Summary
Breast cancer-associated mortality has been significantly reduced since the 1990s, mainly because of early diagnosis and systemic therapeutic interventions. All three therapy components – cytostatic therapy, endocrine therapy and targeted antibody therapy – are at present necessary tools for the curative treatment of primary breast cancer. This article reviews the evidence base for the use of various chemotherapy schedules in patients with primary, node-positive breast cancer, including schedules in combination with targeted HER2/neu therapy.
Key Words: Chemotherapy, Prognosis, Recurrence, Breast cancer, Controversies, Taxanes, Anthracyclines, Trastuzumab
Zusammenfassung
Die Sterblichkeitsrate bei Brustkrebs konnte vor allem dank der Früherkennung und systemischer Therapiemaßnahmen seit den 1990er Jahren signifikant gesenkt werden. Alle drei Therapiesäulen, bestehend aus zytostatischer Therapie, endokriner Therapie und zielgerichteter Antikörpertherapie, sind derzeit notwendige Instrumente zur kurativen Therapie des primären Mammakarzinoms. Dieser Artikel fasst die Evidenzbasis für den Einsatz verschiedener Chemotherapieschemata bei Patientinnen mit einem primären, nodalpositiven Mammakarzinom, auch in Zusammenhang mit einer zielgerichteten HER2/ neu-Therapie, zusammen.
Introduction
The understanding of breast cancer as a primarily local disease has undergone a fundamental change in the last few decades. While throughout most of the previous century breast cancer was still mainly conceived of as a predominantly local disease [1, 2], the radical nature of surgery can now be reduced to a minimum by the use of breast-conserving procedures [3,4,5] and axillary sentinel lymph node excision [6]. The systemic use of cytostatic and endocrine therapeutic modalities has simultaneously provided a major overall survival benefit for patients treated in accordance with current therapeutic standards [7,8,9,10,11].
The therapy recommendations for the use of conventional and dose-dense chemotherapy and trastuzumab have not essentially changed following the St. Gallen Consensus Conference 2007, and are basically in conformity with the S3 guidelines of the German Cancer Society and the German guidelines of the AGO Organkommission Mamma (Working Group for Gynecological Oncology in the Breast Organ Committee), which can be downloaded in detail from http://www.ago-online.org.
Chemotherapy: Anthracycline Therapy versus Taxane Therapy
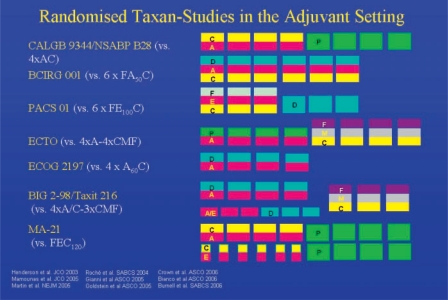
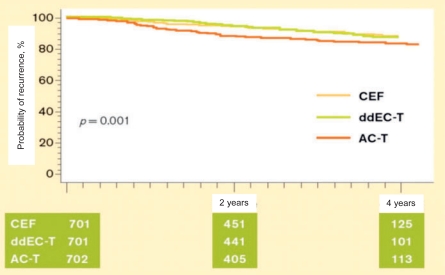
Up to a few years ago, adjuvant standard chemotherapy comprised threefold anthracycline combinations (≥ 30 mg epirubicin/week) for 6 cycles, which was shown to be superior to first-generation chemotherapy regimens (e.g. cyclophosphamide/methotrexate/fluorouracil (CMF) or doxorubicin (adriamycin)/cyclophosphamide (AC)) [11]. However, the results of a good half dozen randomized studies on the use of taxanes in adjuvant therapy of node-positive breast cancer suggest almost without exception that the use of taxanes can improve survival by another 3-6% compared to pure anthracycline therapy (fig. 1). Considered altogether, these data justify the AGO guidelines proposing taxane-containing schedules as a therapeutic option for the adjuvant treatment of node-positive patients. The heterogeneity of the available research findings pertaining to the role of taxanes in the adjuvant therapy of breast cancer, however, still leaves many aspects unresolved. Besides the question as to which patients benefit most from the use of taxanes, the scheduling of the group of substances is still the subject of scientific debate. Sequential taxane therapy appears to have become more established in recent years. The Taxit 216 study of A. Bianco was presented at the American Society of Clinical Oncology (ASCO) 2006 meeting (Abstract no. 520); in this study 972 patients were treated either with 4 cycles of epirubicin 120 mg/m2 q3w followed by 4 cycles of CMF (E-CMF), or with the same schedule plus 4 cycles of docetaxel 100 mg/m2 q3w (E-T-CMF). The interim analysis was performed after a median follow-up of 53 months. The addition of taxane was observed to lead to a reduction in the risk of relapse of 21% (p = 0.05) and in the mortality risk of 28% (not significant). In the BIG 2-98 study presented by J. Crown, sequential taxane therapy with A-T-CMF also provided a significant improvement in relapse-free survival compared to A-CMF (p = 0.035, LBA no. 519), while a combination with AT-CMF did not. At the San Antonio Breast Cancer Symposium (SABCS) 2006, however, the data of the MA-21 study performed to compare FEC120, 4×AC-4xpaclitaxel and a dose-dense anthracycline taxane schedule were greeted with great surprise. While the dose-dense schedule and FEC120 were equally effective, the conventional AC-paclitaxel arm proved inferior both to the FEC120 (p = 0.005) and the dose-dense schedule (p = 0.0006) (fig. 2).
Fig. 1.
Overview of prospective randomized studies comparing adjuvant anthracycline-based and taxane-based chemotherapies in breast cancer.
Fig. 2.
Kaplan-Meier analysis of relapse-free survival in the MA-21 study [20]
Two of the main presentations at the SABCS 2007 were devoted to assessing the value of taxanes as adjuvant therapy. In the TACT Trial, 4162 patients were randomized between anthracycline-based chemotherapy (8×FE60C or E-CMF) and taxane-based chemotherapy (4xFE60C, followed by 4xdocetaxel100). Astonishingly, the use of taxane was not found to provide demonstrable benefit either in terms of relapse-free survival (hazard ratio (HR) 0.67, p = 0.62) or overall survival (HR = 0.98, p = 0.76).
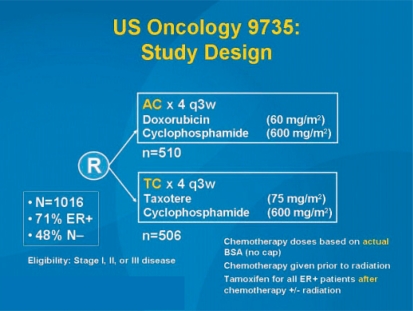
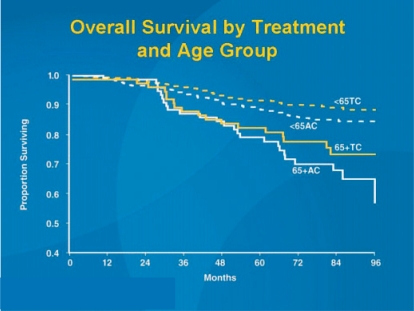
In another study by Jones et al. (US Oncology 9735), adjuvant chemotherapy with 4 cycles of AC was compared with 4 docetaxel 75-cyclophosphamide (fig. 3). While the results of the overall study were already published in 2006 in the Journal of Clinical Oncology [12], the results presented in San Antonio focused on patients aged 65 years or more. As in the overall study, the elderly patients also showed both a significant relapse-free (p = 0.033) and an overall survival benefit (p = 0.032) (fig. 4). However, these results should be interpreted with caution as 4 cycles of AC cannot be regarded as current state of the art. Further studies on anthracycline-free regimens in comparison to modern standard treatment are warranted. Also at SABCS 2007, Sir Richard Peto presented interesting calculation models for the effectiveness of modern taxane-containing chemotherapy. Since modern taxane therapies have only been tested against anthracycline-containing schedules in the adjuvant situation, a direct assessment of the absolute survival benefit provided by this form of chemotherapy has so far not been possible. By statistically combining the individual therapeutic benefits of different chemotherapy schedules, Peto [22] was able to calculate an HR of 0.46 (2p = 0.00001) in the < 50-year age group, i.e. a reduction in the probability of mortality by 54% due to taxane-containing chemotherapy (table 1).
Fig. 3.
US Oncology study 9735: Study flow chart comparing anthracycline-based versus taxane-based chemotherapy [12].
Fig. 4.
US Oncology study 9735: Survival data SABCS 2007 comparing anthracycline-based versus taxane-based chemotherapy [21].
Table 1.
EBCTCG data for approximation of chemotherapy benefit in patients under 50 years with primary breast cancer [22]
| Treatment | HR recurrence years 0–4 | HR breast cancer mortality |
|---|---|---|
| CMF vs. no CT | 0.56 (0.05) | 0.68 (0.05) |
| Anthracyclines vs. CMF | 0.84 (0.05) | 0.81 (0.05) |
| Taxanes vs. anthracyclines | 0.84 (0.04) | 0.86 (0.05) |
| Taxanes vs. no CI (multiplication of 3 RRs) | 0.38 (0.07) | 0.46 (0.08) |
| 2p < 0.00001 | 2p < 0.00001 |
RR = Relative risk.
At the annual ASCO conference 2008, two further studies were presented which complete the overall picture for the comparison between anthracycline-containing schedules with or without taxanes. In the Spanish GEICAM study, 6 cycles of TAC (75/50/500 mg/m2) were compared with 6 cycles of FAC (500/50/500 mg/m2) in patients with node-negative breast cancer. This study confirmed a significant benefit in terms of relapse-free survival (p = 0.02), but not of overall survival. In the German WSG/AGO study, on the other hand, Prof. Dr. U. Nitz was able to demonstrate both a relapse-free (HR 1.77, 95% confidence interval (CI): 1.28–2.46) and an incipient overall survival benefit in patients with 1–3 axillary lymph node metastases. Guidelines will now have to define whether taxane-based regimens should not only be used in node-positive but also in node-negative breast cancer (www.ago-online.org).
Is the Use of Anthracyclines Necessary in Patients with HER2/neu-Negative Breast Cancer?
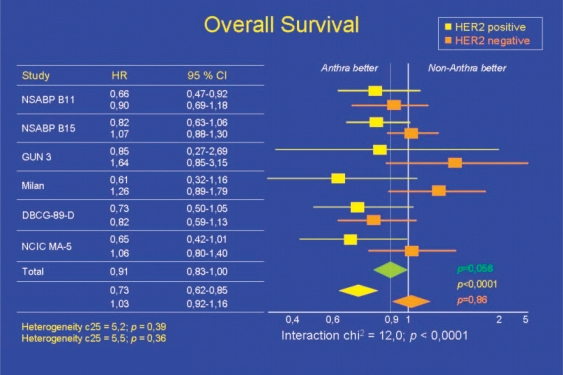
The debate surrounding the need to use anthracyclines in patients with HER2/neu-negative breast cancer was again conducted vigorously at ASCO 2008. The opening meeting of ASCO 2008 devoted to breast cancer addressed the question whether anthracycline-based chemotherapy still constitutes a modern therapeutic approach to HER2/neu-negative breast cancer. At the meeting chaired by Prof. Eric Winer of the Dana-Farber Cancer Institute, this subject was considered from clinical, tumor biological and molecular biological perspectives. Prof. K. Gelmon discussed the survival benefits provided by anthracyclines based on a review of previous anthracycline studies. However, she also reported on the meta-analysis recently published by Gennari et al. in the Journal of the National Cancer Institute (JNCI) which demonstrates that anthracyclines show no superiority in HER2/neu-negative tumors (fig. 5) [13].
Fig. 5.
Meta-analysis of anthracycline efficacy in relation to HER2/neu status [13].
Prof. A. di Leo from Florence emphasized that no topoisomerase IIα overexpression is usually detectable in HER2/neu negative breast cancer and that the target for anthracyclines is therefore absent. The US Oncology 9735 study, which compared 4 cycles of AC with 4 docetaxel 75-cyclophosphamide, was also discussed again. Prof. E. Winer then summarized the undesirable effects of the anthracyclines with reference to several review articles. A risk of long-term myocardial insufficiency of about 2% is generally to be expected, but is higher in elderly patients. Studies to re-assess the value of the anthracyclines are currently being planned both in the USA and in Germany.
Conventional Dosage versus Dose-Dense Chemotherapy or High-Dose Chemotherapy?
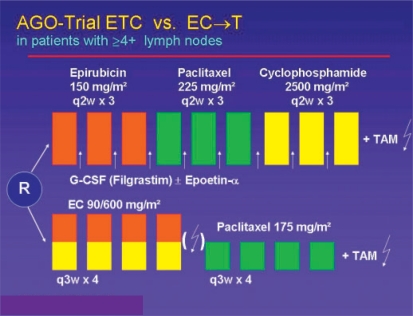
The results of two randomized studies suggest that increasing the dose density, i.e. shortening the time intervals between the cycles, has a positive effect on therapeutic efficacy. An American Intergroup Study and the German ETC study showed that dose-dense treatment resulted in a significant reduction in the risk of relapse [14, 15]. At ASCO 2004, the first survival analysis of the German AGO ETC study was presented by V. Möbus (fig. 6) [16]. This study tested a modified Henderson schedule (4 cycles of EC 90/600 followed by 4 cycles of paclitaxel 175) versus the dose-dense and dose-intensified ETC schedule (3 cycles of epirubicin 150 followed by 3 cycles of paclitaxel 225 and 3 cycles of cyclophosphamide 2500). The data of 1284 patients confirmed the expected high myelotoxicity of the ETC schedule, with a Grade 4 leukopenia rate of 46% despite prophylactic administration of granulocyte colony-stimulating factor (G-CSF). The rate of febrile neutropenia, however, was within a tolerable range at 7%. Without primary prophylactic administration of epoietin-α 28% of the patients, and with epoietin still 12% of the patients, required transfusions because of the ETC chemotherapy. After a 28-month follow-up, a statistically significant advantage was seen in favor of the ETC schedule, both in terms of relapse-free survival (p = 0.0009) and overall survival (p = 0.039). At SABCS 2006, the research group was then able to present the second interim analysis. After a median follow-up of 5.2 years, the significant relapse-free survival benefit in favor of the ETC therapy arm was confirmed with an HR of 0.72 (p = 0.0008). Similar results were produced by the comparison of overall survival (HR 0.76, p = 0.029) [16]. This database was used to design the German GAIN study, which is at present actively recruiting.
Fig. 6.
Study design of the German ETC study on the efficacy of dose-dense/dose-intensive chemotherapy in breast cancer [16].
At SABCS 2005, an update of the American Intergroup Trials INT C9741 was presented which, although still showing a highly significant relapse-free survival benefit for chemotherapy in the 2-week versus the 3-week interval (p = 0.012), also showed a significant but unexpectedly limited overall survival benefit (p = 0.049). Overall, the results suggest that increasing the dose density provides a potential benefit by increasing the dosage in the era of anthracycline and taxane therapy without, however, establishing a new therapy standard at the present time because of the associated toxicity.
At the St. Gallen Consensus Conference 2007 there was no majority in favor of recommending dose-dense therapy, although the convincing data of the German ETC study mentioned above were not discussed. For patients with HER2/neu overexpression in the tumor, dose-dense administration of E/AC-T (32.4%) or FEC-D (36.1%) still received the highest approval rates. A similar pattern was seen for negative HER2/ neu status: dose-dense administration of E/AC-T (36.4%) or FEC-D (25.0%).
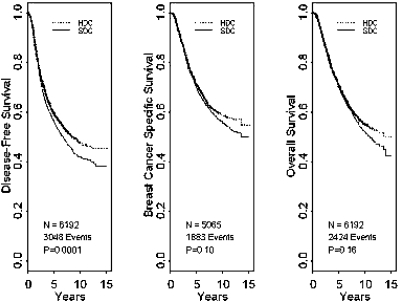
In a meta-analysis of the effectiveness of adjuvant high-dose therapy presented by Berry et al. at the SABCS 2007, the data of 6210 patients from 15 studies were evaluated. Although a disease-free survival benefit with an HR of 0.85 (95% CI 0.81–0.94; p = 0.0001) could be significantly improved by the use of high-dose chemotherapy in the total population, overall survival (p = 0.16) was uninfluenced (fig. 7). Furthermore, in the subgroup analysis, no population could be defined that had benefited more from the use of high-dose chemotherapy. A recommendation for high-dose chemotherapy with stem cell support was already unanimously rejected at the St. Gallen Consensus Conference 2007.
Fig. 7.
Survival curves for adjuvant use of high-dose chemotherapy in a meta-analysis [23].
Antibody Therapy: Soon Herceptin for All?
Targeted humoral therapy with the antibody trastuzumab is probably the most cost intensive but for certain patients also the most effective innovation in the systemic therapy of breast cancer in association with chemotherapy.
The benefits of the adjuvant use of trastuzumab and the cardiotoxicity known to be associated with its use in metastatic breast cancer must be evaluated critically against this background. At ASCO 2005, data on the use of trastuzumab in the primary management of early breast cancer were presented for the first time. A combined analysis of the two American studies NSABP B31 and NCCTG N9831 evaluated therapy with AC followed by paclitaxel, with or without extension of therapy with trastuzumab over 1 year. The use of trastuzumab resulted in a highly impressive, subgroup-independent improvement in relapse-free survival in HER2/neu-positive patients (immunohistochemistry (IHC) +++ or fluorescent in situ hybridization (FISH) +) of 67–85% after 4 years and, after a median follow-up of only 2 years, in an incipiently significant improvement in overall survival (p = 0.015) [17].
In the global HERA study with 4482 patients presented by M. Piccart, a highly significant, absolute benefit in relapse-free survival of 12% after the use of trastuzumab for 1 year was observed after a median follow-up of only 1 year (HR 0.54, 95% CI 0.43–0.67) [18]. This benefit was also seen in all sub-groups, although no overall survival benefit could (yet) be demonstrated. In an update of the HERA study presented by I. Smith at ASCO 2006, the results of ASCO 2005 were not only reproduced, but for the first time a significant overall survival benefit was also demonstrated. The risk of cancer-associated mortality was reduced by 34% (3-year overall survival 92.4% versus 89.7%, HR 0.66, p = 0.012).
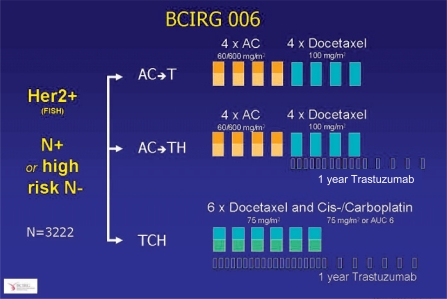
The BCIRG 006 study for the first time evaluated the efficacy of trastuzumab in association with anthracycline-free chemotherapy. In this three-armed study, 3222 patients with primary breast cancer were randomized between an adjuvant therapy with 4 cycles of AC followed by 4 cycles of docetaxel with (AC-TH) or without trastuzumab (AC-T), or a combination of docetaxel with carboplatin and trastuzumab (TCH) (fig. 8). After a median follow-up of 36 months, at the SABCS 2006 D. Slamon presented the second interim analysis of the BCIRG 006 which within a three-arm design is evaluating the efficacy of AC-T versus AC-TH versus TCH in primary, HER2/neu-positive breast cancer. The trastuzumab-containing arms AC-TH and TCH showed practically identical efficacy both in terms of relapse-free (77 versus 83% versus 82%) and overall survival (86 versus 92 versus 91%) with a significant advantage compared to trastuzumab-free therapy. This benefit was present independent of nodal status, hormone receptor status and tumor size. In the anthracycline-free arm, however, there were fewer Grade 3/4 toxicities such as neutropenia, gastrointestinal toxicities and neuropathies. Since higher efficacy of anthracyclines associated with coamplification of topoiso-merase IIα, still assumed in the previous year, was not confirmed, TCH represents a new option in the adjuvant therapy of node-positive breast cancer in the presence of HER2/neu overexpression.
Fig. 8.
Study design of the BCIRG 006 study comparing anthracycline-containing and anthracycline-free chemotherapy in combination with trastuzumab therapy [24].
As anticipated, the panel of experts at the St. Gallen Consensus Conference 2007 followed this convincing evidence and voted 91.9% in favor of a 1-year trial of trastuzumab in patients with chemotherapy and a HER2/neu-overexpressing tumor. A 9-week course of therapy following the FinHer study was approved by only 14.3% of the panelists. 92.1% of the experts considered a positive (+++) immunohistochemical demonstration of HER2/neu overexpression sufficient, while FISH testing (FISH for all?) was rejected by 84.2% [19]. The German AGO guidelines clearly recommend trastuzumab treatment for 1 year in HER2/neu-positive patients undergoing chemotherapy.
Summarizing, patients, and only those with a clearly HER2/ neu-overexpressing tumor and an indication for adjuvant chemotherapy, should receive adjuvant therapy with trastuzumab, at present for 12 months. Patients with a significant history of cardiac illness who fulfill the criteria for chemotherapy and trastuzumab therapy should consider anthracycline-free cytostatic therapy with a combination of a taxane, a platinum derivative and trastuzumab.
Conclusions
Chemotherapy with anthracycline/taxane is regarded as the standard treatment for node-positive women. In these cases, either a combination (e.g. TAC with docetaxel) or a sequential therapy schedule (e.g. EC/AC-T with paclitaxel or FEC-DOC with docetaxel) can be used. High dose frequency regimens such as EC-T with 2-week therapy (with paclitaxel) and dose-intensified ETC (with paclitaxel) are, in the hands of experienced physicians, an evidence-based option in node-positive patients, especially with greater nodal involvement. These schedules are not yet approved and should therefore be used primarily in trials or, in specially justified individual cases, reserved for ‘off-label-use’.
Adjuvant 1-year trastuzumab therapy following primary systemic or adjuvant chemotherapy is standard in HER2/neu-positive breast cancer (HER2 3+ or FISH+). It is approved regardless of age, nodal status or time elapsed since primary surgery. Indication should be assessed independent of nodal status or patient age, provided there is no contraindication to the planned therapy. According to the present state of knowledge, therapy can be started together with taxane chemotherapy or implemented completely after chemotherapy.
References
- 1.Halsted WS. The results of radical operation for the cure of carcinoma of the breast. Ann Surg. 1907;46:1–19. doi: 10.1097/00000658-190707000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beatson GT. On the treatment of inoperable cases of carcinoma of the mamma: suggestions for a new method of treatment, with illustrative cases. Lancet. 1896:74. [PMC free article] [PubMed] [Google Scholar]
- 3.Sarrazin D, Lê MG, Arriagada R, Contesso G, Fontaine F, Spielmann M, Rochard F, Le-Chevalier T, Lacour J. Ten-year results of a randomized trial comparing a conservative treatment to mastectomy in early breast cancer. Radiother Oncol. 1989;14:177–184. doi: 10.1016/0167-8140(89)90165-5. [DOI] [PubMed] [Google Scholar]
- 4.van-Dongen JA, Bartelink H, Fentiman IS, Lerut T, Mignolet F, Olthuis G, van-der-Schueren E, Sylvester R, Winter J, van-Zijl K. Randomized clinical trial to assess the value of breast-conserving therapy in stage I and II breast cancer, EORTC 10801 trial. J Natl Cancer Inst Monogr. 1992:15–18. [PubMed] [Google Scholar]
- 5.Veronesi U, Salvadori B, Luini A, Greco M, Saccozzi R, Del-Vecchio M, Mariani L, Zurrida S, Rilke F. Breast conservation is a safe method in patients with small cancer of the breast. Long-term results of three randomised trials on 1,973 patients. Eur J Cancer. 1995;31A:1574–1579. doi: 10.1016/0959-8049(95)00271-j. [DOI] [PubMed] [Google Scholar]
- 6.Veronesi U, Paganelli G, Viale G, Luini A, Zurrida S, Galimberti V, Intra M, Veronesi P, Robertson C, Maisonneuve P, Renne G, De Cicco C, De Lucia F, Gennari R. A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer. N Engl J Med. 2003;349:546–553. doi: 10.1056/NEJMoa012782. [DOI] [PubMed] [Google Scholar]
- 7.Early Breast Cancer Trialists' Collaborative Group Ovarian ablation for early breast cancer. Cochrane Database Syst Rev. 2000 doi: 10.1002/14651858.CD000485. CD000485. [DOI] [PubMed] [Google Scholar]
- 8.Tamoxifen for early breast cancer. Cochrane Database Syst Rev. 2001 doi: 10.1002/14651858.CD000486. CD000486. [DOI] [PubMed] [Google Scholar]
- 9.Early Breast Cancer Trialists' Collaborative Group Multi-agent chemotherapy for early breast cancer. Cochrane Database Syst Rev. 2002 doi: 10.1002/14651858.CD000487. CD000487. [DOI] [PubMed] [Google Scholar]
- 10.Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans E, Godwin J, Gray R, Hicks C, James S, MacKinnon E, McGale P, McHugh T, Peto R, Taylor C, Wang Y. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366:2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 11.Early Breast Cancer Trialists' Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 12.Jones SE, Savin MA, Holmes FA, O'Shaughnessy JA, Blum JL, Vukelja S, McIntyre KJ, Pippen JE, Bordelon JH, Kirby R, Sandbach J, Hyman WJ, Khandelwal P, Negron AG, Richards DA, Anthony SP, Mennel RG, Boehm KA, Meyer WG, Asmar L. Phase III trial comparing doxorubicin plus cyclophosphamide with docetaxel plus cyclophosphamide as adjuvant therapy for operable breast cancer. J Clin Oncol. 2006;24:5381–5387. doi: 10.1200/JCO.2006.06.5391. [DOI] [PubMed] [Google Scholar]
- 13.Gennari A, Sormani MP, Pronzato P, Puntoni M, Colozza M, Pfeffer U, Bruzzi P. HER2 status and efficacy of adjuvant anthracyclines in early breast cancer: a pooled analysis of randomized trials. J Natl Cancer Inst. 2008;100:14–20. doi: 10.1093/jnci/djm252. [DOI] [PubMed] [Google Scholar]
- 14.Citron ML, Berry DA, Cirrincione C, Hudis C, Winer EP, Gradishar WJ, Davidson NE, Martino S, Livingston R, Ingle JN, Perez EA, Carpenter J, Hurd D, Holland JF, Smith BL, Sartor CI, Leung EH, Abrams J, Schilsky RL, Muss HB, Norton L. Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: first report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. J Clin Oncol. 2003;21:1431–1439. doi: 10.1200/JCO.2003.09.081. [DOI] [PubMed] [Google Scholar]
- 15.Möbus V, Untch M, Du Bois A, Lueck HJ, Thomssen C, Kuhn W, Kurbacher C, Nitz U, Kreienberg R, Jackisch Ch: Dose-dense sequential chemotherapy with epirubicin (E), paclitaxel (T) and cyclophosphamide (C) (ETC) is superior to conventional dosed chemotherapy in high-risk breast cancer patients (? 4 +LN). First results of an AGO-trial. Proc ASCO 2004;22.
- 16.Möbus V, Lueck H-J, Thomssen C, Kuhn W, Kurbacher C, Nitz U, Kreienberg R, Untch M, Jackisch C, Schneeweiss A, Huober J, du Bois A. Dose-dense sequential chemotherapy with epirubicin (E), paclitaxel (T) and cyclophosphamide (C) (ETC) in comparison to conventional dosed chemotherapy in high-risk breast cancer patients (4+ LN). Mature results of an AGO-trial. Breast Cancer Res Treat. 2006;70(suppl 1) abstr 43. [Google Scholar]
- 17.Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE, Jr, Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman PA, Swain SM, Pisansky TM, Fehrenbacher L, Kutteh LA, Vogel VG, Visscher DW, Yothers G, Jenkins RB, Brown AM, Dakhil SR, Mamounas EP, Lingle WL, Klein PM, Ingle JN, Wolmark N. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 18.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, Gianni L, Baselga J, Bell R, Jackisch C, Cameron D, Dowsett M, Barrios CH, Steger G, Huang CS, Andersson M, Inbar M, Lichinitser M, Lang I, Nitz U, Iwata H, Thomssen C, Lohrisch C, Suter TM, Ruschoff J, Suto T, Greatorex V, Ward C, Straehle C, McFadden E, Dolci MS, Gelber RD. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 19.Goldhirsch A, Wood W, Gelber R, Coates A, Thurlimann B, Senn HJ, Members P. Progress and promise: highlights of the international expert consensus on the primary therapy of early breast cancer 2007. Ann Oncol. 2007;18:1133–1144. doi: 10.1093/annonc/mdm271. [DOI] [PubMed] [Google Scholar]
- 20.Burnell M, Chapman JA, Levine MN, Pritchard KI. A randomized trial of CEF versus dose dense EC followed by paclitaxel versus AC followed by paclitaxel in women with node positive or high risk node negative breast cancer, NCIC CTG MA.21: Results of an interim analysis. Breast Cancer Res Treat. 2006;70 suppl 1. abstr 53. [Google Scholar]
- 21.Jones SE, Holmes FA, O'Shaughnessy JA, Savin MA. Extended follow-up and analysis by age of the US Oncology Adjuvant trial 9735: docetaxel/cyclophosphamide is associated with an overall survival benefit compared to doxorubicin/cyclophosphamide and is well-tolerated in women 65 or older. Breast Cancer Res Treat. 2007;71(suppl 1) abstr 12. [Google Scholar]
- 22.Peto R. The worldwide overview: new results for systemic adjuvant therapies. Breast Cancer Res Treat. 2007;71(suppl 1) abstr P1. [Google Scholar]
- 23.Berry D, Ueno N, Demirer T. High-dose chemotherapy with autologous stem-cell support versus standard-dose chemotherapy: meta-analysis of individual patient data from 15 randomized adjuvant breast cancer trials. Breast Cancer Res Treat. 2007;71(suppl 1) abstr 1. [Google Scholar]
- 24.Slamon D, Mackey J, Robert N, Crown J, Martin M, Eiermann W, Pienkowski T, Press M. Role of anthracycline-based therapy in the adjuvant treatment of breast cancer: efficacy analyses determined by molecular subtypes of the disease. Breast Cancer Res Treat. 2007;71(suppl 1) abstr 13. [Google Scholar]