesterol that determines directly the rate at which cholesterol is synthesized by the liver.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEHER W. T., BAKER G. D. Build-up and regression of inhibitory effects of cholic acid on in vivo liver cholesterol synthesis. Proc Soc Exp Biol Med. 1959 Jun;101(2):214–217. doi: 10.3181/00379727-101-24887. [DOI] [PubMed] [Google Scholar]
- BUCHER N. L., OVERATH P., LYNEN F. beta-Hydroxy-beta-methyl-glutaryl coenzyme A reductase, cleavage and condensing enzymes in relation to cholesterol formation in rat liver. Biochim Biophys Acta. 1960 Jun 3;40:491–501. doi: 10.1016/0006-3002(60)91390-1. [DOI] [PubMed] [Google Scholar]
- CHAIKOFF I. L., BLOOM B., SIPERSTEIN M. D., KIYASU J. Y., REINHARDT W. O., DAUBEN W. G., EASTHAM J. F. C14 cholesterol. I. Lymphatic transport of absorbed cholesterol-4-C14. J Biol Chem. 1952 Jan;194(1):407–412. [PubMed] [Google Scholar]
- Danielsson H., Einarsson K., Johansson G. Effect of biliary drainage on individual reactions in the conversion of cholesterol to taurochlic acid. Bile acids and steroids 180. Eur J Biochem. 1967 Jul;2(1):44–49. doi: 10.1111/j.1432-1033.1967.tb00103.x. [DOI] [PubMed] [Google Scholar]
- Dietschy J. M. Effects of bile salts on intermediate metabolism of the intestinal mucosa. Fed Proc. 1967 Nov-Dec;26(6):1589–1598. [PubMed] [Google Scholar]
- Dietschy J. M. Mechanisms for the intestinal absorption of bile acids. J Lipid Res. 1968 May;9(3):297–309. [PubMed] [Google Scholar]
- Dietschy J. M., Salomon H. S., Siperstein M. D. Bile acid metabolism. I. Studies on the mechanisms of intestinal transport. J Clin Invest. 1966 Jun;45(6):832–846. doi: 10.1172/JCI105399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietschy J. M., Siperstein M. D. Cholesterol synthesis by the gastrointestinal tract: localization and mechanisms of control. J Clin Invest. 1965 Aug;44(8):1311–1327. doi: 10.1172/JCI105237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietschy J. M. The role of bile salts in controlling the rate of intestinal cholesterogenesis. J Clin Invest. 1968 Feb;47(2):286–300. doi: 10.1172/JCI105725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietschy J. M., Wilson J. D. Cholesterol synthesis in the squirrel monkey: relative rates of synthesis in various tissues and mechanisms of control. J Clin Invest. 1968 Jan;47(1):166–174. doi: 10.1172/JCI105706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECKLES N. E., TAYLOR C. B., CAMPBELL D. J., GOULD R. G. The origin of plasma cholesterol and the rates of equilibration of liver, plasma, and erythrocyte cholesterol. J Lab Clin Med. 1955 Sep;46(3):359–371. [PubMed] [Google Scholar]
- ECONOMOU S. G., TEWS B. J., TAYLOR C. B., COX G. E. Studies on lipid metabolism in dogs with altered biliary physiology. Surg Forum. 1957;8:218–221. [PubMed] [Google Scholar]
- FREDRICKSON D. S., LOUD A. V., HINKELMAN B. T., SCHNEIDER H. S., FRANTZ I. D., Jr The effect of ligation of the common bile duct on cholesterol synthesis in the rat. J Exp Med. 1954 Jan 1;99(1):43–53. doi: 10.1084/jem.99.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRIEDMAN M., BYERS S. O., MICHAELIS F. Production and excretion of cholesterol in mammals. Iv. Role of liver in restoration of plasma cholesterol after experimentally induced hypocholesteremia. Am J Physiol. 1951 Mar;164(3):789–791. doi: 10.1152/ajplegacy.1951.164.3.789. [DOI] [PubMed] [Google Scholar]
- HUFF J. W., GILFILLAN J. L., HUNT V. M. EFFECT OF CHOLESTYRAMINE, A BILE ACID-BINDING POLYMER ON PLASMA CHOLESTEROL AND FECAL BILE ACID EXCRETION IN THE RAT. Proc Soc Exp Biol Med. 1963 Nov;114:352–355. doi: 10.3181/00379727-114-28674. [DOI] [PubMed] [Google Scholar]
- LACK L., WEINER I. M. In vitro absorption of bile salts by small intestine of rats and guinea pigs. Am J Physiol. 1961 Feb;200:313–317. doi: 10.1152/ajplegacy.1961.200.2.313. [DOI] [PubMed] [Google Scholar]
- LINDSEY C. A., Jr, WILSON J. D. EVIDENCE FOR A CONTRIBUTION BY THE INTESTINAL WALL TO THE SERUM CHOLESTEROL OF THE RAT. J Lipid Res. 1965 Apr;6:173–181. [PubMed] [Google Scholar]
- McGarry J. D., Foster D. W. Ketogenesis and cholesterol synthesis in normal and neoplastic tissues of the rat. J Biol Chem. 1969 Aug 10;244(15):4251–4256. [PubMed] [Google Scholar]
- Moutafis C. D., Myant N. B. Increased hepatic synthesis of cholesterol after ileal by-pass in monkeys. Clin Sci. 1968 Jun;34(3):541–548. [PubMed] [Google Scholar]
- SEITZ W., von BRAND [The effects of conjugated bile acids on the synthesis of fatty acids and cholesterol in the liver]. Klin Wochenschr. 1961 Sep 1;39:891–895. doi: 10.1007/BF01481690. [DOI] [PubMed] [Google Scholar]
- SIPERSTEIN M. D., FAGAN V. M. DELETION OF THE CHOLESTEROL-NEGATIVE FEEDBACK SYSTEM IN LIVER TUMORS. Cancer Res. 1964 Aug;24:1108–1115. [PubMed] [Google Scholar]
- SIPERSTEIN M. D., GUEST M. J. Studies on the site of the feedback control of cholesterol synthesis. J Clin Invest. 1960 Apr;39:642–652. doi: 10.1172/JCI104079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPERRY W. M., WEBB M. A revision of the Schoenheimer-Sperry method for cholesterol determination. J Biol Chem. 1950 Nov;187(1):97–106. [PubMed] [Google Scholar]
- Simmonds W. J., Hofmann A. F., Theodor E. Absorption of cholesterol from a micellar solution: intestinal perfusion studies in man. J Clin Invest. 1967 May;46(5):874–890. doi: 10.1172/JCI105587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siperstein M. D., Fagan V. M., Dietschy J. M. A gas-liquid chromatographic procedure for the measurement of mevalonic acid synthesis. J Biol Chem. 1966 Feb 10;241(3):597–601. [PubMed] [Google Scholar]
- Siperstein M. D., Fagan V. M. Feedback control of mevalonate synthesis by dietary cholesterol. J Biol Chem. 1966 Feb 10;241(3):602–609. [PubMed] [Google Scholar]
- Siperstein M. D., Fagan V. M., Morris H. P. Further studies on the deletion of the cholesterol feedback system in hepatomas. Cancer Res. 1966 Jan;26(1):7–11. [PubMed] [Google Scholar]
- TOMKINS G. M., CHAIKOFF I. L. Cholesterol synthesis by liver. I. Influence of fasting and of diet. J Biol Chem. 1952 May;196(2):569–573. [PubMed] [Google Scholar]
- Wilson J. D. Biosynthetic origin of serum cholesterol in the squirrel monkey: evidence for a contribution by the intestinal wall. J Clin Invest. 1968 Jan;47(1):175–187. doi: 10.1172/JCI105707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J. D., Reinke R. T. Transfer of locally synthesized cholesterol from intestinal wall to intestinal lymph. J Lipid Res. 1968 Jan;9(1):85–92. [PubMed] [Google Scholar]


